Comparative Study of the Effect of Doping ZnTiO3 with Rare Earths (La and Ce) on the Adsorption and Photodegradation of Cyanide in Aqueous Systems
Abstract
1. Introduction
2. Results
2.1. Characterization of the Nanoparticles
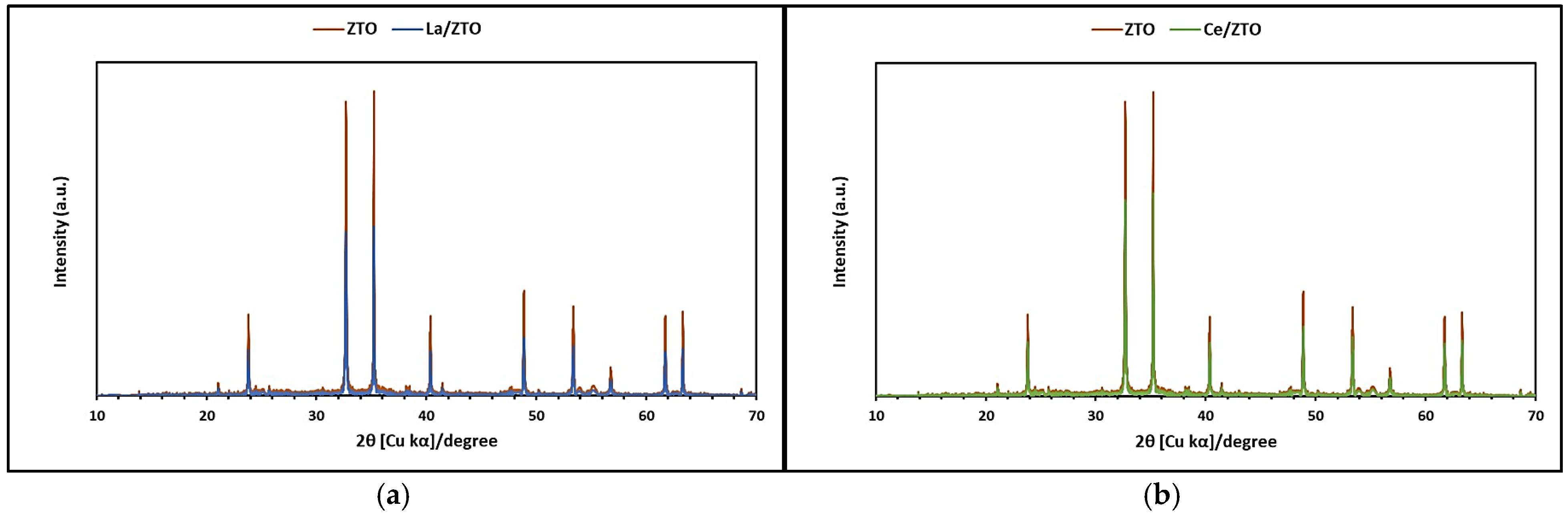
2.1.1. XRD and SSA Analysis
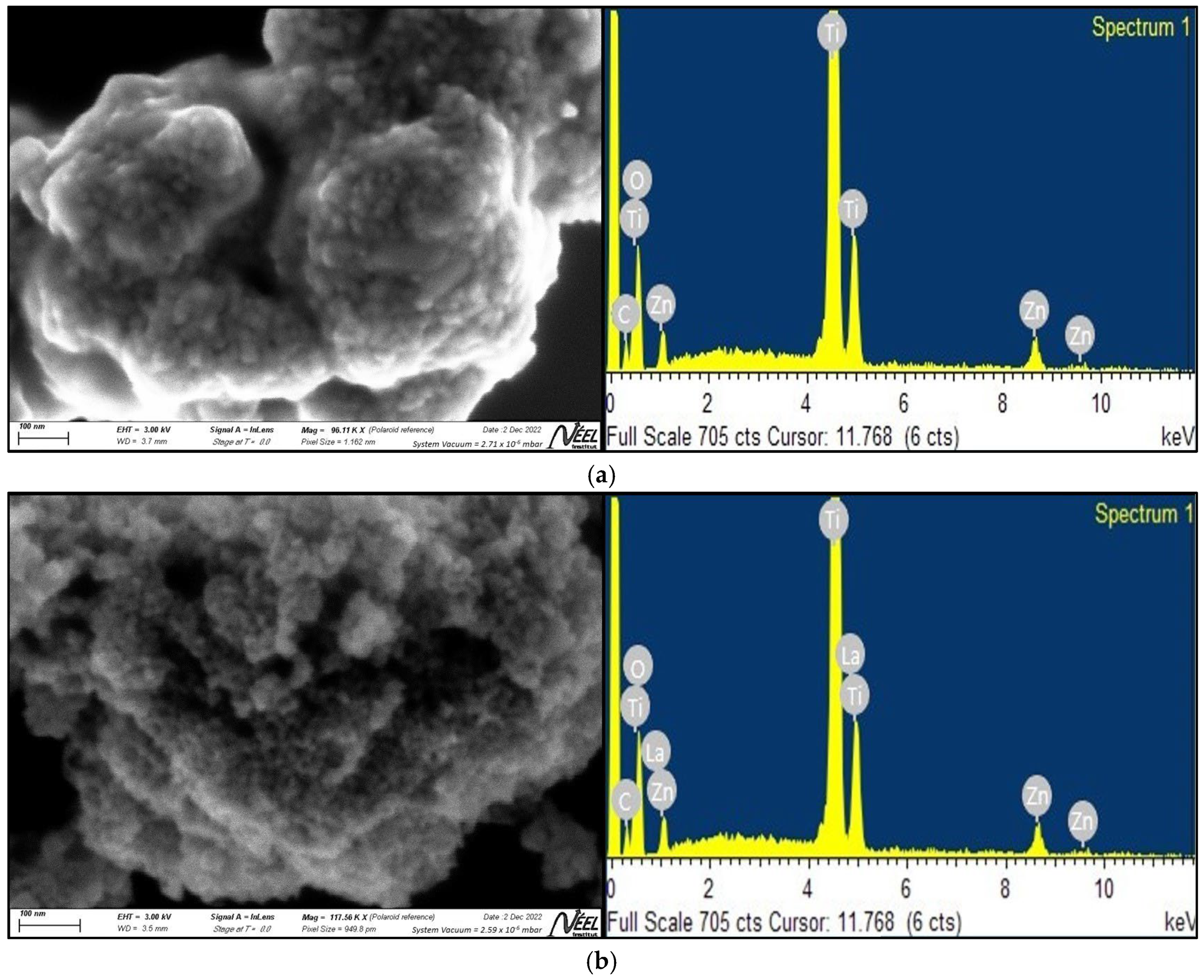
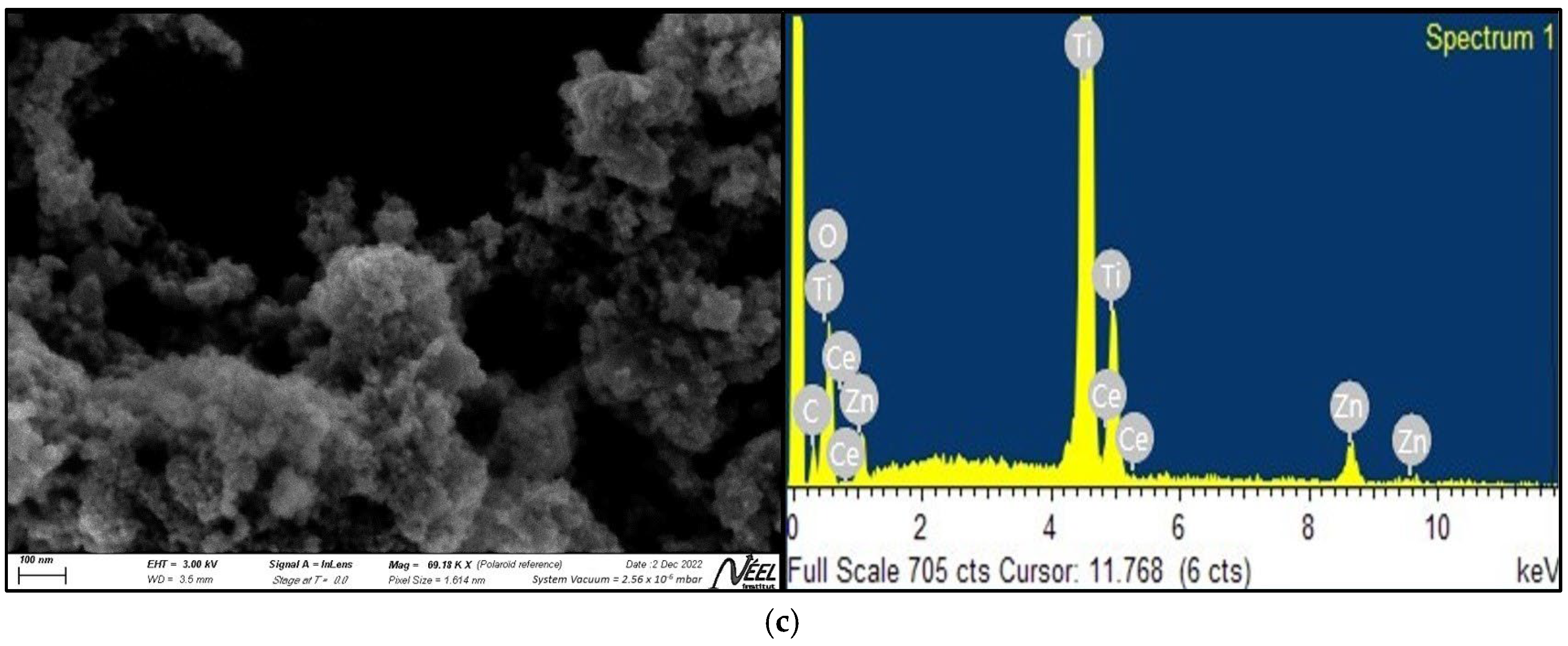
2.1.2. SEM and EDS Analysis
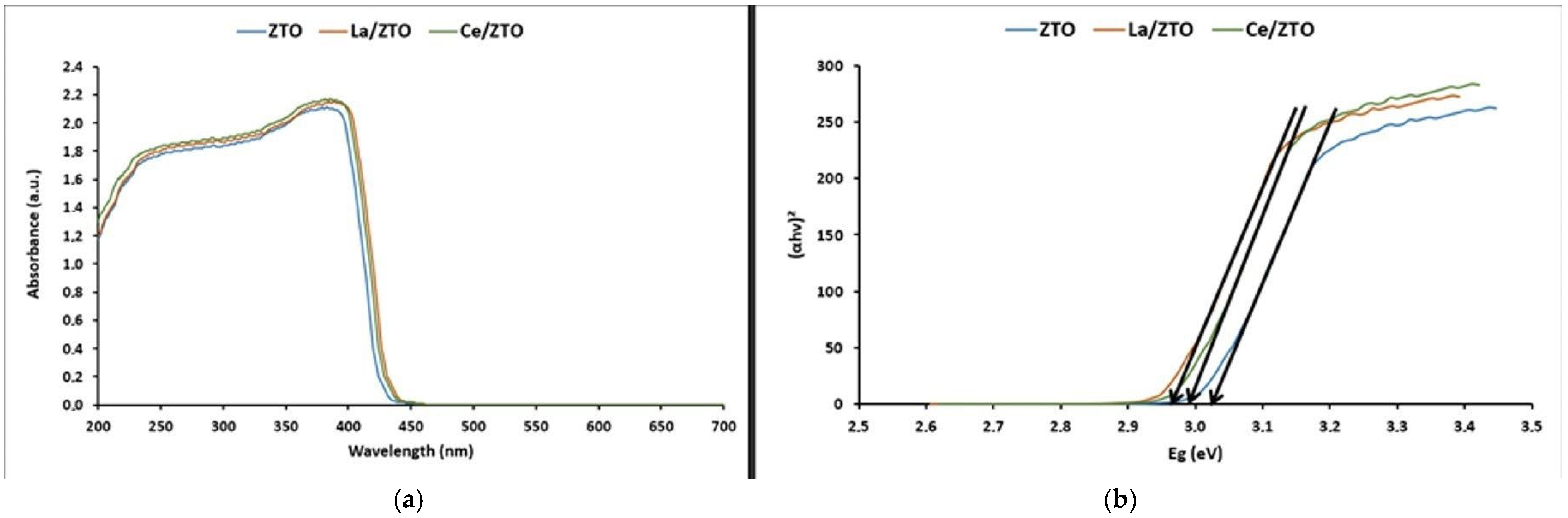
2.1.3. Optical and Photoelectric Properties
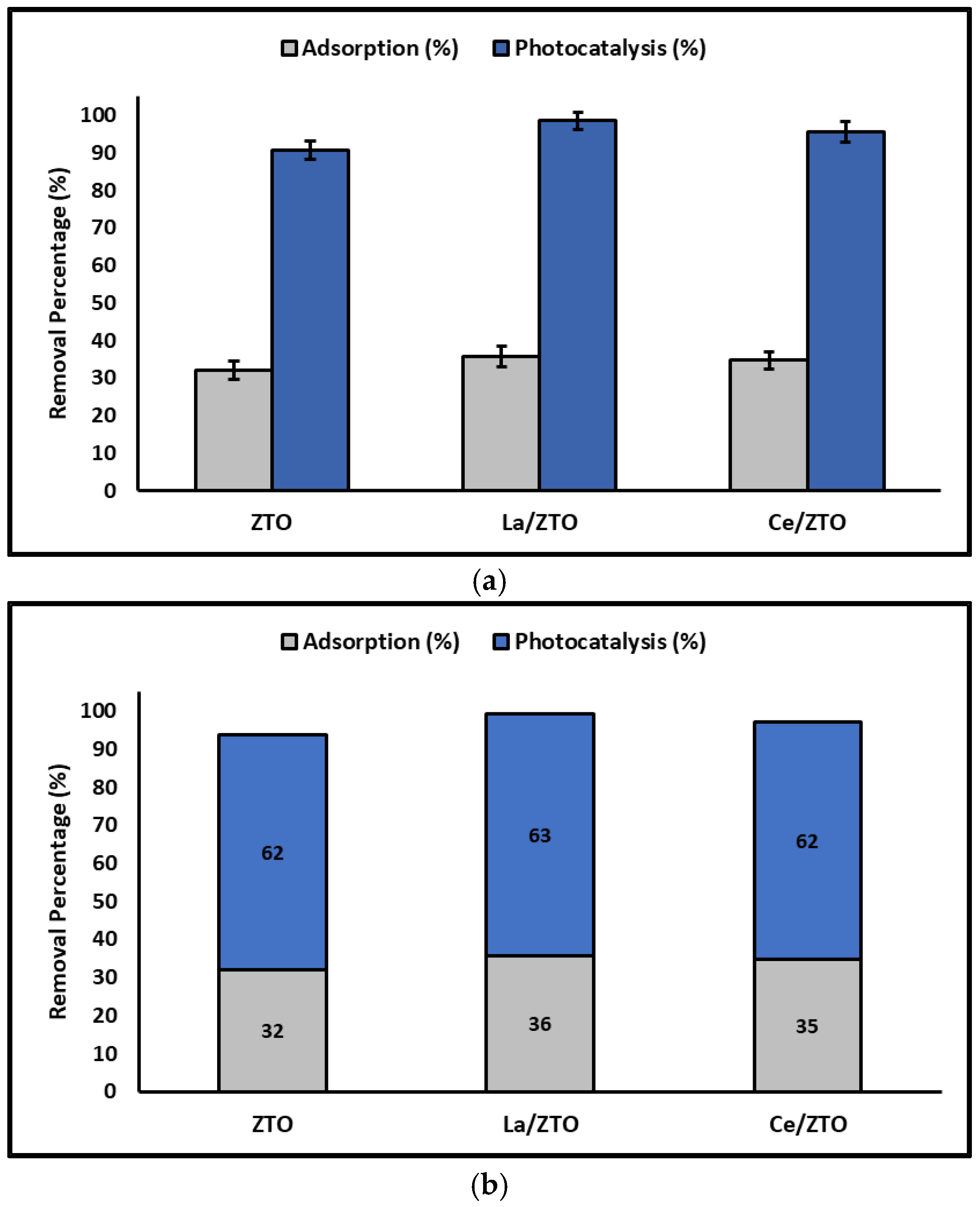
2.2. Effect of Nanoparticles’ Composition
2.3. Adsorption Behaviour
2.3.1. Effect of the pH of the Solution
2.3.2. Adsorption Isotherm
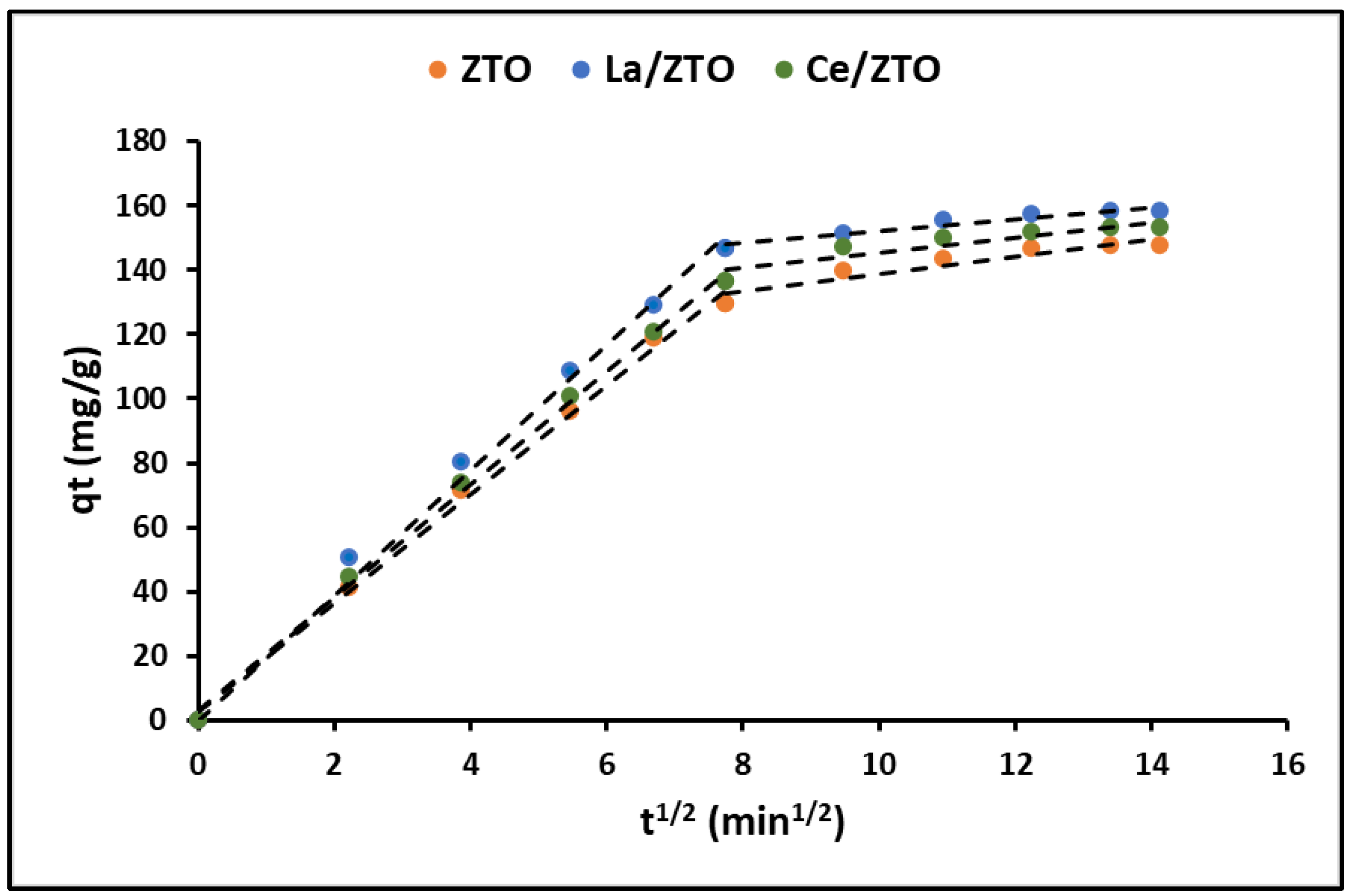
2.3.3. Adsorption Kinetics
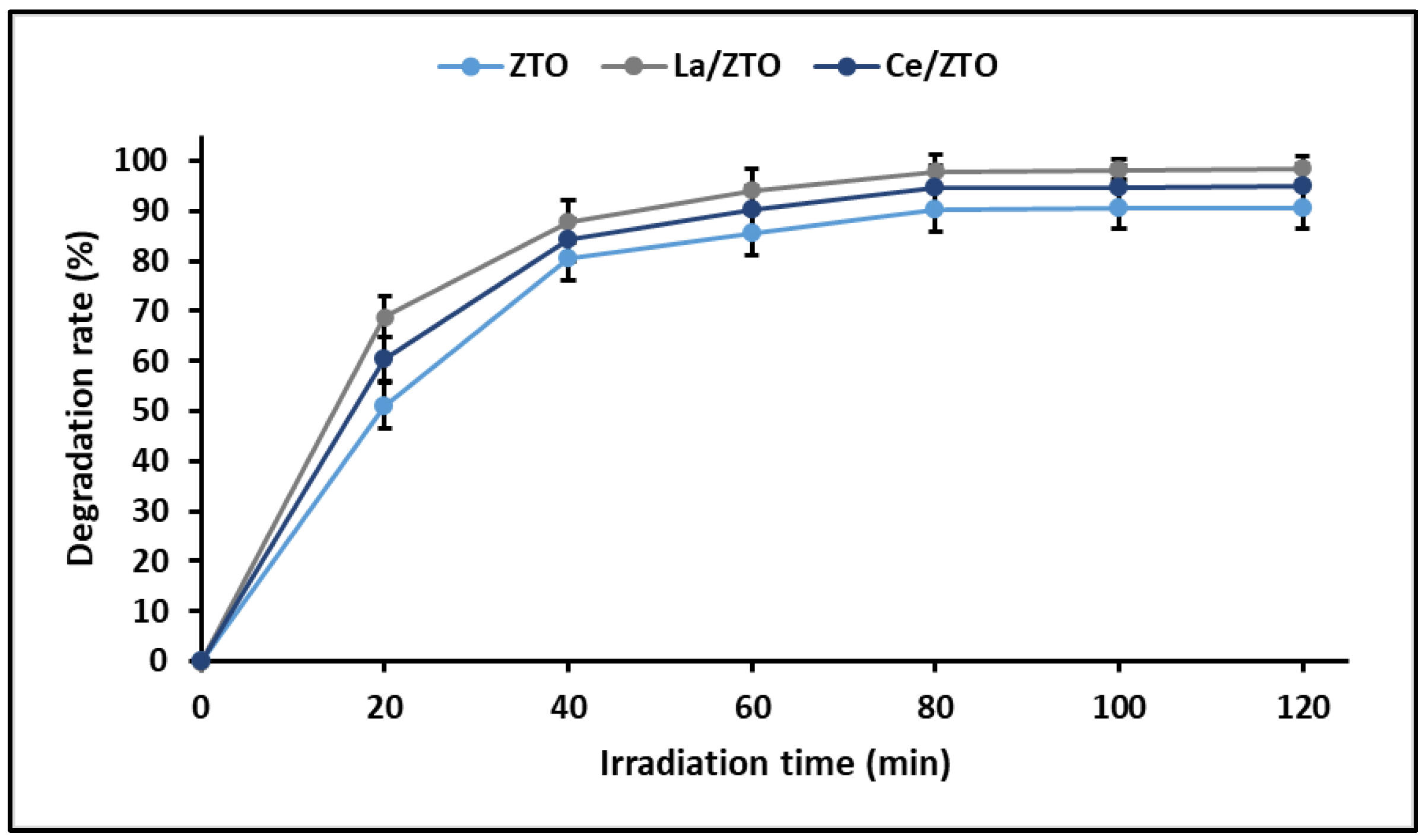
2.4. Photocatalytic Behaviour
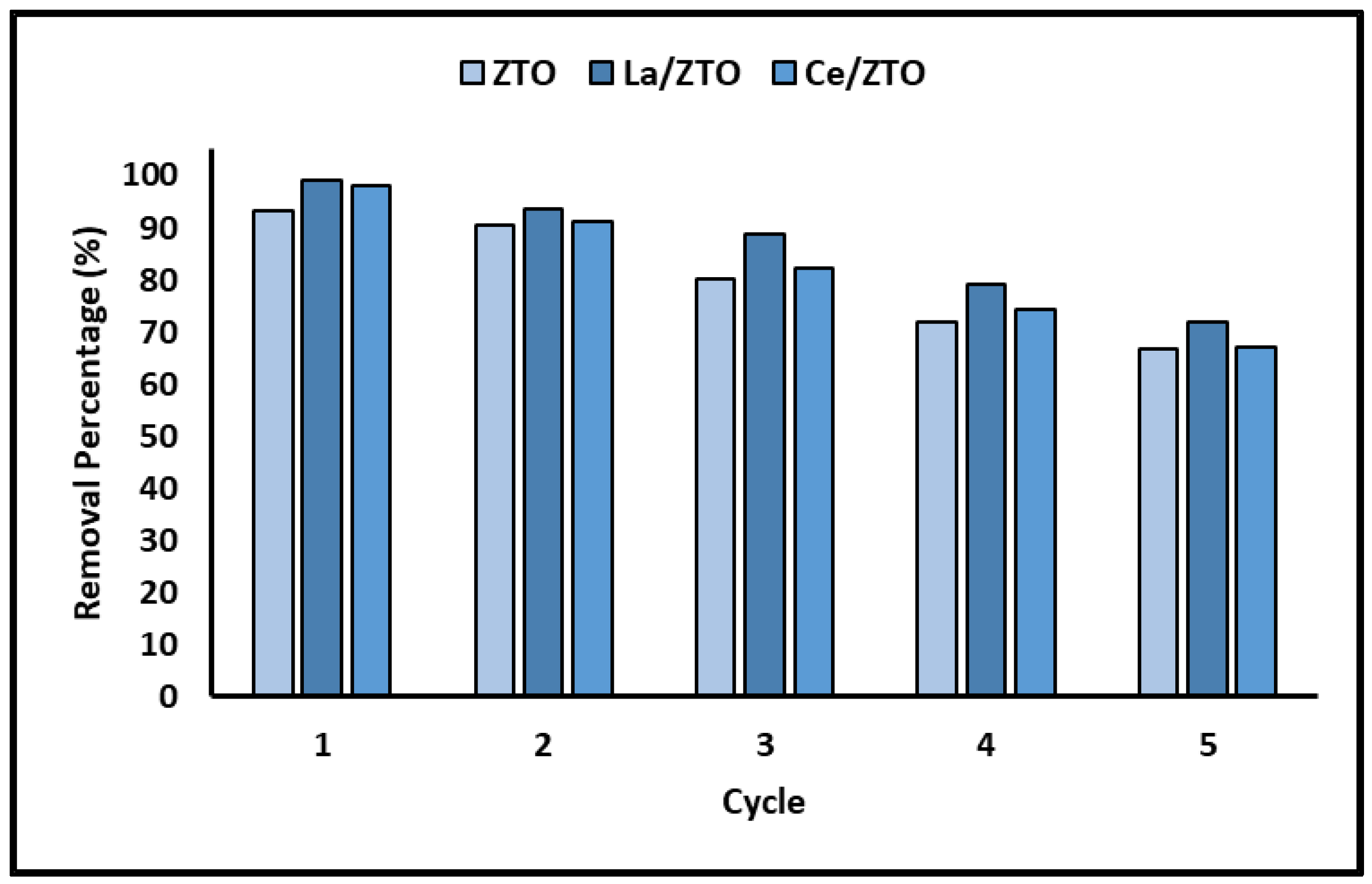
2.5. Reuse of the Nanoparticles
3. Discussion
3.1. Characterization of the Nanoparticles
3.1.1. XRD and SSA Analysis
3.1.2. SEM and EDS Analysis
3.1.3. Optical and Photoelectric Properties
3.2. Effect of Nanoparticles’ Composition
3.3. Adsorption Behaviour
3.3.1. Effect of the pH of the Solution
3.3.2. Adsorption Isotherm
3.3.3. Adsorption Kinetics
3.4. Photocatalytic Behaviour
| Adsorbent | qmax (mg g−1) | Isotherm Model | Kinetic Model | Reference |
|---|---|---|---|---|
| Clay-K | 253.98 | - | Pseudo-second-order | [12] |
| TiO2/Fe2O3 | 124.87 | - | Pseudo-second-order | [12] |
| LTA zeolite modified with HDMTMAB | 24.09 | Langmuir | - | [79] |
| ZnO | 275 | Langmuir | Pseudo-second-order | [84] |
| NiO | 185 | Langmuir | Pseudo-first-order | [84] |
| ZnO-NiO | 320 | Langmuir | Pseudo-second-order | [84] |
| Fe-MFI zeolite | 33.98 | Langmuir | Pseudo-second-order | [86] |
| ZnTiO3 | 57.32 | Langmuir | Pseudo-second-order | In this study |
| La/ZnTiO3 | 59.22 | Langmuir | Pseudo-second-order | In this study |
| Ce/ZnTiO3 | 42.00 | Langmuir | Pseudo-second-order | In this study |
3.5. Reuse of the Nanoparticles
4. Materials and Methods
4.1. Materials
4.2. Synthesis of the Nanoparticles
4.3. Characterization of the Nanoparticles
4.4. Adsorption Behaviour
4.4.1. Effect of the pH of the Solution
4.4.2. Adsorption Isotherm
4.4.3. Adsorption Kinetics
4.5. Photocatalytic Behaviour
4.6. Reuse of the Nanoparticles
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alvillo-Rivera, A.; Garrido-Hoyos, S.; Buitrón, G.; Thangarasu-Sarasvathi, P.; Rosano-Ortega, G. Biological treatment for the degradation of cyanide: A review. J. Mater. Res. Technol. 2021, 12, 1418–1433. [Google Scholar] [CrossRef]
- Han, W.; Yang, H.; Tong, L. Cyanide removal for ultrafine gold cyanide residues by chemical oxidation methods. Trans. Nonferrous Met. Soc. China 2022, 32, 4129–4138. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, Y.; Huang, Y.; Jia, Y.; Chen, L.; Cui, H. Functional Ag-doped coralloid titanosilicate zeolite (CTS-Ag) for efficiently catalytic and photodegradative removal of free cyanides and copper/zinc-cyanide complexes in real wastewater. J. Alloys Compd. 2022, 926, 166848. [Google Scholar] [CrossRef]
- Dong, K.; Xie, F.; Wang, W.; Chang, Y.; Lu, D.; Gu, X.; Chen, C. The detoxification and utilization of cyanide tailings: A critical review. J. Clean. Prod. 2021, 302, 126946. [Google Scholar] [CrossRef]
- Eletta, O.A.A.; Ajayi, O.A.; Ogunleye, O.O.; Akpan, I.C. Adsorption of cyanide from aqueous solution using calcinated eggshells: Equilibrium and optimisation studies. J. Environ. Chem. Eng. 2016, 4, 1367–1375. [Google Scholar] [CrossRef]
- Upadhyay, G.K.; Rajput, J.K.; Pathak, T.K.; Kumar, V.; Purohit, L.P. Synthesis of ZnO:TiO2 nanocomposites for photocatalyst application in visible light. Vacuum 2019, 160, 154–163. [Google Scholar] [CrossRef]
- Ozturk, B.; Soylu, G.S.P. Promoting role of transition metal oxide on ZnTiO3-TiO2 nanocomposites for the photocatalytic activity under solar light irradiation. Ceram. Int. 2016, 42, 11184–11192. [Google Scholar] [CrossRef]
- Stefańska, K.S.; Kubiaka, A.; Piasecki, A.; Goscianska, J.; Nowaczyk, G.; Jurga, S.; Jesionowski, T. TiO2-ZnO Binary Oxide Systems: Comprehensive Characterization and Tests of Photocatalytic Activity. Materials 2018, 11, 841. [Google Scholar] [CrossRef]
- Belver, C.; Hinojosa, M.; Bedia, J.; Tobajas, M.; Alvarez, M.A.; Rodríguez-González, V.; Rodriguez, J.J. Ag-Coated heterostructures of ZnO-TiO2/delaminated montmorillonite as solar photocatalysts. Materials 2017, 10, 960. [Google Scholar] [CrossRef]
- Yu, L.; Yang, X.; He, J.; He, Y.; Wang, D. Synthesis of magnetically separable N, La-doped TiO2 with enhanced photocatalytic activity. Sep. Purif. Technol. 2015, 144, 107–113. [Google Scholar] [CrossRef]
- Sridevi, A.; Ramji, B.R.; Prasanna Venkatesan, G.K.D.; Sugumaran, V.; Selvakumar, P. A facile synthesis of TiO2/BiOCl and TiO2/BiOCl/La2O3 heterostructure photocatalyst for enhanced charge separation efficiency with improved UV-light catalytic activity towards Rhodamine B and Reactive Yellow 86. Inorg. Chem. Commun. 2021, 130, 108715. [Google Scholar] [CrossRef]
- Hermosilla Redondo, D.; Yoon, Y.; Garcia-Costa, A.L.; Margarita Amaro-Medina, B.; Martinez-Luevanos, A.; de Jesus Soria-Aguilar, M.; Antonio Sanchez-Castillo, M.; Estrada-Flores, S.; Raul Carrillo-Pedroza, F. Efficiency of Adsorption and Photodegradation of Composite TiO2/Fe2O3 and Industrial Wastes in Cyanide Removal. Water 2022, 14, 3502. [Google Scholar] [CrossRef]
- Stavropoulos, G.G.; Skodras, G.S.; Papadimitriou, K.G. Effect of solution chemistry on cyanide adsorption in activated carbon. Appl. Therm. Eng. 2015, 74, 182–185. [Google Scholar] [CrossRef]
- Muthirulan, P.; Nirmala Devi, C.; Meenakshi Sundaram, M. Synchronous role of coupled adsorption and photocatalytic degradation on CAC–TiO2 composite generating excellent mineralization of alizarin cyanine green dye in aqueous solution. Arab. J. Chem. 2017, 10, S1477–S1483. [Google Scholar] [CrossRef]
- Aliprandini, P.; Veiga, M.M.; Marshall, B.G.; Scarazzato, T.; Espinosa, D.C.R. Investigation of mercury cyanide adsorption from synthetic wastewater aqueous solution on granular activated carbon. J. Water Process Eng. 2020, 34, 101154. [Google Scholar] [CrossRef]
- Kosmulski, M. The pH dependent surface charging and points of zero charge. VII. Update. Adv. Colloid Interface Sci. 2018, 251, 115–138. [Google Scholar] [CrossRef]
- Li, P.; Li, X.; Dai, S. Adsorption of gold cyanide on quartz. Colloids Surf. A Physicochem. Eng. Asp. 2020, 590, 124514. [Google Scholar] [CrossRef]
- Maulana, I.; Takahashi, F. Cyanide removal study by raw and iron-modified synthetic zeolites in batch adsorption experiments. J. Water Process Eng. 2018, 22, 80–86. [Google Scholar] [CrossRef]
- Li, Q.; Lu, H.; Yin, Y.; Qin, Y.; Tang, A.; Liu, H.; Liu, Y. Synergic effect of adsorption and biodegradation enhance cyanide removal by immobilized Alcaligenes sp. strain DN25. J. Hazard. Mater. 2019, 364, 367–375. [Google Scholar] [CrossRef]
- Li, P.; Zhang, Y.; Huang, Y.; Chen, L. Activity and mechanism of macroporous carbon/nano-TiO2 composite photocatalyst for treatment of cyanide wastewater. Colloids Surf. A Physicochem. Eng. Asp. 2023, 658, 130728. [Google Scholar] [CrossRef]
- Núñez-Salas, R.E.; Hernández-Ramírez, A.; Hinojosa-Reyes, L.; Guzmán-Mar, J.L.; Villanueva-Rodríguez, M.; de Lourdes Maya-Treviño, M. Cyanide degradation in aqueous solution by heterogeneous photocatalysis using boron-doped zinc oxide. Catal. Today 2019, 328, 202–209. [Google Scholar] [CrossRef]
- Pattanayak, D.S.; Mishra, J.; Nanda, J.; Sahoo, P.K.; Kumar, R.; Sahoo, N.K. Photocatalytic degradation of cyanide using polyurethane foam immobilized Fe-TCPP-S-TiO2-rGO nano-composite. J. Environ. Manag. 2021, 297, 113312. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Yang, Q.; Zhang, W.; Cui, S.; Yang, B.; Wang, Q.; Li, S.; Zhang, D. A high sensitivity dual-mode optical thermometry based on charge compensation in ZnTiO3:M (M = Eu3+, Mn4+) hexagonal prisms. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 274, 121101. [Google Scholar] [CrossRef] [PubMed]
- Mofokeng, S.J.; Noto, L.L.; Dhlamini, M.S. Photoluminescence properties of ZnTiO3:Eu3+ phosphor with enhanced red emission by Al3+ charge compensation. J. Lumin. 2020, 228, 117569. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, B.; Wang, Y.S.; Qin, Z.; Ke, S.H. First-principles investigation of the ferroelectric, piezoelectric and nonlinear optical properties of LiNbO3-type ZnTiO3. Sci. Rep. 2019, 9, 17632. [Google Scholar] [CrossRef]
- Djellabi, R.; Ordonez, M.F.; Conte, F.; Falletta, E.; Bianchi, C.L.; Rossetti, I. A Review of Advances in Multifunctional XTiO3 Perovskite-type Oxides as piezo-photocatalysts for Environmental Remediation and Energy Production. J. Hazard. Mater. 2021, 421, 126792. [Google Scholar] [CrossRef]
- Obodo, K.O.; Noto, L.L.; Mofokeng, S.J.; Ouma, C.N.M.; Braun, M.; Dhlamini, M.S. Influence of Tm, Ho and Er dopants on the properties of Yb activated ZnTiO3 perovskite: A density functional theory insight. Mater. Res. Express 2018, 5, 106202. [Google Scholar] [CrossRef]
- Sarkar, M.; Sarkar, S.; Biswas, A.; De, S.; Kumar, P.R.; Mothi, E.M.; Kathiravan, A. Zinc titanate nanomaterials—Photocatalytic studies and sensitization of hydantoin derivatized porphyrin dye. Nano-Struct. Nano-Objects 2020, 21, 100412. [Google Scholar] [CrossRef]
- Kurajica, S.; Minga, I.; Blazic, R.; Muzina, K.; Tominac, P. Adsorption and Degradation Kinetics of Methylene Blue on As-prepared and Calcined Titanate Nanotubes. Athens J. Sci. 2018, 5, 7–22. [Google Scholar] [CrossRef]
- Sahu, A.; Chaurashiya, R.; Hiremath, K.; Dixit, A. Nanostructured zinc titanate wide band gap semiconductor as a photoelectrode material for quantum dot sensitized solar cells. Sol. Energy 2018, 163, 338–346. [Google Scholar] [CrossRef]
- Bhagwat, U.O.; Wu, J.J.; Asiri, A.M.; Anandan, S. Synthesis of ZnTiO3@TiO2 Heterostructure Nanomaterial as a Visible light Photocatalyst. ChemistrySelect 2019, 4, 6106–6112. [Google Scholar] [CrossRef]
- Jaramillo-Fierro, X.; Hernández, K.; González, S. Cu(C3H3N3S3)3 Adsorption onto ZnTiO3/TiO2 for Coordination-Complex Sensitized Photochemical Applications. Materials 2022, 15, 3252. [Google Scholar] [CrossRef]
- Umar, K.; Haque, M.M.; Muneer, M.; Harada, T.; Matsumura, M. Mo, Mn and la doped TiO2: Synthesis, characterization and photocatalytic activity for the decolourization of three different chromophoric dyes. J. Alloys Compd. 2013, 578, 431–438. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, X.; Feng, X.; Zhou, J.; Zhou, S. Preparation of a La/N co-doped TiO2 film electrode with visible light response and its photoelectrocatalytic activity on a Ni substrate. Dye. Pigment. 2016, 125, 375–383. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, G.; Cui, Z.; Zhang, K.; Feng, Y.; Meng, S. Influence of difference quantity La-doped TiO2 photoanodes on the performance of dye-sensitized solar cells: A strategy for choosing an appropriate doping quantity. J. Solid State Chem. 2016, 237, 242–247. [Google Scholar] [CrossRef]
- Raza, W.; Haque, M.M.; Muneer, M.; Fleisch, M.; Hakki, A.; Bahnemann, D. Photocatalytic degradation of different chromophoric dyes in aqueous phase using la and Mo doped TiO2 hybrid carbon spheres. J. Alloys Compd. 2015, 632, 837–844. [Google Scholar] [CrossRef]
- Chai, Y.; Lin, L.; Zhang, K.; Zhao, B.; He, D. Efficient visible-light photocatalysts from Gd-La codoped TiO2 nanotubes. Ceram. Int. 2014, 40, 2691–2696. [Google Scholar] [CrossRef]
- Rafieh, A.I.; Ekanayake, P.; Tan, A.L.; Lim, C.M. Effects of ionic radii of co-dopants (Mg, Ca, Al and La) in TiO2 on performance of dye-sensitized solar cells. Sol. Energy 2017, 141, 249–255. [Google Scholar] [CrossRef]
- Armaković, S.J.; Grujić-Brojčin, M.; Šćepanović, M.; Armaković, S.; Golubović, A.; Babić, B.; Abramović, B.F. Efficiency of La-doped TiO2 calcined at different temperatures in photocatalytic degradation of β-blockers. Arab. J. Chem. 2019, 12, 5355–5369. [Google Scholar] [CrossRef]
- Yu, L.; Yang, X.; He, J.; He, Y.; Wang, D. A fluorine free method to synthesize nitrogen and lanthanum co-doped TiO2 nanocrystals with exposed {0 0 1} facets for enhancing visible-light photocatalytic activity. J. Mol. Catal. A Chem. 2015, 399, 42–47. [Google Scholar] [CrossRef]
- Nie, J.; Mo, Y.; Zheng, B.; Yuan, H.; Xiao, D. Electrochemical fabrication of lanthanum-doped TiO2 nanotube array electrode and investigation of its photoelectrochemical capability. Electrochim. Acta 2013, 90, 589–596. [Google Scholar] [CrossRef]
- Peng, H.; Cui, J.; Zhan, H.; Zhang, X. Improved photodegradation and detoxification of 2,4,6-trichlorophenol by lanthanum doped magnetic TiO2. Chem. Eng. J. 2015, 264, 316–321. [Google Scholar] [CrossRef]
- Dal’Toé, A.T.O.; Colpani, G.L.; Padoin, N.; Fiori, M.A.; Soares, C. Lanthanum doped titania decorated with silver plasmonic nanoparticles with enhanced photocatalytic activity under UV-visible light. Appl. Surf. Sci. 2018, 441, 1057–1071. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, G.; Sun, Z.; Zheng, S.; Frost, R.L. A comparative study about the influence of metal ions (Ce, la and V) doping on the solar-light-induced photodegradation toward rhodamine B. J. Environ. Chem. Eng. 2015, 3, 1444–1451. [Google Scholar] [CrossRef]
- Grujić-Brojčin, M.; Armaković, S.; Tomić, N.; Abramović, B.; Golubović, A.; Stojadinović, B.; Kremenović, A.; Babić, B.; Dohčević-Mitrović, Z.; Šćepanović, M. Surface modification of sol-gel synthesized TiO2 nanoparticles induced by La-doping. Mater. Charact. 2014, 88, 30–41. [Google Scholar] [CrossRef]
- Lan, X.; Wang, L.; Zhang, B.; Tian, B.; Zhang, J. Preparation of lanthanum and boron co-doped TiO2 by modified sol-gel method and study their photocatalytic activity. Catal. Today 2014, 224, 163–170. [Google Scholar] [CrossRef]
- Elsellami, L.; Lachheb, H.; Houas, A. Synthesis, characterization and photocatalytic activity of Li-, Cd-, and La-doped TiO2. Mater. Sci. Semicond. Process. 2015, 36, 103–114. [Google Scholar] [CrossRef]
- Guo, H.; Chen, J.; Weng, W.; Zheng, Z.; Wang, D. Adsorption behavior of Congo red from aqueous solution on La2O3-doped TiO2 nanotubes. J. Ind. Eng. Chem. 2014, 20, 3081–3088. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, H.; Xu, Y.; Zhang, H.; Wang, Y. The effect of lanthanide on the degradation of RB in nanocrystalline Ln/TiO2 aqueous solution. J. Photochem. Photobiol. A Chem. 2005, 3, 279–285. [Google Scholar] [CrossRef]
- Hafez, H.; Wu, J.; Lan, Z.; Li, Q.; Xie, G.; Lin, J.; Huang, M.; Huang, Y.; Abdel-Mottaleb, M.S. Enhancing the photoelectrical performance of dye-sensitized solar cells usingTiO2:Eu3+ nanorods. Nanotechnology 2010, 21, 415201. [Google Scholar] [CrossRef]
- Saif, M. Luminescence based on energy transfer in silica doped with lanthanide titania (Gd2Ti2O7:Ln3+) [Ln3+ = Eu3+ or Dy3+]. J. Photochem. Photobiol. A Chem. 2009, 205, 145–150. [Google Scholar] [CrossRef]
- Priyanka, K.P.; Revathy, V.R.; Rosmin, P.; Thrivedu, B.; Elsa, K.M.; Nimmymol, J.; Balakrishna, K.M.; Varghese, T. Influence of la doping on structural and optical properties of TiO2 nanocrystals. Mater. Charact. 2016, 113, 144–151. [Google Scholar] [CrossRef]
- Khalid, N.R.; Ahmed, E.; Hong, Z.; Ahmad, M. Synthesis and photocatalytic properties of visible light responsive La/TiO2-graphene composites. Appl. Surf. Sci. 2012, 263, 254–259. [Google Scholar] [CrossRef]
- Li, H.; Feng, B. Visible-light-driven composite La2O3/TiO2 nanotube arrays: Synthesis and improved photocatalytic activity. Mater. Sci. Semicond. Process. 2016, 43, 55–59. [Google Scholar] [CrossRef]
- Mazierski, P.; Mikolajczyk, A.; Bajorowicz, B.; Malankowska, A.; Zaleska-Medynska, A.; Nadolna, J. The role of lanthanides in TiO2-based photocatalysis: A review. Appl. Catal. B Environ. 2018, 233, 301–317. [Google Scholar] [CrossRef]
- Tanyi, A.R.; Rafieh, A.I.; Ekaneyaka, P.; Tan, A.L.; Young, D.J.; Zheng, Z.; Chellappan, V.; Subramanian, G.S.; Chandrakanthi, R.L.N. Enhanced efficiency of dye-sensitized solar cells based on Mg and La co-doped TiO2 photoanodes. Electrochim. Acta 2015, 178, 240–248. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Xu, Y.; Wang, Y. Significant effect of lanthanide doping on the texture and properties of nanocrystalline mesoporous TiO2. J. Solid State Chem. 2004, 177, 3490–3498. [Google Scholar] [CrossRef]
- Xu, A.W.; Gao, Y.; Liu, H.Q. The Preparation, Characterization, and their Photocatalytic Activities of Rare-Earth-Doped TiO2 Nanoparticles. J. Catal. 2002, 207, 151–157. [Google Scholar] [CrossRef]
- Ranjit, K.T.; Willner, I.; Bossmann, S.H.; Braun, A.M. Lanthanide oxide-doped titanium dioxide photocatalysts: Novel photocatalysts for the enhanced degradation of p-chlorophenoxyacetic acid. Environ. Sci. Technol. 2001, 35, 1544–1549. [Google Scholar] [CrossRef]
- Razali, N.A.; Conte, M.; McGregor, J. The role of impurities in the La2O3 catalysed carboxylation of crude glycerol. Catal. Lett. 2019, 149, 1403–1414. [Google Scholar] [CrossRef]
- Ma, R.; Jahurul Islam, M.; Amaranatha Reddy, D.; Kim, T.K. Transformation of CeO2 into a mixed phase CeO2/Ce2O3 nanohybrid by liquid phase pulsed laser ablation for enhanced photocatalytic activity through Z-scheme pattern. Ceram. Int. 2016, 42, 18495–18502. [Google Scholar] [CrossRef]
- Holzwarth, U.; Gibson, N. The Scherrer equation versus the “Debye-Scherrer equation”. Nat. Nanotechnol. 2011, 6, 534. [Google Scholar] [CrossRef] [PubMed]
- Mehrabi, M.; Javanbakht, V. Photocatalytic degradation of cationic and anionic dyes by a novel nanophotocatalyst of TiO2/ZnTiO3/αFe2O3 by ultraviolet light irradiation. J. Mater. Sci. Mater. Electron. 2018, 29, 9908–9919. [Google Scholar] [CrossRef]
- Jaramillo-Fierro, X.; González, S.; Medina, F. La-doped zntiO3 /TiO2 nanocomposite supported on ecuadorian diatomaceous earth as a highly efficient photocatalyst driven by solar light. Molecules 2021, 26, 6232. [Google Scholar] [CrossRef] [PubMed]
- Eke-emezie, N.; Etuk, B.R.; Akpan, O.P.; Chinweoke, O.C. Cyanide removal from cassava wastewater onto H3PO4 activated periwinkle shell carbon. Appl. Water Sci. 2022, 12, 157. [Google Scholar] [CrossRef]
- Dash, R.R.; Gaur, A.; Balomajumder, C.; Roshan, R.; Gaur, A.; Balomajumder, C. Cyanide in industrial wastewaters and its removal: A review on biotreatment. J. Hazard. Mater. 2009, 163, 1–11. [Google Scholar] [CrossRef]
- Gupta, N.; Balomajumder, C.; Agarwal, V.K. Adsorption of cyanide ion on pressmud surface: A modeling approach. Chem. Eng. J. 2012, 191, 548–556. [Google Scholar] [CrossRef]
- Adams, M.D. Removal of cyanide from solution using activated carbon. Miner. Eng. 1994, 7, 1165–1177. [Google Scholar] [CrossRef]
- Dash, R.R.; Balomajumder, C.; Kumar, A. Removal of cyanide from water and wastewater using granular activated carbon. Chem. Eng. J. 2009, 146, 408–413. [Google Scholar] [CrossRef]
- Saxena, S.; Prasad, M.; Amritphale, S.S.; Chandra, N. Adsorption of cyanide from aqueous solutions at pyrophyllite surface. Sep. Purif. Technol. 2001, 24, 263–270. [Google Scholar] [CrossRef]
- Fan, S.; Tang, J.; Wang, Y.; Li, H.; Zhang, H.; Tang, J.; Wang, Z.; Li, X. Biochar prepared from co-pyrolysis of municipal sewage sludge and tea waste for the adsorption of methylene blue from aqueous solutions: Kinetics, isotherm, thermodynamic and mechanism. J. Mol. Liq. 2016, 220, 432–441. [Google Scholar] [CrossRef]
- Eskandari, P.; Farhadian, M.; Solaimany Nazar, A.R.; Goshadrou, A. Cyanide adsorption on activated carbon impregnated with ZnO, Fe2O3, TiO2 nanometal oxides: A comparative study. Int. J. Environ. Sci. Technol. 2021, 18, 297–316. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, J.; Zhao, Y.; Han, X. Effect of phase composition, morphology, and specific surface area on the photocatalytic activity of TiO2 nanomaterials. RSC Adv. 2014, 4, 47031–47038. [Google Scholar] [CrossRef]
- He, L.; Meng, J.; Feng, J.; Yao, F.; Zhang, L.; Zhang, Z.; Liu, X.; Zhang, H. Investigation of 4f-Related Electronic Transitions of Rare-Earth Doped ZnO Luminescent Materials: Insights from First-Principles Calculations. ChemPhysChem 2020, 21, 51–58. [Google Scholar] [CrossRef]
- Surendar, T.; Kumar, S.; Shanker, V. Influence of La-doping on phase transformation and photocatalytic properties of ZnTiO3 nanoparticles synthesized via modified sol-gel method. Phys. Chem. Chem. Phys. 2014, 16, 728–735. [Google Scholar] [CrossRef]
- Rouster, P.; Pavlovic, M.; Szilagyi, I. Destabilization of Titania Nanosheet Suspensions by Inorganic Salts: Hofmeister Series and Schulze-Hardy Rule. J. Phys. Chem. B 2017, 121, 6749–6758. [Google Scholar] [CrossRef]
- Muráth, S.; Sáringer, S.; Somosi, Z.; Szilágyi, I. Effect of Ionic Compounds of Different Valences on the Stability of Titanium Oxide Colloids. Colloids Interfaces 2018, 2, 32. [Google Scholar] [CrossRef]
- Prakash, J.; Samriti; Kumar, A.; Dai, H.; Janegitz, B.C.; Krishnan, V.; Swart, H.C.; Sun, S. Novel rare earth metal–doped one-dimensional TiO2 nanostructures: Fundamentals and multifunctional applications. Mater. Today Sustain. 2021, 13, 100066. [Google Scholar] [CrossRef]
- Noroozi, R.; Al-Musawi, T.J.; Kazemian, H.; Kalhori, E.M.; Zarrabi, M. Removal of cyanide using surface-modified Linde Type-A zeolite nanoparticles as an efficient and eco-friendly material. J. Water Process Eng. 2018, 21, 44–51. [Google Scholar] [CrossRef]
- Kumar, V.V.; Naresh, G.; Sudhakar, M.; Tardio, J.; Bhargava, S.K.; Venugopal, A. Role of Brønsted and Lewis acid sites on Ni/TiO2 catalyst for vapour phase hydrogenation of levulinic acid: Kinetic and mechanistic study. Appl. Catal. A Gen. 2015, 505, 217–223. [Google Scholar] [CrossRef]
- Eskandarloo, H.; Badiei, A.; Behnajady, M.A.; Tavakoli, A.; Ziarani, G.M. Ultrasonic-assisted synthesis of Ce doped cubic-hexagonal ZnTiO3 with highly efficient sonocatalytic activity. Ultrason. Sonochem. 2016, 29, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Cho, H.; Kim, D.; Han, S.S.; Lee, K.H.; Cho, S.H.; Song, T.; Choi, H. Defect engineering toward strong photocatalysis of Nb-doped anatase TiO2: Computational predictions and experimental verifications. Appl. Catal. B Environ. 2017, 206, 520–530. [Google Scholar] [CrossRef]
- Shu, Q.; Liu, X.; Huo, Y.; Tan, Y.; Zhang, C.; Zou, L. Construction of a Brönsted-Lewis solid acid catalyst La-PW-SiO2/SWCNTs based on electron withdrawing effect of La(III) on π bond of SWCNTs for biodiesel synthesis from esterification of oleic acid and methanol. Chin. J. Chem. Eng. 2022, 44, 351–362. [Google Scholar] [CrossRef]
- Pirmoradi, M.; Hashemian, S.; Shayesteh, M.R. Kinetics and thermodynamics of cyanide removal by ZnO@NiO nanocrystals. Trans. Nonferrous Met. Soc. China Engl. Ed. 2017, 27, 1394–1403. [Google Scholar] [CrossRef]
- Chaker, H.; Ameur, N.; Saidi-Bendahou, K.; Djennas, M.; Fourmentin, S. Modeling and Box-Behnken design optimization of photocatalytic parameters for efficient removal of dye by lanthanum-doped mesoporous TiO2. J. Environ. Chem. Eng. 2021, 9, 2213–3437. [Google Scholar] [CrossRef]
- Amamou, S.; Cheniti-Belcadhi, L. Tutoring in Project-Based Learning. In Proceedings of the Procedia Computer Science; Elsevier: Amsterdam, The Netherlands, 2018; Volume 126, pp. 176–185. [Google Scholar]
- Golbaz, S.; Jafari, A.J.; Kalantari, R.R. The study of Fenton oxidation process efficiency in the simultaneous removal of phenol, cyanide, and chromium (VI) from synthetic wastewater. Desalination Water Treat. 2013, 51, 5761–5767. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, S.W.; Lee, G.M.; Lee, B.T.; Yun, S.T.; Kim, S.O. Monitoring of TiO2-catalytic UV-LED photo-oxidation of cyanide contained in mine wastewater and leachate. Chemosphere 2016, 143, 106–114. [Google Scholar] [CrossRef]
- Baeissa, E.S. Photocatalytic removal of cyanide by cobalt metal doped on TiO2–SiO2 nanoparticles by photo-assisted deposition and impregnation methods. J. Ind. Eng. Chem. 2014, 20, 3761–3766. [Google Scholar] [CrossRef]
- Faisal, M.; Jalalah, M.; Harraz, F.A.; El-Toni, A.M.; Labis, J.P.; Al-Assiri, M.S. A novel Ag/PANI/ZnTiO3 ternary nanocomposite as a highly efficient visible-light-driven photocatalyst. Sep. Purif. Technol. 2021, 256, 117847. [Google Scholar] [CrossRef]
- Karunakaran, C.; Gomathisankar, P.; Manikandan, G. Preparation and characterization of antimicrobial Ce-doped ZnO nanoparticles for photocatalytic detoxification of cyanide. Mater. Chem. Phys. 2010, 123, 585–594. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, T.; Yao, J.; Li, J.; Jin, Y.; Cheng, J.; Shen, Z. Development of an unmanned device with picric acid strip for on-site rapid detections of sodium cyanide in marine water. IOP Conf. Ser. Earth Environ. Sci. 2021, 734, 012026. [Google Scholar] [CrossRef]
- Pramitha, A.R.; Harijono, H.; Wulan, S.N. Comparison of cyanide content in arbila beans (Phaseolus lunatus L.) of East Nusa Tenggara using picrate and acid hydrolysis methods. IOP Conf. Ser. Earth Environ. Sci. 2021, 924, 012031. [Google Scholar] [CrossRef]
- Castada, H.Z.; Liu, J.; Barringer, S.A.; Huang, X. Cyanogenesis in Macadamia and Direct Analysis of Hydrogen Cyanide in Macadamia Flowers, Leaves, Husks, and Nuts Using Selected Ion Flow Tube–Mass Spectrometry. Foods 2020, 9, 174. [Google Scholar] [CrossRef]
- Nsimba Bakatula, E.; Richard, D.; Neculita, C.M.; Zagury, G.J. Determination of point of zero charge of natural organic materials. Environ. Sci. Pollut. Res. 2018, 25, 7823–7833. [Google Scholar] [CrossRef]
- Sprynskyy, M.; Lebedynets, M.; Zbytniewski, R.; Namieśnik, J.; Buszewski, B. Ammonium removal from aqueous solution by natural zeolite, Transcarpathian mordenite, kinetics, equilibrium and column tests. Sep. Purif. Technol. 2005, 46, 155–160. [Google Scholar] [CrossRef]
- Bettoni, M.; Falcinelli, S.; Rol, C.; Rosi, M.; Sebastiani, G.V. Gas-Phase TiO2 Photosensitized Mineralization of Some VOCs: Mechanistic Suggestions through a Langmuir–Hinshelwood Kinetic Approach. Catalysts 2020, 11, 20. [Google Scholar] [CrossRef]
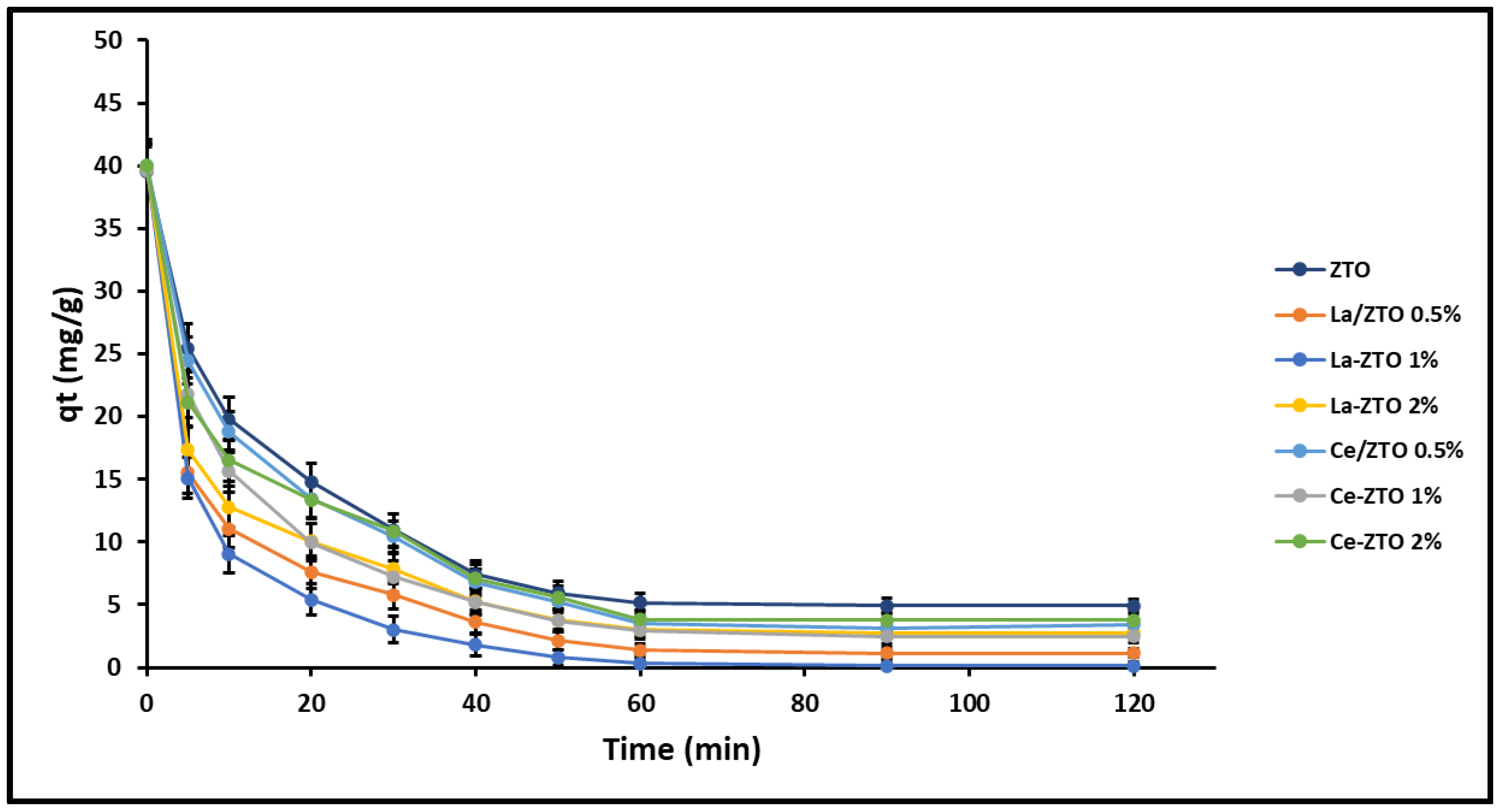
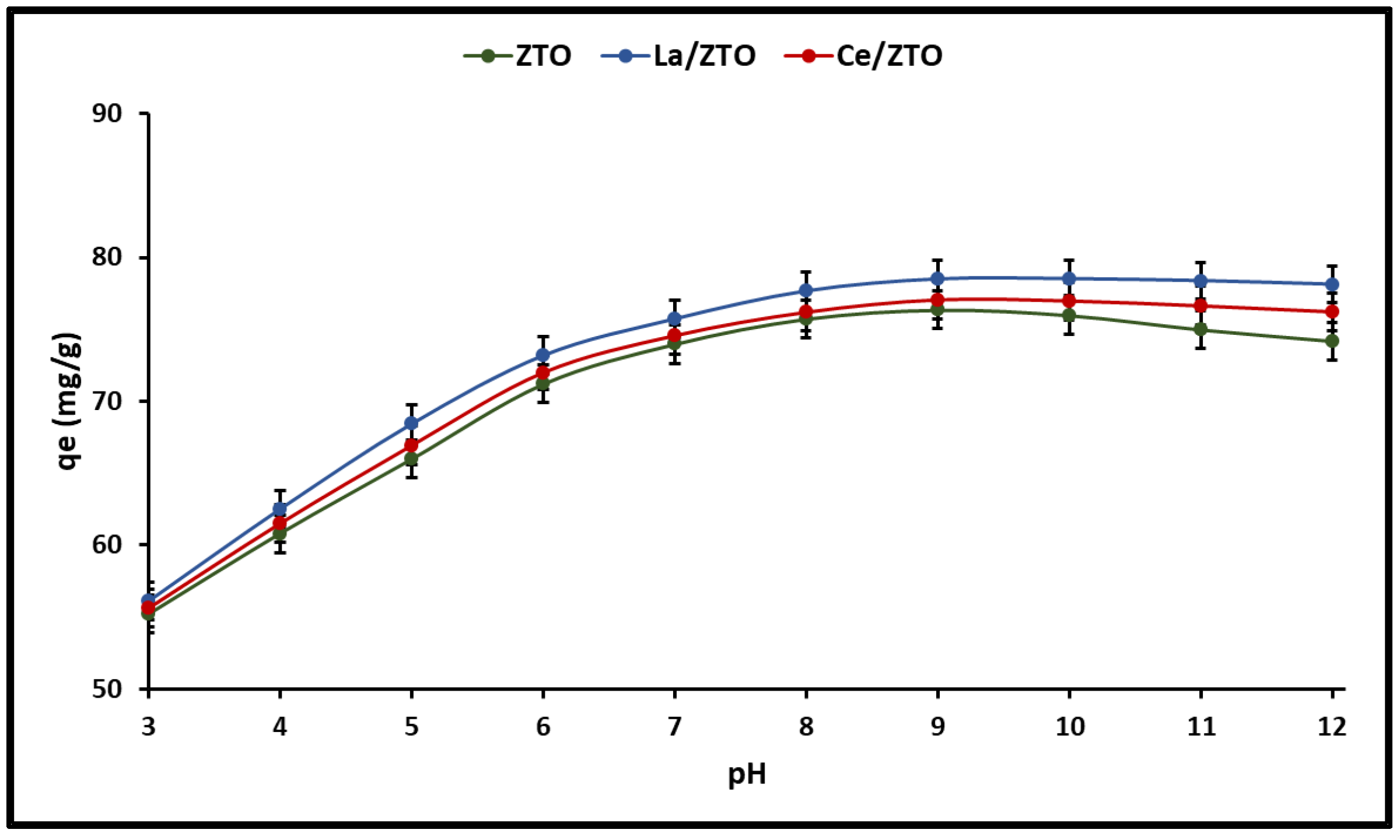
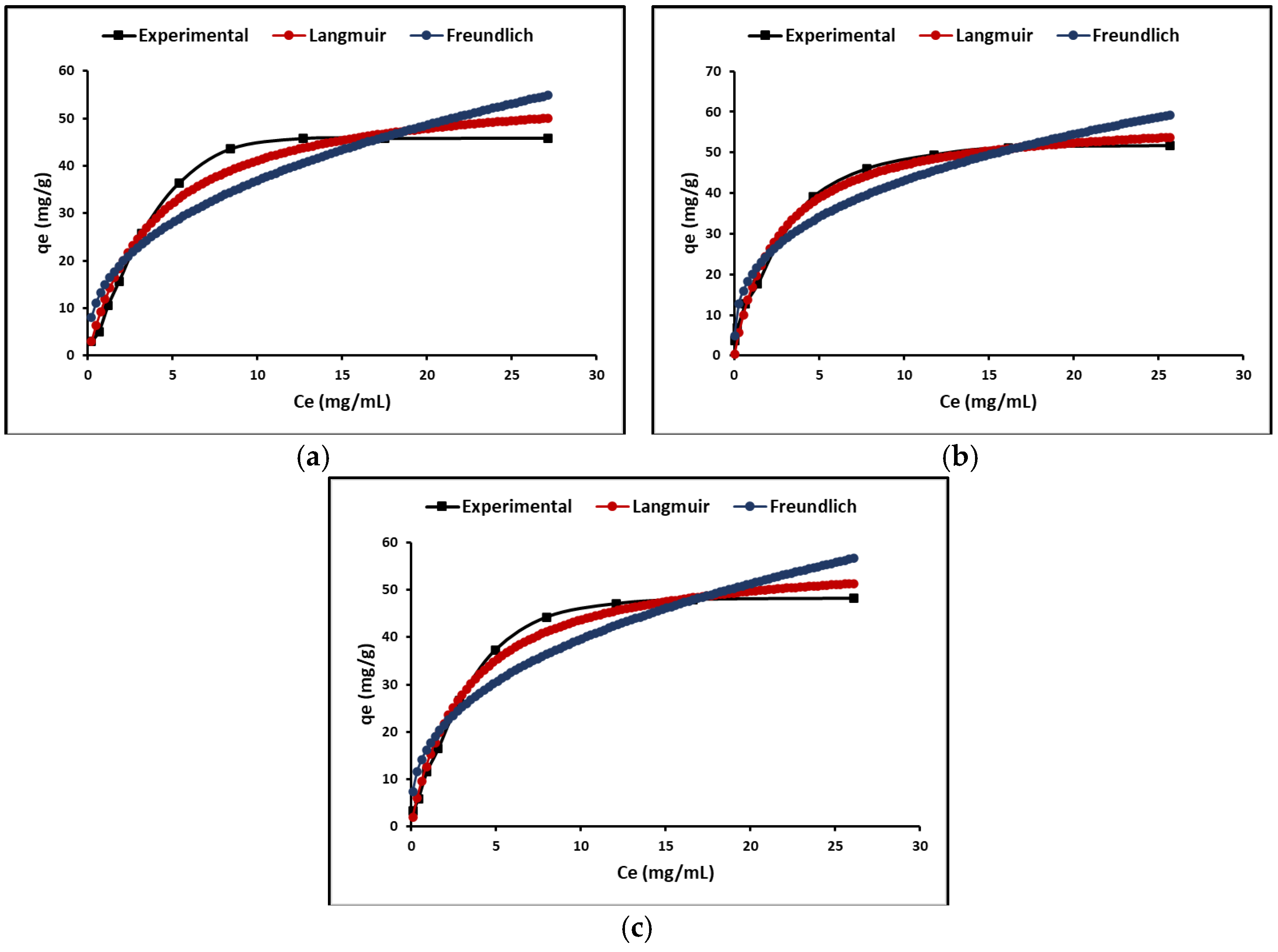
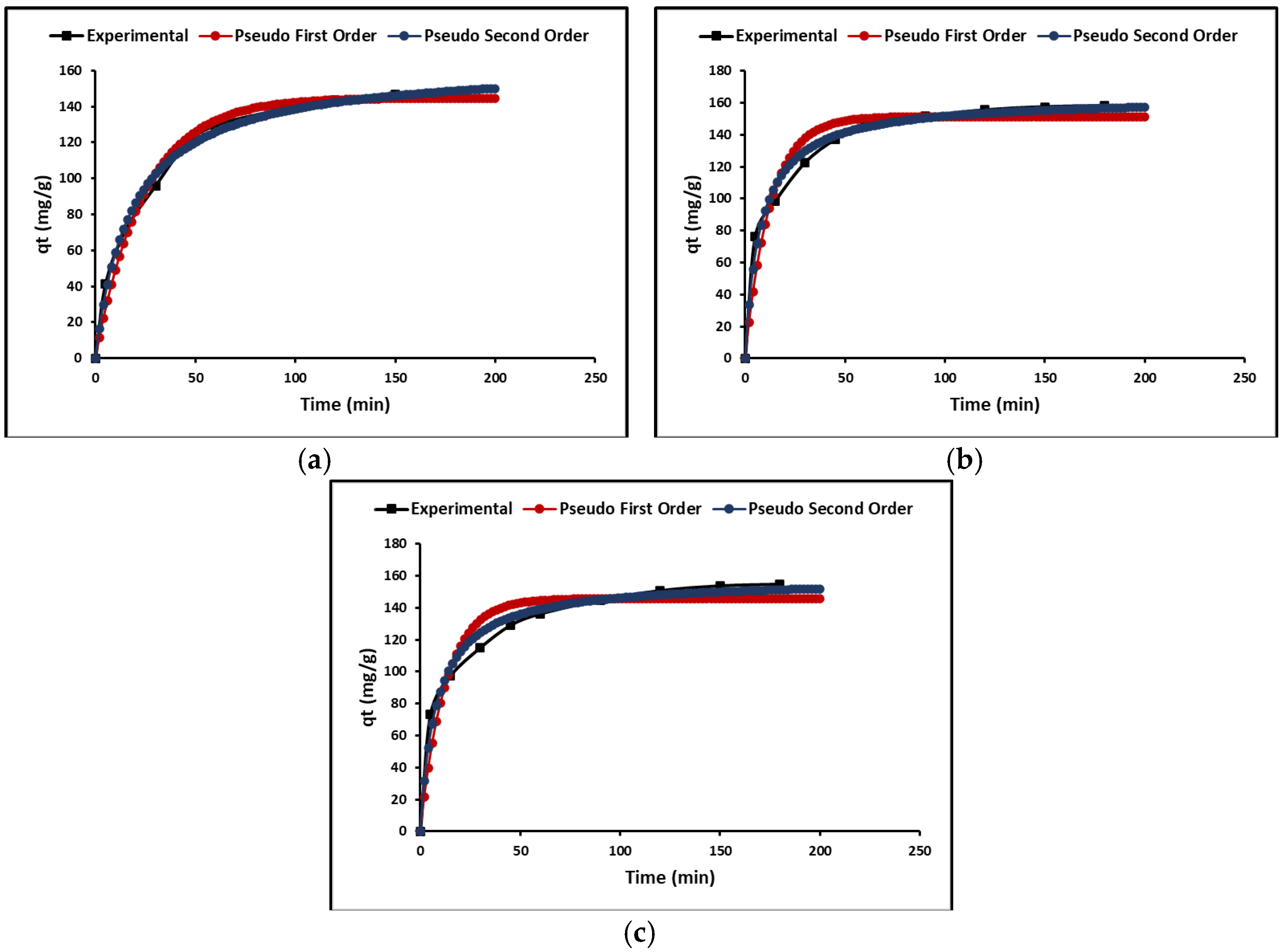
| Nanoparticles’ Composition | HSD Tukey * | Duncan * |
|---|---|---|
| qe (mg g−1) | qe (mg g−1) | |
| La/ZTO (1.0%) | 0.15 ± 0.07 a | 0.15 ± 0.07 a |
| La/ZTO (0.5%) | 1.14 ± 0.15 b | 1.14 ± 0.15 b |
| Ce/ZTO (1.0%) | 2.46 ± 0.32 c | 2.46 ± 0.32 c |
| La/ZTO (2.0%) | 2.78 ± 0.21 c,d | 2.78 ± 0.21 c |
| Ce/ZTO (0.5%) | 3.43 ± 0.48 d,e | 3.43 ± 0.48 d |
| Ce/ZTO (2.0%) | 3.79 ± 0.36 e | 3.79 ± 0.36 d |
| ZTO | 4.93 ± 0.33 f | 4.93 ± 0.33 e |
| p-value | <0.001 | <0.001 |
| Isotherm Parameters | ZTO | La/ZTO | Ce/ZTO | |
|---|---|---|---|---|
| Langmuir | qmax (mg g−1) | 57.32 (±3.53) | 59.22 (±2.12) | 42.00 (±2.26) |
| KL (L mg−1) | 0.25 (±0.05) | 0.38 (±0.04) | 0.31 (±0.04) | |
| RL | 0.16 | 0.12 | 0.14 | |
| χ2 | 6.18 | 5.03 | 4.92 | |
| R2 | 0.97 | 0.99 | 0.99 | |
| Freundlich | KF (L mg−1) | 14.77 (±2.82) | 19.76 (±2.25) | 16.72 (±2.55) |
| n | 2.52 (±0.46) | 2.96 (±0.39) | 2.67 (±0.43) | |
| 1/n | 0.40 | 0.34 | 0.37 | |
| χ2 | 4.15 | 5.96 | 6.78 | |
| R2 | 0.85 | 0.93 | 0.89 | |
| Kinetic Parameters | ZTO | La/ZTO | Ce/ZTO | |
|---|---|---|---|---|
| Pseudo-first-order | qmax (mg g−1) | 144.73 (±2.81) | 151.37 (±2.59) | 145.76 (±2.94) |
| k1 (L mg−1) | 0.04 (±3.35 × 10−3) | 0.08 (±1.51 × 10−2) | 0.08 (±1.38 × 10−2) | |
| χ2 | 14.74 | 15.95 | 16.24 | |
| R2 | 0.99 | 0.94 | 0.93 | |
| Pseudo-second-order | qmax (mg g−1) | 163.60 (±2.60) | 163.33 (±2.19) | 158.07 (±2.44) |
| k2 (L mg−1) | 3.39 × 10−4 (±2.92 × 10−5) | 7.88 × 10−4 (±1.11 × 10−4) | 7.76 × 10−4 (±1.20 × 10−4) | |
| χ2 | 12.31 | 14.33 | 13.78 | |
| R2 | 1.00 | 0.99 | 0.98 | |
| Intraparticle diffusion | k3 (mg g−1 min−1/2) | 10.01 (±0.24) | 10.23 (±0.28) | 10.35 (±0.26) |
| A | 28.91 (±1.75) | 38.52 (±1.25) | 37.65 (±1.57) | |
| R2 | 0.87 | 0.84 | 0.83 | |
| External-film diffusion | Df (m2 min−1) | 1.16 × 10−11 | 1.38 × 10−11 | 1.38 × 10−11 |
| R2 | 0.99 | 0.99 | 0.99 | |
| Internal-pore diffusion | Dp (m2 min−1) | 1.41 × 10−17 | 1.60 × 10−17 | 1.60 × 10−17 |
| R2 | 0.99 | 0.99 | 0.99 | |
| Material | [CN] (mg L−1] | [Catalyst] (g L−1] | Time (min) | Efficiency (%) | Reference |
|---|---|---|---|---|---|
| Cts-Ag | 71.6 | 2.5 | 180 | 98.0 | [3] |
| Blast furnace sludge (BFS) | 750 | 2.0 | 120 | 97.0 | [12] |
| TiO2/Fe2O3/PAC | 300 | 1.4 | 170 | 97.0 | [72] |
| TiO2/Fe2O3/zeolite | 200 | 1.4 | 160 | 89.0 | [72] |
| Fe2+ | 10 | 0.14 | 30 | 86.0 | [87] |
| TiO2 | 30 | 0.05 | 60 | 72.0 | [88] |
| Co/TiO2/SiO2 | 100 | 2.0 | 60 | 55.0 | [89] |
| TiO2/SiO2 | 100 | 1.7 | 180 | 93.0 | [90] |
| Ce/ZnO | 250 | 4.0 | 180 | 84.0 | [91] |
| ZnTiO3 | 20.0 | 0.2 | 90 | 90.7 | In this study |
| La/ZnTiO3 | 20.0 | 0.2 | 90 | 98.5 | In this study |
| Ce/ZnTiO3 | 20.0 | 0.2 | 90 | 95.1 | In this study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaramillo-Fierro, X.; Cuenca, G.; Ramón, J. Comparative Study of the Effect of Doping ZnTiO3 with Rare Earths (La and Ce) on the Adsorption and Photodegradation of Cyanide in Aqueous Systems. Int. J. Mol. Sci. 2023, 24, 3780. https://doi.org/10.3390/ijms24043780
Jaramillo-Fierro X, Cuenca G, Ramón J. Comparative Study of the Effect of Doping ZnTiO3 with Rare Earths (La and Ce) on the Adsorption and Photodegradation of Cyanide in Aqueous Systems. International Journal of Molecular Sciences. 2023; 24(4):3780. https://doi.org/10.3390/ijms24043780
Chicago/Turabian StyleJaramillo-Fierro, Ximena, Guisella Cuenca, and John Ramón. 2023. "Comparative Study of the Effect of Doping ZnTiO3 with Rare Earths (La and Ce) on the Adsorption and Photodegradation of Cyanide in Aqueous Systems" International Journal of Molecular Sciences 24, no. 4: 3780. https://doi.org/10.3390/ijms24043780
APA StyleJaramillo-Fierro, X., Cuenca, G., & Ramón, J. (2023). Comparative Study of the Effect of Doping ZnTiO3 with Rare Earths (La and Ce) on the Adsorption and Photodegradation of Cyanide in Aqueous Systems. International Journal of Molecular Sciences, 24(4), 3780. https://doi.org/10.3390/ijms24043780






