Bacterial Metabolites: A Link between Gut Microbiota and Dermatological Diseases
Abstract
1. Introduction
2. Short-Chain Fatty Acids
2.1. Atopic Dermatitis and Hypersensitivity Reactions
2.2. Psoriasis
2.3. Connective Tissue Diseases
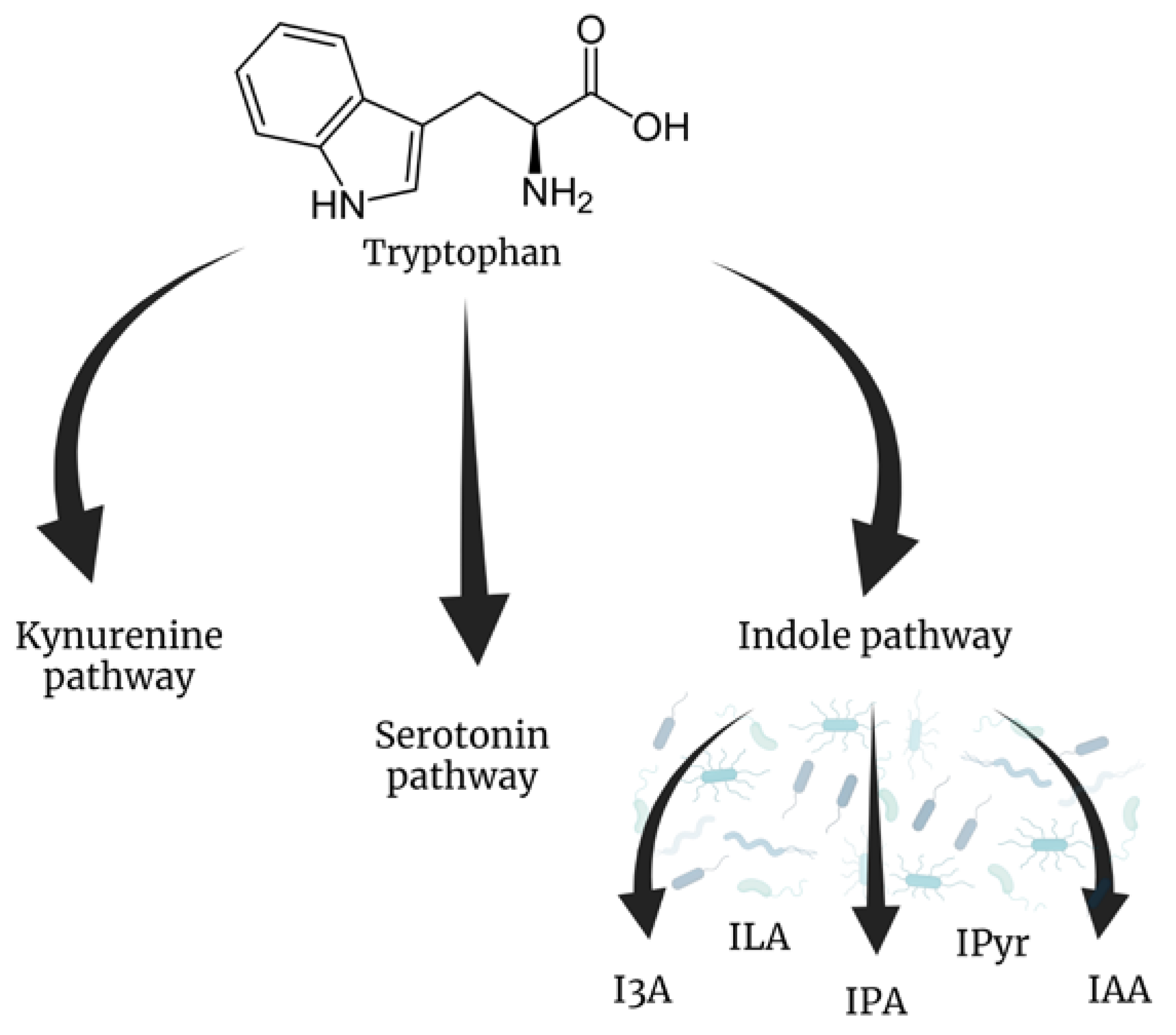
3. Tryptophan Metabolites
3.1. Atopic Dermatitis and Hypersensitivity Reactions
3.2. Lupus
4. Trimethylamine (TMA) and Trimethylamine N-oxide (TMAO)
Dermatological Context
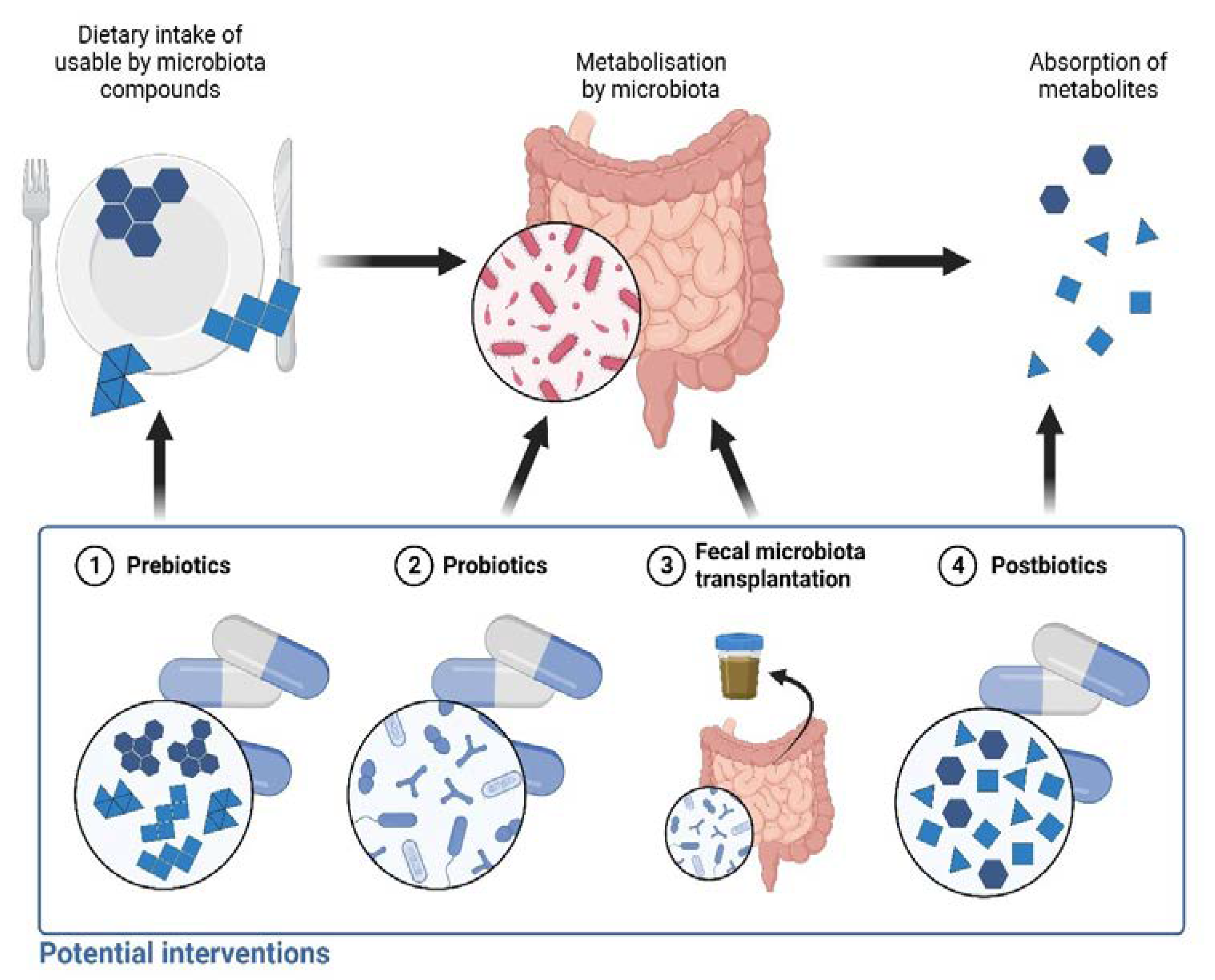
5. Future Perspectives
5.1. Prebiotics
5.2. Probiotics
5.3. Fecal Microbiota Transplantation
5.4. Postbiotics
| References | Disease | Studied Model | Main Findings |
|---|---|---|---|
| Short-chain fatty acids (SCFAs) | |||
| Schwarz et al. [61] | Psoriasis | imiquimod-induced psoriasis-like skin inflammation mouse model, skin biopsies from patients with psoriasis | Topical administration of sodium butyrate (SB) reduced symptoms of psoriasis-like skin inflammation Topical SB increased the number and activity of Treg cells, IL-10 transcription, and decreased IL-17 transcription in the skin of a mouse model SB increased the transcription of IL-10 and decreased the transcription of IL-6 and IL-17 in human skin biopsies |
| Krejner et al. [62] | Psoriasis | skin biopsies from patients with psoriasis | Ex vivo treatment with SB caused a reduction in IL-17 and IL-6, and an upregulation of IL-10 transcription in skin biopsies Skin with psoriasis has decreased expression of GPR109a and GPR43, SB upregulates these receptors |
| Rodríguez-Carrio et al. [67] | Systemic lupus erythematosus | 21 SLE patients, 25 healthy individuals | Fecal acetate and propionate are higher in patients with SLE compared to controls Dysbiosis in SLE patients |
| Sanchez et al. [28] | Systemic lupus erythematosus | MRL/lpr and NZB/W F1 lupus-prone mice | Oral mixture of sodium butyrate and sodium propionate reduced local and systemic antibody responses Orally administered SCFAsT reduced lupus skin lesions and kidney pathology |
| He et al. [68] | Systemic lupus erythematosus | MRL/lpr lupus-prone mice | Reduction in microbial diversity in the SLE mouse model Oral administration of SB reduced renal histopathological changes and increased microbiota diversity |
| Patrone et al. [69] | Systemic sclerosis | 18 SSc patients, 9 healthy subjects | Dysbiosis manifested as a decrease in butyrate-producing genera more prominent in patients with gastrointestinal involvement |
| Park et al. [71] | Systemic sclerosis | bleomycin-induced fibrosis mouse model of SSc, human dermal fibroblasts | SB administered orally or subcutaneously reduced bleomycin-induced dermal and lung fibrosis SB treatment inhibits TGF-β1-induced fibrotic responses in human dermal fibroblasts |
| Reddel et al. [35] | Atopic dermatitis | 19 children with AD and 18 healthy individuals | AD was characterized by dysbiosis, especially manifested in the depletion of butyrate-producing bacteria |
| Nylund et al. [36] | Atopic dermatitis | 28 infants with atopic dermatitis and 11 healthy infants | Less severe eczema was associated with increased butyrate-producing bacterial abundance and microbiome diversity |
| Song et al. [39] | Atopic dermatitis | 90 patients with AD and 42 volunteers without AD | Decreased fecal level of butyrate and propionate in AD patients Some subspecies of Faecalibacterium prausnitzii are linked with AD |
| Lee et al. [38] | Atopic dermatitis | 234 patients with mild to severe AD, 112 non-AD subjects | Diversity of the microbiota in moderate to severe AD was significantly lower than in non-AD Disordered gut microbiota development in AD was associated with dysregulated SCFA production |
| Roduit et al. [40] | Atopic dermatitis | 301 one-year-old children | Children with the highest fecal levels of butyrate and propionate were less prone to atopic sensitization and were less likely to develop asthma between the ages of 3 and 6 Food allergies and allergic rhinitis were less common in children with the highest butyrate levels |
| Cheng et al. [42] | Atopic dermatitis | 75 infants | Low fecal butyric acid was associated with an increased risk of developing atopic dermatitis, food sensitization, and wheezing up to 8 years old |
| Gio-Batta et al. [44] | Atopic dermatitis | 65 infants | A lower level of valeric acid at 3 years of age was associated with a higher prevalence of atopic eczema at the age of 8 years |
| Gio-Batta et al. [45] | Atopic dermatitis | 110 one-year-old children | Eczema at 13 years of age was inversely correlated with the amount of fecal valeric acid at 1 year of age |
| Folkerts et al. [52] | Allergy | Human mast cells | Propionate and butyrate inhibited IgE- and non-IgE-dependent human mast cell degranulation |
| Schwarz et al. [57] | Contact dermatitis | sensitized C57BL/6J mice | Sodium butyrate (SB) administered topically or subcutaneously inhibited both the elicitation phase and ongoing contact hypersensitivity response SB induced the anti-inflammatory response via an increase in the number of skin Treg cells and an increase in IL-10 transcription |
| Trompette et al. [33] | Atopic dermatitis | atopic dermatitis-like skin inflammation mouse model | Fermentable fiber-rich diet or orally administered sodium butyrate alleviate systemic allergen sensitization and disease severity Oral butyrate stimulates terminal differentiation of epidermal keratinocytes and promotes skin barrier function |
| Tryptophan metabolites | |||
| Tsuji et al. [86] | Atopic dermatitis | normal human epidermal keratinocytes | The activation of AHR by tryptophane metabolite significantly increased filaggrin expression FICZ reversed the IL-4-induced downregulation in transcription and protein levels of filaggrin |
| Aoki et al. [89] | UVB-induced skin damage | HR-1 mice, HaCaT keratinocytes | Topical application of indole-3-pyruvate reduced the severity of UVB-induced skin lesions, the augmentation of dermal thickness, and transepithelial water loss Suppression of the overproduction of IL-1b and IL-6 in response to UVB radiation in a mouse model Indole-3-pyruvate improved the survival rate and reduced the expression of IL-1b and IL-6 in UVB-exposed HaCaT keratinocytes |
| Fang et al. [97] | Atopic dermatitis | 87 patients with atopic dermatitis, sensitized female C57BL/6 mice | Bifidobacterium longum probiotic treatment increased serum and fecal indole-3-carbaldehyde, significantly reduced AD symptoms Indole-3-carbaldehyde displayed a significant negative correlation with atopic dermatitis severity measured in both SCORAD and DLQI Oral administration of indole-3-carbaldehyde alleviated AD-like skin lesions in sensitized mice |
| Yu et al. [81] | Atopic dermatitis | 19 patients with AD, 19 healthy volunteers, sensitized C57BL/6 and BALB/c mice | Decreased indole-3-aldehyde was observed in both lesional and non-lesional skin of AD patients Topical and orally administered indole-3-aldehyde attenuated MC903-induced AD-like dermatitis in mouse and decreased expression of IL-4, IL-5, IL-6, IL-13, and TSLP Topically administered indole-3-aldehyde reduced inflammatory cell infiltration in mice |
| Kiyomatsu-Oda et al. [100] | Atopic dermatitis | NC/Nga mice, HaCaT cells and normal human epidermal keratinocytes (NHEKs) | Tryptophan metabolite FICZ improved symptoms of AD-like dermatitis, decreased TEWL, restored filaggrin expression, reduced the number of infiltrated mast cells, and reduced expression of IL-22 and IFN-γ genes in a mouse model FICZ upregulated expression and abundance of filaggrin in HaCaT and NHEKs cells |
| Singh et al. [104] | Delayed-type hypersensitivity | Sensitized C57BL/6 mice | Topical administration of indole-3-carbinol and 3,3′-diindolylmethane alleviated symptoms, triggered induction of Tregs, and suppressed Th17 cells of delayed-type hypersensitivity in a mouse model FICZ exacerbated disease in a mouse model and suppressed Treg cells |
| Shinde et al. [108] | Systemic lupus erythematosus | 48 patients with active SLE, 24 patients with SLE in remission, and 20 control subjects | Serum indole-3-propionic acid was significantly higher than in the control group |
| Trimethylamine N-oxide (TMAO) | |||
| Sikora et al. [127] | Psoriasis | 72 patients with psoriasis and 40 matched controls | In patients with psoriasis, serum TMAO was significantly higher than in the control group TMAO was found to be an independent predictor of cardiovascular risk |
| Sun et al. [128] | Psoriasis | 180 patients with psoriasis, 60 healthy controls | Psoriatic patients had significantly higher serum levels of TMAO compared to controls TMAO had a positive correlation with PASI score |
| Coras et al. [129] | Psoriatic arthritis | 38 patients with psoriatic arthritis | Serum TMAO demonstrated a significant correlation with indicators of disease activity for the skin and peripheral joints |
| Barea et al. [130] | Hidradenitis suppurativa | 35 patients with hidradenitis suppurativa and 35 matched controls | Patients had increased serum TMAO levels compared to controls The level of circulating TMAO correlated positively with the HS Sartorius score also after adjustment for confounding factors Serum TMAO levels and PhA were the two primary indicators of the clinical severity of HS based on a linear regression model |
| Li et al. [133] | Systemic lupus erythematosus | 17 patients with SLE and 17 healthy controls | Serum levels of trimethylamine N-oxide (TMAO) were found to be elevated in lupus patients compared to controls |
| González-Correa et al. [134] | Systemic lupus erythematosus | Imiquimod-induced mouse model of SLE | Plasma TMAO concentrations were significantly elevated in the serum of active systemic lupus erythematosus patients |
| Wu et al. [121] | Graft-versus-host disease | C57BL/6 and BALB/c mice | Induced by oral administration elevation of plasma TMAO was associated with worse course and survival of graft-versus-host disease |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ipci, K.; Altintoprak, N.; Muluk, N.B.; Senturk, M.; Cingi, C. The possible mechanisms of the human microbiome in allergic diseases. Eur. Arch. Otorhinolaryngol. 2017, 274, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Alesa, D.I.; Alshamrani, H.M.; Alzahrani, Y.A.; Alamssi, D.N.; Alzahrani, N.S.; Almohammadi, M.E. The role of gut microbiome in the pathogenesis of psoriasis and the therapeutic effects of probiotics. J. Family Med. Prim. Care 2019, 8, 3496–3503. [Google Scholar] [CrossRef] [PubMed]
- Myers, B.; Brownstone, N.; Reddy, V.; Chan, S.; Thibodeaux, Q.; Truong, A.; Bhutani, T.; Chang, H.W.; Liao, W. The gut microbiome in psoriasis and psoriatic arthritis. Best Pract. Res. Clin. Rheumatol. 2019, 33, 101494. [Google Scholar] [CrossRef] [PubMed]
- Sikora, M.; Stec, A.; Chrabaszcz, M.; Knot, A.; Waskiel-Burnat, A.; Rakowska, A.; Olszewska, M.; Rudnicka, L. Gut Microbiome in Psoriasis: An Updated Review. Pathogens 2020, 9, 463. [Google Scholar] [CrossRef]
- Hevia, A.; Milani, C.; López, P.; Cuervo, A.; Arboleya, S.; Duranti, S.; Turroni, F.; González, S.; Suárez, A.; Gueimonde, M.; et al. Intestinal dysbiosis associated with systemic lupus erythematosus. MBio 2014, 5, e01548-14. [Google Scholar] [CrossRef]
- Lam, S.Y.; Radjabzadeh, D.; Eppinga, H.; Nossent, Y.R.A.; van der Zee, H.H.; Kraaij, R.; Konstantinov, S.R.; Fuhler, G.M.; Prens, E.P.; Thio, H.B.; et al. A microbiome study to explore the gut-skin axis in hidradenitis suppurativa. J. Dermatol. Sci. 2021, 101, 218–220. [Google Scholar] [CrossRef]
- Pothmann, A.; Illing, T.; Wiegand, C.; Hartmann, A.A.; Elsner, P. The Microbiome and Atopic Dermatitis: A Review. Am. J. Clin. Dermatol. 2019, 20, 749–761. [Google Scholar] [CrossRef]
- Volkmann, E.R.; Chang, Y.L.; Barroso, N.; Furst, D.E.; Clements, P.J.; Gorn, A.H.; Roth, B.E.; Conklin, J.L.; Getzug, T.; Borneman, J.; et al. Association of Systemic Sclerosis With a Unique Colonic Microbial Consortium. Arthritis Rheumatol. 2016, 68, 1483–1492. [Google Scholar] [CrossRef]
- Volkmann, E.R.; Hoffmann-Vold, A.M.; Chang, Y.L.; Jacobs, J.P.; Tillisch, K.; Mayer, E.A.; Clements, P.J.; Hov, J.R.; Kummen, M.; Midtvedt, Ø.; et al. Systemic sclerosis is associated with specific alterations in gastrointestinal microbiota in two independent cohorts. BMJ Open Gastroenterol. 2017, 4, e000134. [Google Scholar] [CrossRef]
- Brown, J.; Robusto, B.; Morel, L. Intestinal Dysbiosis and Tryptophan Metabolism in Autoimmunity. Front. Immunol. 2020, 11, 1741. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Takahashi, D.; Takano, S.; Kimura, S.; Hase, K. The Roles of Peyer’s Patches and Microfold Cells in the Gut Immune System: Relevance to Autoimmune Diseases. Front. Immunol. 2019, 10, 2345. [Google Scholar] [CrossRef] [PubMed]
- Garabatos, N.; Santamaria, P. Gut Microbial Antigenic Mimicry in Autoimmunity. Front. Immunol. 2022, 13, 873607. [Google Scholar] [CrossRef]
- Yao, Y.; Cai, X.; Fei, W.; Ye, Y.; Zhao, M.; Zheng, C. The role of short-chain fatty acids in immunity, inflammation and metabolism. Crit. Rev. Food Sci. Nutr. 2022, 62, 1–12. [Google Scholar] [CrossRef]
- Gatarek, P.; Kaluzna-Czaplinska, J. Trimethylamine N-oxide (TMAO) in human health. EXCLI J. 2021, 20, 301–319. [Google Scholar] [CrossRef]
- Kimura, I.; Ichimura, A.; Ohue-Kitano, R.; Igarashi, M. Free Fatty Acid Receptors in Health and Disease. Physiol. Rev. 2020, 100, 171–210. [Google Scholar] [CrossRef] [PubMed]
- Szelest, M.; Walczak, K.; Plech, T. A New Insight into the Potential Role of Tryptophan-Derived AhR Ligands in Skin Physiological and Pathological Processes. Int. J. Mol. Sci. 2021, 22, 1104. [Google Scholar] [CrossRef]
- Chen, S.; Henderson, A.; Petriello, M.C.; Romano, K.A.; Gearing, M.; Miao, J.; Schell, M.; Sandoval-Espinola, W.J.; Tao, J.; Sha, B.; et al. Trimethylamine N-Oxide Binds and Activates PERK to Promote Metabolic Dysfunction. Cell Metab. 2019, 30, 1141–1151.e1145. [Google Scholar] [CrossRef]
- De Pessemier, B.; Grine, L.; Debaere, M.; Maes, A.; Paetzold, B.; Callewaert, C. Gut-Skin Axis: Current Knowledge of the Interrelationship between Microbial Dysbiosis and Skin Conditions. Microorganisms 2021, 9, 353. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [CrossRef]
- Kebede, A.F.; Nieborak, A.; Shahidian, L.Z.; Le Gras, S.; Richter, F.; Gómez, D.A.; Baltissen, M.P.; Meszaros, G.; Magliarelli, H.F.; Taudt, A.; et al. Histone propionylation is a mark of active chromatin. Nat. Struct. Mol. Biol. 2017, 24, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Usami, M.; Kishimoto, K.; Ohata, A.; Miyoshi, M.; Aoyama, M.; Fueda, Y.; Kotani, J. Butyrate and trichostatin A attenuate nuclear factor kappaB activation and tumor necrosis factor alpha secretion and increase prostaglandin E2 secretion in human peripheral blood mononuclear cells. Nutr. Res. 2008, 28, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H. Control of lymphocyte functions by gut microbiota-derived short-chain fatty acids. Cell Mol. Immunol. 2021, 18, 1161–1171. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef]
- Takahashi, D.; Hoshina, N.; Kabumoto, Y.; Maeda, Y.; Suzuki, A.; Tanabe, H.; Isobe, J.; Yamada, T.; Muroi, K.; Yanagisawa, Y.; et al. Microbiota-derived butyrate limits the autoimmune response by promoting the differentiation of follicular regulatory T cells. EBioMedicine 2020, 58, 102913. [Google Scholar] [CrossRef]
- He, J.; Chu, Y.; Li, J.; Meng, Q.; Liu, Y.; Jin, J.; Wang, Y.; Wang, J.; Huang, B.; Shi, L.; et al. Intestinal butyrate-metabolizing species contribute to autoantibody production and bone erosion in rheumatoid arthritis. Sci. Adv. 2022, 8, eabm1511. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef]
- Sanchez, H.N.; Moroney, J.B.; Gan, H.; Shen, T.; Im, J.L.; Li, T.; Taylor, J.R.; Zan, H.; Casali, P. B cell-intrinsic epigenetic modulation of antibody responses by dietary fiber-derived short-chain fatty acids. Nat. Commun. 2020, 11, 60. [Google Scholar] [CrossRef]
- Yao, Y.; Cai, X.; Zheng, Y.; Zhang, M.; Fei, W.; Sun, D.; Zhao, M.; Ye, Y.; Zheng, C. Short-chain fatty acids regulate B cells differentiation via the FFA2 receptor to alleviate rheumatoid arthritis. Br. J. Pharmacol. 2022, 179, 4315–4329. [Google Scholar] [CrossRef]
- Luu, M.; Pautz, S.; Kohl, V.; Singh, R.; Romero, R.; Lucas, S.; Hofmann, J.; Raifer, H.; Vachharajani, N.; Carrascosa, L.C.; et al. The short-chain fatty acid pentanoate suppresses autoimmunity by modulating the metabolic-epigenetic crosstalk in lymphocytes. Nat. Commun. 2019, 10, 760. [Google Scholar] [CrossRef]
- Yuille, S.; Reichardt, N.; Panda, S.; Dunbar, H.; Mulder, I.E. Human gut bacteria as potent class I histone deacetylase inhibitors in vitro through production of butyric acid and valeric acid. PLoS ONE 2018, 13, e0201073. [Google Scholar] [CrossRef]
- Zhu, T.H.; Zhu, T.R.; Tran, K.A.; Sivamani, R.K.; Shi, V.Y. Epithelial barrier dysfunctions in atopic dermatitis: A skin-gut-lung model linking microbiome alteration and immune dysregulation. Br. J. Dermatol. 2018, 179, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Trompette, A.; Pernot, J.; Perdijk, O.; Alqahtani, R.A.A.; Domingo, J.S.; Camacho-Munoz, D.; Wong, N.C.; Kendall, A.C.; Wiederkehr, A.; Nicod, L.P.; et al. Gut-derived short-chain fatty acids modulate skin barrier integrity by promoting keratinocyte metabolism and differentiation. Mucosal Immunol. 2022, 15, 908–926. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G.; Abe, A.; Jin, K.; Higaki, Y.; Kawashima, M.; Hidano, A. Decreased level of ceramides in stratum corneum of atopic dermatitis: An etiologic factor in atopic dry skin? J. Invest. Dermatol. 1991, 96, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Reddel, S.; Del Chierico, F.; Quagliariello, A.; Giancristoforo, S.; Vernocchi, P.; Russo, A.; Fiocchi, A.; Rossi, P.; Putignani, L.; El Hachem, M. Gut microbiota profile in children affected by atopic dermatitis and evaluation of intestinal persistence of a probiotic mixture. Sci. Rep. 2019, 9, 4996. [Google Scholar] [CrossRef] [PubMed]
- Nylund, L.; Nermes, M.; Isolauri, E.; Salminen, S.; de Vos, W.M.; Satokari, R. Severity of atopic disease inversely correlates with intestinal microbiota diversity and butyrate-producing bacteria. Allergy 2015, 70, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Candela, M.; Rampelli, S.; Turroni, S.; Severgnini, M.; Consolandi, C.; De Bellis, G.; Masetti, R.; Ricci, G.; Pession, A.; Brigidi, P. Unbalance of intestinal microbiota in atopic children. BMC Microbiol. 2012, 12, 95. [Google Scholar] [CrossRef]
- Lee, M.J.; Park, Y.M.; Kim, B.; Tae, I.H.; Kim, N.E.; Pranata, M.; Kim, T.; Won, S.; Kang, N.J.; Lee, Y.K.; et al. Disordered development of gut microbiome interferes with the establishment of the gut ecosystem during early childhood with atopic dermatitis. Gut Microbes 2022, 14, 2068366. [Google Scholar] [CrossRef]
- Song, H.; Yoo, Y.; Hwang, J.; Na, Y.C.; Kim, H.S. Faecalibacterium prausnitzii subspecies-level dysbiosis in the human gut microbiome underlying atopic dermatitis. J. Allergy Clin. Immunol. 2016, 137, 852–860. [Google Scholar] [CrossRef]
- Roduit, C.; Frei, R.; Ferstl, R.; Loeliger, S.; Westermann, P.; Rhyner, C.; Schiavi, E.; Barcik, W.; Rodriguez-Perez, N.; Wawrzyniak, M.; et al. High levels of butyrate and propionate in early life are associated with protection against atopy. Allergy 2019, 74, 799–809. [Google Scholar] [CrossRef]
- Wopereis, H.; Sim, K.; Shaw, A.; Warner, J.O.; Knol, J.; Kroll, J.S. Intestinal microbiota in infants at high risk for allergy: Effects of prebiotics and role in eczema development. J. Allergy Clin. Immunol. 2018, 141, 1334–1342.e1335. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.Y.; Chan, J.C.Y.; Yap, G.C.; Huang, C.H.; Kioh, D.Y.Q.; Tham, E.H.; Loo, E.X.L.; Shek, L.P.C.; Karnani, N.; Goh, A.; et al. Evaluation of Stool Short Chain Fatty Acids Profiles in the First Year of Life With Childhood Atopy-Related Outcomes. Front. Allergy 2022, 3, 873168. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.M.; Lee, S.Y.; Kang, M.J.; Kim, B.S.; Lee, M.J.; Jung, S.S.; Yoon, J.S.; Cho, H.J.; Lee, E.; Yang, S.I.; et al. Imbalance of Gut Streptococcus, Clostridium, and Akkermansia Determines the Natural Course of Atopic Dermatitis in Infant. Allergy Asthma Immunol. Res. 2020, 12, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Gio-Batta, M.; Sjöberg, F.; Jonsson, K.; Barman, M.; Lundell, A.C.; Adlerberth, I.; Hesselmar, B.; Sandberg, A.S.; Wold, A.E. Fecal short chain fatty acids in children living on farms and a link between valeric acid and protection from eczema. Sci. Rep. 2020, 10, 22449. [Google Scholar] [CrossRef] [PubMed]
- Gio-Batta, M.; Spetz, K.; Barman, M.; Braback, L.; Norin, E.; Bjorksten, B.; Wold, A.E.; Sandin, A. Low Concentration of Fecal Valeric Acid at 1 Year of Age Is Linked with Eczema and Food Allergy at 13 Years of Age: Findings from a Swedish Birth Cohort. Int. Arch. Allergy Immunol. 2022, 183, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Mubanga, M.; Lundholm, C.; D’Onofrio, B.M.; Stratmann, M.; Hedman, A.; Almqvist, C. Association of Early Life Exposure to Antibiotics With Risk of Atopic Dermatitis in Sweden. JAMA Netw. Open 2021, 4, e215245. [Google Scholar] [CrossRef] [PubMed]
- Slob, E.M.A.; Brew, B.K.; Vijverberg, S.J.H.; Kats, C.; Longo, C.; Pijnenburg, M.W.; van Beijsterveldt, T.; Dolan, C.V.; Bartels, M.; Magnusson, P.; et al. Early-life antibiotic use and risk of asthma and eczema: Results of a discordant twin study. Eur. Respir. J. 2020, 55, 1902021. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, S.H.; Hong, S.J. Antibiotics-Induced Dysbiosis of Intestinal Microbiota Aggravates Atopic Dermatitis in Mice by Altered Short-Chain Fatty Acids. Allergy Asthma Immunol. Res. 2020, 12, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Palomares, O.; Akdis, M.; Martín-Fontecha, M.; Akdis, C.A. Mechanisms of immune regulation in allergic diseases: The role of regulatory T and B cells. Immunol. Rev. 2017, 278, 219–236. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B. Regulation of IgE-Mediated Food Allergy by IL-9 Producing Mucosal Mast Cells and Type 2 Innate Lymphoid Cells. Immune Netw. 2016, 16, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Freeland, D.M.H.; Nadeau, K.C. Food allergy: Immune mechanisms, diagnosis and immunotherapy. Nat. Rev. Immunol. 2016, 16, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Folkerts, J.; Redegeld, F.; Folkerts, G.; Blokhuis, B.; van den Berg, M.P.M.; de Bruijn, M.J.W.; van IJcken, W.F.; Junt, T.; Tam, S.Y.; Galli, S.J.; et al. Butyrate inhibits human mast cell activation via epigenetic regulation of FcepsilonRI-mediated signaling. Allergy 2020, 75, 1966–1978. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Wu, H.; Lin, F.H.; Gong, R.; Xie, F.; Peng, Y.; Feng, J.; Hu, C.H. Sodium butyrate enhances intestinal integrity, inhibits mast cell activation, inflammatory mediator production and JNK signaling pathway in weaned pigs. Innate Immun. 2018, 24, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Yip, W.; Hughes, M.R.; Li, Y.; Cait, A.; Hirst, M.; Mohn, W.W.; McNagny, K.M. Butyrate Shapes Immune Cell Fate and Function in Allergic Asthma. Front. Immunol. 2021, 12, 628453. [Google Scholar] [CrossRef]
- Goldberg, M.R.; Mor, H.; Magid Neriya, D.; Magzal, F.; Muller, E.; Appel, M.Y.; Nachshon, L.; Borenstein, E.; Tamir, S.; Louzoun, Y.; et al. Microbial signature in IgE-mediated food allergies. Genome. Med. 2020, 12, 92. [Google Scholar] [CrossRef]
- Chiu, C.Y.; Cheng, M.L.; Chiang, M.H.; Wang, C.J.; Tsai, M.H.; Lin, G. Integrated metabolic and microbial analysis reveals host-microbial interactions in IgE-mediated childhood asthma. Sci. Rep. 2021, 11, 23407. [Google Scholar] [CrossRef]
- Schwarz, A.; Bruhs, A.; Schwarz, T. The Short-Chain Fatty Acid Sodium Butyrate Functions as a Regulator of the Skin Immune System. J. Invest. Dermatol. 2017, 137, 855–864. [Google Scholar] [CrossRef]
- Nakamura, N.; Tamagawa-Mineoka, R.; Ueta, M.; Kinoshita, S.; Katoh, N. Toll-like receptor 3 increases allergic and irritant contact dermatitis. J. Invest. Dermatol. 2015, 135, 411–417. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Vuillermin, P.J.; Goverse, G.; Vinuesa, C.G.; Mebius, R.E.; Macia, L.; Mackay, C.R. Dietary Fiber and Bacterial SCFA Enhance Oral Tolerance and Protect against Food Allergy through Diverse Cellular Pathways. Cell Rep. 2016, 15, 2809–2824. [Google Scholar] [CrossRef]
- Shi, Y.; Xu, M.; Pan, S.; Gao, S.; Ren, J.; Bai, R.; Li, H.; He, C.; Zhao, S.; Shi, Z.; et al. Induction of the apoptosis, degranulation and IL-13 production of human basophils by butyrate and propionate via suppression of histone deacetylation. Immunology 2021, 164, 292–304. [Google Scholar] [CrossRef]
- Schwarz, A.; Philippsen, R.; Schwarz, T. Induction of Regulatory T Cells and Correction of Cytokine Disbalance by Short-Chain Fatty Acids: Implications for Psoriasis Therapy. J. Invest. Dermatol. 2021, 141, 95–104.e102. [Google Scholar] [CrossRef] [PubMed]
- Krejner, A.; Bruhs, A.; Mrowietz, U.; Wehkamp, U.; Schwarz, T.; Schwarz, A. Decreased expression of G-protein-coupled receptors GPR43 and GPR109a in psoriatic skin can be restored by topical application of sodium butyrate. Arch. Dermatol. Res. 2018, 310, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Mrowietz, U.; Morrison, P.J.; Suhrkamp, I.; Kumanova, M.; Clement, B. The Pharmacokinetics of Fumaric Acid Esters Reveal Their In Vivo Effects. Trends Pharmacol. Sci. 2018, 39, 1–12. [Google Scholar] [CrossRef]
- Tang, H.; Lu, J.Y.; Zheng, X.; Yang, Y.; Reagan, J.D. The psoriasis drug monomethylfumarate is a potent nicotinic acid receptor agonist. Biochem. Biophys. Res. Commun. 2008, 375, 562–565. [Google Scholar] [CrossRef] [PubMed]
- Ota, S.; Sakuraba, H.; Hiraga, H.; Yoshida, S.; Satake, M.; Akemoto, Y.; Tanaka, N.; Watanabe, R.; Takato, M.; Murai, Y.; et al. Cyclosporine protects from intestinal epithelial injury by modulating butyrate uptake via upregulation of membrane monocarboxylate transporter 1 levels. Biochem. Biophys. Rep. 2020, 24, 100811. [Google Scholar] [CrossRef]
- De Luca, F.; Shoenfeld, Y. The microbiome in autoimmune diseases. Clin. Exp. Immunol. 2019, 195, 74–85. [Google Scholar] [CrossRef]
- Rodriguez-Carrio, J.; Lopez, P.; Sanchez, B.; Gonzalez, S.; Gueimonde, M.; Margolles, A.; de Los Reyes-Gavilan, C.G.; Suarez, A. Intestinal Dysbiosis Is Associated with Altered Short-Chain Fatty Acids and Serum-Free Fatty Acids in Systemic Lupus Erythematosus. Front. Immunol. 2017, 8, 23. [Google Scholar] [CrossRef]
- He, H.; Xu, H.; Xu, J.; Zhao, H.; Lin, Q.; Zhou, Y.; Nie, Y. Sodium Butyrate Ameliorates Gut Microbiota Dysbiosis in Lupus-Like Mice. Front. Nutr. 2020, 7, 604283. [Google Scholar] [CrossRef] [PubMed]
- Patrone, V.; Puglisi, E.; Cardinali, M.; Schnitzler, T.S.; Svegliati, S.; Festa, A.; Gabrielli, A.; Morelli, L. Gut microbiota profile in systemic sclerosis patients with and without clinical evidence of gastrointestinal involvement. Sci. Rep. 2017, 7, 14874. [Google Scholar] [CrossRef]
- Tan, T.C.; Noviani, M.; Leung, Y.Y.; Low, A.H.L. The microbiome and systemic sclerosis: A review of current evidence. Best Pract. Res. Clin. Rheumatol. 2021, 35, 101687. [Google Scholar] [CrossRef]
- Park, H.J.; Jeong, O.Y.; Chun, S.H.; Cheon, Y.H.; Kim, M.; Kim, S.; Lee, S.I. Butyrate Improves Skin/Lung Fibrosis and Intestinal Dysbiosis in Bleomycin-Induced Mouse Models. Int. J. Mol. Sci. 2021, 22, 2765. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef] [PubMed]
- Magni, G.; Amici, A.; Emanuelli, M.; Raffaelli, N.; Ruggieri, S. Enzymology of NAD+ synthesis. Adv. Enzymol. Relat. Areas Mol. Biol. 1999, 73, 135–182. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Fink, G.R. Feedback control of morphogenesis in fungi by aromatic alcohols. Genes Dev. 2006, 20, 1150–1161. [Google Scholar] [CrossRef]
- Elleuch, L.; Shaaban, M.; Smaoui, S.; Mellouli, L.; Karray-Rebai, I.; Fourati-Ben Fguira, L.; Shaaban, K.A.; Laatsch, H. Bioactive secondary metabolites from a new terrestrial Streptomyces sp. TN262. Appl. Biochem. Biotechnol. 2010, 162, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Honore, A.H.; Aunsbjerg, S.D.; Ebrahimi, P.; Thorsen, M.; Benfeldt, C.; Knochel, S.; Skov, T. Metabolic footprinting for investigation of antifungal properties of Lactobacillus paracasei. Anal. Bioanal. Chem. 2016, 408, 83–96. [Google Scholar] [CrossRef]
- Landete, J.M.; Rodriguez, H.; De las Rivas, B.; Munoz, R. High-added-value antioxidants obtained from the degradation of wine phenolics by Lactobacillus plantarum. J. Food Prot. 2007, 70, 2670–2675. [Google Scholar] [CrossRef]
- Chimerel, C.; Emery, E.; Summers, D.K.; Keyser, U.; Gribble, F.M.; Reimann, F. Bacterial metabolite indole modulates incretin secretion from intestinal enteroendocrine L cells. Cell Rep. 2014, 9, 1202–1208. [Google Scholar] [CrossRef]
- Fernández-Gallego, N.; Sánchez-Madrid, F.; Cibrian, D. Role of AHR Ligands in Skin Homeostasis and Cutaneous Inflammation. Cells 2021, 10, 3176. [Google Scholar] [CrossRef]
- Esser, C.; Rannug, A. The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology. Pharmacol. Rev. 2015, 67, 259–279. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Luo, Y.; Zhu, Z.; Zhou, Y.; Sun, L.; Gao, J.; Sun, J.; Wang, G.; Yao, X.; Li, W. A tryptophan metabolite of the skin microbiota attenuates inflammation in patients with atopic dermatitis through the aryl hydrocarbon receptor. J. Allergy Clin. Immunol. 2019, 143, 2108–2119.e2112. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, X.; Zhang, J.; Luo, Y.; Xu, B.; Ling, S.; Zhang, Y.; Li, W.; Yao, X. Activation of aryl hydrocarbon receptor in Langerhans cells by a microbial metabolite of tryptophan negatively regulates skin inflammation. J. Dermatol. Sci. 2020, 100, 192–200. [Google Scholar] [CrossRef]
- Jux, B.; Kadow, S.; Luecke, S.; Rannug, A.; Krutmann, J.; Esser, C. The aryl hydrocarbon receptor mediates UVB radiation-induced skin tanning. J. Invest. Dermatol. 2011, 131, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Murai, M.; Yamamura, K.; Hashimoto-Hachiya, A.; Tsuji, G.; Furue, M.; Mitoma, C. Tryptophan photo-product FICZ upregulates AHR/MEK/ERK-mediated MMP1 expression: Implications in anti-fibrotic phototherapy. J. Dermatol. Sci. 2018, 91, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, G.; Hashimoto-Hachiya, A.; Kiyomatsu-Oda, M.; Takemura, M.; Ohno, F.; Ito, T.; Morino-Koga, S.; Mitoma, C.; Nakahara, T.; Uchi, H.; et al. Aryl hydrocarbon receptor activation restores filaggrin expression via OVOL1 in atopic dermatitis. Cell Death Dis. 2017, 8, e2931. [Google Scholar] [CrossRef]
- Poormasjedi-Meibod, M.S.; Hartwell, R.; Kilani, R.T.; Ghahary, A. Anti-scarring properties of different tryptophan derivatives. PLoS ONE 2014, 9, e91955. [Google Scholar] [CrossRef]
- Fritsche, E.; Schafer, C.; Calles, C.; Bernsmann, T.; Bernshausen, T.; Wurm, M.; Hubenthal, U.; Cline, J.E.; Hajimiragha, H.; Schroeder, P.; et al. Lightening up the UV response by identification of the arylhydrocarbon receptor as a cytoplasmatic target for ultraviolet B radiation. Proc. Natl. Acad. Sci. USA 2007, 104, 8851–8856. [Google Scholar] [CrossRef]
- Aoki, R.; Aoki-Yoshida, A.; Suzuki, C.; Takayama, Y. Protective effect of indole-3-pyruvate against ultraviolet b-induced damage to cultured HaCaT keratinocytes and the skin of hairless mice. PLoS ONE 2014, 9, e96804. [Google Scholar] [CrossRef]
- Rynkowska, A.; Stępniak, J.; Karbownik-Lewińska, M. Melatonin and Indole-3-Propionic Acid Reduce Oxidative Damage to Membrane Lipids Induced by High Iron Concentrations in Porcine Skin. Membranes 2021, 11, 571. [Google Scholar] [CrossRef]
- Cervantes-Barragan, L.; Chai, J.N.; Tianero, M.D.; Di Luccia, B.; Ahern, P.P.; Merriman, J.; Cortez, V.S.; Caparon, M.G.; Donia, M.S.; Gilfillan, S.; et al. Lactobacillus reuteri induces gut intraepithelial CD4(+)CD8alphaalpha(+) T cells. Science 2017, 357, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Furue, M.; Hashimoto-Hachiya, A.; Tsuji, G. Aryl Hydrocarbon Receptor in Atopic Dermatitis and Psoriasis. Int. J. Mol. Sci. 2019, 20, 5424. [Google Scholar] [CrossRef] [PubMed]
- Bissonnette, R.; Stein Gold, L.; Rubenstein, D.S.; Tallman, A.M.; Armstrong, A. Tapinarof in the treatment of psoriasis: A review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent. J. Am. Acad. Dermatol. 2021, 84, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cai, L.; Zheng, M. A novel topical treatment for plaque psoriasis: Benvitimod/tapinarof. J. Am. Acad. Dermatol. 2022, 86, e137–e138. [Google Scholar] [CrossRef]
- Keam, S.J. Tapinarof Cream 1%: First Approval. Drugs 2022, 82, 1221–1228. [Google Scholar] [CrossRef]
- Sideris, N.; Paschou, E.; Bakirtzi, K.; Kiritsi, D.; Papadimitriou, I.; Tsentemeidou, A.; Sotiriou, E.; Vakirlis, E. New and Upcoming Topical Treatments for Atopic Dermatitis: A Review of the Literature. J. Clin. Med. 2022, 11, 4974. [Google Scholar] [CrossRef]
- Fang, Z.; Pan, T.; Li, L.; Wang, H.; Zhu, J.; Zhang, H.; Zhao, J.; Chen, W.; Lu, W. Bifidobacterium longum mediated tryptophan metabolism to improve atopic dermatitis via the gut-skin axis. Gut Microbes 2022, 14, 2044723. [Google Scholar] [CrossRef]
- Klonowska, J.; Glen, J.; Nowicki, R.J.; Trzeciak, M. New Cytokines in the Pathogenesis of Atopic Dermatitis-New Therapeutic Targets. Int. J. Mol. Sci. 2018, 19, 3086. [Google Scholar] [CrossRef]
- Smirnova, A.; Wincent, E.; Vikström Bergander, L.; Alsberg, T.; Bergman, J.; Rannug, A.; Rannug, U. Evidence for New Light-Independent Pathways for Generation of the Endogenous Aryl Hydrocarbon Receptor Agonist FICZ. Chem. Res. Toxicol. 2016, 29, 75–86. [Google Scholar] [CrossRef]
- Kiyomatsu-Oda, M.; Uchi, H.; Morino-Koga, S.; Furue, M. Protective role of 6-formylindolo[3,2-b]carbazole (FICZ), an endogenous ligand for arylhydrocarbon receptor, in chronic mite-induced dermatitis. J. Dermatol. Sci. 2018, 90, 284–294. [Google Scholar] [CrossRef]
- Gao, X.K.; Nakamura, N.; Fuseda, K.; Tanaka, H.; Inagaki, N.; Nagai, H. Establishment of allergic dermatitis in NC/Nga mice as a model for severe atopic dermatitis. Biol. Pharm. Bulletin. 2004, 27, 1376–1381. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kobayashi, T.; Nagao, K. Research Techniques Made Simple: Mouse Models of Atopic Dermatitis. J. Investig. Dermatol. 2019, 139, 984–990.e981. [Google Scholar] [CrossRef] [PubMed]
- Zhen, J.; Zhao, P.; Li, Y.; Cai, Y.; Yu, W.; Wang, W.; Zhao, L.; Wang, H.; Huang, G.; Xu, A. The Multiomics Analyses of Gut Microbiota, Urine Metabolome and Plasma Proteome Revealed Significant Changes in Allergy Featured with Indole Derivatives of Tryptophan. J. Asthma. Allergy 2022, 15, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.P.; Singh, U.P.; Rouse, M.; Zhang, J.; Chatterjee, S.; Nagarkatti, P.S.; Nagarkatti, M. Dietary Indoles Suppress Delayed-Type Hypersensitivity by Inducing a Switch from Proinflammatory Th17 Cells to Anti-Inflammatory Regulatory T Cells through Regulation of MicroRNA. J. Immunol. 2016, 196, 1108–1122. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.W.; Fishbein, J.; Hong, J.; Roeser, J.; Furie, R.A.; Aranow, C.; Volpe, B.T.; Diamond, B.; Mackay, M. Quinolinic acid, a kynurenine/tryptophan pathway metabolite, associates with impaired cognitive test performance in systemic lupus erythematosus. Lupus Sci. Med. 2021, 8, e000559. [Google Scholar] [CrossRef]
- Widner, B.; Sepp, N.; Kowald, E.; Ortner, U.; Wirleitner, B.; Fritsch, P.; Baier-Bitterlich, G.; Fuchs, D. Enhanced tryptophan degradation in systemic lupus erythematosus. Immunobiology 2000, 201, 621–630. [Google Scholar] [CrossRef]
- Akesson, K.; Pettersson, S.; Stahl, S.; Surowiec, I.; Hedenstrom, M.; Eketjall, S.; Trygg, J.; Jakobsson, P.J.; Gunnarsson, I.; Svenungsson, E.; et al. Kynurenine pathway is altered in patients with SLE and associated with severe fatigue. Lupus Sci. Med. 2018, 5, e000254. [Google Scholar] [CrossRef]
- Shinde, R.; Hezaveh, K.; Halaby, M.J.; Kloetgen, A.; Chakravarthy, A.; da Silva Medina, T.; Deol, R.; Manion, K.P.; Baglaenko, Y.; Eldh, M.; et al. Apoptotic cell-induced AhR activity is required for immunological tolerance and suppression of systemic lupus erythematosus in mice and humans. Nat. Immunol. 2018, 19, 571–582. [Google Scholar] [CrossRef]
- Brown, J.; Abboud, G.; Ma, L.; Choi, S.C.; Kanda, N.; Zeumer-Spataro, L.; Lee, J.; Peng, W.; Cagmat, J.; Faludi, T.; et al. Microbiota-mediated skewing of tryptophan catabolism modulates CD4(+) T cells in lupus-prone mice. iScience 2022, 25, 104241. [Google Scholar] [CrossRef]
- Choi, S.C.; Brown, J.; Gong, M.; Ge, Y.; Zadeh, M.; Li, W.; Croker, B.P.; Michailidis, G.; Garrett, T.J.; Mohamadzadeh, M.; et al. Gut microbiota dysbiosis and altered tryptophan catabolism contribute to autoimmunity in lupus-susceptible mice. Sci. Transl. Med. 2020, 12, eaax2220. [Google Scholar] [CrossRef]
- Janeiro, M.H.; Ramírez, M.J.; Milagro, F.I.; Martínez, J.A.; Solas, M. Implication of Trimethylamine N-Oxide (TMAO) in Disease: Potential Biomarker or New Therapeutic Target. Nutrients 2018, 10, 1398. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.; Warrier, M. Trimethylamine N-Oxide, the Microbiome, and Heart and Kidney Disease. Annu. Rev. Nutr. 2017, 37, 157–181. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dai, M. Trimethylamine N-Oxide Generated by the Gut Microbiota Is Associated with Vascular Inflammation: New Insights into Atherosclerosis. Mediat. Inflamm. 2020, 2020, 4634172. [Google Scholar] [CrossRef] [PubMed]
- Smits, L.P.; Kootte, R.S.; Levin, E.; Prodan, A.; Fuentes, S.; Zoetendal, E.G.; Wang, Z.; Levison, B.S.; Cleophas, M.C.P.; Kemper, E.M.; et al. Effect of Vegan Fecal Microbiota Transplantation on Carnitine- and Choline-Derived Trimethylamine-N-Oxide Production and Vascular Inflammation in Patients With Metabolic Syndrome. J. Am. Heart Assoc. 2018, 7, e008342. [Google Scholar] [CrossRef] [PubMed]
- Rohrmann, S.; Linseisen, J.; Allenspach, M.; von Eckardstein, A.; Muller, D. Plasma Concentrations of Trimethylamine-N-oxide Are Directly Associated with Dairy Food Consumption and Low-Grade Inflammation in a German Adult Population. J. Nutr. 2016, 146, 283–289. [Google Scholar] [CrossRef]
- Agus, A.; Clement, K.; Sokol, H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut 2021, 70, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Bartikoski, B.J.; De Oliveira, M.S.; Do Espirito Santo, R.C.; Dos Santos, L.P.; Dos Santos, N.G.; Xavier, R.M. A Review of Metabolomic Profiling in Rheumatoid Arthritis: Bringing New Insights in Disease Pathogenesis, Treatment and Comorbidities. Metabolites 2022, 12, 394. [Google Scholar] [CrossRef]
- Yang, S.; Li, X.; Yang, F.; Zhao, R.; Pan, X.; Liang, J.; Tian, L.; Li, X.; Liu, L.; Xing, Y.; et al. Gut Microbiota-Dependent Marker TMAO in Promoting Cardiovascular Disease: Inflammation Mechanism, Clinical Prognostic, and Potential as a Therapeutic Target. Front. Pharmacol. 2019, 10, 1360. [Google Scholar] [CrossRef]
- Chen, K.; Zheng, X.; Feng, M.; Li, D.; Zhang, H. Gut Microbiota-Dependent Metabolite Trimethylamine N-Oxide Contributes to Cardiac Dysfunction in Western Diet-Induced Obese Mice. Front. Physiol. 2017, 8, 139. [Google Scholar] [CrossRef]
- Sun, X.; Jiao, X.; Ma, Y.; Liu, Y.; Zhang, L.; He, Y.; Chen, Y. Trimethylamine N-oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS-TXNIP-NLRP3 inflammasome. Biochem. Biophys. Res. Commun. 2016, 481, 63–70. [Google Scholar] [CrossRef]
- Wu, K.; Yuan, Y.; Yu, H.; Dai, X.; Wang, S.; Sun, Z.; Wang, F.; Fei, H.; Lin, Q.; Jiang, H.; et al. The gut microbial metabolite trimethylamine N-oxide aggravates GVHD by inducing M1 macrophage polarization in mice. Blood 2020, 136, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Yang, X.; Li, J.; Chen, X.; Zhao, X.; Chen, Y.; Wen, Y. Trimethylamine N-oxide prime NLRP3 inflammasome via inhibiting ATG16L1-induced autophagy in colonic epithelial cells. Biochem. Biophys. Res. Commun. 2017, 490, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, R.N.; Jensen, P.N.; Wang, Z.; Fretts, A.M.; McKnight, B.; Nemet, I.; Biggs, M.L.; Sotoodehnia, N.; de Oliveira Otto, M.C.; Psaty, B.M.; et al. Association of Trimethylamine N-Oxide and Related Metabolites in Plasma and Incident Type 2 Diabetes: The Cardiovascular Health Study. JAMA Netw. Open 2021, 4, e2122844. [Google Scholar] [CrossRef] [PubMed]
- Tanase, D.M.; Gosav, E.M.; Neculae, E.; Costea, C.F.; Ciocoiu, M.; Hurjui, L.L.; Tarniceriu, C.C.; Maranduca, M.A.; Lacatusu, C.M.; Floria, M.; et al. Role of Gut Microbiota on Onset and Progression of Microvascular Complications of Type 2 Diabetes (T2DM). Nutrients 2020, 12, 3719. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Nemet, I.; Wang, Z.; Lai, H.T.M.; de Oliveira Otto, M.C.; Lemaitre, R.N.; Fretts, A.M.; Sotoodehnia, N.; Budoff, M.; DiDonato, J.A.; et al. Longitudinal Plasma Measures of Trimethylamine N-Oxide and Risk of Atherosclerotic Cardiovascular Disease Events in Community-Based Older Adults. J. Am. Heart Assoc. 2021, 10, e020646. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, Y.; Zhou, J.; Chen, R.; Li, J.; Zhao, X.; Zhou, P.; Liu, C.; Chen, Y.; Song, L.; et al. Association between the Changes in Trimethylamine N-Oxide-Related Metabolites and Prognosis of Patients with Acute Myocardial Infarction: A Prospective Study. J. Cardiovasc. Dev. Dis. 2022, 9, 380. [Google Scholar] [CrossRef] [PubMed]
- Sikora, M.; Kiss, N.; Stec, A.; Giebultowicz, J.; Samborowska, E.; Jazwiec, R.; Dadlez, M.; Olszewska, M.; Rudnicka, L. Trimethylamine N-Oxide, a Gut Microbiota-Derived Metabolite, Is Associated with Cardiovascular Risk in Psoriasis: A Cross-Sectional Pilot Study. Dermatol. Ther. 2021, 11, 1277–1289. [Google Scholar] [CrossRef]
- Sun, L.; Guo, X.; Qin, Y.; Li, P.; Yu, C.; Gao, X.; Xie, X.; Xu, X. Serum Intestinal Metabolites are Raised in Patients with Psoriasis and Metabolic Syndrome. Clin. Cosmet. Investig. Dermatol. 2022, 15, 879–886. [Google Scholar] [CrossRef]
- Coras, R.; Kavanaugh, A.; Boyd, T.; Huynh, D.; Lagerborg, K.A.; Xu, Y.J.; Rosenthal, S.B.; Jain, M.; Guma, M. Choline metabolite, trimethylamine N-oxide (TMAO), is associated with inflammation in psoriatic arthritis. Clin. Exp. Rheumatol. 2019, 37, 481–484. [Google Scholar]
- Barrea, L.; Muscogiuri, G.; Pugliese, G.; de Alteriis, G.; Maisto, M.; Donnarumma, M.; Tenore, G.C.; Colao, A.; Fabbrocini, G.; Savastano, S. Association of Trimethylamine N-Oxide (TMAO) with the Clinical Severity of Hidradenitis Suppurativa (Acne Inversa). Nutrients 2021, 13, 1997. [Google Scholar] [CrossRef]
- Jiang, S.W.; Whitley, M.J.; Mariottoni, P.; Jaleel, T.; MacLeod, A.S. Hidradenitis Suppurativa: Host-Microbe and Immune Pathogenesis Underlie Important Future Directions. JID Innov. 2021, 1, 100001. [Google Scholar] [CrossRef] [PubMed]
- Korman, N.J. Management of psoriasis as a systemic disease: What is the evidence? Br. J. Dermatol. 2020, 182, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liang, L.; Deng, X.; Zhong, L. Lipidomic and metabolomic profiling reveals novel candidate biomarkers in active systemic lupus erythematosus. Int. J. Clin. Exp. Pathol. 2019, 12, 857–866. [Google Scholar] [PubMed]
- Gonzalez-Correa, C.; Moleon, J.; Minano, S.; Visitacion, N.; Robles-Vera, I.; Gomez-Guzman, M.; Jimenez, R.; Romero, M.; Duarte, J. Trimethylamine N-Oxide Promotes Autoimmunity and a Loss of Vascular Function in Toll-like Receptor 7-Driven Lupus Mice. Antioxidants 2021, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Smolenska, Z.; Zabielska-Kaczorowska, M.; Wojteczek, A.; Kutryb-Zajac, B.; Zdrojewski, Z. Metabolic Pattern of Systemic Sclerosis: Association of Changes in Plasma Concentrations of Amino Acid-Related Compounds With Disease Presentation. Front. Mol. Biosci. 2020, 7, 585161. [Google Scholar] [CrossRef]
- Kim, S.J.; Bale, S.; Verma, P.; Wan, Q.; Ma, F.; Gudjonsson, J.E.; Hazen, S.L.; Harms, P.W.; Tsou, P.S.; Khanna, D.; et al. Gut microbe-derived metabolite trimethylamine N-oxide activates PERK to drive fibrogenic mesenchymal differentiation. iScience 2022, 25, 104669. [Google Scholar] [CrossRef] [PubMed]
- Assassi, S.; Wu, M.; Tan, F.K.; Chang, J.; Graham, T.A.; Furst, D.E.; Khanna, D.; Charles, J.; Ferguson, E.C.; Feghali-Bostwick, C.; et al. Skin gene expression correlates of severity of interstitial lung disease in systemic sclerosis. Arthritis Rheum. 2013, 65, 2917–2927. [Google Scholar] [CrossRef] [PubMed]
- Chadli, L.; Sotthewes, B.; Li, K.; Andersen, S.N.; Cahir-McFarland, E.; Cheung, M.; Cullen, P.; Dorjee, A.; de Vries-Bouwstra, J.K.; Huizinga, T.W.J.; et al. Identification of regulators of the myofibroblast phenotype of primary dermal fibroblasts from early diffuse systemic sclerosis patients. Sci. Rep. 2019, 9, 4521. [Google Scholar] [CrossRef]
- Skaug, B.; Khanna, D.; Swindell, W.R.; Hinchcliff, M.E.; Frech, T.M.; Steen, V.D.; Hant, F.N.; Gordon, J.K.; Shah, A.A.; Zhu, L.; et al. Global skin gene expression analysis of early diffuse cutaneous systemic sclerosis shows a prominent innate and adaptive inflammatory profile. Ann. Rheum. Dis. 2020, 79, 379–386. [Google Scholar] [CrossRef]
- Shi, Z.; Wu, X.; Wu, C.Y.; Singh, S.P.; Law, T.; Yamada, D.; Huynh, M.; Liakos, W.; Yang, G.; Farber, J.M.; et al. Bile Acids Improve Psoriasiform Dermatitis through Inhibition of IL-17A Expression and CCL20-CCR6-Mediated Trafficking of T Cells. J. Invest. Dermatol. 2022, 142, 1381–1390.e1311. [Google Scholar] [CrossRef]
- Paine, A.; Brookes, P.S.; Bhattacharya, S.; Li, D.; De La Luz Garcia-Hernandez, M.; Tausk, F.; Ritchlin, C. Dysregulation of Bile Acids, Lipids, and Nucleotides in Psoriatic Arthritis Revealed by Unbiased Profiling of Serum Metabolites. Arthritis Rheumatol. 2023, 75, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Sipka, S.; Bruckner, G. The immunomodulatory role of bile acids. Int. Arch. Allergy Immunol. 2014, 165, 1–8. [Google Scholar] [CrossRef]
- Sorokin, A.V.; Domenichiello, A.F.; Dey, A.K.; Yuan, Z.X.; Goyal, A.; Rose, S.M.; Playford, M.P.; Ramsden, C.E.; Mehta, N.N. Bioactive Lipid Mediator Profiles in Human Psoriasis Skin and Blood. J. Invest. Dermatol. 2018, 138, 1518–1528. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Ma, Y.; Yan, B.; Pei, W.; Wu, Q.; Ding, C.; Huang, C. The promotion mechanism of prebiotics for probiotics: A review. Front. Nutr. 2022, 9, 1000517. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lin, T.; Meng, Y.; Hu, M.; Shu, L.; Jiang, H.; Gao, R.; Ma, J.; Wang, C.; Zhou, X. FOS/GOS attenuates high-fat diet induced bone loss via reversing microbiota dysbiosis, high intestinal permeability and systemic inflammation in mice. Metabolism 2021, 119, 154767. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Sobh, M.; Montroy, J.; Daham, Z.; Sibbald, S.; Lalu, M.; Stintzi, A.; Mack, D.; Fergusson, D.A. Tolerability and SCFA production after resistant starch supplementation in humans: A systematic review of randomized controlled studies. Am. J. Clin. Nutr. 2022, 115, 608–618. [Google Scholar] [CrossRef]
- Cuello-Garcia, C.; Fiocchi, A.; Pawankar, R.; Yepes-Nunez, J.J.; Morgano, G.P.; Zhang, Y.; Agarwal, A.; Gandhi, S.; Terracciano, L.; Schunemann, H.J.; et al. Prebiotics for the prevention of allergies: A systematic review and meta-analysis of randomized controlled trials. Clin. Exp. Allergy 2017, 47, 1468–1477. [Google Scholar] [CrossRef]
- Shibata, R.; Kimura, M.; Takahashi, H.; Mikami, K.; Aiba, Y.; Takeda, H.; Koga, Y. Clinical effects of kestose, a prebiotic oligosaccharide, on the treatment of atopic dermatitis in infants. Clin. Exp. Allergy 2009, 39, 1397–1403. [Google Scholar] [CrossRef]
- Koga, Y.; Tokunaga, S.; Nagano, J.; Sato, F.; Konishi, K.; Tochio, T.; Murakami, Y.; Masumoto, N.; Tezuka, J.I.; Sudo, N.; et al. Age-associated effect of kestose on Faecalibacterium prausnitzii and symptoms in the atopic dermatitis infants. Pediatr Res. 2016, 80, 844–851. [Google Scholar] [CrossRef]
- Sestito, S.; D’Auria, E.; Baldassarre, M.E.; Salvatore, S.; Tallarico, V.; Stefanelli, E.; Tarsitano, F.; Concolino, D.; Pensabene, L. The Role of Prebiotics and Probiotics in Prevention of Allergic Diseases in Infants. Front. Pediatr. 2020, 8, 583946. [Google Scholar] [CrossRef] [PubMed]
- Cuello-Garcia, C.A.; Fiocchi, A.; Pawankar, R.; Yepes-Nunez, J.J.; Morgano, G.P.; Zhang, Y.; Ahn, K.; Al-Hammadi, S.; Agarwal, A.; Gandhi, S.; et al. World Allergy Organization-McMaster University Guidelines for Allergic Disease Prevention (GLAD-P): Prebiotics. World Allergy Organ. J. 2016, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Alirezaei, P.; Doosti-Irani, A.; Mehrpooya, M.; Nouri, F. The Efficacy of Lactocare(R) Synbiotic on the Clinical Symptoms in Patients with Psoriasis: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Dermatol. Res. Pract. 2022, 2022, 4549134. [Google Scholar] [CrossRef] [PubMed]
- Widhani, A.; Djauzi, S.; Suyatna, F.D.; Dewi, B.E. Changes in Gut Microbiota and Systemic Inflammation after Synbiotic Supplementation in Patients with Systemic Lupus Erythematosus: A Randomized, Double-Blind, Placebo-Controlled Trial. Cells 2022, 11, 3419. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- De Simone, C. The Unregulated Probiotic Market. Clin. Gastroenterol. Hepatol. 2019, 17, 809–817. [Google Scholar] [CrossRef]
- Voigt, J.; Lele, M. Lactobacillus rhamnosus Used in the Perinatal Period for the Prevention of Atopic Dermatitis in Infants: A Systematic Review and Meta-Analysis of Randomized Trials. Am. J. Clin. Dermatol. 2022, 23, 801–811. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, B.; Guo, J.; Cao, Z.; Shen, M. The efficacy of probiotics supplementation for the treatment of atopic dermatitis in adults: A systematic review and meta-analysis. J. Dermatolog. Treat. 2022, 33, 2800–2809. [Google Scholar] [CrossRef]
- Tan-Lim, C.S.C.; Esteban-Ipac, N.A.R.; Mantaring, J.B.V., 3rd; Chan Shih Yen, E.; Recto, M.S.T.; Sison, O.T.; Alejandria, M.M. Comparative effectiveness of probiotic strains for the treatment of pediatric atopic dermatitis: A systematic review and network meta-analysis. Pediatr. Allergy Immunol. 2021, 32, 124–136. [Google Scholar] [CrossRef]
- Umborowati, M.A.; Damayanti, D.; Anggraeni, S.; Endaryanto, A.; Surono, I.S.; Effendy, I.; Prakoeswa, C.R.S. The role of probiotics in the treatment of adult atopic dermatitis: A meta-analysis of randomized controlled trials. J. Health Popul. Nutr. 2022, 41, 37. [Google Scholar] [CrossRef]
- Fiocchi, A.; Cabana, M.D.; Mennini, M. Current Use of Probiotics and Prebiotics in Allergy. J. Allergy Clin. Immunol. Pract. 2022, 10, 2219–2242. [Google Scholar] [CrossRef] [PubMed]
- Uwaezuoke, S.N.; Ayuk, A.C.; Eze, J.N.; Odimegwu, C.L.; Ndiokwelu, C.O.; Eze, I.C. Postnatal probiotic supplementation can prevent and optimize treatment of childhood asthma and atopic disorders: A systematic review of randomized controlled trials. Front. Pediatr. 2022, 10, 956141. [Google Scholar] [CrossRef] [PubMed]
- Askari, G.; Ghavami, A.; Shahdadian, F.; Moravejolahkami, A.R. Effect of synbiotics and probiotics supplementation on autoimmune diseases: A systematic review and meta-analysis of clinical trials. Clin. Nutr. 2021, 40, 3221–3234. [Google Scholar] [CrossRef] [PubMed]
- Chaiyasut, C.; Sivamaruthi, B.S.; Lailerd, N.; Sirilun, S.; Khongtan, S.; Fukngoen, P.; Peerajan, S.; Saelee, M.; Chaiyasut, K.; Kesika, P.; et al. Probiotics Supplementation Improves Intestinal Permeability, Obesity Index and Metabolic Biomarkers in Elderly Thai Subjects: A Randomized Controlled Trial. Foods 2022, 11, 268. [Google Scholar] [CrossRef]
- Kwon, H.K.; Kim, G.C.; Kim, Y.; Hwang, W.; Jash, A.; Sahoo, A.; Kim, J.E.; Nam, J.H.; Im, S.H. Amelioration of experimental autoimmune encephalomyelitis by probiotic mixture is mediated by a shift in T helper cell immune response. Clin. Immunol. 2013, 146, 217–227. [Google Scholar] [CrossRef]
- Moludi, J.; Khedmatgozar, H.; Saiedi, S.; Razmi, H.; Alizadeh, M.; Ebrahimi, B. Probiotic supplementation improves clinical outcomes and quality of life indicators in patients with plaque psoriasis: A randomized double-blind clinical trial. Clin. Nutr. ESPEN 2021, 46, 33–39. [Google Scholar] [CrossRef]
- Zangrilli, A.; Diluvio, L.; Di Stadio, A.; Di Girolamo, S. Improvement of Psoriasis Using Oral Probiotic Streptococcus salivarius K-12: A Case-Control 24-Month Longitudinal Study. Probiotics Antimicrob. Proteins 2022, 14, 573–578. [Google Scholar] [CrossRef]
- Moludi, J.; Fathollahi, P.; Khedmatgozar, H.; Pourteymour Fard Tabrizi, F.; Ghareaghaj Zare, A.; Razmi, H.; Amirpour, M. Probiotics Supplementation Improves Quality of Life, Clinical Symptoms, and Inflammatory Status in Patients With Psoriasis. J. Drugs Dermatol. 2022, 21, 637–644. [Google Scholar] [CrossRef]
- Marighela, T.F.; Arismendi, M.I.; Marvulle, V.; Brunialti, M.K.C.; Salomao, R.; Kayser, C. Effect of probiotics on gastrointestinal symptoms and immune parameters in systemic sclerosis: A randomized placebo-controlled trial. Rheumatology 2019, 58, 1985–1990. [Google Scholar] [CrossRef]
- Huang, H.L.; Xu, H.M.; Liu, Y.D.; Shou, D.W.; Nie, Y.Q.; Chen, H.T.; Zhou, Y.J. Fecal microbiota transplantation as a novel approach for the treatment of atopic dermatitis. J. Dermatol. 2021, 48, e574–e576. [Google Scholar] [CrossRef]
- Mashiah, J.; Karady, T.; Fliss-Isakov, N.; Sprecher, E.; Slodownik, D.; Artzi, O.; Samuelov, L.; Ellenbogen, E.; Godneva, A.; Segal, E.; et al. Clinical efficacy of fecal microbial transplantation treatment in adults with moderate-to-severe atopic dermatitis. Immun. Inflamm. Dis. 2022, 10, e570. [Google Scholar] [CrossRef]
- Zou, B.; Liu, S.-X.; Li, X.-S.; He, J.-Y.; Dong, C.; Ruan, M.-L.; Xu, L.; Bai, T.; Huang, Z.-H.; Shu, S.-N. Long-term safety and efficacy of fecal microbiota transplantation in 74 children: A single-center retrospective study. Front. Pediatr. 2022, 10, 964154. [Google Scholar] [CrossRef]
- Huang, C.; Yi, P.; Zhu, M.; Zhou, W.; Zhang, B.; Yi, X.; Long, H.; Zhang, G.; Wu, H.; Tsokos, G.C.; et al. Safety and efficacy of fecal microbiota transplantation for treatment of systemic lupus erythematosus: An EXPLORER trial. J. Autoimmun. 2022, 130, 102844. [Google Scholar] [CrossRef]
- Fretheim, H.; Chung, B.K.; Didriksen, H.; Baekkevold, E.S.; Midtvedt, O.; Brunborg, C.; Holm, K.; Valeur, J.; Tennoe, A.H.; Garen, T.; et al. Fecal microbiota transplantation in systemic sclerosis: A double-blind, placebo-controlled randomized pilot trial. PLoS ONE 2020, 15, e0232739. [Google Scholar] [CrossRef]
- Selvanderan, S.P.; Goldblatt, F.; Nguyen, N.Q.; Costello, S.P. Faecal microbiota transplantation for Clostridium difficile infection resulting in a decrease in psoriatic arthritis disease activity. Clin. Exp. Rheumatol. 2019, 37, 514–515. [Google Scholar]
- Kragsnaes, M.S.; Kjeldsen, J.; Horn, H.C.; Munk, H.L.; Pedersen, J.K.; Just, S.A.; Ahlquist, P.; Pedersen, F.M.; de Wit, M.; Moller, S.; et al. Safety and efficacy of faecal microbiota transplantation for active peripheral psoriatic arthritis: An exploratory randomised placebo-controlled trial. Ann. Rheum. Dis. 2021, 80, 1158–1167. [Google Scholar] [CrossRef]
- Yin, G.; Li, J.F.; Sun, Y.F.; Ding, X.; Zeng, J.Q.; Zhang, T.; Peng, L.H.; Yang, Y.S.; Zhao, H. Fecal microbiota transplantation as a novel therapy for severe psoriasis. Zhonghua Nei Ke Za Zhi 2019, 58, 782–785. [Google Scholar] [CrossRef]
- Cleophas, M.C.P.; Ratter, J.M.; Bekkering, S.; Quintin, J.; Schraa, K.; Stroes, E.S.; Netea, M.G.; Joosten, L.A.B. Effects of oral butyrate supplementation on inflammatory potential of circulating peripheral blood mononuclear cells in healthy and obese males. Sci. Rep. 2019, 9, 775. [Google Scholar] [CrossRef]
- Abramczyk, R.; Queller, J.N.; Rachfal, A.W.; Schwartz, S.S. Diabetes and Psoriasis: Different Sides of the Same Prism. Diabetes Metab. Syndr. Obes. 2020, 13, 3571–3577. [Google Scholar] [CrossRef]
- Soltani-Arabshahi, R.; Wong, B.; Feng, B.J.; Goldgar, D.E.; Duffin, K.C.; Krueger, G.G. Obesity in early adulthood as a risk factor for psoriatic arthritis. Arch. Dermatol. 2010, 146, 721–726. [Google Scholar] [CrossRef]
- Roshanravan, N.; Alamdari, N.M.; Jafarabadi, M.A.; Mohammadi, A.; Shabestari, B.R.; Nasirzadeh, N.; Asghari, S.; Mansoori, B.; Akbarzadeh, M.; Ghavami, A.; et al. Effects of oral butyrate and inulin supplementation on inflammation-induced pyroptosis pathway in type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Cytokine 2020, 131, 155101. [Google Scholar] [CrossRef]
- Roshanravan, N.; Mahdavi, R.; Alizadeh, E.; Ghavami, A.; Rahbar Saadat, Y.; Mesri Alamdari, N.; Alipour, S.; Dastouri, M.R.; Ostadrahimi, A. The effects of sodium butyrate and inulin supplementation on angiotensin signaling pathway via promotion of Akkermansia muciniphila abundance in type 2 diabetes; A randomized, double-blind, placebo-controlled trial. J. Cardiovasc. Thorac. Res. 2017, 9, 183–190. [Google Scholar] [CrossRef]
- Roshanravan, N.; Mahdavi, R.; Alizadeh, E.; Jafarabadi, M.A.; Hedayati, M.; Ghavami, A.; Alipour, S.; Alamdari, N.M.; Barati, M.; Ostadrahimi, A. Effect of Butyrate and Inulin Supplementation on Glycemic Status, Lipid Profile and Glucagon-Like Peptide 1 Level in Patients with Type 2 Diabetes: A Randomized Double-Blind, Placebo-Controlled Trial. Horm. Metab. Res. 2017, 49, 886–891. [Google Scholar] [CrossRef]
- Chambers, E.S.; Byrne, C.S.; Morrison, D.J.; Murphy, K.G.; Preston, T.; Tedford, C.; Garcia-Perez, I.; Fountana, S.; Serrano-Contreras, J.I.; Holmes, E.; et al. Dietary supplementation with inulin-propionate ester or inulin improves insulin sensitivity in adults with overweight and obesity with distinct effects on the gut microbiota, plasma metabolome and systemic inflammatory responses: A randomised cross-over trial. Gut 2019, 68, 1430–1438. [Google Scholar] [CrossRef]
- Chambers, E.S.; Viardot, A.; Psichas, A.; Morrison, D.J.; Murphy, K.G.; Zac-Varghese, S.E.; MacDougall, K.; Preston, T.; Tedford, C.; Finlayson, G.S.; et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 2015, 64, 1744–1754. [Google Scholar] [CrossRef]
- Todesco, T.; Rao, A.V.; Bosello, O.; Jenkins, D.J. Propionate lowers blood glucose and alters lipid metabolism in healthy subjects. Am. J. Clin. Nutr. 1991, 54, 860–865. [Google Scholar] [CrossRef]
- Chambers, E.S.; Byrne, C.S.; Aspey, K.; Chen, Y.; Khan, S.; Morrison, D.J.; Frost, G. Acute oral sodium propionate supplementation raises resting energy expenditure and lipid oxidation in fasted humans. Diabetes Obes. Metab. 2018, 20, 1034–1039. [Google Scholar] [CrossRef]
- Gertsman, I.; Gangoiti, J.A.; Nyhan, W.L.; Barshop, B.A. Perturbations of tyrosine metabolism promote the indolepyruvate pathway via tryptophan in host and microbiome. Mol. Genet. Metab. 2015, 114, 431–437. [Google Scholar] [CrossRef]
- Katz, E.; Nisani, S.; Chamovitz, D.A. Indole-3-carbinol: A plant hormone combatting cancer. F1000Res. 2018, 7, 689. [Google Scholar] [CrossRef]
- Naik, R.; Nixon, S.; Lopes, A.; Godfrey, K.; Hatem, M.H.; Monaghan, J.M. A randomized phase II trial of indole-3-carbinol in the treatment of vulvar intraepithelial neoplasia. Int. J. Gynecol. Cancer 2006, 16, 786–790. [Google Scholar] [CrossRef]
- Boltežar, I.H.; Bahar, M.S.; Zargi, M.; Gale, N.; Matičič, M.; Poljak, M. Adjuvant therapy for laryngeal papillomatosis. Acta Dermatovenerol. Alp. Pannonica. Adriat. 2011, 20, 175–180. [Google Scholar]
- Ashrafian, L.; Sukhikh, G.; Kiselev, V.; Paltsev, M.; Drukh, V.; Kuznetsov, I.; Muyzhnek, E.; Apolikhina, I.; Andrianova, E. Double-blind randomized placebo-controlled multicenter clinical trial (phase IIa) on diindolylmethane’s efficacy and safety in the treatment of CIN: Implications for cervical cancer prevention. EPMA J. 2015, 6, 25. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stec, A.; Sikora, M.; Maciejewska, M.; Paralusz-Stec, K.; Michalska, M.; Sikorska, E.; Rudnicka, L. Bacterial Metabolites: A Link between Gut Microbiota and Dermatological Diseases. Int. J. Mol. Sci. 2023, 24, 3494. https://doi.org/10.3390/ijms24043494
Stec A, Sikora M, Maciejewska M, Paralusz-Stec K, Michalska M, Sikorska E, Rudnicka L. Bacterial Metabolites: A Link between Gut Microbiota and Dermatological Diseases. International Journal of Molecular Sciences. 2023; 24(4):3494. https://doi.org/10.3390/ijms24043494
Chicago/Turabian StyleStec, Albert, Mariusz Sikora, Magdalena Maciejewska, Karolina Paralusz-Stec, Milena Michalska, Ewa Sikorska, and Lidia Rudnicka. 2023. "Bacterial Metabolites: A Link between Gut Microbiota and Dermatological Diseases" International Journal of Molecular Sciences 24, no. 4: 3494. https://doi.org/10.3390/ijms24043494
APA StyleStec, A., Sikora, M., Maciejewska, M., Paralusz-Stec, K., Michalska, M., Sikorska, E., & Rudnicka, L. (2023). Bacterial Metabolites: A Link between Gut Microbiota and Dermatological Diseases. International Journal of Molecular Sciences, 24(4), 3494. https://doi.org/10.3390/ijms24043494







