A Small Non-Coding RNA Mediates Transcript Stability and Expression of Cytochrome bd Ubiquinol Oxidase Subunit I in Rickettsia conorii
Abstract
1. Introduction
2. Results
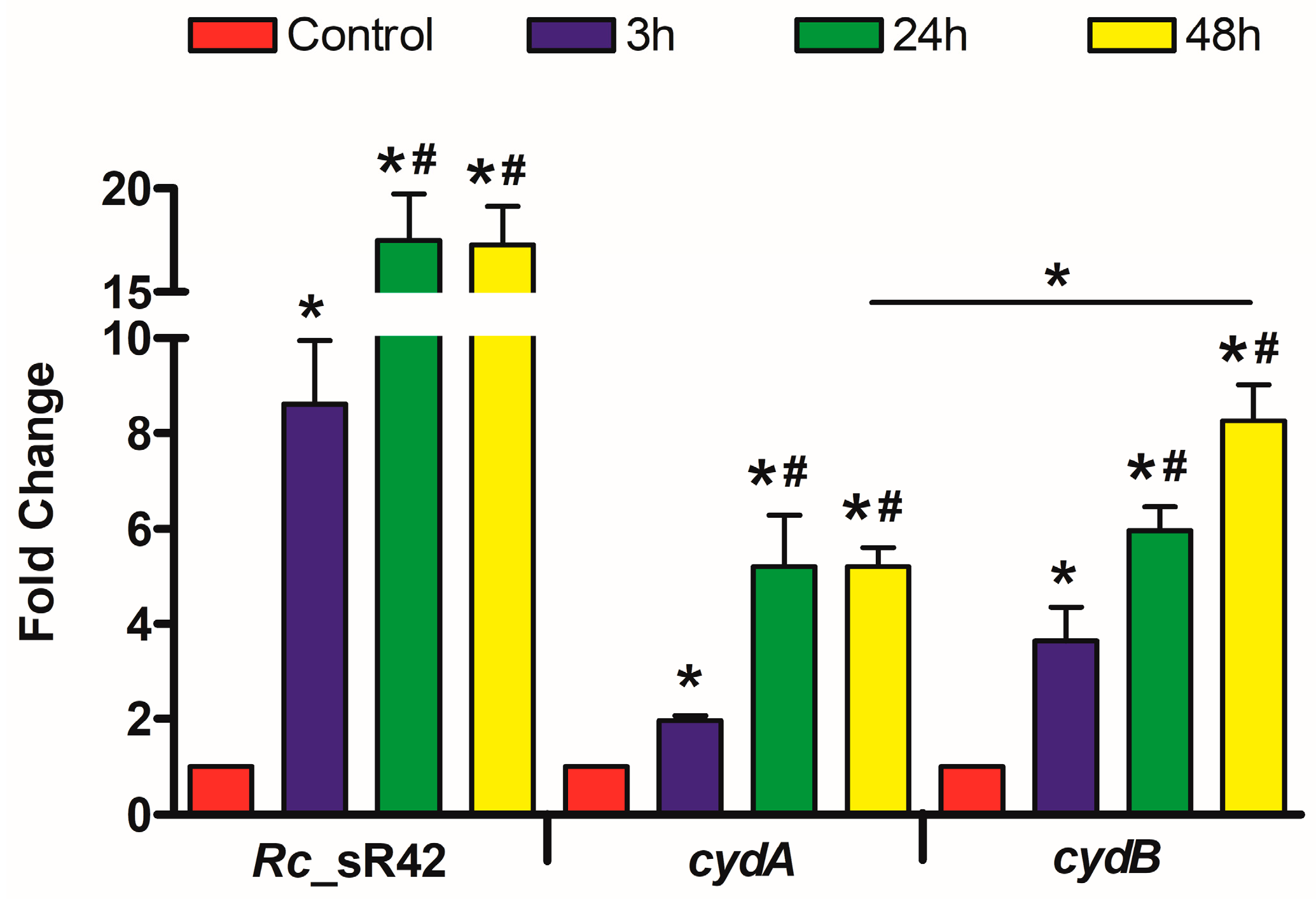
2.1. Transcriptional Changes in R. conorii Rc_sR42, cydA, and cydB during Infection of Mouse b.End3 Cells In Vitro
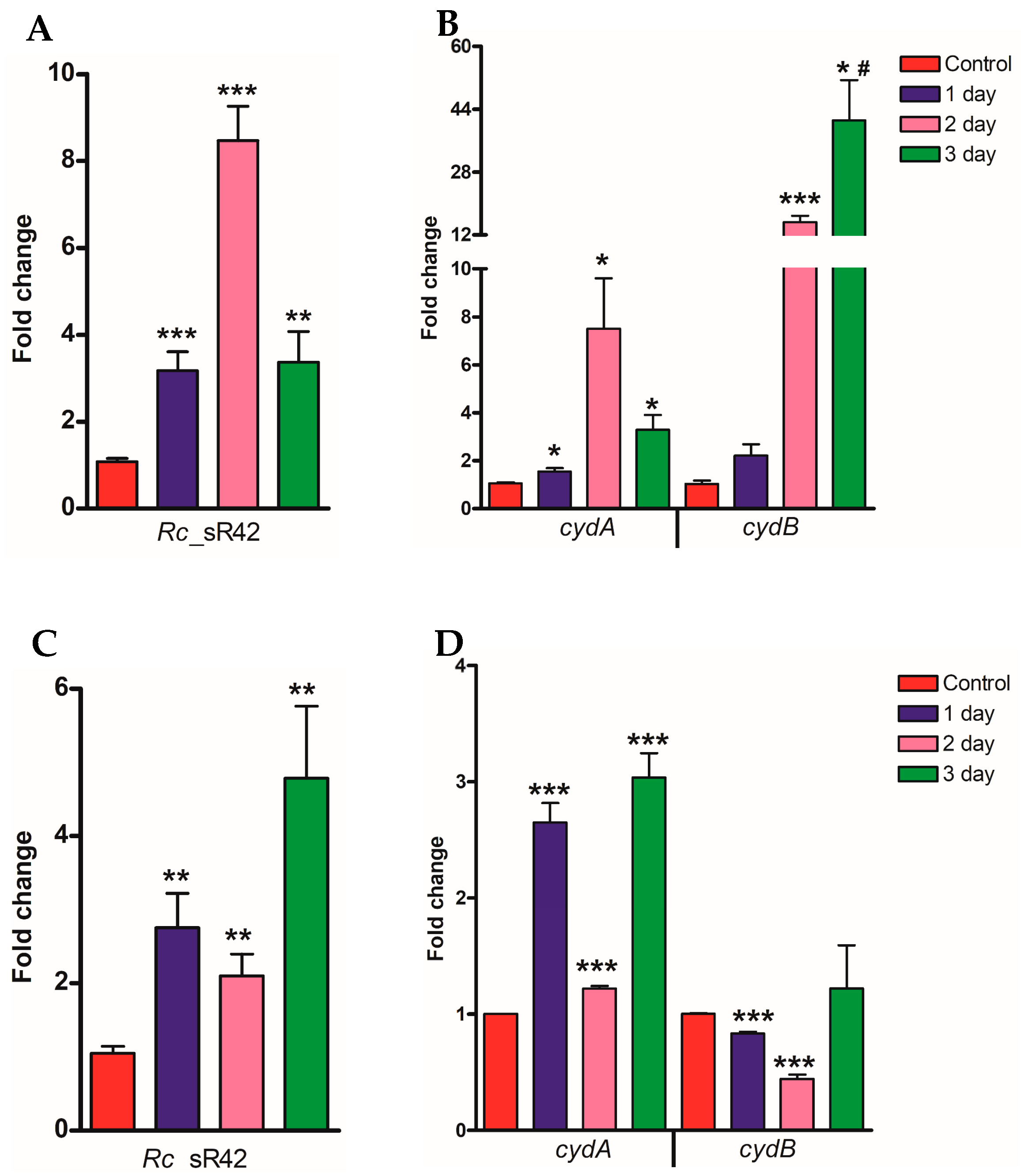
2.2. Expression Profile of Rc_sR42, cydA, and cydB in Lung and Brain Tissues of Mice during R. conorii Infection In Vivo
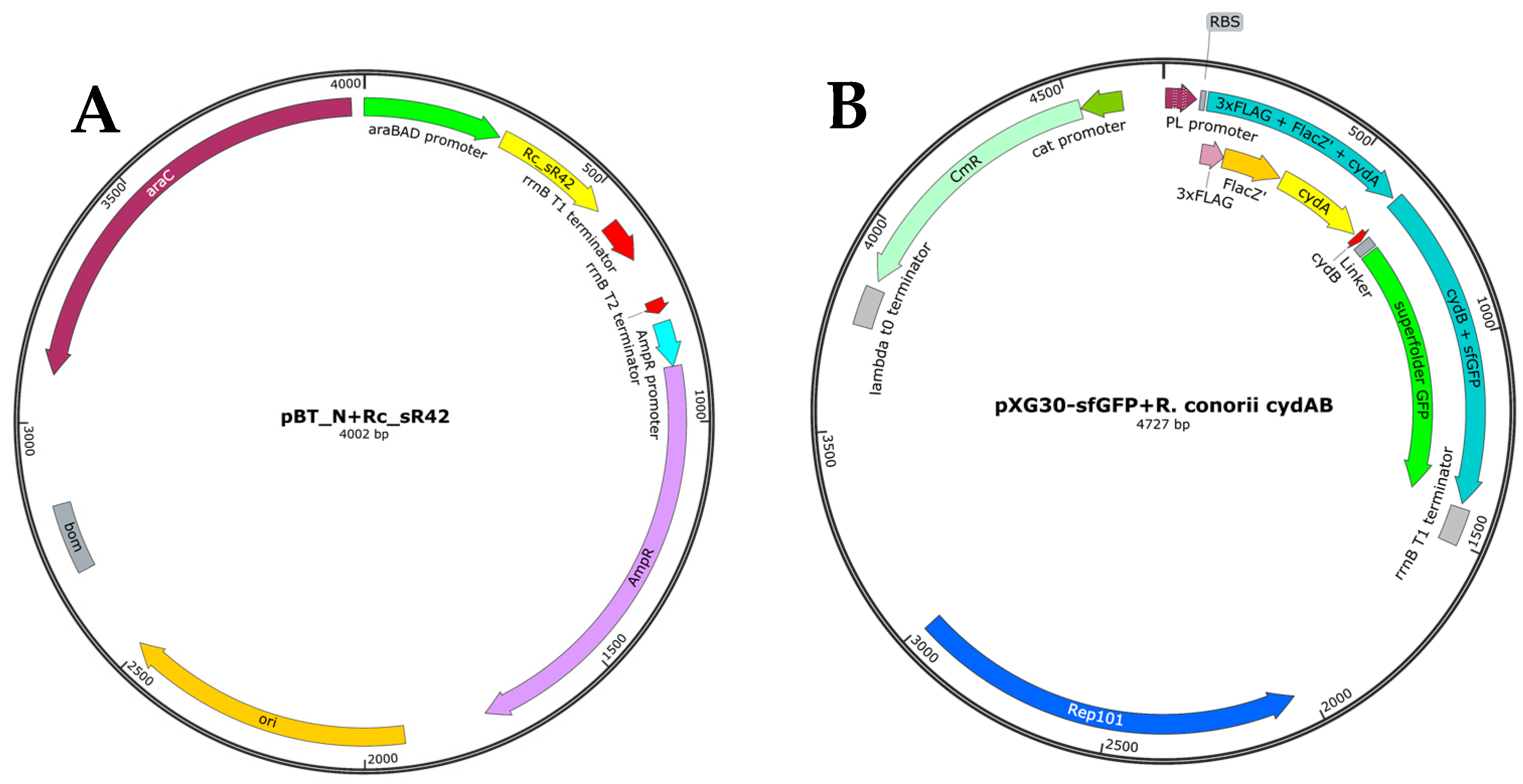
2.3. Generation of Plasmid Constructs for Expression of Small RNAs and Their Cognate Target Gene Seed Regions
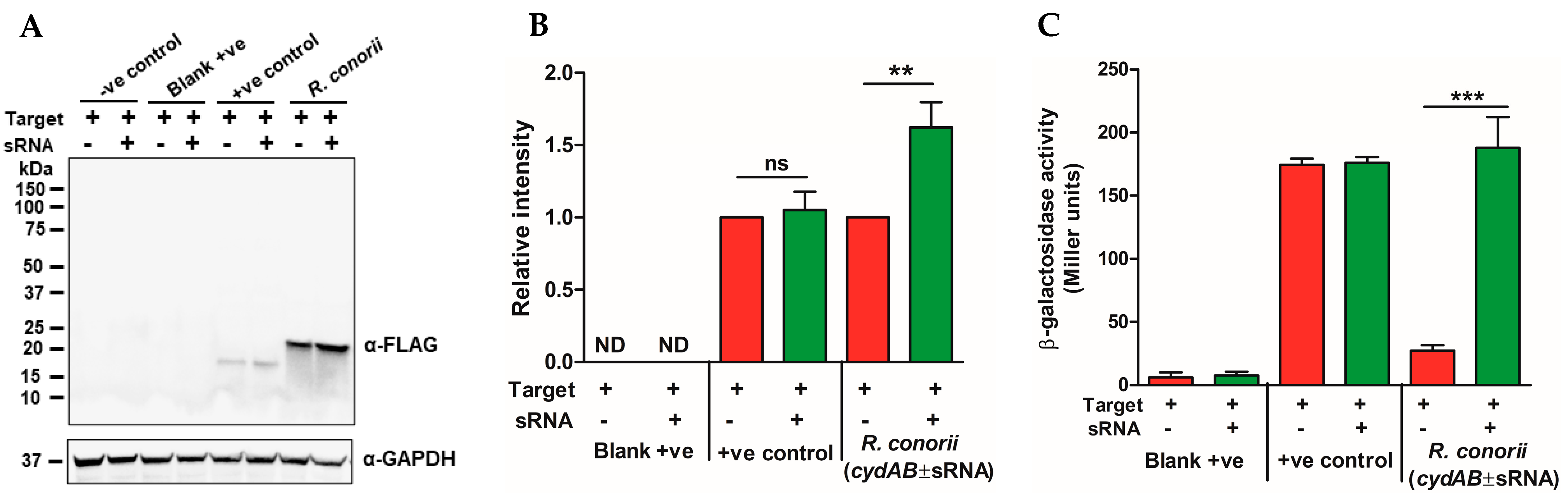
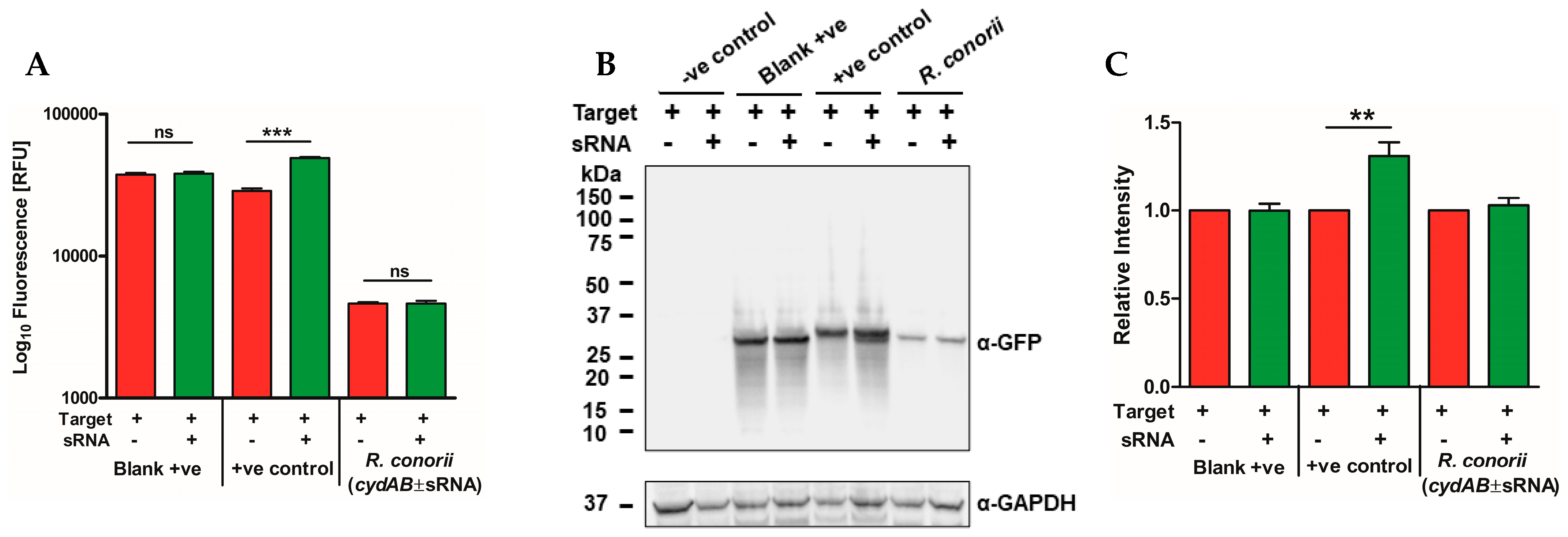
2.4. The R. conorii sRNA Rc_sR42 Is Required for Transcript Stabilization and Expression of cydA
2.5. The R. conorii sRNA Rc_sR42 Is Not Involved in the Regulation of cydB
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Generation of Plasmid Constructs
4.3. Induction of Bacterial Cultures
4.4. Measurement of GFP Fluorescence in Live Bacterial Cultures
4.5. β-Galactosidase Assay
4.6. Western Blotting
4.7. Cell Culture and Infection
4.8. Quantitative Real-Time PCR
4.9. Animal Studies
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Narra, H.P.; Sahni, A.; Walker, D.H.; Sahni, S.K. Recent research milestones in the pathogenesis of human rickettsioses and opportunities ahead. Future Microbiol. 2020, 15, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Ortuno, A.; Sanfeliu, I.; Nogueras, M.M.; Pons, I.; Lopez-Claessens, S.; Castella, J.; Anton, E.; Segura, F. Detection of Rickettsia massiliae/Bar29 and Rickettsia conorii in red foxes (Vulpes vulpes) and their Rhipicephalus sanguineus complex ticks. Ticks Tick Borne Dis. 2018, 9, 629–631. [Google Scholar] [CrossRef] [PubMed]
- Curto, P.; Simoes, I.; Riley, S.P.; Martinez, J.J. Differences in intracellular fate of two spotted fever group Rickettsia in macrophage-like cells. Front Cell Infect. Microbiol. 2016, 6, 80. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Paddock, C.D.; Socolovschi, C.; Labruna, M.B.; Mediannikov, O.; Kernif, T.; Abdad, M.Y.; Stenos, J.; Bitam, I.; Fournier, P.E.; et al. Update on tick-borne rickettsioses around the world: A geographic approach. Clin. Microbiol. Rev. 2013, 26, 657–702. [Google Scholar] [CrossRef] [PubMed]
- Helminiak, L.; Mishra, S.; Keun Kim, H. Pathogenicity and virulence of Rickettsia. Virulence 2022, 13, 1752–1771. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K. Rickettsia-host-tick interactions: Knowledge advances and gaps. Infect. Immun. 2022, 90, e0062121. [Google Scholar] [CrossRef] [PubMed]
- Sahni, S.K.; Narra, H.P.; Sahni, A.; Walker, D.H. Recent molecular insights into rickettsial pathogenesis and immunity. Future Microbiol. 2013, 8, 1265–1288. [Google Scholar] [CrossRef] [PubMed]
- Felden, B.; Augagneur, Y. Diversity and versatility in small RNA-mediated regulation in bacterial pathogens. Front Microbiol. 2021, 12, 719977. [Google Scholar] [CrossRef]
- Svensson, S.L.; Sharma, C.M. Small RNAs in bacterial virulence and communication. Microbiol. Spectr. 2016, 4, 169–212. [Google Scholar] [CrossRef]
- Hor, J.; Matera, G.; Vogel, J.; Gottesman, S.; Storz, G. Trans-acting small RNAs and their effects on gene expression in Escherichia coli and Salmonella enterica. EcoSal. Plus 2020, 9. [Google Scholar]
- Gottesman, S.; Storz, G. Bacterial small RNA regulators: Versatile roles and rapidly evolving variations. Cold Spring Harb. Perspect. Biol. 2011, 3, a003798. [Google Scholar] [CrossRef] [PubMed]
- Storz, G.; Vogel, J.; Wassarman, K.M. Regulation by small RNAs in bacteria: Expanding frontiers. Mol. Cell 2011, 43, 880–891. [Google Scholar] [CrossRef] [PubMed]
- Iosub, I.A.; van Nues, R.W.; McKellar, S.W.; Nieken, K.J.; Marchioretto, M.; Sy, B.; Tree, J.J.; Viero, G.; Granneman, S. Hfq CLASH uncovers sRNA-target interaction networks linked to nutrient availability adaptation. eLife 2020, 9, e54655. [Google Scholar] [CrossRef] [PubMed]
- Faigenbaum-Romm, R.; Reich, A.; Gatt, Y.E.; Barsheshet, M.; Argaman, L.; Margalit, H. Hierarchy in Hfq chaperon occupancy of small RNA targets plays a major role in their regulation. Cell Rep. 2020, 30, 3127–3138.e6. [Google Scholar] [CrossRef] [PubMed]
- Venkat, K.; Hoyos, M.; Haycocks, J.R.; Cassidy, L.; Engelmann, B.; Rolle-Kampczyk, U.; von Bergen, M.; Tholey, A.; Grainger, D.C.; Papenfort, K. A dual-function RNA balances carbon uptake and central metabolism in Vibrio cholerae. EMBO J. 2021, 40, e108542. [Google Scholar] [CrossRef] [PubMed]
- Wadler, C.S.; Vanderpool, C.K. A dual function for a bacterial small RNA: SgrS performs base pairing-dependent regulation and encodes a functional polypeptide. Proc. Natl. Acad. Sci. USA 2007, 104, 20454–20459. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, C.R.; Park, S.; Fei, J.; Vanderpool, C.K. The small protein SgrT controls transport activity of the glucose-specific phosphotransferase system. J. Bacteriol. 2017, 199, e00869-16. [Google Scholar] [CrossRef]
- Thairu, M.W.; Hansen, A.K. It’s a small, small world: Unravelling the role and evolution of small RNAs in organelle and endosymbiont genomes. FEMS Microbiol. Lett. 2019, 366, fnz049. [Google Scholar] [CrossRef]
- Thairu, M.W.; Hansen, A.K. Changes in aphid host plant diet influence the small-RNA expression profiles of its obligate nutritional symbiont, Buchnera. mBio 2019, 10, e01733-19. [Google Scholar] [CrossRef]
- Warrier, I.; Hicks, L.D.; Battisti, J.M.; Raghavan, R.; Minnick, M.F. Identification of novel small RNAs and characterization of the 6S RNA of Coxiella burnetii. PLoS ONE 2014, 9, e100147. [Google Scholar] [CrossRef]
- Wachter, S.; Bonazzi, M.; Shifflett, K.; Moses, A.S.; Raghavan, R.; Minnick, M.F. A CsrA-binding, trans-acting sRNA of Coxiella burnetii is necessary for optimal intracellular growth and vacuole formation during early infection of host cells. J. Bacteriol. 2019, 201, e00524-19. [Google Scholar] [CrossRef] [PubMed]
- Thairu, M.W.; Meduri, V.R.S.; Degnan, P.H.; Hansen, A.K. Natural selection shapes maintenance of orthologous sRNAs in divergent host-restricted bacterial genomes. Mol. Biol. Evol. 2021, 38, 4778–4791. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, C.L.; Narra, H.P.; Rojas, M.; Sahni, A.; Patel, J.; Khanipov, K.; Wood, T.G.; Fofanov, Y.; Sahni, S.K. Bacterial small RNAs in the Genus Rickettsia. BMC Genom. 2015, 16, 1075. [Google Scholar] [CrossRef] [PubMed]
- Narra, H.P.; Schroeder, C.L.; Sahni, A.; Rojas, M.; Khanipov, K.; Fofanov, Y.; Sahni, S.K. Small regulatory RNAs of Rickettsia conorii. Sci. Rep. 2016, 6, 36728. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, C.L.; Narra, H.P.; Sahni, A.; Rojas, M.; Khanipov, K.; Patel, J.; Shah, R.; Fofanov, Y.; Sahni, S.K. Identification and characterization of novel small RNAs in Rickettsia prowazekii. Front. Microbiol. 2016, 7, 859. [Google Scholar] [CrossRef]
- Schroeder, C.L.C.; Narra, H.P.; Sahni, A.; Khanipov, K.; Patel, J.; Fofanov, Y.; Sahni, S.K. Transcriptional profiling of Rickettsia prowazekii coding and non-coding transcripts during in vitro host-pathogen and vector-pathogen interactions. Ticks Tick Borne Dis. 2017, 8, 827–836. [Google Scholar] [CrossRef]
- Narra, H.P.; Sahni, A.; Alsing, J.; Schroeder, C.L.C.; Golovko, G.; Nia, A.M.; Fofanov, Y.; Khanipov, K.; Sahni, S.K. Comparative transcriptomic analysis of Rickettsia conorii during in vitro infection of human and tick host cells. BMC Genom. 2020, 21, 665. [Google Scholar] [CrossRef]
- Corcoran, C.P.; Podkaminski, D.; Papenfort, K.; Urban, J.H.; Hinton, J.C.; Vogel, J. Superfolder GFP reporters validate diverse new mRNA targets of the classic porin regulator, MicF RNA. Mol. Microbiol. 2012, 84, 428–445. [Google Scholar] [CrossRef]
- Urban, J.H.; Vogel, J. Two seemingly homologous noncoding RNAs act hierarchically to activate glmS mRNA translation. PLoS Biol. 2008, 6, e64. [Google Scholar] [CrossRef]
- Borisov, V.B.; Siletsky, S.A.; Paiardini, A.; Hoogewijs, D.; Forte, E.; Giuffre, A.; Poole, R.K. Bacterial oxidases of the cytochrome bd family: Redox enzymes of unique structure, function, and utility as drug targets. Antioxid. Redox Signal 2021, 34, 1280–1318. [Google Scholar] [CrossRef]
- Arutyunyan, A.M.; Sakamoto, J.; Inadome, M.; Kabashima, Y.; Borisov, V.B. Optical and magneto-optical activity of cytochrome bd from Geobacillus thermodenitrificans. Biochim. Biophys. Acta 2012, 1817, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Duc, K.M.; Kang, B.G.; Lee, C.; Park, H.J.; Park, Y.M.; Joung, Y.H.; Bang, I.S. The small protein CydX is required for cytochrome bd quinol oxidase stability and function in Salmonella enterica Serovar Typhimurium: A phenotypic study. J. Bacteriol. 2020, 202, e00348-19. [Google Scholar] [CrossRef] [PubMed]
- VanOrsdel, C.E.; Bhatt, S.; Allen, R.J.; Brenner, E.P.; Hobson, J.J.; Jamil, A.; Haynes, B.M.; Genson, A.M.; Hemm, M.R. The Escherichia coli CydX protein is a member of the CydAB cytochrome bd oxidase complex and is required for cytochrome bd oxidase activity. J. Bacteriol. 2013, 195, 3640–3650. [Google Scholar] [CrossRef] [PubMed]
- Mascolo, L.; Bald, D. Cytochrome bd in Mycobacterium tuberculosis: A respiratory chain protein involved in the defense against antibacterials. Prog. Biophys. Mol. Biol. 2020, 152, 55–63. [Google Scholar] [CrossRef]
- Wall, D.; Delaney, J.M.; Fayet, O.; Lipinska, B.; Yamamoto, T.; Georgopoulos, C. arc-dependent thermal regulation and extragenic suppression of the Escherichia coli cytochrome d operon. J. Bacteriol. 1992, 174, 6554–6562. [Google Scholar] [CrossRef]
- Forte, E.; Borisov, V.B.; Falabella, M.; Colaco, H.G.; Tinajero-Trejo, M.; Poole, R.K.; Vicente, J.B.; Sarti, P.; Giuffre, A. The terminal oxidase cytochrome bd promotes sulfide-resistant bacterial respiration and growth. Sci. Rep. 2016, 6, 23788. [Google Scholar] [CrossRef]
- Teysseire, N.; Boudier, J.A.; Raoult, D. Rickettsia conorii entry into Vero cells. Infect. Immun. 1995, 63, 366–374. [Google Scholar] [CrossRef]
- Bovarnick, M.R.; Snyder, J.C. Respiration of typhus rickettsiae. J. Exp. Med. 1949, 89, 561–565. [Google Scholar] [CrossRef]
- Renesto, P.; Ogata, H.; Audic, S.; Claverie, J.M.; Raoult, D. Some lessons from Rickettsia genomics. FEMS Microbiol. Rev. 2005, 29, 99–117. [Google Scholar] [CrossRef]
- Driscoll, T.P.; Verhoeve, V.I.; Guillotte, M.L.; Lehman, S.S.; Rennoll, S.A.; Beier-Sexton, M.; Rahman, M.S.; Azad, A.F.; Gillespie, J.J. Wholly Rickettsia! reconstructed metabolic profile of the quintessential bacterial parasite of eukaryotic cells. MBio 2017, 8, e00859-1. [Google Scholar] [CrossRef]
- Krab, K.; Kempe, H.; Wikstrom, M. Explaining the enigmatic K(M) for oxygen in cytochrome c oxidase: A kinetic model. Biochim. Biophys. Acta 2011, 1807, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Berney, M.; Cook, G.M. Unique flexibility in energy metabolism allows mycobacteria to combat starvation and hypoxia. PLoS ONE 2010, 5, e8614. [Google Scholar] [CrossRef] [PubMed]
- Beebout, C.J.; Robertson, G.L.; Reinfeld, B.I.; Blee, A.M.; Morales, G.H.; Brannon, J.R.; Chazin, W.J.; Rathmell, W.K.; Rathmell, J.C.; Gama, V.; et al. Uropathogenic Escherichia coli subverts mitochondrial metabolism to enable intracellular bacterial pathogenesis in urinary tract infection. Nat. Microbiol. 2022, 7, 1348–1360. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.J.; Lee, Y.; N, M.P.A.; Wang, X.; Chattoraj, D.K.; Lim, H.M. sRNA-mediated regulation of gal mRNA in E. coli: Involvement of transcript cleavage by RNase E together with Rho-dependent transcription termination. PLoS Genet. 2021, 17, e1009878. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.H.; Blanton, L.S.; Laroche, M.; Fang, R.; Narra, H.P. A vaccine for canine Rocky Mountain spotted fever: An unmet one health need. Vaccines 2022, 10, 1626. [Google Scholar] [CrossRef]
- Tree, J.J.; Granneman, S.; McAteer, S.P.; Tollervey, D.; Gally, D.L. Identification of bacteriophage-encoded anti-sRNAs in pathogenic Escherichia coli. Mol. Cell 2014, 55, 199–213. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H.; Wang, H.; Wang, J.; Bi, Y.; Wang, X.; Yang, R.; Han, Y. Intrinsic plasmids influence MicF-mediated translational repression of ompF in Yersinia pestis. Front Microbiol. 2015, 6, 862. [Google Scholar] [CrossRef]
- Masse, E.; Vanderpool, C.K.; Gottesman, S. Effect of RyhB small RNA on global iron use in Escherichia coli. J. Bacteriol. 2005, 187, 6962–6971. [Google Scholar] [CrossRef]
- Alhassan, A.; Liu, H.; McGill, J.; Cerezo, A.; Jakkula, L.; Nair, A.D.S.; Winkley, E.; Olson, S.; Marlow, D.; Sahni, A.; et al. Rickettsia rickettsii whole cell antigens offer protection against Rocky Mountain spotted fever in the canine host. Infect. Immun. 2018, 87, e00628-18. [Google Scholar] [CrossRef]
- Narra, H.P.; Sahni, A.; Sepuru, K.M.; Alsing, J.; Sahni, S.K. Sensing the messenger: Potential roles of cyclic-di-GMP in rickettsial pathogenesis. Int. J. Mol. Sci. 2022, 23, 3853. [Google Scholar] [CrossRef]
- Griffith, K.L.; Wolf, R.E., Jr. Measuring beta-galactosidase activity in bacteria: Cell growth, permeabilization, and enzyme assays in 96-well arrays. Biochem. Biophys. Res. Commun. 2002, 290, 397–402. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Sahni, A.; Narra, H.P.; Sahni, S.K. MicroRNA-424 regulates the expression of CX3CL1 (fractalkine) in human microvascular endothelial cells during Rickettsia rickettsii infection. Biochem. Biophys. Rep. 2021, 25, 100897. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narra, H.P.; Alsing, J.; Sahni, A.; Montini, M.; Zafar, Y.; Sahni, S.K. A Small Non-Coding RNA Mediates Transcript Stability and Expression of Cytochrome bd Ubiquinol Oxidase Subunit I in Rickettsia conorii. Int. J. Mol. Sci. 2023, 24, 4008. https://doi.org/10.3390/ijms24044008
Narra HP, Alsing J, Sahni A, Montini M, Zafar Y, Sahni SK. A Small Non-Coding RNA Mediates Transcript Stability and Expression of Cytochrome bd Ubiquinol Oxidase Subunit I in Rickettsia conorii. International Journal of Molecular Sciences. 2023; 24(4):4008. https://doi.org/10.3390/ijms24044008
Chicago/Turabian StyleNarra, Hema P., Jessica Alsing, Abha Sahni, Michelle Montini, Yasim Zafar, and Sanjeev K. Sahni. 2023. "A Small Non-Coding RNA Mediates Transcript Stability and Expression of Cytochrome bd Ubiquinol Oxidase Subunit I in Rickettsia conorii" International Journal of Molecular Sciences 24, no. 4: 4008. https://doi.org/10.3390/ijms24044008
APA StyleNarra, H. P., Alsing, J., Sahni, A., Montini, M., Zafar, Y., & Sahni, S. K. (2023). A Small Non-Coding RNA Mediates Transcript Stability and Expression of Cytochrome bd Ubiquinol Oxidase Subunit I in Rickettsia conorii. International Journal of Molecular Sciences, 24(4), 4008. https://doi.org/10.3390/ijms24044008




