Analysis of Bacteriophage Behavior of a Human RNA Virus, SARS-CoV-2, through the Integrated Approach of Immunofluorescence Microscopy, Proteomics and D-Amino Acid Quantification
Abstract
1. Introduction
2. Results
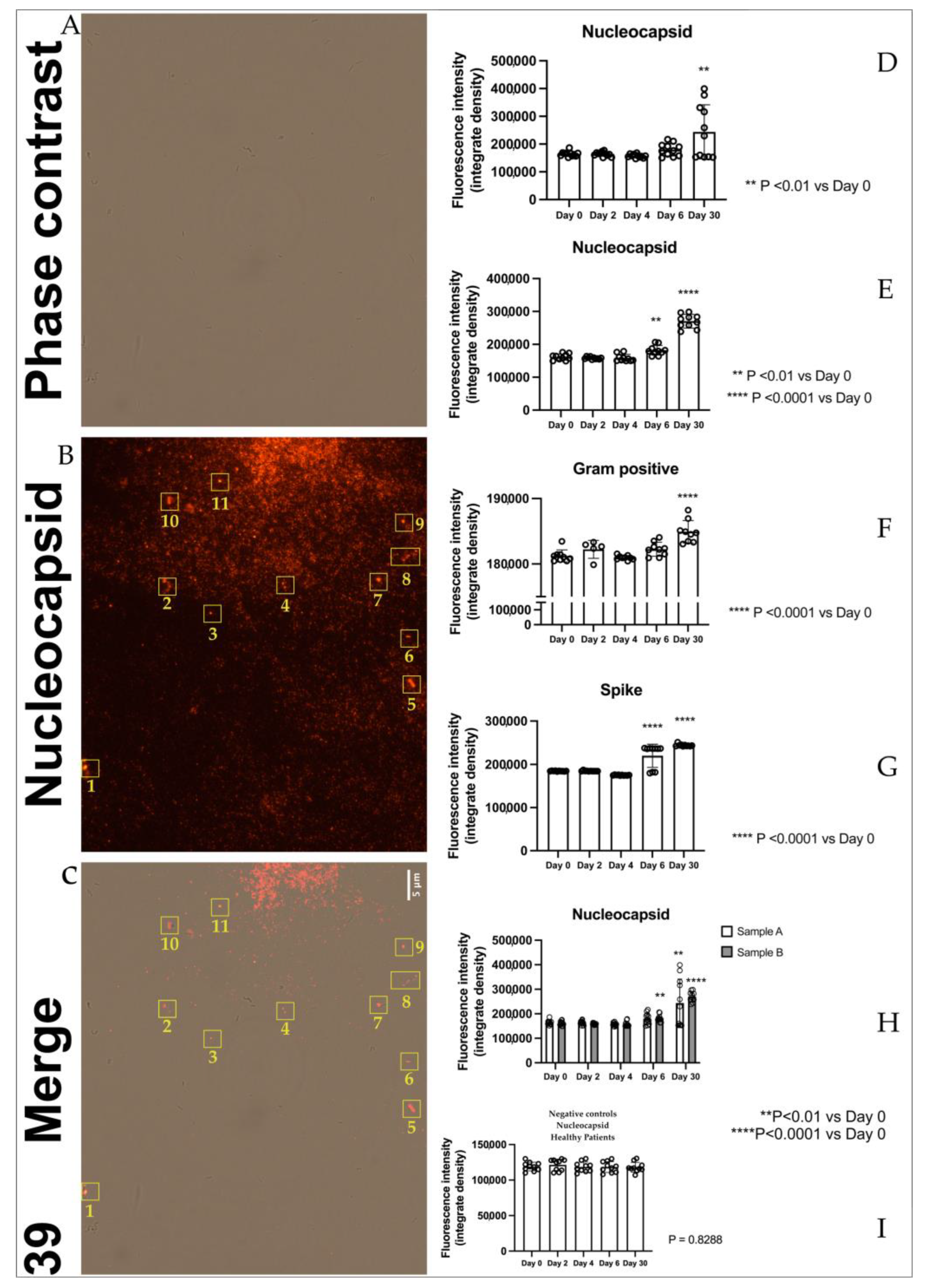
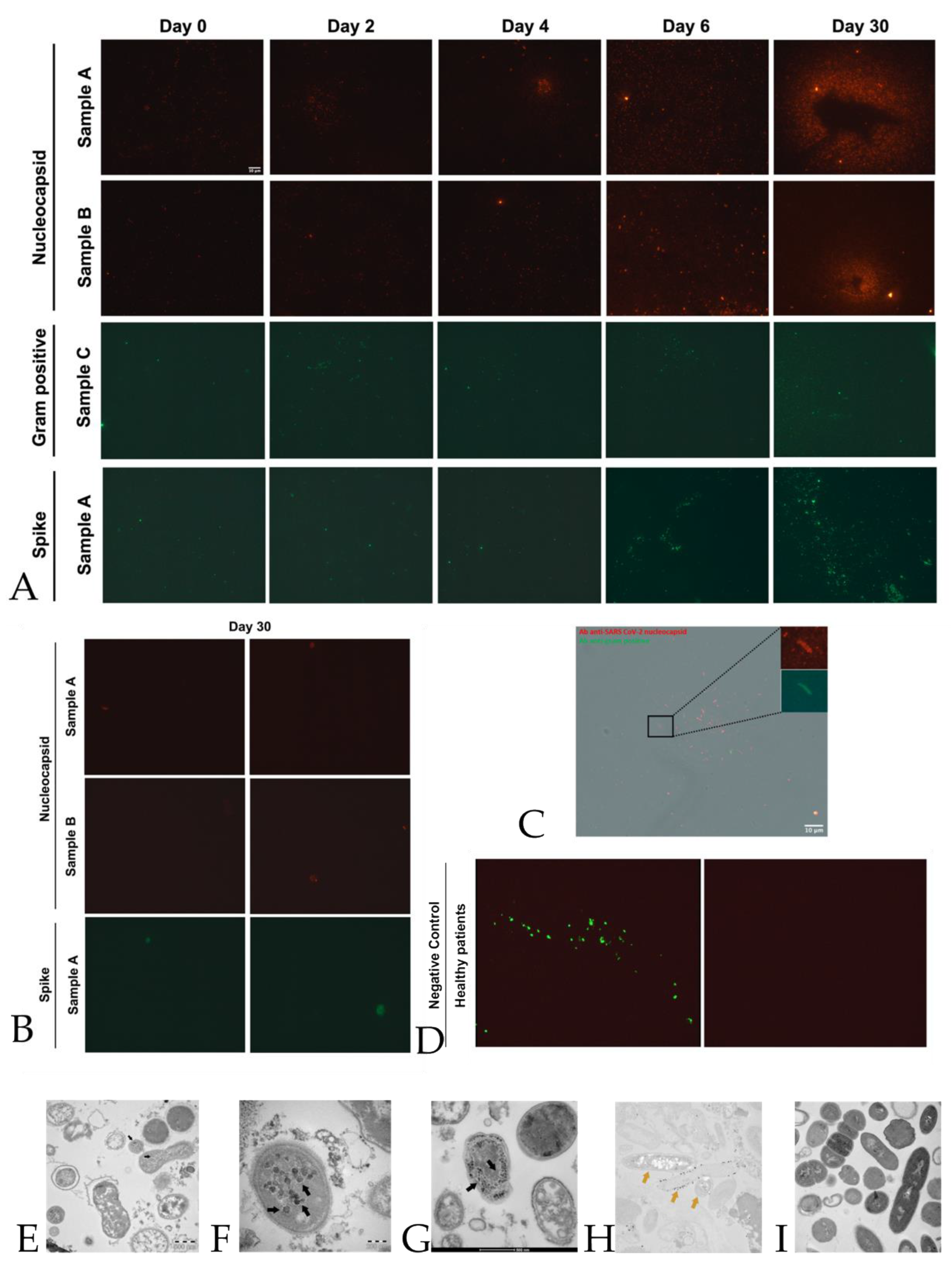
2.1. Immunofluorescence
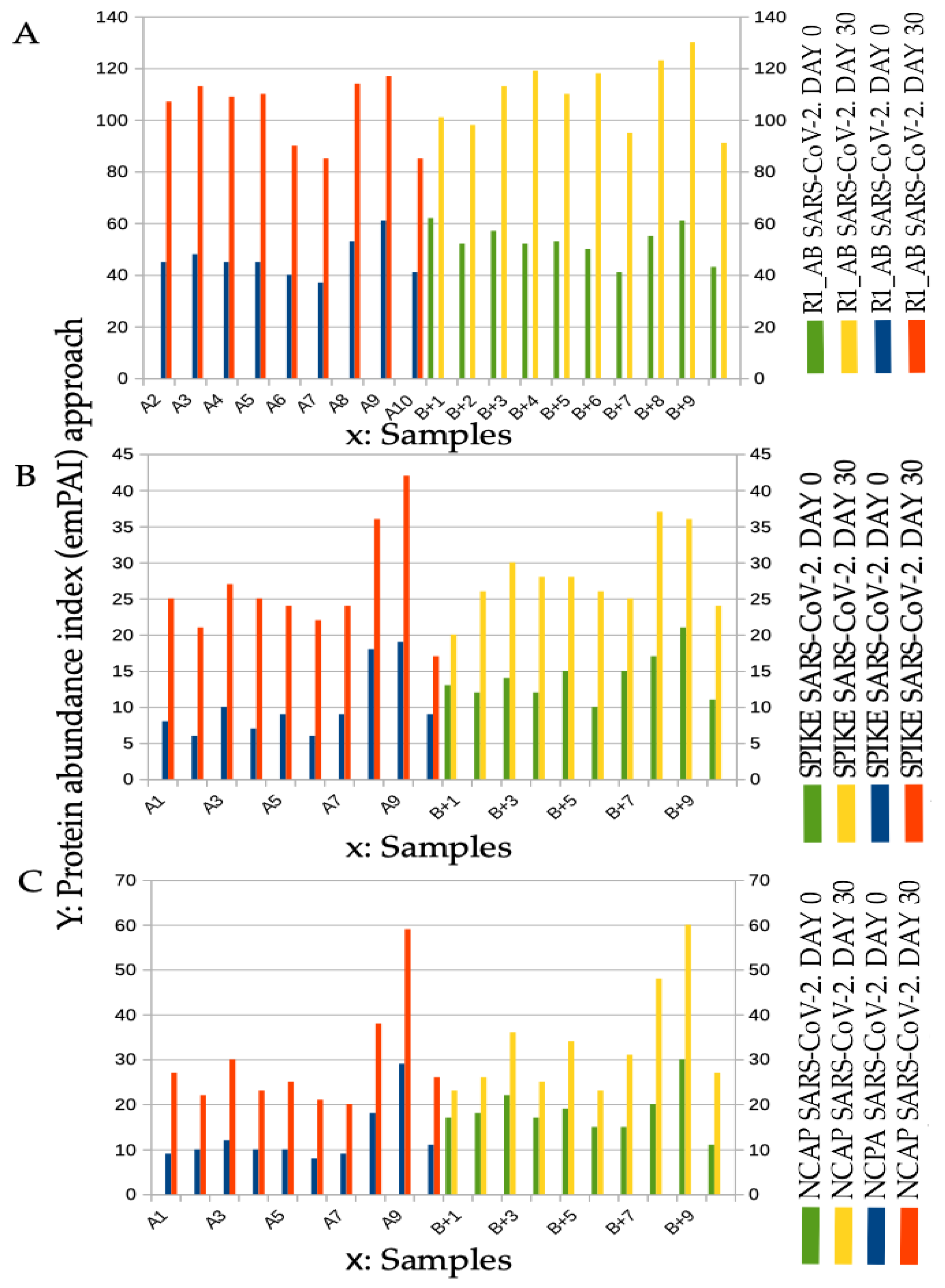
2.2. Spectral Counting Quantification Peptides of SARS-CoV-2 at Mass Spectrometry Analysis
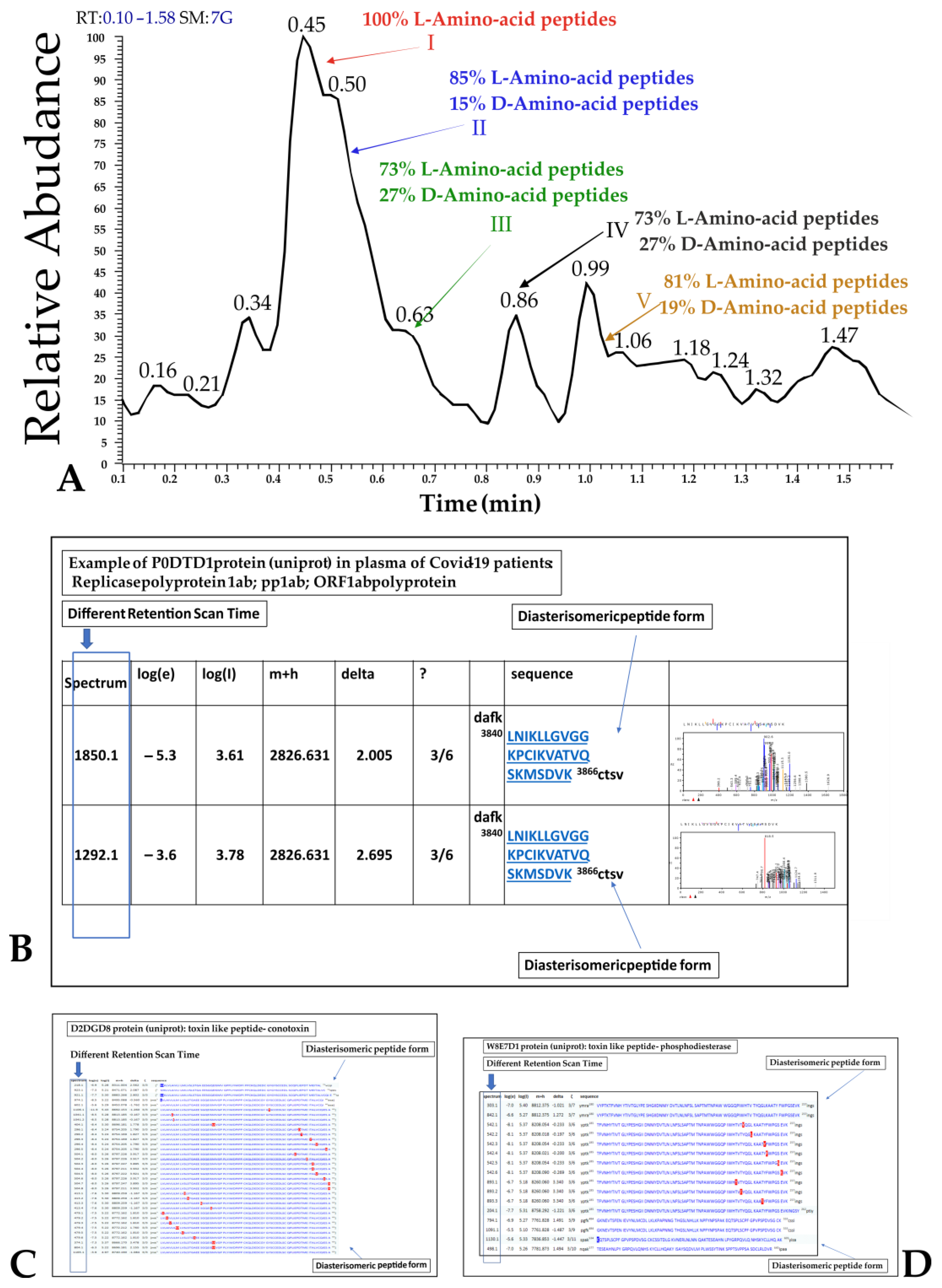
2.3. D-Amino Acid Presence at Mass Spectrometry in Plasma
2.4. Electron Microscopy
3. Discussion
4. Materials and Methods
4.1. Virus Growth in Bacterial Cultures and Sample Preparation
4.1.1. Collection and Storage of Fecal Samples
4.1.2. Initial Stool Samples A and neg-B Processing
4.1.3. Medium Preparation
4.1.4. Bacterial Growth
4.1.5. Nucleic Acid Extraction
4.1.6. Evaluation of SARS-CoV-2 RNA Load
4.1.7. Creation of Samples B and C
4.1.8. Supernatant Collection
4.1.9. Dorea formicigenerans Strains Growth
4.1.10. Plasma Collection [37]
4.2. Immunofluorescence
4.3. Proteomic Profiling Analysis of Bacteria Cultures for Spectral Counting and for D-Amino Acid Presence Observation
4.3.1. Preparation of Buffers for Each Supernatant of Bacterial Culture Aliquot
4.3.2. Preparation of Reagents
4.3.3. Liquid Chromatography Surface-Activated Chemical Ionization—Cloud Ion Mobility Mass Spectrometry (LC-SACI-CIMS) Instrumentation [77,78,79,80]
4.3.4. Plasma Preparation
4.4. Internal Control with Electron Microscopy, Figure 2-Panel E–G
Immunogold Labelling Technique, Figure 2-Panel E–G
4.5. Statistical Immunofluorescence Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mardian, Y.; Kosasih, H.; Karyana, M.; Neal, A.; Lau, C.-Y. Review of Current COVID-19 Diagnostics and Opportunities for Further Development. Front. Med. 2021, 8, 615099. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Chang, L.; Wang, L. Laboratory testing of SARS-CoV, MERS-CoV, and SARS-CoV-2 (2019-nCoV): Current status, challenges, and countermeasures. Rev. Med. Virol. 2020, 30, e2106. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Cheng, M.; Chen, X.; Zhu, J. Current approaches in laboratory testing for SARS-CoV-2. Int. J. Infect. Dis. 2020, 100, 7–9. [Google Scholar] [CrossRef]
- Laboratory Testing for 2019 Novel Coronavirus (2019-nCoV) in Suspected Human Cases. Available online: https://www.who.int/publications-detail-redirect/10665-331501 (accessed on 17 January 2020).
- Tsujimoto, Y.; Teradaa, J.; Kimurab, M.; Moriyac, A.; Motohashic, A.; Izumia, S.; Kawajiria, K.; Hakkakua, K.; Morishitaa, M.; Saitoa, S.; et al. Diagnostic accuracy of nasopharyngeal swab, nasal swab and saliva swab samples for the detection of SARS-CoV-2 using RT-PCR. Infect. Dis. 2021, 53, 581–589. [Google Scholar] [CrossRef]
- Porte, L.; Legarraga, P.; Vollrath, V.; Aguilera, X.; Munita, J.M.; Araos, R.; Pizarro, G.; Vial, P.; Iruretagoyena, M.; Dittrich, S.; et al. Evaluation of a novel antigen-based rapid detection test for the diagnosis of SARS-CoV-2 in respiratory samples. Int. J. Infect. Dis. 2020, 99, 328–333. [Google Scholar] [CrossRef] [PubMed]
- LeGoff, J.; Kernéis, S.; Elie, C.; Mercier-Delarue, S.; Gastli, N.; Choupeaux, L.; Fourgeaud, J.; Alby, M.-L.; Quentin, P.; Pavie, J.; et al. Evaluation of a saliva molecular point of care for the detection of SARS-CoV-2 in ambulatory care. Sci. Rep. 2021, 11, 21126. [Google Scholar] [CrossRef]
- Amendola, A.; Sberna, G.; Lalle, E.; Colavita, F.; Castilletti, C.; Menchinelli, G.; Posteraro, B.; Sanguinetti, M.; Ippolito, G.; Bordi, L.; et al. Saliva Is a Valid Alternative to Nasopharyngeal Swab in Chemiluminescence-Based Assay for Detection of SARS-CoV-2 Antigen. J. Clin. Med. 2021, 10, 1471. [Google Scholar] [CrossRef]
- Sun, Q.; Li, J.; Ren, H.; Pastor, L.; Loginova, Y.; Madej, R.; Taylor, K.; Wong, J.K.; Zhang, Z.; Zhang, A.; et al. Saliva as a testing specimen with or without pooling for SARS-CoV-2 detection by multiplex RT-PCR test. PLoS ONE 2021, 16, e0243183. [Google Scholar] [CrossRef]
- Peña, M. Performance of SARS-CoV-2 rapid antigen test compared with real-time RT-PCR in asymptomatic individuals. Int. J. Infect. Dis. 2021, 107, 201–204. [Google Scholar] [CrossRef]
- Rahimi, A.; Mirzazadeh, A.; Tavakolpour, S. Genetics and genomics of SARS-CoV-2: A review of the literature with the special focus on genetic diversity and SARS-CoV-2 genome detection. Genomics 2021, 113, 1221–1232. [Google Scholar] [CrossRef]
- Van Balken JA, M.; De Leeuw, P.W.; Ellens, D.J.; Straver, P.J. Detection of coronavirus in calf faeces with a haemadsorption-elution-haemagglutination assay (HEHA). Vet. Microbiol. 1979, 3, 205–211. [Google Scholar] [CrossRef]
- Foster, L.G.; Peterson, M.W.; Spendlove, R.S. Fluorescent virus precipitin test. Proc. Soc. Exp. Biol. Med. 1975, 150, 155–160. [Google Scholar] [CrossRef]
- Peterson, M.W.; Spendlove, R.S.; Smart, R.A. Detection of neonatal calf diarrhea virus, infant reovirus-like diarrhea virus, and a coronavirus using the fluorescent virus precipitin test. J. Clin. Microbiol. 1976, 3, 376–377. [Google Scholar] [CrossRef]
- Roy, S.S.; Hajnóczky, G. Calcium, mitochondria and apoptosis studied by fluorescence measurements. Methods 2008, 46, 213–223. [Google Scholar] [CrossRef]
- Astruc, T.; Marinova, P.; Labas, R.; Gatellier, P.; Santé-Lhoutellier, V. Detection and localization of oxidized proteins in muscle cells by fluorescence microscopy. J. Agric. Food Chem. 2007, 55, 9554–9558. [Google Scholar] [CrossRef] [PubMed]
- Petrillo, F.; Iervolino, A.; Angrisano, T.; Jele, S.; Costanzo, V.; D’Acierno, M.; Cheng, L.; Wu, Q.; Guerriero, I.; Mazzarella, M.C.; et al. Dysregulation of Principal Cell miRNAs Facilitates Epigenetic Regulation of AQP2 and Results in Nephrogenic Diabetes Insipidus. J. Am. Soc. Nephrol. JASN 2021, 32, 1339–1354. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, V.; D’Apolito, L.; Sardella, D.; Iervolino, A.; La Manna, G.; Capasso, G.; Frische, S.; Trepiccion, F. Single nephron glomerular filtration rate measured by linescan multiphoton microscopy compared to conventional micropuncture. Pflug. Arch. Eur. J. Physiol. 2022, 474, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Engbjerg, J.S.; Costanzo, V.; Sardella, D.; Bordoni, L.; Jakobsen, S.; D’Apolito, L.; Frøkiær, J.; Trepiccione, F.; Capasso, G.; Frische, S. The Probe for Renal Organic Cation Secretion (4-Dimethylaminostyryl)-N-Methylpyridinium (ASP+)) Shows Amplified Fluorescence by Binding to Albumin and Is Accumulated In Vivo. Molecularimaging 2022, 2022, 7908357. [Google Scholar] [CrossRef]
- Chagnon, F.; Fournier, C.; Charette, P.G.; Moleski, L.; Payet, M.D.; Dobbs, L.G.; Lesur, O. In vivo intravital endoscopic confocal fluorescence microscopy of normal and acutely injured rat lungs. Lab. Investig. A J. Tech. Methods Pathol. 2010, 90, 824–834. [Google Scholar] [CrossRef]
- Prosperi, F.; Suzumoto, Y.; i Marzuillo, P.; Costanzo, V.; Jelen, S.; Iervolino, A.; Guarino, S.; La Manna, A.; Del Giudice, E.M.; Perna, A.; et al. Characterization of five novel vasopressin V2 receptor mutants causing nephrogenic diabetes insipidus reveals a role of tolvaptan for M272R-V2R mutation. Sci. Rep. 2020, 10, 16383. [Google Scholar] [CrossRef]
- Fujimura, H.; Dekura, E.; Kurabe, M.; Shimazu, N.; Koitabashi, M.; Toriumi, W. Cell-based fluorescence assay for evaluation of new-drugs potential for phospholipidosis in an early stage of drug development. Exp. Toxicol. Pathol. Off. J. Ges. Fur Toxikol. Pathol. 2007, 58, 375–382. [Google Scholar] [CrossRef]
- Gomes, I. SARS-CoV-2 infection of the central nervous system in a 14-month-old child: A case report of a complete autopsy. Lancet Reg. Health –Am. 2021, 2, 100046. [Google Scholar] [CrossRef] [PubMed]
- Ogando, N.S.; Dalebout, T.J.; Zevenhoven-Dobbe, J.C.; Limpens, R.W.A.L.; van der Meer, Y.; Caly, L.; Druce, J.; de Vries, J.J.C.; Kikkert, M.; Bárcena, M.; et al. SARS-coronavirus-2 replication in Vero E6 cells: Replication kinetics, rapid adaptation and cytopathology. J. Gen. Virol. 2020, 101, 925–940. [Google Scholar] [CrossRef]
- Putlyaeva, L.V.; Lukyanov, K.A. Studying SARS-CoV-2 with Fluorescence Microscopy. Int. J. Mol. Sci. 2021, 22, 6558. [Google Scholar] [CrossRef]
- Mondeja, B.; Valdes, O.; Resik, S.; Vizcaino, A.; Acosta, E.; Montalván, A.; Paez, A.; Mune, M.; Rodríguez, R.; Valdés, J.; et al. SARS-CoV-2: Preliminary study of infected human nasopharyngeal tissue by high resolution microscopy. Virol. J. 2021, 18, 149. [Google Scholar] [CrossRef]
- Petrillo, M.; Brogna, C.; Cristoni, S.; Querci, M.; Piazza, O.; Van den Eede, G. Increase of SARS-CoV-2 RNA load in faecal samples prompts for rethinking of SARS-CoV-2 biology and COVID-19 epidemiology. F1000Research 2021, 10, 370. [Google Scholar] [CrossRef]
- Brogna, C. Could SARS-CoV-2 Have Bacteriophage Behavior or Induce the Activity of Other Bacteriophages? Vaccines 2022, 10, 708. [Google Scholar] [CrossRef]
- Ács, N.; Gambino, M.; Brøndsted, L. Bacteriophage Enumeration and Detection Methods. Front. Microbiol. 2020, 11, 594868. [Google Scholar] [CrossRef]
- Lundgren, D.H.; Hwang, S.I.; Wu, L.; Han, D.K. Role of spectral counting in quantitative proteomics. Expert. Rev. Proteomics. 2010, 7, 39–53. [Google Scholar] [CrossRef]
- Arike, L.; Peil, L. Spectral counting label-free proteomics. Methods Mol. Biol. 2014, 1156, 213–222. [Google Scholar] [CrossRef]
- Miyamoto, T.; Hiroshi, H. D-Amino acid metabolism in bacteria. J. Biochem. 2021, 170, 5–13. [Google Scholar] [CrossRef]
- Alvarez, L.; Aliashkevich, A.; de Pedro, M.A.; Cava, F. Bacterial secretion of D-arginine controls environmental microbial biodiversity. ISME J. 2018, 12, 438–450. [Google Scholar] [CrossRef] [PubMed]
- McKevitt, M.T.; Bryant, K.M.; Shakir, S.M.; Larabee, J.L.; Blanke, S.R.; Lovchik, J.; Lyons, C.R.; Ballard, J.D. Effects of endogenous D-alanine synthesis and autoinhibition of Bacillus anthracis germination on in vitro and in vivo infections. Infect. Immun. 2007, 75, 5726–5734. [Google Scholar] [CrossRef]
- Miyamoto, T.; Katane, M.; Saitoh, Y.; Sekine, M.; Homma, H. ystathionine β-lyase is involved in D-amino acid metabolism. Biochem. J. 2018, 475, 1397–1410. [Google Scholar] [CrossRef]
- Brogna, C.; Cristoni, S.; Petrillo, M.; Bisaccia, D.R.; Lauritano, F.; Montano, L.; Prisco, M.; Piscopo, M. The first report on detecting SARS-CoV-2 inside bacteria of the human gut microbiome: A case series on asymptomatic family members and a child with COVID-19 [version 2; peer review: 1 approved with reservations, 1 not approved]. F1000Research 2022, 11, 135. [Google Scholar] [CrossRef]
- Brogna, C.; Cristoni, S.; Petrillo, M.; Querci, M.; Piazza, O.; Van den Eede, G. Toxin-like peptides in plasma, urine and faecal samples from COVID-19 patients. F1000Research 2021, 10, 550. [Google Scholar] [CrossRef]
- Brogna, C.; Cristoni, S.; Brogna, B.; Bisaccia, D.R.; Marino, G.; Viduto, V.; Montano, L.; Piscopo, M. Toxin-like Peptides from the Bacterial Cultures Derived from Gut Microbiome Infected by SARS-CoV-2—New Data for a Possible Role in the Long COVID Pattern. Biomedicines 2023, 11, 87. [Google Scholar] [CrossRef]
- Petrillo, M.; Querci, M.; Brogna, C.; Ponti, J.; Cristoni, C.; Markov, P.V.; Valsesia, A.; Leoni, G.; Alessandro Benedetti, A.; Wiss, T.; et al. Evidence of SARS-CoV-2 bacteriophage potential in human gut microbiota [version 1; peer review: 1 approved with reservations]. F1000Research 2022, 11, 292. [Google Scholar] [CrossRef]
- Sokolowska, T.; Biernat, J. The chromatographic separation of diastereoisomeric dipeptides. J. Chromatogr. 1964, 13, 269–270. [Google Scholar] [CrossRef]
- Larsen, B.; Viswanatha, V.; Chang, S.Y.; Hruby, V.J. Reverse phase high pressure liquid chromatography for the separation of peptide hormone diastereoisomers. J. Chromatogr. Sci. 1978, 16, 207–210. [Google Scholar] [CrossRef]
- Ha, S.; Kim, I.; Takata, T.; Kinouchi, T.; Isoyama, M.; Suzuki, M.; Fujii, N. Identification of d-amino acid-containing peptides in human serum. PLoS ONE 2017, 12, e0189972. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, A.; Zlitni, S.; Brooks, E.F.; Vance, S.E.; Dahlen, A.; Hedlin, H.; Park, R.M.; Han, A.; Schmidtke, D.T.; Verma, R.; et al. Gastrointestinal symptoms and fecal shedding of SARS-CoV-2 RNA suggest prolonged gastrointestinal infection. Med 2022, 3, 371–387.e9. [Google Scholar] [CrossRef]
- Wang, Y.; Lenoch, J.; Kohler, D.; De Liberto, T.J.; Tang, C.; Li, T.; Tao, Y.J.; Guan, M.; Compton, S.; Zeiss, C.; et al. SARS-CoV-2 exposure in Norwegian rats (Rattus norvegicus) from New York City. bioRxiv 2022. [Google Scholar] [CrossRef]
- First Case of Postmortem Study in a Patient Vaccinated against SARS-CoV-2—International Journal of Infectious Diseases. Available online: https://www.ijidonline.com/article/S1201-9712(21)00364-7/fulltext (accessed on 16 April 2021).
- Brogna, B.; Bignardi, E.; Brogna, C.; Capasso, C.; Gagliardi, G.; Martino, A.; Musto, L.A. COVID-19 Pneumonia in Vaccinated Population: A Six Clinical and Radiological Case Series. Medicina 2021, 57, 891. [Google Scholar] [CrossRef]
- Jones, D.L.; Baluja, M.Q.; Graham, D.W.; Corbishley, A.; McDonald, J.E.; Malham, S.K.; Hillary, L.S.; Connor, T.R.; Gaze, W.H.; Moura, I.B.; et al. Shedding of SARS-CoV-2 in feces and urine and its potential role in person-to-person transmission and the environment-based spread of COVID-19. Sci. Total Environ. 2020, 749, 141364. [Google Scholar] [CrossRef]
- Brogna, B.; Brogna, C.; Petrillo, M.; Conte, A.M.; Benincasa, G.; Montano, L.; Piscopo, M. SARS-CoV-2 Detection in Fecal Sample from a Patient with Typical Findings of COVID-19 Pneumonia on CT but Negative to Multiple SARS-CoV-2 RT-PCR Tests on Oropharyngeal and Nasopharyngeal Swab Samples. Medicina 2021, 57, 290. [Google Scholar] [CrossRef]
- Brogna, C.; Brogna, B.; Bisaccia, D.R.; Giuliano, M.; Montano, L.; Cristoni, S.; Petrillo, M.; Piscopo, M. SARS-CoV-2: Reinfection after 18 Months of a Previous Case with Multiple Negative Nasopharyngeal Swab Tests and Positive Fecal Molecular Test. Medicina 2022, 58, 642. [Google Scholar] [CrossRef]
- Xie, G.; Yu, J.; Duan, Z. New strategy for virus discovery: Viruses identified in human feces in the last decade. Sci. China Life Sci. 2013, 56, 688–696. [Google Scholar] [CrossRef]
- Chen, Y.; Yao, H.; Fu, J.; Shu, Q.; Chen, Z.; Wu, N.; Ye, S.; Wang, W.; Ni, Y.; Shang, S.; et al. The low contagiousness and new A958D mutation of SARS-CoV-2 in children: An observational cohort study. Int. J. Infect. Dis. 2021, 111, 347–353. [Google Scholar] [CrossRef]
- Geva-Zatorskyl, N.; Sefik, E.; Kua, L.; Pasman, L.; Tan, T.G.; Ortiz-Lopez, A.; Yanortsang, T.B.; Yang, L.; Jupp, R.; Mathis, D.; et al. Mining the human gut microbiota for immunomodulatory organisms. Cell 2017, 168, 928–943.e11. [Google Scholar] [CrossRef] [PubMed]
- Sundararaman, A.; Ray, M.; Ravindra, P.V.; Halami, P.M. Role of probiotics to combat viral infections with emphasis on COVID-19. Appl. Microbiol. Biotechnol. 2020, 104, 8089–8104. [Google Scholar] [CrossRef]
- Bernard-Raichon, L.; Venzon, M.; Klein, J.; Axelrad, J.E.; Zhang, C.; Sullivan, A.P.; Hussey, G.A.; Casanovas-Massana, A.; Noval, M.G.; Valero-Jimenez, A.M.; et al. Gut microbiome dysbiosis in antibiotic-treated COVID-19 patients is associated with microbial translocation and bacteremia. Nat. Commun. 2022, 13, 5926. [Google Scholar] [CrossRef]
- Blanchard, A.; Montagnier, L. AIDS-associated mycoplasmas. Annu. Rev. Microbiol. 1994, 48, 687–712. [Google Scholar] [CrossRef]
- Brenner, C.; Neyrolles, O.; Blanchard, A. Mycoplasmas and HIV infection: From epidemiology to their interaction with immune cells. Front. Biosci. A J. Virtual Libr. 1996, 1, e42–e54. [Google Scholar] [CrossRef]
- Honarmand Ebrahimi, K. SARS-CoV-2 spike glycoprotein-binding proteins expressed by upper respiratory tract bacteria may prevent severe viral infection. FEBS Lett. 2020, 594, 1651–1660. [Google Scholar] [CrossRef]
- Langford, B.J. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef]
- Singh, V.; Upadhyay, P.; Reddy, J.; Granger, J. SARS-CoV-2 respiratory co-infections: Incidence of viral and bacterial co-pathogens. Int. J. Infect. Dis. 2021, 105, 617–620. [Google Scholar] [CrossRef]
- Russell, M.W.; Moldoveanu, Z.; Ogra, P.L.; Mestecky, J. Mucosal Immunity in COVID-19: A Neglected but Critical Aspect of SARS-CoV-2 Infection. Front. Immunol. 2020, 11, 611337. [Google Scholar] [CrossRef]
- Tugizov, S.M.; Herrera, R.; Chin-Hong, P.; Veluppillai, P.; Greenspan, D.; Michael Berry, J.; Pilcher, C.D.; Shiboski, C.H.; Jay, N.; Rubin, M.; et al. HIV-associated disruption of mucosal epithelium facilitates paracellular penetration by human papillomavirus. Virology 2013, 446, 378–388. [Google Scholar] [CrossRef]
- Shafran, N.; Shafran, I.; Ben-Zvi, H.; Sofer, S.; Sheena, L.; Krause, I.; Shlomai, A.; Goldberg, E.; Sklan, E.H. Secondary bacterial infection in COVID-19 patients is a stronger predictor for death compared to influenza patients. Sci. Rep. 2021, 11, 12703. [Google Scholar] [CrossRef]
- Zajac, V.; Matelova, L.; Liskova, A.; Mego, M.; Holec, V.; Adamcikova, Z.; Stevurkova, V.; Shahum, A.; Krcmery, V. Confirmation of HIV-like sequences in respiratory tract bacteria of Cambodian and Kenyan HIV-positive pediatric patients. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2011, 17, CR154–CR158. [Google Scholar] [CrossRef]
- Hainova, K.; Adamcikova, Z.; Ciernikova, S.; Stevurkova, V.; Krčméry, V.; Zajac, V. Detection of protein homologs with HIV-1 antigens in bacteria of positive patients—Phase II. Neuro Endocrinol. Lett. 2014, 35, 110–115. [Google Scholar]
- Barré-Sinoussi, F.; Chermann, J.C.; Rey, F.; Nugeyre, M.T.; Chamaret, S.; Gruest, J.; Dauguet, C.; Axler-Blin, B.; Vézinet-Brun, F.; Rouzioux, C.; et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 1983, 220, 868–871. [Google Scholar] [CrossRef]
- Kuss, S.K.; Best, G.T.; Etheredge, C.A.; Pruijssers, A.J.; Frierson, J.M.; Hooper, L.V.; Dermody, T.S.; Pfeiffer, J.K. Intestinal microbiota promote enteric virus replication and systemic pathogenesis. Science 2011, 334, 249–252. [Google Scholar] [CrossRef]
- Borgognone, A.; Noguera-Julian, M.; Oriol, B.; Noël-Romas, L.; Ruiz-Riol, M.; Guillén, Y.; Parera, M.; Casadellà, M.; Duran, C.; Puertas, M.C.; et al. Gut microbiome signatures linked to HIV-1 reservoir size and viremia control. Microbiome 2022, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Sabin, A.B. Pathogenesis of poliomyelitis; reappraisal in the light of new data. Science 1956, 123, 1151–1157. [Google Scholar] [CrossRef]
- Sabin, A.B. Behaviour of Chimpanzee-avirulent Poliomyelitis Viruses in Experimentally Infected Human Volunteers. Br. Med. J. 1955, 2, 160–162. [Google Scholar] [CrossRef] [PubMed]
- Sabin, A.B. Present status of attenuated live virus poliomyelitis vaccine. Bull. N. Y. Acad. Med. 1957, 33, 17–39. [Google Scholar] [CrossRef]
- Sabin, A.B. Oral Poliovirus Vaccine: Recent results and recommendations for optimum use. R. Soc. Health J. 1962, 82, 51–59. [Google Scholar] [CrossRef]
- Nomoto, A.; Omata, T.; Toyoda, H.; Kuge, S.; Horie, H.; Kataoka, Y.; Genba, Y.; Nakano, Y.; Imura, N. Complete nucleotide sequence of the attenuated poliovirus Sabin 1 strain genome. Proc. Natl. Acad. Sci. USA 1982, 79, 5793–5797. [Google Scholar] [CrossRef]
- Floridia, M.; Cristoni, S. PROSAD: A powerful platform for instrument calibration and quantification. Rapid Commun. Mass Spectrom. 2014, 28, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Kohda, K.; Li, X.; Soga, N.; Nagura, R.; Duerna, T.; Nakajima, S.; Nakagawa, I.; Ito, M.; Ikeuchi, A. An In Vitro Mixed Infection Model With Commensal and Pathogenic Staphylococci for the Exploration of Interspecific Interactions and Their Impacts on Skin Physiology. Front. Cell Infect. Microbiol. 2021, 11, 712360. [Google Scholar] [CrossRef] [PubMed]
- Kameli, N.; Borman, R.; Lpez-Iglesias, C.; Savelkoul, P.; Stassen, F.R.M. Characterization of Feces-Derived Bacterial Membrane Vesicles and the Impact of Their Origin on the Inflammatory Response. Front. Cell Infect. Microbiol. 2021, 11, 667987. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Ni, C.; Gao, R.; Wang, Y.; Yang, L.; Wei, J.; Lv, T.; Liang, J.; Zhang, Q.; Xu, W. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell 2020, 11, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Arzoni, A.; Bernardi, L.R.; Cristoni, S. In-source cloud ion mobility mass spectrometry. Rapid Commun. Mass Spectrom. 2015, 29, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Cristoni, S.; Dusi, G.; Brambilla, P.; Albini, A.; Conti, M.; Brambilla, M.; Bruno, A.; Di Gaudio, F.; Ferlin, L.; Tazzari, V.; et al. SANIST: Optimization of a technology for compound identification based on the European Union directive with applications in forensic, pharmaceutical and food analyses. J. Mass Spectrom. 2017, 52, 16–21. [Google Scholar] [CrossRef]
- Cristoni, S.; Rossi Bernardi, L.; Larini, M.; Natale, G.; Didomenico, N.; Varell, i.M.; Conti, M.; Dorna, I.; Puccio, G. Predicting and preventing intestinal dysbiosis on the basis of pharmacological gut microbiota metabolism. Rapid Commun. Mass Spectrom. 2019, 33, 1221–1225. [Google Scholar] [CrossRef]
- Albini, A.; Briga, D.; Conti, M.; Bruno, A.; Farioli, D.; Canali, S.; Sogno, I.; D’Ambrosio, G.; Consonni, P.; Noonan, D.M. SANIST: A rapid mass spectrometric SACI/ESI data acquisition and elaboration platform for verifying potential candidate biomarkers. Rapid Commun. Mass Spectrom. 2015, 29, 1703–1710. [Google Scholar] [CrossRef]
| Time (Minutes) | %C | Flow mL/min |
|---|---|---|
| 0 | 2 | 0.250 |
| 2.5 | 2 | 0.250 |
| 3 | 80 | 0.250 |
| 7 | 80 | 0.250 |
| 8 | 2 | 0.250 |
| 15 | 2 | 0.250 |
| ID Protein | Full Name |
|---|---|
| P0DTD1 | R1AB_SARS2 Replicase polyprotein 1ab[…] rep, 1a-1b Severe acute respiratory syndrome coronavirus 2 (2019-nCoV) (SARS-CoV-2) |
| P0DTC9 | NCAP_SARS2 Nucleoprotein[...] N Severe acute respiratory syndrome coronavirus 2 (2019-nCoV) (SARS-CoV-2) |
| P0DTC2 | SPIKE_SARS2 Spike glycoprotein[...] S, 2 Severe acute respiratory syndrome Coronavirus 2 (2019-nCoV) (SARS-CoV-2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brogna, C.; Costanzo, V.; Brogna, B.; Bisaccia, D.R.; Brogna, G.; Giuliano, M.; Montano, L.; Viduto, V.; Cristoni, S.; Fabrowski, M.; et al. Analysis of Bacteriophage Behavior of a Human RNA Virus, SARS-CoV-2, through the Integrated Approach of Immunofluorescence Microscopy, Proteomics and D-Amino Acid Quantification. Int. J. Mol. Sci. 2023, 24, 3929. https://doi.org/10.3390/ijms24043929
Brogna C, Costanzo V, Brogna B, Bisaccia DR, Brogna G, Giuliano M, Montano L, Viduto V, Cristoni S, Fabrowski M, et al. Analysis of Bacteriophage Behavior of a Human RNA Virus, SARS-CoV-2, through the Integrated Approach of Immunofluorescence Microscopy, Proteomics and D-Amino Acid Quantification. International Journal of Molecular Sciences. 2023; 24(4):3929. https://doi.org/10.3390/ijms24043929
Chicago/Turabian StyleBrogna, Carlo, Vincenzo Costanzo, Barbara Brogna, Domenico Rocco Bisaccia, Giancarlo Brogna, Marino Giuliano, Luigi Montano, Valentina Viduto, Simone Cristoni, Mark Fabrowski, and et al. 2023. "Analysis of Bacteriophage Behavior of a Human RNA Virus, SARS-CoV-2, through the Integrated Approach of Immunofluorescence Microscopy, Proteomics and D-Amino Acid Quantification" International Journal of Molecular Sciences 24, no. 4: 3929. https://doi.org/10.3390/ijms24043929
APA StyleBrogna, C., Costanzo, V., Brogna, B., Bisaccia, D. R., Brogna, G., Giuliano, M., Montano, L., Viduto, V., Cristoni, S., Fabrowski, M., & Piscopo, M. (2023). Analysis of Bacteriophage Behavior of a Human RNA Virus, SARS-CoV-2, through the Integrated Approach of Immunofluorescence Microscopy, Proteomics and D-Amino Acid Quantification. International Journal of Molecular Sciences, 24(4), 3929. https://doi.org/10.3390/ijms24043929








