Voghera Sweet Pepper: A Potential Ally against Oxidative Stress and Aging
Abstract
1. Introduction
2. Results
2.1. Chemical Characterization
2.2. Validation of the Replicative Senescent Model
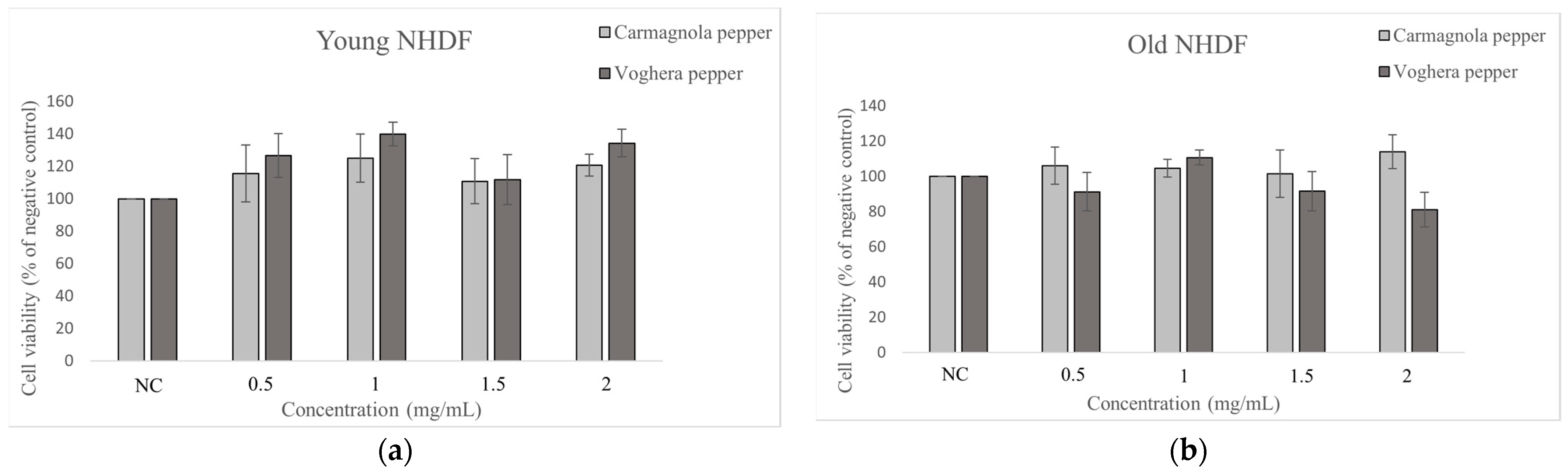
2.3. MTT Assay
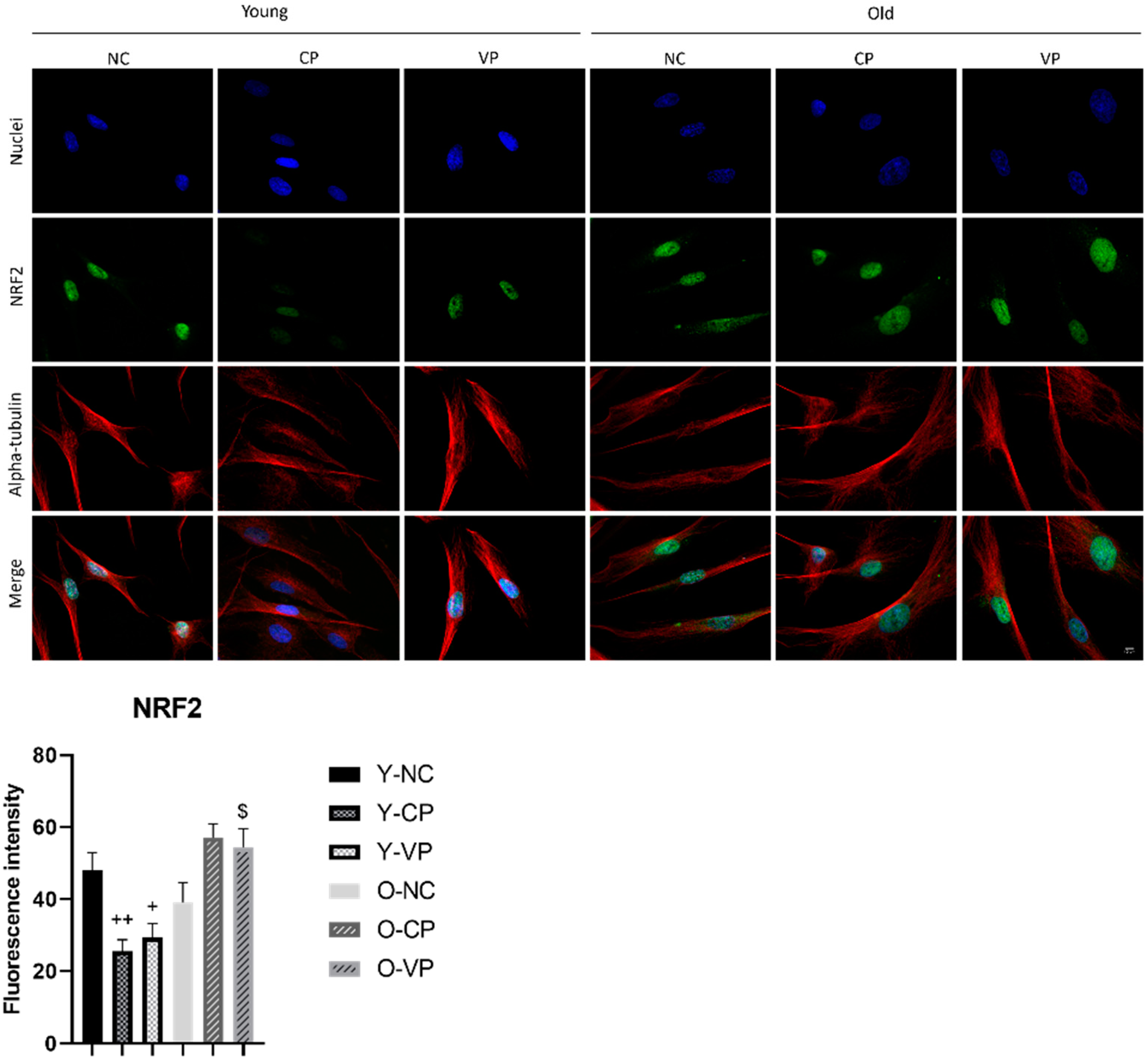
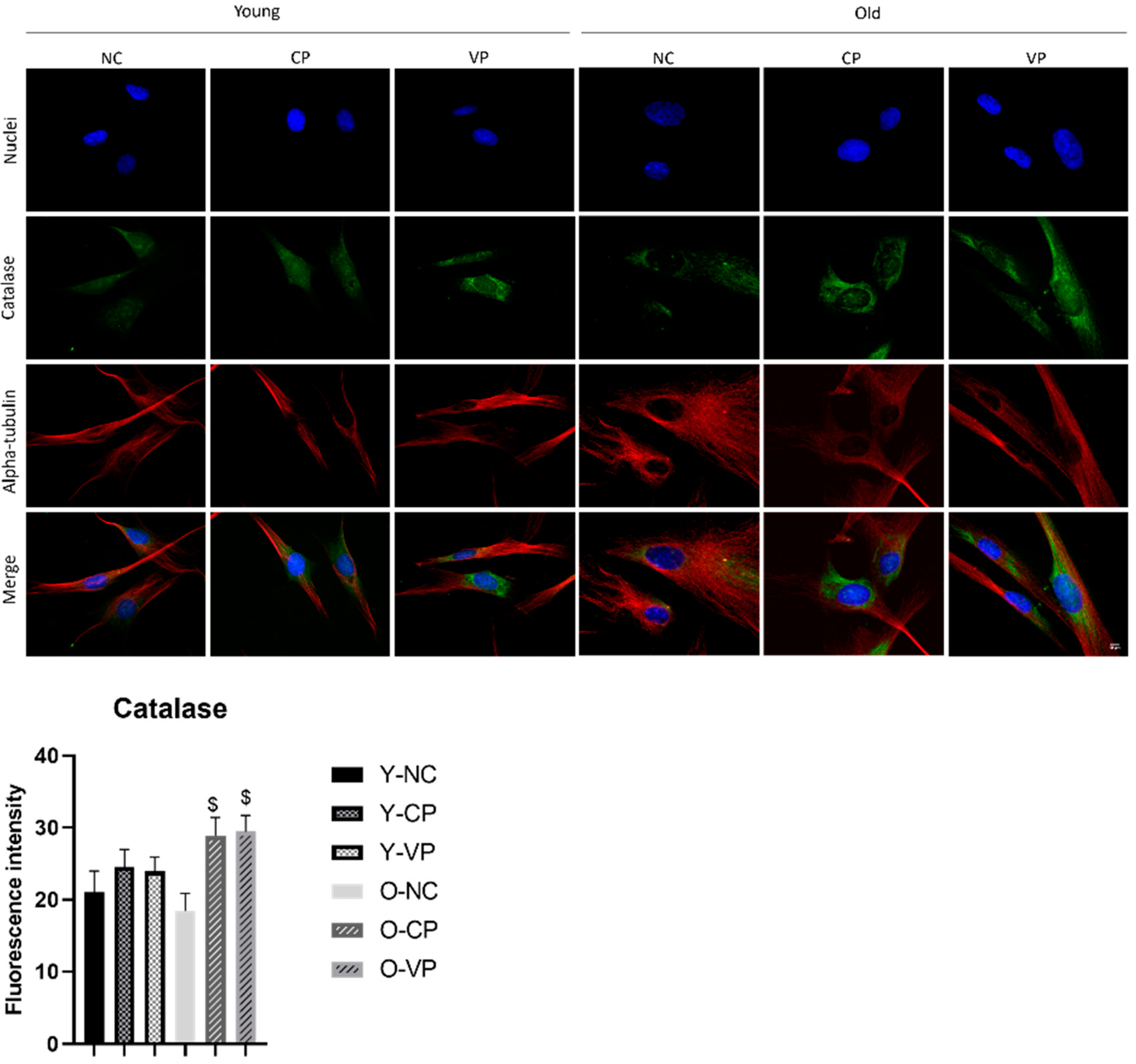
2.4. Antioxidant Activity
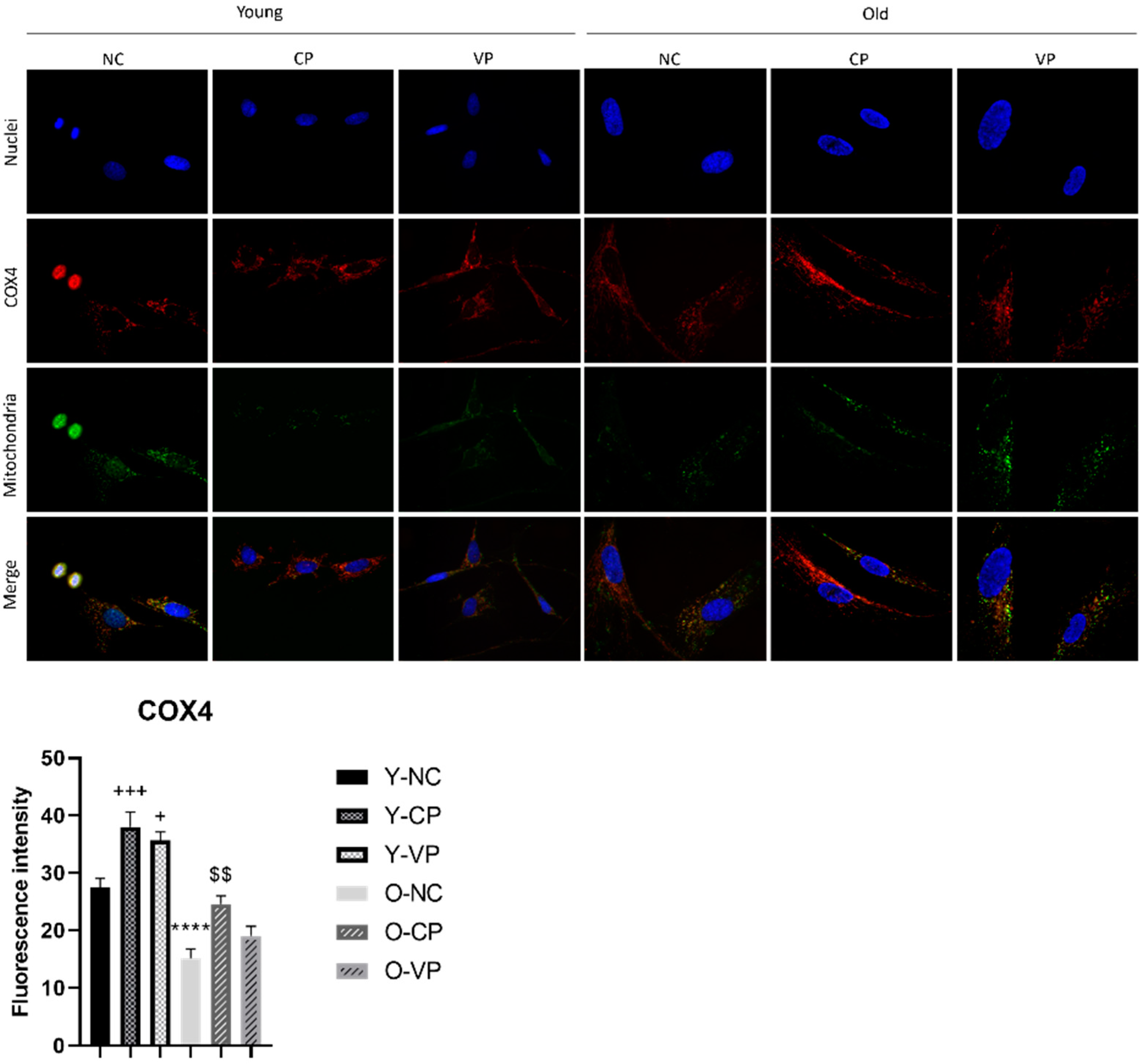
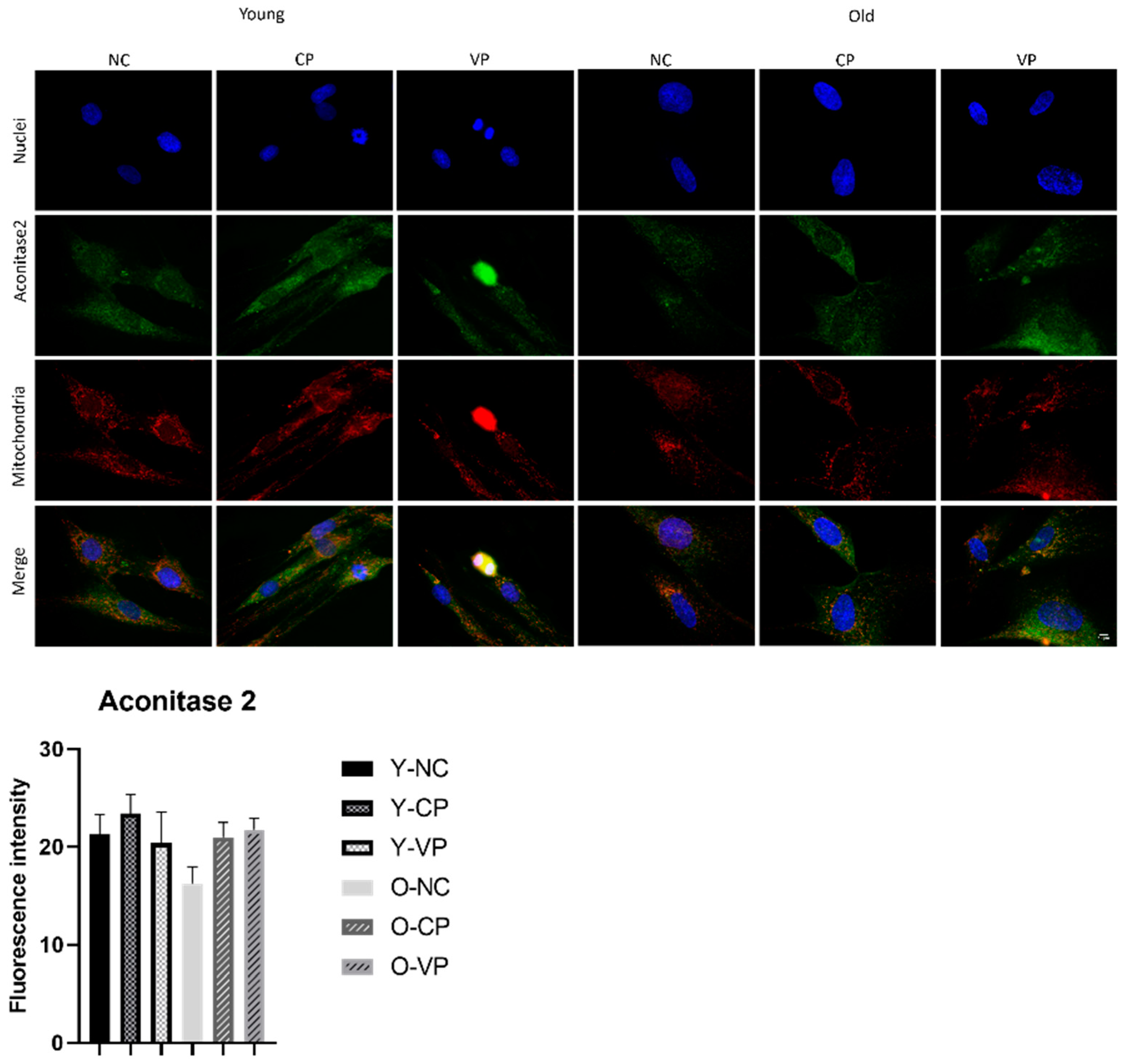
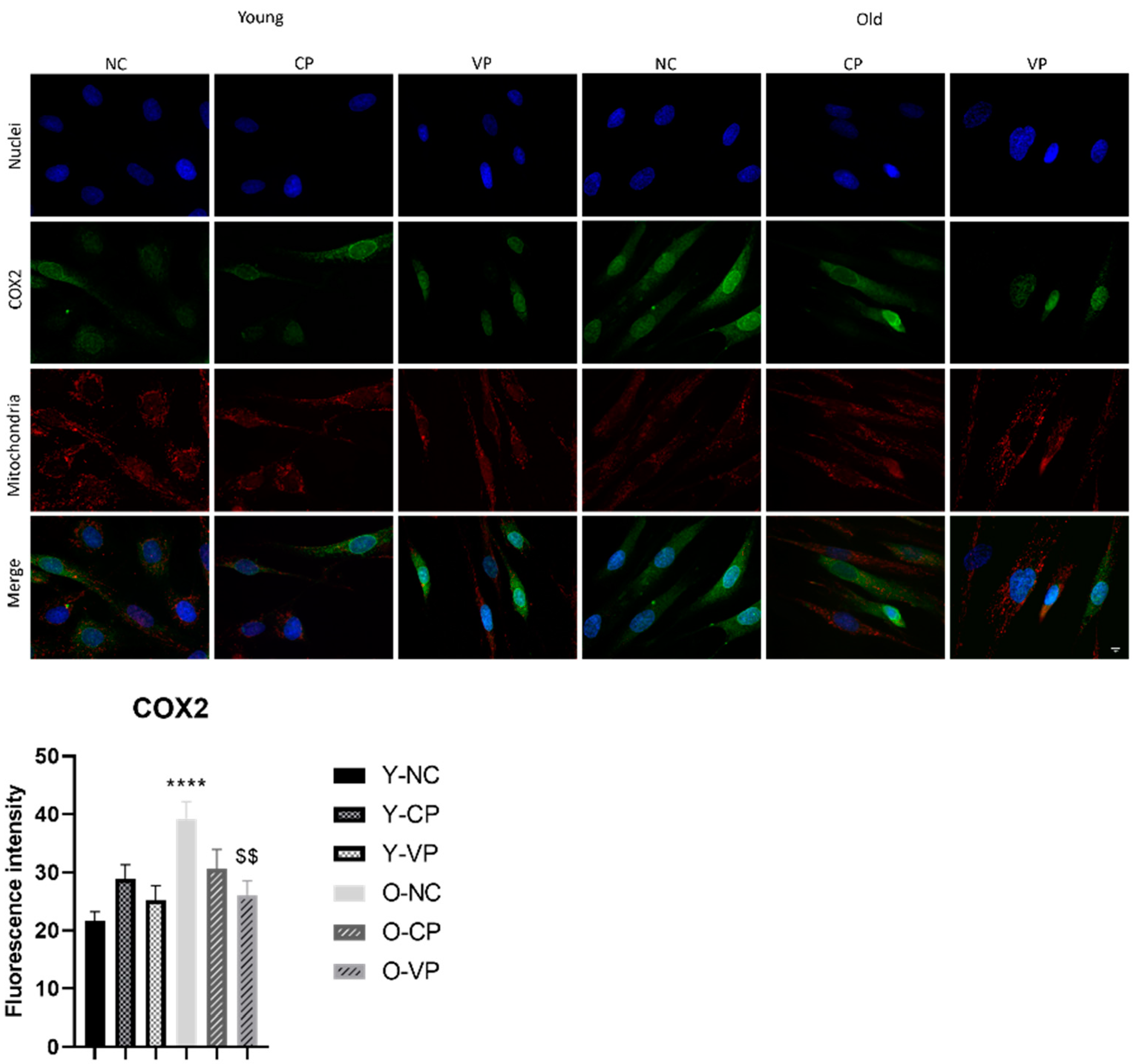
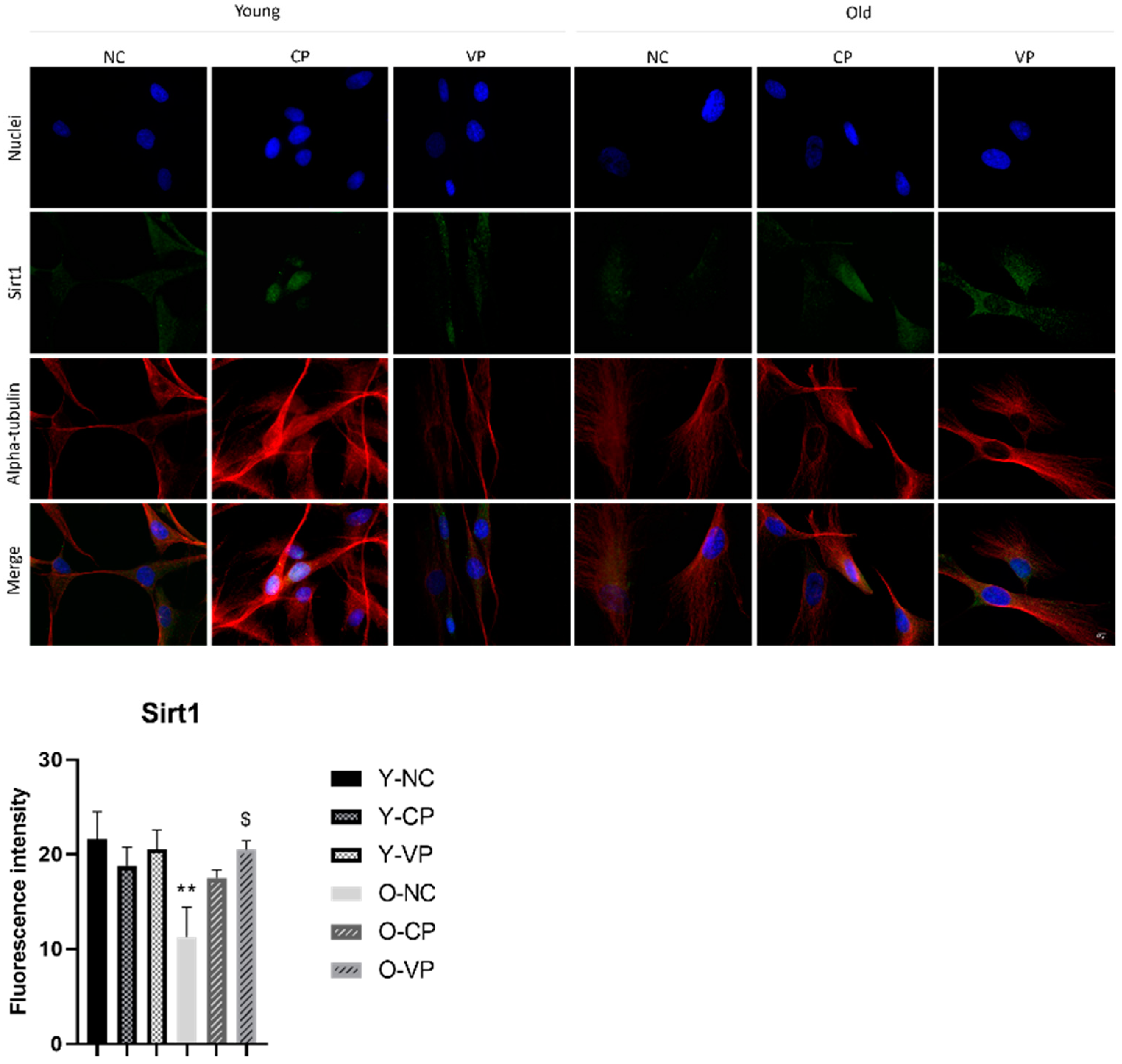
2.5. Evaluation of the Antiaging Properties
3. Discussion
4. Materials and Methods
4.1. Extracts Preparation and Characterization
4.2. Cell Cultures and Treatments
4.3. Cell Viability
4.4. Immunofluorescence Reactions
4.5. Immunofluorescence Quantification
4.6. Statistical Analyzes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Da Costa, J.P.; Vitorino, R.; Silva, G.M.; Vogel, C.; Duarte, A.C.; Rocha-Santos, T.A. Synopsis on aging-Theories, mechanisms and future prospects. Ageing Res. Rev. 2016, 29, 90–112. [Google Scholar] [CrossRef] [PubMed]
- Cencioni, C.; Spallotta, F.; Martelli, F.; Valente, S.; Mai, A.; Zeiher, A.M.; Gaetano, C. Oxidative stress and epigenetic regulation in ageing and age-related diseases. Int. J. Mol. Sci. 2013, 14, 17643–17663. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Zhou, T.; Pannell, B.K.; Ziegler, A.C.; Best, T.M. Biological and physiological role of reactive oxygen species—The good, the bad and the ugly. Acta Physiol. 2015, 214, 329–348. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.; Rahman, H.S. Antioxidant and oxidative stress: A mutual interplay in age-related diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Cino, E.A.; Wong-ekkabut, J.; Karttunen, M.; Choy, W.Y. Microsecond molecular dynamics simulations of intrinsically disordered proteins involved in the oxidative stress response. PLoS ONE 2011, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Scandalios, J.G. Oxidative stress: Molecular perception and transduction of signals triggering antioxidant gene defenses. Braz. J. Med. Biol. Res. 2005, 38, 7. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Arancibia-Hernández, Y.L.; Hernández-Cruz, E.Y.; Pedraza-Chaverri, J. RONS and Oxidative Stress: An Overview of Basic Concepts. Oxygen 2022, 2, 437–478. [Google Scholar] [CrossRef]
- Gladyshev, V.N. The free radical theory of aging is dead. Long live the damage theory! Antioxid. Redox Signal. 2014, 20, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Sobenin, I.A.; Revin, V.V.; Orekhov, A.N.; Bobryshev, Y.V. Mitochondrial aging and age-related dysfunction of mitochondria. BioMed Res. Int. 2014, 2014, 238463. [Google Scholar] [CrossRef] [PubMed]
- Belenguer-Varea, Á.; Tarazona-Santabalbina, F.J.; Avellana-Zaragoza, J.A.; Martínez-Reig, M.; Mas-Bargues, C.; Inglés, M. Oxidative stress and exceptional human longevity: Systematic review. Free Radic. Biol. Med. 2020, 149, 51–63. [Google Scholar] [CrossRef] [PubMed]
- El Assar, M.; Angulo, J.; Rodríguez-Mañas, L. Frailty as a phenotypic manifestation of underlying oxidative stress. Free Radic. Biol. Med. 2020, 149, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Si, H.; Jia, Z.; Liu, D. Dietary Anti-Aging Polyphenols and Potential Mechanisms. Antioxidants 2021, 10, 283. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Shanaida, M.; Lysiuk, R.; Butnariu, M.; Peana, M.; Sarac, I.; Strus, O.; Smetanina, K.; Chirumbolo, S. Natural Compounds and Products from an Anti-Aging Perspective. Molecules 2022, 27, 7084. [Google Scholar] [CrossRef]
- Rietjens, I.M.; Boersma, M.G.; de Haan, L.; Spenkelink, B.; Awad, H.M.; Cnubben, N.H.P.; van Zanden, J.J.; van der Woude, H.; Alink, G.M.; Koeman, J.H. The pro-oxidant chemistry of the natural antioxidants vitamin C, vitamin E, carotenoids and flavonoids. Environ. Toxicol. Pharmacol. 2002, 11, 321–333. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, J.; Shin, D.W. The Molecular Mechanism of Polyphenols with Anti-Aging Activity in Aged Human Dermal Fibroblasts. Molecules 2022, 27, 4351. [Google Scholar] [CrossRef]
- Rolt, A.; Cox, L.S. Structural basis of the anti-ageing effects of polyphenolics: Mitigation of oxidative stress. BMC Chem. 2020, 14, 50. [Google Scholar] [CrossRef]
- Chávez-Mendoza, C.; Sánchez, E.; Munõz-Márquez, E.; Sida-Arreola, J.P.; Flores-Cordova, M. Bioactive compounds and antioxidant activity in different grafted varieties of bell pepper. Antioxidants 2015, 4, 427–446. [Google Scholar] [CrossRef]
- Martínez-Ispizua, E.; Martínez-Cuenca, M.R.; Marsal, J.I.; Díez, M.J.; Soler, S.; Valcárcel, J.V.; Calatayud, Á. Bioactive Compounds and Antioxidant Capacity of Valencian Pepper Landraces. Molecules 2021, 26, 1031. [Google Scholar] [CrossRef]
- Jain, S.M.; Gupta, S.D. Biotechnology of Neglected and Underutilized Crops; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Samuels, J. The Solanaceae-novel crops with high potential. Org. Grow. 2009, 9, 32–34. [Google Scholar]
- Cavagna, P.; Camerini, G.; Fibiani, M.; Andreani, L.; Cella, R.; Concia, L.; Lo Scalzo, R. Characterization of the Rescued “Voghera” Sweet Pepper Landrace Grown in Northern Italy. Span. J. Agric. Res. 2012, 10, 1059. [Google Scholar] [CrossRef]
- Rossi, G.; Guzzon, F.; Canella, M.; Tazzari, E.R.; Cauzzi, P.; Bodino, S.; Ardenghi, N.M.G. Le varietà agronomiche lombarde tradizionali a rischio di estinzione o di erosione genetica. In Ortive e Cerealicole: Uno Sguardo D’insieme; Pavia University Press: Lombardia, Italy, 2019. [Google Scholar]
- Thuphairo, K.; Sornchan, P.; Suttisansanee, U. Bioactive Compounds, Antioxidant Activity and Inhibition of Key Enzymes Relevant to Alzheimer’s Disease from Sweet Pepper (Capsicum annuum) Extracts. Prev. Nutr. Food Sci. 2019, 24, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.R.; Azevedo, J.; Pereira, M.J.; Valentão, P.; Andrade, P.B. Chemical assessment and antioxidant capacity of pepper (Capsicum annuum L.) seeds. Food Chem. Toxicol. 2013, 53, 240–248. [Google Scholar] [CrossRef]
- Sun, T.; Xu, Z.; Wu, C.T.; Janes, M.; Prinyawiwatkul, W.; No, H.K. Antioxidant activities of different colored sweet bell peppers (Capsicum annuum L.). J. Food Sci. 2007, 72, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Al Sulaiman, K.; Al Juhani, O.; Alhammad, A.; Al Aamer, K.; Alshehri, S.; Alhuwahmel, A.; Vishwakarma, R. The Role of Adjunctive Ascorbic Acid in the Prevention of Colistin Induced Nephrotoxicity in Critically Ill Patients: A Retrospective Study. Pharm. J. 2022, 30, 1748–1754. [Google Scholar] [CrossRef]
- Fernández-García, E.; Carvajal-Lérida, I.; Pérez-Gálvez, A. Carotenoids exclusively synthesized in red pepper (capsanthin and capsorubin) protect human dermal fibroblasts against UVB induced DNA damage. Photochem. Photobiol. Sci. 2016, 9, 1204–1211. [Google Scholar] [CrossRef]
- Luo, J.; Mills, K.; le Cessie, S.; Noordam, R.; van Heemst, D. Ageing, age-related diseases and oxidative stress: What to do next? Ageing Res. Rev. 2020, 57, 100982. [Google Scholar] [CrossRef]
- Gurău, F.; Baldoni, S.; Prattichizzo, F.; Espinosa, E.; Amenta, F.; Procopio, A.D.; Albertini, M.C.; Bonafè, M.; Olivieri, F. Anti-senescence compounds: A potential nutraceutical approach to healthy aging. Ageing Res. Rev. 2018, 46, 14–31. [Google Scholar] [CrossRef]
- Schmidlin, C.J.; Dodson, M.B.; Madhavan, L.; Zhang, D.D. Redox regulation by NRF2 in aging and disease. Free Radic. Biol. Med. 2019, 134, 702–707. [Google Scholar] [CrossRef]
- Hwang, W.S.; Park, S.H.; Kim, H.S.; Kang, H.J.; Kim, M.J.; Oh, S.J.; Park, J.B.; Kim, J.; Kim, S.C.; Lee, J.Y. Ascorbic acid extends replicative life span of human embryonic fibroblast by reducing DNA and mitochondrial damages. Nutr. Res. Pract. 2007, 2, 105–112. [Google Scholar] [CrossRef]
- Chae, S.Y.; Park, S.Y.; Park, G. Lutein protects human retinal pigment epithelial cells from oxidative stress-induced cellular senescence. Mol. Med. Rep. 2018, 6, 5182–5190. [Google Scholar] [CrossRef] [PubMed]
- Tigges, J.; Krutmann, J.; Fritsche, E.; Haendeler, J.; Schaal, H.; Fischer, J.W.; Kalfalah, F.; Reinke, H.; Reifenberger, G.; Stühler, K.; et al. The hallmarks of fibroblast ageing. Mech. Ageing Dev. 2014, 138, 26–44. [Google Scholar] [CrossRef]
- Zandi, M.; Masoumian, M.; Shariatinia, A.; Sanjabi, M.R. Optimal Concentrations and Synergistic Effects of Some Herbal Extracts on Viability of Dermal Fibroblasts. Gene, Cell Tissue 2016, 3, e40458. [Google Scholar] [CrossRef]
- Han, J.H.; Roh, M.S.; Park, C.H.; Park, K.C.; Cho, K.H.; Kim, K.H.; Eun, H.C.; Chung, J.H. Selective COX-2 inhibitor, NS-398, inhibits the replicative senescence of cultured dermal fibroblasts. Mech. Ageing Dev. 2004, 125, 359–366. [Google Scholar] [CrossRef]
- Menicacci, B.; Cipriani, C.; Margheri, F.; Mocali, A.; Giovannelli, L. Modulation of the Senescence-Associated Inflammatory Phenotype in Human Fibroblasts by Olive Phenols. Int. J. Mol. Sci. 2017, 18, 2275. [Google Scholar] [CrossRef]
- Park, Y.I.; Cha, Y.E.; Jang, M.; Park, R.; Namkoong, S.; Kwak, J.; Jang, I.S.; Park, J. The Flower Extract of Abelmoschus manihot (Linn.) Increases Cyclin D1 Expression and Activates Cell Proliferation. J. Microbiol. Biotechnol. 2020, 30, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; An, C.; Hwang, S.D.; Kim, Y.S. Ceriporia lacerata mycelium culture medium as a novel anti-aging microbial material for cosmeceutical application. Cosmetics 2021, 8, 101. [Google Scholar] [CrossRef]
- Meng, Q.; Wong, Y.T.; Chen, J.; Ruan, R. Age-related changes in mitochondrial function and antioxidative enzyme activity in fischer 344 rats. Mech. Ageing Dev. 2007, 128, 286–292. [Google Scholar] [CrossRef]
- Zhang, H.; Davies, K.J.A.; Forman, H.J. Oxidative stress response and Nrf2 signaling in aging. Free Radic. Biol. Med. 2015, 88, 314–336. [Google Scholar] [CrossRef]
- Silva-Palacios, A.; Ostolga-Chavarría, M.; Zazueta, C.; Königsberg, M. Nrf2: Molecular and epigenetic regulation during aging. Ageing Res. Rev. 2018, 47, 31–40. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Abramov, A.Y. The emerging role of Nrf2 in mitochondrial function. Free. Radic. Biol. Med. 2015, 88, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Park, J.S.; Deng, J.H.; Bai, Y. Cytochrome c oxidase subunit IV is essential for assembly and respiratory function of the enzyme complex. J. Bioenerg. Biomembr. 2006, 38, 283–291. [Google Scholar] [CrossRef]
- Ciccarone, F.; Di Leo, L.; Lazzarino, G.; Maulucci, G.; Di Giacinto, F.; Tavazzi, B.; Ciriolo, M.R. Aconitase 2 inhibits the proliferation of MCF-7 cells promoting mitochondrial oxidative metabolism and ROS/FoxO1-mediated autophagic response. Br. J. Cancer 2020, 122, 182–193. [Google Scholar] [CrossRef]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Thayyullathil, F. Reactive oxygen species and cancer paradox: To promote or to suppress? Radic. Biol. Med. 2017, 104, 144–164. [Google Scholar] [CrossRef]
- Sarubbo, F.; Esteban, S.; Miralles, A.; Moranta, D. Effects of Resveratrol and other Polyphenols on Sirt1: Relevance to Brain Function During Aging. Curr. Neuropharmacol. 2018, 16, 126–136. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, J.H.; Lee, J.H.; Min, K.-J. Sirtuin signaling in cellular senescence and aging. BMB Rep. 2019, 52, 24–34. [Google Scholar] [CrossRef]
- Xie, L.; Huang, R.; Liu, S.; Wu, W.; Su, A.; Li, R.; Liu, X.; Lei, Y.; Sun, H.; Liu, X.; et al. A positive feedback loop of SIRT1 and miR17HG promotes the repair of DNA double-stranded breaks. Cell Cycle 2019, 17, 2110–2123. [Google Scholar] [CrossRef]
- Qin, W.; Yang, T.; Ho, L.; Zhao, Z.; Wang, J.; Chen, L.; Pasinetti, G.M. Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J. Biol. Chem. 2006, 281, 21745–21754. [Google Scholar] [CrossRef] [PubMed]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Bartolini, D.; Dallaglio, K.; Torquato, P.; Piroddi, M.; Galli, F. Nrf2-p62 autophagy pathway and its response to oxidative stress in hepatocellular carcinoma. Transl. Res. 2018, 193, 54–71. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Matsuki, N. Measurement of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction activity and lactate dehydrogenase release using MTT. Neurosci. Res. 2000, 38, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Chilczuk, B.; Marciniak, B.; Stochmal, A.; Pecio, Ł.; Kontek, R.; Jackowska, I.; Materska, M. Anticancer Potential and Capsianosides Identification in Lipophilic Fraction of Sweet Pepper (Capsicum annuum L.). Molecules 2020, 25, 3097. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, B.; Urselli, F.; Gilodi, M.M.; Camuso, S.; Priori, E.C.; Rangone, B.; Ravera, M.; Veneroni, P.; Zanellato, I.; Roda, E.; et al. New Platinum-Based Prodrug Pt(IV)Ac-POA: Antitumour Effects in Rat C6 Glioblastoma Cells. Neurotox. Res. 2020, 37, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Gaiaschi, L.; Roda, E.; Favaron, C.; Gola, F.; Gabano, E.; Ravera, M.; Rossi, P.; Bottone, M.G. The power of a novel combined anticancer therapy: Challenge and opportunity of micotherapy in the treatment of Glioblastoma Multiforme. Biomed. Pharmacother. 2022, 155, 113729. [Google Scholar] [CrossRef]


| Bioactive Compounds (μg/g) | Voghera Pepper (VP) | Carmagnola Pepper (CP) |
|---|---|---|
| Carotene derivatives | n.d. | 21.8 ± 0.7 |
| α-carotene | 11.3 ± 0.2 | 13.9 ± 0.2 |
| β-carotene | 9.6 ± 0.1 | 21.4 ± 0.7 |
| Trans-β-carotene | n.d. | 14.4 ± 0.2 |
| Lutein | 16.7 ± 0.2 | 11.5 ± 0.02 |
| Zeaxanthin | 9.6 ± 0.6 | 15.4 ± 0.1 |
| Zeaxanthin derivatives | n.d. | 14.1 ± 0.2 |
| Total carotenoids | 55.4 | 112.5 |
| Ascorbic Acid | 724.2 ± 0.37 | 378.5 ± 0.25 |
| Dehydroascorbic acid (DHAA) | 17.5 ± 0.50 | 13.4 ± 0.44 |
| Antigen | Primary Antibody | Dilution in PBS |
|---|---|---|
| PCNA | Mouse monoclonal anti-PCNA (Calbiochem, San Diego, CA, USA) | 1:200 |
| P16INK4a/CDKN2A | Mouse monoclonal anti- p16INK4a/CDKN2A (GeneTex, Irvine, CA, USA) | 1:250 |
| Sirt1 | Rabbit polyclonal anti-Sirt1 (Abcam, Cambridge, UK) | 1:100 |
| SOD2 | Rabbit monoclonal anti-SOD2 (Cell Signaling Technology, Danvers, MA, USA) | 1:200 |
| Catalase | Rabbit monoclonal anti-Catalase (GeneTex, Irvine, CA, USA) | 1:250 |
| NRF2 | Rabbit polyclonal anti-NRF2 (Abcam, Cambridge, UK) | 1:200 |
| COX4 | Mouse monoclonal [20E8C12] anti-COX4 (Abcam, Cambridge, UK) | 1:200 |
| ACO2 | Rabbit polyclonal anti-Aconitase2 (Abcam, Cambridge, UK) | 1:200 |
| Mitochondria | Human autoimmune serum recognizing the 70 kDa E2 subunit of pyruvate dehydrogenase complex | 1:300 |
| α-tubulin | Mouse monoclonal anti-Alpha-tubulin (Cell Signaling Technology, Danvers, MA, USA) | 1:1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gola, F.; Gaiaschi, L.; Roda, E.; De Luca, F.; Ferulli, F.; Vicini, R.; Rossi, P.; Bottone, M.G. Voghera Sweet Pepper: A Potential Ally against Oxidative Stress and Aging. Int. J. Mol. Sci. 2023, 24, 3782. https://doi.org/10.3390/ijms24043782
Gola F, Gaiaschi L, Roda E, De Luca F, Ferulli F, Vicini R, Rossi P, Bottone MG. Voghera Sweet Pepper: A Potential Ally against Oxidative Stress and Aging. International Journal of Molecular Sciences. 2023; 24(4):3782. https://doi.org/10.3390/ijms24043782
Chicago/Turabian StyleGola, Federica, Ludovica Gaiaschi, Elisa Roda, Fabrizio De Luca, Federica Ferulli, Riccardo Vicini, Paola Rossi, and Maria Grazia Bottone. 2023. "Voghera Sweet Pepper: A Potential Ally against Oxidative Stress and Aging" International Journal of Molecular Sciences 24, no. 4: 3782. https://doi.org/10.3390/ijms24043782
APA StyleGola, F., Gaiaschi, L., Roda, E., De Luca, F., Ferulli, F., Vicini, R., Rossi, P., & Bottone, M. G. (2023). Voghera Sweet Pepper: A Potential Ally against Oxidative Stress and Aging. International Journal of Molecular Sciences, 24(4), 3782. https://doi.org/10.3390/ijms24043782









