Detection and Genotypic Analysis of Anaplasma bovis and A. phagocytophilum in Horse Blood and Lung Tissue
Abstract
1. Introduction
2. Results
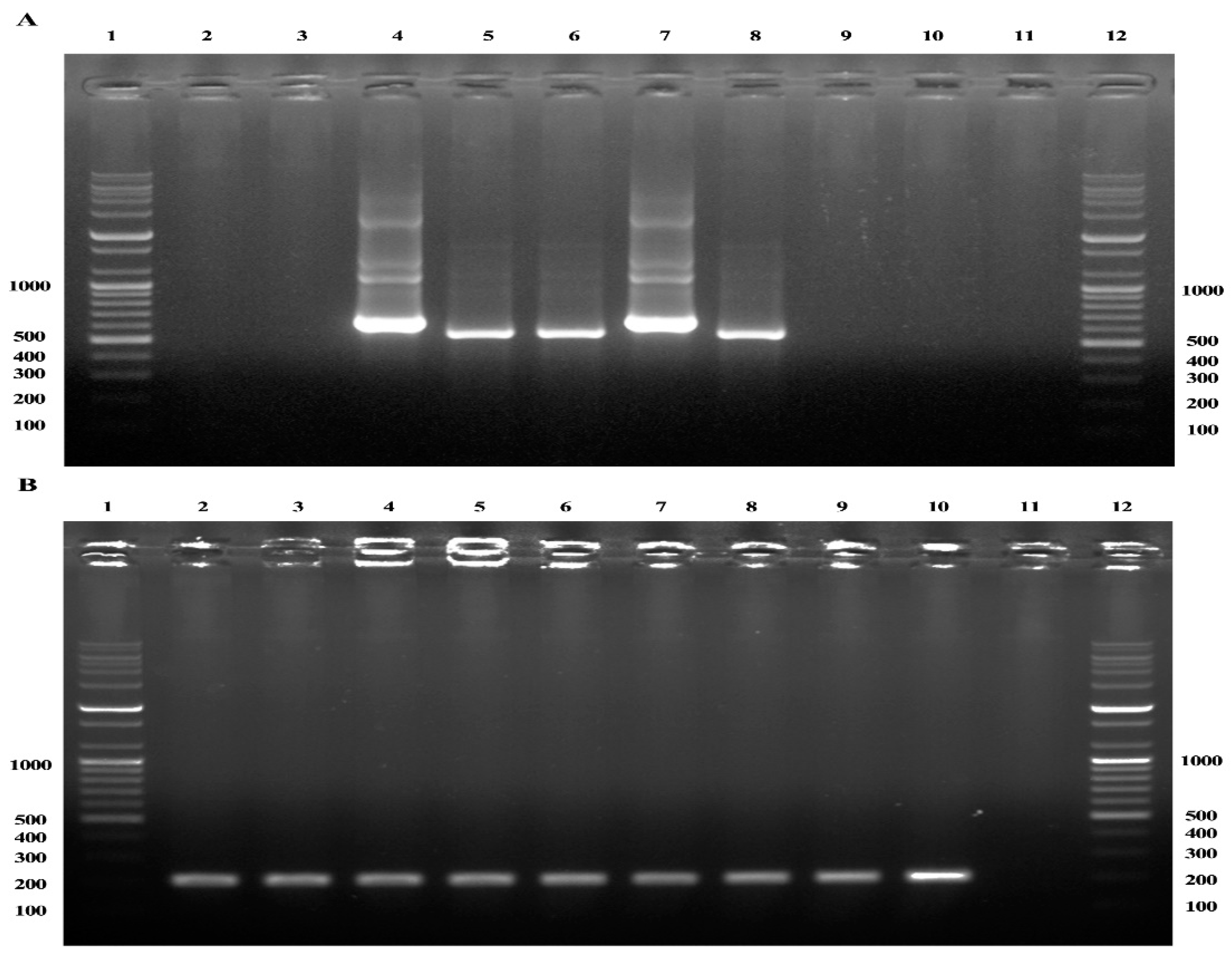
2.1. Nested PCR and Restriction Fragment Length Polymorphism
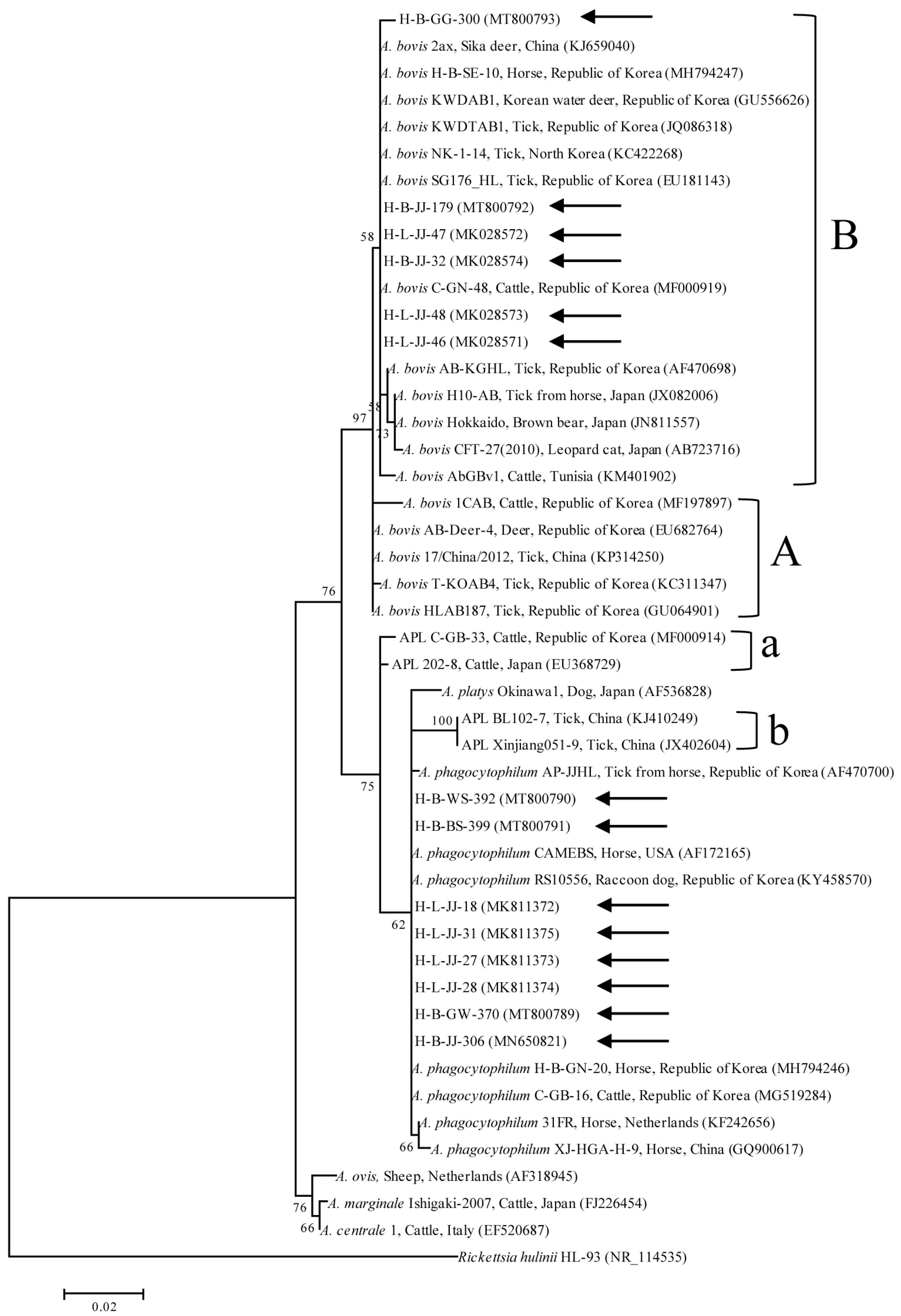
2.2. Cloning, Sequencing, and Phylogeny
3. Discussion
4. Materials and Methods
4.1. Ethical Approval
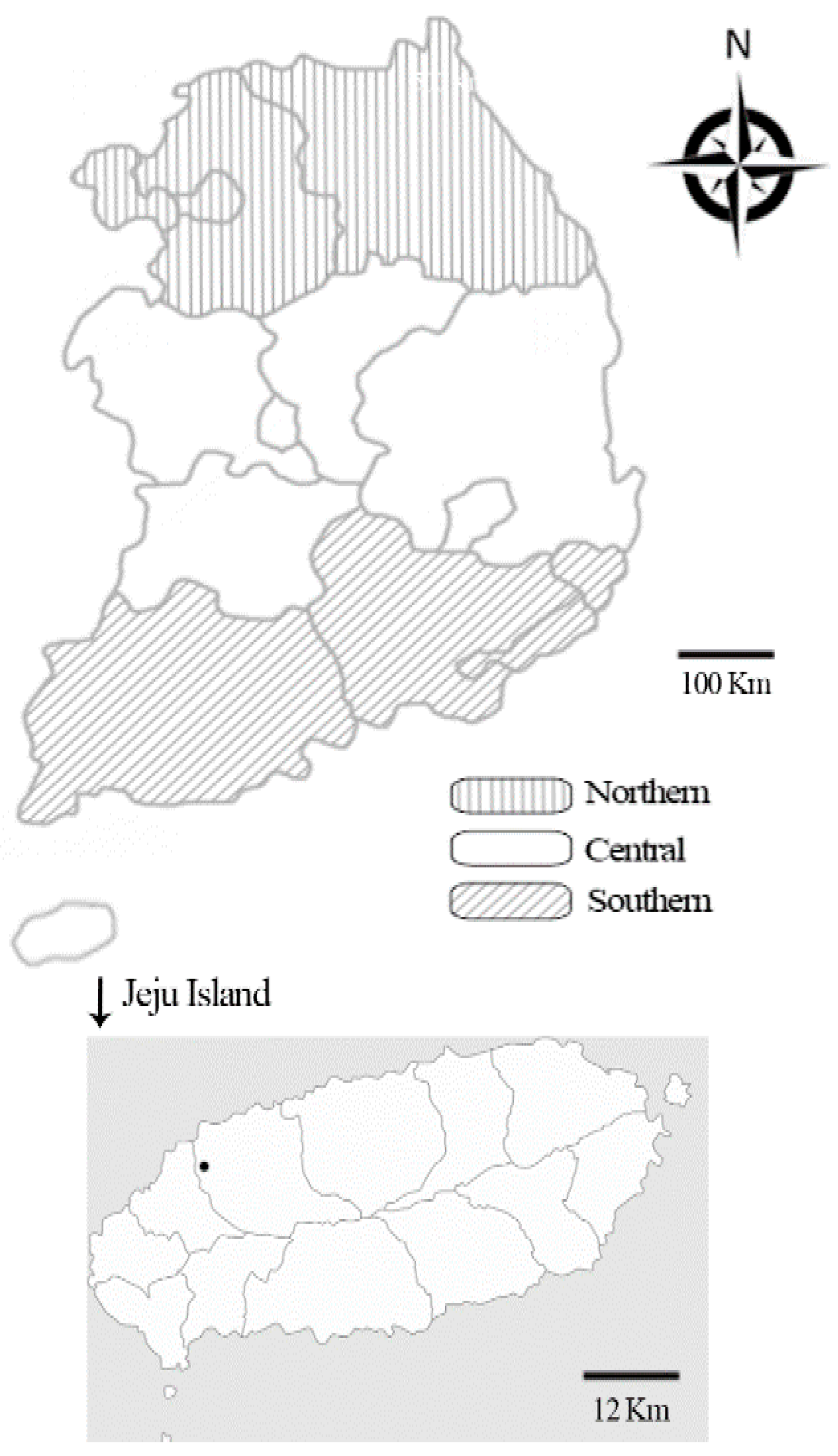
4.2. Sample Size Determination and Sample Collection
4.3. DNA Extraction and PCR
4.4. RFLP
4.5. DNA Cloning
4.6. DNA Sequencing and Phylogenetic Analysis
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dumler, J.S.; Barbet, A.F.; Bekker, C.P.; Dasch, G.A.; Palmer, G.H.; Ray, S.C.; Rikihisa, Y.; Rurangirwa, F.R. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: Unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol. 2001, 51, 2145–2165. [Google Scholar] [CrossRef] [PubMed]
- Madigan, J.E.; Pusterla, N. Ehrlichial diseases. Vet. Clin. N. Am. Equine Pract. 2000, 16, 487–499. [Google Scholar] [CrossRef]
- Seo, M.G.; Kwon, O.D.; Kwak, D. Molecular detection and phylogenetic analysis of canine tick-borne pathogens from Korea. Ticks Tick Borne Dis. 2020, 11, 101357. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Moon, B.-C.; Bae, B.; Shin, E.H.; Ko, Y.-H.; Kim, Y.-J.; Park, Y.; Chae, J.-S. Genetic identification and phylogenetic analysis of Anaplasma and Ehrlichia species in Haemaphysalis longicornis collected from Jeju Island, Korea. J. Bacteriol. Virol. 2009, 39, 257–267. [Google Scholar] [CrossRef]
- Seo, M.G.; Kwon, O.D.; Kwak, D. Molecular detection of Rickettsia raoultii, Rickettsia tamurae, and associated pathogens from ticks parasitizing water deer (Hydropotes inermis argyropus) in South Korea. Ticks Tick Borne Dis. 2021, 12, 101712. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.G.; Kwon, O.D.; Kwak, D. Genotypic analysis of piroplasms and associated pathogens from ticks infesting cattle in Korea. Microorganisms 2020, 8, 728. [Google Scholar] [CrossRef]
- Park, J.; Han, D.G.; Ryu, J.H.; Chae, J.B.; Chae, J.S.; Yu, D.H.; Park, B.K.; Kim, H.C.; Choi, K.S. Molecular detection of Anaplasma bovis in Holstein cattle in the Republic of Korea. Acta Vet. Scand. 2018, 60, 15. [Google Scholar] [CrossRef]
- Seo, M.G.; Ouh, I.O.; Kwon, O.D.; Kwak, D. Molecular detection of Anaplasma phagocytophilum-like Anaplasma spp. and pathogenic A. Phagocytophilum in cattle from South Korea. Mol. Phylogenet. Evol. 2018, 126, 23–30. [Google Scholar] [CrossRef]
- Seo, M.G.; Ouh, I.O.; Lee, H.; Geraldino, P.J.L.; Rhee, M.H.; Kwon, O.D.; Kwak, D. Differential identification of Anaplasma in cattle and potential of cattle to serve as reservoirs of Anaplasma capra, an emerging tick-borne zoonotic pathogen. Vet. Microbiol. 2018, 226, 15–22. [Google Scholar] [CrossRef]
- Lee, M.; Seo, M.G.; Lee, S.H.; Ouh, I.O.; Kim, Y.H.; Kim, J.K.; Goo, Y.K.; Rhee, M.H.; Kim, T.H.; Kwon, O.D.; et al. Molecular detection and phylogenetic analysis of tick-borne pathogens in wild Korean water deer and farmed elk in Gyeongbuk and Gangwon Provinces of Korea. J. Vet. Med. Sci. 2018, 80, 1473–1478. [Google Scholar] [CrossRef]
- Seo, M.G.; Ouh, I.O.; Choi, E.; Kwon, O.D.; Kwak, D. Molecular Detection and Phylogenetic Analysis of Anaplasma phagocytophilum in Horses in Korea. Korean J. Parasitol. 2018, 56, 559–565. [Google Scholar] [CrossRef]
- Seo, M.G.; Kwon, O.D.; Kwak, D. Anaplasma bovis infection in a horse: First clinical report and molecular analysis. Vet. Microbiol. 2019, 233, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Agriculture, Food and Rural Affairs, South Korea (MAFRA). Report of “A Fact Finding Survey of Horse Industry in South Korea during 2017”; Ministry of Agriculture, Food and Rural Affairs: Sejong-Si, Republic of Korea, 2018.
- Silaghi, C.; Santos, A.S.; Gomes, J.; Christova, I.; Matei, I.A.; Walder, G.; Domingos, A.; Bell-Sakyi, L.; Sprong, H.; von Loewenich, F.D.; et al. Guidelines for the direct detection of Anaplasma spp. in diagnosis and epidemiological studies. Vector Borne Zoonot. Dis. 2017, 17, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Lepidi, H.; Bunnell, J.E.; Martin, M.E.; Madigan, J.E.; Stuen, S.; Dumler, J.S. Comparative pathology, and immunohistology associated with clinical illness after Ehrlichia phagocytophila-group infections. Am. J. Trop. Med. Hyg. 2000, 62, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Hoar, B.R.; Nieto, N.C.; Rhodes, D.M.; Foley, J.E. Evaluation of sequential coinfection with Anaplasma phagocytophilum and Anaplasma marginale in cattle. Am. J. Vet. Res. 2008, 69, 1171–1178. [Google Scholar] [CrossRef]
- Wamsley, H.L.; Alleman, A.R.; Johnson, C.M.; Barbet, A.F.; Abbott, J.R. Investigation of endothelial cells as an in vivo nidus of Anaplasma marginale infection in cattle. Vet. Microbiol. 2011, 153, 264–273. [Google Scholar] [CrossRef]
- Härtwig, V.; von Loewenich, F.D.; Schulze, C.; Straubinger, R.K.; Daugschies, A.; Dyachenko, V. Detection of Anaplasma phagocytophilum in red foxes (Vulpes vulpes) and raccoon dogs (Nyctereutes procyonoides) from Brandenburg, Germany. Ticks Tick Borne Dis. 2014, 5, 277–280. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Davis, E.G.; Bai, J. Determination of internal control for gene expression studies in equine tissues and cell culture using quantitative RT-PCR. Vet. Immunol. Immunopathol. 2009, 130, 114–119. [Google Scholar] [CrossRef]
- Brito, A.B.; Lima, J.S.; Brito, D.C.; Santana, L.N.; Costa, N.N.; Miranda, M.S.; Ohashi, O.M.; Santos, R.R.; Domingues, S.F. Validation of reference genes for ovarian tissue from capuchin monkeys (Cebus apella). Zygote 2013, 21, 167–171. [Google Scholar] [CrossRef]
- Huggett, J.; Dheda, K.; Bustin, S.; Zumla, A. Real-time RT-PCR normalisation; strategies and considerations. Genes Immun. 2005, 6, 279–284. [Google Scholar] [CrossRef]
- Figueiredo, M.D.; Salter, C.E.; Andrietti, A.L.; Vandenplas, M.L.; Hurley, D.J.; Moore, J.N. Validation of a reliable set of primer pairs for measuring gene expression by real-time quantitative RT-PCR in equine leukocytes. Vet. Immunol. Immunopathol. 2009, 131, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Stuen, S.; Granquist, E.G.; Silaghi, C. Anaplasma phagocytophilum—A widespread multi-host pathogen with highly adaptive strategies. Front. Cell Infect. Microbiol. 2013, 3, 31. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Chen, X.P.; Liu, N.; Dumler, S.J.; Zhang, Y.Z. Co-circulation of multiple species of Rickettsiales bacteria in one single species of hard ticks in Shenyang, China. Ticks Tick Borne Dis. 2014, 5, 727–733. [Google Scholar] [CrossRef]
- Parola, P.; Paddock, C.D.; Raoult, D. Tick-borne rickettsioses around the world: Emerging diseases challenging old concepts. Clin. Microbiol. Rev. 2005, 18, 719–756. [Google Scholar] [CrossRef]
- Hwang, J.; Oh, D.H.; Lee, H.; Chun, M.S. Anaplasma sp. and hemoplasma infection in leopard cats (Prionailurus bengalensis euptilurus) from Korea. J. Vet. Sci. 2015, 16, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Ybañez, A.P.; Sashika, M.; Inokuma, H. The phylogenetic position of Anaplasma bovis and inferences on the phylogeny of the genus Anaplasma. J. Vet. Med. Sci. 2014, 76, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Masuzawa, T.; Uchishima, Y.; Fukui, T.; Okamoto, Y.; Pan, M.J.; Kadosaka, T.; Takada, N. Detection of Anaplasma phagocytophilum and Anaplasma bovis in small wild mammals from Taichung and Kinmen Island, Taiwan. Jpn. J. Infect. Dis. 2014, 67, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Sashika, M.; Abe, G.; Matsumoto, K.; Inokuma, H. Molecular survey of Anaplasma and Ehrlichia infections of feral raccoons (Procyon lotor) in Hokkaido, Japan. Vector Borne Zoonot. Dis. 2011, 11, 349–354. [Google Scholar] [CrossRef]
- Lu, M.; Chen, Q.; Qin, X.; Lyu, Y.; Teng, Z.; Li, K.; Yu, L.; Jin, X.; Chang, H.; Wang, W.; et al. Anaplasma bovis infection in fever and thrombocytopenia patients—Anhui Province, China, 2021. China CDC Wkly. 2022, 4, 249–253. [Google Scholar] [CrossRef]
- Doan, H.T.; Noh, J.H.; Choe, S.E.; Yoo, M.S.; Kim, Y.H.; Reddy, K.E.; Quyen, D.V.; Nguyen, L.T.; Nguyen, T.T.; Kweon, C.H.; et al. Molecular detection and phylogenetic analysis of Anaplasma bovis from Haemaphysalis longicornis feeding on grazing cattle in Korea. Vet. Parasitol. 2013, 196, 478–481. [Google Scholar] [CrossRef]
- Seong, G.; Han, Y.J.; Chae, J.B.; Chae, J.S.; Yu, D.H.; Lee, Y.S.; Park, J.; Park, B.K.; Yoo, J.G.; Choi, K.S. Detection of Anaplasma sp. in Korean native goats (Capra aegagrus hircus) on Jeju Island, Korea. Korean J. Parasitol. 2015, 53, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ma, M.; Wang, Z.; Wang, J.; Peng, Y.; Li, Y.; Guan, G.; Luo, J.; Yin, H. Molecular survey and genetic identification of Anaplasma species in goats from central and southern China. Appl. Environ. Microbiol. 2012, 78, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Belkahia, H.; Ben Said, M.; Alberti, A.; Abdi, K.; Issaoui, Z.; Hattab, D.; Gharbi, M.; Messadi, L. First molecular survey and novel genetic variants’ identification of Anaplasma marginale, A. centrale and A. bovis in cattle from Tunisia. Infect. Genet. Evol. 2015, 34, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Stuen, S.; Van De Pol, I.; Bergström, K.; Schouls, L.M. Identification of Anaplasma phagocytophila (formerly Ehrlichia phagocytophila) variants in blood from sheep in Norway. J. Clin. Microbiol. 2002, 40, 3192–3197. [Google Scholar] [CrossRef]
- Stuen, S.; Casey, A.N.; Woldehiwet, Z.; French, N.P.; Ogden, N.H. Detection by the polymerase chain reaction of Anaplasma phagocytophilum in tissues of persistently infected sheep. J. Comp. Pathol. 2006, 134, 101–104. [Google Scholar] [CrossRef]
- Kocan, K.M.; de la Fuente, J.; Blouin, E.F.; Coetzee, J.F.; Ewing, S.A. The natural history of Anaplasma marginale. Vet. Parasitol. 2010, 167, 95–107. [Google Scholar] [CrossRef]
- Remy, V.; Hansmann, Y.; De Martino, S.; Christmann, D.; Brouqui, P. Human anaplasmosis presenting as atypical pneumonitis in France. Clin. Infect. Dis. 2003, 37, 846–848. [Google Scholar] [CrossRef]
- Kang, J.G.; Ko, S.; Smith, W.B.; Kim, H.C.; Lee, I.Y.; Chae, J.S. Prevalence of Anaplasma, Bartonella and Borrelia Species in Haemaphysalis longicornis collected from goats in North Korea. J. Vet. Sci. 2016, 17, 207–216. [Google Scholar] [CrossRef]
- Li, H.; Zheng, Y.C.; Ma, L.; Jia, N.; Jiang, B.G.; Jiang, R.R.; Huo, Q.B.; Wang, Y.W.; Liu, H.B.; Chu, Y.L.; et al. Human infection with a novel tick-borne Anaplasma species in China: A surveillance study. Lancet Infect. Dis. 2015, 15, 663–670. [Google Scholar] [CrossRef]
- Chochlakis, D.; Ioannou, I.; Tselentis, Y.; Psaroulaki, A. Human anaplasmosis and Anaplasma ovis variant. Emerg. Infect. Dis. 2010, 16, 1031–1032. [Google Scholar] [CrossRef]
- Thrusfield, M.V.; Christley, R. Veterinary Epidemiology, 4th ed.; Wiley: Hoboken, NJ, USA, 2018. [Google Scholar]
- Seo, M.G.; Lee, S.H.; VanBik, D.; Ouh, I.O.; Yun, S.H.; Choi, E.; Park, Y.S.; Lee, S.E.; Kim, J.W.; Cho, G.J.; et al. Detection and genotyping of Coxiella burnetii and Coxiella-like bacteria in horses in South Korea. PLoS ONE 2016, 11, e0156710. [Google Scholar] [CrossRef] [PubMed]

| Sample | Group | No. Tested | A. bovis | A. phagocytophilum | |||||
|---|---|---|---|---|---|---|---|---|---|
| No. Positive (%) | 95% CI † | p-Value | No. Positive (%) | 95% CI † | p-Value | ||||
| Blood | Sex | Female | 756 | 2 (0.3) | 0–0.6 | 0.6040 | 3 (0.4) | 0–0.8 | 0.5021 |
| Male | 354 | 0 | 0 | 0 | 0 | ||||
| Castrated | 323 | 1 (0.3) | 0–0.9 | 1 (0.3) | 0–0.9 | ||||
| Age (year) | <5 | 460 | 0 | 0 | 0.3204 | 0 | 0 | 0.3752 | |
| 5–10 | 537 | 1 (0.2) | 0–0.6 | 2 (0.4) | 0–0.9 | ||||
| >10 | 436 | 2 (0.5) | 0–1.1 | 2 (0.5) | 0–1.1 | ||||
| Breed | Thoroughbred | 456 | 2 (0.4) | 0–1.0 | 0.4324 | 3 (0.7) | 0–1.4 | 0.2361 | |
| Warmblood | 308 | 1 (0.3) | 0–1.0 | 1 (0.3) | 0–1.0 | ||||
| Native Korean Pony | 416 | 0 | 0 | 0 | 0 | ||||
| Mixed | 253 | 0 | 0 | 0 | 0 | ||||
| Type of activity | Broodmare | 549 | 2 (0.4) | 0–0.9 | 0.6730 | 1 (0.2) | 0–0.5 | 0.2843 | |
| Leisure | 452 | 1 (0.2) | 0–0.7 | 3 (0.7) | 0–1.4 | ||||
| Race | 210 | 0 | 0 | 0 | 0 | ||||
| Breeding | 222 | 0 | 0 | 0 | 0 | ||||
| Region | Northern | 301 | 1 (0.3) | 0–1.0 | 0.8016 | 1 (0.3) | 0–1.0 | 0.0253 * | |
| Central | 216 | 0 | 0 | 0 | 0 | ||||
| Southern | 123 | 0 | 0 | 2 (1.6) | 0–3.9 | ||||
| Jeju Island | 793 | 2 (0.3) | 0–0.6 | 1 (0.1) | 0–0.4 | ||||
| Subtotal | 1433 | 3 (0.2) | 0–0.4 | 4 (0.3) | 0–0.6 | ||||
| Lung | 263 | 26 (9.9) | 6.3–13.5 | 27 (10.3) | 6.6–13.9 | ||||
| Total | 1696 | 29 (1.7) | 1.1–2.3 | 31 (1.8) | 1.2–2.5 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, M.-G.; Ouh, I.-O.; Kwak, D. Detection and Genotypic Analysis of Anaplasma bovis and A. phagocytophilum in Horse Blood and Lung Tissue. Int. J. Mol. Sci. 2023, 24, 3239. https://doi.org/10.3390/ijms24043239
Seo M-G, Ouh I-O, Kwak D. Detection and Genotypic Analysis of Anaplasma bovis and A. phagocytophilum in Horse Blood and Lung Tissue. International Journal of Molecular Sciences. 2023; 24(4):3239. https://doi.org/10.3390/ijms24043239
Chicago/Turabian StyleSeo, Min-Goo, In-Ohk Ouh, and Dongmi Kwak. 2023. "Detection and Genotypic Analysis of Anaplasma bovis and A. phagocytophilum in Horse Blood and Lung Tissue" International Journal of Molecular Sciences 24, no. 4: 3239. https://doi.org/10.3390/ijms24043239
APA StyleSeo, M.-G., Ouh, I.-O., & Kwak, D. (2023). Detection and Genotypic Analysis of Anaplasma bovis and A. phagocytophilum in Horse Blood and Lung Tissue. International Journal of Molecular Sciences, 24(4), 3239. https://doi.org/10.3390/ijms24043239





