Abstract
Over the past two decades, it was discovered that introducing synthetic small interfering RNAs (siRNAs) into the cytoplasm facilitates effective gene-targeted silencing. This compromises gene expression and regulation by repressing transcription or stimulating sequence-specific RNA degradation. Substantial investments in developing RNA therapeutics for disease prevention and treatment have been made. We discuss the application to proprotein convertase subtilisin/kexin type 9 (PCSK9), which binds to and degrades the low-density lipoprotein cholesterol (LDL-C) receptor, interrupting the process of LDL-C uptake into hepatocytes. PCSK9 loss-of-function modifications show significant clinical importance by causing dominant hypocholesterolemia and lessening the risk of cardiovascular disease (CVD). Monoclonal antibodies and small interfering RNA (siRNA) drugs targeting PCSK9 are a significant new option for managing lipid disorders and improving CVD outcomes. In general, monoclonal antibodies are restricted to binding with cell surface receptors or circulating proteins. Similarly, overcoming the intracellular and extracellular defenses that prevent exogenous RNA from entering cells must be achieved for the clinical application of siRNAs. N-acetylgalactosamine (GalNAc) conjugates are a simple solution to the siRNA delivery problem that is especially suitable for treating a broad spectrum of diseases involving liver-expressed genes. Inclisiran is a GalNAc-conjugated siRNA molecule that inhibits the translation of PCSK9. The administration is only required every 3 to 6 months, which is a significant improvement over monoclonal antibodies for PCSK9. This review provides an overview of siRNA therapeutics with a focus on detailed profiles of inclisiran, mainly its delivery strategies. We discuss the mechanisms of action, its status in clinical trials, and its prospects.
1. Introduction
The different origins and structures of small non-coding RNAs have led to the identification of three main categories: piwi-interacting RNA (piRNAs), small interfering RNA (siRNAs), and microRNAs (miRNAs). Development of siRNA molecules is reasonably undemanding once a target mRNA sequence has been determined; hence it is a popular tool for controlling gene expression. siRNA is a double-stranded RNA molecule, which is non-coding, short and well-defined (~21–25 base pairs long), with hydroxylated 3′ and phosphorylated 5′ ends. Since the first discovery of siRNA and their role in post-transcriptional gene silencing (PTGS) in plants, it has played a significant role in many functional studies for the post-human-genome era. In the early 2000s, it was anticipated that knowledge of the human proteome would identify more than 3000 potentially druggable proteins and 600–1500 promising small-molecule drug targets [,,,,,]. Conventional small molecules acting as therapeutic agents function at the protein level, necessitating a higher structural accuracy and, thus, a more complex and challenging developmental process []. Among the many limitations, one must consider various side effects, early drug elimination, non-specific cytotoxicity, high dosing levels, multi-drug resistance, and low bioavailability. Monoclonal antibodies have emerged as the leading class of therapeutic agents in the treatment of numerous human diseases, such as cancers, immunological, infectious (such as cytomegalovirus, hepatitis A and B viruses) [], and metabolic disorders. The market for monoclonal antibodies has experienced exponential growth in recent years, even though therapeutic monoclonal antibodies are restricted to binding with cell surface receptors or circulating proteins. As a result, finding and developing alternative therapeutic approaches, such as RNA techniques, is an ongoing quest for academic research chemists and pharmaceutical companies.
Small interfering RNAs (siRNAs) enable gene-targeted silencing. This compromises gene expression and regulation by cleaving mRNA or repressing its translation []. Effective knockdowns of disease-associated genes with siRNAs open the door for new therapeutic techniques, such as chemical modifications of siRNA and direct and indirect mutation targeting, among others [,,]. siRNA technology faces many barriers. The first challenge is overcoming several intracellular and extracellular defenses designed to prevent exogenous RNA from entering cells [,,,]. More generally, in the systematic administration of siRNA, many physiological barriers must be overcome to achieve the following: (a) the crossing of the vascular endothelium to reach target tissues, (b) nuclease stability, (c) delivery to the specified target, (d) renal filtration, (e) reaching the cytoplasm of the target cells, and (f) incorporation into the RNA interference machinery [,,,,,,,,,]. Strategies for intracellular administration of siRNA are expected to be non-toxic and to have good stability at the site of action [,]. Advances in the distribution of siRNA drugs continue as investigators maximize the specificity of siRNA administration while lowering the associated toxicity and degradation effects that reduce drug effectiveness []. There are two distinct approaches that enable the delivery of siRNAs to target tissues that have been reported in the literature: lipid nanoparticles (LNPs) and conjugates []. For example, patisiran (Onpattro), an RNAi therapeutic drug that uses LNP-based delivery. Patisiran may not be used in combination with additional ribonucleic acid interfering drugs or transthyretin stabilizers that are used to treat hereditary transthyretin-mediated amyloidosis (hATTR). Further, siRNAs are becoming novel nucleic acid drugs for undruggable targets to manage terminal illnesses such as cancers [,]. However, due to the systemic administration that is needed in most incidents, there are vital challenges in developing siRNAs for cancer therapies []. Givosiran was approved to treat acute hepatic porphyria (AHP) in adults []. Vutrisiran can treat hATTR in adult patients with polyneuropathy. At the end of 2021, Novartis announced the US Food and Drug Administration (FDA) approval of a new drug, inclisiran (Leqvio), as the first and thus-far only siRNA therapy that decreases low-density lipoprotein cholesterol (bad cholesterol or LDL-C) [,]. Notably, inclisiran is a drug that is produced using N-acetylgalactosamine (GalNAc) conjugates (see Table 1).

Table 1.
Reported approved/Phase III clinical trials of siRNA-based drugs.
2. Lipid Nanoparticles (LNPs) and GalNAc Conjugates for siRNA Delivery
2.1. Lipid Nanoparticles (LNPs)-siRNA
LNPs have appeared primarily as a preferred carrier to transport a variety of therapeutic agents. They are clinically promising to enhance nonviral and FDA-approved nanomedicines and to deliver siRNAs to a specific target [,]. LNPs have many pros, such as formulation simplicity, biodegradability, biocompatibility, high bioavailability, the ability to transport large payloads, and a series of physicochemical properties that can be structured to regulate their biological features [,,,,]. Enroute to their terminus, siRNAs that are encapsulated in LNPs are effectively protected against breaking down by ubiquitous nucleases. Degradation is delayed, thus promoting the drug’s efficacy and presenting long-lasting effects for any target. Ionizable lipids are protonated at a low pH, which makes them positively charged, but they remain uncharged under physiological conditions to impede rapid sequestering by immune cells during systemic circulation (positive nanoparticles are quickly segregated by Kupffer cells in the liver and splenic macrophages) [,,,,]. LNPs can be loaded with nucleic acid polymers at pH values lower than pKa of the ionizable lipid, which is positively charged. Sensitivity to the pH of ionizable lipids significantly influences the administration of mRNA in vivo, as neutral lipids have a reduced association with the anionic membranes of blood and cells, thereby increasing the biocompatibility of LNPs [,,,,]. Notably, the pKa of an ionizable lipid should be sufficiently low for it to remain unprotonated during circulation but reasonably high to be protonated in the initial or deferred endosome to maintain efficacy and reduce toxicity []. Currently, lipids, cationic ionizable lipid, cholesterol, phosphatidylcholine, and a poly(ethylene glycol) (PEG) lipid (Figure 1) are the critical components for formulations. Also, the effective delivery of siRNA in LNPs has demonstrated favorable outcomes in different phases of clinical studies.
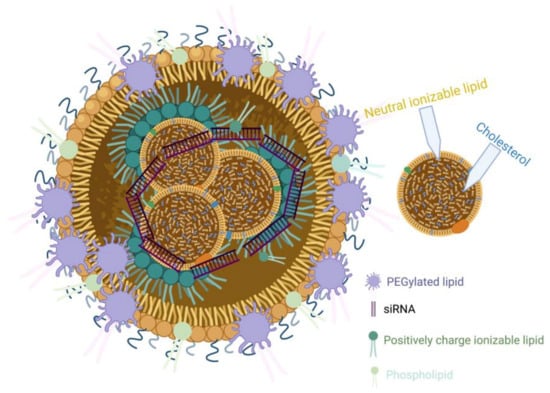
Figure 1.
Schematic illustration of LNPs containing siRNA and other key lipid components.
2.1.1. Ionizable Cationic Lipids
The ionizable cationic lipid has been enhanced for RNA encapsulation and facilitates delivery [,]. Cationic lipids that are used for gene therapy are composed of three basic domains: a positively charged group, a hydrophobic tail, and an amine spacer to bridge the polar and non-polar motif. A sequence of ionizable lipids has been designed and synthesized for siRNA delivery, with specific pKa and physical properties. Namely, 1,2-dioleoyl-3-dimethylaminopropane (DODAP), 1,2-dilinoleoyl-3-dimethylaminopropane, 1,2-dilinoleyloxy-3-dimethylaminopropane (DLin-DMA), 2,2-dilinoleyl-4-dimethylaminomethyl -dioxolane (DLin-KC2-DMA), 2,2-dilinoleyl-4-(2-dimethylaminoethyl)-dioxolane (DLin-KC2-DMA), and (6Z,9Z,28Z,31Z)-heptatriaconta-6,9,28,31-tetraen-19-yl-4-(dimethylamino)-butanoate (Dlin-MC3-DMA) with pKa values of 6.6, 6.8, 5.9, and 6.4, respectively (Table 2). DODAP is the first example of an ionizable lipid, and rapid mixing with other lipids such as PEG-lipid, cholesterol, and distearoylphosphatidylcholine (DSPC) and oligonucleotides in ethanol allowed high encapsulation efficiencies [,]. Meanwhile, DLinDMA is the first ionizable lipid that caused substantial hepatocyte gene silencing in vivo; the formulations demonstrated minimal effectiveness with high doses of siRNA []. Despite the efficacy of DODAP and prolonged hepatocyte knockdown using DLin-DMA, they never succeeded in extending studies to the clinical trial. This may be due to their unacceptable toxicity, poor tolerability and potency, and unspecific association to negatively charged cellular and extracellular components. Figure 2 displays the three basic domains of DODAP. The polar and hydrophobic tails of the cationic lipids are an essential component resulting in favorable effects on both the transfection and toxicity of the lipid.

Table 2.
Chemical structures and names of ionizable lipids.
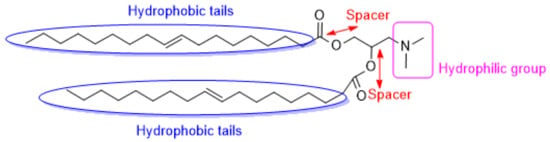
Figure 2.
Chemical structure of DODAP, a cationic ionizable lipid.
Moreover, the variation of the spacer is an essential factor to consider for delivery efficiency. The introduction of a ketal linker significantly influences the delivery efficacy with good in vivo activity. Reduced efficacy is observed when the ketal is substituted with the ester or alkoxy functional group []. In designing a new library of ionizable lipids and increasing the delivery system’s efficacy, the linker and the hydrophobic regions of DLinDMA were modified, resulting in the identification of DLin-KC2-DMA. Meanwhile, modifying the amine functional group led to the production of DLin-MC3-DMA. Jayaraman and co-workers reported that Dlin-MC3-DMA (ED50~0.03 mg/kg) was 10-fold superior compared to Dlin-KC2-DMA (ED50~0.3 mg/kg) for hepatic gene silencing in vivo [,]. DLin-MC3-DMA is currently the most promising cationic lipid for therapeutic genetic drug carriers that is approved by the FDA and a fundamental delivery constituent of Onpattro, the first FDA-approved siRNA drug. It can be concluded that the significant factors influencing cationic lipid power include acyl chain unsaturation, ether bonds, and the pKa of the cationic lipid amino headgroup [].
2.1.2. Poly (Ethylene Glycol) (PEG)
PEG-lipids can have multiple effects on the properties of LNP [,,,,] and PEG-lipids have been engineered to control LNP size and produce higher transfection efficiencies [,]. In addition, the type and amount of PEG-lipid utilized are proportional to the size, and this significantly affects the gene-silencing potency of LNP siRNA systems [,]. Further, it provides drugs with more significant physical and thermal stability and inhibits drug aggregation in vivo []. PEG-lipids such as 1,2-dimyristoyl-rac-glycero-3-methoxypolyethylene glycol-2000 (DMG-PEG2000) and 1,2-distearoyl-rac-glycero-3-methoxypolyethylene glycol-2000 (DSG-PEG2000) are uncharged with an alkyl chain length of C14 and C18, respectively (Figure 3). In addition, the length of lipid chains that are attached to the PEG affects how long it stays on liposomes. DSG-PEG2000 demonstrated longer retention times compared to DMG-PEG2000. PEG lipids with short (C14) lipid tails tend to break free from LNP-siRNA in vivo with about a half-time of 1 h in LNPs–siRNA formulations. However, PEG lipids with increased (C18) alkyl chains display dissociation rates of days or longer [,]. Increasing the number of carbon chains did not satisfactorily change the hepatic gene silencing, delivery efficacy, and pharmacokinetic features of LNPs [,,]. This suggests the likelihood that LNP stabilization by PEG lipids is a functionality of short alkyl chains that quickly desorb after application which may be advantageous for LNP cellular uptake and endosomal escape [,,,,].
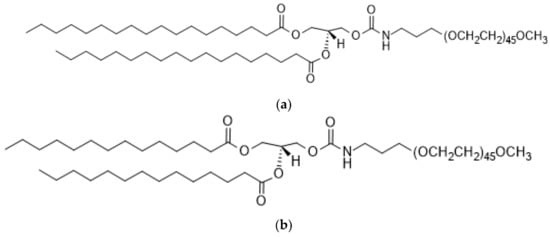
Figure 3.
Chemical structure of PEG-lipids, (a) DMG-PEG2000 and (b) DSG-PEG2000. (a) 1,2-dimyristoyl-rac-glycero-3-methoxypolyethylene glycol-2000 (DMG-PEG 2000). (b) 1,2-distearoyl- rac-glycero-3-methoxypolyethylene glycol-2000 (DSG-PEG 2000).
2.1.3. Cholesterol and Saturated Phosphatidylcholine (PC)
Liposomes comprise one or several lipid bilayers, between 20 and ~1000 nm in size, that serve as suitable delivery cargo for therapeutic agents [,,]. Liposomes can encapsulate hydrophobic and hydrophilic agents in the lipid membrane and the aqueous core. In contrast, the hydrophilic fragment can defend the loaded drugs from the host bodies’ damaging features that eventually decrease the undesirable side effects [,,,,,,,,]. The development of liposomes as a drug carrier system has been advanced in the last few years to improve pharmacokinetics. Their capacity to deposit their cargo enhances drug efficacy and minimizes side effects [,]. Cholesterol and saturated phosphatidylcholines (PC), such as distearoyl-PC(DSPC) or dipalmitoyl-PC (DPPC), are commonly employed liposome substituents that favorably influence the strength of the LNP formulation. However, inadequate evidence obscures the purpose behind the use of cholesterol from the perspective of nucleic acid delivery systems. A mixture of unsaturated phospholipids in a lipid delivery system was reported to enhance the capacity of the phosphor-lipid membrane to form a stable bicontinuous cubic phase at physiological conditions, hence improving fusogenicity []. In particular, the development of inverted bi-continual cubic phases is directly related to lipid membrane fusion [,]. Notably, there must be a focus on critical issues such as cost-effectiveness, off-target effects, and toxicity as we advance, despite the rapid silencing, expression, or modification of genes in human patients using LNPs technology. Based on the understanding that is gained through the design, development, and use of the first FDA-approved and marketed LNP−siRNA drug, the technology can be improved for future applications [].
2.2. N-Acetylgalactosamine (GalNAc)-siRNA
Asialoglycoprotein receptor (ASGPR), also known as hepatic binding protein or the Ashwell–Morell is a C-type lectin and the first animal lectin that was identified during turnover studies of a serum glycoprotein, ceruloplasmin [,,,,,]. Several studies have shown hepatic lectin as a promising candidate target as a drug and gene carrier into hepatocytes [,]. ASGPR is comprised of two homologous units: a significant subunit (ASGPR-1) 48 kDa and a minor 40 kDa subunit (ASGPR-2). This heterooligomeric receptor is amply articulated on the sinusoidal (i.e., basolateral) surface of the liver hepatocytes [,,,,,,]. ASGPR mediates binding and internalization, followed by clearance of glycoproteins enfolding terminal galactose or N-acetylgalactosamine (GalNAc) residues from the circulation [,]. It has been reported that in the past three decades, asialoglycoprotein, galactoside, galactosamine, galactan, and galactose derivatives, namely GalNac, have been extensively studied to deliver naturally active glycopeptides [], antisense peptide nucleic acid (asPNA) [], glycolipids [], small molecules [], nucleoside analogues [,], plasma DNA [,], and ASOs [,,] to the central functional parenchymal tissue of the liver. Also, a molecular hybrid of siRNA with GalNAc hastens the uptake of hepatocytes by binding with the asialoglycoprotein receptor (ASGPR) [,]. Patisiran is used for treating hereditary TTR amyloidosis and it utilizes LNP for liver delivery. However, due to the intricate formulation of LNP and the challenge in administration routes, GalNAc technology, which is uncomplicated, smaller, and with a distinct formation methodology, is currently emerging and superseding LNP as a delivery vehicle of small interfering RNA (siRNA) to target tissues.
GalNAc-siRNA and GalNAc-ASO (antisense oligonucleotides) conjugate delivery studies have shown exquisite superiority overall for this delivery approach. ASOs are short, chemically-modified oligonucleotides that bind to the RNA in cells via Watson–Crick base-pairing and modulate RNA function to cause a pharmacological effect []. The Watson–Crick molecular detection provides the antisense field more flexibility in RNA-based drug design. It accelerates development and is vital for targeting a myriad of rare and genetic diseases [,]. Covalently linked ASO-triantennary GalNAc, was shown to provide favorable gene silencing []. The ED50 of GalNAc conjugated 5-10-5 MOE gapmer targeting SRB1 mRNA in mouse liver was 3.3 mg/kg and superior to unconjugated gapmer ASO with ED50 of 18.3 mg/kg []. The replacement of the MOE nucleotide sugar wings in gapmer ASO with constrained ethyl (cEt)-bridged ribonucleotides led to a ~60-fold augmentation in relation to the parent MOE (2′-O-methoxyethyl RNA) []. The amalgamation of three GalNAc moieties to one siRNA with a functional spacer is shown in Figure 4. The methodology favors the high affinity of the interaction between ASGPR and the GalNAc ligand and the optimal efficiency of siRNA delivery to hepatocytes via subcutaneous injection. Further, the enhanced stabilization alteration allows the amalgamation of GalNAc- siRNA to remain in the circulation system and cytoplasm for more than 100 days []. Remarkably, this enables long-term gene silencing and therapeutic effects in humans after a single dose [,,].
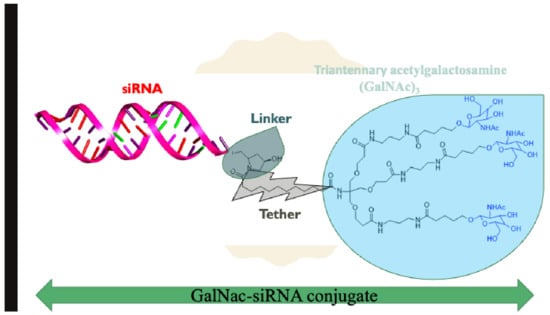
Figure 4.
Chemical structure of triantennary acetylgalactosamine (GalNAc)3 hybridized with siRNA.
A synthetic route to GalNAc conjugates has been obtained together with a method to covalently link GalNAc to siRNAs []. GalNAc-conjugated siRNAs confer high-affinity binding to the ASGPRs, which is highly efficient, hepatocyte-specific, and clathrin-mediated endocytic. Trivalent GalNAc clusters have higher affinity, efficacy, and apparent advantages than divalent, trivalent, and tetravalent galactose-based conjugates [,,,]. The GalNAc3 arranged in the triantennary approach, as shown in Figure 5, was found to be the most favorable chemically improved and metabolically stable GalNAc conjugates for ASGPR-mediated siRNA uptake. It is a long-acting treatment for different diseases relating to liver-expressed genes []. In mice, the TTR gene was silenced in the liver with a single-dose median effective dose (ED50) of ∼1 mg/kg subcutaneously). Chronic weekly medication of GalNAc-siRNA conjugate resulted in continued dose-dependent gene silencing for >9 months with no after-effects in mice. Alnylam Pharmaceuticals uses triantennary GalNAc-siRNA conjugates as a delivery vehicle, and they have designed and synthesized more than five novel GalNAc-siRNA hybrids to target different diseases. For example, givosiran is a GalNAc-conjugated siRNA therapeutic agent targeting 5′-aminolevulinate synthase 1 (ALAS1) that is approved for the treatment of acute hepatic porphyria (AHP) in the United States and in the European Union [].
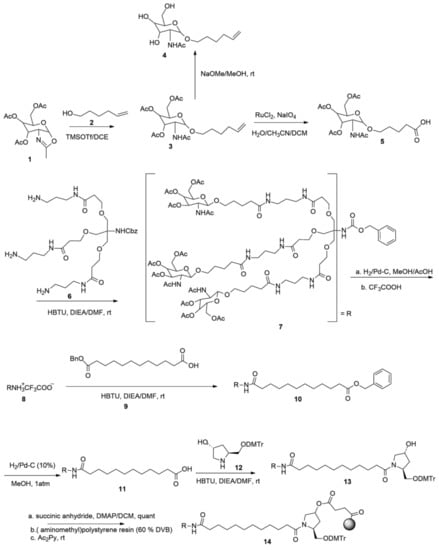
Figure 5.
Synthetic route to triantennary acetylgalactosamine (GalNAc)3.
Inclisiran is also an approved GalNAc-conjugated siRNA containing 2′-F, 2′-OMe and PS modifications. As mentioned above, inclisiran is used in a specified maximum-tolerated statin therapy for adults with ASCVD or heterozygous familial hypercholesterolemia (HeFH), which require further lowering of LDL-C. Meanwhile, CVD is one of the leading global causes of mortality. In order to reduce the death rate from CVD, the U.K.’s National Health Service (NHS) has recommended the use of inclisiran to treat patients with ASCVD [].
3. Inclisiran
3.1. Some Selected Cholesterol-Lowering Drugs before Approval of Inclisiran
Hypercholesterolemia is a leading risk factor for CVD. A high concentration of low-density lipoprotein cholesterol (LDL-C) is associated with a greater risk of heart attack, stroke, and ensuing complications []. The reduction of LDL-C has been clearly shown to lead to a substantial decrease in CVD incidence. Conventional drugs that are used to reduce high cholesterol before approval of inclisiran include statins, niacin, bile acid drugs, fibrates, and antibodies acting as PCSK9 inhibitors. Statins were the first widely used high-cholesterol therapies (for example, lipitor, lescol, pravachol, crestor, and zocor). They prevent hardening of the arteries, decrease the risk of heart attacks or strokes, and enhance the reduction of LDL by obstructing vital enzymes that are imperative for cholesterol assembly. Niacin (niaspan and nicoar), a vitamin B, is orally administered in appropriate concentrations for therapeutic applications. Niacin can effectively lower LDL and has benefits in raising high-density lipoprotein (HDL) cholesterol and lowering triglyceride (TG) levels and is often used as a combination therapy with statins [,,]. Fenofibrate and gemfibrozil are fibrates that reduce the production of triglycerides. Both treatments exert their effects on HDL through peroxisome proliferator-activated receptor (PPARα) [,]. Bile acid acts by binding to the bile of the liver. Examples of approved bile acid sequestrants include cholestyramine (questran), colestipol (colestid), and colesevelam. Colesevelam has superior binding and affinity for biliary acids than cholestyramine and colestipol. It can be administered at considerably lower doses to obtain minimal side effects []. Notably, the reduction of the LDL-C level depends on the specific drug and the quantity that is administered; bile acid sequestrants can be administered as monotherapy (10% reduction of LDL-C) or as combination therapy with statins (25% reduction of LDL-C) [,]. Ezetimibe (zetia) as a combination therapy with a statin can successfully impede cholesterol absorption, leading to a reduction in LDL-C levels without interfering with the absorption of triglycerides, fatty acids, bile acids, or fat-soluble vitamins such as vitamin D and K []. PCSK9 inhibitors were first approved in 2015 and are more effective in lowering serum cholesterol levels than statins []. These inhibitors act by binding to PCSK9 with high affinity, deactivating PCSK9 in the blood and interfering with its ability to reduce LDL cholesterol (bad cholesterol) uptake by hepatocytes—all without apparent negative health consequences. Notably, the combination of a PCSK9 inhibitor and a (low dose) statin may further increase the lipid-lowering effects while preventing the side effects [,,,].
PCSK9 was initially known as neural apoptosis-regulated convertase 1 because levels of this protein are high in cerebral neurons during apoptosis [,,]. It was subsequently discovered that PCSK9 loss-of-function modifications were clinically significant and cause dominant hypocholesterolemia while reducing CVD risk. This is because PCSK9 binds to (and degrades) the LDL-C receptor at the surface of hepatocytes, thus impeding LDL uptake from blood [,,]. Monoclonal antibodies against PCSK9 are currently available: alirocumab (praluent) and evolocumab (repatha). Evolocumab was FDA approved for adults with HeFH including patients with significant atherosclerotic CVD who require further reduction of LDL C after undergoing a controlled diet and maximum tolerated statin therapy. Alirocumab and evolocumab, either in monotherapy or in combination with lipid-lowering therapies, significantly reduced LDL-C levels by up to 60% in patients with high cholesterol. Such as those with familial hypercholesterolemia, moderate to very high cardiovascular risk, and statin intolerance [,,,,,,].
Most of these drugs have proven effective in lowering high cholesterol and decreasing mortalities and complications from CVD. However, the adverse effects of these drugs cannot be overlooked. For example, despite optimal statin therapy or statin intolerance, many patients fail to achieve LDL-C treatment targets []. Statins and fibrates have been reported to cause severe myopathy [,]. The drug interactions involving statins result from inducing or inhibiting different CYP isoenzymes leading to an increase in the risk of myopathy, rhabdomyolysis, diarrhea, joint pain, fatigue, and other severe adverse events []. Niacin is effective in the treatment of children with hypercholesterolemia []. The adverse effects include muscle pain, liver problem, tiredness or weakness, loss of appetite, upper stomach pain, and itching. PCSK9 blockers, administered once or twice a month, reduce circulating PCSK9 levels and decrease LDL cholesterol levels without severe adverse effects. Long-term therapy has a lower incidence of CVD than controls. However, PCSK9 antibodies have a short effect interval, requiring recurrent intravenous injections. Hence, there is a necessity for additional therapy that brings the possibility of lowering LDL cholesterol levels with minimal adverse effects.
3.2. Development of Inclisiran
Inclisiran (Figure 6) is a chemically-modified small interfering RNA (siRNA) molecule that is conjugated to GalNAc to target PCSK9. It is the first siRNA therapeutic class across the medical spectrum to be subjected to exhaustive assessment in clinical trials to determine its efficacy in reducing LDL-C and precluding CVD. Inclisiran sodium has a molecular formula of C529H664F12N176Na43O316P43S6 and a molecular weight of 17,284.75 g/mol, both parameters in salt form. Inclisiran sodium was formed by synthesizing two single-strand oligonucleotides, sense and antisense strands, by exploring the established solid-phase approach []. The 3′-O-(2-cyanoethyl) phosphoramidite with the 5′-hydroxyls protected with 4,4′--dimethoxytriphenylmethyl (DMT) moiety and fluoro-, OMe-, or deoxy-changes at the 2′-position was utilized. However, the sense constituent is synthesized on protected GalNAc-based ligand such as L96-loaded polymer support and the antisense constituent on 2′-OMe A-loaded controlled pore glass (CPG) support. The sense strand is coupled with GalNAc to accelerate fast hepatocyte uptake with high specificity only by the liver after subcutaneous low-volume injection. The sense strand consists of 21 and 23 nucleotides in the antisense strand. The single-strand oligonucleotides are cleaved from the solid support, deprotected, and subsequently ultrafiltrated, followed by HPLC to eliminate impurities. Purification was also performed after the second ultrafiltration to eliminate the substances from the mobile phases that are used in chromatography. Afterward, the two single strands were mixed in an equimolar ratio during annealing, followed by concentration and freeze-drying []. The chemical structure of inclisiran consists of 1 2′-deoxy, 11 2′-fluoro-, and 32 2′-O-methyl-modified nucleotides, and a triantennary N-acetylgalactosamine (GalNAc) that coupled with the 3′-end of the passenger strand.
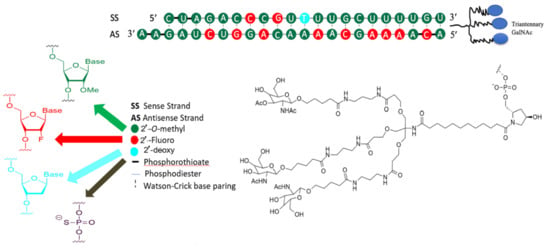
Figure 6.
Chemical structure of inclisiran.
Since unmodified siRNAs lack stability because of the high susceptibility of ribonucleotides to exonuclease, they are susceptible to degradation in serum [,,]. There are five alterations that are utilized, or more specifically, phosphorothioate, 2′-deoxy, 2′-fluoro-RNA, 2′-O-methyl-RNA, and triantennary GalNAc–all without loss of efficacy. The amalgamation of the 2-fluoro and 2-O-methyl changes permits a significant stabilization of inclisiran without obstructing the siRNAs from immediately entering the RNA-induced silencer complex (RISC) []. The RISC protein is crucial for refining transcriptional profiles in cells, improving the stabilization of siRNAs. The terminal phosphodiester bonds are improved with phosphorothioates and provide further stabilization against exonuclease degradation and potentially influence the cellular internalization of the compound. It is worth pointing out that modifying 2′-fluoro and 2′-O-methyl and the additional four phosphorothioates to the inclisiran are liable for lessening the degradation of the molecule in the bloodstream [].
3.3. Clinical Trials of Inclisiran
The ALN-PCS molecule, a precursor to inclisiran, was incorporated into a lipid nanoparticle. It decreased PCSK9 mRNA and protein concentrations by 70% and 60% in LDL-C concentrations over 3 weeks in an in vivo study []. The molecule caused a 70% decrease in PCSK9 protein after a 60-minute infusion and a 40% decrease in LDL-C levels on the third day of follow-up in a clinical trial []. The ALN-PCS molecule was later introduced by N-acetylgalactosamine (GalNAc) to the molecule to produce ALN-PCSSC. This improved its clinical efficacy and extent of action by increasing the uptake of the hepatocyte cell membrane and liver cell specificity. The results of clinical studies of ALN-PCS (Table 3) [] suggest that inhibiting PCSK9 synthesis through RNA interference (RNAi) gives an effective, safe mechanism for lowering the concentration of LDL cholesterol in healthy patients with elevated cholesterol. These findings support further evaluation of ALN-PCS in individuals with hypercholesterolemia, comprising those receiving statin therapy. This first report shows the drastic influence of RNAi drugs on the clinically confirmed endpoint (i.e., LDL-C) in humans. Liver function tests, troponin, or inflammatory markers (cytokines and C-reactive protein) had no clinically significant changes.

Table 3.
The current registered clinical studies on inclisiran [] (* recruiting (green color), ** active, not recruiting (red color), *** completed (blue color), **** not-recruiting (brown color), ***** enrolling by invitation (purple color)).
A multiple-dose study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of subcutaneously administered ALN PCSSC in subjects with elevated LDL cholesterol was then conducted []. The results of this study demonstrated clinical evidence for the use of PCSK9 as a therapeutic target for a significant reduction in LDL cholesterol, which can be effective for at least six months after starting treatment. This is highly attractive when compared to the endorsed regimens of PCSK9 antibodies that are administered once or twice monthly. Notably, inclisiran is different from anti-PCSK9 antibodies. Anti-PCSK9 antibodies attach to extracellular PCSK9 and block it from interacting with the LDL receptor, while inclisiran impedes the production of the PCSK9 protein precisely in the liver. Secondly, the pharmacodynamic properties of inclisiran differ significantly from that of anti-PCSK9 antibodies.
ORION-1 was a Phase 2, placebo-controlled, double-blind, randomized trial of volunteer participants with ASCVD or ASCVD-risk equivalents such as diabetes and FH as well as participants with elevated LDL-C, despite a maximum tolerated dose of LDL-C lowering therapies. This is to measure the efficacy, safety, and tolerability of ALN-PCSSC injection(s) []. The results showed that PCSK9 levels were decreased from baseline levels by a mean of 59.6 to 68.7% throughout the range of inclisiran doses from 100 mg to 500 mg after 14 days of a single injection. Further, at day 180, average reductions from reference concentrations of PCSK9 in patients on a two-dose diet ranged from 53.2% to 69.1%. The most common adverse events in inclisiran-treated and placebo-treated individuals (>2% of patients) were myalgia, headache, fatigue, nasopharyngitis, back pain, hypertension, diarrhea, and dizziness []. Immune activation, typically found in RNA therapies, was occasionally associated with inclisiran. There have been flu-like symptoms but no observed elevated C-reactive protein.
The ORION-3, open-label, non-randomized, active comparator extension trial was registered to assess the efficacy, safety, and tolerability of long-term dosing of inclisiran and evolocumab given as subcutaneous injections in participants with high cardiovascular risk and elevated low-density lipoprotein cholesterol (LDL-C). On day 210 of the ORION-3 test, LDL-C concentrations were reduced by an average of 51%, while PCSK9 concentrations were reduced by 77%. A long-term effect of 300 mg of inclisiran on lowering LDL-C was observed in ORION-3 for approximately 22 months. Further, the LDL-C was reduced over time by about 60 mg/dL. Meanwhile, no changes were made to the overall safety profile for a minimum of three years of monitoring. No laboratory abnormalities that were associated with therapy, including liver and renal function tests, were observed since the successful study of the Phase 2 clinical trial. The Phase 3 trial focused on evaluating the use of inclisiran in a large group of adult participants with HeFH who had been treated with a maximally accepted dose of statin therapy.
A total of three Phase 3 studies, i.e., the ORION-9 (NCT03397121), ORION-10 (NCT03399370), and the ORION-11 (NCT03400800) trial, two randomized, double-blind, placebo-controlled, parallel-group, were registered [,,]. This was done to determine the efficacy, safety, and adverse-event profile of inclisiran over 18 months in patients, either at elevated or excessive CV risk with an LDL-C ≥ 70 mg/dl (in a patient with ASCVD) or ≥100 mg/dl (in patients with an ASCVD risk equivalent). Even though the patients are given statin therapy at the maximal dose tolerated with or without ezetimibe, the results of the analysis identified 284 mg of inclisiran exhibited statistically considerable developments compared to placebo by reducing LDL-C concentrations in adult patients with HeFH or nFH with ASCVD who received the maximum tolerated dose of a statin or who were statin intolerant. The cluster variances in percentage change in LDL-C from baseline to day 510 were −49.52 in ORION-9; −57.64 in ORION-10, and −53.5 in ORION-11 (all p < 0.0001). Adverse occurrences that were reported for ORION-9 were 76.8% in the inclisiran group and 71.7% in the placebo group. Many reported occurrences were mild or moderate. A greater number of patients in the inclisiran group than in the placebo group reacted at the protocol-defined injection site, at 17.0% compared to 1.7%. In the ORION-9 trial, 90.2% of events were classified as mild, and none were reported as severe or persistent. The number of severe adverse events that were associated with inclisiran was lower than those that were associated with the placebo (7.5% compared to 13.8%). Lowering LDL-C levels in circulating plasma reduces CVD risk. This will improve CVD outcomes because high cholesterol is an important risk factor for cardiovascular disease.
3.4. Inclisiran-Mechanism of Action
Inclisiran is a chemically synthesized siRNA that is conjugated with carbohydrates N-acetylgalactosamine triantennary and not only interacts but binds to ASGPR, a specific liver receptor. This promotes the absorption of inclisiran in hepatocytes. ASGPR is a transmembrane protein that is composed of two subunits, a significant subunit, ASGR1, and a minor subunit, ASGR2, expressed primarily through hepatocytes []. It has been shown that ASGR1 expression is wholly associated with protein levels in the liver, which could affect drug administration by ASGPR. []. In particular, the ASGR1 subunit was found to be the most severely implicated sub-unit. Human models using variations in ASGR1 may be used to evaluate the ability of ASGPR to administer drugs [,]. Subsequently, inclisiran binds with the cytosolic RISC, a ribonucleoprotein complex that acts as a model for identifying the complementary target mRNA. Thus, the activated RNAse cleaves the target mRNA, which ejects one strand, exiting the antisense strand to bind to the target mRNA and facilitate its sequence-specific cleavage by the RISC RNase Argonaute. As the incorporation of inclisiran RISC tolerates the drug to bind to the mRNA PCSK9, it further obstructs the translation of PCSK9, and hence the degradation of hepatic production of PCSK9. Meanwhile, the lower the synthesizing of PCSK9 protein, the more the recycling of LDL receptors, thus causing a reduction in the levels of LDL-C in the blood. In summary, inclisiran binds to the precursor of the mRNA of PCSK9, inhibiting its translation and production of PCSK9, and subsequent PCSK9 protein reduction promotes LDLR recycling, increasing absorption and degradation of plasma LDL-C and decreasing plasma LDL-C levels [,,] (see Figure 7).
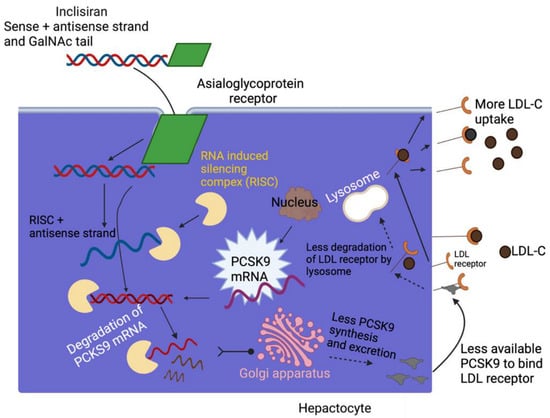
Figure 7.
Schematic of the mechanism of action of inclisiran. Figure created with BioRender.com (accessed on September 29, 2022). Adapted from Cupido AJ, Kastelein JJP. Cardiovasc Res. 2020;116(11):e136-e139. GalNAc—N-acetylgalactosamine; LDL—low-density lipoprotein; LDL-C—LDL cholesterol; LDL-R—LDL receptor; PCSK9—proprotein convertase subtilisin/kexin type 9; RISC, RNA—induced silencing complex; siRNA—small interfering RNA.
3.5. Clinical Benefits of Inclisiran
Administration of inclisiran twice a year has been established to significantly improve treatment adherence and clinical efficacy, such as lowering the level of LDL-C for a more extended period. This is an undeniable benefit of inclisiran compared to other drugs that lower LDL-C. Most require daily administration, increasing the likelihood of poor adherence to medical treatment, which severely compromises optimal outcomes, leading to high rates of withdrawal from treatment, reduced achievement of LDL-C targets, and lesser medical effects []. Further, an efficient and continuous decrease can be afforded using inclisiran for LDL-C levels up to 52% compared with placebo for some patients with ASCVD on treatment with statins to the maximum tolerated [,].
4. Other Critical Facts on siRNA Therapeutic and Inclisiran
RNA technology has been able to resolve the issues of stability, delivery system, translation efficiency, and immunogenicity to accomplish effective, safe, and non-toxic delivery of siRNA. However, despite the significant improvement of the inclisiran therapy in treating high cholesterol and inhibiting CV events, some questions remain unanswered. The review of the age group of volunteers who took part in the completed clinical trial on inclisiran showed that it is not yet known if inclisiran is safe enough to be used in young patients with homozygous family hypercholesterolemia (HoFH). However, the worldwide Phase III placebo-controlled trials ORION-13 and ORION-16 are currently enrolling patients to evaluate and determine the short-term effectiveness, safety, and tolerability of inclisiran in adolescents with HoFH and HeFH, respectively, and high LDL-C on the stable standard of hypolipidemia therapy. This could be one of the drawbacks of inclisiran if this current study does not answer these issues. Furthermore, there is still a lack of long-term data on participants who have experienced major adverse cardiovascular events (MACE). There are two current trials that are registered to examine the effects of inclisiran on major adverse cardiovascular events (ORION-4 and VICTORION-2P). The present VICTORION-2P study aims to assess that inclisiran sodium therapy should be administered on day 1, day 90, and every six months (30 mg). After that, in addition to statin therapy in patients with ASCVD, the risk of CV mortality, non-fatal myocardial infarction (MI), and non-fatal ischemic stroke is substantially lessened. This is comparable to placebo in adjuvant to well-tolerated high-intensity statin therapy. At the same time, ORION-4 trials aim to determine if inclisiran will safely reduce the risk of a heart attack, including strokes, in individuals who have already had any of these conditions or who have had an operation or procedure to treat clogged arteries. However, some uncertainty remains whether treatment with inclisiran will translate into a decrease in MACE rates that are akin to when statins or PCSK9 inhibitors are used. Also, long-term observation of a drug is critical because some potential side effects may not have had time to occur. The semi-annual administration of inclisiran may impact the patient’s desire to take the medication for longer-term therapy. Although this hypothesis has not yet been proven, a long-term extension of Phase III (ORION 8) has been registered to appraise the consequence of long-term dosing of inclisiran in subjects with high CV risk and raised LDL-C (ORION-8). This will strengthen confidence related to the safety profile of inclisiran therapy. This also indicates that opportunities for improvement and novelties cannot be underestimated. Thus, several dominating companies in the RNA biopharma sector will become apparent, and new small biotech start-ups and academic groups with transformative concepts will continue to propagate []. Some of the challenges in the siRNA therapeutic still need urgent attention by providing a better and more efficient delivery vehicle, regulation of the activated immune system, structure-based antigen design, and delivery system optimization to enhance stability and degradation []. The modification or optimization of the siRNA delivery strategies can support transporting siRNAs to a wide range of targets. Notably, GalNAc-siRNA conjugates have considerably higher plasma clearance due to more efficient hepatocyte uptake than ASOs. It is imperative to understand how much these variables contribute to drug clearance and how they are affected by various diseases [,]. We also hope that research on the delivery of GalNAc-siRNA drugs by oral instead of subcutaneous administration can be explicitly achieved when treating patients with chronic disease. In summary, although this technology still faces several challenges, its rapid emergence and success in a wide range of liver-based conditions have absolutely changed the standpoint for siRNA therapies targeting hepatocytes.
Author Contributions
Conceptualization, G.K.-S.W. and J.A.T.; methodology, O.E. and G.K.-S.W.; formal analysis, O.E.; writing—original draft preparation, O.E.; writing—review and editing, O.E., P.C., G.K.-S.W. and J.A.T.; funding acquisition, J.A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Olivier Lampron for his careful reading of our manuscript and for providing insightful comments and suggestions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hopkins, A.L.; Groom, C.R. The druggable genome. Nat. Rev. Drug Discov. 2002, 1, 727–730. [Google Scholar] [CrossRef]
- Overington, J.P.; Al-Lazikani, B.; Hopkins, A.L. How many drug targets are there? Nat. Rev. Drug Discov. 2006, 5, 993–996. [Google Scholar] [CrossRef] [PubMed]
- Russ, A.P.; Lampel, S. The druggable genome: An update. Drug Discov. Today 2005, 10, 1607–1610. [Google Scholar] [CrossRef]
- Hambly, K.; Danzer, J.; Muskal, S.; Debe, D.A. Interrogating the druggable genome with structural informatics. Mol. Divers. 2006, 10, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Cimermancic, P.; Weinkam, P.; Rettenmaier, T.J.; Bichmann, L.; Keedy, D.A.; Woldeyes, R.A.; Schneidman-Duhovny, D.; Demerdash, O.N.; Mitchell, J.C.; Wells, J.A.; et al. CryptoSite: Expanding the Druggable Proteome by Characterization and Prediction of Cryptic Binding Sites. J. Mol. Biol. 2016, 428, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Bartoszewski, R.; Sikorski, A.F. Editorial focus: Understanding off-target effects as the key to successful RNAi therapy. Cell. Mol. Biol. Lett. 2019, 24, 69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.M.; Bahal, R.; Rasmussen, T.P.; Manautou, J.E.; Zhong, X.B. The growth of siRNA-based therapeutics: Updated clinical studies. Biochem. Pharmacol. 2021, 189, 114432. [Google Scholar] [CrossRef]
- Flego, M.; Ascione, A.; Cianfriglia, M.; Vella, S. Clinical development of monoclonal antibody-based drugs in HIV and HCV diseases. BMC Med. 2013, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Weng, Y.; Xia, X.H.; Liang, X.J.; Huang, Y. Clinical advances of siRNA therapeutics. J. Gene Med. 2019, 21, e3097. [Google Scholar] [CrossRef] [PubMed]
- Schütze, N. siRNA technology. Mol. Cell. Endocrinol. 2004, 213, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Michler, T.; Merkel, O.M. siRNA Therapeutics against Respiratory Viral Infections-What Have We Learned for Potential COVID-19 Therapies? Adv. Healthc. Mater. 2021, 10, e2001650. [Google Scholar] [CrossRef] [PubMed]
- Ryther, R.C.; Flynt, A.S.; Phillips, J.A., 3rd; Patton, J.G. siRNA therapeutics: Big potential from small RNAs. Gene Ther. 2005, 12, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Dowdy, S.F. Overcoming cellular barriers for RNA therapeutics. Nat. Biotechnol. 2017, 35, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Juliano, R.L. The delivery of therapeutic oligonucleotides. Nucleic Acids Res. 2016, 44, 6518–6548. [Google Scholar] [CrossRef] [PubMed]
- Crooke, S.T.; Witztum, J.L.; Bennett, C.F.; Baker, B.F. RNA-Targeted Therapeutics. Cell Metab. 2018, 27, 714–739. [Google Scholar] [CrossRef]
- Springer, A.D.; Dowdy, S.F. GalNAc-siRNA Conjugates: Leading the Way for Delivery of RNAi Therapeutics. Nucleic Acid Ther. 2018, 28, 109–118. [Google Scholar] [CrossRef]
- Whitehead, K.A.; Langer, R.; Anderson, D.G. Knocking down barriers: Advances in siRNA delivery. Nat. Rev. Drug Discov. 2009, 8, 129–138. [Google Scholar] [CrossRef]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef]
- Petros, R.A.; DeSimone, J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef]
- Kanasty, R.L.; Whitehead, K.A.; Vegas, A.J.; Anderson, D.G. Action and reaction: The biological response to siRNA and its delivery vehicles. Mol. Ther. 2012, 20, 513–524. [Google Scholar] [CrossRef]
- Kanasty, R.; Dorkin, J.R.; Vegas, A.; Anderson, D. Delivery materials for siRNA therapeutics. Nat. Mater. 2013, 12, 967–977. [Google Scholar] [CrossRef]
- Chen, X.; Mangala, L.S.; Rodriguez-Aguayo, C.; Kong, X.; Lopez-Berestein, G.; Sood, A.K. RNA interference-based therapy and its delivery systems. Cancer Metastasis Rev. 2018, 37, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Tiemann, K.; Rossi, J.J. RNAi-based therapeutics-current status, challenges and prospects. EMBO Mol. Med. 2009, 1, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, Z.; Wientjes, M.G.; Au, J.L. Delivery of siRNA therapeutics: Barriers and carriers. AAPS J. 2010, 12, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, P.S.; Rudra, A.; Miao, L.; Anderson, D.G. Delivering the Messenger: Advances in Technologies for Therapeutic mRNA Delivery. Mol. Ther. 2019, 27, 710–728. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi Chalbatani, G.; Dana, H.; Gharagouzloo, E.; Grijalvo, S.; Eritja, R.; Logsdon, C.D.; Memari, F.; Miri, S.R.; Rad, M.R.; Marmari, V. Small interfering RNAs (siRNAs) in cancer therapy: A nano-based approach. Int. J. Nanomed. 2019, 14, 3111–3128. [Google Scholar] [CrossRef] [PubMed]
- Burnett, J.C.; Rossi, J.J.; Tiemann, K. Current progress of siRNA/shRNA therapeutics in clinical trials. Biotechnol. J. 2011, 6, 1130–1146. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://investors.alnylam.com/press-release?id=26816 (accessed on 26 September 2022).
- Landen, C.N., Jr.; Chavez-Reyes, A.; Bucana, C.; Schmandt, R.; Deavers, M.T.; Lopez-Berestein, G.; Sood, A.K. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer Res. 2005, 65, 6910–6918. [Google Scholar] [CrossRef]
- Pal, A.; Ahmad, A.; Khan, S.; Sakabe, I.; Zhang, C.; Kasid, U.N.; Ahmad, I. Systemic delivery of RafsiRNA using cationic cardiolipin liposomes silences Raf-1 expression and inhibits tumor growth in xenograft model of human prostate cancer. Int. J. Oncol. 2005, 26, 1087–1091. [Google Scholar] [CrossRef]
- Oh, Y.K.; Park, T.G. siRNA delivery systems for cancer treatment. Adv. Drug Deliv. Rev. 2009, 61, 850–862. [Google Scholar] [CrossRef]
- Balwani, M.; Sardh, E.; Ventura, P.; Peiro, P.A.; Rees, D.C.; Stolzel, U.; Bissell, D.M.; Bonkovsky, H.L.; Windyga, J.; Anderson, K.E.; et al. Phase 3 Trial of RNAi Therapeutic Givosiran for Acute Intermittent Porphyria. N. Engl. J. Med. 2020, 382, 2289–2301. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-add-therapy-lower-cholesterol-among-certain-high-risk-adults (accessed on 26 September 2022).
- Available online: https://www.novartis.com/news/media-releases/fda-approves-novartis-leqvio-inclisiran-first-class-sirna-lower-cholesterol-and-keep-it-low-two-doses-year (accessed on 4 February 2013).
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef] [PubMed]
- Fenton, O.S.; Olafson, K.N.; Pillai, P.S.; Mitchell, M.J.; Langer, R. Advances in Biomaterials for Drug Delivery. Adv. Mater. 2018, 30, e1705328. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Santos, B.; Gremiao, M.P.; Chorilli, M. Nanotechnology-based drug delivery systems for the treatment of Alzheimer’s disease. Int. J. Nanomed. 2015, 10, 4981–5003. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ray, S.; Thakur, R.S. Solid lipid nanoparticles: A modern formulation approach in drug delivery system. Indian J. Pharm. Sci. 2009, 71, 349–358. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: Applications, advantages and disadvantages. Res. Pharm. Sci. 2018, 13, 288–303. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Hajj, K.A.; Whitehead, K.A. Tools for translation: Non-viral materials for therapeutic mRNA delivery. Nat. Rev. Mater. 2017, 2, 17056. [Google Scholar] [CrossRef]
- Meng, C.; Chen, Z.; Li, G.; Welte, T.; Shen, H. Nanoplatforms for mRNA therapeutics. Adv. Ther. 2021, 4, 2000099. [Google Scholar] [CrossRef]
- Bailey, A.L.; Cullis, P.R. Modulation of membrane fusion by asymmetric transbilayer distributions of amino lipids. Biochemistry 1994, 33, 12573–12580. [Google Scholar] [CrossRef] [PubMed]
- Schlich, M.; Palomba, R.; Costabile, G.; Mizrahy, S.; Pannuzzo, M.; Peer, D.; Decuzzi, P. Cytosolic delivery of nucleic acids: The case of ionizable lipid nanoparticles. Bioeng. Transl. Med. 2021, 6, e10213. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Pelc, R.S.; Muramatsu, H.; Andersen, H.; DeMaso, C.R.; Dowd, K.A.; Sutherland, L.L.; Scearce, R.M.; Parks, R.; et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature 2017, 543, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Oberli, M.A.; Reichmuth, A.M.; Dorkin, J.R.; Mitchell, M.J.; Fenton, O.S.; Jaklenec, A.; Anderson, D.G.; Langer, R.; Blankschtein, D. Lipid Nanoparticle Assisted mRNA Delivery for Potent Cancer Immunotherapy. Nano Lett. 2017, 17, 1326–1335. [Google Scholar] [CrossRef]
- Liang, F.; Lindgren, G.; Lin, A.; Thompson, E.A.; Ols, S.; Rohss, J.; John, S.; Hassett, K.; Yuzhakov, O.; Bahl, K.; et al. Efficient Targeting and Activation of Antigen-Presenting Cells In Vivo after Modified mRNA Vaccine Administration in Rhesus Macaques. Mol. Ther. 2017, 25, 2635–2647. [Google Scholar] [CrossRef]
- Patel, S.; Ashwanikumar, N.; Robinson, E.; DuRoss, A.; Sun, C.; Murphy-Benenato, K.E.; Mihai, C.; Almarsson, O.; Sahay, G. Boosting Intracellular Delivery of Lipid Nanoparticle-Encapsulated mRNA. Nano Lett. 2017, 17, 5711–5718. [Google Scholar] [CrossRef]
- Leung, A.K.; Tam, Y.Y.; Cullis, P.R. Lipid nanoparticles for short interfering RNA delivery. Adv. Genet. 2014, 88, 71–110. [Google Scholar] [CrossRef]
- Jayaraman, M.; Ansell, S.M.; Mui, B.L.; Tam, Y.K.; Chen, J.; Du, X.; Butler, D.; Eltepu, L.; Matsuda, S.; Narayanannair, J.K.; et al. Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angew. Chem. Int. Ed. Engl. 2012, 51, 8529–8533. [Google Scholar] [CrossRef]
- Zimmermann, T.S.; Lee, A.C.; Akinc, A.; Bramlage, B.; Bumcrot, D.; Fedoruk, M.N.; Harborth, J.; Heyes, J.A.; Jeffs, L.B.; John, M.; et al. RNAi-mediated gene silencing in non-human primates. Nature 2006, 441, 111–114. [Google Scholar] [CrossRef]
- Semple, S.C.; Klimuk, S.K.; Harasym, T.O.; Dos Santos, N.; Ansell, S.M.; Wong, K.F.; Maurer, N.; Stark, H.; Cullis, P.R.; Hope, M.J.; et al. Efficient encapsulation of antisense oligonucleotides in lipid vesicles using ionizable aminolipids: Formation of novel small multilamellar vesicle structures. Biochim. Biophys. Acta 2001, 1510, 152–166. [Google Scholar] [CrossRef]
- Maurer, N.; Wong, K.F.; Stark, H.; Louie, L.; McIntosh, D.; Wong, T.; Scherrer, P.; Semple, S.C.; Cullis, P.R. Spontaneous entrapment of polynucleotides upon electrostatic interaction with ethanol-destabilized cationic liposomes. Biophys. J. 2001, 80, 2310–2326. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, J.A.; Witzigmann, D.; Chen, S.; Cullis, P.R.; van der Meel, R. Lipid Nanoparticle Technology for Clinical Translation of siRNA Therapeutics. Acc. Chem. Res. 2019, 52, 2435–2444. [Google Scholar] [CrossRef] [PubMed]
- Heyes, J.; Palmer, L.; Bremner, K.; MacLachlan, I. Cationic lipid saturation influences intracellular delivery of encapsulated nucleic acids. J. Control. Release 2005, 107, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Semple, S.C.; Akinc, A.; Chen, J.; Sandhu, A.P.; Mui, B.L.; Cho, C.K.; Sah, D.W.; Stebbing, D.; Crosley, E.J.; Yaworski, E.; et al. Rational design of cationic lipids for siRNA delivery. Nat. Biotechnol. 2010, 28, 172–176. [Google Scholar] [CrossRef]
- Coelho, T.; Adams, D.; Silva, A.; Lozeron, P.; Hawkins, P.N.; Mant, T.; Perez, J.; Chiesa, J.; Warrington, S.; Tranter, E.; et al. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N. Engl. J. Med. 2013, 369, 819–829. [Google Scholar] [CrossRef]
- Akinc, A.; Maier, M.A.; Manoharan, M.; Fitzgerald, K.; Jayaraman, M.; Barros, S.; Ansell, S.; Du, X.; Hope, M.J.; Madden, T.D.; et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat. Nanotechnol. 2019, 14, 1084–1087. [Google Scholar] [CrossRef]
- Zhi, D.; Bai, Y.; Yang, J.; Cui, S.; Zhao, Y.; Chen, H.; Zhang, S. A review on cationic lipids with different linkers for gene delivery. Adv. Colloid Interface Sci. 2018, 253, 117–140. [Google Scholar] [CrossRef]
- Cullis, P.R.; Hope, M.J. Lipid Nanoparticle Systems for Enabling Gene Therapies. Mol. Ther. 2017, 25, 1467–1475. [Google Scholar] [CrossRef]
- Kim, J.; Eygeris, Y.; Gupta, M.; Sahay, G. Self-assembled mRNA vaccines. Adv. Drug Deliv. Rev. 2021, 170, 83–112. [Google Scholar] [CrossRef]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. Engl. 2010, 49, 6288–6308. [Google Scholar] [CrossRef]
- Akinc, A.; Goldberg, M.; Qin, J.; Dorkin, J.R.; Gamba-Vitalo, C.; Maier, M.; Jayaprakash, K.N.; Jayaraman, M.; Rajeev, K.G.; Manoharan, M.; et al. Development of lipidoid-siRNA formulations for systemic delivery to the liver. Mol. Ther. 2009, 17, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Tao, W.; Liu, D.; Wu, J.; Guo, Z.; Ji, X.; Bharwani, Z.; Zhao, L.; Zhao, X.; Farokhzad, O.C.; et al. Surface De-PEGylation Controls Nanoparticle-Mediated siRNA Delivery In Vitro and In Vivo. Theranostics 2017, 7, 1990–2002. [Google Scholar] [CrossRef] [PubMed]
- Heyes, J.; Hall, K.; Tailor, V.; Lenz, R.; MacLachlan, I. Synthesis and characterization of novel poly(ethylene glycol)-lipid conjugates suitable for use in drug delivery. J. Control. Release 2006, 112, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Ambegia, E.; Ansell, S.; Cullis, P.; Heyes, J.; Palmer, L.; MacLachlan, I. Stabilized plasmid-lipid particles containing PEG-diacylglycerols exhibit extended circulation lifetimes and tumor selective gene expression. Biochim. Biophys. Acta 2005, 1669, 155–163. [Google Scholar] [CrossRef]
- Belliveau, N.M.; Huft, J.; Lin, P.J.; Chen, S.; Leung, A.K.; Leaver, T.J.; Wild, A.W.; Lee, J.B.; Taylor, R.J.; Tam, Y.K.; et al. Microfluidic Synthesis of Highly Potent Limit-size Lipid Nanoparticles for In Vivo Delivery of siRNA. Mol. Ther. Nucleic Acids 2012, 1, e37. [Google Scholar] [CrossRef]
- Mui, B.L.; Tam, Y.K.; Jayaraman, M.; Ansell, S.M.; Du, X.; Tam, Y.Y.; Lin, P.J.; Chen, S.; Narayanannair, J.K.; Rajeev, K.G.; et al. Influence of Polyethylene Glycol Lipid Desorption Rates on Pharmacokinetics and Pharmacodynamics of siRNA Lipid Nanoparticles. Mol. Ther. Nucleic Acids 2013, 2, e139. [Google Scholar] [CrossRef]
- Parr, M.J.; Ansell, S.M.; Choi, L.S.; Cullis, P.R. Factors influencing the retention and chemical stability of poly(ethylene glycol)-lipid conjugates incorporated into large unilamellar vesicles. Biochim. Biophys. Acta 1994, 1195, 21–30. [Google Scholar] [CrossRef]
- Chen, S.; Tam, Y.Y.C.; Lin, P.J.C.; Sung, M.M.H.; Tam, Y.K.; Cullis, P.R. Influence of particle size on the in vivo potency of lipid nanoparticle formulations of siRNA. J. Control. Release 2016, 235, 236–244. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Riley, R.S.; Kashyap, M.V.; Billingsley, M.M.; White, B.; Alameh, M.G.; Bose, S.K.; Zoltick, P.W.; Li, H.; Zhang, R.; Cheng, A.Y.; et al. Ionizable lipid nanoparticles for in utero mRNA delivery. Sci. Adv. 2021, 7, eaba1028. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles-From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Daraee, H.; Etemadi, A.; Kouhi, M.; Alimirzalu, S.; Akbarzadeh, A. Application of liposomes in medicine and drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef]
- Nakhaei, P.; Margiana, R.; Bokov, D.O.; Abdelbasset, W.K.; Jadidi Kouhbanani, M.A.; Varma, R.S.; Marofi, F.; Jarahian, M.; Beheshtkhoo, N. Liposomes: Structure, Biomedical Applications, and Stability Parameters with Emphasis on Cholesterol. Front. Bioeng. Biotechnol. 2021, 9, 705886. [Google Scholar] [CrossRef] [PubMed]
- Çağdaş, M.; Sezer, A.D.; Bucak, S. Liposomes as potential drug carrier systems for drug delivery. Appl. Nanotechnol. Drug Deliv. 2014, 1, 1–50. [Google Scholar]
- Nisini, R.; Poerio, N.; Mariotti, S.; De Santis, F.; Fraziano, M. The Multirole of Liposomes in Therapy and Prevention of Infectious Diseases. Front. Immunol. 2018, 9, 155. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A.; Caccamo, M.T. Smart nanoparticles for drug delivery application: Development of versatile nanocarrier platforms in biotechnology and nanomedicine. J. Nanomater. 2019, 2019, e3702518. [Google Scholar] [CrossRef]
- Shafiei, M.; Ansari, M.N.M.; Razak, S.I.A.; Khan, M.U.A. A Comprehensive Review on the Applications of Exosomes and Liposomes in Regenerative Medicine and Tissue Engineering. Polymers 2021, 13, 2529. [Google Scholar] [CrossRef]
- Monteiro, N.; Martins, A.; Reis, R.L.; Neves, N.M. Liposomes in tissue engineering and regenerative medicine. J. R. Soc. Interface 2014, 11, 20140459. [Google Scholar] [CrossRef]
- Xu, Q.; Tanaka, Y.; Czernuszka, J.T. Encapsulation and release of a hydrophobic drug from hydroxyapatite coated liposomes. Biomaterials 2007, 28, 2687–2694. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.A.; Reynolds, L.; Deitcher, S.R. Pharmacokinetics and pharmacodynamics of vincristine sulfate liposome injection (VSLI) in adults with acute lymphoblastic leukemia. J. Clin. Pharmacol. 2013, 53, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, B.G.; MacDonald, R.C.; Siegel, D.P. Cubic phases in phosphatidylcholine-cholesterol mixtures: Cholesterol as membrane “fusogen”. Biophys. J. 2006, 91, 2508–2516. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D. The relationship between bicontinuous inverted cubic phases and membrane fusion. Surfactant Sci. Ser. 2005, 127, 59. [Google Scholar]
- Ashwell, G.; Harford, J. Carbohydrate-specific receptors of the liver. Annu. Rev. Biochem. 1982, 51, 531–554. [Google Scholar] [CrossRef] [PubMed]
- Grewal, P.K. The Ashwell-Morell receptor. Methods Enzym. 2010, 479, 223–241. [Google Scholar] [CrossRef]
- Grozovsky, R.; Begonja, A.J.; Liu, K.; Visner, G.; Hartwig, J.H.; Falet, H.; Hoffmeister, K.M. The Ashwell-Morell receptor regulates hepatic thrombopoietin production via JAK2-STAT3 signaling. Nat. Med. 2015, 21, 47–54. [Google Scholar] [CrossRef]
- Rigopoulou, E.I.; Roggenbuck, D.; Smyk, D.S.; Liaskos, C.; Mytilinaiou, M.G.; Feist, E.; Conrad, K.; Bogdanos, D.P. Asialoglycoprotein receptor (ASGPR) as target autoantigen in liver autoimmunity: Lost and found. Autoimmun. Rev. 2012, 12, 260–269. [Google Scholar] [CrossRef]
- Morell, A.G.; Gregoriadis, G.; Scheinberg, I.H.; Hickman, J.; Ashwell, G. The role of sialic acid in determining the survival of glycoproteins in the circulation. J. Biol. Chem. 1971, 246, 1461–1467. [Google Scholar] [CrossRef]
- Taylor, M.E.; Drickamer, K.; Imberty, A.; van Kooyk, Y.; Schnaar, R.L.; Etzler, M.E.; Varki, A. Discovery and Classification of Glycan-Binding Proteins. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Mohnen, D., Kinoshita, T., Packer, N.H., Eds.; Cold Spring Harbor: Woodbury, NY, USA, 2022; pp. 375–386. [Google Scholar]
- Wu, J.; Nantz, M.H.; Zern, M.A. Targeting hepatocytes for drug and gene delivery: Emerging novel approaches and applications. Front. Biosci. 2002, 7, d717–d725. [Google Scholar] [CrossRef]
- Lee, Y.C. Biochemistry of carbohydrate-protein interaction. FASEB J. 1992, 6, 3193–3200. [Google Scholar] [CrossRef] [PubMed]
- Spiess, M. The asialoglycoprotein receptor: A model for endocytic transport receptors. Biochemistry 1990, 29, 10009–10018. [Google Scholar] [CrossRef] [PubMed]
- Stockert, R.J. The asialoglycoprotein receptor: Relationships between structure, function, and expression. Physiol. Rev. 1995, 75, 591–609. [Google Scholar] [CrossRef] [PubMed]
- Diao, J.; Michalak, T.I. Composition, antigenic properties and hepatocyte surface expression of the woodchuck asialoglycoprotein receptor. J. Recept. Signal Transduct. 1996, 16, 243–271. [Google Scholar] [CrossRef] [PubMed]
- Morell, A.G.; Scheinberg, I.H. Solubilization of hepatic binding sites for asialo-glycoproteins. Biochem. Biophys. Res. Commun. 1972, 48, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, K.; Kuhlenschmidt, M.; Roseman, S.; Lee, Y.C. Synthesis of some cluster galactosides and their effect on the hepatic galactose-binding system. Arch. Biochem. Biophys. 1980, 205, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Meier, M.; Bider, M.D.; Malashkevich, V.N.; Spiess, M.; Burkhard, P. Crystal structure of the carbohydrate recognition domain of the H1 subunit of the asialoglycoprotein receptor. J. Mol. Biol. 2000, 300, 857–865. [Google Scholar] [CrossRef]
- D’Souza, A.A.; Devarajan, P.V. Asialoglycoprotein receptor mediated hepatocyte targeting-strategies and applications. J. Control. Release 2015, 203, 126–139. [Google Scholar] [CrossRef]
- Roggenbuck, D.; Mytilinaiou, M.G.; Lapin, S.V.; Reinhold, D.; Conrad, K. Asialoglycoprotein receptor (ASGPR): A peculiar target of liver-specific autoimmunity. Autoimmun. Highlights 2012, 3, 119–125. [Google Scholar] [CrossRef]
- Baenziger, J.U.; Fiete, D. Galactose and N-acetylgalactosamine-specific endocytosis of glycopeptides by isolated rat hepatocytes. Cell 1980, 22, 611–620. [Google Scholar] [CrossRef]
- Biessen, E.A.; Sliedregt-Bol, K.; PA, T.H.; Prince, P.; Van der Bilt, E.; Valentijn, A.R.; Meeuwenoord, N.J.; Princen, H.; Bijsterbosch, M.K.; Van der Marel, G.A.; et al. Design of a targeted peptide nucleic acid prodrug to inhibit hepatic human microsomal triglyceride transfer protein expression in hepatocytes. Bioconjugate Chem. 2002, 13, 295–302. [Google Scholar] [CrossRef]
- Rensen, P.C.; van Leeuwen, S.H.; Sliedregt, L.A.; van Berkel, T.J.; Biessen, E.A. Design and synthesis of novel N-acetylgalactosamine-terminated glycolipids for targeting of lipoproteins to the hepatic asialoglycoprotein receptor. J. Med. Chem. 2004, 47, 5798–5808. [Google Scholar] [CrossRef]
- Seymour, L.W.; Ferry, D.R.; Anderson, D.; Hesslewood, S.; Julyan, P.J.; Poyner, R.; Doran, J.; Young, A.M.; Burtles, S.; Kerr, D.J.; et al. Hepatic drug targeting: Phase I evaluation of polymer-bound doxorubicin. J. Clin. Oncol. 2002, 20, 1668–1676. [Google Scholar] [CrossRef]
- Fiume, L.; Mattioli, A.; Balboni, P.G.; Tognon, M.; Barbanti-Brodano, G.; de Vries, J.; Wieland, T. Enhanced inhibition of virus DNA synthesis in hepatocytes by trifluorothymidine coupled to asialofetuin. FEBS Lett. 1979, 103, 47–51. [Google Scholar] [CrossRef]
- Fiume, L.; Mattioli, A.; Busi, C.; Balboni, P.G.; Barbanti-Brodano, G.; De Vries, J.; Altmann, R.; Wieland, T. Selective inhibition of Ectromelia virus DNA synthesis in hepatocytes by adenine-9-beta-D-arabinofuranoside (ara-A) and adenine-9-beta-D-arabinofuranoside 5’-monophosphate (ara-AMP) conjugated to asialofetuin. FEBS Lett. 1980, 116, 185–188. [Google Scholar] [CrossRef]
- Wu, G.Y.; Wu, C.H. Receptor-mediated in vitro gene transformation by a soluble DNA carrier system. J. Biol. Chem. 1987, 262, 4429–4432. [Google Scholar] [CrossRef] [PubMed]
- Plank, C.; Zatloukal, K.; Cotten, M.; Mechtler, K.; Wagner, E. Gene transfer into hepatocytes using asialoglycoprotein receptor mediated endocytosis of DNA complexed with an artificial tetra-antennary galactose ligand. Bioconjugate Chem. 1992, 3, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Hangeland, J.J.; Levis, J.T.; Lee, Y.C.; Ts’o, P.O. Cell-type specific and ligand specific enhancement of cellular uptake of oligodeoxynucleoside methylphosphonates covalently linked with a neoglycopeptide, YEE(ah-GalNAc)3. Bioconjugate Chem. 1995, 6, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Prakash, T.P.; Graham, M.J.; Yu, J.; Carty, R.; Low, A.; Chappell, A.; Schmidt, K.; Zhao, C.; Aghajan, M.; Murray, H.F.; et al. Targeted delivery of antisense oligonucleotides to hepatocytes using triantennary N-acetyl galactosamine improves potency 10-fold in mice. Nucleic Acids Res. 2014, 42, 8796–8807. [Google Scholar] [CrossRef]
- Debacker, A.J.; Voutila, J.; Catley, M.; Blakey, D.; Habib, N. Delivery of Oligonucleotides to the Liver with GalNAc: From Research to Registered Therapeutic Drug. Mol. Ther. 2020, 28, 1759–1771. [Google Scholar] [CrossRef]
- Shen, X.; Corey, D.R. Chemistry, mechanism and clinical status of antisense oligonucleotides and duplex RNAs. Nucleic Acids Res. 2018, 46, 1584–1600. [Google Scholar] [CrossRef]
- Nair, J.K.; Willoughby, J.L.; Chan, A.; Charisse, K.; Alam, M.R.; Wang, Q.; Hoekstra, M.; Kandasamy, P.; Kel’in, A.V.; Milstein, S.; et al. Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J. Am. Chem. Soc. 2014, 136, 16958–16961. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.F.; Swayze, E.E. RNA targeting therapeutics: Molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 259–293. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, J.C.; Kowalski, P.S.; Anderson, D.G. Advances in the delivery of RNA therapeutics: From concept to clinical reality. Genome Med. 2017, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Dhuri, K.; Bechtold, C.; Quijano, E.; Pham, H.; Gupta, A.; Vikram, A.; Bahal, R. Antisense Oligonucleotides: An Emerging Area in Drug Discovery and Development. J. Clin. Med. 2020, 9, 2004. [Google Scholar] [CrossRef]
- Prakash, T.P.; Yu, J.; Migawa, M.T.; Kinberger, G.A.; Wan, W.B.; Østergaard, M.E.; Carty, R.L.; Vasquez, G.; Low, A.; Chappell, A.; et al. Comprehensive Structure-Activity Relationship of Triantennary N-Acetylgalactosamine Conjugated Antisense Oligonucleotides for Targeted Delivery to Hepatocytes. J. Med. Chem. 2016, 59, 2718–2733. [Google Scholar] [CrossRef]
- Castellanos-Rizaldos, E.; Brown, C.R.; Dennin, S.; Kim, J.; Gupta, S.; Najarian, D.; Gu, Y.; Aluri, K.; Enders, J.; Brown, K. Reverse transcription quantitative polymerase chain reaction methods to support pharmacokinetics and drug mechanism of action to advance development of RNA interference therapeutics. Nucleic Acid Ther. 2020, 30, 133–142. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, M.; Hu, B.; Huang, Y. siRNA Design and GalNAc-Empowered Hepatic Targeted Delivery. Methods Mol. Biol. 2021, 2282, 77–100. [Google Scholar] [CrossRef]
- Ray, K.K.; Wright, R.S.; Kallend, D.; Koenig, W.; Leiter, L.A.; Raal, F.J.; Bisch, J.A.; Richardson, T.; Jaros, M.; Wijngaard, P.L.J.; et al. Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol. N. Engl. J. Med. 2020, 382, 1507–1519. [Google Scholar] [CrossRef]
- Fitzgerald, K.; White, S.; Borodovsky, A.; Bettencourt, B.R.; Strahs, A.; Clausen, V.; Wijngaard, P.; Horton, J.D.; Taubel, J.; Brooks, A.; et al. A Highly Durable RNAi Therapeutic Inhibitor of PCSK9. N. Engl. J. Med. 2017, 376, 41–51. [Google Scholar] [CrossRef]
- Kinberger, G.A.; Prakash, T.P.; Yu, J.; Vasquez, G.; Low, A.; Chappell, A.; Schmidt, K.; Murray, H.M.; Gaus, H.; Swayze, E.E.; et al. Conjugation of mono and di-GalNAc sugars enhances the potency of antisense oligonucleotides via ASGR mediated delivery to hepatocytes. Bioorganic Med. Chem. Lett. 2016, 26, 3690–3693. [Google Scholar] [CrossRef]
- Zhou, Y.; Teng, P.; Montgomery, N.T.; Li, X.; Tang, W. Development of Triantennary N-Acetylgalactosamine Conjugates as Degraders for Extracellular Proteins. ACS Cent. Sci. 2021, 7, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Sardh, E.; Harper, P.; Balwani, M.; Stein, P.; Rees, D.; Bissell, D.M.; Desnick, R.; Parker, C.; Phillips, J.; Bonkovsky, H.L.; et al. Phase 1 Trial of an RNA Interference Therapy for Acute Intermittent Porphyria. N. Engl. J. Med. 2019, 380, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.gov.uk/government/news/uk-government-tackles-heart-disease-with-new-partnership (accessed on 29 September 2022).
- Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 15 August 2022).
- Investigators, A.-H.; Boden, W.E.; Probstfield, J.L.; Anderson, T.; Chaitman, B.R.; Desvignes-Nickens, P.; Koprowicz, K.; McBride, R.; Teo, K.; Weintraub, W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N. Engl. J. Med. 2011, 365, 2255–2267. [Google Scholar] [CrossRef] [PubMed]
- Fazio, S.; Guyton, J.R.; Polis, A.B.; Adewale, A.J.; Tomassini, J.E.; Ryan, N.W.; Tershakovec, A.M. Long-term safety and efficacy of triple combination ezetimibe/simvastatin plus extended-release niacin in patients with hyperlipidemia. Am. J. Cardiol. 2010, 105, 487–494. [Google Scholar] [CrossRef]
- Bays, H. Safety of niacin and simvastatin combination therapy. Am. J. Cardiol. 2008, 101, 3B–8B. [Google Scholar] [CrossRef]
- Staels, B.; Dallongeville, J.; Auwerx, J.; Schoonjans, K.; Leitersdorf, E.; Fruchart, J.C. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation 1998, 98, 2088–2093. [Google Scholar] [CrossRef]
- Insua, A.; Massari, F.; Rodriguez Moncalvo, J.J.; Ruben Zanchetta, J.; Insua, A.M. Fenofibrate of gemfibrozil for treatment of types IIa and IIb primary hyperlipoproteinemia: A randomized, double-blind, crossover study. Endocr. Pract. 2002, 8, 96–101. [Google Scholar] [CrossRef]
- Aldridge, M.A.; Ito, M.K. Colesevelam hydrochloride: A novel bile acid-binding resin. Ann. Pharmacother. 2001, 35, 898–907. [Google Scholar] [CrossRef]
- Heel, R.C.; Brogden, R.N.; Pakes, G.E.; Speight, T.M.; Avery, G.S. Colestipol: A review of its pharmacological properties and therapeutic efficacy in patients with hypercholesterolaemia. Drugs 1980, 19, 161–180. [Google Scholar] [CrossRef]
- Insull, W., Jr. Clinical utility of bile acid sequestrants in the treatment of dyslipidemia: A scientific review. South. Med. J. 2006, 99, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R. Cholesterol Lowering Drugs. [Updated 2021 Mar 30]. In Endotext [Internet]; Feingold, K.R., Boyce, A.B.A., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK395573/ (accessed on 29 September 2022).
- Chung, B.H.Y.; Chau, J.F.T.; Wong, G.K. Rare versus common diseases: A false dichotomy in precision medicine. NPJ Genom. Med. 2021, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Garg, J.; Shah, N.; Sumner, A. PCSK9 inhibitors: A new era of lipid lowering therapy. World J. Cardiol. 2017, 9, 76–91. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Colon, E.; Daum, A.; Yosefy, C. Statins and PCSK9 inhibitors: A new lipid-lowering therapy. Eur. J. Pharmacol. 2020, 878, 173114. [Google Scholar] [CrossRef]
- Olsson, A. PCSK9 inhibition—A new era in cholesterol treatment. Lakartidningen 2015, 112, DAAF. [Google Scholar]
- Cicero, A.F.; Fogacci, F.; Zambon, A.; Toth, P.P.; Borghi, C. Efficacy and safety of inclisiran a newly approved FDA drug: A systematic review and pooled analysis of available clinical studies. Am. Heart J. Plus Cardiol. Res. Pract. 2022, 13, 100127. [Google Scholar] [CrossRef]
- Abifadel, M.; Varret, M.; Rabes, J.P.; Allard, D.; Ouguerram, K.; Devillers, M.; Cruaud, C.; Benjannet, S.; Wickham, L.; Erlich, D.; et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 2003, 34, 154–156. [Google Scholar] [CrossRef]
- Allard, D.; Amsellem, S.; Abifadel, M.; Trillard, M.; Devillers, M.; Luc, G.; Krempf, M.; Reznik, Y.; Girardet, J.P.; Fredenrich, A.; et al. Novel mutations of the PCSK9 gene cause variable phenotype of autosomal dominant hypercholesterolemia. Hum. Mutat. 2005, 26, 497. [Google Scholar] [CrossRef]
- Santos, R.D.; Rocha, V.Z. Cholesterol Lowering with Inclisiran: A New Chapter in the PCSK9 Story Book; Oxford University Press US: Oxford, UK, 2023; Volume 44, pp. 139–141. [Google Scholar]
- Ruscica, M.; Ferri, N.; Macchi, C.; Meroni, M.; Lanti, C.; Ricci, C.; Maggioni, M.; Fracanzani, A.L.; Badiali, S.; Fargion, S.; et al. Liver fat accumulation is associated with circulating PCSK9. Ann. Med. 2016, 48, 384–391. [Google Scholar] [CrossRef]
- Guo, Y.L.; Zhang, W.; Li, J.J. PCSK9 and lipid lowering drugs. Clin. Chim. Acta 2014, 437, 66–71. [Google Scholar] [CrossRef]
- Handelsman, Y.; Lepor, N.E. PCSK9 Inhibitors in Lipid Management of Patients With Diabetes Mellitus and High Cardiovascular Risk: A Review. J. Am. Heart Assoc. 2018, 7, e008953. [Google Scholar] [CrossRef] [PubMed]
- McKenney, J.M.; Koren, M.J.; Kereiakes, D.J.; Hanotin, C.; Ferrand, A.C.; Stein, E.A. Safety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy. J. Am. Coll. Cardiol. 2012, 59, 2344–2353. [Google Scholar] [CrossRef] [PubMed]
- Roth, E.M.; McKenney, J.M.; Hanotin, C.; Asset, G.; Stein, E.A. Atorvastatin with or without an antibody to PCSK9 in primary hypercholesterolemia. N. Engl. J. Med. 2012, 367, 1891–1900. [Google Scholar] [CrossRef] [PubMed]
- Stein, E.A.; Gipe, D.; Bergeron, J.; Gaudet, D.; Weiss, R.; Dufour, R.; Wu, R.; Pordy, R. Effect of a monoclonal antibody to PCSK9, REGN727/SAR236553, to reduce low-density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: A phase 2 randomised controlled trial. Lancet 2012, 380, 29–36. [Google Scholar] [CrossRef]
- Stroes, E.; Colquhoun, D.; Sullivan, D.; Civeira, F.; Rosenson, R.S.; Watts, G.F.; Bruckert, E.; Cho, L.; Dent, R.; Knusel, B.; et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: The GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J. Am. Coll. Cardiol. 2014, 63, 2541–2548. [Google Scholar] [CrossRef]
- Nissen, S.E.; Stroes, E.; Dent-Acosta, R.E.; Rosenson, R.S.; Lehman, S.J.; Sattar, N.; Preiss, D.; Bruckert, E.; Ceska, R.; Lepor, N.; et al. Efficacy and Tolerability of Evolocumab vs Ezetimibe in Patients with Muscle-Related Statin Intolerance: The GAUSS-3 Randomized Clinical Trial. JAMA 2016, 315, 1580–1590. [Google Scholar] [CrossRef]
- Roth, E.M.; Taskinen, M.R.; Ginsberg, H.N.; Kastelein, J.J.; Colhoun, H.M.; Robinson, J.G.; Merlet, L.; Pordy, R.; Baccara-Dinet, M.T. Monotherapy with the PCSK9 inhibitor alirocumab versus ezetimibe in patients with hypercholesterolemia: Results of a 24 week, double-blind, randomized Phase 3 trial. Int. J. Cardiol. 2014, 176, 55–61. [Google Scholar] [CrossRef]
- Roth, E.M.; Diller, P. Alirocumab for hyperlipidemia: Physiology of PCSK9 inhibition, pharmacodynamics and Phase I and II clinical trial results of a PCSK9 monoclonal antibody. Future Cardiol. 2014, 10, 183–199. [Google Scholar] [CrossRef]
- Matzno, S.; Tazuya-Murayama, K.; Tanaka, H.; Yasuda, S.; Mishima, M.; Uchida, T.; Nakabayashi, T.; Matsuyama, K. Evaluation of the synergistic adverse effects of concomitant therapy with statins and fibrates on rhabdomyolysis. J. Pharm. Pharmacol. 2003, 55, 795–802. [Google Scholar] [CrossRef]
- Banerjee, Y.; Pantea Stoian, A.; Cicero, A.F.G.; Fogacci, F.; Nikolic, D.; Sachinidis, A.; Rizvi, A.A.; Janez, A.; Rizzo, M. Inclisiran: A small interfering RNA strategy targeting PCSK9 to treat hypercholesterolemia. Expert Opin. Drug Saf. 2022, 21, 9–20. [Google Scholar] [CrossRef]
- Brar, K.S. Ezetimibe (Zetia). Med. J. Armed Forces India 2004, 60, 388–389. [Google Scholar] [CrossRef] [PubMed]
- Colletti, R.B.; Neufeld, E.J.; Roff, N.K.; McAuliffe, T.L.; Baker, A.L.; Newburger, J.W. Niacin treatment of hypercholesterolemia in children. Pediatrics 1993, 92, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.ema.europa.eu/en/documents/assessment-report/leqvio-epar-public-assessment-report_en.pdf (accessed on 29 September 2022).
- Tsui, N.B.; Ng, E.K.; Lo, Y.M. Stability of endogenous and added RNA in blood specimens, serum, and plasma. Clin. Chem. 2002, 48, 1647–1653. [Google Scholar] [CrossRef] [PubMed]
- Czauderna, F.; Fechtner, M.; Dames, S.; Aygun, H.; Klippel, A.; Pronk, G.J.; Giese, K.; Kaufmann, J. Structural variations and stabilising modifications of synthetic siRNAs in mammalian cells. Nucleic Acids Res. 2003, 31, 2705–2716. [Google Scholar] [CrossRef]
- Choung, S.; Kim, Y.J.; Kim, S.; Park, H.O.; Choi, Y.C. Chemical modification of siRNAs to improve serum stability without loss of efficacy. Biochem. Biophys. Res. Commun. 2006, 342, 919–927. [Google Scholar] [CrossRef]
- Khvorova, A. Oligonucleotide Therapeutics-A New Class of Cholesterol-Lowering Drugs. N. Engl. J. Med. 2017, 376, 4–7. [Google Scholar] [CrossRef]
- Scicchitano, P.; Milo, M.; Mallamaci, R.; De Palo, M.; Caldarola, P.; Massari, F.; Gabrielli, D.; Colivicchi, F.; Ciccone, M.M. Inclisiran in lipid management: A Literature overview and future perspectives. Biomed. Pharmacother. 2021, 143, 112227. [Google Scholar] [CrossRef]
- Frank-Kamenetsky, M.; Grefhorst, A.; Anderson, N.N.; Racie, T.S.; Bramlage, B.; Akinc, A.; Butler, D.; Charisse, K.; Dorkin, R.; Fan, Y.; et al. Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates. Proc. Natl. Acad. Sci. USA 2008, 105, 11915–11920. [Google Scholar] [CrossRef]
- Fitzgerald, K.; Frank-Kamenetsky, M.; Shulga-Morskaya, S.; Liebow, A.; Bettencourt, B.R.; Sutherland, J.E.; Hutabarat, R.M.; Clausen, V.A.; Karsten, V.; Cehelsky, J.; et al. Effect of an RNA interference drug on the synthesis of proprotein convertase subtilisin/kexin type 9 (PCSK9) and the concentration of serum LDL cholesterol in healthy volunteers: A randomised, single-blind, placebo-controlled, phase 1 trial. Lancet 2014, 383, 60–68. [Google Scholar] [CrossRef]
- Available online: https://clinicaltrials.gov/ct2/results?cond=&term=inclisiran&cntry=&state=&city=&dist= (accessed on 29 September 2022).
- Ray, K.K.; Landmesser, U.; Leiter, L.A.; Kallend, D.; Dufour, R.; Karakas, M.; Hall, T.; Troquay, R.P.; Turner, T.; Visseren, F.L.; et al. Inclisiran in Patients at High Cardiovascular Risk with Elevated LDL Cholesterol. N. Engl. J. Med. 2017, 376, 1430–1440. [Google Scholar] [CrossRef]
- Kosmas, C.E.; Munoz Estrella, A.; Skavdis, A.; Pena Genao, E.; Martinez, I.; Guzman, E. Inclisiran for the Treatment of Cardiovascular Disease: A Short Review on the Emerging Data and Therapeutic Potential. Ther. Clin. Risk Manag. 2020, 16, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Raal, F.J.; Kallend, D.; Ray, K.K.; Turner, T.; Koenig, W.; Wright, R.S.; Wijngaard, P.L.J.; Curcio, D.; Jaros, M.J.; Leiter, L.A.; et al. Inclisiran for the Treatment of Heterozygous Familial Hypercholesterolemia. N. Engl. J. Med. 2020, 382, 1520–1530. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.K.; Kallend, D.; Leiter, L.A.; Raal, F.J.; Koenig, W.; Jaros, M.J.; Schwartz, G.G.; Landmesser, U.; Garcia Conde, L.; Wright, R.S. Effect of inclisiran on lipids in primary prevention: The ORION-11 trial. Eur. Heart J. 2022, 43, 5047–5057. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, J.; Lodish, H.F. Two asialoglycoprotein receptor polypeptides in human hepatoma cells. J. Biol. Chem. 1987, 262, 11825–11832. [Google Scholar] [CrossRef] [PubMed]
- Witzigmann, D.; Quagliata, L.; Schenk, S.H.; Quintavalle, C.; Terracciano, L.M.; Huwyler, J. Variable asialoglycoprotein receptor 1 expression in liver disease: Implications for therapeutic intervention. Hepatol. Res. 2016, 46, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, J.L.S.; Chan, A.; Sehgal, A.; Butler, J.S.; Nair, J.K.; Racie, T.; Shulga-Morskaya, S.; Nguyen, T.; Qian, K.; Yucius, K.; et al. Evaluation of GalNAc-siRNA Conjugate Activity in Pre-clinical Animal Models with Reduced Asialoglycoprotein Receptor Expression. Mol. Ther. 2018, 26, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Cupido, A.J.; Kastelein, J.J.P. Inclisiran for the treatment of hypercholesterolaemia: Implications and unanswered questions from the ORION trials. Cardiovasc. Res. 2020, 116, e136–e139. [Google Scholar] [CrossRef]
- Dyrbuś, K.; Gąsior, M.; Penson, P.; Ray, K.K.; Banach, M. Inclisiran-New hope in the management of lipid disorders? J. Clin. Lipidol. 2020, 14, 16–27. [Google Scholar] [CrossRef]
- Hardy, J.; Niman, S.; Pereira, E.; Lewis, T.; Reid, J.; Choksi, R.; Goldfaden, R.F. A Critical Review of the Efficacy and Safety of Inclisiran. Am. J. Cardiovasc. Drugs 2021, 21, 629–642. [Google Scholar] [CrossRef]
- Pirillo, A.; Catapano, A.L. Inclisiran: How Widely and When Should We Use It? Curr. Atheroscler. Rep. 2022, 24, 803–811. [Google Scholar] [CrossRef]
- Damase, T.R.; Sukhovershin, R.; Boada, C.; Taraballi, F.; Pettigrew, R.I.; Cooke, J.P. The Limitless Future of RNA Therapeutics. Front. Bioeng. Biotechnol. 2021, 9, 628137. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Tang, X.; Chen, Y.; Chen, K.; Fan, N.; Xiao, W.; Zheng, Q.; Li, G.; Teng, Y.; Wu, M.; et al. mRNA-based therapeutics: Powerful and versatile tools to combat diseases. Signal Transduct. Target. Ther. 2022, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.M.; Donner, A.J.; Blank, E.E.; Egger, A.W.; Kellar, B.M.; Østergaard, M.E.; Seth, P.P.; Harris, E.N. Stabilin-1 and Stabilin-2 are specific receptors for the cellular internalization of phosphorothioate-modified antisense oligonucleotides (ASOs) in the liver. Nucleic Acids Res. 2016, 44, 2782–2794. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).