The Relationship between Iron and LRRK2 in a 6-OHDA-Induced Parkinson’s Disease Model
Abstract
1. Introduction
2. Results
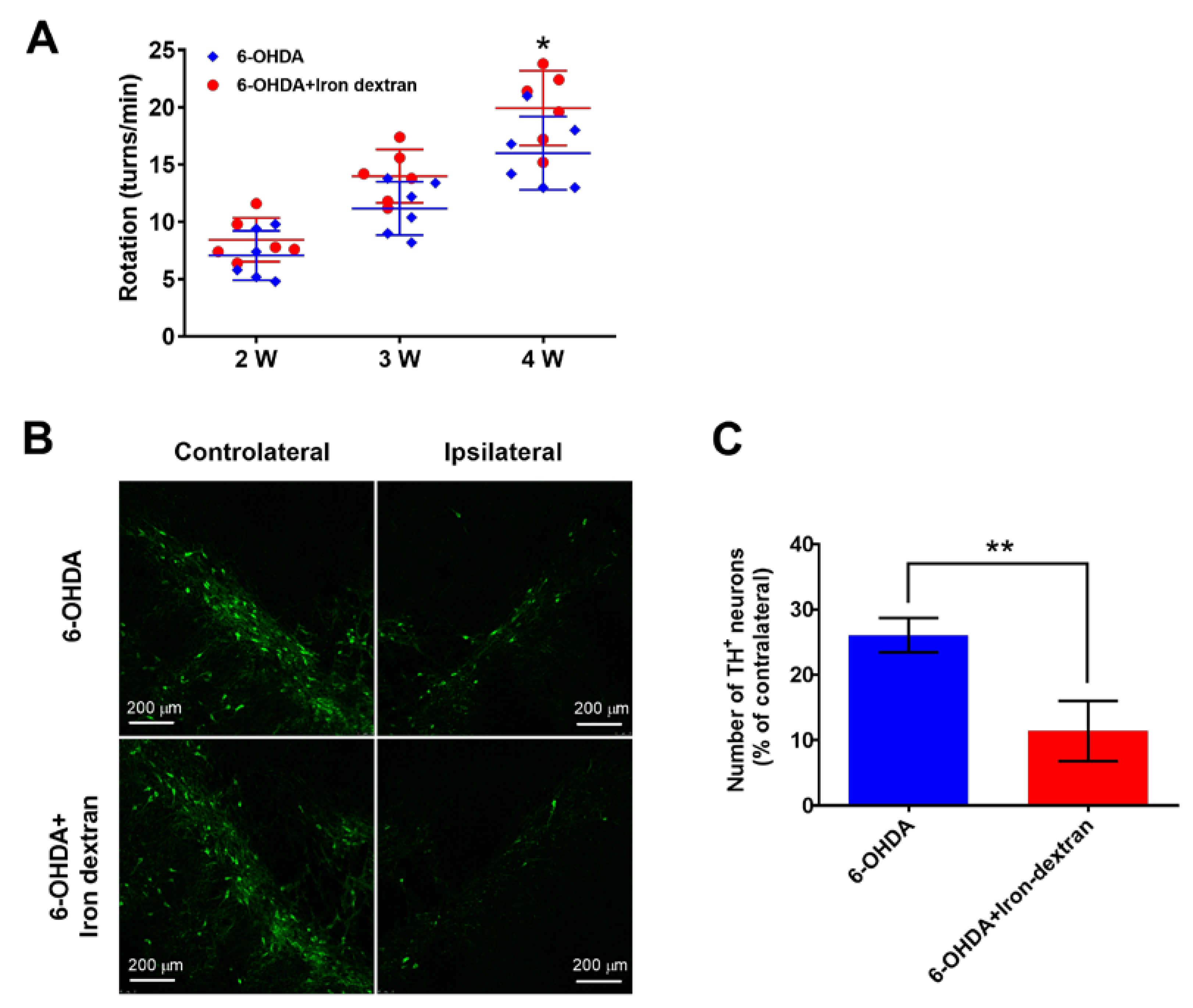
2.1. Iron Dextran Exacerbates Loss of Dopaminergic Neurons in Substantia Nigra of a 6-OHDA-Induced Rat Model of Parkinson’s Disease
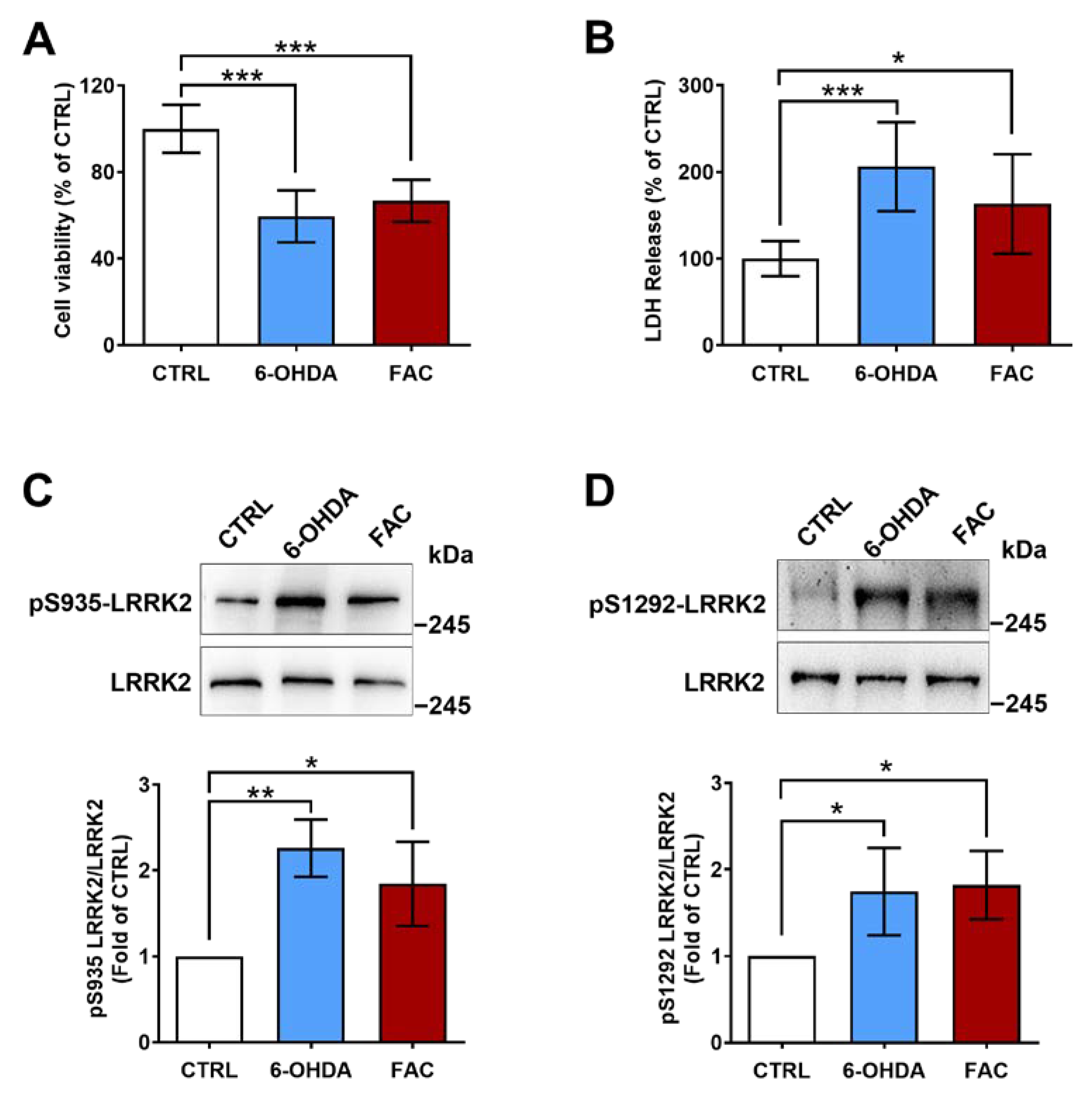
2.2. 6-OHDA and Ferric Ammonium Citrate Induce LRRK2 Activation and a Decrease in Viability of SH-SY5Y Cells
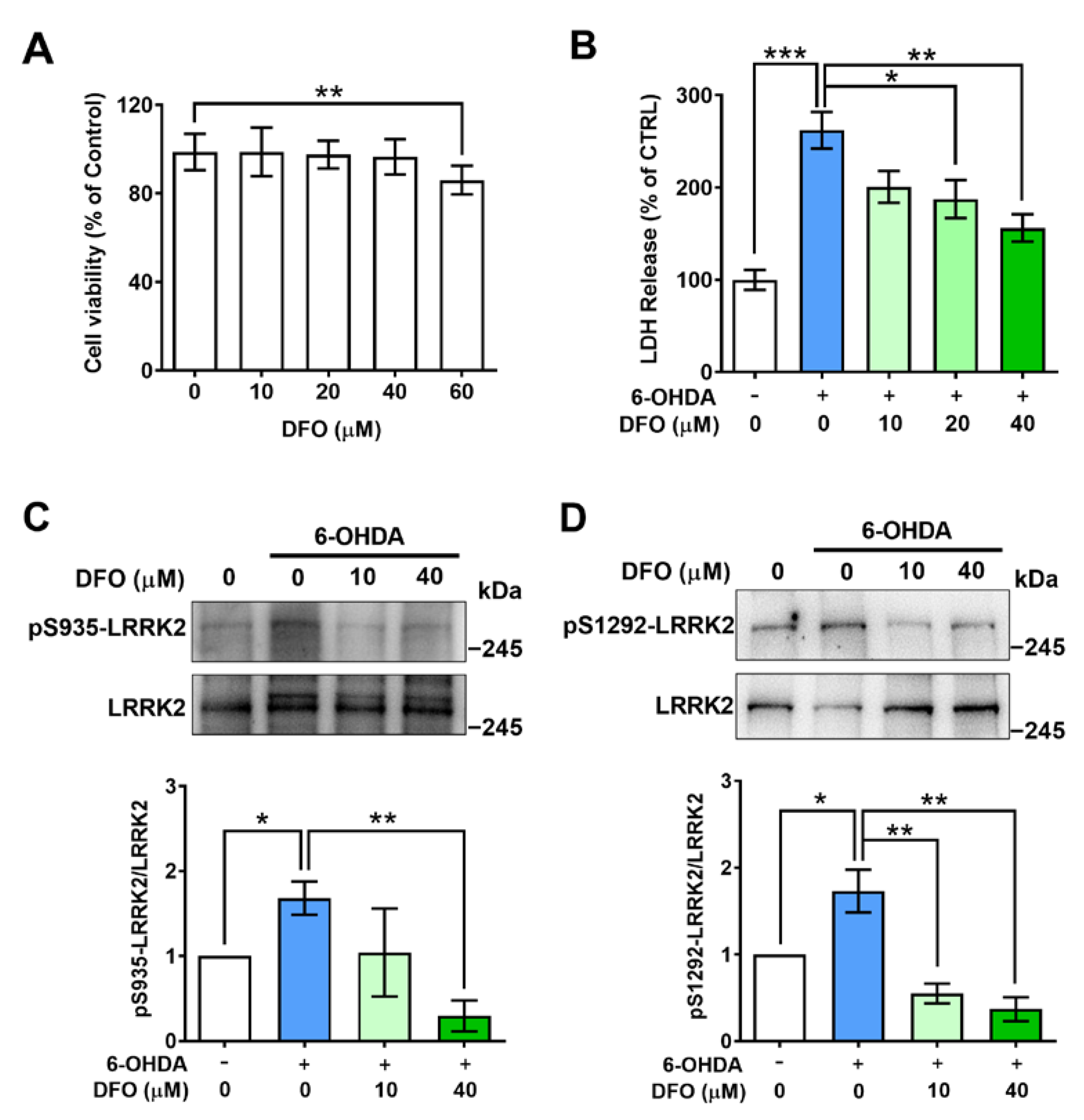
2.3. Desferrioxamine Attenuates LRRK2 Activation and Cell Damage Induced by 6-OHDA
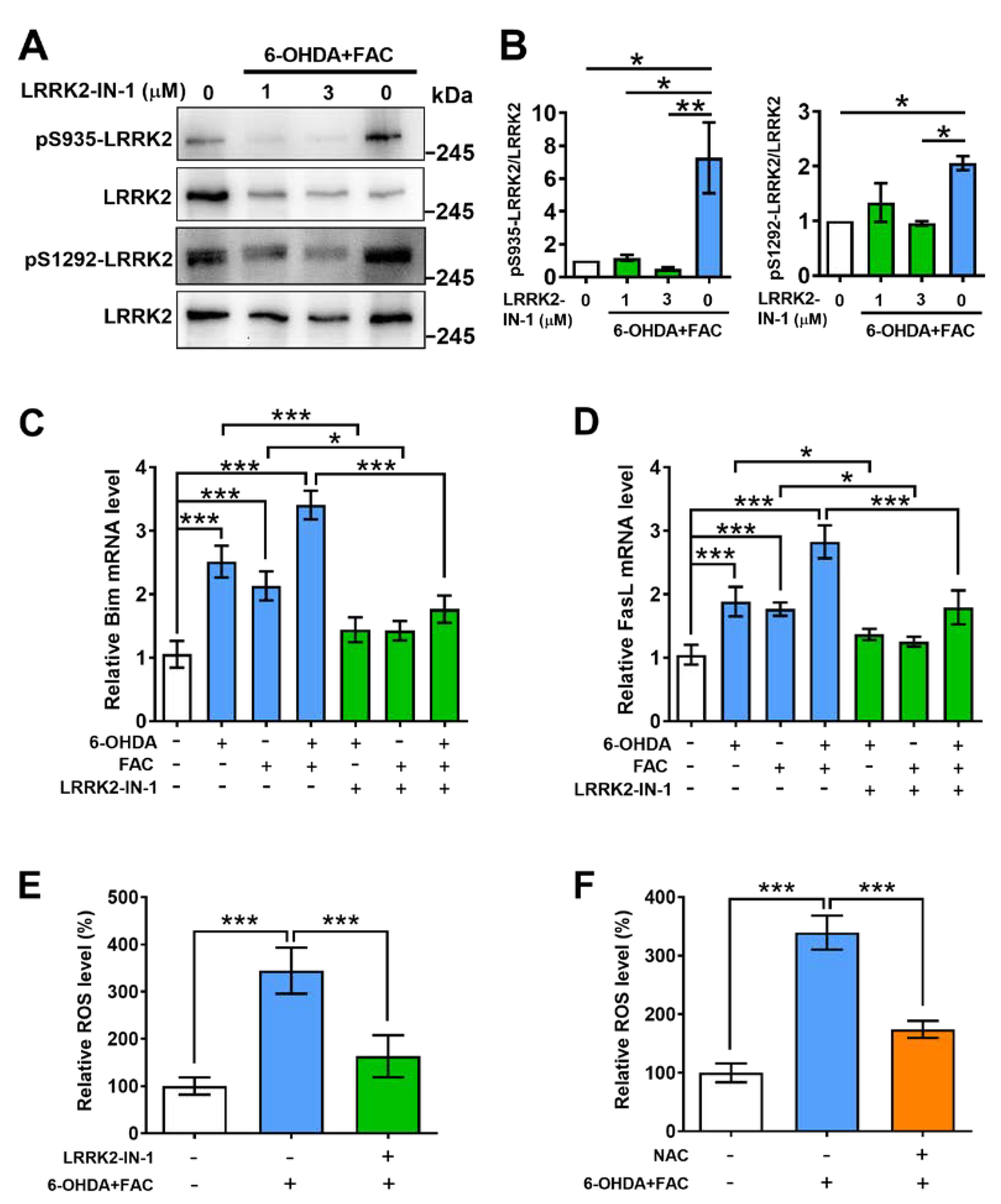
2.4. Inhibitor of LRRK2 Prevents Phosphorylation of LRRK2, Expression of Pro-Apoptotic Molecules, and Production of ROS Induced by 6-OHDA and Ferric Ammonium Citrate
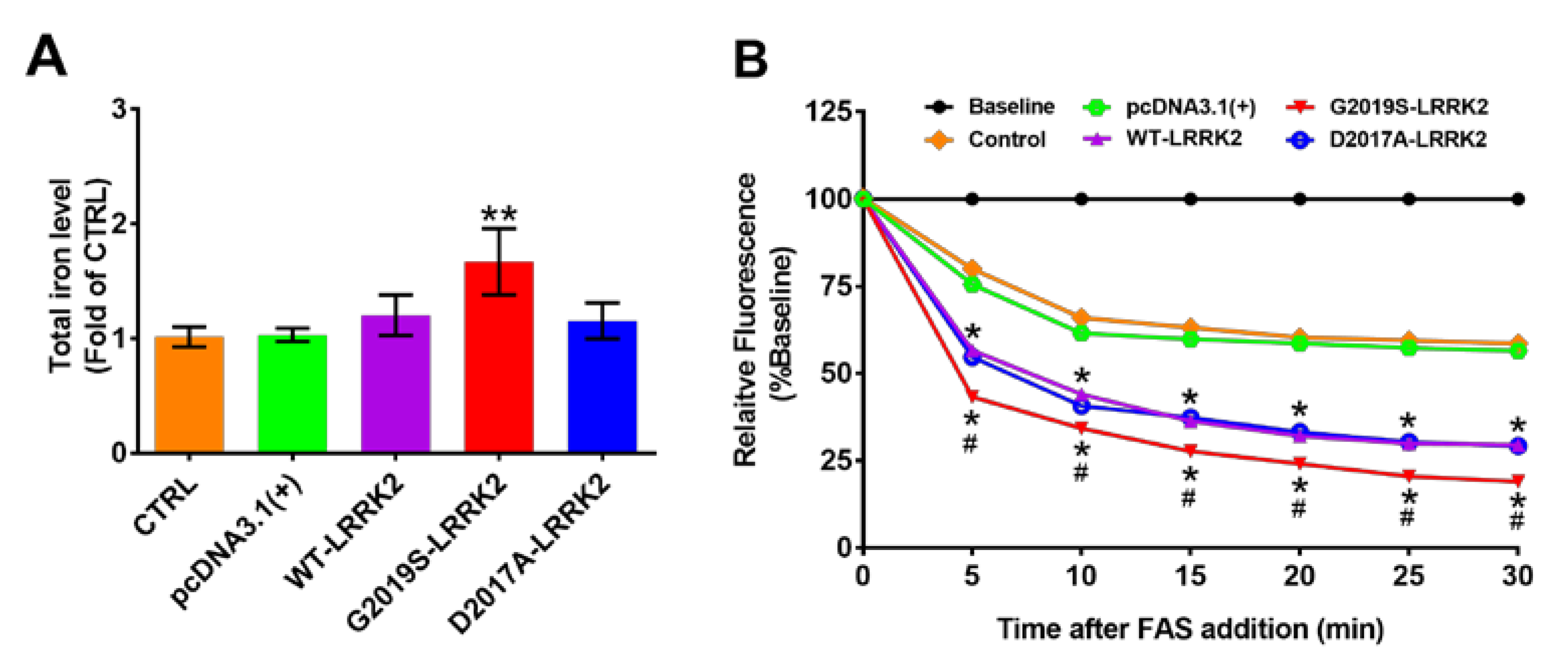
2.5. Activated LRRK2 Increases Total Iron Content and Ferrous Iron Uptake in SH-SY5Y Cells
3. Discussion
4. Materials and Methods
4.1. 6-OHD- Induced PD Model and Iron Dextran Administration
4.2. Immunohistochemistry
4.3. Measurement of Cell Viability
4.4. qPCR Assay
4.5. Western Blot Analysis
4.6. Measurement of Reactive Oxygen Species (ROS)
4.7. Total Iron Content Assay
4.8. Ferrous Iron Uptake Assay
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, H.M.; Hong, J.S. Gene-environment interactions: Key to unraveling the mystery of Parkinson’s disease. Prog. Neurobiol. 2011, 94, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.R.; Lee, B.D. Pathological Functions of LRRK2 in Parkinson’s Disease. Cells 2020, 9, 2565. [Google Scholar] [CrossRef] [PubMed]
- Konno, T.; Ross, O.A.; Puschmann, A.; Dickson, D.W.; Wszolek, Z.K. Autosomal dominant Parkinson’s disease caused by SNCA duplications. Park. Relat. Disord. 2016, 22 (Suppl. S1), S1–S6. [Google Scholar] [CrossRef] [PubMed]
- Pajarillo, E.; Rizor, A.; Lee, J.; Aschner, M.; Lee, E. The role of posttranslational modifications of α-synuclein and LRRK2 in Parkinson’s disease: Potential contributions of environmental factors. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2019, 1865, 1992–2000. [Google Scholar] [CrossRef] [PubMed]
- Mandel, S.A.; Fishman, T.; Youdim, M.B. Gene and protein signatures in sporadic Parkinson’s disease and a novel genetic model of PD. Park. Relat. Disord. 2007, 13 (Suppl. S3), S242–S247. [Google Scholar] [CrossRef] [PubMed]
- Wu-Chou, Y.H.; Chen, Y.T.; Yeh, T.H.; Chang, H.C.; Weng, Y.H.; Lai, S.C.; Huang, C.L.; Chen, R.S.; Huang, Y.Z.; Chen, C.C.; et al. Genetic variants of SNCA and LRRK2 genes are associated with sporadic PD susceptibility: A replication study in a Taiwanese cohort. Park. Relat. Disord. 2013, 19, 251–255. [Google Scholar] [CrossRef]
- Cookson, M.R. The role of leucine-rich repeat kinase 2 (LRRK2) in Parkinson’s disease. Nat. Rev. Neurosci. 2010, 11, 791–797. [Google Scholar] [CrossRef]
- MacLeod, D.; Dowman, J.; Hammond, R.; Leete, T.; Inoue, K.; Abeliovich, A. The familial Parkinsonism gene LRRK2 regulates neurite process morphology. Neuron 2006, 52, 587–593. [Google Scholar] [CrossRef]
- Puschmann, A.; Englund, E.; Ross, O.A.; Vilariño-Güell, C.; Lincoln, S.J.; Kachergus, J.M.; Cobb, S.A.; Törnqvist, A.L.; Rehncrona, S.; Widner, H.; et al. First neuropathological description of a patient with Parkinson’s disease and LRRK2 p.N1437H mutation. Park. Relat. Disord. 2012, 18, 332–338. [Google Scholar] [CrossRef]
- Sen, S.; Webber, P.J.; West, A.B. Dependence of leucine-rich repeat kinase 2 (LRRK2) kinase activity on dimerization. J. Biol. Chem. 2009, 284, 36346–36356. [Google Scholar] [CrossRef]
- Rocha, E.M.; Keeney, M.T.; Di Maio, R.; De Miranda, B.R.; Greenamyre, J.T. LRRK2 and idiopathic Parkinson’s disease. Trends Neurosci. 2022, 45, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Di Maio, R.; Hoffman, E.K.; Rocha, E.M.; Keeney, M.T.; Sanders, L.H.; De Miranda, B.R.; Zharikov, A.; Van Laar, A.; Stepan, A.F.; Lanz, T.A.; et al. LRRK2 activation in idiopathic Parkinson’s disease. Sci. Transl. Med. 2018, 10, eaar5429. [Google Scholar] [CrossRef] [PubMed]
- Kluss, J.H.; Conti, M.M.; Kaganovich, A.; Beilina, A.; Melrose, H.L.; Cookson, M.R.; Mamais, A. Detection of endogenous S1292 LRRK2 autophosphorylation in mouse tissue as a readout for kinase activity. NPJ Park. Dis. 2018, 4, 13. [Google Scholar] [CrossRef]
- Tolosa, E.; Vila, M.; Klein, C.; Rascol, O. LRRK2 in Parkinson disease: Challenges of clinical trials. Nat. Rev. Neurol. 2020, 16, 97–107. [Google Scholar] [CrossRef]
- Jennings, D.; Huntwork-Rodriguez, S.; Henry, A.G.; Sasaki, J.C.; Meisner, R.; Diaz, D.; Solanoy, H.; Wang, X.; Negrou, E.; Bondar, V.V.; et al. Preclinical and clinical evaluation of the LRRK2 inhibitor DNL201 for Parkinson’s disease. Sci. Transl. Med. 2022, 14, eabj2658. [Google Scholar] [CrossRef]
- Medeiros, M.S.; Schumacher-Schuh, A.; Cardoso, A.M.; Bochi, G.V.; Baldissarelli, J.; Kegler, A.; Santana, D.; Chaves, C.M.; Schetinger, M.R.; Moresco, R.N.; et al. Iron and Oxidative Stress in Parkinson’s Disease: An Observational Study of Injury Biomarkers. PLoS ONE 2016, 11, e0146129. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhou, Y.; Tang, G.; Wong, N.K.; Yang, M.; Tan, D.; Xiao, Y. Chemoproteomics Reveals the Antiproliferative Potential of Parkinson’s Disease Kinase Inhibitor LRRK2-IN-1 by Targeting PCNA Protein. Mol. Pharm. 2018, 15, 3252–3259. [Google Scholar] [CrossRef]
- Chang, K.H.; Chen, C.M. The Role of Oxidative Stress in Parkinson’s Disease. Antioxidants 2020, 9, 597. [Google Scholar] [CrossRef]
- Pyatigorskaya, N.; Sharman, M.; Corvol, J.C.; Valabregue, R.; Yahia-Cherif, L.; Poupon, F.; Cormier-Dequaire, F.; Siebner, H.; Klebe, S.; Vidailhet, M.; et al. High nigral iron deposition in LRRK2 and Parkin mutation carriers using R2* relaxometry. Mov. Disord. Off. J. Mov. Disord. Soc. 2015, 30, 1077–1084. [Google Scholar] [CrossRef]
- Berwick, D.C.; Harvey, K. LRRK2 signaling pathways: The key to unlocking neurodegeneration? Trends Cell Biol. 2011, 21, 257–265. [Google Scholar] [CrossRef]
- Yoon, J.-H.; Mo, J.-S.; Kim, M.-Y.; Ann, E.-J.; Ahn, J.-S.; Jo, E.-H.; Lee, H.-J.; Lee, Y.C.; Seol, W.; Yarmoluk, S.M.; et al. LRRK2 functions as a scaffolding kinase of ASK1-mediated neuronal cell death. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2017, 1864, 2356–2368. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Weng, Y.H.; Chien, K.Y.; Lin, K.J.; Yeh, T.H.; Cheng, Y.P.; Lu, C.S.; Wang, H.L. (G2019S) LRRK2 activates MKK4-JNK pathway and causes degeneration of SN dopaminergic neurons in a transgenic mouse model of PD. Cell Death Differ. 2012, 19, 1623–1633. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.; Qi, X.J.; Wildey, G.M.; Robinson, J.; Molkentin, J.; Letterio, J.; Howe, P.H. TGF beta-mediated BIM expression and apoptosis are regulated through SMAD3-dependent expression of the MAPK phosphatase MKP2. EMBO Rep. 2008, 9, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Li, T.; Liu, Z.; Arbez, N.; Yan, J.; Moran, T.H.; Ross, C.A.; Smith, W.W. LRRK2 kinase activity mediates toxic interactions between genetic mutation and oxidative stress in a Drosophila model: Suppression by curcumin. Neurobiol. Dis. 2012, 47, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, K.; Wang, X.; Smith, A.M.; Ning, B.; Liu, Z.; Liu, C.; Ross, C.A.; Smith, W.W. Curcumin Reduced H(2)O(2)- and G2385R-LRRK2-Induced Neurodegeneration. Front. Aging Neurosci. 2021, 13, 754956. [Google Scholar] [CrossRef] [PubMed]
- Martin, I.; Kim, J.W.; Dawson, V.L.; Dawson, T.M. LRRK2 pathobiology in Parkinson’s disease. J. Neurochem. 2014, 131, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Oun, A.; Soliman, A.; Trombetta-Lima, M.; Tzepapadaki, A.; Tsagkari, D.; Kortholt, A.; Dolga, A.M. LRRK2 protects immune cells against erastin-induced ferroptosis. Neurobiol. Dis. 2022, 175, 105917. [Google Scholar] [CrossRef] [PubMed]
- Mamais, A.; Kluss, J.H.; Bonet-Ponce, L.; Landeck, N.; Langston, R.G.; Smith, N.; Beilina, A.; Kaganovich, A.; Ghosh, M.C.; Pellegrini, L.; et al. Mutations in LRRK2 linked to Parkinson disease sequester Rab8a to damaged lysosomes and regulate transferrin-mediated iron uptake in microglia. PLoS Biol. 2021, 19, e3001480. [Google Scholar] [CrossRef]
- Xie, L.; Hu, L.F.; Teo, X.Q.; Tiong, C.X.; Tazzari, V.; Sparatore, A.; Del Soldato, P.; Dawe, G.S.; Bian, J.S. Therapeutic effect of hydrogen sulfide-releasing L-Dopa derivative ACS84 on 6-OHDA-induced Parkinson’s disease rat model. PLoS ONE 2013, 8, e60200. [Google Scholar] [CrossRef]
- Martí, M.J.; Saura, J.; Burke, R.E.; Jackson-Lewis, V.; Jiménez, A.; Bonastre, M.; Tolosa, E. Striatal 6-hydroxydopamine induces apoptosis of nigral neurons in the adult rat. Brain Res. 2002, 958, 185–191. [Google Scholar] [CrossRef]
- Wu, X.M.; Qian, C.; Zhou, Y.F.; Yan, Y.C.; Luo, Q.Q.; Yung, W.H.; Zhang, F.L.; Jiang, L.R.; Qian, Z.M.; Ke, Y. Bi-directionally protective communication between neurons and astrocytes under ischemia. Redox Biol. 2017, 13, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Riemer, J.; Hoepken, H.H.; Czerwinska, H.; Robinson, S.R.; Dringen, R. Colorimetric ferrozine-based assay for the quantitation of iron in cultured cells. Anal. Biochem. 2004, 331, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, D.; Wang, X.T.; Lu, Y.P.; Zhu, L. The roles of hypoxia-inducible Factor-1 and iron regulatory protein 1 in iron uptake induced by acute hypoxia. Biochem. Biophys. Res. Commun. 2018, 507, 128–135. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, R.; Liu, Y.; Shuai, K.; Zhou, C.; Chen, L.; Zhu, L.; Wu, X.-M. The Relationship between Iron and LRRK2 in a 6-OHDA-Induced Parkinson’s Disease Model. Int. J. Mol. Sci. 2023, 24, 3709. https://doi.org/10.3390/ijms24043709
Jia R, Liu Y, Shuai K, Zhou C, Chen L, Zhu L, Wu X-M. The Relationship between Iron and LRRK2 in a 6-OHDA-Induced Parkinson’s Disease Model. International Journal of Molecular Sciences. 2023; 24(4):3709. https://doi.org/10.3390/ijms24043709
Chicago/Turabian StyleJia, Ruru, Yanling Liu, Ke Shuai, Cheng Zhou, Lei Chen, Li Zhu, and Xiao-Mei Wu. 2023. "The Relationship between Iron and LRRK2 in a 6-OHDA-Induced Parkinson’s Disease Model" International Journal of Molecular Sciences 24, no. 4: 3709. https://doi.org/10.3390/ijms24043709
APA StyleJia, R., Liu, Y., Shuai, K., Zhou, C., Chen, L., Zhu, L., & Wu, X.-M. (2023). The Relationship between Iron and LRRK2 in a 6-OHDA-Induced Parkinson’s Disease Model. International Journal of Molecular Sciences, 24(4), 3709. https://doi.org/10.3390/ijms24043709



