Bio-Derived Furanic Compounds with Natural Metabolism: New Sustainable Possibilities for Selective Organic Synthesis
Abstract
1. Introduction
2. Results and Discussion
2.1. Oxidation Level of 2,5-Dimethylfuran (OL = 0%)

2.2. Oxidation Level of Methyl(Hydroxymethyl)furan (OL = 17%)
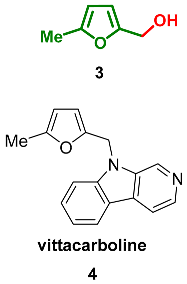
2.3. Oxidation Level of 2,5-Bis(Hydroxymethyl)furan (OL = 33%)
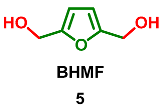
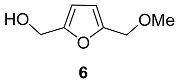
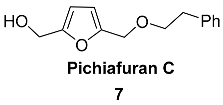

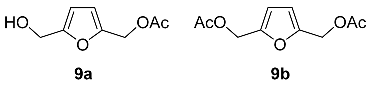
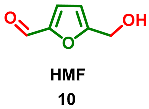
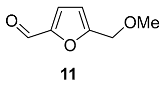
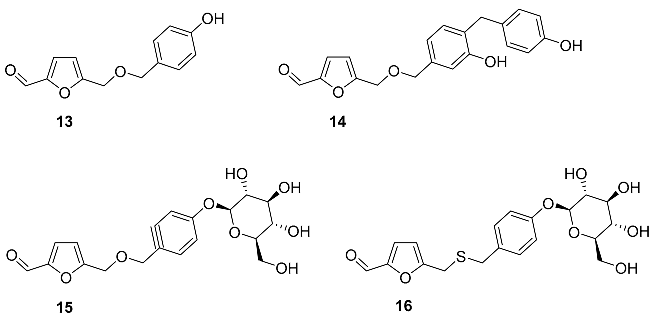
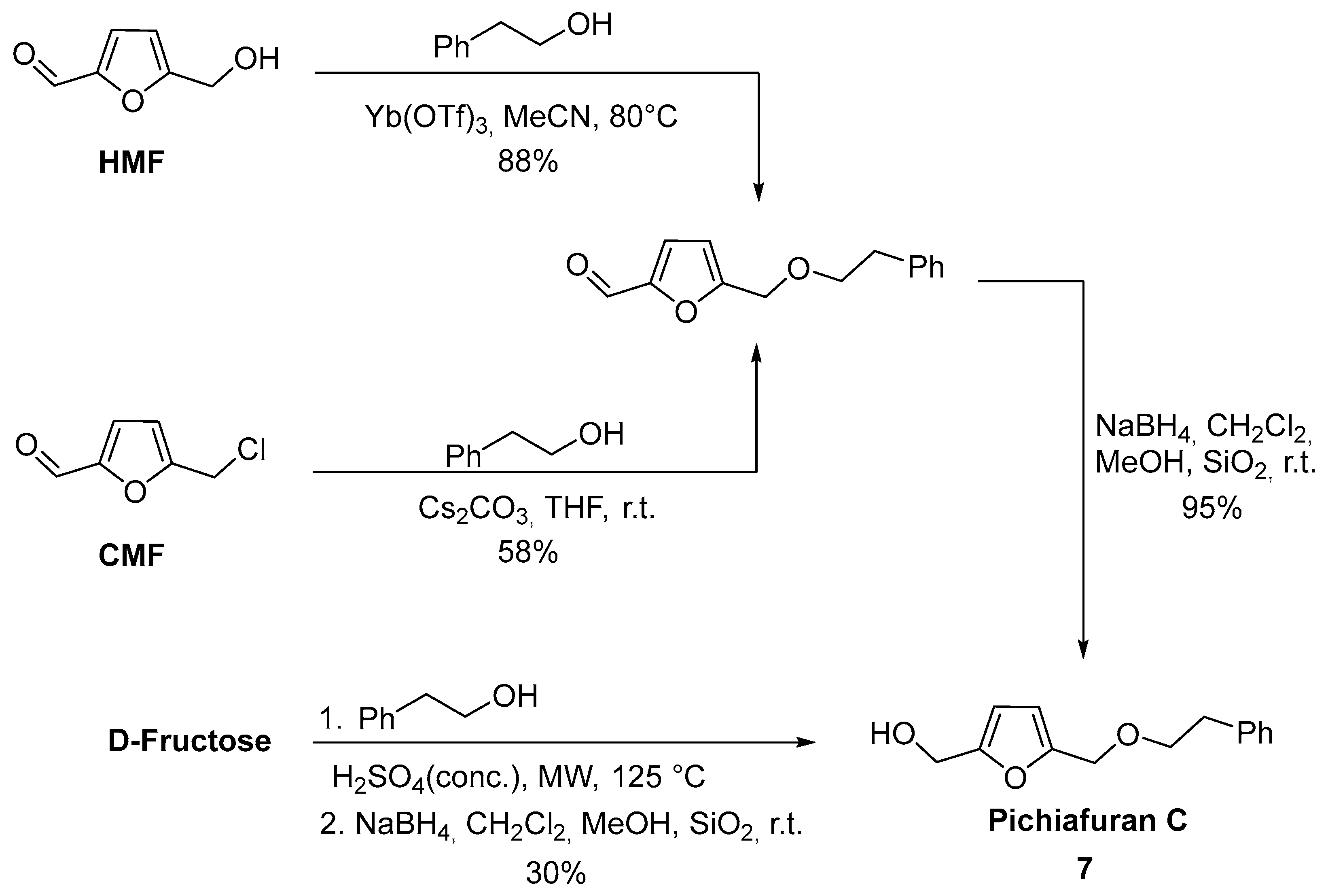
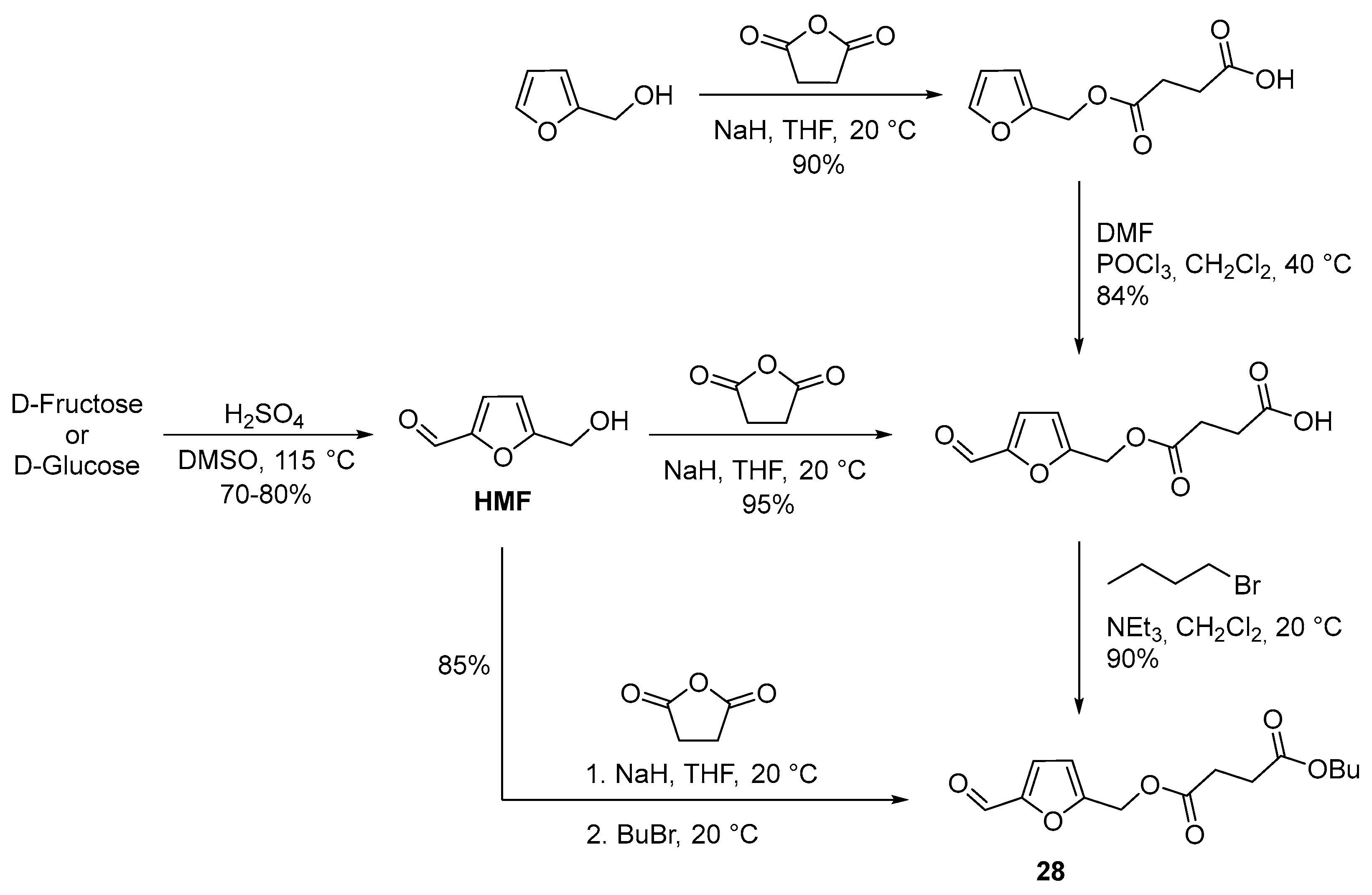
2.4. Oxidation Level of 5-(Hydroxymethyl)furfural (OL = 50%)

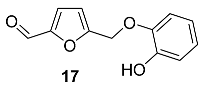

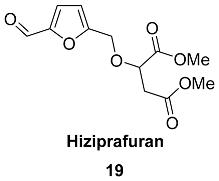
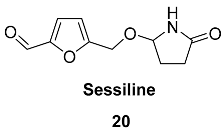

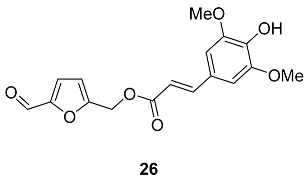
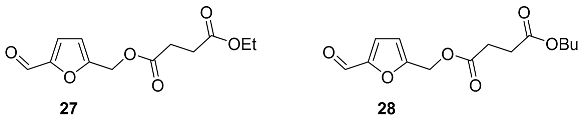
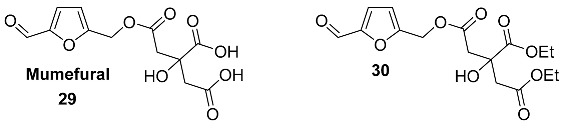
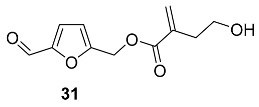

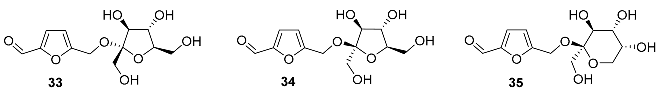
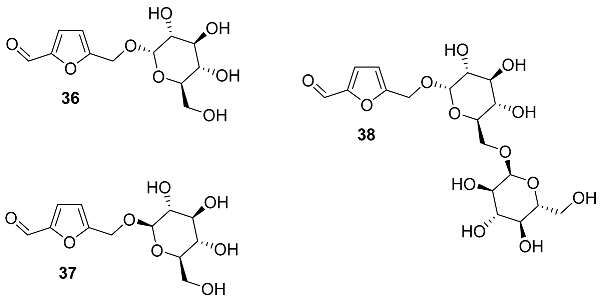
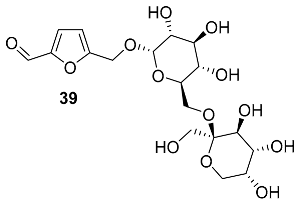
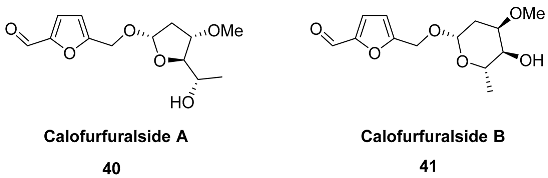
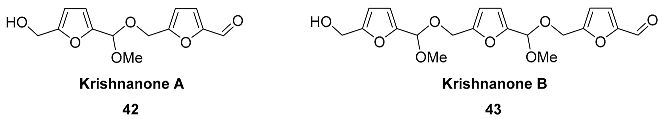

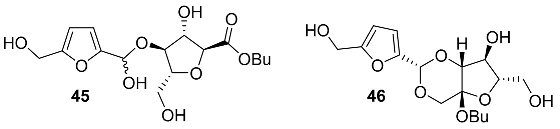
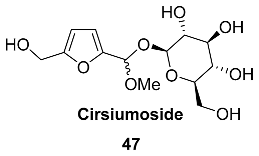
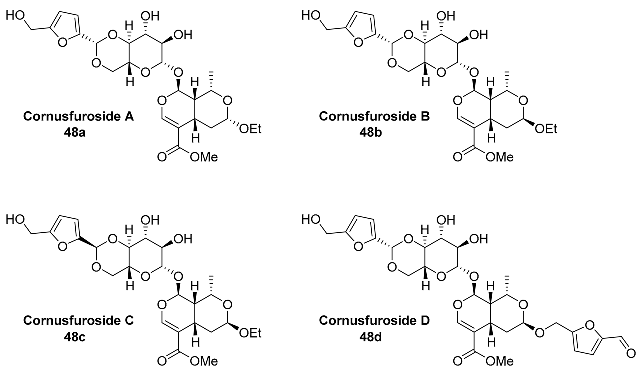
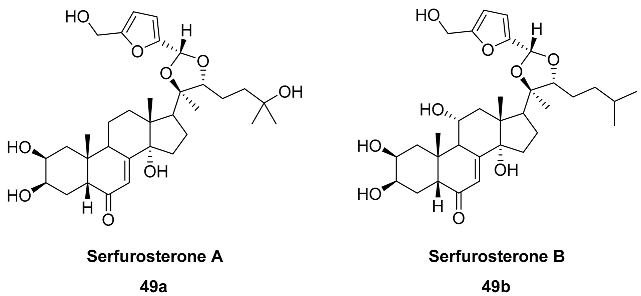
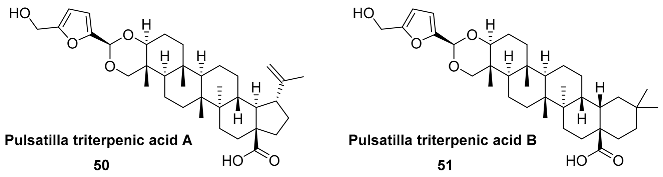


2.5. Oxidation Level of 5-Methylfuran-2-carboxylic Acid (OL = 50%)

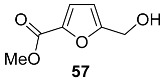
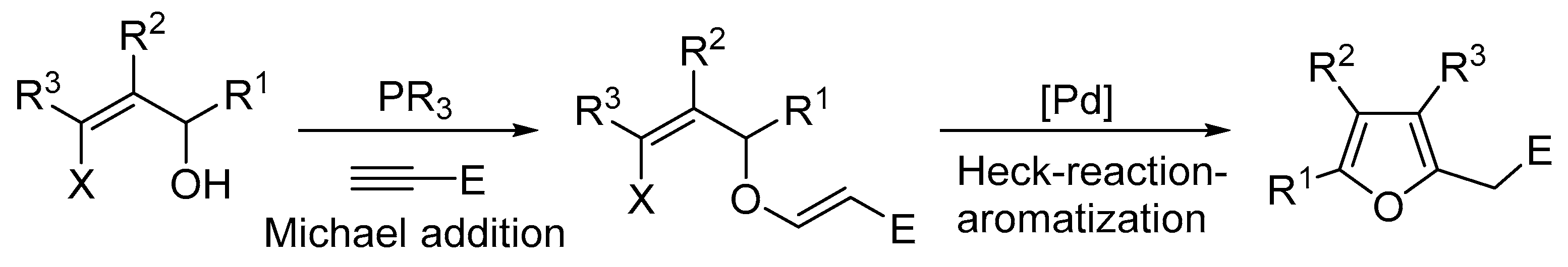
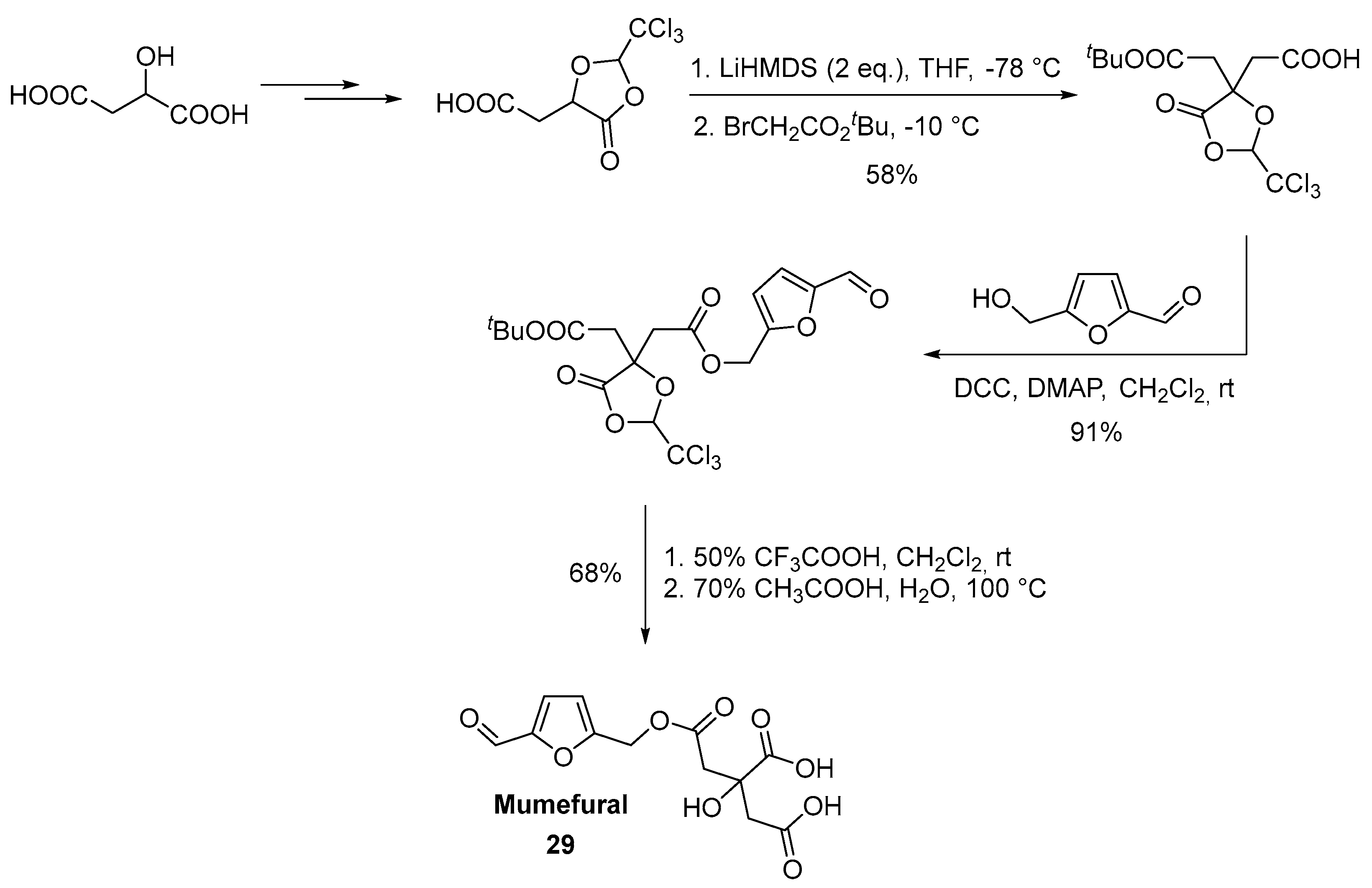
2.6. Oxidation Level of 5-(Hydroxymethyl)furan-2-carboxylic Acid (OL = 67%)

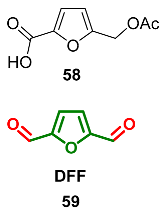
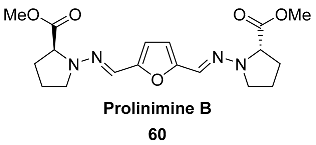
2.7. Oxidation Level of 2,5-Diformylfuran (OL = 67%)
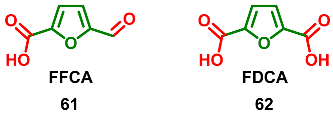
2.8. Oxidation Levels of 5-Formylfuran-2-carboxylic Acid and Furan-2,5-dicarboxylic Acid (OL = 83% and 100%)
3. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Putten, R.-J.; van der Waal, J.C.; de Jong, E.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural, A Versatile Platform Chemical Made from Renewable Resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef] [PubMed]
- Lewkowski, J. Synthesis, chemistry and applications of 5-hydroxymethyl-furfural and its derivatives. Arkivoc 2001, 2001, 17–54. [Google Scholar] [CrossRef]
- Kuster, B.F.M. 5-Hydroxymethylfurfural (HMF). A Review Focussing on its Manufacture. Starke 1990, 42, 314–321. [Google Scholar] [CrossRef]
- Fan, W.; Verrier, C.; Queneau, Y.; Popowycz, F. 5-Hydroxymethylfurfural (HMF) in Organic Synthesis: A Review of its Recent Applications Towards Fine Chemicals. Curr. Org. Synth. 2019, 16, 583–614. [Google Scholar] [CrossRef] [PubMed]
- Kucherov, F.A.; Romashov, L.V.; Galkin, K.I.; Ananikov, V.P. Chemical Transformations of Biomass-Derived C6-Furanic Platform Chemicals for Sustainable Energy Research, Materials Science, and Synthetic Building Blocks. ACS Sustain. Chem. Eng. 2018, 6, 8064–8092. [Google Scholar] [CrossRef]
- Wang, Y.; Brown, C.A.; Chen, R. Industrial production, application, microbial biosynthesis and degradation of furanic compound, hydroxymethylfurfural (HMF). AIMS Microbiol. 2018, 4, 261–273. [Google Scholar] [CrossRef]
- Dutta, S.; Bhat, N.S. Catalytic Transformation of Biomass-Derived Furfurals to Cyclopentanones and Their Derivatives: A Review. ACS Omega 2021, 6, 35145–35172. [Google Scholar] [CrossRef]
- Galkin, K.I.; Ananikov, V.P. Intermolecular Diels-Alder Cycloadditions of Furfural-Based Chemicals from Renewable Resources: A Focus on the Regio- and Diastereoselectivity in the Reaction with Alkenes. Int. J. Mol. Sci. 2021, 22, 11856. [Google Scholar] [CrossRef]
- Kucherov, F.A.; Romashov, L.V.; Averochkin, G.M.; Ananikov, V.P. Biobased C6-Furans in Organic Synthesis and Industry: Cycloaddition Chemistry as a Key Approach to Aromatic Building Blocks. ACS Sustain. Chem. Eng. 2021, 9, 3011–3042. [Google Scholar] [CrossRef]
- Xiong, S.; Guan, Y.; Luo, C.; Zhu, L.; Wang, S. Critical Review on the Preparation of Platform Compounds from Biomass or Saccharides via Hydrothermal Conversion over Carbon-Based Solid Acid Catalysts. Energy Fuels 2021, 35, 14462–14483. [Google Scholar] [CrossRef]
- Davidson, M.G.; Elgie, S.; Parsons, S.; Young, T.J. Production of HMF, FDCA and their derived products: A review of life cycle assessment (LCA) and techno-economic analysis (TEA) studies. Green Chem. 2021, 23, 3154–3171. [Google Scholar] [CrossRef]
- Rosatella, A.A.; Simeonov, S.P.; Frade, R.F.M.; Afonso, C.A.M. 5-Hydroxymethylfurfural (HMF) as a building block platform: Biological properties, synthesis and synthetic applications. Green Chem. 2011, 13, 754–793. [Google Scholar] [CrossRef]
- Tong, X.; Ma, Y.; Li, Y. Biomass into chemicals: Conversion of sugars to furan derivatives by catalytic processes. Appl. Catal. A Gen. 2010, 385, 1–13. [Google Scholar] [CrossRef]
- Mukherjee, A.; Dumont, M.-J.; Raghavan, V. Review: Sustainable production of hydroxymethylfurfural and levulinic acid: Challenges and opportunities. Biomass Bioenergy 2015, 72, 143–183. [Google Scholar] [CrossRef]
- Turkin, A.A.; Makshina, E.V.; Sels, B.F. Catalytic Hydroconversion of 5-HMF to Value-Added Chemicals: Insights into the Role of Catalyst Properties and Feedstock Purity. ChemSusChem 2022, 15, e202200412. [Google Scholar] [CrossRef] [PubMed]
- Galkin, K.I.; Krivodaeva, E.A.; Romashov, L.V.; Zalesskiy, S.S.; Kachala, V.V.; Burykina, J.V.; Ananikov, V.P. Critical Influence of 5-Hydroxymethylfurfural Aging and Decomposition on the Utility of Biomass Conversion in Organic Synthesis. Angew. Chem. Int. Ed. 2016, 55, 8338–8342. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. The E factor 25 years on: The rise of green chemistry and sustainability. Green Chem. 2017, 19, 18–43. [Google Scholar] [CrossRef]
- Sheldon, R.A. The E Factor: Fifteen years on. Green Chem. 2007, 9, 1273–1283. [Google Scholar] [CrossRef]
- Sheldon, R.A. Metrics of Green Chemistry and Sustainability: Past, Present, and Future. ACS Sustain. Chem. Eng. 2018, 6, 32–48. [Google Scholar] [CrossRef]
- Jimenez-Gonzalez, C.C.; Lund, C. Green metrics in pharmaceutical development. Curr. Opin. Green Sustain. Chem. 2022, 33, 100564. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Bode, M.L.; Akakios, S.G. Metrics of green chemistry: Waste minimization. Curr. Opin. Green Sustain. Chem. 2022, 33, 100569. [Google Scholar] [CrossRef]
- Horváth, I.T. Introduction: Sustainable Chemistry. Chem. Rev. 2018, 118, 369–371. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. Utilisation of biomass for sustainable fuels and chemicals: Molecules, methods and metrics. Catal. Today 2011, 167, 3–13. [Google Scholar] [CrossRef]
- Corma, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Musolino, M.; Andraos, J.; Aricò, F. An Easy Scalable Approach to HMF Employing DMC as Reaction Media: Reaction Optimization and Comparative Environmental Assessment. ChemistrySelect 2018, 3, 2359–2365. [Google Scholar] [CrossRef]
- Fiorentino, G.; Ripa, M.; Ulgiati, S. Chemicals from biomass: Technological versus environmental feasibility. A review. Biofuels Bioprod. Biorefining 2016, 11, 195–214. [Google Scholar] [CrossRef]
- de Carvalho, E.G.L.; Rodrigues, F.d.A.; Monteiro, R.S.; Ribas, R.M.; da Silva, M.J. Experimental design and economic analysis of 5-hydroxymethylfurfural synthesis from fructose in acetone-water system using niobium phosphate as catalyst. Biomass Convers. Biorefinery 2018, 8, 635–646. [Google Scholar] [CrossRef]
- Torres, A.I.; Daoutidis, P.; Tsapatsis, M. Continuous production of 5-hydroxymethylfurfural from fructose: A design case study. Energy Environ. Sci. 2010, 3, 1560–1572. [Google Scholar] [CrossRef]
- Tuck, C.O.; Pérez, E.; Horváth, I.T.; Sheldon, R.A.; Poliakoff, M. Valorization of Biomass: Deriving More Value from Waste. Science 2012, 337, 695–699. [Google Scholar] [CrossRef]
- Sheldon, R.A. The Road to Biorenewables: Carbohydrates to Commodity Chemicals. ACS Sustain. Chem. Eng. 2018, 6, 4464–4480. [Google Scholar] [CrossRef]
- Sun, Z.; Fridrich, B.; de Santi, A.; Elangovan, S.; Barta, K. Bright Side of Lignin Depolymerization: Toward New Platform Chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef] [PubMed]
- Anastas, P.T.; Zimmerman, J.B. Chapter 2 The twelve principles of green engineering as a foundation for sustainability. In Sustainability Science and Engineering; Abraham, M.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 1, pp. 11–32. [Google Scholar]
- Zechendorf, B. Sustainable development: How can biotechnology contribute? Trends Biotechnol. 1999, 17, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Mannion, A.M. Sustainable Development and Biotechnology. Environ. Conserv. 2009, 19, 297–306. [Google Scholar] [CrossRef]
- Escobar, N.; Laibach, N. Sustainability check for bio-based technologies: A review of process-based and life cycle approaches. Renew. Sustain. Energy Rev. 2021, 135, 110213. [Google Scholar] [CrossRef]
- Strohmann, M.; Bordet, A.; Vorholt, A.J.; Leitner, W. Tailor-made biofuel 2-butyltetrahydrofuran from the continuous flow hydrogenation and deoxygenation of furfuralacetone. Green Chem. 2019, 21, 6299–6306. [Google Scholar] [CrossRef]
- Leitner, W.; Lercher, J.A. From Biomass to Fuels: Homogeneous and Heterogeneous Approaches. Chem. Ing. Tech. 2014, 86, 1391. [Google Scholar] [CrossRef]
- Leitner, W.; Klankermayer, J.; Pischinger, S.; Pitsch, H.; Kohse-Höinghaus, K. Advanced Biofuels and Beyond: Chemistry Solutions for Propulsion and Production. Angew. Chem. Int. Ed. 2017, 56, 5412–5452. [Google Scholar] [CrossRef]
- Yasir, M.; Chowdhury, S.; Mansor, N.; Mohamed, N.M.; Uemura, Y. Upgrading of Pyrolysis Bio-Oil to Fuel over Supported Nanomaterials—A Review. Appl. Mech. Mater. 2014, 625, 357–360. [Google Scholar] [CrossRef]
- Jacobson, K.; Maheria, K.C.; Kumar Dalai, A. Bio-oil valorization: A review. Renew. Sustain. Energy Rev. 2013, 23, 91–106. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Pyrolysis of Wood/Biomass for Bio-oil: A Critical Review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Jones, M.R. Biomass for Energy. In Biomass Handbookp; Gordon and Breach Science Publication: New York, NY, USA, 1989. [Google Scholar]
- Demirbas, A. Combustion Efficiency Impacts of Biofuels. Energy Sources Part A 2009, 31, 602–609. [Google Scholar] [CrossRef]
- Ahmad, F.B.; Kalam, M.A.; Zhang, Z.; Masjuki, H.H. Sustainable production of furan-based oxygenated fuel additives from pentose-rich biomass residues. Energy Convers. Manag. X 2022, 14, 100222. [Google Scholar] [CrossRef]
- Jing, Y.; Guo, Y.; Xia, Q.; Liu, X.; Wang, Y. Catalytic Production of Value-Added Chemicals and Liquid Fuels from Lignocellulosic Biomass. Chem 2019, 5, 2520–2546. [Google Scholar] [CrossRef]
- Wang, C.; Xu, H.; Daniel, R.; Ghafourian, A.; Herreros, J.M.; Shuai, S.; Ma, X. Combustion characteristics and emissions of 2-methylfuran compared to 2,5-dimethylfuran, gasoline and ethanol in a DISI engine. Fuel 2013, 103, 200–211. [Google Scholar] [CrossRef]
- Fischer, G.; Schwalbe, R.; Möller, M.; Ostrowski, R.; Dott, W. Species-specific production of microbial volatile organic compounds (MVOC) by airborne fungi from a compost facility. Chemosphere 1999, 39, 795–810. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, G.S.; Poll, L. Determination of Odor Active Aroma Compounds in Freshly Cut Leek (Allium ampeloprasum Var. Bulga) and in Long-Term Stored Frozen Unblanched and Blanched Leek Slices by Gas Chromatography Olfactometry Analysis. J. Agric. Food. Chem. 2004, 52, 1642–1646. [Google Scholar] [CrossRef]
- Shen, Y.-C.; Prakash, C.V.S.; Kuo, Y.-H. Three New Furan Derivatives and a New Fatty Acid from a Taiwanese Marine Sponge Plakortis simplex. J. Nat. Prod. 2001, 64, 324–327. [Google Scholar] [CrossRef]
- Chen, V.Y.; Kwon, O. Unified Approach to Furan Natural Products via Phosphine-Palladium Catalysis. Angew. Chem. Int. Ed. 2021, 60, 8874–8881. [Google Scholar] [CrossRef]
- Youssef, D.T.A. Alkaloids of the Flowers of Hippeastrum vittatum. J. Nat. Prod. 2001, 64, 839–841. [Google Scholar] [CrossRef]
- Song, K.-S.; Cho, S.-M.; Ka, K.-S.; Han, M.-W.; Yoo, I.-D. Secondary metabolites from the mycelial culture broth of Phellinus linteus. Han’guk Nonghwa Hakhoechi 1994, 37, 100–107. [Google Scholar]
- Schneider, G.; Anke, H.; Sterner, O. Xylaramide, a New Antifungal Compound, and Other Secondary Metabolites from Xylaria longipes. Z. Naturforsch. C 1996, 51, 802–806. [Google Scholar] [CrossRef] [PubMed]
- Mancilla, G.; Jiménez-Teja, D.; Femenía-Ríos, M.; Macías-Sánchez, A.J.; Collado, I.G.; Hernández-Galán, R. Novel Macrolide from Wild Strains of the Phytopathogen Fungus Colletotrichum Acutatum. Nat. Prod. Commun. 2009, 4, 1934578X0900400316. [Google Scholar] [CrossRef]
- Mosadeghzad, Z.; Zuriati, Z.; Asmat, A.; Gires, U.; Wickneswari, R.; Pittayakhajonwut, P.; Farahani, G.H.N. Chemical components and bioactivity of the marine-derived fungus Paecilomyces sp. Collected from Tinggi Island, Malaysia. Chem. Nat. Compd. 2013, 49, 621–625. [Google Scholar] [CrossRef]
- Mukku, V.J.R.V.; Maskey, R.P.; Monecke, P.; Grün-Wollny, I.; Laatsch, H. 5-(2-Methylphenyl)-4-pentenoic Acid from a Terrestrial Streptomycete. Z. Naturforsch. B 2002, 57, 335–337. [Google Scholar] [CrossRef]
- Elbandy, M.; Shinde, P.B.; Dang, H.T.; Hong, J.; Bae, K.S.; Jung, J.H. Furan Metabolites from the Sponge-Derived Yeast Pichia membranifaciens. J. Nat. Prod. 2008, 71, 869–872. [Google Scholar] [CrossRef]
- Quiroz-Florentino, H.; Hernández-Benitez, R.I.; Aviña, J.A.; Burgueño-Tapia, E.; Tamariz, J. Total Synthesis of Naturally Occurring Furan Compounds 5-{[(4-Hydroxybenzyl)oxy]methyl}-2-furaldehyde and Pichiafuran C. Synthesis 2011, 2011, 1106–1112. [Google Scholar]
- Du, X.-M.; Sun, N.-Y.; Irino, N.; Shoyama, Y. Glycosidic Constituents from in Vitro Anoectochilus formosanus. Chem. Pharm. Bull. 2000, 48, 1803–1804. [Google Scholar] [CrossRef]
- Fotso, S.; Maskey, R.P.; Schröder, D.; Ferrer, A.S.; Grün-Wollny, I.; Laatsch, H. Furan Oligomers and β-Carbolines from Terrestrial Streptomycetes. J. Nat. Prod. 2008, 71, 1630–1633. [Google Scholar] [CrossRef]
- Antal, M.J.; Mok, W.S.L.; Richards, G.N. Mechanism of formation of 5-(hydroxymethyl)-2-furaldehyde from d-fructose and sucrose. Carbohydr. Res. 1990, 199, 91–109. [Google Scholar] [CrossRef]
- Kus, S.; Gogus, F.; Eren, S. Hydroxymethyl Furfural Content of Concentrated Food Products. Int. J. Food Prop. 2005, 8, 367–375. [Google Scholar] [CrossRef]
- Xu, Q.; Li, Y.H.; Lü, X.Y. Investigation on influencing factors of 5-HMF content in Schisandra. J. Zhejiang Univ. Sci. B 2007, 8, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.X.; Li, Y.; Qian, Z.J.; Kim, M.M.; Kim, S.K. In vitro antioxidant activity of 5-HMF isolated from marine red alga Laurencia undulata in free-radical-mediated oxidative systems. J. Microbiol. Biotechnol. 2009, 19, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.-m.; Mu, Q.-z. New Furans from Cirsium chlorolepis. Planta Med. 1990, 56, 472–474. [Google Scholar] [CrossRef] [PubMed]
- Podesta, F.; Fajardo, V.; Freyer, A.J.; Shamma, M. 5-Methoxymethyl-2-furaldehyde: A Natural Furanoid from Jaborosa Magellanica (Solanaceae) 5-Methoxymethyl-furfural: Ein natürliches Furanoid aus Jaborosa magellanica (Colanaceae). Arch. Pharm. 1988, 321, 949. [Google Scholar] [CrossRef]
- Kobayashi, T.; Tomimori, T.; Nakajima, T.; Yahagi, N. Yakugaku Kenkyu 1958, 30, 477.
- Zheng, R.-X.; Xu, X.-D.; Tian, Z.; Yang, J.-S. Chemical constituents from the fruits of Hippophae rhamnoides. Nat. Prod. Res. 2009, 23, 1451–1456. [Google Scholar] [CrossRef]
- Xiang, T.; Xiong, Q.-B.; Ketut, A.I.; Tezuka, Y.; Nagaoka, T.; Wu, L.-J.; Kadota, S. Studies on the Hepatocyte Protective Activity and the Structure-Activity Relationships of Quinic Acid and Caffeic Acid Derivatives from the Flower Buds of Lonicera bournei. Planta Med. 2001, 67, 322–325. [Google Scholar] [CrossRef]
- Choi, H.; Pyo, M.; Park, K. Cirsiumaldehyde from Gastrodia elata. Nat. Prod. Sci. 1997, 3, 104–105. [Google Scholar]
- Lee, Y.-K.; Woo, M.-H.; Kim, C.-H.; Kim, Y.; Lee, S.-H.; Jeong, B.-S.; Chang, H.-W.; Son, J.-K. Two New Benzofurans from Gastrodia elata and their DNA Topoisomerases I and II Inhibitory Activities. Planta Med. 2007, 73, 1287–1291. [Google Scholar] [CrossRef]
- Huang, L.-Q.; Li, Z.-F.; Wang, Q.; Cui, Y.-R.; Ou, Y.-H.; Xiong, S.-S.; Huang, W.-P.; Feng, Y.-L.; Yang, S.-L. Two new furaldehyde compounds from the rhizomes of Gastrodia elata. J. Asian Nat. Prod. Res. 2015, 17, 352–356. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Q.; Ouyang, H.; Huang, L.; Feng, Y.; Wang, R.; Yang, S. New compounds with neuroprotective activities from Gastrodia elata. Phytochem. Lett. 2016, 15, 94–97. [Google Scholar] [CrossRef]
- Xu, H.; Hsu, H.Y. Oriental Materia Medica: A Concise Guide; Oriental Healing Arts Institute: Long Beach, CA, USA, 1986. [Google Scholar]
- Tang, W.; Eisenbrand, G. Gastrodia elata Bl. In Chinese Drugs of Plant Origin: Chemistry, Pharmacology, and Use in Traditional and Modern Medicine; Springer: Berlin/Heidelberg, Germany, 1992; pp. 545–548. [Google Scholar]
- Hayashi, J.; Sekine, T.; Deguchi, S.; Lin, Q.; Horie, S.; Tsuchiya, S.; Yano, S.; Watanabe, K.; Ikegami, F. Phenolic compounds from Gastrodia rhizome and relaxant effects of related compounds on isolated smooth muscle preparation. Phytochemistry 2002, 59, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.-T.; Wu, C.-R.; Chen, C.-F. Gastrodin and p-hydroxybenzyl alcohol facilitate memory consolidation and retrieval, but not acquisition, on the passive avoidance task in rats. J. Ethnopharmacol. 1997, 56, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-R.; Hsieh, M.-T.; Huang, S.-C.; Peng, W.-H.; Chang, Y.-S.; Chen, C.-F. Effects of Gastrodia elata and its Active Constituents on Scopolamine-Induced Amnesia in Rats. Planta Med. 1996, 62, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-H.; Lee, P.-H.; Wein, Y.-S. Four New Compounds from the Seeds of Cassia fistula. J. Nat. Prod. 2002, 65, 1165–1167. [Google Scholar] [CrossRef]
- Ni, G.; Shi, G.-R.; Zhang, D.; Fu, N.-J.; Yang, H.-Z.; Chen, X.-G.; Yu, D.-Q. Cytotoxic Lignans and Sesquiterpenoids from the Rhizomes of Acorus tatarinowii. Planta Med. 2016, 82, 632–638. [Google Scholar] [CrossRef]
- Yang, R.; Yan, Z.; Chen, C.; Liu, J. Constituents and Activities of Acorus tatarinowii. Med. Res. Arch. 2017, 5. [Google Scholar] [CrossRef]
- Boukamcha, H.; Jannet, H.B.; Bouazizi, Y.; Mighri, Z. Isolation and Structure Determination of a Novel Furanic Ester from the Aerial Part of Prasium Majus. Nat. Prod. Res. 2003, 17, 63–66. [Google Scholar] [CrossRef]
- Sanghyun, L.; Jun, J.; Kuk, H.S.; Bak-Kwang, K. Sessiline, A New Nitrogenous Compound from the Fruits of Acanthopanax sessiliflorus. Planta Med. 2002, 68, 939–941. [Google Scholar]
- Ilkei, V.; Faragó, K.; Sánta, Z.; Dékány, M.; Hazai, L.; Szántay Jr, C.; Szántay, C.; Kalaus, G. The First Synthesis of Sessiline. Int. J. Org. Chem. 2014, 4, 309–313. [Google Scholar] [CrossRef][Green Version]
- Kaweetripob, W.; Mahidol, C.; Prachyawarakorn, V.; Prawat, H.; Ruchirawat, S. 5-Formylfurfuryl esters from Duabanga grandiflora. Phytochemistry 2012, 76, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, B.S.; Perwaiz, S.; Begum, S.; Ali, S.T. Three new constituents, latifolinal, latifolidin and cordicinol, from the fruits and leaves of Cordia latifolia. Nat. Prod. Res. 2010, 24, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.-X.; Feng, B.-M.; Chen, F.; Liu, J.-Y.; Li, F.; Wang, Y.-Q.; Pei, Y.-H. Sinapic acid derivatives from the seeds of Raphanus nussatirus L. J. Asian Nat. Prod. Res. 2007, 9, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.-X.; Zhi, D.-J.; Ren, H.; Li, Z.-Y.; Hu, Q.-L.; Liu, Y.-H.; Zhang, Z.-X.; Fei, D.-Q. A New Succinate Derivative from Ajuga decumbens. Nat. Prod. Commun. 2016, 11, 1934578X1601100. [Google Scholar] [CrossRef]
- Samoylenko, V.; Zhao, J.; Dunbar, D.C.; Khan, I.A.; Rushing, J.W.; Muhammad, I. New Constituents from Noni (Morinda citrifolia) Fruit Juice. J. Agric. Food. Chem. 2006, 54, 6398–6402. [Google Scholar] [CrossRef]
- Quiroz-Florentino, H.; García, A.; Burgueño-Tapia, E.; Tamariz, J. Total synthesis of the natural succinate derivative of 5-(hydroxymethyl)furfural isolated from the Noni fruit (Morinda citrifolia). Nat. Prod. Res. 2009, 23, 1355–1362. [Google Scholar] [CrossRef]
- Chuda, Y.; Ono, H.; Ohnishi-Kameyama, M.; Matsumoto, K.; Nagata, T.; Kikuchi, Y. Mumefural, Citric Acid Derivative Improving Blood Fluidity from Fruit-Juice Concentrate of Japanese Apricot (Prunus mume Sieb. et Zucc). J. Agric. Food. Chem. 1999, 47, 828–831. [Google Scholar] [CrossRef]
- Sriwilaijaroen, N.; Kadowaki, A.; Onishi, Y.; Gato, N.; Ujike, M.; Odagiri, T.; Tashiro, M.; Suzuki, Y. Mumefural and related HMF derivatives from Japanese apricot fruit juice concentrate show multiple inhibitory effects on pandemic influenza A (H1N1) virus. Food Chem. 2011, 127, 1–9. [Google Scholar] [CrossRef]
- Sugimura, H.; Kikuchi, M.; Kato, S.; Sekita, W.; Sasaki, I. Practical synthesis of mumefural, a component of Japanese apricot juice concentrate. Tetrahedron 2016, 72, 7638–7641. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.-Q.; Kang, J.; Liu, C.; Chen, R.-Y. Studies on chemical constituents from fruits of Morus alba L. Acta Pharmacol. Sin. 2014, 49, 504–506. [Google Scholar]
- Li, J.; Zeng, K.-W.; Shi, S.-P.; Jiang, Y.; Tu, P.-F. Anti-neuroinflammatory constituents from Polygala tricornis Gagnep. Fitoterapia 2012, 83, 896–900. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Sato, A.; Ono, T.; Goto, K.; Maeda, T.; Takanari, J.; Nishioka, H.; Komatsu, K.; Matsuura, H. Isolation, Structural Elucidation, and Biological Evaluation of a 5-Hydroxymethyl-2-furfural Derivative, Asfural, from Enzyme-Treated Asparagus Extract. J. Agric. Food. Chem. 2013, 61, 9155–9159. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.-M.; Zhan, Z.-L.; Yang, Y.-N.; Jiang, J.-S.; Zhang, P.-C. New heterocyclic compounds from Ranunculus ternatus Thunb. Bioorg. Chem. 2017, 74, 10–14. [Google Scholar] [CrossRef]
- Lin, A.-S.; Qian, K.; Usami, Y.; Lin, L.; Itokawa, H.; Hsu, C.; Morris-Natschke, S.L.; Lee, K.-H. 5-Hydroxymethyl-2-furfural, a clinical trials agent for sickle cell anemia, and its mono/di-glucosides from classically processed steamed Rehmanniae Radix. J. Nat. Med. 2008, 62, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, F.W.; Martin, D.; Weber, T.; Schiweck, H. Studies on Ketoses, 7 – 5-(α-D-Glucosyloxymethyl)furfural: Preparation from Isomaltulose and Exploration of Its Ensuing Chemistry. Liebigs Ann. 1993, 1993, 967–974. [Google Scholar] [CrossRef]
- Ruß, C.; Luff, C.; Begli, A.H.; Koenig, B. Solvent-Free Preparation of 5-(α-D-Glucosyloxymethyl)furfural from Isomaltulose–Choline Chloride Melts. Synth. Commun. 2012, 42, 3112–3116. [Google Scholar] [CrossRef]
- Yan, X.-T.; Li, W.; Sun, Y.-N.; Yang, S.Y.; Song, G.Y.; Kim, Y.H. A New Furfural Diglycoside and Other Carbohydrate Derivatives from Fermented Beverage of Prunus mume Fruit. Bull. Korean Chem. Soc. 2014, 35, 2162–2164. [Google Scholar] [CrossRef]
- Nguyen, K.D.H.; Dang, P.H.; Nguyen, H.X.; Nguyen, M.T.T.; Awale, S.; Nguyen, N.T. Phytochemical and cytotoxic studies on the leaves of Calotropis gigantea. Bioorg. Med. Chem. Lett. 2017, 27, 2902–2906. [Google Scholar] [CrossRef]
- Ding, L.-J.; Gu, B.-B.; Jiao, W.-H.; Yuan, W.; Li, Y.-X.; Tang, W.-Z.; Yu, H.-B.; Liao, X.-J.; Han, B.-N.; Li, Z.-Y.; et al. New Furan and Cyclopentenone Derivatives from the Sponge-Associated Fungus Hypocrea Koningii PF04. Mar. Drugs 2015, 13, 5579–5592. [Google Scholar] [CrossRef]
- Lian, L.; Fan, X.-M.; Chen, G.; Li, W.; Lu, X.; Ma, H.-M.; Shen, M.-X.; Pei, Y.-H. Two new compounds from the fruits of Trichosanthes kirilowii Maxim. J. Asian Nat. Prod. Res. 2012, 14, 64–67. [Google Scholar] [CrossRef]
- He, J.; Ye, X.-S.; Wang, X.-X.; Yang, Y.-N.; Zhang, P.-C.; Ma, B.-Z.; Zhang, W.-K.; Xu, J.-K. Four new iridoid glucosides containing the furan ring from the fruit of Cornus officinalis. Fitoterapia 2017, 120, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.-h.; Ding, Y.-x.; Zhang, L.; Li, L. Cornel iridoid glycoside improves memory ability and promotes neuronal survival in fimbria–fornix transected rats. Eur. J. Pharmacol. 2010, 647, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Angewandte Chemie International Edition China Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2010. [Google Scholar]
- Liktor-Busa, E.; Simon, A.; Tóth, G.; Báthori, M. The first two ecdysteroids containing a furan ring from Serratula wolffii. Tetrahedron Lett. 2008, 49, 1738–1740. [Google Scholar] [CrossRef]
- Shu, Z.; Chen, Z.; Ding, X.-J.; Lu, B.-Q.; Ji, C.-J.; Xu, Q.-M.; Li, X.-R.; Yang, S.-L. Three New Triterpenoids from Pulsatilla chinensis (Bunge) Regel and Their Cytotoxic Activities. Heterocycles 2011, 83, 2365–2371. [Google Scholar] [CrossRef]
- Ye, W.C.; Ji, N.N.; Zhao, S.X.; Liu, J.H.; Ye, T.; McKervey, M.A.; Stevenson, P. Triterpenoids from Pulsatilla chinensis. Phytochemistry 1996, 42, 799–802. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, H.; Yan, M.-L.; Xing, X.-D.; Zhang, Y.-Y.; Wei, N.; Yang, B.-Y.; Wang, Q.-H.; Kuang, H.-X. A new phytoecdysteroid from the roots of Achyranthes bidentata Bl. Nat. Prod. Res. 2017, 31, 1073–1079. [Google Scholar] [CrossRef]
- Mohamed, O.G.; Khalil, Z.G.; Capon, R.J. Prolinimines: N-Amino-l-Pro-methyl Ester (Hydrazine) Schiff Bases from a Fish Gastrointestinal Tract-Derived Fungus, Trichoderma sp. CMB-F563. Org. Lett. 2018, 20, 377–380. [Google Scholar] [CrossRef]
- Citron, C.A.; Rabe, P.; Dickschat, J.S. The Scent of Bacteria: Headspace Analysis for the Discovery of Natural Products. J. Nat. Prod. 2012, 75, 1765–1776. [Google Scholar] [CrossRef]
- Munekata, M.; Tamura, G. Antitumor activity of 5-hydroxymethyl-2-furoic acid. Agric. Biol. Chem. 1981, 45, 2149–2150. [Google Scholar]
- Abdel-Lateff, A.; Fisch, K.M.; Wright, A.D.; König, G.M. A New Antioxidant Isobenzofuranone Derivative from the Algicolous Marine Fungus Epicoccum sp. Planta Med. 2003, 69, 831–834. [Google Scholar]
- Abdel-Lateff, A.; Klemke, C.; König, G.M.; Wright, A.D. Two New Xanthone Derivatives from the Algicolous Marine Fungus Wardomyces anomalus. J. Nat. Prod. 2003, 66, 706–708. [Google Scholar] [CrossRef] [PubMed]
- Jadulco, R.; Proksch, P.; Wray, V.; Sudarsono; Berg, A.; Gräfe, U. New Macrolides and Furan Carboxylic Acid Derivative from the Sponge-Derived Fungus Cladosporium herbarum. J. Nat. Prod. 2001, 64, 527–530. [Google Scholar] [CrossRef]
- Mrochek, J.E.; Rainey, W.T., Jr. Identification and Biochemical Significance of Substituted Furans in Human Urine. Clin. Chem. 1972, 18, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Lawson, A.M.; Chalmers, R.A.; Watts, R.W. Urinary organic acids in man. I. Normal patterns. Clin. Chem. 1976, 22, 1283–1287. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, L.; Jiang, X.; Huang, X.; Chen, J. A new furanoid toxin produced by Curvularia lunata, the causal agent of maize Curvularia leaf spot. Can. J. Plant Pathol. 2009, 31, 22–27. [Google Scholar] [CrossRef]
- Wu, J.; Uchida, K.; Ridwan, A.Y.; Kondo, M.; Choi, J.-H.; Hirai, H.; Kawagishi, H. Erinachromanes A and B and Erinaphenol A from the Culture Broth of Hericium erinaceus. J. Agric. Food. Chem. 2019, 67, 3134–3139. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, K.S.; Romashov, L.V.; Ananikov, V.P. A tunable precious metal-free system for selective oxidative esterification of biobased 5-(hydroxymethyl)furfural. Green Chem. 2019, 21, 3464–3468. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romashov, L.V.; Kucherov, F.A.; Kozlov, K.S.; Ananikov, V.P. Bio-Derived Furanic Compounds with Natural Metabolism: New Sustainable Possibilities for Selective Organic Synthesis. Int. J. Mol. Sci. 2023, 24, 3997. https://doi.org/10.3390/ijms24043997
Romashov LV, Kucherov FA, Kozlov KS, Ananikov VP. Bio-Derived Furanic Compounds with Natural Metabolism: New Sustainable Possibilities for Selective Organic Synthesis. International Journal of Molecular Sciences. 2023; 24(4):3997. https://doi.org/10.3390/ijms24043997
Chicago/Turabian StyleRomashov, Leonid V., Fedor A. Kucherov, Kirill S. Kozlov, and Valentine P. Ananikov. 2023. "Bio-Derived Furanic Compounds with Natural Metabolism: New Sustainable Possibilities for Selective Organic Synthesis" International Journal of Molecular Sciences 24, no. 4: 3997. https://doi.org/10.3390/ijms24043997
APA StyleRomashov, L. V., Kucherov, F. A., Kozlov, K. S., & Ananikov, V. P. (2023). Bio-Derived Furanic Compounds with Natural Metabolism: New Sustainable Possibilities for Selective Organic Synthesis. International Journal of Molecular Sciences, 24(4), 3997. https://doi.org/10.3390/ijms24043997






