Memory Precursors and Short-Lived Effector T cell Subsets Have Different Sensitivities to TGFβ
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. In Vitro Activation
2.3. Antibodies and Flow Cytometry
2.4. Bioinformatics
2.5. Chromatin IP
3. Results and Discussion
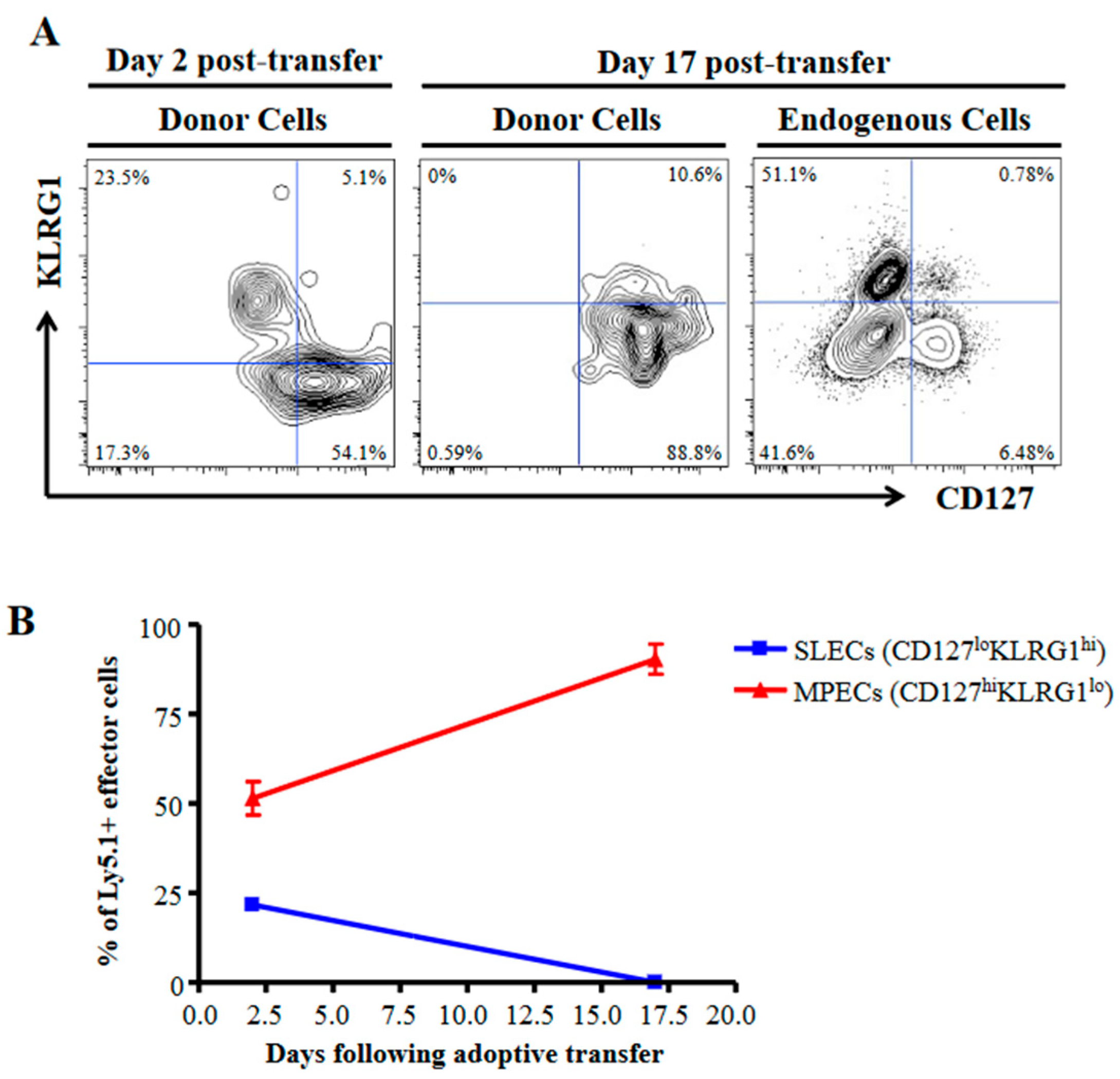
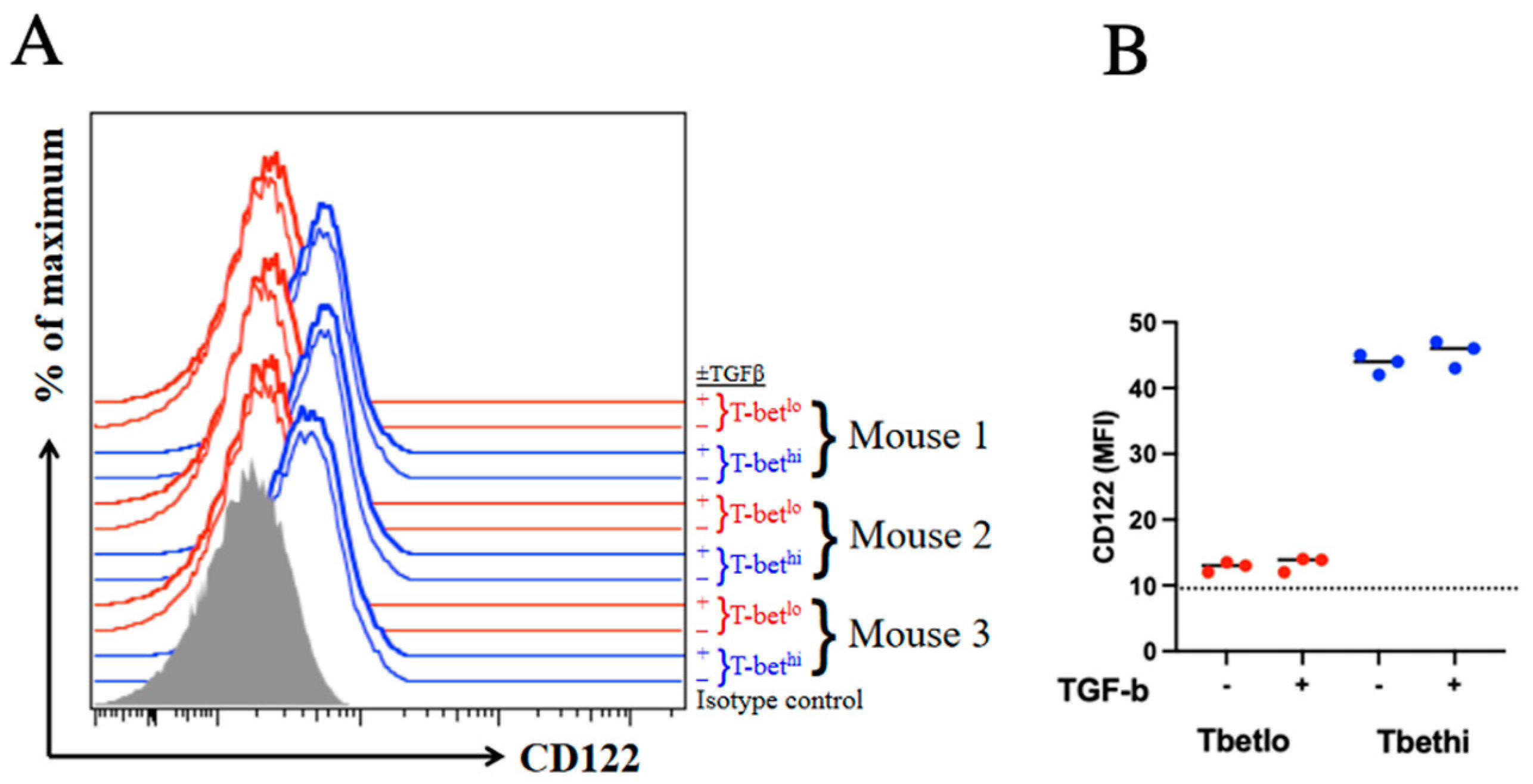
3.1. SLECs Exhibit a More TGFβ-Sensitive Phenotype
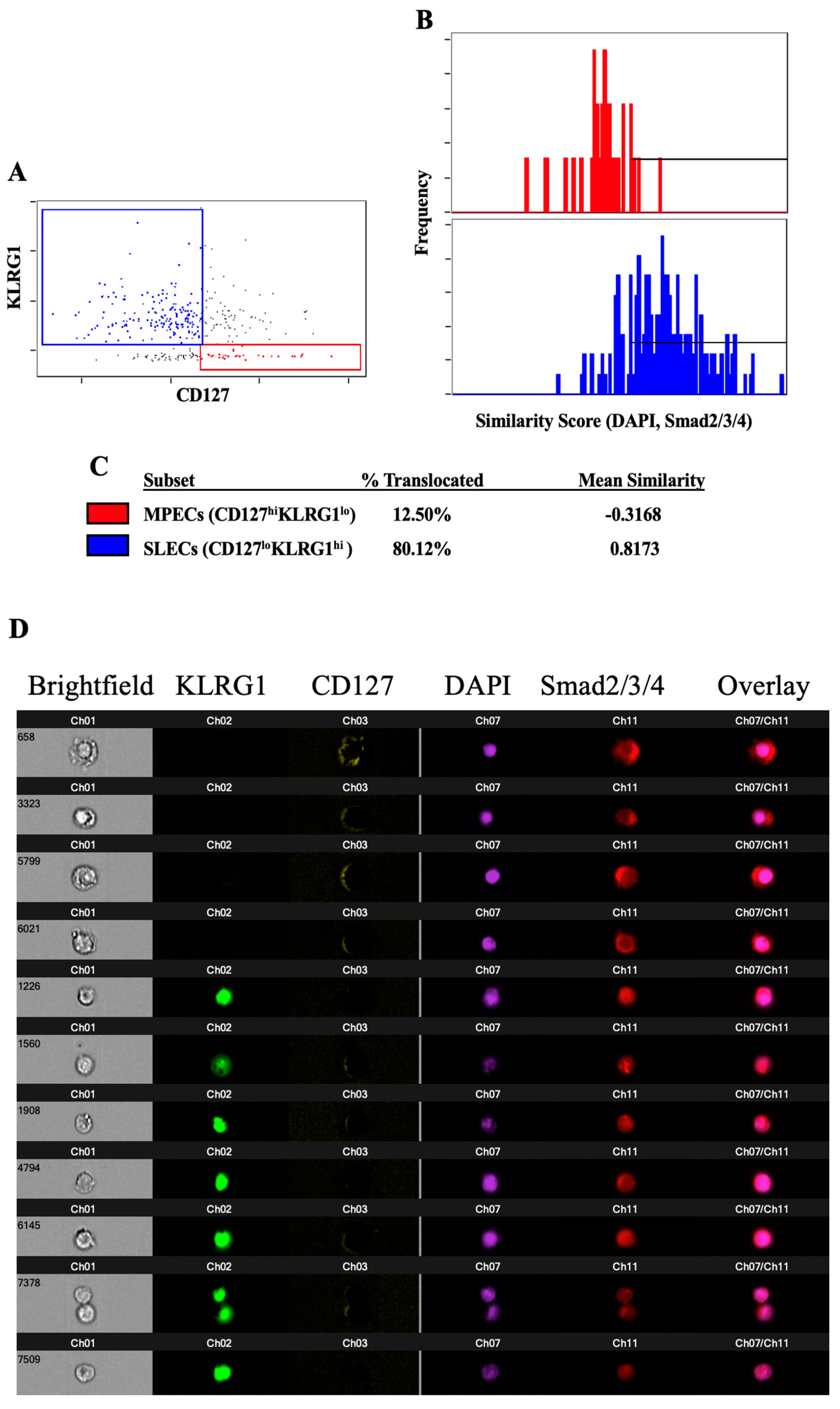
3.2. TGFβ-Induced Smad Nuclear Translocation Is Decreased in MPECs Relative to SLECs
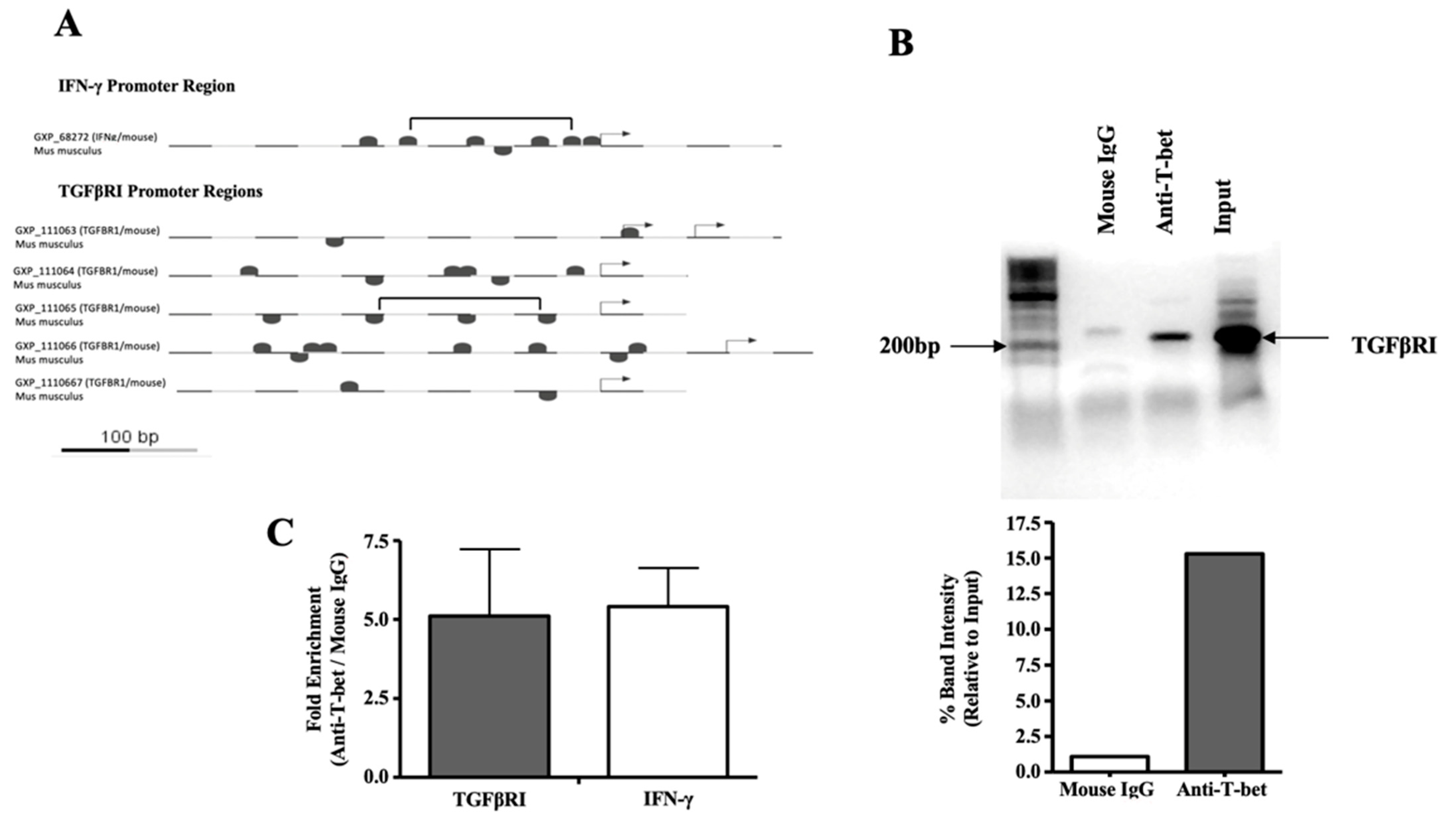
3.3. T-Bet Binds to the TGFβRI Promoter
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DAPI | 4’,6-diamidino-2-phenylindole |
| KLRG1 | killer cell lectin-like receptor subfamily G member 1 |
| mAb | monoclonal antibody |
| MPEC | memory progenitor effector cell |
| RGS3 | regulator of G protein signaling 3 |
| SLEC | short-lived effector cell |
| T-bet | T-box expressed in T cells |
| TGFβ | transforming growth factor β |
| TGFβRI | TGFβ receptor I |
| TGFβRII | TGFβ receptor II |
| Th1/Th2 | T helper c regulatory T cell ell type 1/2 |
| Treg | regulatory T cell |
References
- Sanjabi, S.; Mosaheb, M.M.; Flavell, R.A. Opposing effects of TGF-beta and IL-15 cytokines control the number of short-lived effector CD8+ T cells. Immunity 2009, 31, 131–144. [Google Scholar] [CrossRef]
- Blobe, G.C.; Schiemann, W.P.; Lodish, H.F. Role of transforming growth factor beta in human disease. N. Engl. J. Med. 2000, 342, 1350–1358. [Google Scholar] [CrossRef]
- Chen, W.; Jin, W.; Hardegen, N.; Lei, K.J.; Li, L.; Marinos, N.; McGrady, G.; Wahl, S.M. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 2003, 198, 1875–1886. [Google Scholar] [CrossRef]
- Kulkarni, A.B.; Huh, C.G.; Becker, D.; Geiser, A.; Lyght, M.; Flanders, K.C.; Roberts, A.B.; Sporn, M.B.; Ward, J.M.; Karlsson, S. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc. Natl. Acad. Sci. USA 1993, 90, 770–774. [Google Scholar] [CrossRef]
- Li, M.O.; Wan, Y.Y.; Sanjabi, S.; Robertson, A.K.; Flavell, R.A. Transforming growth factor-beta regulation of immune responses. Annu. Rev. Immunol. 2006, 24, 99–146. [Google Scholar] [CrossRef]
- Mangan, P.R.; Harrington, L.E.; O’Quinn, D.B.; Helms, W.S.; Bullard, D.C.; Elson, C.O.; Hatton, R.D.; Wahl, S.M.; Schoeb, T.R.; Weaver, C.T. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature 2006, 441, 231–234. [Google Scholar] [CrossRef]
- Massague, J. How cells read TGF-beta signals. Nat. Rev. Mol. Cell Biol. 2000, 1, 169–178. [Google Scholar] [CrossRef]
- Shi, Y.; Massague, J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 2003, 113, 685–700. [Google Scholar] [CrossRef]
- Yau, D.M.; Sethakorn, N.; Taurin, S.; Kregel, S.; Sandbo, N.; Camoretti-Mercado, B.; Sperling, A.I.; Dulin, N.O. Regulation of Smad-mediated gene transcription by RGS3. Mol. Pharmacol. 2008, 73, 1356–1361. [Google Scholar] [CrossRef]
- Dulin, N.O.; Sorokin, A.; Reed, E.; Elliott, S.; Kehrl, J.H.; Dunn, M.J. RGS3 inhibits G protein-mediated signaling via translocation to the membrane and binding to Galpha11. Mol. Cell. Biol. 1999, 19, 714–723. [Google Scholar] [CrossRef]
- Zloza, A.; Jagoda, M.C.; Lyons, G.E.; Graves, M.C.; Kohlhapp, F.J.; O’Sullivan, J.A.; Lacek, A.T.; Nishimura, M.I.; Guevara-Patino, J.A. CD8 Co-receptor promotes susceptibility of CD8(+) T cells to transforming growth factor-beta (TGF-beta)-mediated suppression. Cancer Immunol. Immunother. 2011, 60, 291–297. [Google Scholar] [CrossRef]
- Joshi, N.S.; Cui, W.; Chandele, A.; Lee, H.K.; Urso, D.R.; Hagman, J.; Gapin, L.; Kaech, S.M. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity 2007, 27, 281–295. [Google Scholar] [CrossRef]
- Yeo, C.J.; Fearon, D.T. T-bet-mediated differentiation of the activated CD8+ T cell. Eur. J. Immunol. 2011, 41, 60–66. [Google Scholar] [CrossRef]
- Rao, J.N.; Li, L.; Bass, B.L.; Wang, J.Y. Expression of the TGF-beta receptor gene and sensitivity to growth inhibition following polyamine depletion. Am. J. Physiol. Cell Physiol. 2000, 279, C1034–C1044. [Google Scholar] [CrossRef]
- Reif, K.; Cyster, J.G. RGS molecule expression in murine B lymphocytes and ability to down-regulate chemotaxis to lymphoid chemokines. J. Immunol. 2000, 164, 4720–4729. [Google Scholar] [CrossRef]
- Shi, G.X.; Harrison, K.; Han, S.B.; Moratz, C.; Kehrl, J.H. Toll-like receptor signaling alters the expression of regulator of G protein signaling proteins in dendritic cells: Implications for G protein-coupled receptor signaling. J. Immunol. 2004, 172, 5175–5184. [Google Scholar] [CrossRef]
- Gronroos, E.; Hellman, U.; Heldin, C.H.; Ericsson, J. Control of Smad7 stability by competition between acetylation and ubiquitination. Mol. Cell 2002, 10, 483–493. [Google Scholar] [CrossRef]
- Kim, B.C.; Lee, H.J.; Park, S.H.; Lee, S.R.; Karpova, T.S.; McNally, J.G.; Felici, A.; Lee, D.K.; Kim, S.J. Jab1/CSN5, a component of the COP9 signalosome, regulates transforming growth factor beta signaling by binding to Smad7 and promoting its degradation. Mol. Cell. Biol. 2004, 24, 2251–2262. [Google Scholar] [CrossRef]
- Koinuma, D.; Shinozaki, M.; Komuro, A.; Goto, K.; Saitoh, M.; Hanyu, A.; Ebina, M.; Nukiwa, T.; Miyazawa, K.; Imamura, T.; et al. Arkadia amplifies TGF-beta superfamily signalling through degradation of Smad7. EMBO J. 2003, 22, 6458–6470. [Google Scholar] [CrossRef]
- Holzer, U.; Rieck, M.; Buckner, J.H. Lineage and signal strength determine the inhibitory effect of transforming growth factor beta1 (TGF-beta1) on human antigen-specific Th1 and Th2 memory cells. J. Autoimmun. 2006, 26, 241–251. [Google Scholar] [CrossRef]
- Ludviksson, B.R.; Seegers, D.; Resnick, A.S.; Strober, W. The effect of TGF-beta1 on immune responses of naive versus memory CD4+ Th1/Th2 T cells. Eur. J. Immunol. 2000, 30, 2101–2111. [Google Scholar] [CrossRef]
- Cho, J.Y.; Grigura, V.; Murphy, T.L.; Murphy, K. Identification of cooperative monomeric Brachyury sites conferring T-bet responsiveness to the proximal IFN-gamma promoter. Int. Immunol. 2003, 15, 1149–1160. [Google Scholar] [CrossRef]
- Intlekofer, A.M.; Takemoto, N.; Wherry, E.J.; Longworth, S.A.; Northrup, J.T.; Palanivel, V.R.; Mullen, A.C.; Gasink, C.R.; Kaech, S.M.; Miller, J.D.; et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat. Immunol. 2005, 6, 1236–1244. [Google Scholar] [CrossRef]
- Gorelik, L.; Constant, S.; Flavell, R.A. Mechanism of transforming growth factor beta-induced inhibition of T helper type 1 differentiation. J. Exp. Med. 2002, 195, 1499–1505. [Google Scholar] [CrossRef]
- Neurath, M.F.; Weigmann, B.; Finotto, S.; Glickman, J.; Nieuwenhuis, E.; Iijima, H.; Mizoguchi, A.; Mizoguchi, E.; Mudter, J.; Galle, P.R.; et al. The transcription factor T-bet regulates mucosal T cell activation in experimental colitis and Crohn’s disease. J. Exp. Med. 2002, 195, 1129–1143. [Google Scholar] [CrossRef]
- Gattinoni, L.; Klebanoff, C.A.; Palmer, D.C.; Wrzesinski, C.; Kerstann, K.; Yu, Z.; Finkelstein, S.E.; Theoret, M.R.; Rosenberg, S.A.; Restifo, N.P. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J. Clin. Investig. 2005, 115, 1616–1626. [Google Scholar] [CrossRef]
- Yang, J.; Brook, M.O.; Carvalho-Gaspar, M.; Zhang, J.; Ramon, H.E.; Sayegh, M.H.; Wood, K.J.; Turka, L.A.; Jones, N.D. Allograft rejection mediated by memory T cells is resistant to regulation. Proc. Natl. Acad. Sci. USA 2007, 104, 19954–19959. [Google Scholar] [CrossRef]
- Liu, S.; Etto, T.; Rodriguez-Cruz, T.; Li, Y.; Wu, C.; Fulbright, O.J.; Hwu, P.; Radvanyi, L.; Lizee, G. TGF-beta1 induces preferential rapid expansion and persistence of tumor antigen-specific CD8+ T cells for adoptive immunotherapy. J. Immunother 2010, 33, 371–381. [Google Scholar] [CrossRef]
- McKinney, E.F.; Lyons, P.A.; Carr, E.J.; Hollis, J.L.; Jayne, D.R.; Willcocks, L.C.; Koukoulaki, M.; Brazma, A.; Jovanovic, V.; Kemeny, D.M.; et al. A CD8+ T cell transcription signature predicts prognosis in autoimmune disease. Nat. Med. 2010, 16, 586–591. [Google Scholar] [CrossRef]
- Haring, J.S.; Jing, X.; Bollenbacher-Reilley, J.; Xue, H.H.; Leonard, W.J.; Harty, J.T. Constitutive expression of IL-7 receptor alpha does not support increased expansion or prevent contraction of antigen-specific CD4 or CD8 T cells following Listeria monocytogenes infection. J. Immunol. 2008, 180, 2855–2862. [Google Scholar] [CrossRef]
- Petschner, F.; Zimmerman, C.; Strasser, A.; Grillot, D.; Nunez, G.; Pircher, H. Constitutive expression of Bcl-xL or Bcl-2 prevents peptide antigen-induced T cell deletion but does not influence T cell homeostasis after a viral infection. Eur. J. Immunol. 1998, 28, 560–569. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Sullivan, J.A.; Kohlhapp, F.J.; Zloza, A.; Plaza-Rojas, L.; Burke, B.; Dulin, N.O.; Guevara-Patiño, J.A. Memory Precursors and Short-Lived Effector T cell Subsets Have Different Sensitivities to TGFβ. Int. J. Mol. Sci. 2023, 24, 3930. https://doi.org/10.3390/ijms24043930
O’Sullivan JA, Kohlhapp FJ, Zloza A, Plaza-Rojas L, Burke B, Dulin NO, Guevara-Patiño JA. Memory Precursors and Short-Lived Effector T cell Subsets Have Different Sensitivities to TGFβ. International Journal of Molecular Sciences. 2023; 24(4):3930. https://doi.org/10.3390/ijms24043930
Chicago/Turabian StyleO’Sullivan, Jeremy A., Frederick J. Kohlhapp, Andrew Zloza, Lourdes Plaza-Rojas, Brianna Burke, Nickolai O. Dulin, and José A. Guevara-Patiño. 2023. "Memory Precursors and Short-Lived Effector T cell Subsets Have Different Sensitivities to TGFβ" International Journal of Molecular Sciences 24, no. 4: 3930. https://doi.org/10.3390/ijms24043930
APA StyleO’Sullivan, J. A., Kohlhapp, F. J., Zloza, A., Plaza-Rojas, L., Burke, B., Dulin, N. O., & Guevara-Patiño, J. A. (2023). Memory Precursors and Short-Lived Effector T cell Subsets Have Different Sensitivities to TGFβ. International Journal of Molecular Sciences, 24(4), 3930. https://doi.org/10.3390/ijms24043930






