Quantification of Xanthone and Anthocyanin in Mangosteen Peel by UPLC-MS/MS and Preparation of Nanoemulsions for Studying Their Inhibition Effects on Liver Cancer Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. Analysis of Xanthones and Anthocyanins in Mangosteen Peel by UPLC-MS/MS
2.2. Method Validation
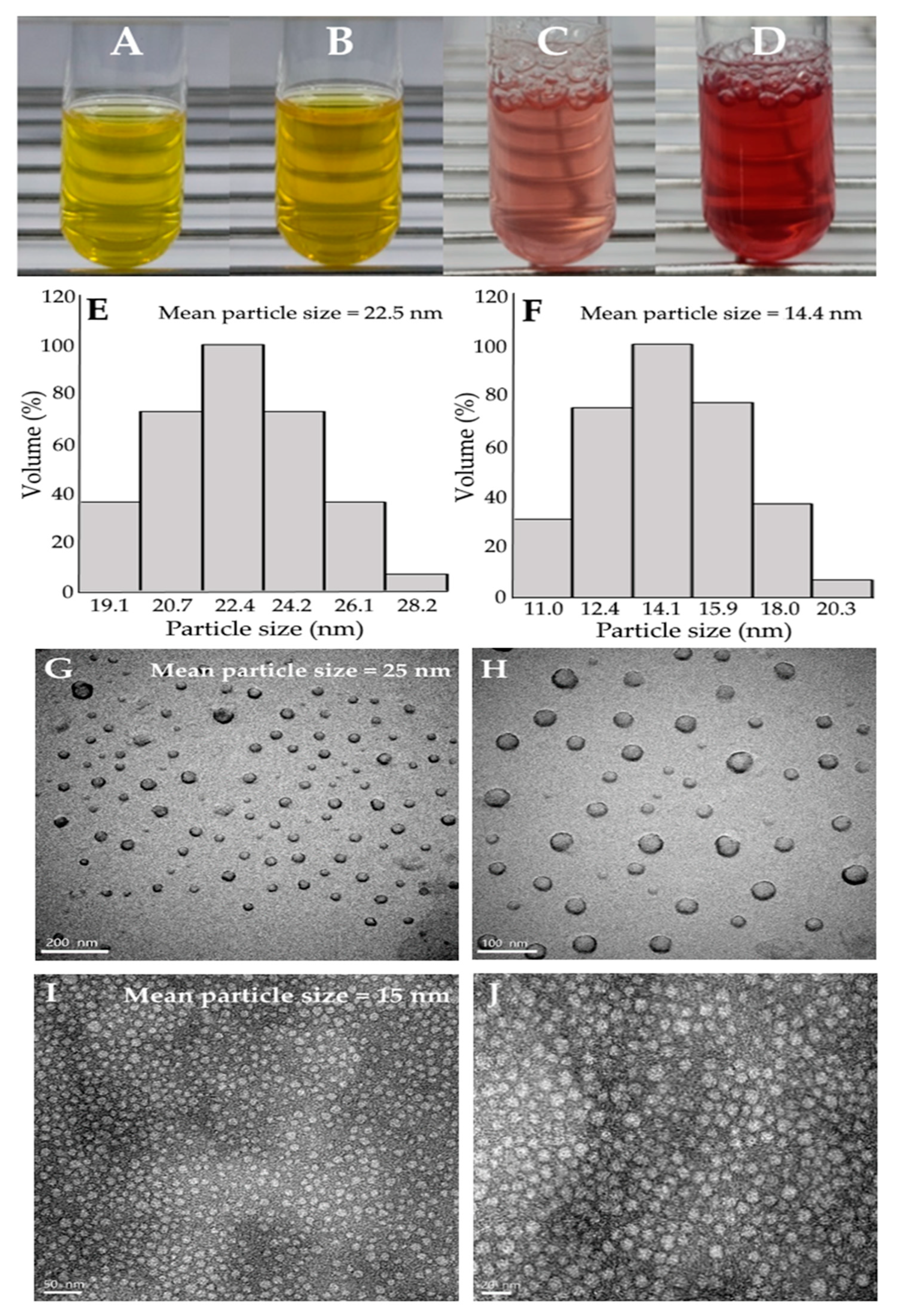
2.3. Preparation and Characterization of Xanthone and Anthocyanin Nanoemulsions
2.4. Stability of Xanthone and Anthocyanin Nanoemulsions
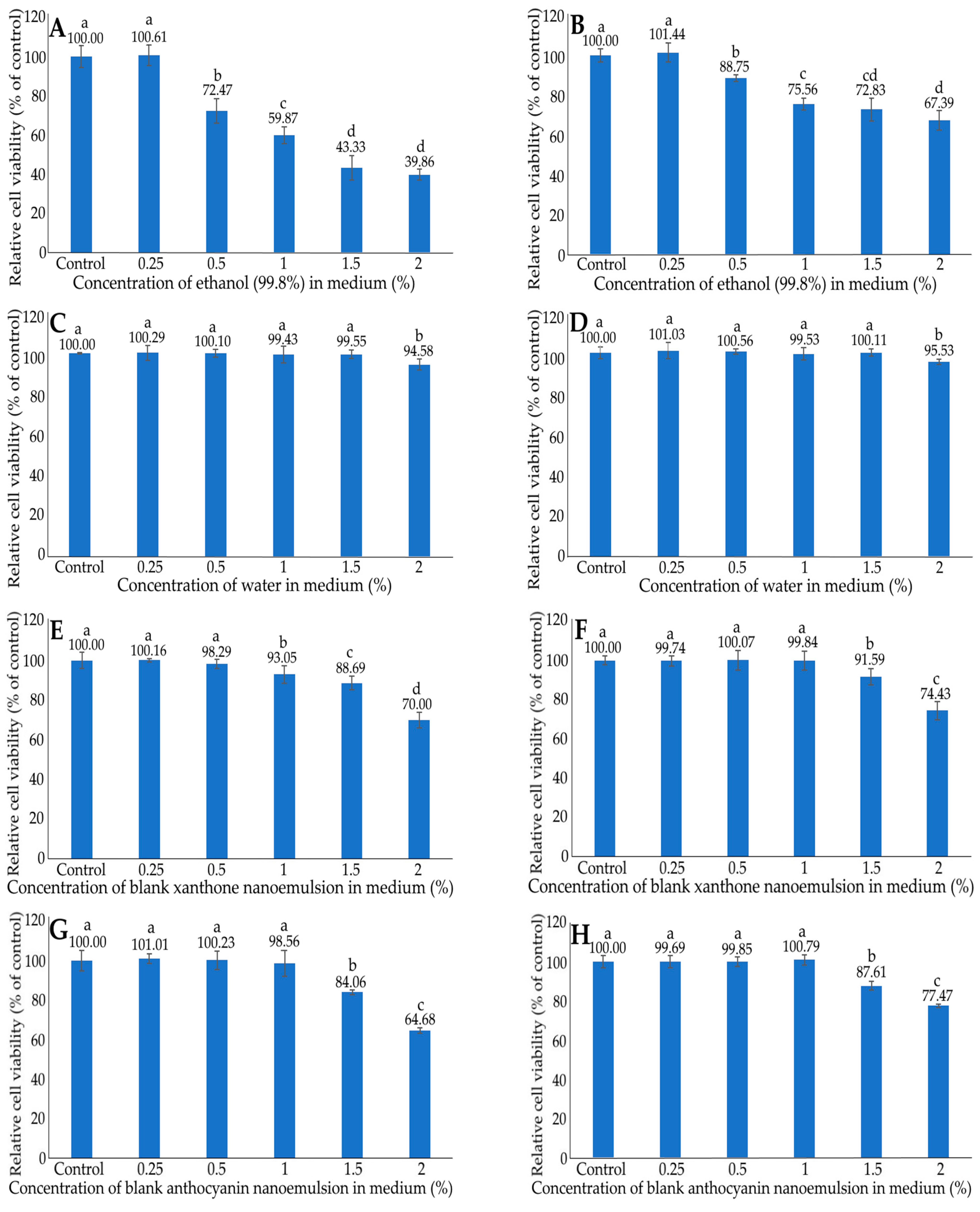
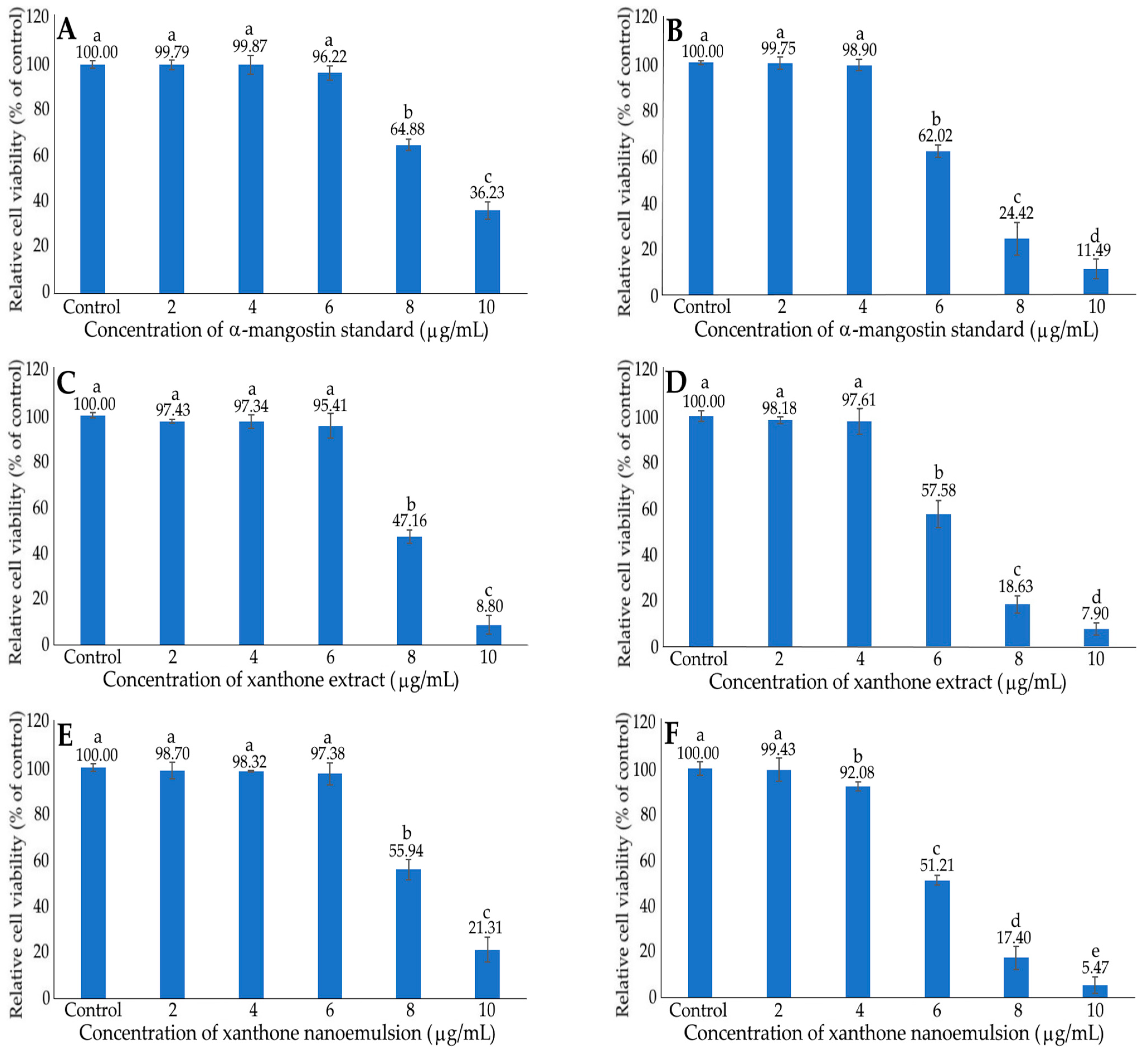
2.5. MTT Assay
2.6. Inhibition of HepG2 Cells by α-Mangostin Standard, Xanthone Extract and Nanoemulsion
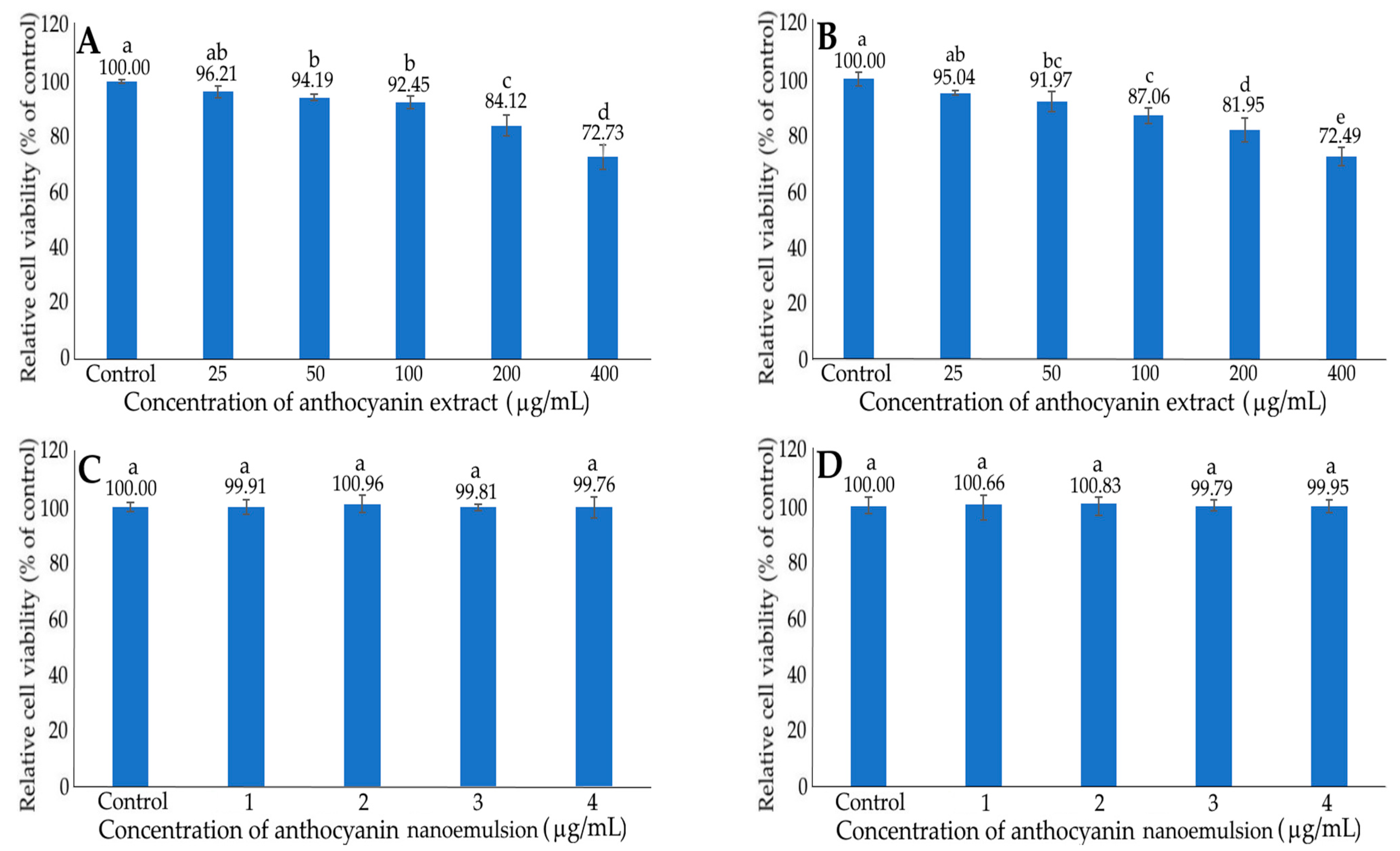
2.7. Inhibition of HepG2 Cells by Anthocyanin Extract and Nanoemulsion
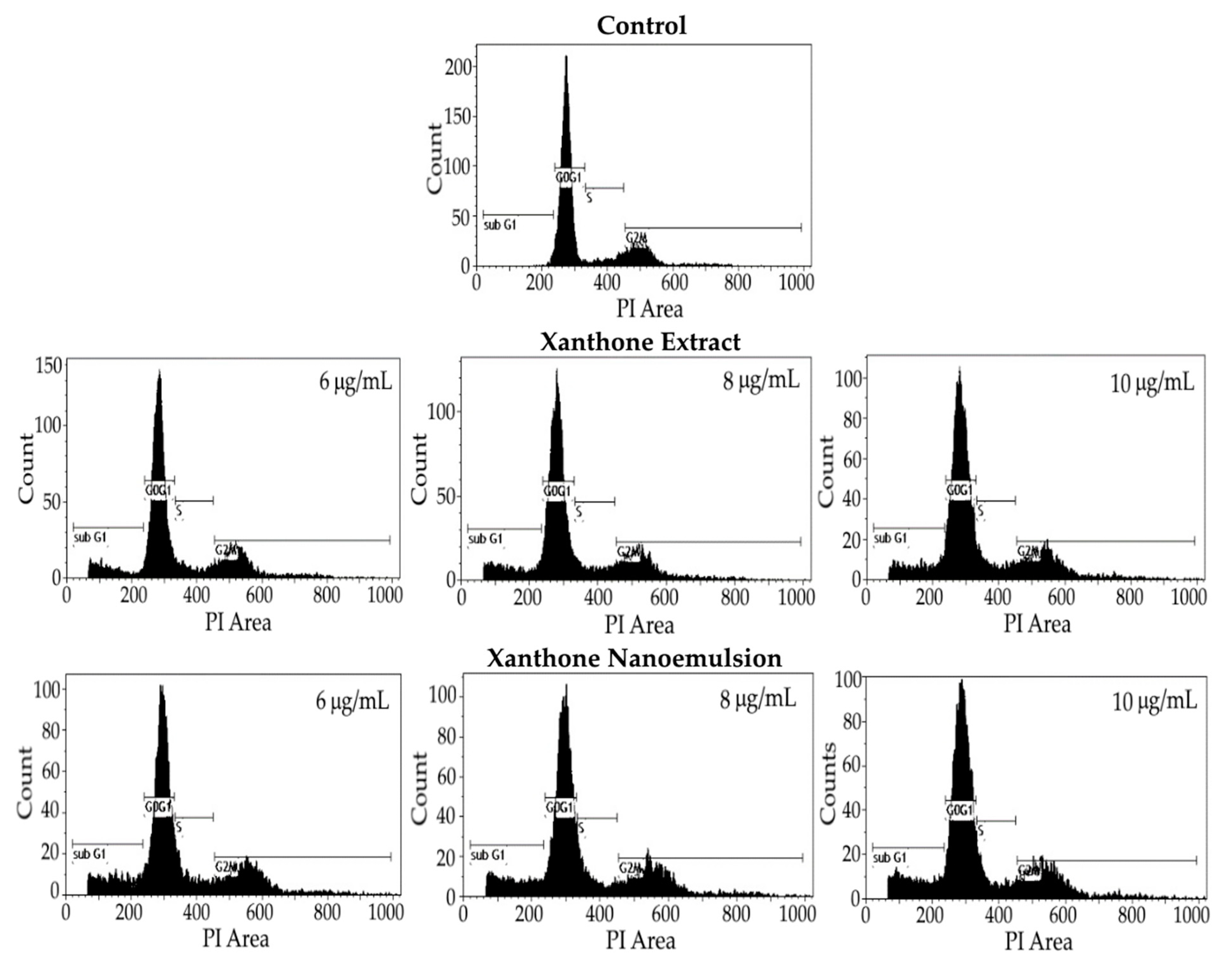
2.8. Cell Cycle Analysis
2.9. Analysis of Apoptosis of Liver Cancer Cells
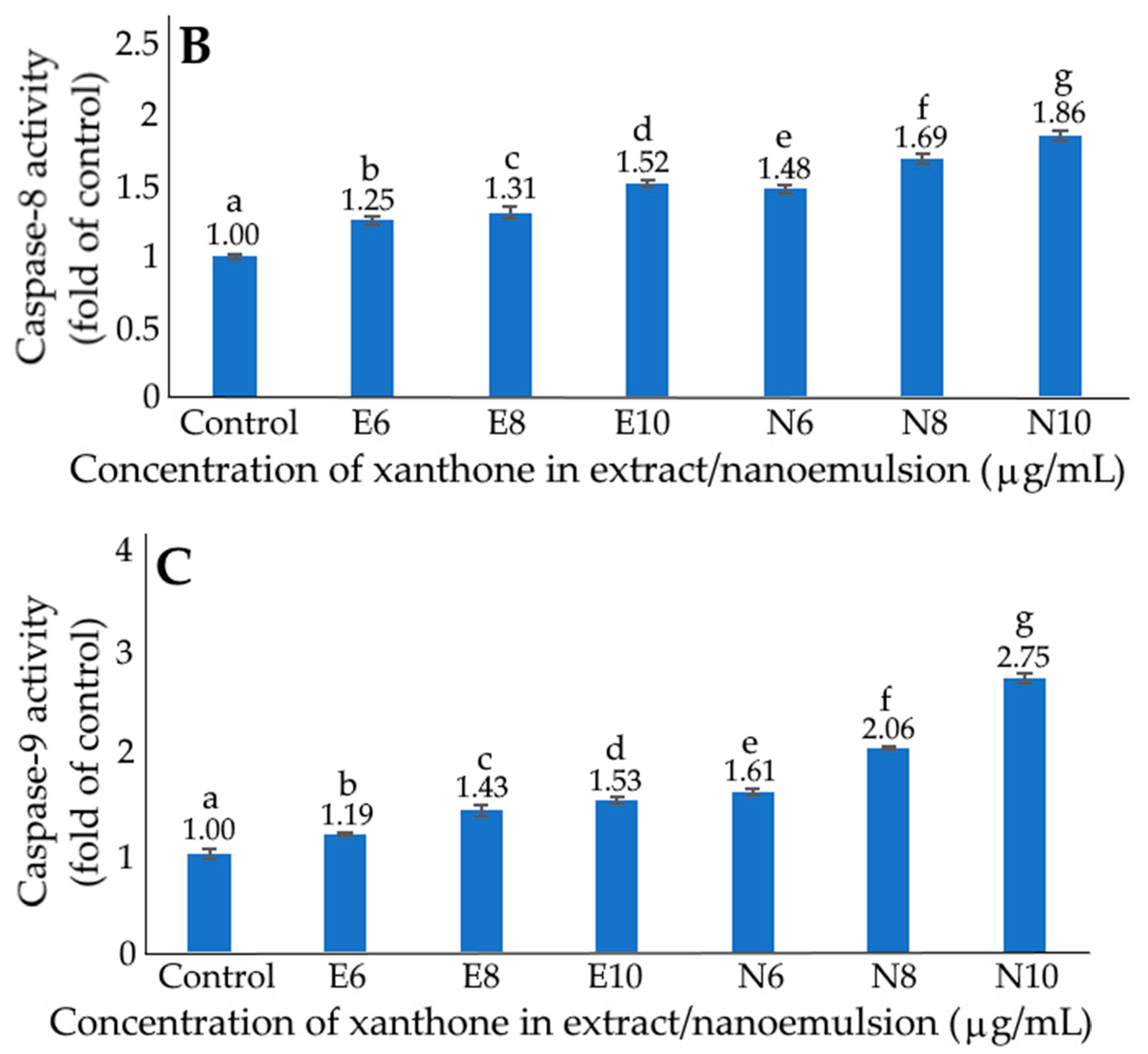
2.10. Activities of Caspase-3, Caspase-8 and Caspase-9
3. Materials and Methods
3.1. Materials
3.2. Sample Pretreatment
3.3. Extraction of Xanthones and Anthocyanins
3.4. Separation and Identification of Xanthones and Anthocyanins by UPLC-MS/MS
3.5. Method Validation
3.6. Quantitation
3.7. Preparation of Xanthone and Anthocyanin Nanoemulsions
3.8. Determination of Nanoemulsion Characteristics
3.9. Stability of Xanthone and Anthocyanin Nanoemulsions
3.10. Protection of Xanthone and Anthocyanin Extracts/Nanoemulsions
3.11. Cell Culture Experiment
3.12. MTT Experiment
3.13. Cell Cycle Experiment
3.14. FITC-Annexin V/PI Experiment
3.15. Determination of Caspase-3, Caspase-8 and Caspase-9 Activities
3.16. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaivisuthangkura, A.; Malaikaew, Y.; Chaovanalikit, A.; Jaratrungtawee, A.; Panseeta, P.; Ratananukul, P.; Suksamrarn, S. Prenylated xanthone composition of Garcinia mangostana (mangosteen) fruit hull. Chromatographia 2009, 69, 315–318. [Google Scholar] [CrossRef]
- Rizaldy, D.; Hartati, R.; Nadhifa, T.; Fidrianny, I. Chemical compounds and pharmacological activities of mangosteen (Garcinia mangostana L.)-Updated review. Biointerface Res. Appl. Chem. 2022, 12, 2503–2516. [Google Scholar]
- Corley, H. Fruits for the Future 9. Mangosteen (Garcinia mangostana). By M.B Osman and R. Milan. Southampton Centre for Underutilised Crops: Southampton, United Kingdom, 2006, pp. 170, available free on request to national scientists of developing countries. ISBN 0854328173. Exp. Agric. 2007, 43, 130–131. [Google Scholar]
- Palapol, Y.; Ketsa, S.; Stevenson, D.; Cooney, J.; Allan, A.; Ferguson, I. Colour development and quality of mangosteen (Garcinia mangostana L.) fruit during ripening and after harvest. Postharvest Biol. Technol. 2009, 51, 349–353. [Google Scholar] [CrossRef]
- Ansori, A.N.M.; Fadholly, A.; Hayaza, S.; Susilo, R.J.K.; Inayatillah, B.; Winarni, D.; Husen, S.A. A review on medicinal properties of mangosteen (Garcinia mangostana L.). Res. J. Pharm. Technol. 2020, 13, 974–982. [Google Scholar] [CrossRef]
- Walker, E.B. HPLC analysis of selected xanthones in mangosteen fruit. J. Sep. Sci. 2007, 30, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Li, Y.; Wang, W.; Deng, L. Bioactivity and pharmacological properties of α-mangostin from the mangosteen fruit: A review. Expert Opin. Ther. Pat. 2018, 28, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Ovalle-Magallanes, B.; Eugenio-Pérez, D.; Pedraza-Chaverri, J. Medicinal properties of mangosteen (Garcinia mangostana L.): A comprehensive update. Food Chem. Toxicol. 2017, 109, 102–122. [Google Scholar]
- Tousian Shandiz, H.; Razavi, B.M.; Hosseinzadeh, H. Review of Garcinia mangostana and its xanthones in metabolic syndrome and related complications. Phytother. Res. 2017, 31, 1173–1182. [Google Scholar]
- Muchtaridi, M.; Wijaya, C.A. Anticancer potential of α-mangostin. Asian J. Pharm. Clin. Res. 2017, 10, 440. [Google Scholar] [CrossRef]
- Chien, H.J.; Ying, T.H.; Hsieh, S.C.; Lin, C.L.; Yu, Y.L.; Kao, S.H.; Hsieh, Y.H. α-Mangostin attenuates stemness and enhances cisplatin-induced cell death in cervical cancer stem-like cells through induction of mitochondrial-mediated apoptosis. J. Cell. Physiol. 2020, 235, 5590–5601. [Google Scholar] [CrossRef]
- Aizat, W.M.; Ahmad-Hashim, F.H.; Jaafar, S.N.S. Valorization of mangosteen, “The Queen of Fruits,” and new advances in postharvest and in food and engineering applications: A review. J. Adv. Res. 2019, 20, 61–70. [Google Scholar] [PubMed]
- Ongkowijoyo, P.; Luna-Vital, D.A.; de Mejia, E.G. Extraction techniques and analysis of anthocyanins from food sources by mass spectrometry: An update. Food Chem. 2018, 250, 113–126. [Google Scholar] [PubMed]
- Moldovan, B.; David, L.; Chişbora, C.; Cimpoiu, C. Degradation kinetics of anthocyanins from European cranberrybush (Viburnum opulus L.) fruit extracts: Effects of temperature, pH and storage solvent. Molecules 2012, 17, 11655–11666. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [PubMed]
- Contreras-Lopez, E.; Casta, A.; González-Olivares, L.G.; Jaimez-Ordaz, J. Effect of light on stability of anthocyanins in ethanolic extracts of Rubus fruticosus. Food Nutr. Sci. 2014, 5, 488–494. [Google Scholar]
- Wu, H.Y.; Yang, K.M.; Chiang, P.Y. Roselle anthocyanins: Antioxidant properties and stability to heat and pH. Molecules 2018, 23, 1357. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Yun, S.M.; Song, M.Y.; Jung, K.; Kim, E.H. Cyanidin chloride induces apoptosis by inhibiting NF-κB signaling through activation of Nrf2 in colorectal cancer cells. Antioxidants 2020, 9, 285. [Google Scholar] [CrossRef] [PubMed]
- Lage, N.N.; Layosa, M.A.A.; Arbizu, S.; Chew, B.; Pedrosa, M.L.; Mertens-Talcott, S.; Talcott, S.; Noratto, G.D. Dark sweet cherry (Prunus avium) phenolics enriched in anthocyanins exhibit enhanced activity against the most aggressive breast cancer subtypes without toxicity to normal breast cells. J. Funct. Foods 2020, 64, 103710. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A comprehensive review of their chemical properties and health effects on cardiovascular and neurodegenerative diseases. Molecules 2020, 25, 3809. [Google Scholar]
- Malode, M.G.P.; Chauhan, S.A.; Bartare, S.A.; Malode, L.M.; Manwar, J.V.; Bakal, R.L. A critical review on nanoemulsion: Advantages, techniques and characterization. J. Appl. Pharm. Sci. Res. 2022, 4, 6–12. [Google Scholar] [CrossRef]
- Rosso, F.; Quagliariello, V.; Tortora, C.; Lazzaro, A.D.; Barbarisi, A.; Iaffaioli, R.V. Cross-linked hyaluronic acid sub-micron particles: In vitro and in vivo biodistribution study in cancer xenograft model. J. Mater. Sci. Mater. Med. 2013, 24, 1473–1481. [Google Scholar] [PubMed]
- Vecchione, R.; Quagliariello, V.; Giustetto, P.; Calabria, D.; Sathya, A.; Marotta, R.; Profeta, M.; Nitti, S.; Silvestri, N.; Pellegrino, T.; et al. Oil/water nano-emulsion loaded with cobalt ferrite oxide nanocubes for photo-acoustic and magnetic resonance dual imaging in cancer: In vitro and preclinical studies. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 275–286. [Google Scholar]
- WHO. Fact Sheet on Cancer; World Health Organization: Geneva, Switzerland, 2022; Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 15 August 2022).
- MOHW. Cause of Death Statistics in Taiwan. 2020. Available online: https://www.mohw.gov.tw/cp-5256-63399-2.html (accessed on 23 August 2021).
- NCI. Liver Cancer; National Cancer Institute: Rockville, MD, USA, 2021. Available online: https://www.cancer.gov/types/liver/what-is-liver-cancer (accessed on 23 August 2021).
- Lasfar, A. Liver Cancer; Intechopen Publishers: London, UK, 2018. [Google Scholar]
- Liang, X.; Hu, Y.; Li, J.; Chang, A.K.; Tao, X.; Li, Y.; Liu, W.; Pi, K.; Yuan, J.; Jiang, Z. Identification and pharmacokinetics of quinone reductase 2 inhibitors after oral administration of Garcinia mangostana L. extract in rat by LC-MS/MS. J. Agric. Food Chem. 2020, 68, 11975–11986. [Google Scholar] [PubMed]
- Wittenauer, J.; Falk, S.; Schweiggert-Weisz, U.; Carle, R. Characterisation and quantification of xanthones from the aril and pericarp of mangosteens (Garcinia mangostana L.) and a mangosteen containing functional beverage by HPLC–DAD–MSn. Food Chem. 2012, 134, 445–452. [Google Scholar] [CrossRef]
- Crupi, P.; Genghi, R.; Antonacci, D. In-time and in-space tandem mass spectrometry to determine the metabolic profiling of flavonoids in a typical sweet cherry (Prunus avium L.) cultivar from Southern Italy. J. Mass Spectrom. 2014, 49, 1025–1034. [Google Scholar] [CrossRef]
- TFDA. Method Validation Report; Taiwan Food and Drug Administration: Taipei, Taiwan, 2021.
- McClements, D.J.; Jafari, S.M. Improving emulsion formation, stability and performance using mixed emulsifiers: A review. Adv. Colloid Interface Sci. 2018, 251, 55–79. [Google Scholar]
- Hsu, H.Y.; Chen, B.H. A comparative study on inhibition of breast cancer cells and tumors in mice by carotenoid extract and nanoemulsion prepared from sweet potato (Ipomoea batatas L.) peel. Pharmaceutics 2022, 14, 980. [Google Scholar] [CrossRef]
- Lakshmi, P.; Kumar, G.A. Nanosuspension technology: A review. Int. J. Pharm. Pharm. Sci. 2010, 2, 35–40. [Google Scholar]
- Clogston, J.D.; Patri, A.K. Zeta potential measurement. In Characterization of Nanoparticles Intended for Drug Discovery—Methods in Molecular Biology; Mc Neil, S.E., Ed.; Humana Press: Totowa, NJ, USA, 2011; Volume 697, pp. 63–70. [Google Scholar]
- Jusril, N.A.; Abu Bakar, S.I.; Khalil, K.A.; Md Saad, W.M.; Wen, N.K.; Adenan, M.I. Development and optimization of nanoemulsion from ethanolic extract of Centella asiatica (NanoSECA) using D-optimal mixture design to improve blood-brain barrier permeability. Evid.-Based Complement. Altern. Med. 2022, 2022, 3483511. [Google Scholar] [CrossRef]
- Bamba, B.S.B.; Shi, J.; Tranchant, C.C.; Xue, S.J.; Forney, C.F.; Lim, L.T.; Xu, W.; Xu, G. Coencapsulation of polyphenols and anthocyanins from blueberry pomace by double emulsion stabilized by whey proteins: Effect of homogenization parameters. Molecules 2018, 23, 2525. [Google Scholar] [CrossRef]
- Garcia, N.O.; Fernandes, C.P.; da Conceição, E.C. Is it possible to obtain nanodispersions with jaboticaba peel’s extract using low energy methods and absence of any high cost equipment? Food Chem. 2019, 276, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Cai, N.; Xie, S.J.; Qiu, D.B.; Jia, C.C.; Du, C.; Liu, W.; Chen, J.J.; Zhang, Q. Potential effects of α-mangostin in the prevention and treatment of hepatocellular carcinoma. J. Funct. Foods 2016, 26, 309–318. [Google Scholar] [CrossRef]
- Zhang, H.; Tan, Y.P.; Zhao, L.; Wang, L.; Fu, N.I.; Zheng, S.P.; Shen, X.F. Anticancer activity of dietary xanthone α-mangostin against hepatocellular carcinoma by inhibition of STAT3 signalling via stabilization of SHP1. Cell Death Dis. 2020, 11, 63. [Google Scholar] [CrossRef]
- Su, Q.G.; Liu, Y.; Cai, Y.C.; Sun, Y.L.; Wang, B.; Xian, L.J. Anti-tumour effects of xanthone derivatives and the possible mechanisms of action. Investig. New Drugs 2011, 29, 1230–1240. [Google Scholar] [CrossRef] [PubMed]
- Forbes-Hernández, T.Y.; Gasparrini, M.; Afrin, S.; Cianciosi, D.; González-Paramás, A.M.; Santos-Buelga, C.; Mezzetti, B.; Quiles, J.L.; Battino, M.; Giampieri, F. Strawberry (cv. Romina) methanolic extract and anthocyanin-enriched fraction improve lipid profile and antioxidant status in HepG2 cells. Int. J. Mol. Sci. 2017, 18, 1149. [Google Scholar] [CrossRef] [PubMed]
- Watanapokasin, R.; Jarinthanan, F.; Nakamura, Y.; Sawasjirakij, N.; Jaratrungtawee, A.; Suksamrarn, S. Effects of α-mangostin on apoptosis induction of human colon cancer. World J. Gastroenterol. 2011, 17, 2086. [Google Scholar] [CrossRef]
- Jin, S.; Shi, K.; Liu, L.; Chen, Y.; Yang, G. Xanthones from the bark of Garcinia xanthochymus and the mechanism of induced apoptosis in human hepatocellular carcinoma HepG2 cells via the mitochondrial pathway. Int. J. Mol. Sci. 2019, 20, 4803. [Google Scholar] [CrossRef]
- Abu Bakar, M.F.; Ahmad, N.E.; Suleiman, M.; Rahmat, A.; Isha, A. Garcinia dulcis fruit extract induced cytotoxicity and apoptosis in HepG2 liver cancer cell line. BioMed Res. Int. 2015, 2015, 916902. [Google Scholar] [CrossRef]
- Wudtiwai, B.; Pitchakarn, P.; Banjerdpongchai, R. Alpha-mangostin, an active compound in Garcinia mangostana, abrogates anoikis-resistance in human hepatocellular carcinoma cells. Toxicol. Vitr. 2018, 53, 222–232. [Google Scholar] [CrossRef]
- Quagliariello, V.; Vecchione, R.; Coppola, C.; Cicco, C.D.; Capua, A.D.; Piscopo, G.; Paciello, R.; Narciso, V.; Formisano, C.; Taglialatela-Scafati, O.; et al. Cardioprotective effects of nanoemulsions loaded with anti-inflammatory nutraceuticals against doxorubicin-induced cardiotoxicity. Nutrients 2018, 10, 1304. [Google Scholar] [CrossRef] [PubMed]
- Liobikas, J.; Skemiene, K.; Trumbeckaite, S.; Borutaite, V. Anthocyanins in cardioprotection: A path through mitochondria. Pharmacol. Res. 2016, 113, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Deb, P.K.; Priya, S.; Medina, K.D.; Devi, R.; Walode, S.G.; Rudrapal, M. Dietary flavonoids: Cardioprotective potential with antioxidant effects and their pharmacokinetic, toxicological and therapeutic concerns. Molecules 2021, 26, 4021. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, P.; Guo, Q.Q.; Wang, Z.Y. Anthocyanin composition and content of the Vaccinium uliginosum berry. Food Chem. 2011, 125, 116–120. [Google Scholar] [CrossRef]
- Yang, C.C.; Hung, C.F.; Chen, B.H. Preparation of coffee oil-algae oil-based nanoemulsions and the study of their inhibition effect on UVA-induced skin damage in mice and melanoma cell growth. Int. J. Nanomed. 2017, 12, 6559–6580. [Google Scholar] [CrossRef]
- SAS. SAS Procedures and SAS/Graph User’s Guide, Version 6; Statistical Analysis System Institute: Cary, NC, USA, 2019. [Google Scholar]
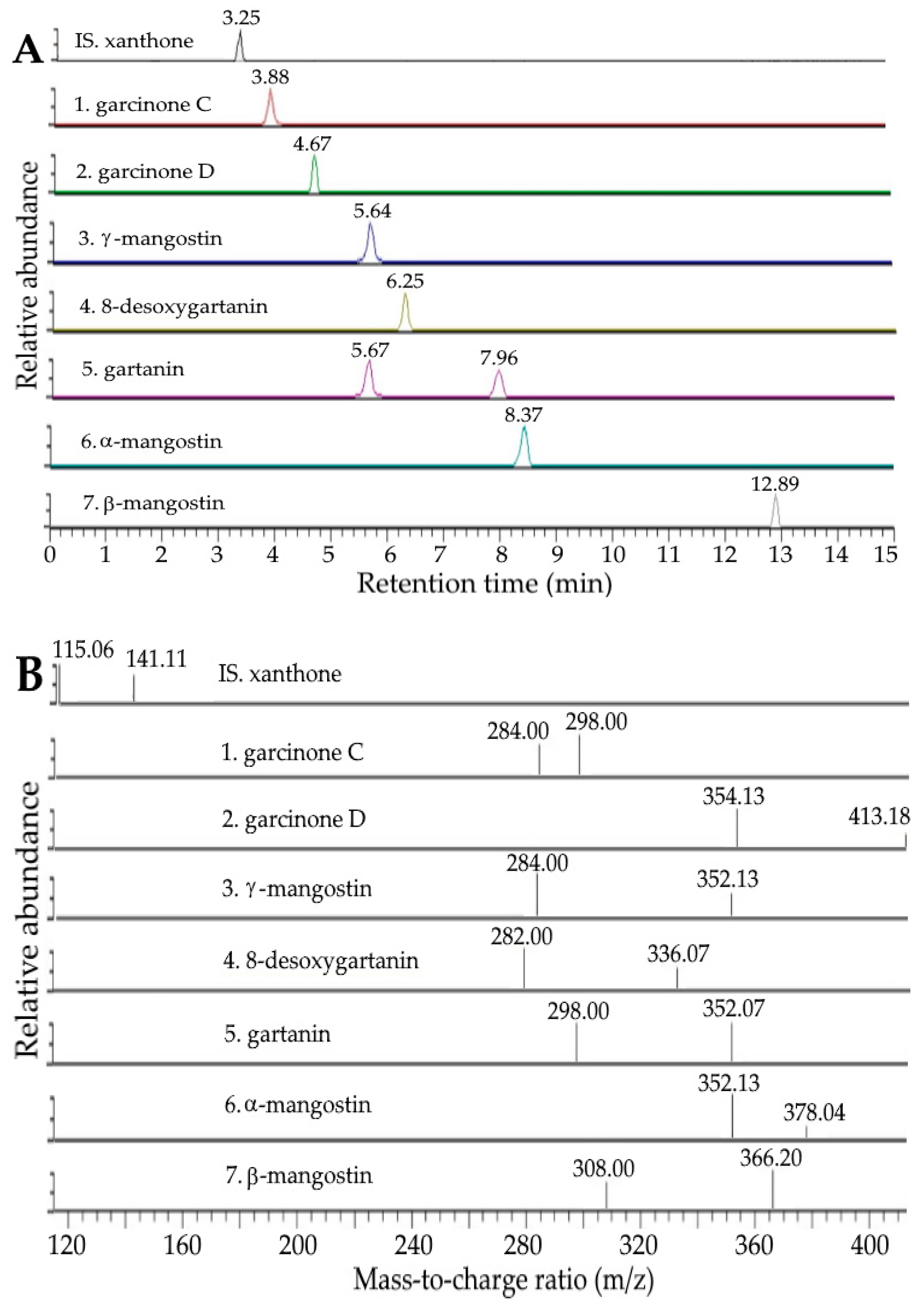
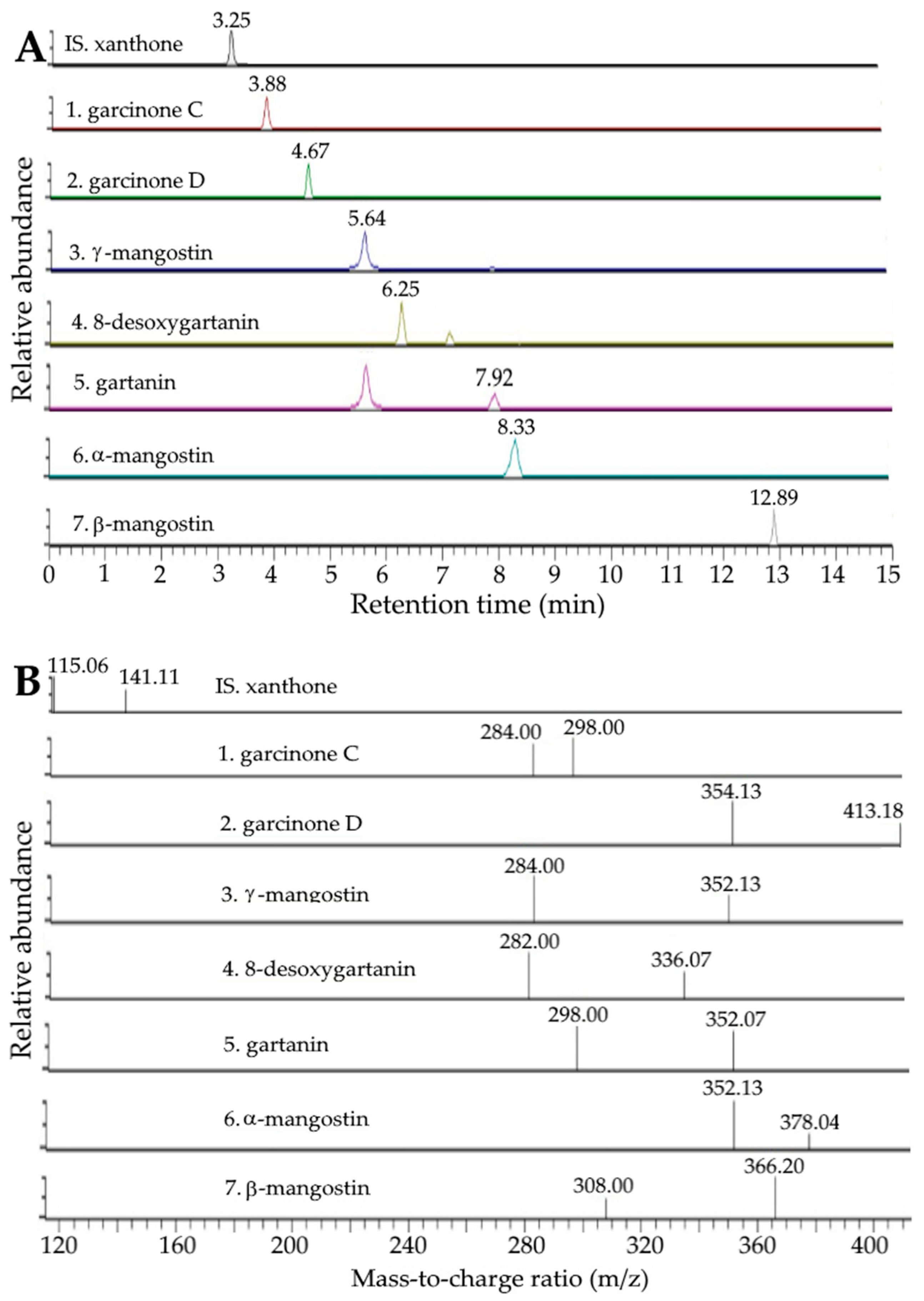



| Peak No. | Compound a | Retention Time (min) | MS/MS (m/z) | Content (μg/g) e,f,h | Content (μg/g) e,g,h | |||
|---|---|---|---|---|---|---|---|---|
| Standard | Sample | Precursor Ion | Product Ion | Reported | ||||
| Xanthone | ||||||||
| 1 | Garcinone C | 3.88 | 3.88 | 413 | 297, 283 | 413, 395, 369, 357, 339, 297, 283, 271 b | 513.06 ± 8.25 A | 526.12 ± 9.73 A |
| 2 | Garcinone D | 4.67 | 4.67 | 427 | 353, 412 | 427, 395, 369, 357, 353, 339, 283 b | 469.82 ± 4.68 A | 475.38 ± 5.74 A |
| 3 | γ-mangostin | 5.64 | 5.64 | 395 | 283, 351 | 395, 283,339,326,297, 351, 271 b | 11,100.72 ± 365.36 A | 10,984.56 ± 343.27 A |
| 4 | 8-desoxygartanin | 6.25 | 6.25 | 379 | 281, 335 | 379,363, 335, 321, 281, 269 b | 1490.61 ± 15.03 A | 1385.45 ± 18.21 A |
| 5 | Gartanin | 7.96 | 7.92 | 395 | 297, 351 | 395, 340, 297, 380, 351,337 c | 2398.96 ± 34.71 A | 2526.34 ± 32.18 A |
| 6 | α-mangostin | 8.37 | 8.33 | 409 | 351, 377 | 409, 351, 394, 377, 339 b | 51,062.21 ± 838.41 A | 50,971.24 ± 440.50 A |
| 7 | β-mangostin | 12.89 | 12.89 | 423 | 365, 307 | 423, 408, 391, 365, 353, 307, 295 b | 1508.01 ± 5.75 A | 1453.39 ± 7.26 A |
| Total | 68,543.39 ± 1257.20 A | 68,322.48 ± 856.89 A | ||||||
| Anthocyanin | ||||||||
| 1 | Cyanidin-3- sophoroside | 7.25 | 7.24 | 611 | 287, 483 | 611, 287 d | 2889.85 ± 45.65 A | 1482.12 ± 30.13 B |
| 2 | Cyanidin-3- glucoside | 7.67 | 7.66 | 449 | 287, 136 | 449, 287 d | 19.72 ± 0.4 A | 16.35 ± 0.61 B |
| Total | 2909.57 ± 46.08 A | 1498.47 ± 30.74 B | ||||||
| Compound | Recovery | Intra-Day Variability c | Inter-Day Variability c | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Original (μg) | Spiked (μg) | Found (μg) | Recovery a (%) | Mean ± SD (%) | RSD b (%) | Contents (μg/g) | RSD (%) b | Contents (μg/g) | RSD (%) b | |
| Xanthone | ||||||||||
| Garcinone C | 519.06 | 200 | 698.06 | 89.50 | 91.64 ± 3.02 | 3.30 | 503.66 ± 12.74 | 2.53 | 516.47 ± 18.96 | 3.67 |
| 2000 | 2394.58 | 93.78 | ||||||||
| Garcinone D | 467.83 | 200 | 656.34 | 94.25 | 95.93 ± 2.37 | 2.47 | 466.47 ± 6.19 | 1.33 | 475.17 ± 9.48 | 1.99 |
| 2000 | 2419.92 | 97.60 | ||||||||
| γ-mangostin | 11,145.09 | 4000 | 14,980.29 | 95.88 | 96.68 ± 1.12 | 1.16 | 10,715.21 ± 307.69 | 2.87 | 11,441.87 ± 237.96 | 2.08 |
| 40,000 | 50,133.09 | 97.47 | ||||||||
| 8-desoxygartanin | 1492.26 | 400 | 1878.43 | 96.54 | 97.66 ± 1.57 | 1.61 | 1474.83 ± 16.79 | 1.14 | 1504.75 ± 24.03 | 1.60 |
| 4000 | 5443.07 | 98.77 | ||||||||
| Gartanin | 2392.29 | 400 | 2761.47 | 92.30 | 93.98 ± 2.38 | 2.53 | 2368.08 ± 61.95 | 2.62 | 2436.52 ± 38.09 | 1.56 |
| 4000 | 6218.75 | 95.66 | ||||||||
| α-mangostin | 51,044.48 | 5000 | 55,805.98 | 95.23 | 96.54 ± 1.85 | 1.91 | 50,232.81 ± 1102.29 | 2.19 | 51,909.35 ± 1180.10 | 2.27 |
| 55,000 | 104,856.48 | 97.84 | ||||||||
| β-mangostin | 1512.00 | 400 | 1866.35 | 88.59 | 90.34 ± 2.48 | 2.74 | 1510.61 ± 14.12 | 0.93 | 1501.42 ± 27.69 | 1.84 |
| 4000 | 5195.66 | 92.09 | ||||||||
| Anthocyanin | ||||||||||
| Cyanidin-3- sophoroside | 2903.88 | 500 | 3362.03 | 91.63 | 93.15 ± 2.14 | 2.30 | 2838.83 ± 97.40 | 3.43 | 2926.84 ± 108.23 | 3.70 |
| 5000 | 7636.88 | 94.66 | ||||||||
| Cyanidin-3- glucoside | 19.76 | 10 | 28.81 | 90.48 | 88.37 ± 2.99 | 3.38 | 19.27 ± 0.57 | 2.94 | 20.12 ± 0.52 | 2.60 |
| 100 | 106.01 | 86.25 | ||||||||
| Temperature (°C) | Particle Size (nm) | Zeta-Potential (mV) | Polydispersity Index (PDI) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.5 h | 1 h | 1.5 h | 2 h | 0.5 h | 1 h | 1.5 h | 2 h | 0.5 h | 1 h | 1.5 h | 2 h | |
| Xanthone Nanoemulsion | ||||||||||||
| Control (unheated) | 22.4 | −89.3 | 0.229 | |||||||||
| 40 °C | 22.3 | 22.6 | 22.7 | 22.8 | −89.1 | −89.2 | −89.1 | −87.5 | 0.227 | 0.231 | 0.236 | 0.243 |
| 60 °C | 22.3 | 22.5 | 22.8 | 23.0 | −88.9 | −88.4 | −87.9 | −86.9 | 0.229 | 0.230 | 0.243 | 0.252 |
| 80 °C | 22.7 | 22.9 | 23.1 | 23.5 | −88.0 | −87.2 | −86.3 | −84.9 | 0.232 | 0.246 | 0.254 | 0.267 |
| 100 °C | 22.8 | 23.3 | 25.8 | 29.8 | −83.6 | −81.2 | −76.7 | −73.0 | 0.235 | 0.256 | 0.279 | 0.305 |
| Anthocyanin Nanoemulsion | ||||||||||||
| pH = 2 | ||||||||||||
| Control (unheated) | 14.2 | −39.4 | 0.153 | |||||||||
| 40 °C | 14.5 | 15.3 | 15.9 | 16.2 | −38.9 | −37.4 | −35.5 | −33.6 | 0.154 | 0.155 | 0.153 | 0.159 |
| 60 °C | 15.2 | 15.8 | 16.3 | 17.5 | −38.5 | −36.7 | −34.6 | −32.7 | 0.154 | 0.168 | 0.183 | 0.186 |
| 80 °C | 15.6 | 16.4 | 17.6 | 20.2 | −38.1 | −35.1 | −33.2 | −32.2 | 0.162 | 0.173 | 0.199 | 0.217 |
| 100 °C | 15.9 | 18.0 | 20.2 | 23.1 | −37.0 | −34.7 | −32.6 | −30.7 | 0.176 | 0.200 | 0.231 | 0.251 |
| pH = 3 | ||||||||||||
| Control (unheated) | 14.4 | −61.5 | 0.151 | |||||||||
| 40 °C | 14.4 | 15.1 | 15.5 | 16.1 | −60.9 | −60.1 | −59.3 | −58.4 | 0.148 | 0.152 | 0.153 | 0.151 |
| 60 °C | 14.6 | 15.3 | 16.5 | 17.2 | −60.8 | −59.6 | −58.5 | −57.2 | 0.153 | 0.158 | 0.162 | 0.164 |
| 80 °C | 15.1 | 16.2 | 16.9 | 18.8 | −59.9 | −59.1 | −58.4 | −57.1 | 0.161 | 0.169 | 0.174 | 0.185 |
| 100 °C | 15.2 | 16.7 | 18.3 | 19.8 | −58.7 | −57.9 | −56.2 | −53.9 | 0.162 | 0.171 | 0.190 | 0.206 |
| Storage Time (Day) | Particle Size (nm) A | Polydispersity Index (PDI) A | Zeta Potential (mV) A |
|---|---|---|---|
| Xanthone Nanoemulsion | |||
| 0 | 22.0 ± 0.3 a | 0.225 ± 0.019 a | −87.7 ± 1.5 a |
| 15 | 22.5 ± 0.3 ab | 0.237 ± 0.014 ab | −87.6 ± 0.4 ab |
| 30 | 22.1 ± 0.7 a | 0.240 ± 0.002 ab | −87.3 ± 0.1 ab |
| 45 | 22.4 ± 0.3 ab | 0.240 ± 0.007 ab | −87.0 ± 0.4 ab |
| 60 | 22.7 ± 0.2 ab | 0.242 ± 0.005 ab | −86.5 ± 0.6 ab |
| 75 | 22.5 ± 0.3 ab | 0.243 ± 0.005 ab | −85.9 ± 1.0 b |
| 90 | 22.9 ± 0.2 b | 0.246 ± 0.011 b | −82.9 ± 1.2 c |
| Anthocyanin Nanoemulsion | |||
| 0 | 14.1 ± 0.5 a | 0.148 ± 0.011 a | −61.5 ± 0.7 a |
| 15 | 14.5 ± 0.2 ab | 0.150 ± 0.009 a | −60.1 ± 1.8 ab |
| 30 | 14.4 ± 0.1 ab | 0.155 ± 0.009 ab | −58.8 ± 0.7 b |
| 45 | 14.6 ± 0.2 abc | 0.171 ± 0.008 b | −57.0 ± 0.2 c |
| 60 | 14.9 ± 0.4 bc | 0.189 ± 0.008 c | −55.2 ± 0.4 d |
| 75 | 15.2 ± 0.3 cd | 0.217 ± 0.013 d | −53.3 ± 0.6 e |
| 90 | 15.8 ± 0.6 d | 0.254 ± 0.010 e | −50.7 ± 0.3 f |
| Concentration (μg/mL) | Sub-G1 (%) | G0/G1 (%) | S (%) | G2/M (%) |
|---|---|---|---|---|
| Control | 1.10 ± 0.51 a | 67.52 ± 0.01 a | 8.74 ± 1.16 a | 21.76 ± 0.11 a |
| Extract | ||||
| 6 | 10.66 ± 0.58 b | 56.60 ± 0.16 b | 10.22 ± 1.94 b | 21.63 ± 2.25 a |
| 8 | 11.61 ± 0.74 c | 56.11 ± 0.41 b | 10.47 ± 1.62 b | 20.70 ± 1.00 a |
| 10 | 11.50 ± 0.16 c | 53.94 ± 1.33 c | 11.82 ± 2.40 b | 21.33 ± 1.09 a |
| Nanoemulsion | ||||
| 6 | 14.12 ± 0.30 d | 50.35 ± 0.39 d | 11.44 ± 0.56 b | 22.05 ± 1.36 a |
| 8 | 15.73 ± 0.02 e | 48.35 ± 1.61 e | 11.85 ± 0.76 b | 23.16 ± 1.80 a |
| 10 | 16.53 ± 0.10 f | 47.68 ± 0.28 e | 11.95 ± 1.89 b | 22.25 ± 1.60 a |
| Concentration (μg/mL) | Necrosis Cells A→+) (%) | Late Apoptosis Cells (A+ +) (%) | Viable Cells A→−) (%) | Early Apoptosis Cells (A+ −) (%) |
|---|---|---|---|---|
| Control | 4.19 ± 0.07 a | 1.94 ± 0.61 a | 88.54 ± 2.84 a | 5.36 ± 2.30 a |
| Extract | ||||
| 6 | 11.10 ± 1.59 b | 27.79 ± 4.21 b | 3.86 ± 3.01 b | 57.27 ± 0.38 b |
| 8 | 13.79 ± 3.25 c | 30.11 ± 5.49 c | 1.70 ± 1.33 c | 54.42 ± 0.91 c |
| 10 | 12.68 ± 3.91 d | 54.88 ± 5.71 d | 0.40 ± 0.32 d | 32.05 ± 1.49 d |
| Nanoemulsion | ||||
| 6 | 13.93 ± 4.70 e | 48.07 ± 5.89 e | 0.15 ± 0.10 e | 37.87 ± 1.09 e |
| 8 | 8.23 ± 3.50 f | 81.91 ± 4.69 f | 0.07 ± 0.05 f | 9.81 ± 1.15 f |
| 10 | 7.05 ± 2.96 g | 85.82 ± 3.77 g | 0.05 ± 0.04 g | 7.10 ± 0.77 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Inbaraj, B.S.; Chen, B.-H. Quantification of Xanthone and Anthocyanin in Mangosteen Peel by UPLC-MS/MS and Preparation of Nanoemulsions for Studying Their Inhibition Effects on Liver Cancer Cells. Int. J. Mol. Sci. 2023, 24, 3934. https://doi.org/10.3390/ijms24043934
Li R, Inbaraj BS, Chen B-H. Quantification of Xanthone and Anthocyanin in Mangosteen Peel by UPLC-MS/MS and Preparation of Nanoemulsions for Studying Their Inhibition Effects on Liver Cancer Cells. International Journal of Molecular Sciences. 2023; 24(4):3934. https://doi.org/10.3390/ijms24043934
Chicago/Turabian StyleLi, Rui, Baskaran Stephen Inbaraj, and Bing-Huei Chen. 2023. "Quantification of Xanthone and Anthocyanin in Mangosteen Peel by UPLC-MS/MS and Preparation of Nanoemulsions for Studying Their Inhibition Effects on Liver Cancer Cells" International Journal of Molecular Sciences 24, no. 4: 3934. https://doi.org/10.3390/ijms24043934
APA StyleLi, R., Inbaraj, B. S., & Chen, B.-H. (2023). Quantification of Xanthone and Anthocyanin in Mangosteen Peel by UPLC-MS/MS and Preparation of Nanoemulsions for Studying Their Inhibition Effects on Liver Cancer Cells. International Journal of Molecular Sciences, 24(4), 3934. https://doi.org/10.3390/ijms24043934







