Facile Synthesis of 2D/2D Ti2C3/ZnIn2S4 Heterostructure for Enhanced Photocatalytic Hydrogen Generation
Abstract
1. Introduction
2. Results
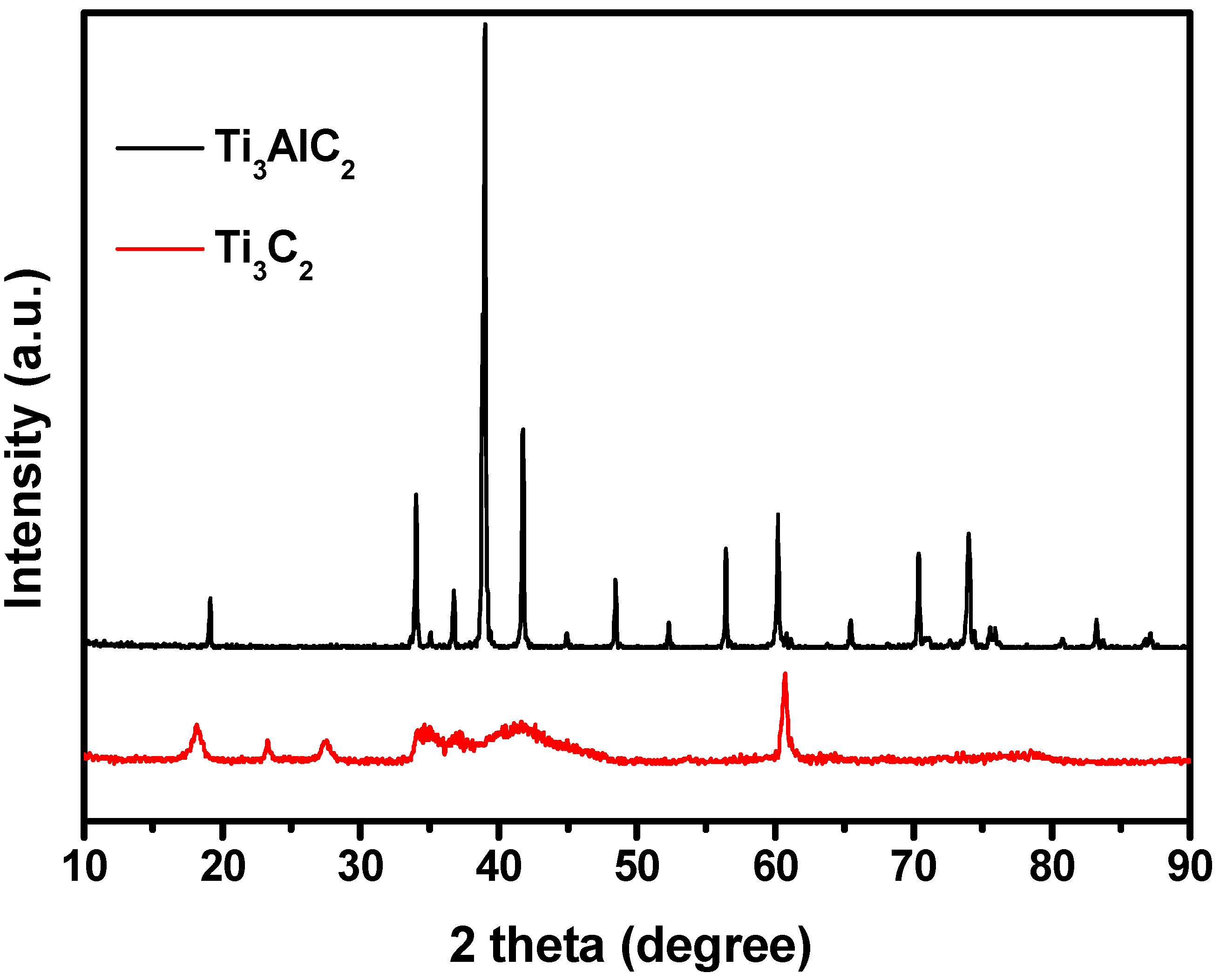
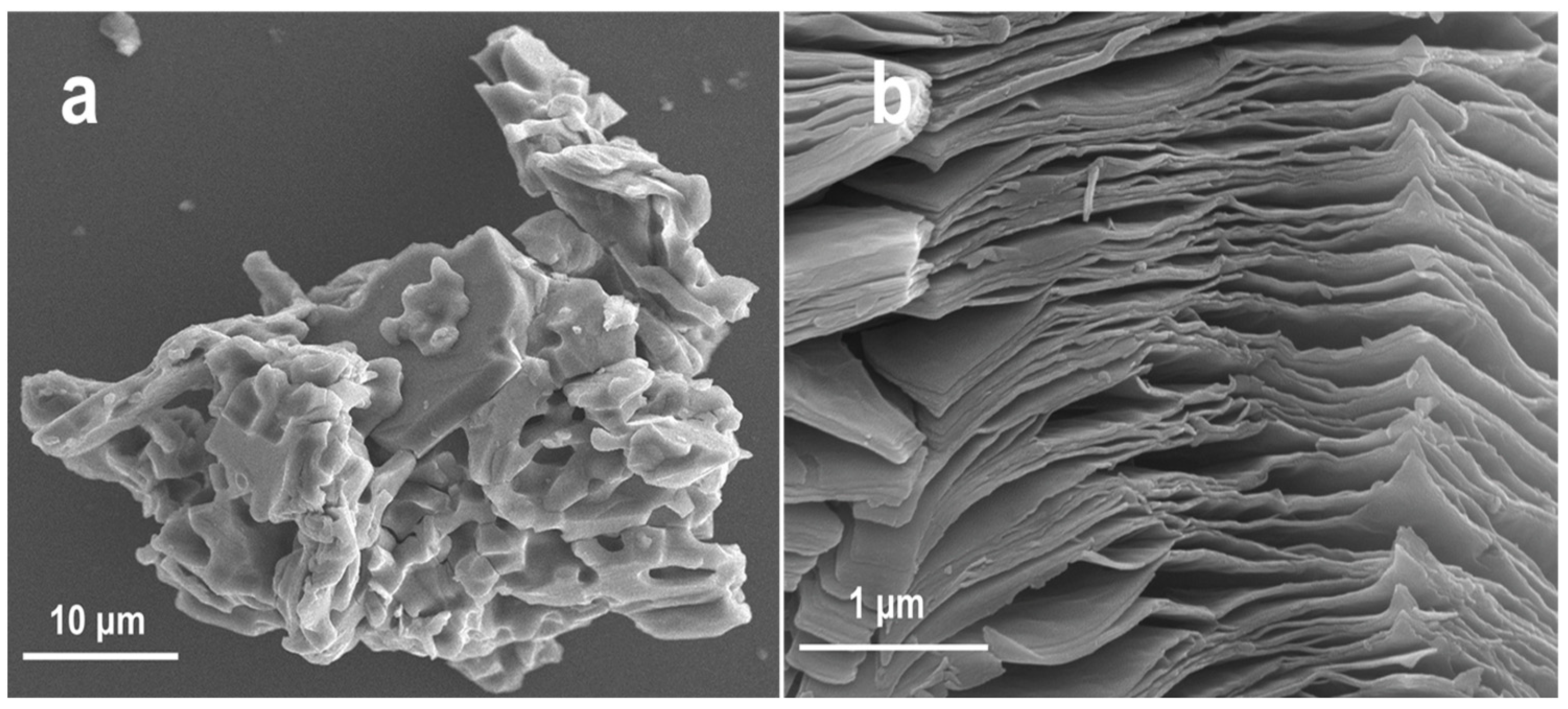
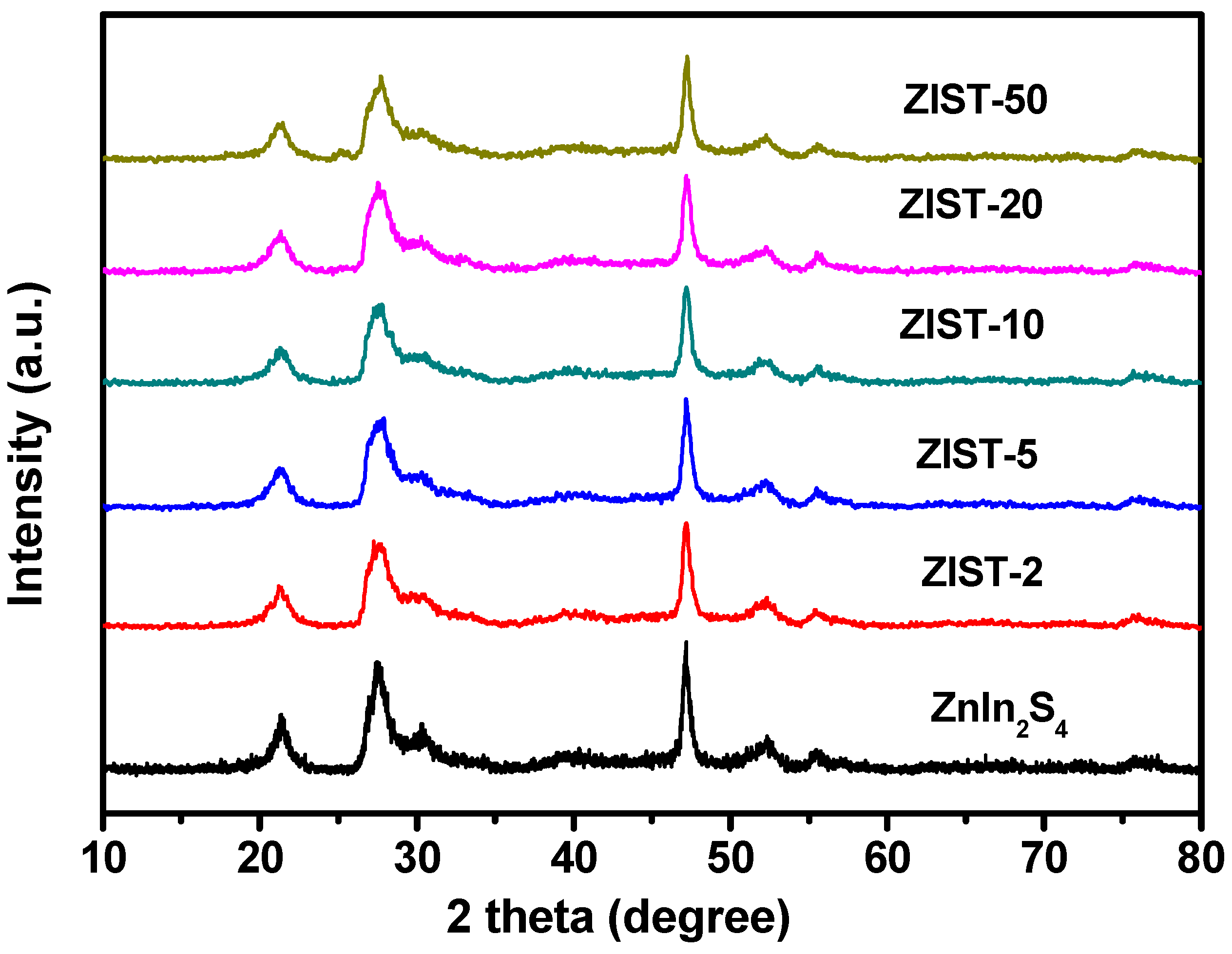
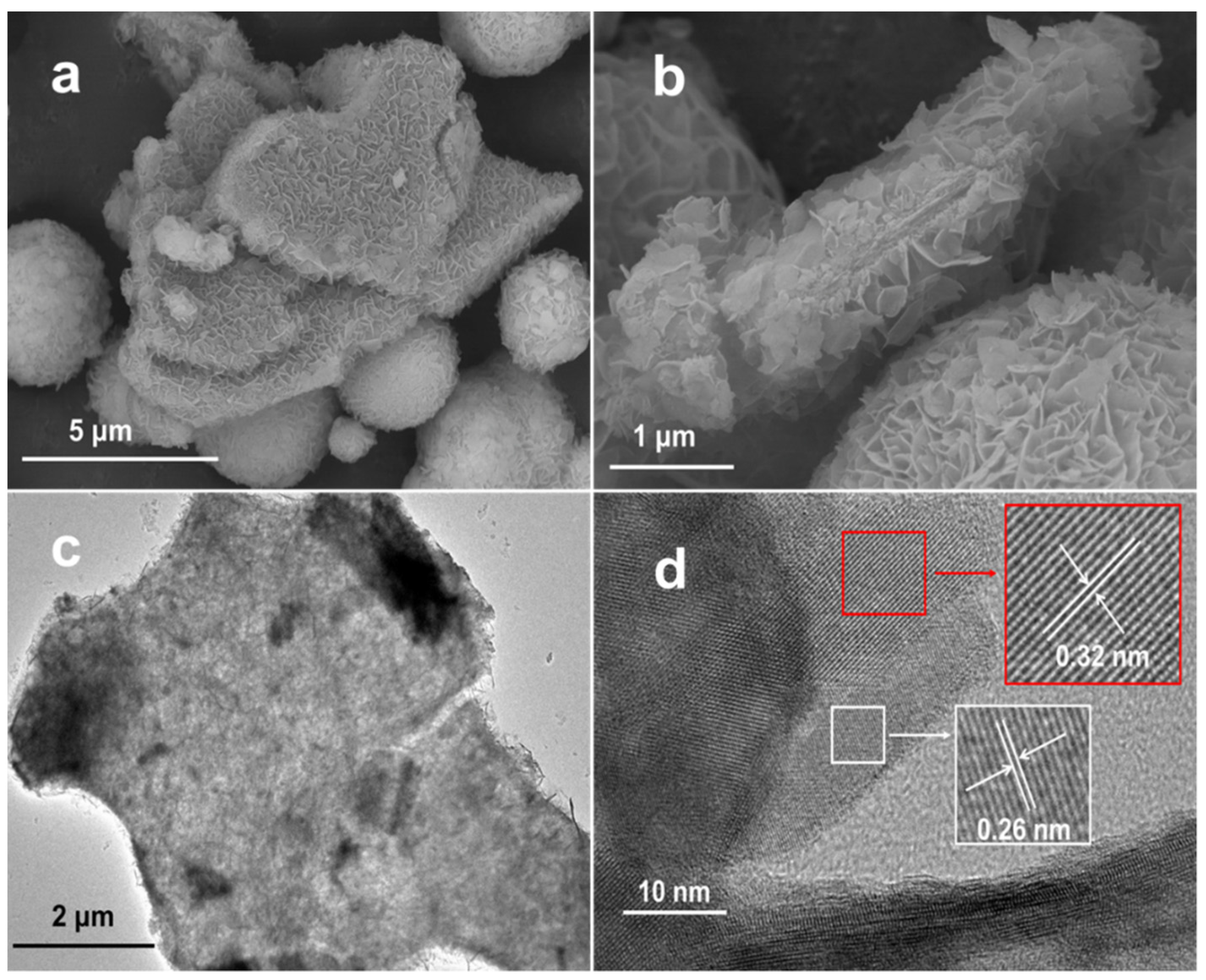
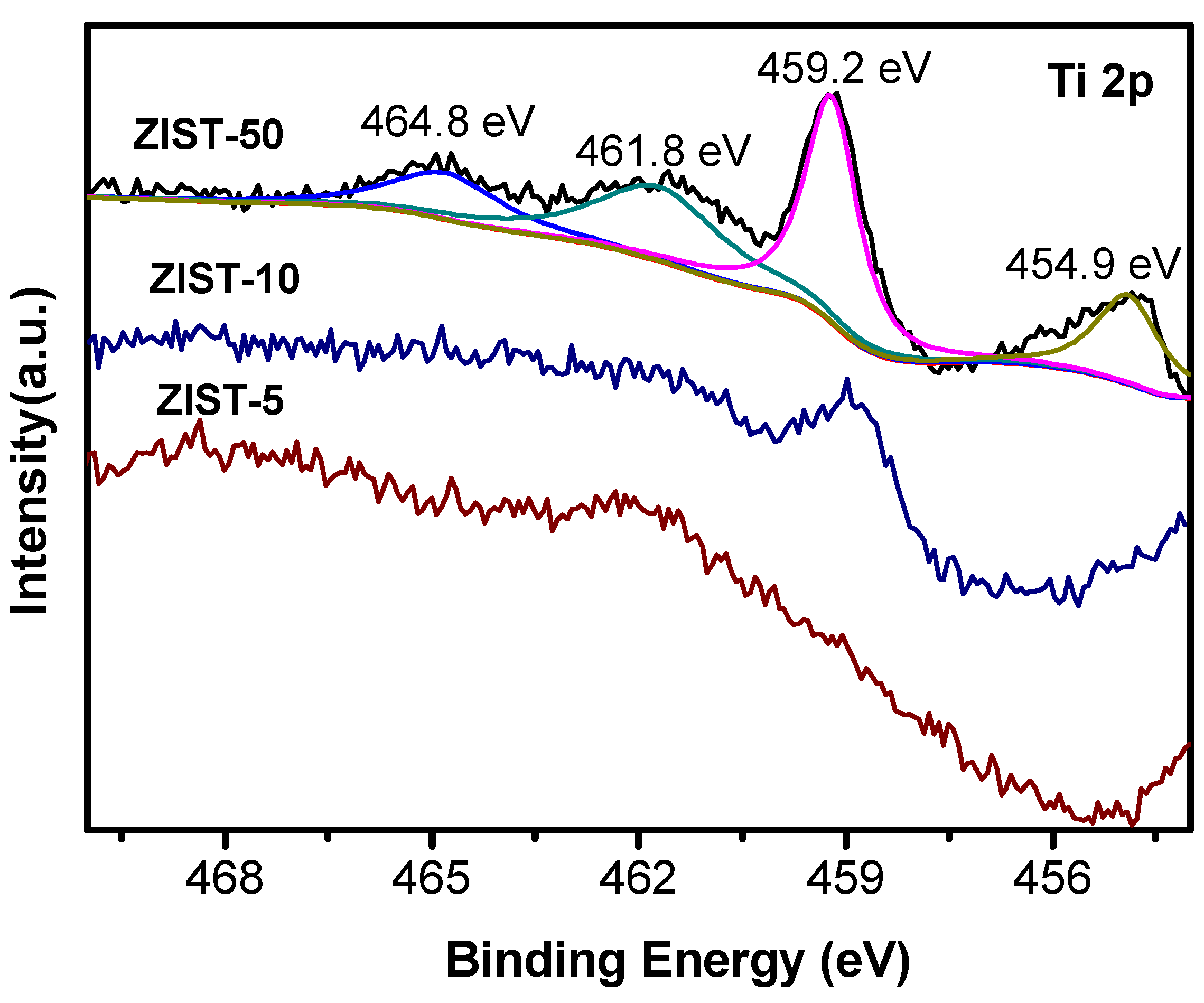
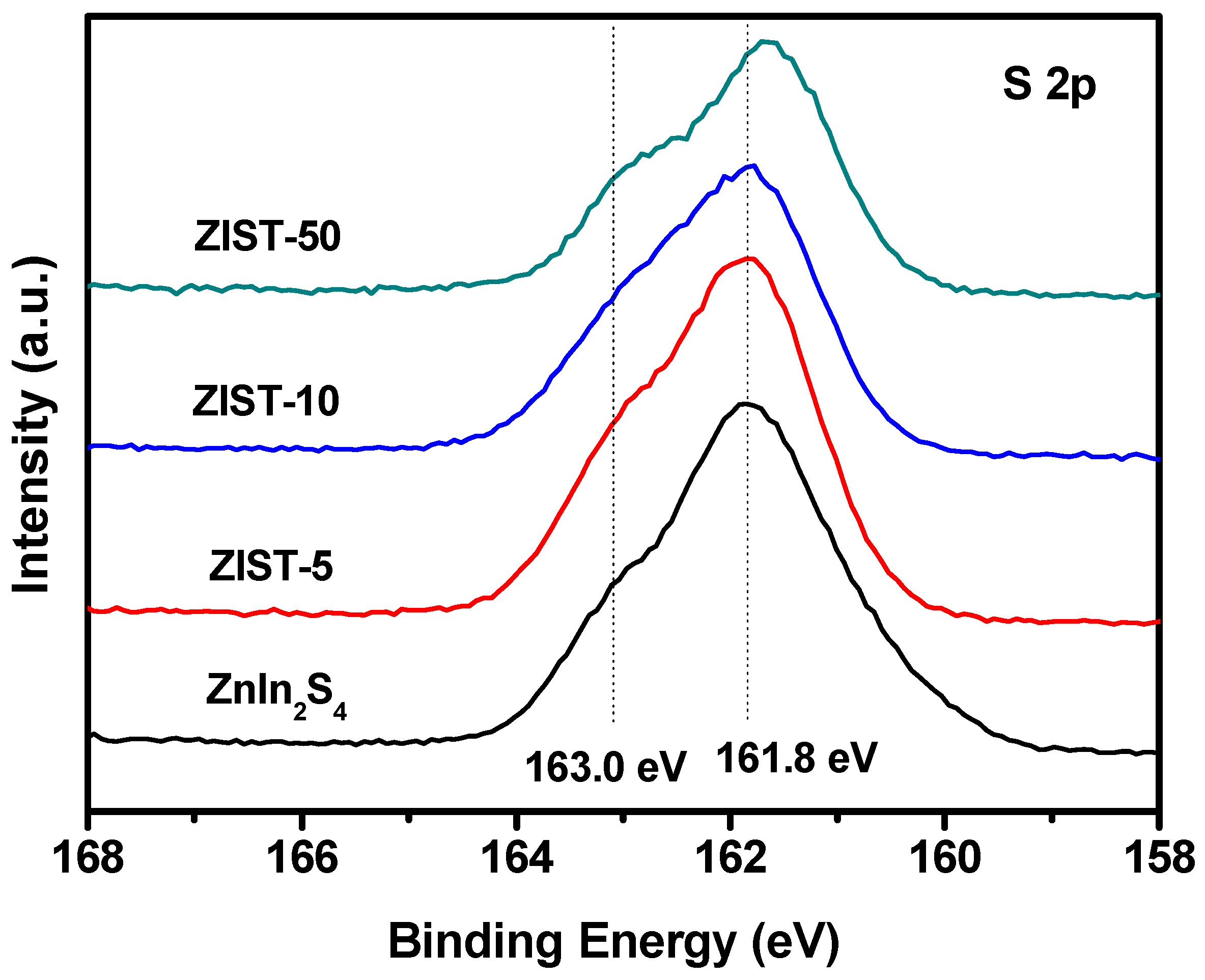
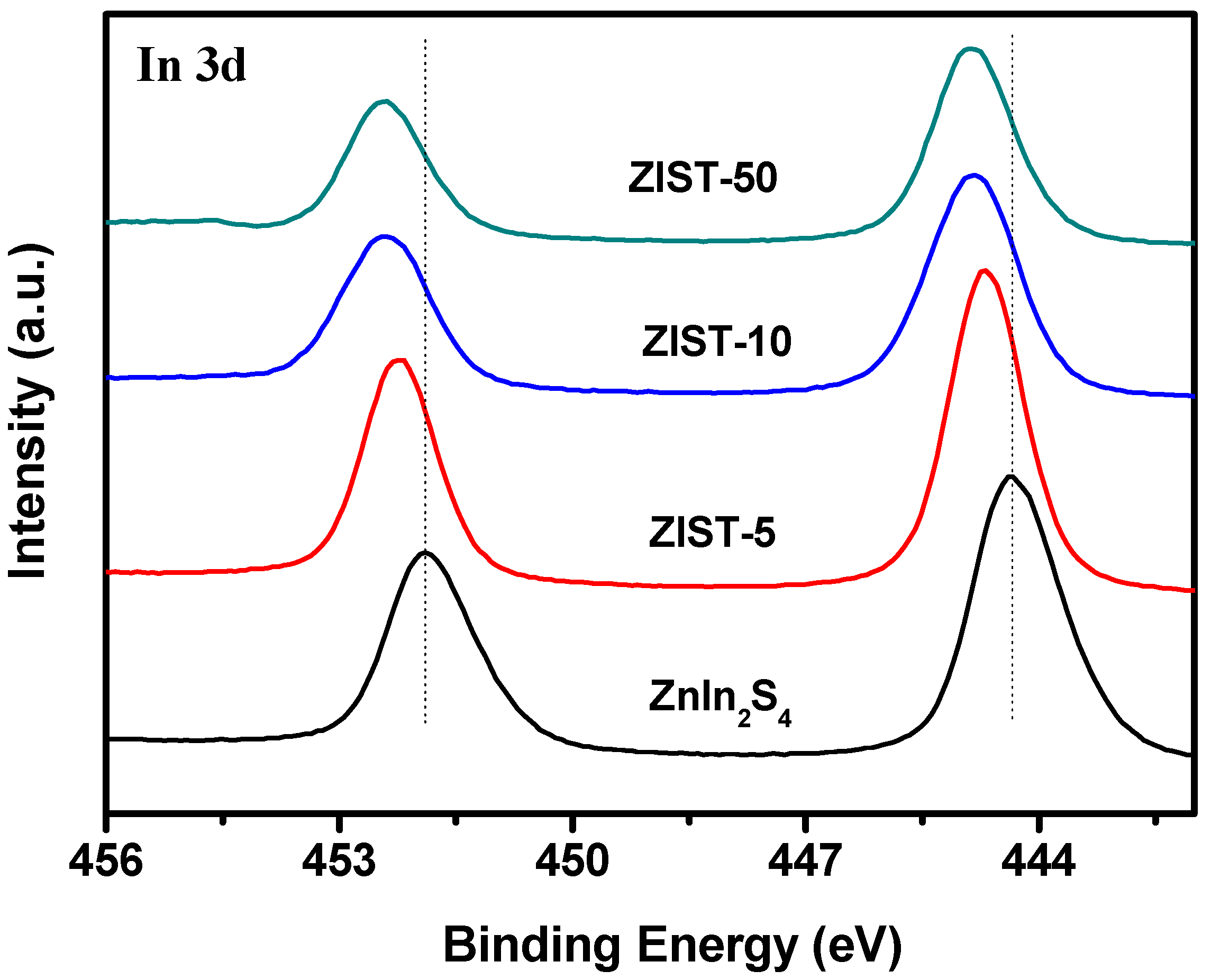
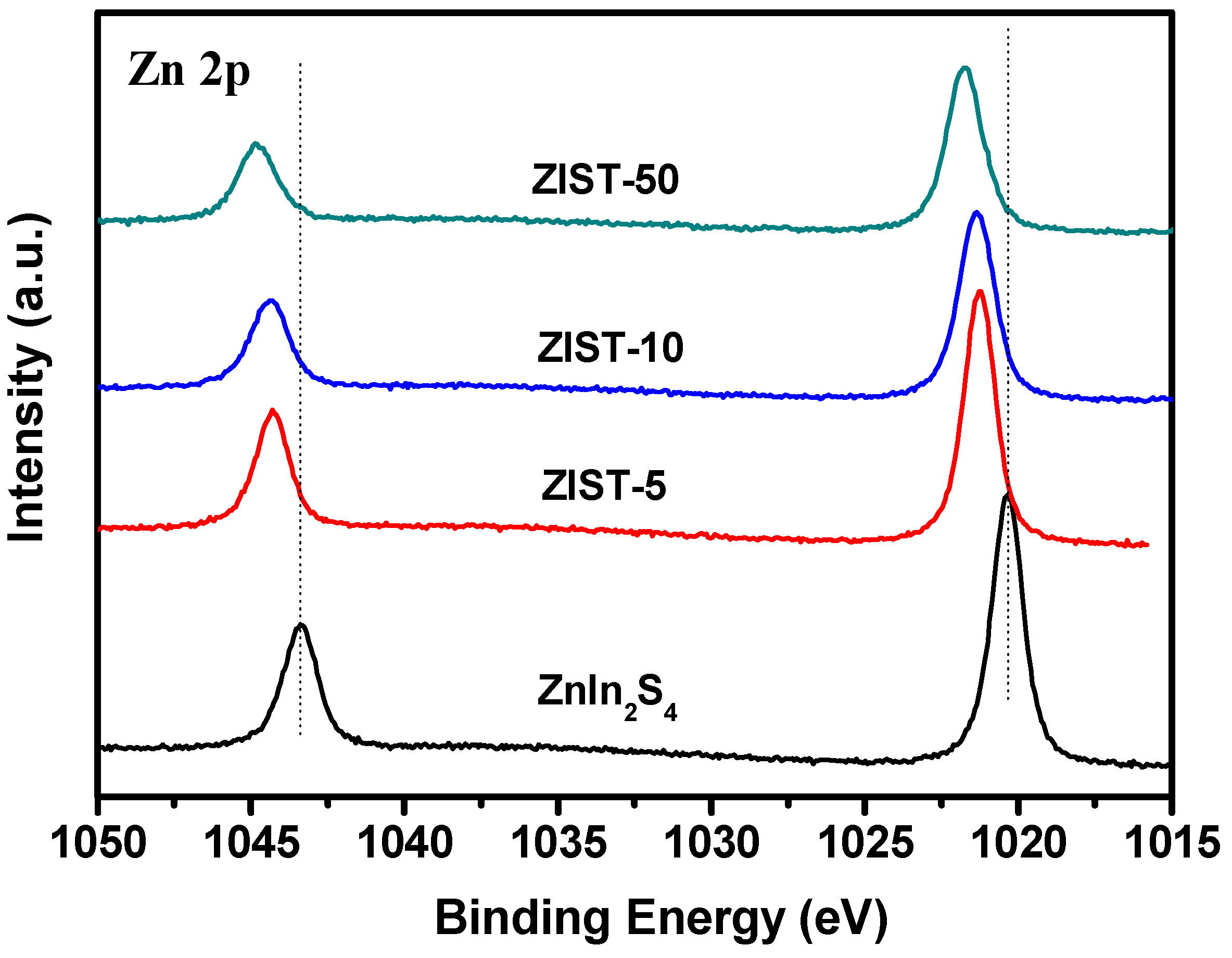
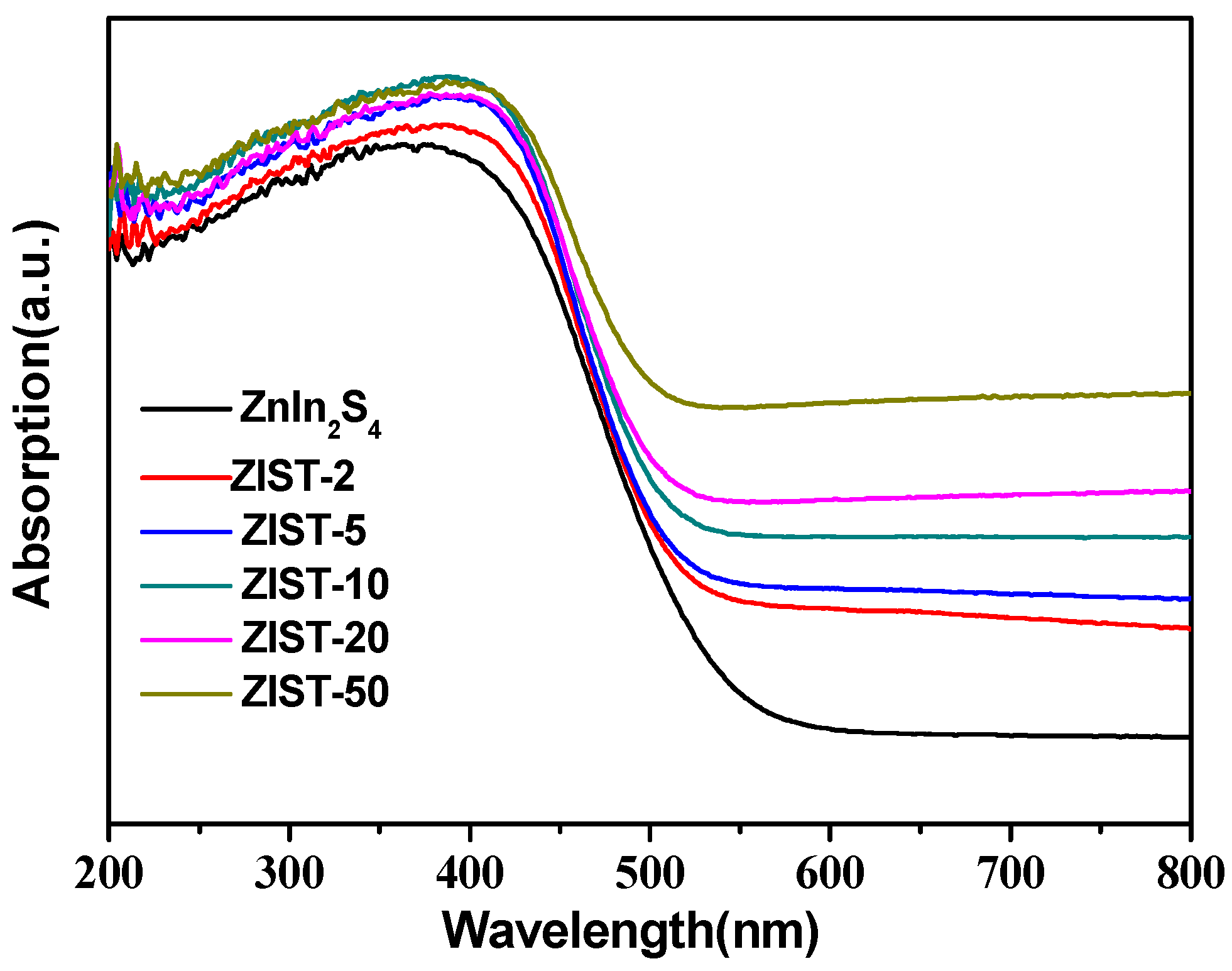
2.1. Characterizations
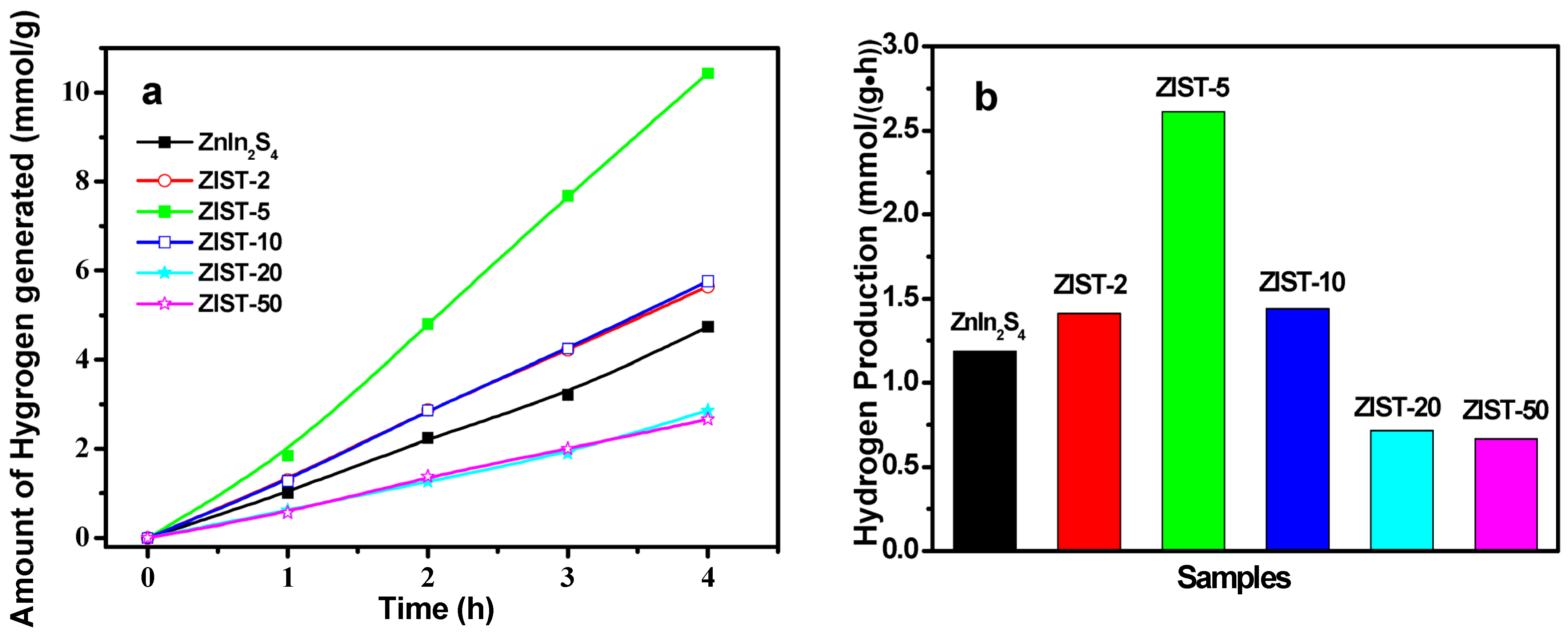
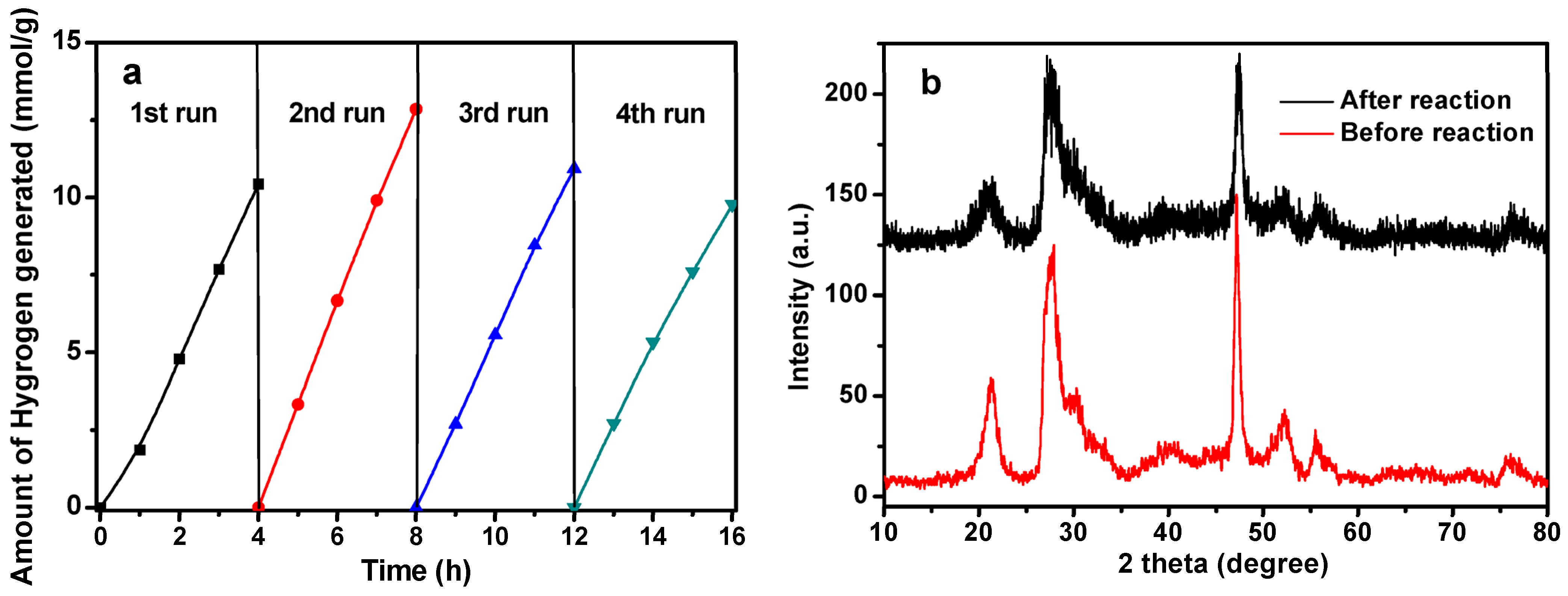
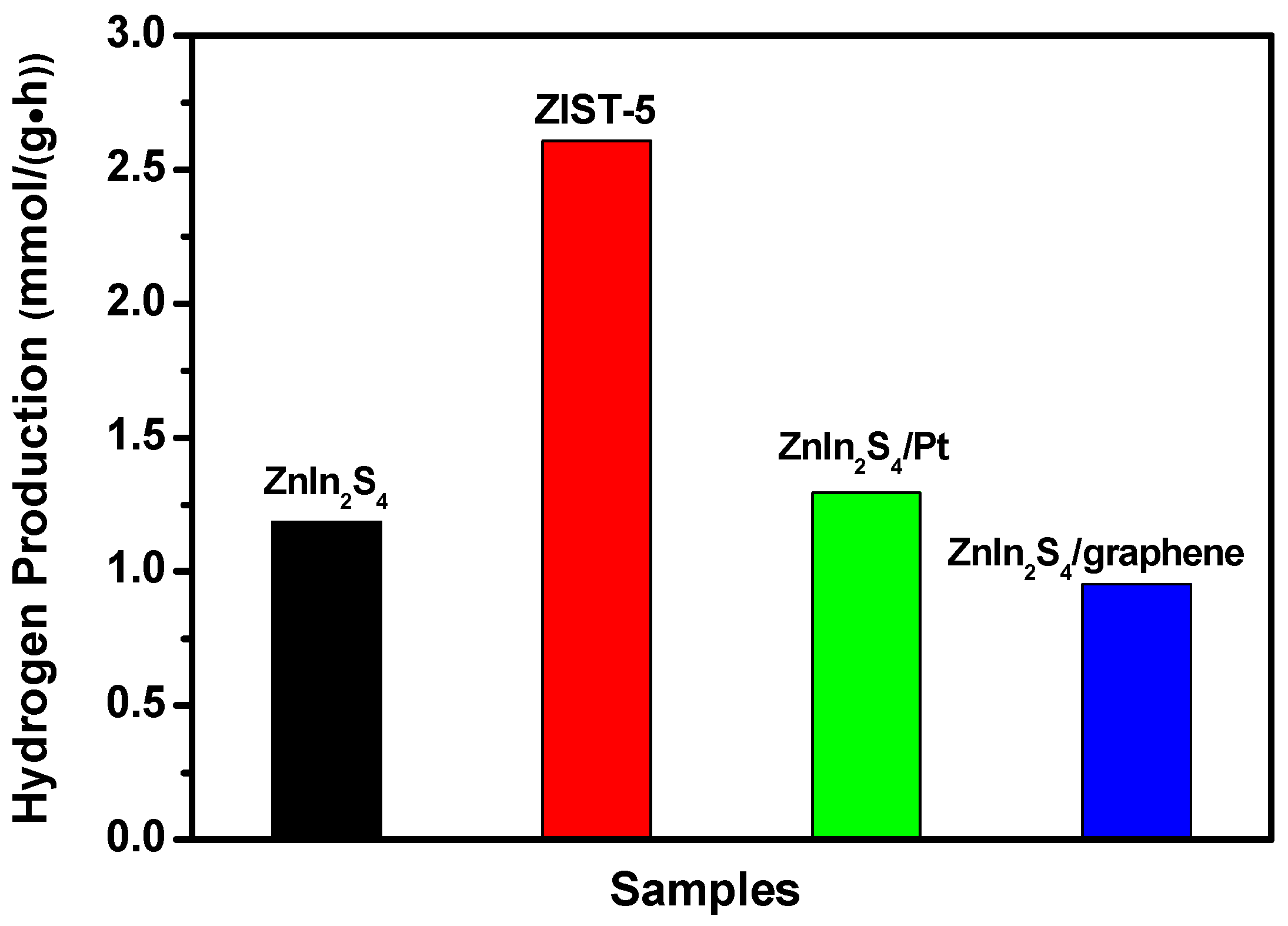
2.2. Photocatalytic Activity
3. Discussion
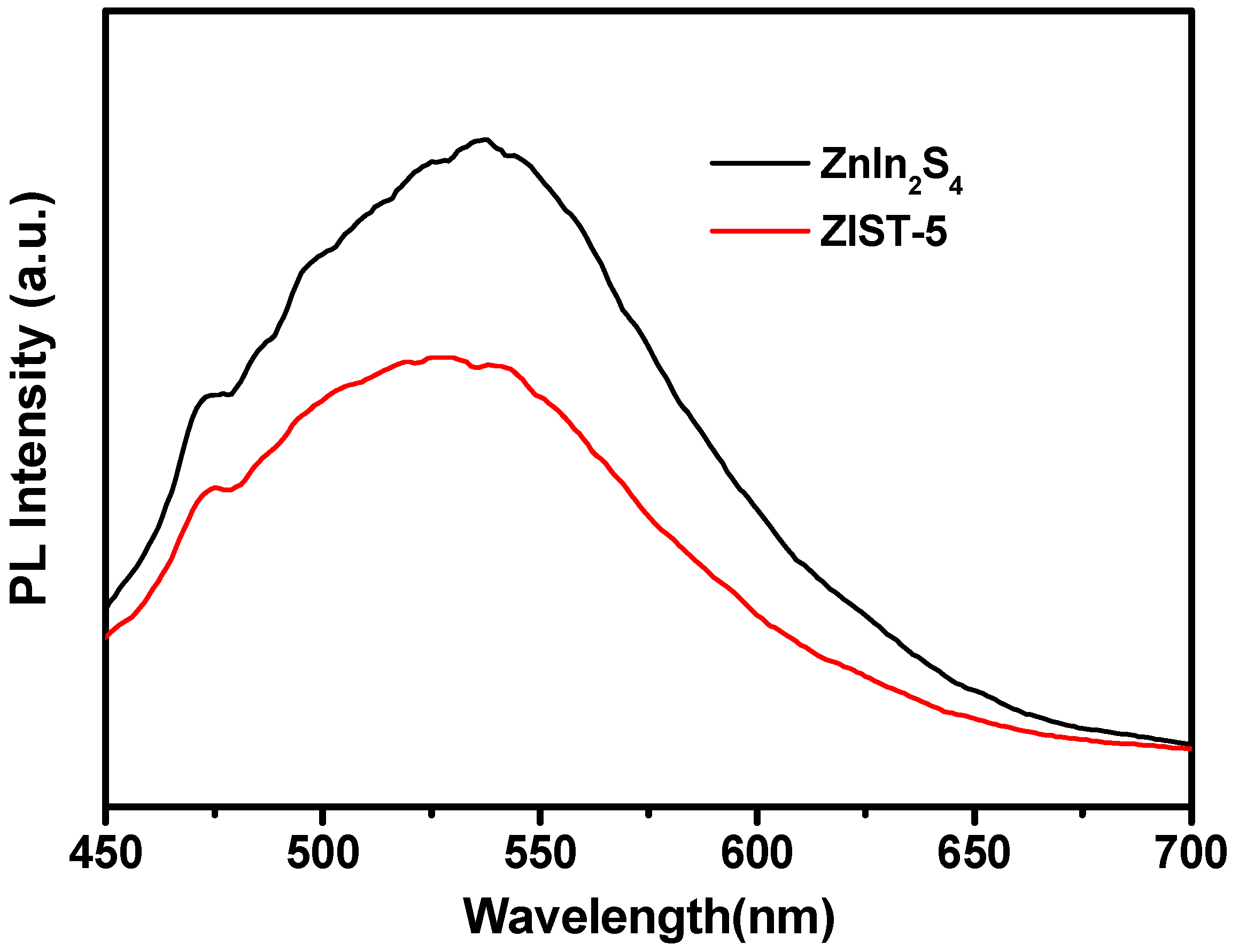
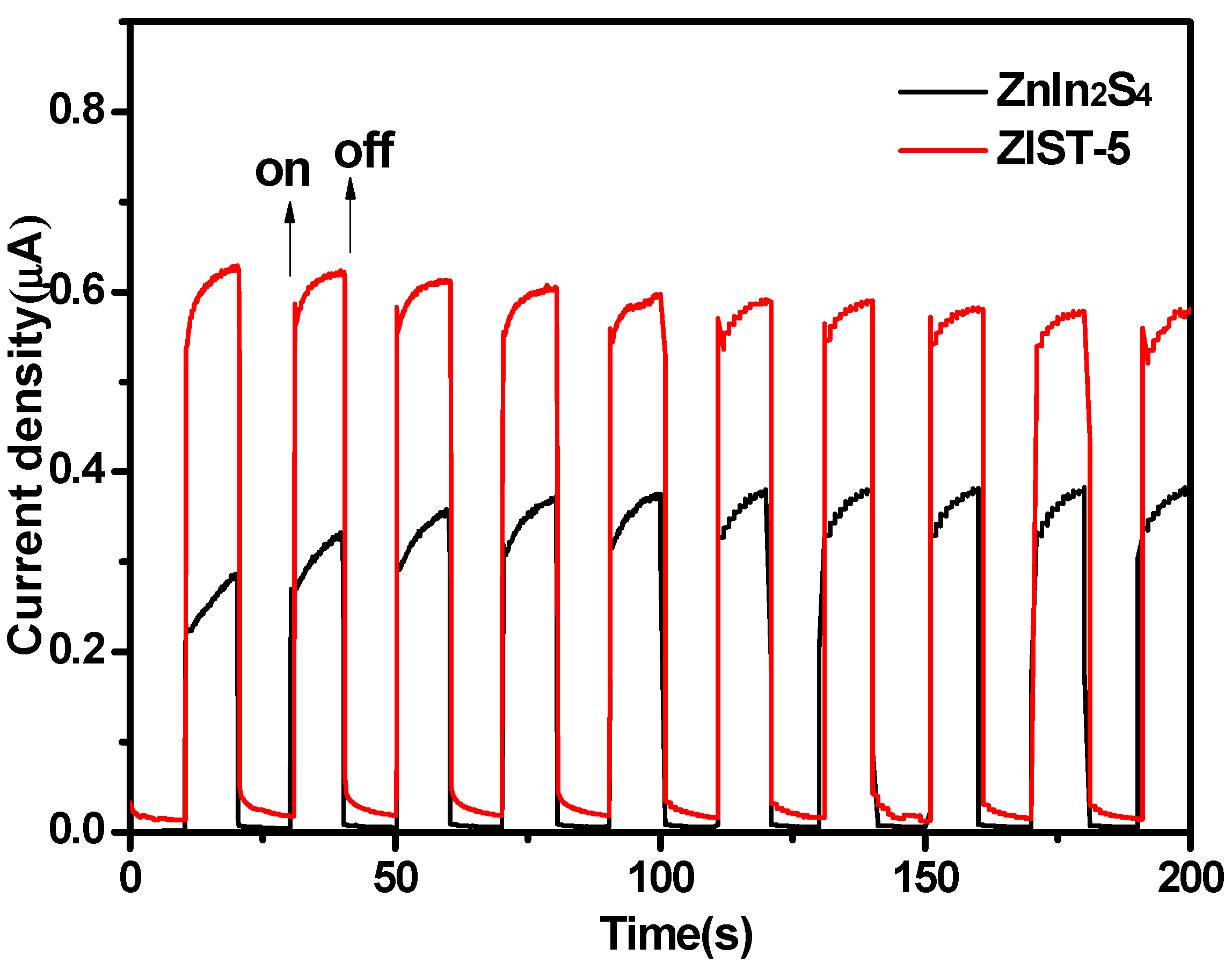
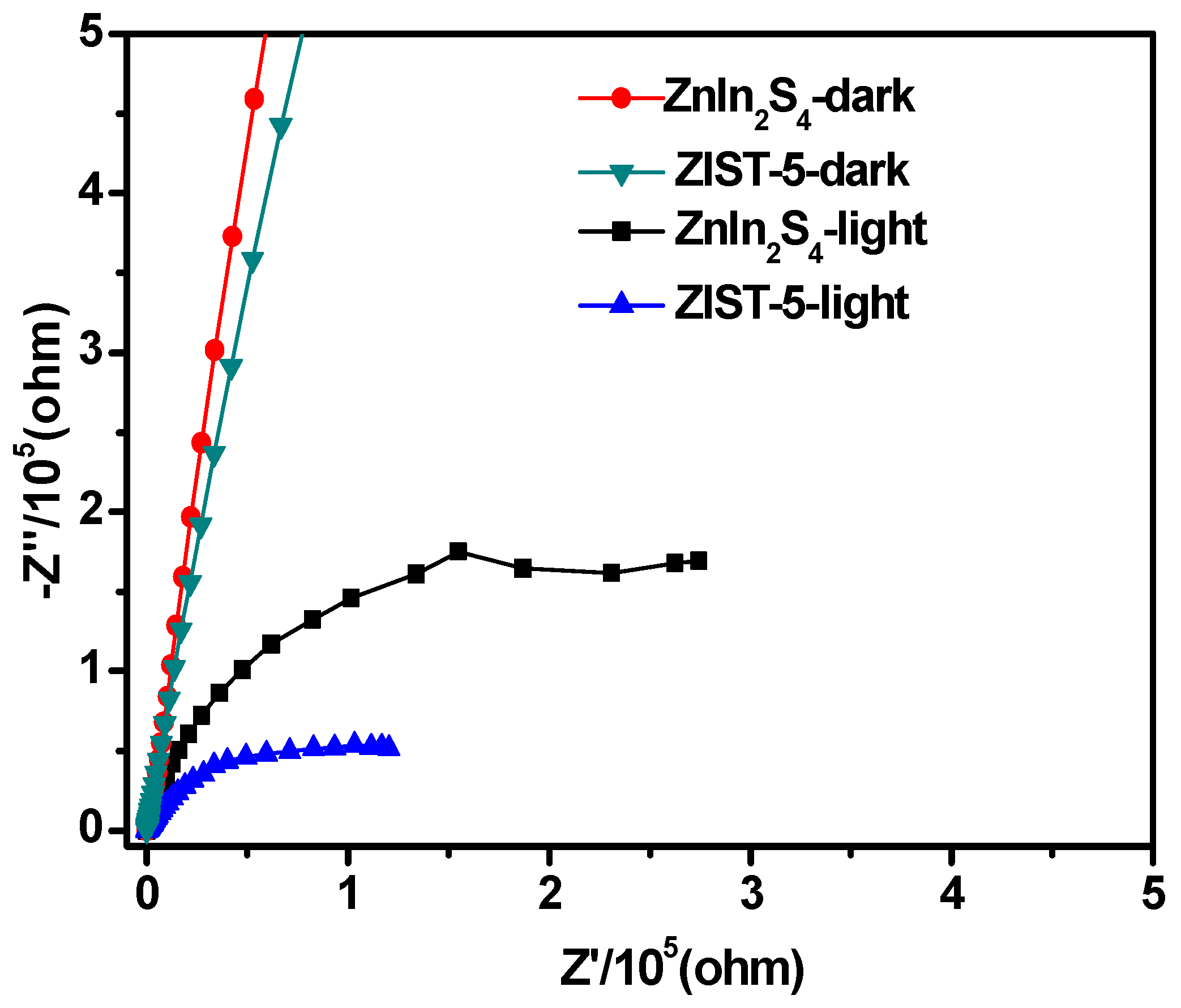
3.1. The Photoluminescence Spectra and Electrochemical Impedance Spectra
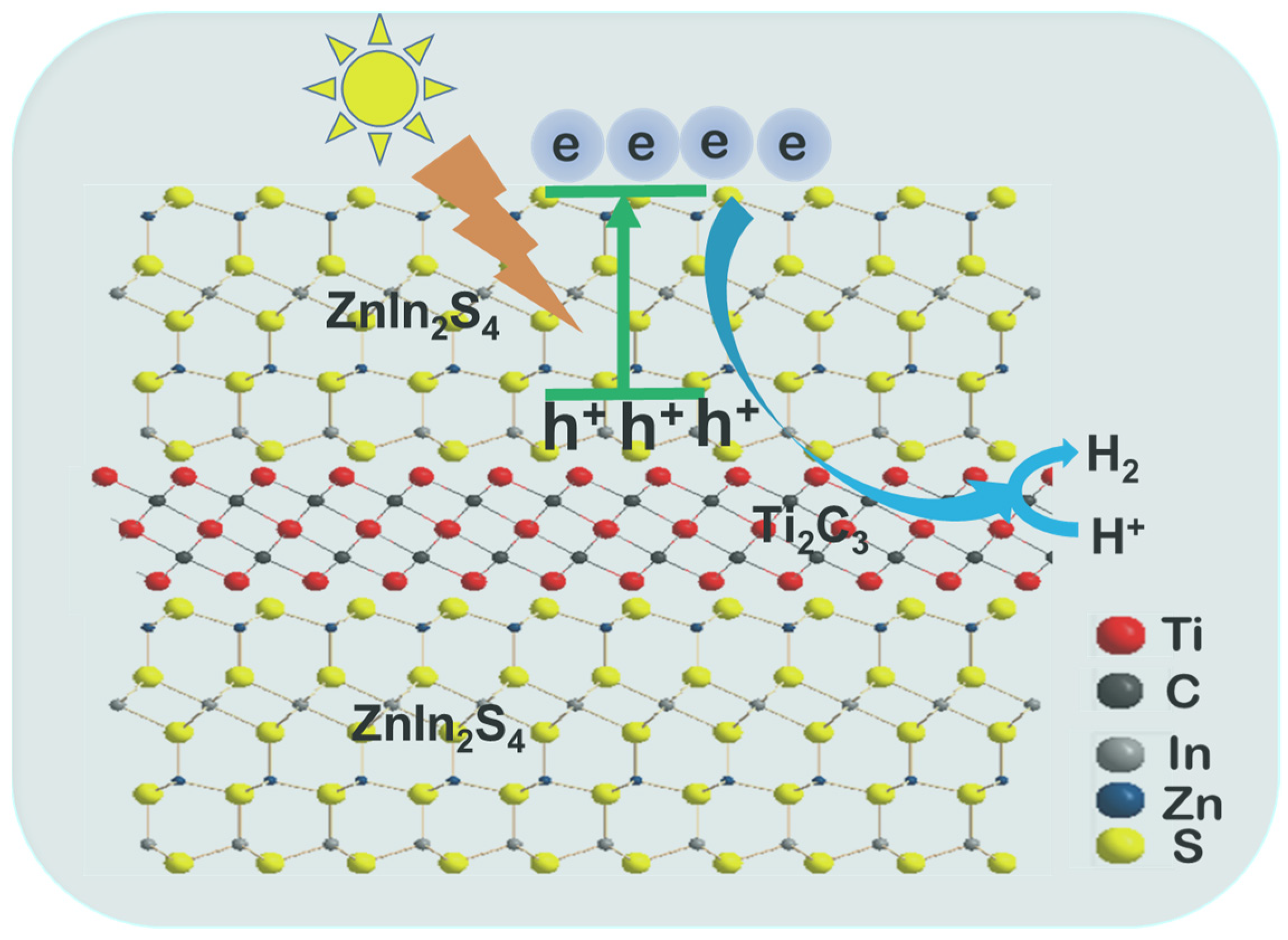
3.2. The Photocatalytic Mechanism
4. Materials and Methods
4.1. Synthesis of Samples
4.2. Characterizations
4.3. Photocatalytic H2 Generation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Z.; Yan, W.; Guo, Z.; Ruan, M. Decorating non-noble metal plasmonic Al on a TiO2/Cu2O photoanode to boost performance in photoelectrochemical water splitting. Chin. J. Catal. 2020, 41, 1884–1893. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, X. Efficient photoelectrochemical water splitting of CaBi6O10 decorated with Cu2O and NiOOH for improved photogenerated carriers. Int. J. Hydrogen Energy 2018, 43, 13276–13283. [Google Scholar] [CrossRef]
- Fang, G.; Liu, Z.; Han, C. Enhancing the PEC water splitting performance of BiVO4 co-modifying with NiFeOOH and Co-Pi double layer cocatalysts. Appl. Surf. Sci. 2020, 515, 146095. [Google Scholar] [CrossRef]
- Chen, Y.; Ge, H.; Wei, L.; Li, Z.; Yuan, R.; Liu, P.; Fu, X. Reduction degree of reduced graphene oxide (RGO) dependence of photocatalytic hydrogen evolution performance over RGO/ZnIn2S4 nanocomposites. Catal. Sci. Technol. 2013, 3, 1712–1717. [Google Scholar] [CrossRef]
- Wei, L.; Chen, Y.; Lin, Y.; Wu, H.; Yuan, R.; Li, Z. MoS2 as non-noble-metal co-catalyst for photocatalytic hydrogen evolution over hexagonal ZnIn2S4 under visible light irradiations. Appl. Catal. B 2014, 144, 521–527. [Google Scholar] [CrossRef]
- Shang, L.; Zhou, C.; Bian, T.; Yu, H.; Wu, L.-Z.; Tung, C.-H.; Zhang, T. Facile synthesis of hierarchical ZnIn2S4 submicrospheres composed of ultrathin mesoporous nanosheets as a highly efficient visible-light-driven photocatalyst for H2 production. J. Mater. Chem. A 2013, 1, 4552–4558. [Google Scholar] [CrossRef]
- Fang, F.; Chen, L.; Chen, Y.-B.; Wu, L.-M. Synthesis and Photocatalysis of ZnIn2S4 Nano/Micropeony. J. Phys. Chem. C 2010, 114, 2393–2397. [Google Scholar] [CrossRef]
- Huang, J.; Cheuk, W.; Wu, Y.; Lee, F.S.C.; Ho, W. Template-free synthesis of ternary sulfides submicrospheres as visible light photocatalysts by ultrasonic spray pyrolysis. Catal. Sci. Technol. 2012, 2, 1825–1827. [Google Scholar] [CrossRef]
- Chen, Z.; Li, D.; Zhang, W.; Chen, C.; Li, W.; Sun, M.; He, Y.; Fu, X. Low-Temperature and Template-Free Synthesis of ZnIn2S4 Microspheres. Inorg. Chem. 2008, 47, 9766–9772. [Google Scholar] [CrossRef]
- Yang, M.-Q.; Xu, Y.-J.; Lu, W.; Zeng, K.; Zhu, H.; Xu, Q.-H.; Ho, G.W. Self-surface charge exfoliation and electrostatically coordinated 2D hetero-layered hybrids. Nat. Commun. 2017, 8, 14224. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Zhao, L.; Guo, L. Morphology, structure and photocatalytic performance of ZnIn2S4 synthesized via a solvothermal/hydrothermal route in different solvents. J. Phys. Chem. Solids 2008, 69, 2426–2432. [Google Scholar] [CrossRef]
- Bai, X.; Li, J. Photocatalytic hydrogen generation over porous ZnIn2S4 microspheres synthesized via a CPBr-assisted hydrothermal method. Mater. Res. Bull. 2011, 46, 1028–1034. [Google Scholar] [CrossRef]
- Shen, S.; Zhao, L.; Zhou, Z.; Guo, L. Enhanced Photocatalytic Hydrogen Evolution over Cu-Doped ZnIn2S4 under Visible Light Irradiation. J. Phys. Chem. C 2008, 112, 16148–16155. [Google Scholar] [CrossRef]
- Shen, S.; Zhao, L.; Guan, X.; Guo, L. Improving visible-light photocatalytic activity for hydrogen evolution over ZnIn2S4: A case study of alkaline-earth metal doping. J. Phys. Chem. Solids 2012, 73, 79–83. [Google Scholar] [CrossRef]
- Wang, Y.; Kong, X.; Jiang, M.; Zhang, F.; Lei, X. A Z-scheme ZnIn2S4/Nb2O5 nanocomposite: Constructed and used as an efficient bifunctional photocatalyst for H2 evolution and oxidation of 5-hydroxymethylfurfural. Inorg. Chem. Front. 2020, 7, 437–446. [Google Scholar] [CrossRef]
- Dang, X.; Xie, M.; Dai, F.; Guo, J.; Liu, J.; Lu, X. The in situ construction of ZnIn2S4/CdIn2S4 2D/3D nano hetero-structure for an enhanced visible-light-driven hydrogen production. J. Mater. Chem. A 2021, 9, 14888–14896. [Google Scholar] [CrossRef]
- Hao, M.; Deng, X.; Xu, L.; Li, Z. Noble metal Free MoS2/ZnIn2S4 nanocomposite for acceptorless photocatalytic semi-dehydrogenation of 1,2,3,4-tetrahydroisoquinoline to produce 3,4-dihydroisoquinoline. Appl. Catal. B 2019, 252, 18–23. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, Z.; Li, J.; Yan, W.; Ya, J.; Wu, X. Piezoelectric polarization assisted WO3/CdS photoanode improved carrier separation efficiency via CdS phase regulation. Int. J. Hydrogen Energy 2021, 46, 36113–36123. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Li, J.; Ruan, M.; Guo, Z. An effective strategy of constructing a multi-junction structure by integrating a heterojunction and a homojunction to promote the charge separation and transfer efficiency of WO3. J. Mater. Chem. A 2020, 8, 6256–6267. [Google Scholar] [CrossRef]
- Zuo, G.; Wang, Y.; Teo, W.L.; Xie, A.; Guo, Y.; Dai, Y.; Zhou, W.; Jana, D.; Xian, Q.; Dong, W.; et al. Ultrathin ZnIn2S4 Nanosheets Anchored on Ti3C2TX MXene for Photocatalytic H2 Evolution. Angew. Chem. Int. Ed. 2020, 59, 11287–11292. [Google Scholar] [CrossRef]
- Cao, S.; Shen, B.; Tong, T.; Fu, J.; Yu, J. 2D/2D Heterojunction of Ultrathin MXene/Bi2WO6 Nanosheets for Improved Photocatalytic CO2 Reduction. Adv. Funct. Mater. 2018, 28, 1800136. [Google Scholar] [CrossRef]
- Su, T.; Hood, Z.D.; Naguib, M.; Bai, L.; Luo, S.; Rouleau, C.M.; Ivanov, I.N.; Ji, H.; Qin, Z.; Wu, Z. 2D/2D heterojunction of Ti3C2/g-C3N4 nanosheets for enhanced photocatalytic hydrogen evolution. Nanoscale 2019, 11, 8138–8149. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, S.; Liu, W.; Chen, X.; Wu, L.; Wang, X.; Liu, P.; Li, Z. Controlled syntheses of cubic and hexagonal ZnIn2S4 nanostructures with different visible-light photocatalytic performance. Dalton Trans. 2011, 40, 2607–2613. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cai, J.; Hao, M.; Li, Z. Visible light initiated hydrothiolation of alkenes and alkynes over ZnIn2S4. Green Chem. 2019, 21, 2345–2351. [Google Scholar] [CrossRef]
- Cai, T.; Wang, L.; Liu, Y.; Zhang, S.; Dong, W.; Chen, H.; Yi, X.; Yuan, J.; Xia, X.; Liu, C.; et al. Ag3PO4/Ti3C2 MXene interface materials as a Schottky catalyst with enhanced photocatalytic activities and anti-photocorrosion performance. Appl. Catal. B 2018, 239, 545–554. [Google Scholar] [CrossRef]
- Chen, R.; Wang, P.; Chen, J.; Wang, C.; Ao, Y. Synergetic effect of MoS2 and MXene on the enhanced H2 evolution performance of CdS under visible light irradiation. Appl. Surf. Sci. 2019, 473, 11–19. [Google Scholar] [CrossRef]
- Ran, J.; Gao, G.; Li, F.; Ma, T.; Du, A.; Qiao, S. Ti3C2 MXene co-catalyst on metal sulfide photo-absorbers for enhanced visible-light photocatalytic hydrogen production. Nat. Commun. 2017, 8, 13907. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Wang, X.; Yu, L.; Chen, Y.; Shi, J. Two-Dimensional Ultrathin MXene Ceramic Nanosheets for Photothermal Conversion. Nano Lett. 2017, 17, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Hood, Z.D.; Naguib, M.; Bai, L.; Luo, S.; Rouleau, C.M.; Ivanoy, I.N.; Ji, H.; Qin, Z.; Wu, Z. Monolayer Ti3C2Tx as an Effective Co-catalyst for Enhanced Photocatalytic Hydrogen Production over TiO2. ACS Appl. Energy Mater. 2019, 2, 4640–4651. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Liu, C.; Luo, S.; Wang, L.; Cai, T.; Zeng, Y.; Yuan, J.; Dong, W.; Pei, Y.; et al. MoS2 Quantum Dot Growth Induced by S Vacancies in a ZnIn2S4 Monolayer: Atomic-Level Heterostructure for Photocatalytic Hydrogen Production. ACS Nano 2018, 12, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yin, Z.; Ji, G.; Liang, Z.; Xue, Y.; Guo, Y.; Tian, J.; Wang, X.; Cui, H. 2D/2D/2D heterojunction of Ti3C2 MXene/MoS2 nanosheets/TiO2 nanosheets with exposed (001) facets toward enhanced photocatalytic hydrogen production activity. Appl. Catal. B 2019, 246, 12–20. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Ge, Y.; Wu, C.; Tang, H.; Luo, X.; He, J.; Jiang, L.; Yan, Z.; Wang, J. Facile Synthesis of 2D/2D Ti2C3/ZnIn2S4 Heterostructure for Enhanced Photocatalytic Hydrogen Generation. Int. J. Mol. Sci. 2023, 24, 3936. https://doi.org/10.3390/ijms24043936
Chen Y, Ge Y, Wu C, Tang H, Luo X, He J, Jiang L, Yan Z, Wang J. Facile Synthesis of 2D/2D Ti2C3/ZnIn2S4 Heterostructure for Enhanced Photocatalytic Hydrogen Generation. International Journal of Molecular Sciences. 2023; 24(4):3936. https://doi.org/10.3390/ijms24043936
Chicago/Turabian StyleChen, Yongjuan, Yanfang Ge, Chunling Wu, Hua Tang, Xiu Luo, Jiao He, Liang Jiang, Zhiying Yan, and Jiaqiang Wang. 2023. "Facile Synthesis of 2D/2D Ti2C3/ZnIn2S4 Heterostructure for Enhanced Photocatalytic Hydrogen Generation" International Journal of Molecular Sciences 24, no. 4: 3936. https://doi.org/10.3390/ijms24043936
APA StyleChen, Y., Ge, Y., Wu, C., Tang, H., Luo, X., He, J., Jiang, L., Yan, Z., & Wang, J. (2023). Facile Synthesis of 2D/2D Ti2C3/ZnIn2S4 Heterostructure for Enhanced Photocatalytic Hydrogen Generation. International Journal of Molecular Sciences, 24(4), 3936. https://doi.org/10.3390/ijms24043936






