Impairment of Endogenous Synthesis of Omega-3 DHA Exacerbates T-Cell Inflammatory Responses
Abstract
1. Introduction
2. Results
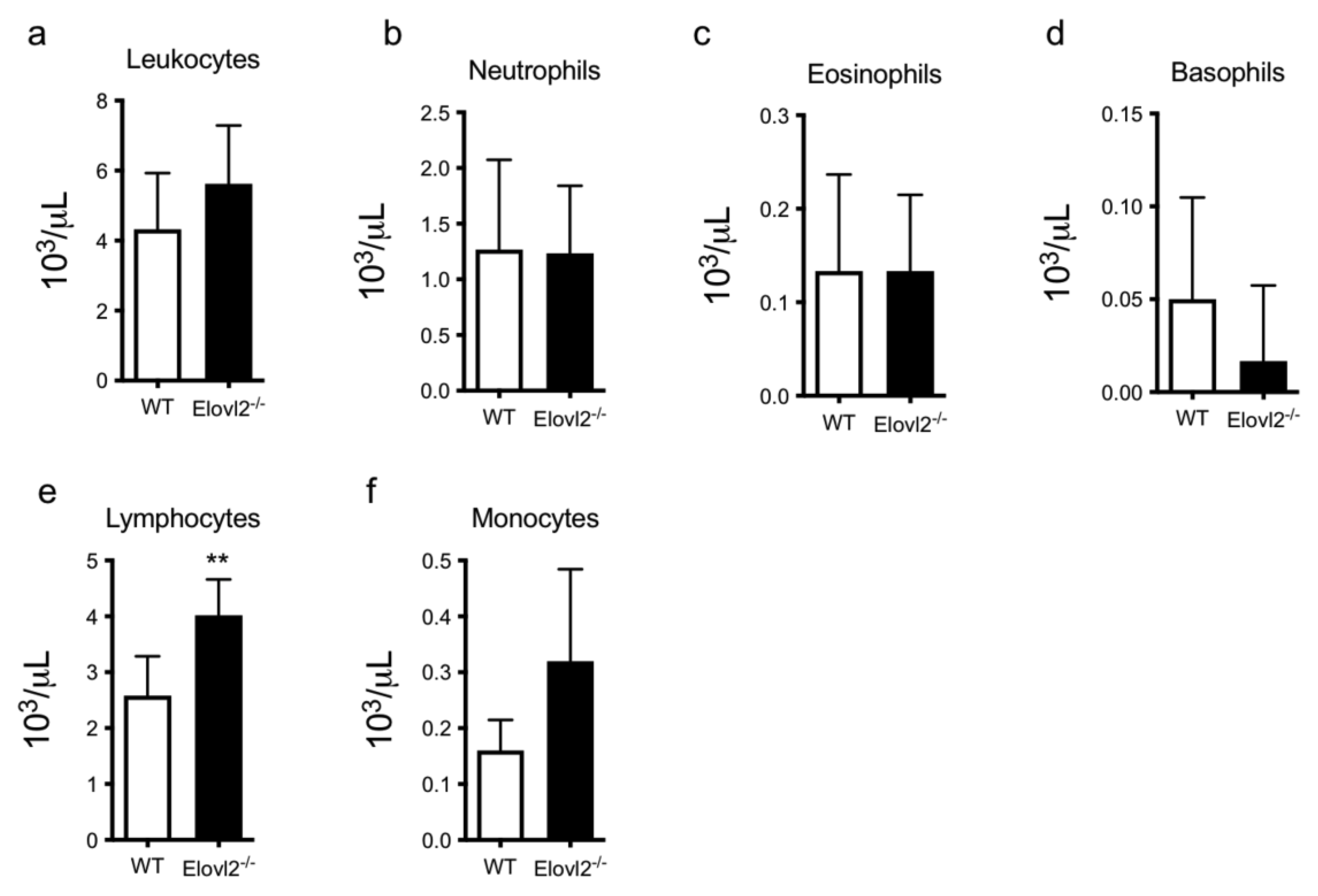
2.1. DHA Deficiency Increases Lymphocyte Cell Count
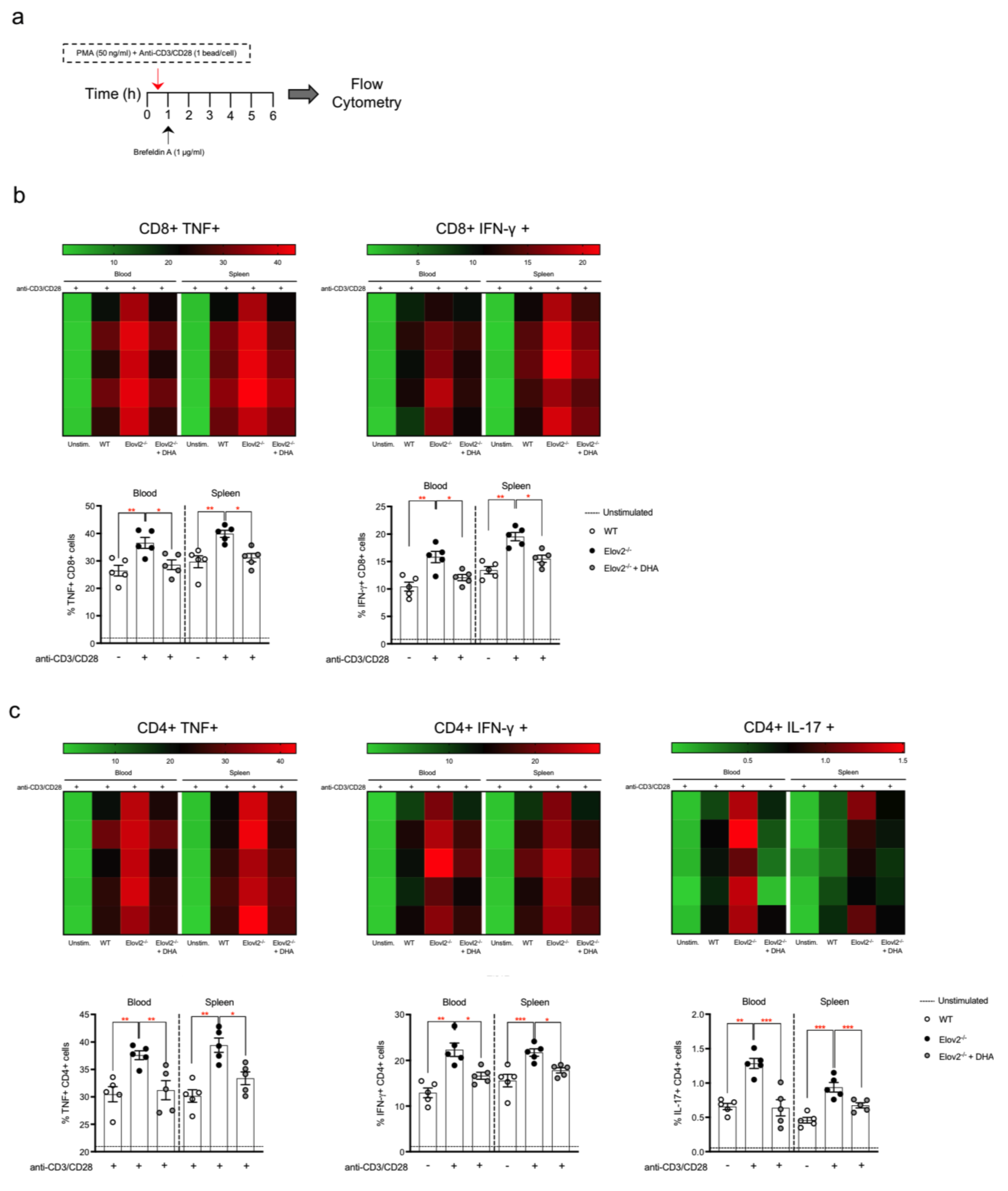
2.2. DHA Deficiency Exacerbates the Pro-Inflammatory Responses of T Lymphocytes In Vitro
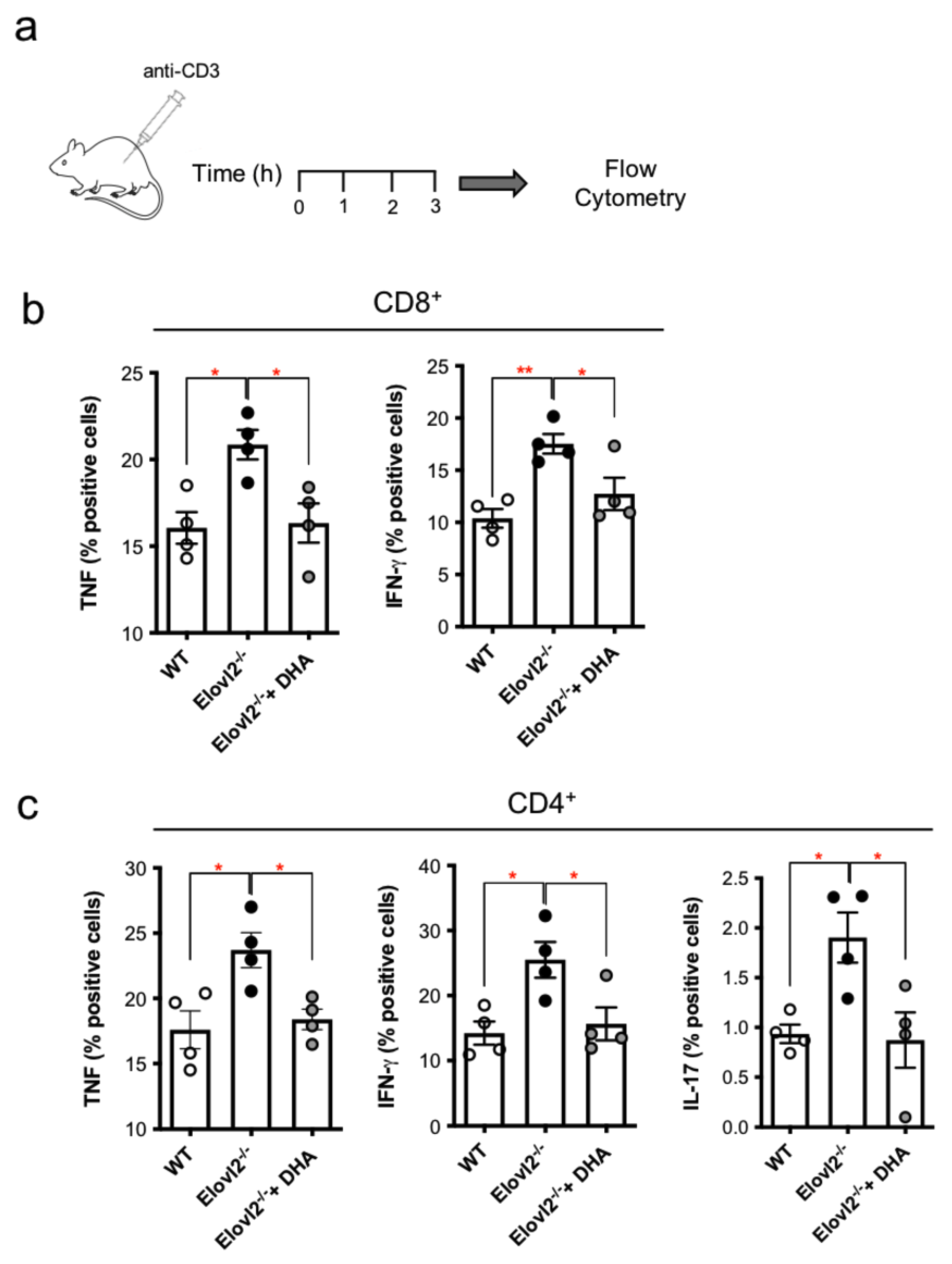
2.3. DHA Deficiency Exacerbates the Pro-Inflammatory Responses of T Lymphocytes In Vivo
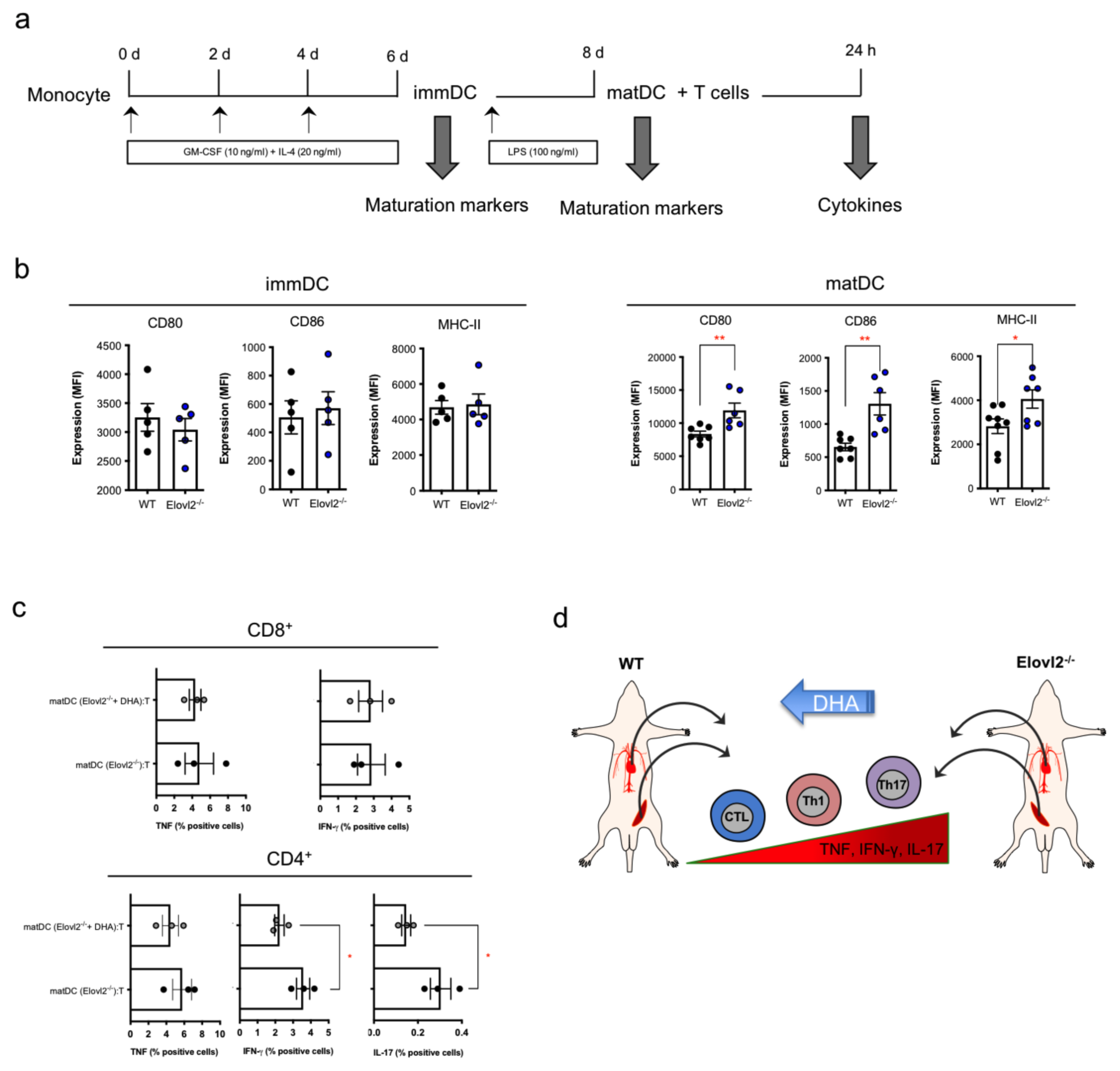
2.4. Impacts of DHA Deficiency on DC-T Cell Cross-Talks
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Animals and Tissue Processing
5.2. In Vitro and In Vivo Stimulation of T Cells
5.3. Isolation and Culture of Bone Marrow Dendritic Cells
5.4. Co-Cultures between Dendritic Cells and T Cells
5.5. Flow Cytometry
5.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shimizu, T. Lipid Mediators in Health and Disease: Enzymes and Receptors as Therapeutic Targets for the Regulation of Immunity and Inflammation. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 123–150. [Google Scholar] [CrossRef]
- Salem, N., Jr.; Litman, B.; Kim, H.Y.; Gawrisch, K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids 2001, 36, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Guillou, H.; Zadravec, D.; Martin, P.G.; Jacobsson, A. The key roles of elongases and desaturases in mammalian fatty acid metabolism: Insights from transgenic mice. Prog. Lipid. Res. 2010, 49, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Pauter, A.M.; Olsson, P.; Asadi, A.; Herslöf, B.; Csikasz, R.I.; Zadravec, D.; Jacobsson, A. Elovl2 ablation demonstrates that systemic DHA is endogenously produced and is essential for lipid homeostasis in mice. J. Lipid. Res. 2014, 55, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. The Salutary Effe.cts of DHA Dietary Supplementation on Cognition, Neuroplasticity, and Membrane Homeostasis after Brain Trauma. J. Neurotrauma 2011, 28, 2113–2122. [Google Scholar] [CrossRef]
- Kim, K.-B.; Nam, Y.A.; Kim, H.S.; Hayes, A.W.; Lee, B.-M. α-Linolenic acid: Nutraceutical, pharmacological and toxicological evaluation. Food Chem. Toxicol. 2014, 70, 163–178. [Google Scholar] [CrossRef]
- Labrousse, V.F.; Nadjar, A.; Joffre, C.; Costes, L.; Aubert, A.; Grégoire, S.; Bretillon, L.; Layé, S. Short-Term Long Chain Omega3 Diet Protects from Neuroinflammatory Processes and Memory Impairment in Aged Mice. PLoS ONE 2012, 7, e36861. [Google Scholar] [CrossRef]
- Moranis, A.; Delpech, J.C.; De Smedt-Peyrusse, V.; Aubert, A.; Guesnet, P.; Lavialle, M.; Joffre, C.; Layé, S. Long term adequate n-3 polyunsaturated fatty acid diet protects from depressive-like behavior but not from working memory disruption and brain cytokine expression in aged mice. Brain Behav. Immun. 2012, 26, 721–731. [Google Scholar] [CrossRef]
- Fedorova, I.; Hussein, N.; Di Martino, C.; Moriguchi, T.; Hoshiba, J.; Majchrzak, S.; Salem, N., Jr. An n-3 fatty acid deficient diet affects mouse spatial learning in the Barnes circular maze. Prostaglandins Leukot. Essent. Fat. Acids 2007, 77, 269–277. [Google Scholar] [CrossRef]
- Harauma, A.; Moriguchi, T. Dietary n-3 fatty acid deficiency in mice enhances anxiety induced by chronic mild stress. Lipids 2011, 46, 409–416. [Google Scholar] [CrossRef]
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef]
- Serhan, C.N.; Gupta, S.K.; Perretti, M.; Godson, C.; Brennan, E.; Li, Y.; Soehnlein, O.; Shimizu, T.; Werz, O.; Chiurchiù, V.; et al. The Atlas of Inflammation Resolution (AIR). Mol. Aspects Med. 2020, 74, 100894. [Google Scholar] [CrossRef]
- Tiberi, M.; Chiurchiù. Specialized Pro-resolving Lipid Mediators and Glial Cells: Emerging Candidates for Brain Homeostasis and Repair. Front. Cell Neurosci. 2021, 15, 673549. [Google Scholar] [CrossRef]
- Perez-Hernandez, J.; Chiurchiù, V.; Perruche, S.; You, S. Regulation of T-Cell Immune Responses by Pro-Resolving Lipid Mediators. Front. Immunol. 2021, 12, 768133. [Google Scholar] [CrossRef]
- Talamonti, E.; Sasso, V.; To, H.; Haslam, R.P.; Napier, J.A.; Ulfhake, B.; Pernold, K.; Asadi, A.; Hessa, T.; Jacobsson, A.; et al. Impairment of DHA synthesis alters the expression of neuronal plasticity markers and the brain inflammatory status in mice. FASEB J. 2020, 34, 2024–2040. [Google Scholar] [CrossRef]
- Talamonti, E.; Pauter, A.M.; Asadi, A.; Fischer, A.W.; Chiurchiù, V.; Jacobsson, A. Impairment of systemic DHA synthesis affects macrophage plasticity and polarization: Implications for DHA supplementation during inflammation. Cell Mol. Life Sci. 2017, 74, 2815–2826. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Choi, Y.R.; Kim, M.; Park, J.M.; Kang, M.; Oh, J.; Lee, C.J.; Park, S.; Kang, S.M.; Manabe, I.; et al. Common and differential effects of docosahexaenoic acid and eicosapentaenoic acid on helper T-cell responses and associated pathways. BMB Rep. 2021, 54, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Teague, H.; Rockett, B.D.; Harris, M.; Brown, D.A.; Shaikh, S.R. Dendritic cell activation, phagocytosis and CD69 expression on cognate T cells are suppressed by n-3 long-chain polyunsaturated fatty acids. Immunology 2013, 139, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Raza Shaikh, S. Diet-induced docosahexaenoic acid non-raft domains and lymphocyte function. Prostaglandins Leukot Essent Fat. Acids 2010, 82, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Pauter, A.M.; Trattner, S.; Gonzalez-Bengtsson, A.; Talamonti, E.; Asadi, A.; Dethlefsen, O.; Jacobsson, A. Both maternal and offspring Elovl2 genotypes determine systemic DHA levels in perinatal mice. J. Lipid. Res. 2017, 58, 111–123. [Google Scholar] [CrossRef]
- Sinclair, A.J.; Wang, Y.; Li, D. What Is the Evidence for Dietary-Induced DHA Deficiency in Human Brains? Nutrients 2022, 15, 161. [Google Scholar] [CrossRef]
- Singhal, A.; Lanigan, J.; Storry, C.; Low, S.; Birbara, T.; Lucas, A.; Deanfield, J. Docosahexaenoic acid supplementation, vascular function and risk factors for cardiovascular disease: A randomized controlled trial in young adults. J. Am. Heart Assoc. 2013, 2, e000283. [Google Scholar] [CrossRef]
- Maskrey, B.H.; Megson, I.L.; Rossi, A.G.; Whitfield, P.D. Emerging importance of omega-3 fatty acids in the innate immune response: Molecular mechanisms and lipidomic strategies for their analysis. Mol. Nutr. Food Res. 2013, 57, 1390–1400. [Google Scholar] [CrossRef]
- Calder, P.C. The relationship between the fatty acid composition of immune cells and their function. Prostaglandins Leukot. Essent. Fat. Acids 2008, 79, 101–108. [Google Scholar] [CrossRef]
- Barry, M.; Bleackley, R.C. Cytotoxic T lymphocytes: All roads lead to death. Nat. Rev. Immunol. 2002, 2, 401–409. [Google Scholar] [CrossRef]
- Romagnoli, P.A.; Premenko-Lanier, M.F.; Loria, G.D.; Altman, J.D. CD8 T cell memory recall is enhanced by novel direct interactions with CD4 T cells enabled by MHC class II transferred from APCs. PLoS ONE 2013, 8, e56999. [Google Scholar] [CrossRef] [PubMed]
- Raphael, I.; Nalawade, S.; Eagar, T.N.; Forsthuber, T.G. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine 2015, 74, 5–17. [Google Scholar] [CrossRef]
- Steinman, L. A brief history of TH17, the first major revision in the TH1/TH2 hypothesis of T cellmediated tissue damage. Nat. Med. 2007, 13, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Korn, T.; Bettelli, E.; Oukka, M.; Kuchroo, V.K. IL-17 and Th17 Cells. Annu. Rev. Immunol. 2009, 27, 485–517. [Google Scholar] [CrossRef] [PubMed]
- Ishnamoorthy, N.; Burkett, P.R.; Dalli, J.; Abdulnour, R.-E.E.; Colas, R.; Ramon, S.; Phipps, R.P.; Petasis, N.A.; Kuchroo, V.K.; Serhan, C.N.; et al. Cutting edge: Maresin-1 engages regulatory T cells to limit type 2 innate lymphoid cell activation and promote resolution of lung inflammation. J. Immunol. 2015, 194, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Ergas DEilat, E.; Mendlovic, S.; Sthoeger, Z.M. n-3 fatty acids and the immune system in autoimmunity. Isr. Med. Assoc. J. 2002, 4, 34–38. [Google Scholar]
- Kooij, G.; Troletti, C.D.; Leuti, A.; Norris, P.C.; Riley, I.; Albanese, M.; Ruggieri, S.; Libreros, S.; van der Pol, S.M.; van Het Hof, B.; et al. Specialized pro-resolving lipid mediators are differentially altered in peripheral blood of patients with multiple sclerosis and attenuate monocyte and blood-brain barrier dysfunction. Haematologica 2020, 105, 2056–2070. [Google Scholar] [CrossRef]
- Ghasemi Darestani, N.; Bahrami, A.; Mozafarian, M.R.; Esmalian Afyouni, N.; Akhavanfar, R.; Abouali, R.; Moradian, A.; Lorase, S. Association of Polyunsaturated Fatty Acid Intake on Inflammatory Gene Expression and Multiple Sclerosis: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 4627. [Google Scholar] [CrossRef] [PubMed]
- Merzouk, S.A.; Saker, M.; Reguig, K.B.; Soulimane, N.; Merzouk, H.; Guermouche, B.; Berrouiguet, A.Y.; Hichami, A.; Narce, M.; Khan, N.A. N-3 polyunsaturated fatty acids modulate in-vitro T cell function in type I diabetic patients. Lipids 2008, 43, 485–497. [Google Scholar] [CrossRef]
- Yan, S.C.; Wang, Y.J.; Li, Y.J.; Cai, W.Y.; Weng, X.G.; Li, Q.; Chen, Y.; Yang, Q.; Zhu, X.X. Dihydroartemisinin Regulates the Th/Treg Balance by Inducing Activated CD4+ T cell Apoptosis via Heme Oxygenase-1 Induction in Mouse Models of Inflammatory Bowel Disease. Molecules 2019, 24, 2475. [Google Scholar] [CrossRef]
- Elsayed, H.R.H.; Anbar, H.S.; Rabei, M.R.; Adel, M.; El-Gamal, R. Eicosapentaenoic and docosahexaenoic acids attenuate methotrexate-induced apoptosis and suppression of splenic T, B-Lymphocytes and macrophages with modulation of expression of CD3, CD20 and CD68. Tissue Cell 2021, 72, 101533. [Google Scholar] [CrossRef]
- Monk, J.M.; Hou, T.Y.; Turk, H.F.; McMurray, D.N.; Chapkin, R.S. n3 PUFAs reduce mouse CD4+ T-cell ex vivo polarization into Th17 cells. J. Nutr. 2013, 143, 1501–1508. [Google Scholar] [CrossRef]
- Cucchi, D.; Camacho-Muñoz, D.; Certo, M.; Niven, J.; Smith, J.; Nicolaou, A.; Mauro, C. Omega-3 polyunsaturated fatty acids impinge on CD4+ T cell motility and adipose tissue distribution via direct and lipid mediator-dependent effects. Cardiovasc. Res. 2020, 116, 1006–1020. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Jalal, Z.; Guo, P. Medicine Adherence and Associated Factors in Immigrants and Refugees: A Systematic Review. Int. J. Clin. Pract. 2022, 2022, 1993066. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, J.; Meng, Y.; Shi, N.; Wang, X.; Zhou, M.; Li, G.; Yang, Y. DHA Sensor GPR120 in Host Defense Exhibits the Dual Characteristics of Regulating Dendritic Cell Function and Skewing the Balance of Th17/Tregs. Int. J. Biol. Sci. 2020, 16, 374–387. [Google Scholar] [CrossRef] [PubMed]
- Spyrantis, A.; Krieger, J.; Stifter, K.; Boehm, B.O.; Schirmbeck, R. A dominant insulin-specific and islet-destructive T-cell response is sufficient to activate CD8 T cells directed against the fatty-acid receptor GPR40. Cell Mol. Immunol. 2020, 17, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zheng, Y.; Miao, Y.M.; Yan, W.X.; Geng, Y.Z.; Dai, Y.; Wei, Z.F. Bergenin, a PPARγ agonist, inhibits Th17 differentiation and subsequent neutrophilic asthma by preventing GLS1-dependent glutaminolysis. Acta Pharmacol. Sin. 2022, 43, 963–976. [Google Scholar] [CrossRef] [PubMed]
- Bapat, S.P.; Whitty, C.; Mowery, C.T.; Liang, Y.; Yoo, A.; Jiang, Z.; Peters, M.C.; Zhang, L.J.; Vogel, I.; Zhou, C.; et al. Obesity alters pathology and treatment response in inflammatory disease. Nature 2022, 604, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiù, V.; Leuti, A.; Dalli, J.; Jacobsson, A.; Battistini, L.; Maccarrone, M.; Serhan, C.N. Proresolving lipid mediators resolvin D1, resolvin D2, and maresin 1 are critical in modulating T cell responses. Sci. Transl. Med. 2016, 8, 353ra111. [Google Scholar] [CrossRef] [PubMed]
- Derada Troletti, C.; Enzmann, G.; Chiurchiù, V.; Kamermans, A.; Tietz, S.M.; Norris, P.C.; Jahromi, N.H.; Leuti, A.; van der Pol, S.M.A.; Schouten, M.; et al. Pro-resolving lipid mediator lipoxin A4 attenuates neuro-inflammation by modulating T cell responses and modifies the spinal cord lipidome. Cell Rep. 2021, 35, 109201. [Google Scholar] [CrossRef] [PubMed]
- Zadravec, D.; Tvrdik, P.; Guillou, H.; Haslam, R.; Kobayashi, T.; Napier, J.A.; Capecchi, M.R.; Jacobsson, A. ELOVL2 controls the level of n-6 28:5 and 30:5 fatty acids in testis, a prerequisite for male fertility and sperm maturation in mice. J. Lipid. Res. 2011, 52, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Tiberi, M.; Evron, T.; Saracini, S.; Boffa, L.; Mercuri, N.B.; Chintalacharuvu, S.R.; Atamas, S.P.; Chiurchiù. Potent T cell-mediated anti-inflammatory role of the selective CB2 agonist lenabasum in multiple sclerosis. Neuropathol. Appl. Neurobiol. 2022, 48, e12768. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiù, V.; Rapino, C.; Talamonti, E.; Leuti, A.; Lanuti, M.; Gueniche, A.; Jourdain, R.; Breton, L.; Maccarrone, M. Anandamide Suppresses Proinflammatory T Cell Responses In Vitro through Type-1 Cannabinoid Receptor-Mediated mTOR Inhibition in Human Keratinocytes. J. Immunol. 2016, 197, 3545–3553. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiù, V.; Cencioni, M.T.; Bisicchia, E.; De Bardi, M.; Gasperini, C.; Borsellino, G.; Centonze, D.; Battistini, L.; Maccarrone, M. Distinct modulation of human myeloid and plasmacytoid dendritic cells by anandamide in multiple sclerosis. Ann. Neurol. 2013, 73, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiù, V.; Leuti, A.; Smoum, R.; Mechoulam, R.; Maccarrone, M. Bioactive lipids ALIAmides differentially modulate inflammatory responses of distinct subsets of primary human T lymphocytes. FASEB J. 2018, 32, 5716–5723. [Google Scholar] [CrossRef] [PubMed]
| Fatty Acid | Not DHA-Enriched Diet D12450H | DHA-Enriched Diet D13021002 |
|---|---|---|
| C12:0 | 0.09 | 0.05 |
| C14:0 | 0.80 | 0.48 |
| C15:0 | 0.05 | 0.05 |
| C16:0 | 16.83 | 12.48 |
| C16:1n-9 | 0.17 | 0.09 |
| C16:1n-7 | 0.86 | 0.51 |
| C18:0 | 8.43 | 5.35 |
| C18:1n-9 | 27.87 | 21.35 |
| C18:1n-7 | 1.66 | 1.29 |
| C18:2n-6 * | 37.43 | 35.64 |
| C18:3n-6 | data | data |
| C18:3n-3 ** | 4.24 | 4.46 |
| C18:4n-3 | 0.05 | 0 |
| C20:0 | 0.30 | 0.23 |
| C20:1n-9 | 0.37 | 0.25 |
| C20:2n-6 | 0.31 | 0.19 |
| C20:4n-6 | 0.11 | 0.24 |
| C20:4n-3 | 0 | 0.03 |
| C20:5n-6 | 0 | 0.71 |
| C22:0 | 0.24 | 0.20 |
| C22:3n-3 | 0 | 0.08 |
| C22:4n-6 | 0 | 1.04 |
| C22:5n-3 | 0 | 0.57 |
| C22:6n-3 | 0 | 14.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talamonti, E.; Jacobsson, A.; Chiurchiù, V. Impairment of Endogenous Synthesis of Omega-3 DHA Exacerbates T-Cell Inflammatory Responses. Int. J. Mol. Sci. 2023, 24, 3717. https://doi.org/10.3390/ijms24043717
Talamonti E, Jacobsson A, Chiurchiù V. Impairment of Endogenous Synthesis of Omega-3 DHA Exacerbates T-Cell Inflammatory Responses. International Journal of Molecular Sciences. 2023; 24(4):3717. https://doi.org/10.3390/ijms24043717
Chicago/Turabian StyleTalamonti, Emanuela, Anders Jacobsson, and Valerio Chiurchiù. 2023. "Impairment of Endogenous Synthesis of Omega-3 DHA Exacerbates T-Cell Inflammatory Responses" International Journal of Molecular Sciences 24, no. 4: 3717. https://doi.org/10.3390/ijms24043717
APA StyleTalamonti, E., Jacobsson, A., & Chiurchiù, V. (2023). Impairment of Endogenous Synthesis of Omega-3 DHA Exacerbates T-Cell Inflammatory Responses. International Journal of Molecular Sciences, 24(4), 3717. https://doi.org/10.3390/ijms24043717





