The Involvement of Photobiology in Contemporary Dentistry—A Narrative Review
Abstract
1. Introduction
1.1. Concepts
1.2. Historical Perspectives on Light Theories
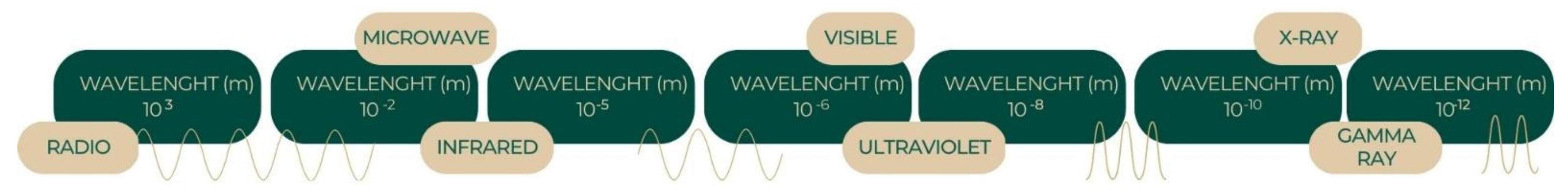
2. Spectrum of Light: From Visible to Invisible
- The number of photons per unit time indicates the amplitude of light;
- The frequency of photons in a ray of light shows its intensity and color;
- The direction of spin of photons in a ray of light indicates its polarity.
3. Light in Modern Dentistry
3.1. Infrared Light and Near Infrared Light
3.2. High-Energy Visible (HEV) Light
3.3. Ultraviolet Light
3.4. Shortest Wavelengths-X-ray
4. Light-Sources in Dentistry Lasers
4.1. Mechanism of Action
4.2. Types of Lasers
4.2.1. Carbon Dioxide Laser (CO2)
4.2.2. Neodymium Yttrium Aluminum Garnet Laser (Nd:YAG)
4.2.3. Erbium Lasers
4.2.4. Diode Lasers
5. Dental Therapeutic Strategies
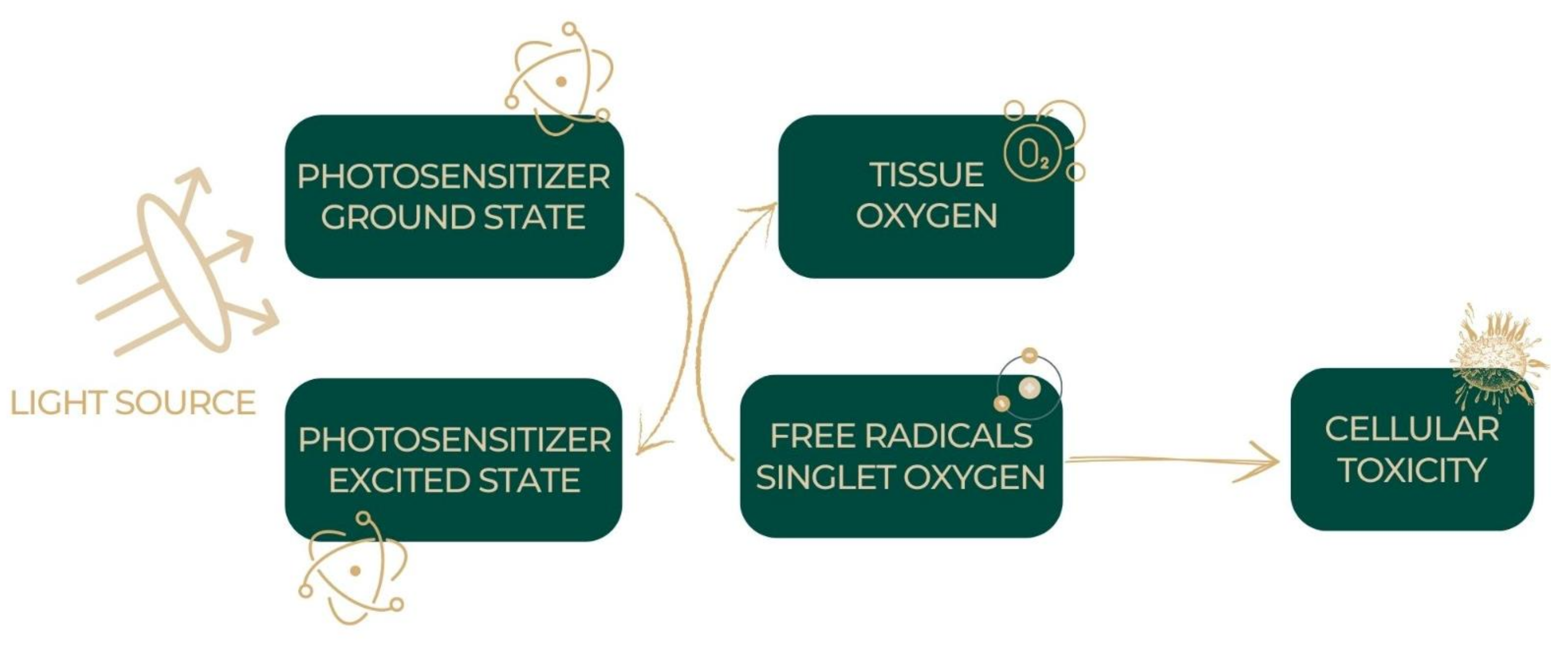
5.1. Photodynamic Therapy
5.2. Mechanism of the Photodynamic Action
5.3. PDT in Dentistry
5.4. Photobiomodulation (PBM)
5.5. Photodynamic Inactivation
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kern, H.E.; Lewy, A.J. Corrections and additions to the history of light therapy and seasonal affective disorder. Arch. Gen. Psychiatry 1990, 47, 90–91. [Google Scholar] [CrossRef] [PubMed]
- Sabra, A.I. Theories of Light: From Descartes to Newton; Cambridge University Press: Cambridge, UK, 1981. [Google Scholar]
- Subbarayappa, B.V. The roots of ancient medicine: An historical outline. J. Biosci. 2001, 26, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.B. Nature, Custom, and Reason as the Explanatory and Practical Principles of Aristotelian Political Science. Rev. Politics 2002, 64, 469–495. [Google Scholar] [CrossRef]
- Metwaly, A.M.; Ghoneim, M.M.; Eissa, I.H.; Elsehemy, I.A.; Mostafa, A.E.; Hegazy, M.M.; Afifi, W.M.; Dou, D. Traditional ancient Egyptian medicine: A review. Saudi J. Biol. Sci. 2021, 28, 5823–5832. [Google Scholar] [CrossRef]
- Rueggeberg, F.A.; Giannini, M.; Arrais, C.A.G.; Price, R.B.T. Light curing in dentistry and clinical implications: A literature review. Braz. Oral Res. 2017, 31, e61. [Google Scholar] [CrossRef]
- Cocilovo, A. Colored light therapy: Overview of its history, theory, recent developments and clinical applications combined with acupuncture. Am. J. Acupunct. 1999, 27, 71–83. [Google Scholar]
- Liebert, A.; Kiat, H. The history of light therapy in hospital physiotherapy and medicine with emphasis on Australia: Evolution into novel areas of practice. Physiother. Theory Pract. 2021, 37, 389–400. [Google Scholar] [CrossRef]
- Qureshi, N.A.; Ali, G.I.; Abushanab, T.S.; El-Olemy, A.T.; Alqaed, M.S.; El-Subai, I.S.; Al-Bedah, A.M.N. History of cupping (Hijama): A narrative review of literature. J. Integr. Med. 2017, 15, 172–181. [Google Scholar] [CrossRef]
- Rkein, A.M.; Ozog, D.M. Photodynamic therapy. Dermatol. Clin. 2014, 32, 415–425. [Google Scholar] [CrossRef]
- Voit, F.; Schäfer, J.; Kienle, A. Light scattering by multiple spheres: Comparison between Maxwell theory and radiative-transfer-theory calculations. Opt. Lett. 2009, 34, 2593–2595. [Google Scholar] [CrossRef]
- Hohmann, A.; Voit, F.; Schäfer, J.; Kienle, A. Multiple scattering of polarized light: Influence of absorption. Phys. Med. Biol. 2014, 59, 2583–2597. [Google Scholar] [CrossRef] [PubMed]
- Jacques, S.L. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, R37–R61. [Google Scholar] [CrossRef] [PubMed]
- Nothelfer, S.; Bergmann, F.; Liemert, A.; Reitzle, D.; Kienle, A. Spatial frequency domain imaging using an analytical model for separation of surface and volume scattering. J. Biomed. Opt. 2018, 24, 071604. [Google Scholar] [CrossRef] [PubMed]
- Wiest, J.; Bodenschatz, N.; Brandes, A.; Liemert, A.; Kienle, A. Polarization influence on reflectance measurements in the spatial frequency domain. Phys. Med. Biol. 2015, 60, 5717–5732. [Google Scholar] [CrossRef]
- Sliney, D.H.; Wangemann, R.T.; Franks, J.K.; Wolbarsht, M.L. Visual sensitivity of the eye to infrared laser radiation. J. Opt. Soc. Am. 1976, 66, 339–341. [Google Scholar] [CrossRef]
- Yeh, S.W.; Hong, C.H.; Shih, M.C.; Tam, K.W.; Huang, Y.H.; Kuan, Y.C. Low-Level Laser Therapy for Fibromyalgia: A Systematic Review and Meta-Analysis. Pain Physician 2019, 22, 241–254. [Google Scholar]
- Fried, D.; Featherstone, J.D.; Darling, C.L.; Jones, R.S.; Ngaotheppitak, P.; Bühler, C.M. Early caries imaging and monitoring with near-infrared light. Dent. Clin. N. Am. 2005, 49, 771–793. [Google Scholar] [CrossRef]
- Jones, R.S.; Huynh, G.D.; Jones, G.C.; Fried, D. Near-infrared transillumination at 1310-nm for the imaging of early dental decay. Opt. Express 2003, 11, 2259–2265. [Google Scholar] [CrossRef]
- Buhler, C.M.; Ngaotheppitak, P.; Fried, D. Imaging of occlusal dental caries (decay) with near-IR light at 1310-nm. Opt. Express 2005, 13, 573–582. [Google Scholar] [CrossRef]
- Abogazalah, N.; Ando, M. Alternative methods to visual and radiographic examinations for approximal caries detection. J. Oral Sci. 2017, 59, 315–322. [Google Scholar] [CrossRef]
- Yin, R.; Dai, T.; Avci, P.; Jorge, A.E.; de Melo, W.C.; Vecchio, D.; Huang, Y.Y.; Gupta, A.; Hamblin, M.R. Light based anti-infectives: Ultraviolet C irradiation, photodynamic therapy, blue light, and beyond. Curr. Opin. Pharmacol. 2013, 13, 731–762. [Google Scholar] [CrossRef] [PubMed]
- Cabral, J.; Ag, R. Blue Light Disinfection in Hospital Infection Control: Advantages, Drawbacks, and Pitfalls. Antibiotics 2019, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Gupta, A.; Murray, C.K.; Vrahas, M.S.; Tegos, G.P.; Hamblin, M.R. Blue light for infectious diseases: Propionibacterium acnes, Helicobacter pylori, and beyond? Drug Resist. Updat. 2012, 15, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Feuerstein, O.; Ginsburg, I.; Dayan, E.; Veler, D.; Weiss, E.I. Mechanism of visible light phototoxicity on Porphyromonas gingivalis and Fusobacterium nucleatum. Photochem. Photobiol. 2005, 81, 1186–1189. [Google Scholar] [CrossRef]
- Spranley, T.J.; Winkler, M.; Dagate, J.; Oncale, D.; Strother, E. Curing light burns. Gen. Dent. 2012, 60, 210–214. [Google Scholar]
- Price, R.B.; Strassler, H.E.; Price, H.L.; Seth, S.; Lee, C.J. The effectiveness of using a patient simulator to teach light-curing skills. J. Am. Dent. Assoc. 2014, 145, 32–43. [Google Scholar] [CrossRef]
- Price, R.B.; Shortall, A.C.; Palin, W.M. Contemporary issues in light curing. Oper. Dent. 2014, 39, 4–14. [Google Scholar] [CrossRef]
- Jandt, K.D.; Mills, R.W. A brief history of LED photopolymerization. Dent. Mater. 2013, 29, 605–617. [Google Scholar] [CrossRef]
- Yoshino, F.; Yoshida, A. Effects of blue-light irradiation during dental treatment. Jpn. Dent. Sci. Rev. 2018, 54, 160–168. [Google Scholar] [CrossRef]
- Oliveira, D.; Rocha, M.G. Dental Light-Curing-Assessing the Blue-Light Hazard. Dent. Clin. N. Am. 2022, 66, 537–550. [Google Scholar] [CrossRef]
- Fluent, M.T.; Ferracane, J.L.; Mace, J.G.; Shah, A.R.; Price, R.B. Shedding light on a potential hazard: Dental light-curing units. J. Am. Dent. Assoc. 2019, 150, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Maclean, M.; Anderson, J.G.; MacGregor, S.J.; White, T.; Atreya, C.D. A New Proof of Concept in Bacterial Reduction: Antimicrobial Action of Violet-Blue Light (405 nm) in Ex Vivo Stored Plasma. J. Blood Transfus. 2016, 2016, 2920514. [Google Scholar] [CrossRef] [PubMed]
- Grober, U.; Spitz, J.; Reichrath, J.; Kisters, K.; Holick, M.F. Vitamin D: Update 2013: From rickets prophylaxis to general preventive healthcare. Derm.-Endocrinol. 2013, 5, 331–347. [Google Scholar] [CrossRef] [PubMed]
- Gerba, C.P. Use of an ultraviolet light at point-of-dispense faucet to eliminate pseudomonas aeruginosa. Am. J. Infect. Control 2015, 43, 528–529. [Google Scholar] [CrossRef]
- Dai, T.; Tegos, G.P.; Denis, T.G.S.; Anderson, D.; Sinofsky, E.; Hamblin, M.R. Ultraviolet-C irradiation for prevention of central venous catheter-related infections: An in vitro study. Photochem. Photobiol. 2011, 87, 250–255. [Google Scholar] [CrossRef]
- Wang, D.; Kuzma, M.L.; Tan, X.; He, T.C.; Dong, C.; Liu, Z.; Yang, J. Phototherapy and optical waveguides for the treatment of infection. Adv. Drug Deliv. Rev. 2021, 179, 114036. [Google Scholar] [CrossRef] [PubMed]
- Conner-Kerr, T.A.; Sullivan, P.K.; Gaillard, J.; Franklin, M.E.; Jones, R.M. The effects of ultraviolet radiation on antibiotic-resistant bacteria in vitro. Ostomy Wound Manag. 1998, 44, 50–56. [Google Scholar]
- Thai, T.P.; Keast, D.H.; Campbell, K.E.; Woodbury, M.G.; Houghton, P.E. Effect of ultraviolet light C on bacterial colonization in chronic wounds. Ostomy Wound Manag. 2005, 51, 32–45. [Google Scholar]
- De Brito, L.C.N.; Doolittle-Hall, J.; Lee, C.T.; Moss, K.; Bambirra, W., Jr.; Tavares, W.L.F.; Ribeiro Sobrinho, A.P.; Teles, F.R.F. The apical root canal system microbial communities determined by next-generation sequencing. Sci. Rep. 2020, 10, 10932. [Google Scholar] [CrossRef]
- Delikan, E.; Caliskan, S.; Cankilic, M.Y.; Aksu, S.; Kesim, B.; Ulger, S.T. Microbiota of endodontically infected primary and permanent teeth. Pediatr. Dent. 2021, 43, 102–110. [Google Scholar]
- Metzger, Z.; Better, H.; Abramovitz, I. Immediate root canal disinfection with ultraviolet light: An ex vivo feasibility study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 104, 425–433. [Google Scholar] [CrossRef]
- Stajer, A.; Kajari, S.; Gajdacs, M.; Musah-Eroje, A.; Barath, Z. Utility of photodynamic therapy in dentistry: Current concepts. Dent. J. 2020, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Morio, K.; Thayer, E.L.; Bates, A.M.; Brogden, K.A. 255-nm lightemitting diode kills Enterococcus faecalis and induces the production of cellular biomarkers in human embryonic palatal mesenchyme cells and gingival fibroblasts. J. Endod. 2019, 45, 774–783.e6. [Google Scholar] [CrossRef] [PubMed]
- Metzger, Z.; Dotan, M.; Better, H.; Abramovitz, I. Sensitivity of oral bacteria to 254 nm ultraviolet light. Int. Endod. J. 2007, 40, 120–127. [Google Scholar] [CrossRef]
- Reed, N.G. The history of ultraviolet germicidal irradiation for air disinfection. Public Health Rep. 2010, 125, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Bruls, W.A.; Slaper, H.; Van der Leun, J.C.; Berrens, L. Transmission of human epidermis and stratum corneum as a function of thickness in ultraviolet and visible wavelengths. Photochem. Photobiol. 1984, 40, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Schafer, M.P.; Kujundzic, E.; Moss, C.E.; Miller, S.L. Method for estimating ultraviolet germicidal fluence rates in a hospital room. Infect. Control Hosp. Epidemiol. 2008, 29, 1042–1047. [Google Scholar] [CrossRef] [PubMed]
- Yamano, N.; Kunisada, M.; Kaidzu, S.; Sugihara, K.; Nishiaki-Sawada, A.; Ohashi, H.; Yoshioka, A.; Igarashi, T.; Ohira, A.; Tanito, M.; et al. Long-term effects of 222-nm ultraviolet radiation C sterilizing lamps on mice susceptible to ultraviolet radiation. Photochem. Photobiol. 2020, 96, 853–862. [Google Scholar] [CrossRef]
- Devlin, H.; Soltani, P. COVID-19 and Dentistry. Encyclopedia 2021, 1, 496–504. [Google Scholar] [CrossRef]
- Giraudeau, N. Teledentistry and COVID-19: Be Mindful of Bogus “Good” Ideas! Inquiry 2021, 58, 469580211015050. [Google Scholar] [CrossRef]
- Goriuc, A.; Sandu, D.; Tatarciuc, M.; Luchian, I. The Impact of the COVID-19 Pandemic on Dentistry and Dental Education: A Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 2537. [Google Scholar] [CrossRef] [PubMed]
- Gianfaldoni, S.; Gianfaldoni, R.; Wollina, U.; Lotti, J.; Tchernev, G.; Lotti, T. An Overview on Radiotherapy: From Its History to Its Current Applications in Dermatology. Open Access Maced. J. Med. Sci. 2017, 5, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Cognetta, A.; Howard, B.; Heaton, H.; Stoddard, E.R.; Hong, H.G.; Green, W.H. Superficial X-ray in the treatment of basal and squamous cell carcinoma: A viable option in select patients. J. Am. Acad. Dermatol. 2012, 67, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.J.; Hermann, T.R. History of lasers. World J. Urol. 2007, 25, 217–220. [Google Scholar] [CrossRef]
- Walsh, L.J. The current status of laser applications in dentistry. Aust. Dent. J. 2003, 48, 146–155. [Google Scholar] [CrossRef]
- Theodoro, L.H.; Marcantonio, R.A.C.; Wainwright, M.; Garcia, V.G. LASER in periodontal treatment: Is it an effective treatment or science fiction? Braz. Oral Res. 2021, 35, e099. [Google Scholar] [CrossRef]
- Kikuchi, T.; Hayashi, J.I.; Mitani, A. Next-Generation Examination, Diagnosis, and Personalized Medicine in Periodontal Disease. J. Pers. Med. 2022, 12, 1743. [Google Scholar] [CrossRef]
- Fujiyama, K.; Deguchi, T.; Murakami, T.; Fujii, A.; Kushima, K.; Takano-Yamamoto, T. Clinical effect of CO2 laser in reducing pain in orthodontics. Angle Orthod. 2008, 78, 299–303. [Google Scholar] [CrossRef]
- Sağlam, M.; Köseoğlu, S.; Taşdemir, I.; Erbak Yılmaz, H.; Savran, L.; Sütçü, R. Combined application of Er:YAG and Nd:YAG lasers in treatment of chronic periodontitis. A split-mouth, single-blind, randomized controlled trial. J. Periodontal Res. 2017, 52, 853–862. [Google Scholar] [CrossRef]
- Aoki, A.; Mizutani, K.; Takasaki, A.A.; Sasaki, K.M.; Nagai, S.; Schwarz, F.; Yoshida, I.; Eguro, T.; Zeredo, J.L.; Izumi, Y. Current status of clinical laser applications in periodontal therapy. Gen. Dent. 2008, 56, 674–687. [Google Scholar]
- Oliveira, G.J.; Theodoro, L.H.; Marcantonio, E., Jr.; Sampaio, J.E.; Marcantonio, R.A. Effect of Er,Cr:YSGG and Er:YAG laser irradiation on the adhesion of blood components on the root surface and on root morphology. Braz. Oral Res. 2012, 26, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Theodoro, L.H.; Garcia, V.G.; Haypek, P.; Zezell, D.M.; Eduardo, C.P. Morphologic analysis, by means of scanning electron microscopy, of the effect of Er: YAG laser on root surfaces submitted to scaling and root planing. Pesqui. Odontol. Bras. 2002, 16, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Lesniewski, A.; Estrin, N.; Romanos, G.E. Comparing the Use of Diode Lasers to Light-Emitting Diode Phototherapy in Oral Soft and Hard Tissue Procedures: A Literature Review. Photobiomodul. Photomed. Laser Surg. 2022, 40, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Pawelczyk-Madalińska, M.; Benedicenti, S.; Sălăgean, T.; Bordea, I.R.; Hanna, R. Impact of Adjunctive Diode Laser Application to Non-Surgical Periodontal Therapy on Clinical, Microbiological and Immunological Outcomes in Management of Chronic Periodontitis: A Systematic Review of Human Randomized Controlled Clinical Trials. J. Inflamm. Res. 2021, 14, 2515–2545. [Google Scholar] [CrossRef]
- Slot, D.E.; Kranendonk, A.A.; Paraskevas, S.; Van der Weijden, F. The effect of a pulsed Nd:YAG laser in non-surgical periodontal therapy. J. Periodontol. 2009, 80, 1041–1056. [Google Scholar] [CrossRef]
- Inchingolo, A.M.; Malcangi, G.; Ferrara, I.; Viapiano, F.; Netti, A.; Buongiorno, S.; Latini, G.; Azzollini, D.; De Leonardis, N.; de Ruvo, E.; et al. Laser Surgical Approach of Upper Labial Frenulum: A Systematic Review. Int. J. Environ. Res. Public Health 2023, 20, 1302. [Google Scholar] [CrossRef]
- Sperandio, F.F.; Huang, Y.Y.; Hamblin, M.R. Antimicrobial photodynamic therapy to kill Gram-negative bacteria. Recent Pat. Antiinfect. Drug Discov. 2013, 8, 108–120. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy–mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- D’Ercole, S.; Carlesi, T.; Dotta, T.C.; Pierfelice, T.V.; D’Amico, E.; Tripodi, D.; Iezzi, G.; Piattelli, A.; Petrini, M. 5-Aminolevulinic Acid and Red Led in Endodontics: A Narrative Review and Case Report. Gels 2022, 8, 697. [Google Scholar] [CrossRef]
- Warrier, A.; Mazumder, N.; Prabhu, S.; Satyamoorthy, K.; Murali, T.S. Photodynamic Therapy to Control Microbial Biofilms. Photodiagn. Photodyn. Ther. 2021, 33, 102090. [Google Scholar] [CrossRef]
- Takasaki, A.A.; Aoki, A.; Mizutani, K.; Schwarz, F.; Sculean, A.; Wang, C.Y.; Koshy, G.; Romanos, G.; Ishikawa, I.; Izumi, Y. Application of Antimicrobial Photodynamic Therapy in Periodontal and Peri-Implant Diseases. Periodontology 2009, 51, 109–140. [Google Scholar] [CrossRef] [PubMed]
- Rossoni, R.D.; Junqueira, J.C.; Santos, E.L.S.; Costa, A.C.B.; Jorge, A.O.C. Comparison of the Efficacy of Rose Bengal and Erythrosin in Photodynamic Therapy against Enterobacteriaceae. Lasers Med. Sci. 2010, 25, 581–586. [Google Scholar] [CrossRef]
- O’Riordan, K.; Sharlin, D.S.; Gross, J.; Chang, S.; Errabelli, D.; Akilov, O.E.; Kosaka, S.; Nau, G.J.; Hasan, T. Photoinactivation of Mycobacteria in Vitro and in a New Murine Model of Localized Mycobacterium Bovis BCG-Induced Granulomatous Infection. Antimicrob. Agents Chemother. 2006, 50, 1828–1834. [Google Scholar] [CrossRef] [PubMed]
- Späth, A.; Leibl, C.; Cieplik, F.; Lehner, K.; Regensburger, J.; Hiller, K.A.; Bäumler, W.; Schmalz, G.; Maisch, T. Improving Photodynamic Inactivation of Bacteria in Dentistry: Highly Effective and Fast Killing of Oral Key Pathogens with Novel Tooth-Colored Type-II Photosensitizers. J. Med. Chem. 2014, 57, 5157–5168. [Google Scholar] [CrossRef] [PubMed]
- Bergmans, L.; Moisiadis, P.; Huybrechts, B.; van Meerbeek, B.; Quirynen, M.; Lambrechts, P. Effect of Photo-Activated Disinfection on Endodontic Pathogens Ex Vivo. Int. Endod. J. 2008, 41, 227–239. [Google Scholar] [CrossRef]
- Xu, Y.; Young, M.J.; Battaglino, R.A.; Morse, L.R.; Fontana, C.R.; Pagonis, T.C.; Kent, R.; Soukos, N.S. Endodontic antimicrobial photodynamic therapy: Safety assessment in mammalian cell cultures. J. Endod. 2009, 35, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Foschi, F.; Fontana, C.R.; Ruggiero, K.; Riahi, R.; Vera, A.; Doukas, A.G.; Pagonis, T.C.; Kent, R.; Stashenko, P.P.; Soukos, N.S. Photodynamic inactivation of Enterococcus faecalis in dental root canals in vitro. Lasers Surg. Med. 2007, 39, 782–787. [Google Scholar] [CrossRef]
- Garcez, A.S.; Arantes-Neto, J.G.; Sellera, D.P.; Fregnani, E.R. Effects of antimicrobial photodynamic therapy and surgical endodontic treatment on the bacterial load reduction and periapical lesion healing. Three years follow up. Photodiagn. Photodyn. Ther. 2015, 12, 575–580. [Google Scholar] [CrossRef]
- Vohra, F.; Akram, Z.; Safii, S.H.; Vaithilingam, R.D.; Ghanem, A.; Sergis, K.; Javed, F. Role of antimicrobial photodynamic therapy in the treatment of aggressive periodontitis: A systematic review. Photodiagn. Photodyn. Ther. 2016, 13, 139–147. [Google Scholar] [CrossRef]
- Zambon, J.J.; Christersson, L.A.; Slots, J. Actinobacillus actinomycetemcomitans in human periodontal disease. Prevalence in patient groups and distribution of biotypes and serotypes within families. J. Periodontol. 1983, 54, 707–711. [Google Scholar] [CrossRef]
- Park, D.; Choi, E.J.; Weon, K.Y.; Lee, W.; Lee, S.H.; Choi, J.S.; Park, G.H.; Lee, B.; Byun, M.R.; Baek, K.; et al. Non-Invasive Photodynamic Therapy against -Periodontitis-causing Bacteria. Sci. Rep. 2019, 9, 8248. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Rispoli, L.; Lopez, M.A.; Netti, A.; Petrini, M.; Piattelli, A. Photodynamic Therapy by Mean of 5-Aminolevulinic Acid for the Management of Periodontitis and Peri-Implantitis: A Retrospective Analysis of 20 Patients. Antibiotics 2022, 11, 1267. [Google Scholar] [CrossRef] [PubMed]
- Salvi, G.E.; Stähli, A.; Schmidt, J.C.; Ramseier, C.A.; Sculean, A.; Walter, C. Adjunctive laser or antimicrobial photodynamic therapy to non-surgical mechanical instrumentation in patients with untreated periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2020, 47, 176–198. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.R.; Luca, R.E.; Mateas, M.; Darawsha, L.D.; Boia, S.; Boia, E.R.; Todea, C.D. The Efficiency of Photodynamic Therapy in the Bacterial Decontamination of Periodontal Pockets and Its Impact on the Patient. Diagnostics 2022, 12, 3026. [Google Scholar] [CrossRef]
- Lopez, M.A.; Passarelli, P.C.; Marra, M.; Lopez, A.; Moffa, A.; Casale, M.; D’Addona, A. Antimicrobial efficacy of photodynamic therapy (PDT) in periodontitis and peri-implantitis: A systematic review. J. Biol. Regul. Homeost. Agents 2020, 34, 59–65. [Google Scholar]
- Sgolastra, F.; Petrucci, A.; Gatto, R.; Marzo, G.; Monaco, A. Photodynamic therapy in the treatment of chronic periodontitis: A systematic review and meta-analysis. Lasers Med. Sci. 2013, 28, 669–682. [Google Scholar] [CrossRef]
- Park, D.; Kim, M.; Choi, J.W.; Baek, J.H.; Lee, S.H.; Baek, K. Antimicrobial photodynamic therapy efficacy against specific pathogenic periodontitis bacterial species. Photodiagn. Photodyn. Ther. 2020, 30, 101688. [Google Scholar] [CrossRef]
- Hu, X.; Huang, Y.Y.; Wang, Y.; Wang, X.; Hamblin, M.R. Antimicrobial Photodynamic Therapy to Control Clinically Relevant Biofilm Infections. Front. Microbiol. 2018, 9, 1299. [Google Scholar] [CrossRef]
- Huang, L.; Xuan, Y.; Koide, Y.; Zhiyentayev, T.; Tanaka, M.; Hamblin, M.R. Type I and Type II mechanisms of antimicrobial photodynamic therapy: An in vitro study on gram-negative and gram-positive bacteria. Lasers Surg. Med. 2012, 44, 490–499. [Google Scholar] [CrossRef]
- Whaley, S.G.; Berkow, E.L.; Rybak, J.M.; Nishimoto, A.T.; Barker, K.S.; Rogers, P.D. Azole antifungal resistance in Candida albicans and emerging non-albicans Candida species. Front. Microbiol. 2016, 7, 2173. [Google Scholar] [CrossRef]
- Senna, A.M.; Vieira, M.M.F.; Machado-de-Sena, R.M.; Bertolin, A.O.; Núñez, S.C.; Ribeiro, M.S. Photodynamic inactivation of Candida ssp. on denture stomatitis. A clinical trial involving palatal mucosa and prosthesis disinfection. Photodiagn. Photodyn. Ther. 2018, 22, 212–216. [Google Scholar] [CrossRef]
- Alrabiah, M.; Alsahhaf, A.; Alofi, R.S.; Al-Aali, K.A.; Abduljabbar, A.; Vohra, F. Efficacy of photodynamic therapy versus local nystatin in the treatment of denture stomatitis: A randomized clinical study. Photodiagn. Photodyn. Ther. 2019, 28, 98–101. [Google Scholar] [CrossRef]
- Etcheverry, M.E.; Pasquale, M.A.; Garavaglia, M. Photodynamic therapy of HeLa cell cultures by using LED or laser sources. J. Photochem. Photobiol. B 2016, 160, 271–277. [Google Scholar] [CrossRef]
- Javed, F.; Samaranayake, L.P.; Romanos, G.E. Treatment of oral fungal infections using antimicrobial photodynamic therapy: A systematic review of currently available evidence. Photochem. Photobiol. Sci. 2014, 13, 726–734. [Google Scholar] [CrossRef]
- Afroozi, B.; Zomorodian, K.; Lavaee, F.; Shahrabadi, Z.Z.; Mardani, M. Comparison of the efficacy of indocyanine green-mediated photodynamic therapy and nystatin therapy in treatment of denture stomatitis. Photodiagn. Photodyn. Ther. 2019, 27, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Vendramini, Y.; Salles, A.; Portella, F.F.; Brew, M.C.; Steier, L.; de Figueiredo, J.A.P.; Bavaresco, C.S. Antimicrobial effect of photodynamic therapy on intracanal biofilm: A systematic review of in vitro studies. Photodiagn. Photodyn. Ther. 2020, 32, 102025. [Google Scholar] [CrossRef] [PubMed]
- Plotino, G.; Grande, N.M.; Mercade, M. Photodynamic therapy in endodontics. Int. Endod. J. 2019, 52, 760–774. [Google Scholar] [CrossRef] [PubMed]
- Mester, A.; Mester, A. The History of Photobiomodulation: Endre Mester (1903–1984). Photomed. Laser Surg. 2017, 35, 393–394. [Google Scholar] [CrossRef] [PubMed]
- Anders, J.J.; Lanzafame, R.J.; Arany, P.R. Low-level light/laser therapy versus photobiomodulation therapy. Photomed. Laser Surg. 2015, 33, 183–184. [Google Scholar] [CrossRef] [PubMed]
- Nejat, A.H.; Eshghpour, M.; Danaeifar, N.; Abrishami, M.; Vahdatinia, F.; Fekrazad, R. Effect of Photobiomodulation on the Incidence of Alveolar Osteitis and Postoperative Pain following Mandibular Third Molar Surgery: A Double-Blind Randomized Clinical Trial. Photochem. Photobiol. 2021, 97, 1129–1135. [Google Scholar] [CrossRef]
- Zadik, Y.; Arany, P.R.; Fregnani, E.R.; Bossi, P.; Antunes, H.S.; Bensadoun, R.J.; Gueiros, L.A.; Majorana, A.; Nair, R.G.; Ranna, V.; et al. Systematic review of photobiomodulation for the management of oral mucositis in cancer patients and clinical practice guidelines. Support Care Cancer 2019, 27, 3969–3983. [Google Scholar] [CrossRef] [PubMed]
- Badeaux, J.; Bonanno, L.; Au, H. Effectiveness of ondansetron as an adjunct to lidocaine intravenous regional anesthesia on tourniquet pain and postoperative pain in patients undergoing elective hand surgery: A systematic review protocol. JBI Database Syst. Rev. Implement. Rep. 2015, 13, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Modrak, M.; Talukder, M.A.H.; Gurgenashvili, K.; Noble, M.; Elfar, J.C. Peripheral nerve injury and myelination: Potential therapeutic strategies. J. Neurosci. Res. 2020, 98, 780–795. [Google Scholar] [CrossRef] [PubMed]
- Dadjoo, S.; Michelogiannakis, D.; Rossouw, P.E.; Javed, F. Potential adjunct therapies for the management of temporomandibular disorders: An evidence-based review. Cranio J. Craniomandib. Pract. 2022, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Yang, K. Clinical research: Low-level laser therapy in accelerating orthodontic tooth movement. BMC Oral Health 2021, 21, 324. [Google Scholar] [CrossRef]
- Carneiro, A.M.P.; Barros, A.P.O.; de Oliveira, R.P.; de Paula, B.L.F.; Silva, A.M.; de Melo Alencar, C.; Silva, C.M. The effect of photobiomodulation using low-level laser therapy on tooth sensitivity after dental bleaching: A systematic review. Lasers Med. Sci. 2022, 37, 2791–2804. [Google Scholar] [CrossRef]
- Costa, L.; Alves, E.; Carvalho, C.M.; Tomé, J.P.; Faustino, M.A.; Neves, M.G.; Tomé, A.C.; Cavaleiro, J.A.; Cunha, A.; Almeida, A. Sewage bacteriophage photoinactivation by cationic porphyrins: A study of charge effect. Photochem. Photobiol. Sci. 2008, 7, 415–422. [Google Scholar] [CrossRef]
- Schultz, E.W. Inactivation of Staphyloccus Bacteriophage by Methylene Blue. Proc. Soc. Exp. Biol. Med. 1928, 26, 100–101. [Google Scholar] [CrossRef]
| Lasers Used in Dentistry | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Argon Laser | Potassium-Titanyl-Phosphate (KTP) Laser | Helium-Neon Laser | Low-Level Laser (LLL) | Diode Laser (DL) | Nd: YAG Laser | Er,Cr: YSGG Laser | Er:YAG Laser | CO2 Laser | |
| Wavelength [nm] | 488, 515 | 532 | 633 | 632–904 | 635, 670, 810, 830, 980 | 1064 | 2780 | 2940 | 9300, 10,600 |
| Authors | PDT Uses Groups |
|---|---|
| Warrier et al., 2021 [71] | Photodynamic antimicrobial therapy (aPDT) |
| Takasaki et al., 2009 [72] | Photodynamic antimicrobial chemotherapy (PACT) |
| Rossoni et al., 2010 [73] | Photodynamic disinfection (PDI) |
| O’Riordan et al., 2006 [74] | Antimicrobial photodynamic inactivation (aPDI) |
| Späth et al., 2014 [75] | Lethal photosensitization |
| Bergmans et al., 2008 [76] | Photoactivated disinfection (PAD) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luchian, I.; Budală, D.G.; Baciu, E.-R.; Ursu, R.G.; Diaconu-Popa, D.; Butnaru, O.; Tatarciuc, M. The Involvement of Photobiology in Contemporary Dentistry—A Narrative Review. Int. J. Mol. Sci. 2023, 24, 3985. https://doi.org/10.3390/ijms24043985
Luchian I, Budală DG, Baciu E-R, Ursu RG, Diaconu-Popa D, Butnaru O, Tatarciuc M. The Involvement of Photobiology in Contemporary Dentistry—A Narrative Review. International Journal of Molecular Sciences. 2023; 24(4):3985. https://doi.org/10.3390/ijms24043985
Chicago/Turabian StyleLuchian, Ionut, Dana Gabriela Budală, Elena-Raluca Baciu, Ramona Gabriela Ursu, Diana Diaconu-Popa, Oana Butnaru, and Monica Tatarciuc. 2023. "The Involvement of Photobiology in Contemporary Dentistry—A Narrative Review" International Journal of Molecular Sciences 24, no. 4: 3985. https://doi.org/10.3390/ijms24043985
APA StyleLuchian, I., Budală, D. G., Baciu, E.-R., Ursu, R. G., Diaconu-Popa, D., Butnaru, O., & Tatarciuc, M. (2023). The Involvement of Photobiology in Contemporary Dentistry—A Narrative Review. International Journal of Molecular Sciences, 24(4), 3985. https://doi.org/10.3390/ijms24043985












