Preliminary Toxicity Evaluation of a Porphyrin Photosensitizer in an Alternative Preclinical Model
Abstract
1. Introduction
2. Results
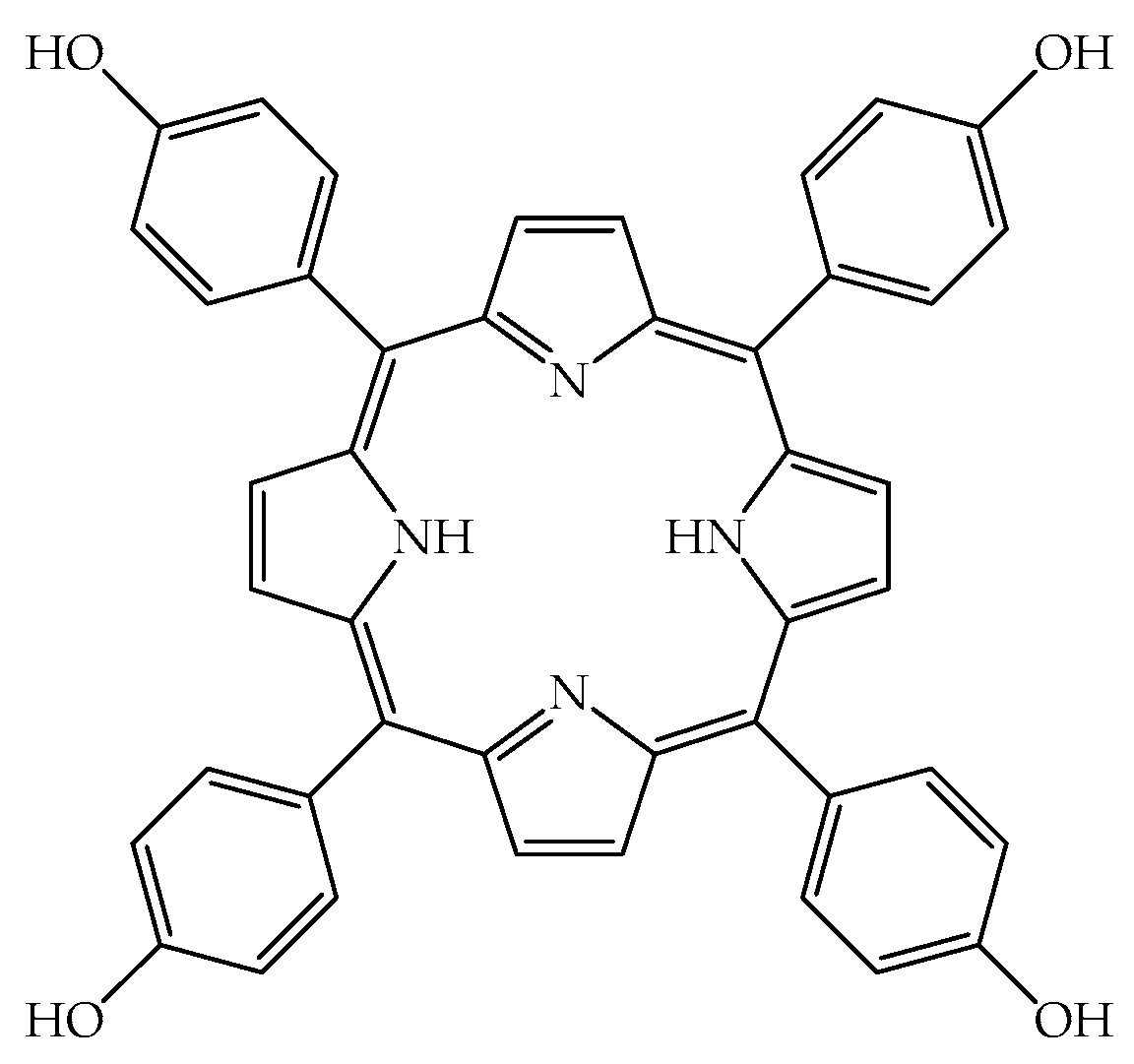
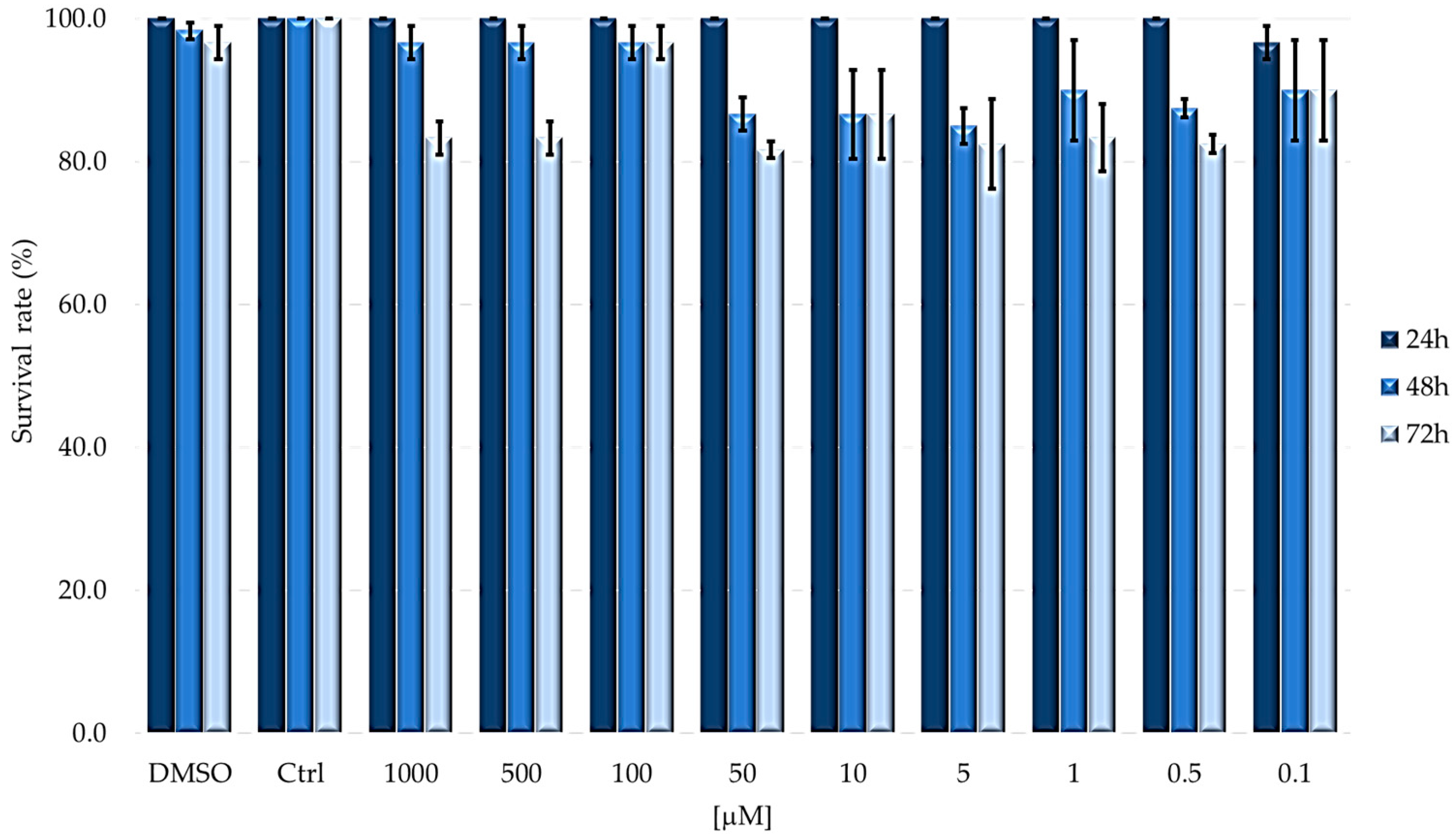
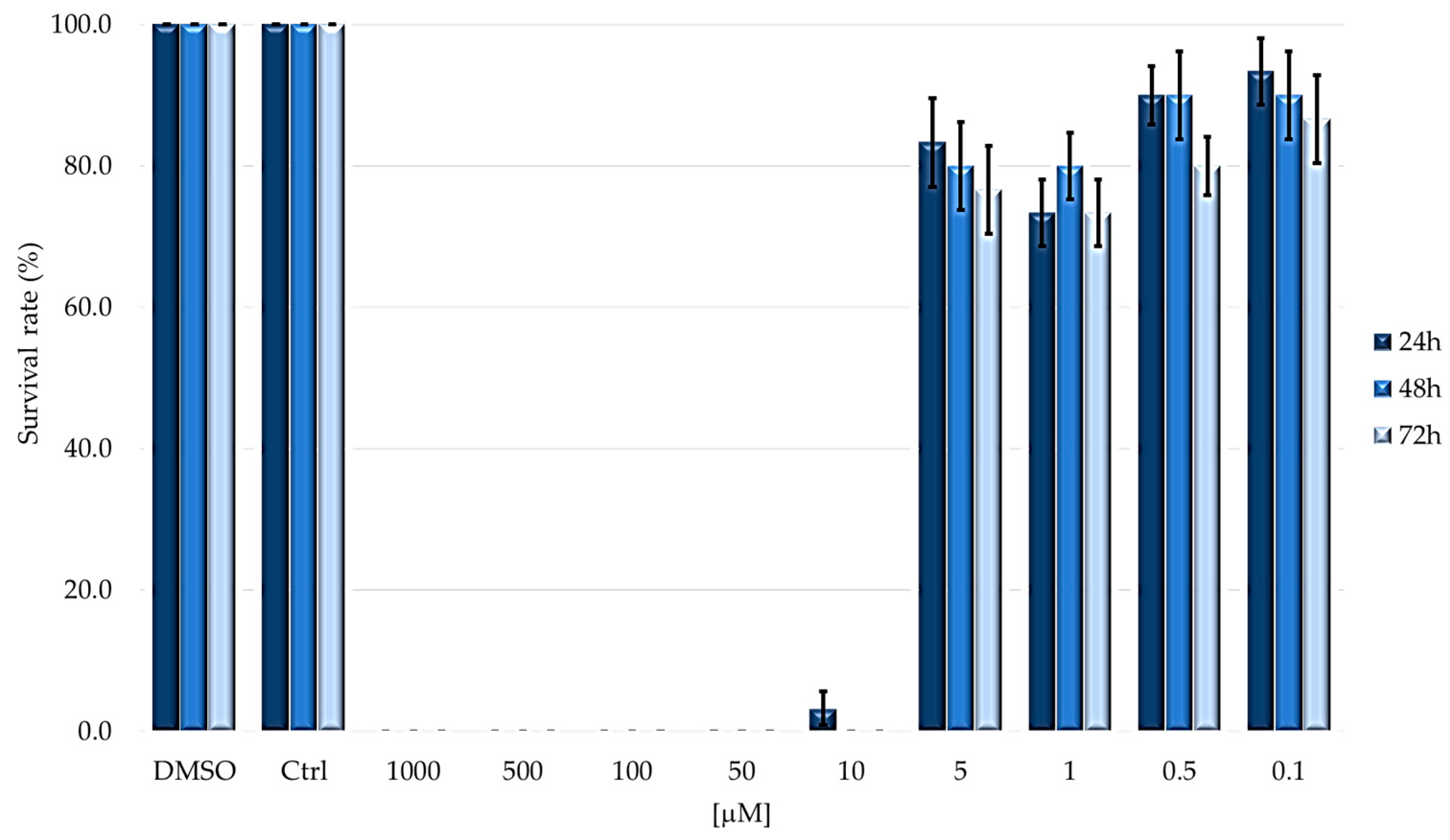
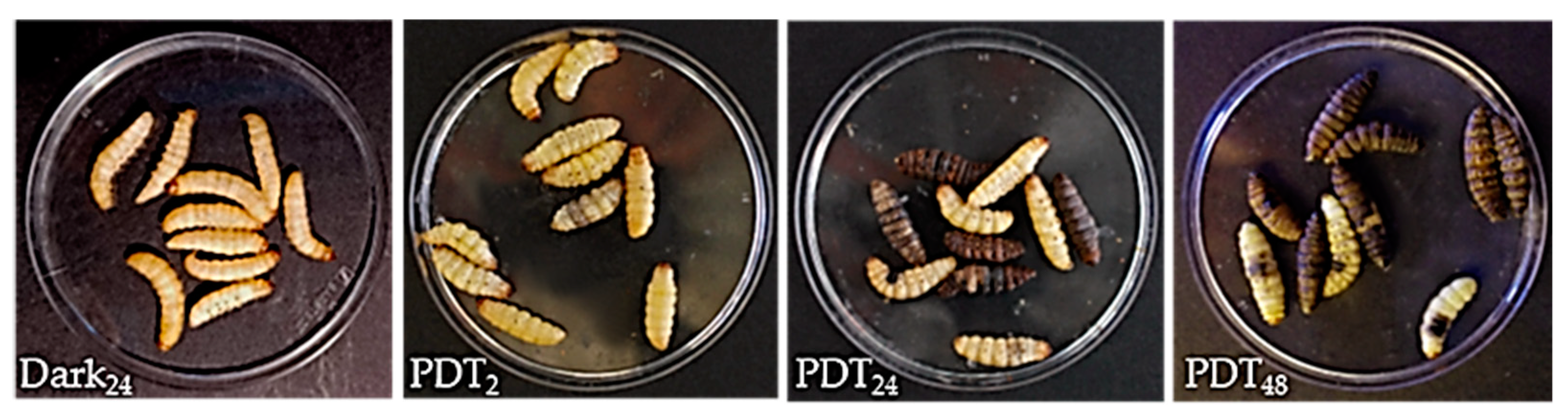
2.1. TPPOH Toxicity in G. mellonella Larvae
2.2. Cytotoxicity on Immunocompetent Cells (Hemocytes)
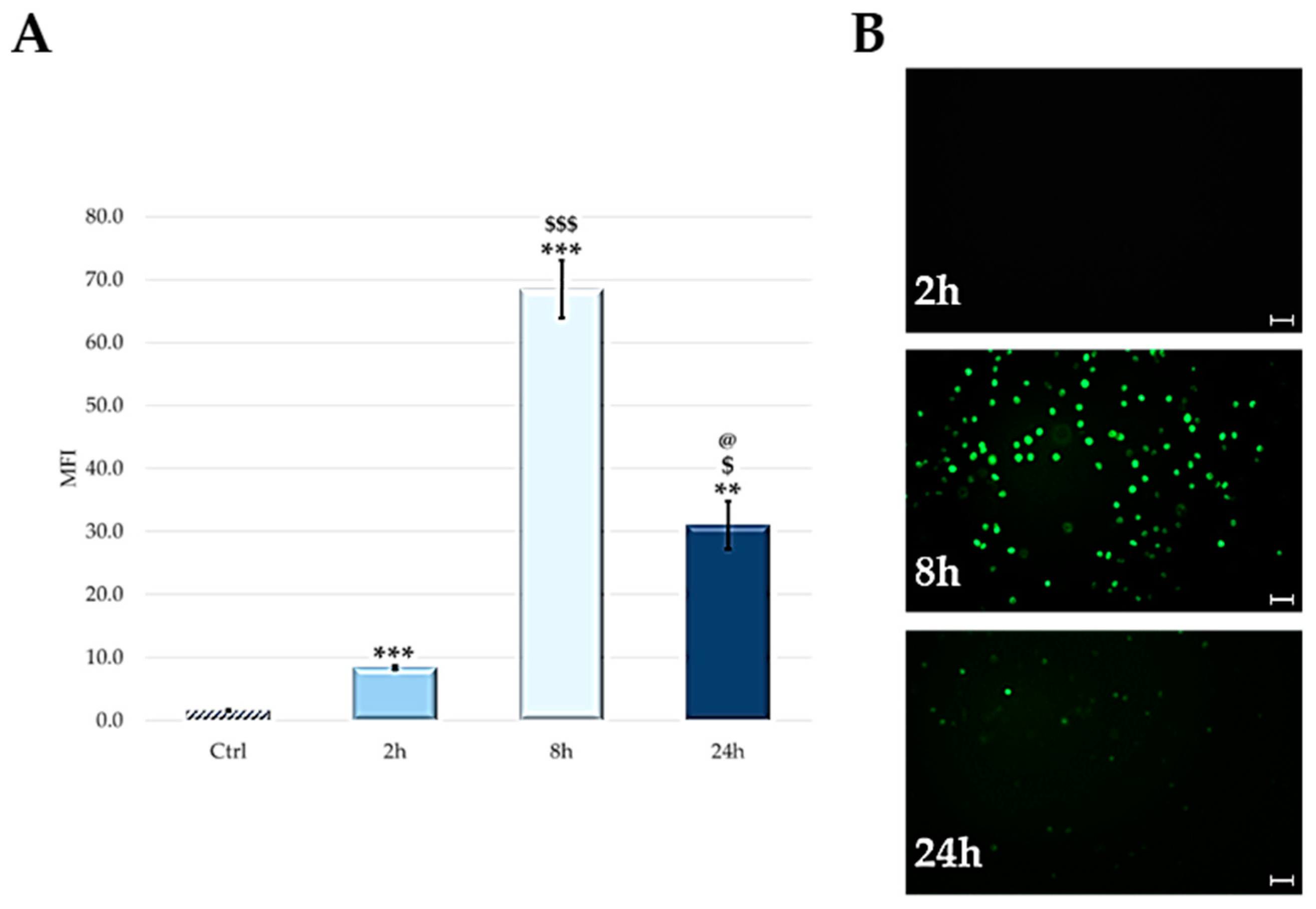
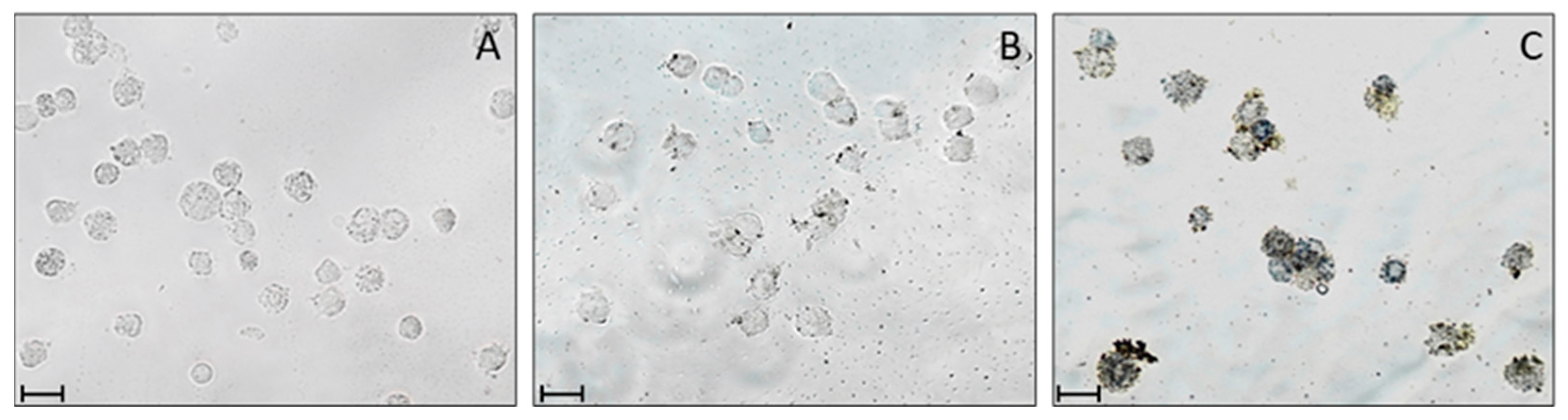
2.3. Cellular Uptake: Flow Cytometry and Microscopy
2.4. ROS Detection after PDT Treatment
3. Discussion
4. Materials and Methods
4.1. General
4.2. Phototoxicity Assays
4.3. Cytotoxicity Assay and Total Hemocytes Counts
4.4. Cellular Uptake: Flow Cytometric Analysis and Microscopy Technique
4.5. ROS Detection after PDT Treatment by NBT Reduction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hopper, C. Photodynamic therapy: A clinical reality in the treatment of cancer. Lancet Oncol. 2000, 1, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Plaetzer, K.; Krammer, B.; Berlanda, J.; Berr, F.; Kiesslich, T. Photophysics and photochemistry of photodynamic therapy: Fundamental aspects. Lasers Med. Sci. 2009, 24, 259–268. [Google Scholar] [CrossRef]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, A.F.; Terra, L.F.; Wailemann, R.A.; Oliveira, T.C.; Gomes, V.D.M.; Mineiro, M.F.; Meotti, F.C.; Bruni-Cardoso, A.; Baptista, M.S.; Labriola, L. Methylene blue photodynamic therapy induces selective and massive cell death in human breast cancer cells. BMC Cancer 2017, 17, 194–209. [Google Scholar] [CrossRef] [PubMed]
- Ledoux-Lebards, C. Annales de l’Institut Pasteur; Masson Et Cie: Paris, France, 1902. [Google Scholar]
- Schmidt, R. Photosensitized Generation of Singlet Oxygen. Photochem. Photobiol. 2007, 82, 1161–1177. [Google Scholar] [CrossRef]
- Kessel, D.; Oleinick, N.L. Initiation of autophagy by photodynamic therapy. Methods Enzymol. 2009, 453, 1–16. [Google Scholar] [CrossRef]
- Dewaele, M.; Maes, H.; Agostinis, P. ROS-mediated mechanisms of autophagy stimulation and their relevance in cancer therapy. Autophagy 2010, 6, 838–854. [Google Scholar] [CrossRef]
- Chevalier, S.; Anidjar, M.; Scarlata, E.; Hamel, L.; Scherz, A.; Ficheux, H.; Borenstein, N.; Fiette, L.; Elhilali, M. Preclinical study of the novel vascular occluding agent, WST11, for photodynamic therapy of the canine prostate. J. Urol. 2011, 186, 302–309. [Google Scholar] [CrossRef]
- Dovigo, L.N.; Pavarina, A.C.; Ribeiro, A.P.; Brunetti, I.L.; Costa, C.A.; Jacomassi, D.P.; Bagnato, V.S.; Kurachi, C. Investigation of the photodynamic effects of curcumin against Candida albicans. Photochem. Photobiol. 2011, 87, 895–903. [Google Scholar] [CrossRef]
- Josefsen, L.B.; Boyle, R.W. Unique diagnostic and therapeutic roles of porphyrins and phthalocyanines in photodynamic therapy, imaging and theranostics. Theranostics 2012, 2, 916–966. [Google Scholar] [CrossRef]
- Ericsson, A.C.; Crim, M.J.; Franklin, C.L. A brief history of animal modeling. Mo. Med. 2013, 110, 201–205. [Google Scholar]
- Pound, P.; Ebrahim, S.; Sandercock, P.; Bracken, M.B.; Roberts, I. Where is the evidence that animal research benefits humans? BMJ 2004, 328, 514–517. [Google Scholar] [CrossRef] [PubMed]
- Burden, N.; Chapman, K.; Sewell, F.; Robinson, V. Pioneering better science through the 3Rs: An introduction to the national centre for the replacement, refinement, and reduction of animals in research (NC3Rs). J. Am. Assoc. Lab. Anim. Sci. 2015, 54, 198–208. [Google Scholar] [PubMed]
- Reduce, refine, replace. Nat. Immunol. 2010, 11, 971. [CrossRef] [PubMed]
- Robinson, N.B.; Krieger, K.; Khan, F.M.; Huffman, W.; Chang, M.; Naik, A.; Yongle, R.; Hameed, I.; Krieger, K.; Girardi, L.N.; et al. The current state of animal models in research: A review. Int. J. Surg. 2019, 72, 9–13. [Google Scholar] [CrossRef]
- Singkum, P.; Suwanmanee, S.; Pumeesat, P.; Luplertlop, N. A powerful in vivo alternative model in scientific research: Galleria mellonella. Acta Microbiol. Immunol. Hung. 2019, 66, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Doke, S.K.; Dhawale, S.C. Alternatives to animal testing: A review. Saudi Pharm. J. 2015, 23, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Wilson-Sanders, S.E. Invertebrate models for biomedical research, testing, and education. ILAR J. 2011, 52, 126–152. [Google Scholar] [CrossRef]
- Wojda, I.; Staniec, B.; Sulek, M.; Kordaczuk, J. The greater wax moth Galleria mellonella: Biology and use in immune studies. Pathog. Dis. 2020, 78, ftaa057. [Google Scholar] [CrossRef]
- Hamilos, G.; Samonis, G.; Kontoyiannis, D.P. Recent Advances in the Use of Drosophila melanogaster as a Model to Study Immunopathogenesis of Medically Important Filamentous Fungi. Int. J. Microbiol. 2012, 2012, 583792. [Google Scholar] [CrossRef]
- Jacobsen, I.D.; Große, K.; Hube, B. Embryonated Chicken Eggs as Alternative Infection Model for Pathogenic Fungi. In Host-Fungus Interactions: Methods and Protocols; Brand, A.C., MacCallum, D.M., Eds.; Humana Press: Totowa, NJ, USA, 2012; pp. 487–496. [Google Scholar] [CrossRef]
- Muhammed, M.; Coleman, J.J.; Mylonakis, E. Caenorhabditis elegans: A Nematode Infection Model for Pathogenic Fungi. In Host-Fungus Interactions: Methods and Protocols; Brand, A.C., MacCallum, D.M., Eds.; Humana Press: Totowa, NJ, USA, 2012; pp. 447–454. [Google Scholar] [CrossRef]
- Harding, C.R.; Schroeder, G.N.; Collins, J.W.; Frankel, G. Use of Galleria mellonella as a model organism to study Legionella pneumophila infection. J. Vis. Exp. 2013, 81, e50964. [Google Scholar] [CrossRef]
- Kristensen, N.P.; Scoble, M.J.; Karsholt, O.L.E. Lepidoptera phylogeny and systematics: The state of inventorying moth and butterfly diversity. Zootaxa 2007, 1668, 699–747. [Google Scholar] [CrossRef]
- Ellis, J.D.; Graham, J.R.; Mortensen, A. Standard methods for wax moth research. J. Apic. Res. 2015, 52, 1–17. [Google Scholar] [CrossRef]
- Romera, D.; Aguilera-Correa, J.J.; Garcia-Coca, M.; Mahillo-Fernandez, I.; Vinuela-Sandoval, L.; Garcia-Rodriguez, J.; Esteban, J. The Galleria mellonella infection model as a system to investigate the virulence of Candida auris strains. Pathog. Dis. 2020, 78, ftaa067. [Google Scholar] [CrossRef]
- Bruchmann, S.; Feltwell, T.; Parkhill, J.; Short, F.L. Identifying virulence determinants of multidrug-resistant Klebsiella pneumoniae in Galleria mellonella. Pathog. Dis. 2021, 79, ftab009. [Google Scholar] [CrossRef]
- Staczek, S.; Zdybicka-Barabas, A.; Wiater, A.; Pleszczynska, M.; Cytrynska, M. Activation of cellular immune response in insect model host Galleria mellonella by fungal alpha-1,3-glucan. Pathog. Dis. 2020, 78, ftaa062. [Google Scholar] [CrossRef] [PubMed]
- DeSarno, A.E.; Parcell, B.J.; Coote, P.J. Repurposing the anti-viral drug zidovudine (AZT) in combination with meropenem as an effective treatment for infections with multi-drug resistant, carbapenemase-producing strains of Klebsiella pneumoniae. Pathog. Dis. 2020, 78, ftaa063. [Google Scholar] [CrossRef]
- Vergis, J.; Malik, S.V.S.; Pathak, R.; Kumar, M.; Kurkure, N.V.; Barbuddhe, S.B.; Rawool, D.B. Exploring Galleria mellonella larval model to evaluate antibacterial efficacy of Cecropin A (1-7)-Melittin against multi-drug resistant enteroaggregative Escherichia coli. Pathog. Dis. 2021, 79, ftab010. [Google Scholar] [CrossRef]
- Kwadha, C.A.; Mutunga, J.M.; Irungu, J.; Ongamo, G.; Ndegwa, P.; Raina, S.; Fombong, A.T. Decanal as a major component of larval aggregation pheromone of the greater wax moth, Galleria mellonella. J. Appl. Entomol. 2019, 143, 417–429. [Google Scholar] [CrossRef]
- Tsai, C.J.; Loh, J.M.; Proft, T. Galleria mellonella infection models for the study of bacterial diseases and for antimicrobial drug testing. Virulence 2016, 7, 214–229. [Google Scholar] [CrossRef]
- Wojda, I. Immunity of the greater wax moth Galleria mellonella. Insect Sci. 2017, 24, 342–357. [Google Scholar] [CrossRef] [PubMed]
- Maravilla, E.; Le, D.P.; Tran, J.J.; Chiu, M.H.; Prenner, E.J.; Weers, P.M.M. Apolipophorin III interaction with phosphatidylglycerol and lipopolysaccharide: A potential mechanism for antimicrobial activity. Chem. Phys. Lipids 2020, 229, 104909. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.F.; Rossi, C.C.; da Silva, G.C.; Rosa, J.N.; Bazzolli, D.M.S. Galleria mellonella as an infection model: An in-depth look at why it works and practical considerations for successful application. Pathog. Dis. 2020, 78, ftaa056. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.L.; Kavanagh, O. Galleria mellonella as a Novel In Vivo Model to Screen Natural Product-Derived Modulators of Innate Immunity. Appl. Sci. 2022, 12, 6587. [Google Scholar] [CrossRef]
- Ignasiak, K.; Maxwell, A. Galleria mellonella (greater wax moth) larvae as a model for antibiotic susceptibility testing and acute toxicity trials. BMC Res. Notes 2017, 10, 428. [Google Scholar] [CrossRef]
- Junqueira, J.C.; Mylonakis, E.; Borghi, E. Galleria mellonella experimental model: Advances and future directions. Pathog. Dis. 2021, 79, ftab021. [Google Scholar] [CrossRef]
- Piatek, M.; Sheehan, G.; Kavanagh, K. Galleria mellonella: The Versatile Host for Drug Discovery, In Vivo Toxicity Testing and Characterising Host-Pathogen Interactions. Antibiotics 2021, 10, 1545. [Google Scholar] [CrossRef]
- Banfi, S.; Caruso, E.; Buccafurni, L.; Murano, R.; Monti, E.; Gariboldi, M.; Papa, E.; Gramatica, P. Comparison between 5,10,15,20-tetraaryl- and 5,15-diarylporphyrins as photosensitizers: Synthesis, photodynamic activity, and quantitative structure-activity relationship modeling. J. Med. Chem. 2006, 49, 3293–3304. [Google Scholar] [CrossRef]
- Banfi, S.; Caruso, E.; Caprioli, S.; Mazzagatti, L.; Canti, G.; Ravizza, R.; Gariboldi, M.; Monti, E. Photodynamic effects of porphyrin and chlorin photosensitizers in human colon adenocarcinoma cells. Bioorg. Med. Chem. 2004, 12, 4853–4860. [Google Scholar] [CrossRef]
- Glupov, V.V.; Khvoshevskaya, M.F.; Lozinskaya, Y.L.; Dubovski, I.M.; Martemyanov, V.V.; Sokolova, J.Y. Application of the nitroblue tetrazolium-reduction method for studies on the production of reactive oxygen species in insect haemocytes. Cytobios 2001, 106 (Suppl. S2), 165–178. [Google Scholar]
- Nappi, A.J.; Vass, E. Melanogenesis and the generation of cytotoxic molecules during insect cellular immune reactions. Pigment Cell Res. 1993, 6, 117–126. [Google Scholar] [CrossRef]
- Nappi, A.J.; Vass, E.; Frey, F.; Carton, Y. Superoxide anion generation in Drosophila during melanotic encapsulation of parasites. Eur. J. Cell Biol. 1995, 68, 450–456. [Google Scholar]
- Menard, L.M.; Wood, N.B.; Vigoreaux, J.O. Secondary Structure of the Novel Myosin Binding Domain WYR and Implications within Myosin Structure. Biology 2021, 10, 603. [Google Scholar] [CrossRef]
- Cools, F.; Torfs, E.; Aizawa, J.; Vanhoutte, B.; Maes, L.; Caljon, G.; Delputte, P.; Cappoen, D.; Cos, P. Optimization and Characterization of a Galleria mellonella Larval Infection Model for Virulence Studies and the Evaluation of Therapeutics Against Streptococcus pneumoniae. Front. Microbiol. 2019, 10, 311. [Google Scholar] [CrossRef]
- Piatek, M.; Sheehan, G.; Kavanagh, K. Utilising Galleria mellonella larvae for studying in vivo activity of conventional and novel antimicrobial agents. Pathog. Dis. 2020, 78, ftaa059. [Google Scholar] [CrossRef] [PubMed]
- Hubrecht, R.C.; Carter, E. The 3Rs and Humane Experimental Technique: Implementing Change. Animals 2019, 9, 754. [Google Scholar] [CrossRef]
- Cook, S.M.; McArthur, J.D. Developing Galleria mellonella as a model host for human pathogens. Virulence 2013, 4, 350–353. [Google Scholar] [CrossRef]
- Brennan, M.; Thomas, D.Y.; Whiteway, M.; Kavanagh, K. Correlation between virulence of Candida albicans mutants in mice and Galleria mellonella larvae. FEMS Immunol. Med. Microbiol. 2002, 34, 153–157. [Google Scholar] [CrossRef]
- Jander, G.; Rahme, L.G.; Ausubel, F.M. Positive correlation between virulence of Pseudomonas aeruginosa mutants in mice and insects. J. Bacteriol. 2000, 182, 3843–3845. [Google Scholar] [CrossRef] [PubMed]
- Caruso, E.; Malacarne, M.C.; Banfi, S.; Gariboldi, M.B.; Orlandi, V.T. Cationic diarylporphyrins: In vitro versatile anticancer and antibacterial photosensitizers. J. Photochem. Photobiol. B 2019, 197, 111548. [Google Scholar] [CrossRef] [PubMed]
- Malacarne, M.C.; Banfi, S.; Rugiero, M.; Caruso, E. Drug delivery systems for the photodynamic application of two photosensitizers belonging to the porphyrin family. Photochem. Photobiol. Sci. 2021, 20, 1011–1025. [Google Scholar] [CrossRef] [PubMed]
- Malacarne, M.C.; Banfi, S.; Atherton, A.S.; Caruso, E. Photodynamic activity of new photosensitizers obtained from 5,10,15,20-tetrapentafluorophenylporphyrin. J. Porphyr. Phthalocyanines 2019, 23, 1047–1056. [Google Scholar] [CrossRef]
- Gariboldi, M.B.; Marras, E.; Vaghi, I.; Margheritis, A.; Malacarne, M.C.; Caruso, E. Phototoxicity of two positive-charged diaryl porphyrins in multicellular tumor spheroids. J. Photochem. Photobiol. B 2021, 225, 112353. [Google Scholar] [CrossRef] [PubMed]
- Caruso, E.; Cerbara, M.; Malacarne, M.C.; Marras, E.; Monti, E.; Gariboldi, M.B. Synthesis and photodynamic activity of novel non-symmetrical diaryl porphyrins against cancer cell lines. J. Photochem. Photobiol. B 2019, 195, 39–50. [Google Scholar] [CrossRef] [PubMed]
- van Straten, D.; Mashayekhi, V.; de Bruijn, H.S.; Oliveira, S.; Robinson, D.J. Oncologic Photodynamic Therapy: Basic Principles, Current Clinical Status and Future Directions. Cancers 2017, 9, 19. [Google Scholar] [CrossRef]
- Ormond, A.B.; Freeman, H.S. Dye Sensitizers for Photodynamic Therapy. Materials 2013, 6, 817–840. [Google Scholar] [CrossRef]
- Cerenius, L.; Soderhall, K. Immune properties of invertebrate phenoloxidases. Dev. Comp. Immunol. 2021, 122, 104098. [Google Scholar] [CrossRef]
- Clark, K.D. Insect Hemolymph Immune Complexes. Subcell Biochem. 2020, 94, 123–161. [Google Scholar] [CrossRef]
- Hanson, M.A.; Lemaitre, B. New insights on Drosophila antimicrobial peptide function in host defense and beyond. Curr. Opin. Immunol. 2020, 62, 22–30. [Google Scholar] [CrossRef]
- Hultmark, D. Insect lysozymes. EXS 1996, 75, 87–102. [Google Scholar] [CrossRef]
- Wu, B.; Luo, L.; Gao, X.J. Cas9-triggered chain ablation of cas9 as a gene drive brake. Nat. Biotechnol. 2016, 34, 137–138. [Google Scholar] [CrossRef] [PubMed]
- Esfandiari, N.; Sharma, R.K.; Saleh, R.A.; Thomas, A.J., Jr.; Agarwal, A. Utility of the nitroblue tetrazolium reduction test for assessment of reactive oxygen species production by seminal leukocytes and spermatozoa. J. Androl. 2003, 24, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Tabachnick, B.G.; Fidell, L.S.; Ullman, J.B. Using Multivariate Statistics, 7th ed.; Pearson Education: Boston, MA, USA, 2019. [Google Scholar]
- Mastore, M.; Caramella, S.; Quadroni, S.; Brivio, M.F. Drosophila suzukii Susceptibility to the Oral Administration of Bacillus thuringiensis, Xenorhabdus nematophila and Its Secondary Metabolites. Insects 2021, 12, 635. [Google Scholar] [CrossRef] [PubMed]
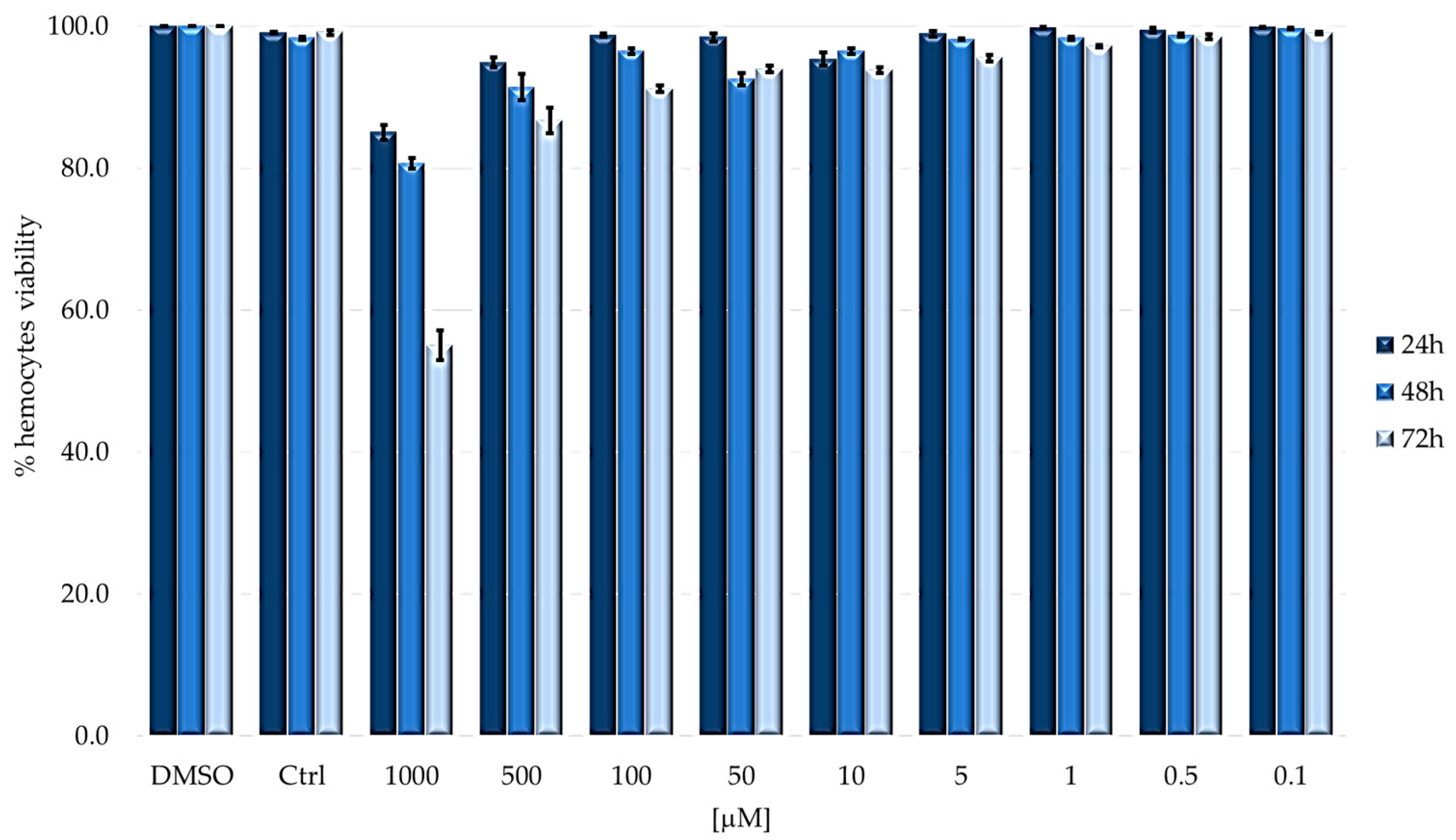

| 24 h | 48 h | 72 h | |
|---|---|---|---|
| LD50 [μM] | 5.247 ± 0.932 | 5.262 ± 1.079 | 3.936 ± 0.865 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malacarne, M.C.; Mastore, M.; Gariboldi, M.B.; Brivio, M.F.; Caruso, E. Preliminary Toxicity Evaluation of a Porphyrin Photosensitizer in an Alternative Preclinical Model. Int. J. Mol. Sci. 2023, 24, 3131. https://doi.org/10.3390/ijms24043131
Malacarne MC, Mastore M, Gariboldi MB, Brivio MF, Caruso E. Preliminary Toxicity Evaluation of a Porphyrin Photosensitizer in an Alternative Preclinical Model. International Journal of Molecular Sciences. 2023; 24(4):3131. https://doi.org/10.3390/ijms24043131
Chicago/Turabian StyleMalacarne, Miryam Chiara, Maristella Mastore, Marzia Bruna Gariboldi, Maurizio Francesco Brivio, and Enrico Caruso. 2023. "Preliminary Toxicity Evaluation of a Porphyrin Photosensitizer in an Alternative Preclinical Model" International Journal of Molecular Sciences 24, no. 4: 3131. https://doi.org/10.3390/ijms24043131
APA StyleMalacarne, M. C., Mastore, M., Gariboldi, M. B., Brivio, M. F., & Caruso, E. (2023). Preliminary Toxicity Evaluation of a Porphyrin Photosensitizer in an Alternative Preclinical Model. International Journal of Molecular Sciences, 24(4), 3131. https://doi.org/10.3390/ijms24043131










