Exploration of the Core Pathways and Potential Targets of Luteolin Treatment on Late-Onset Depression Based on Cerebrospinal Fluid Proteomics
Abstract
1. Introduction
2. Results
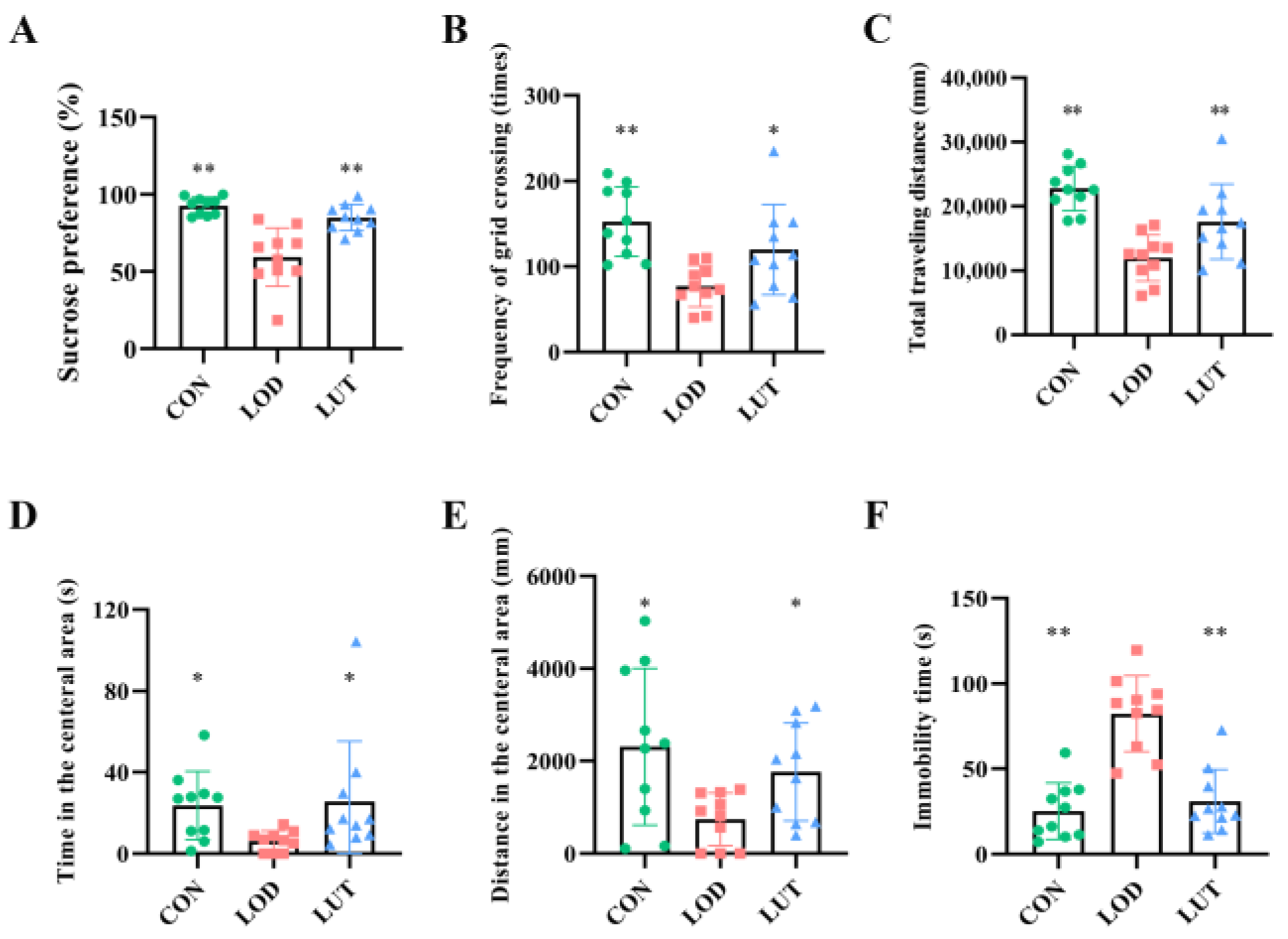
2.1. LUT Could Improve Depression-like Behaviors in LOD Rats
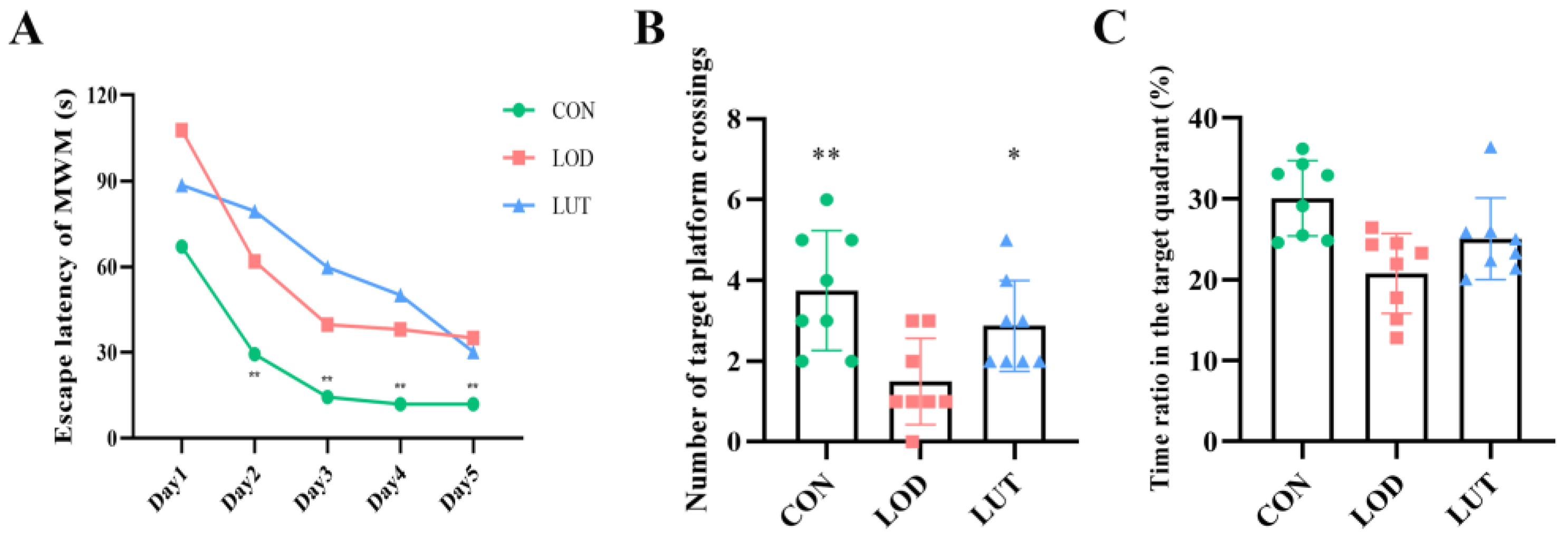
2.2. LUT Could Improve the Cognition of LOD Rats
2.3. Analysis of GSEA–KEGG Pathway in LOD Treatment with LUT
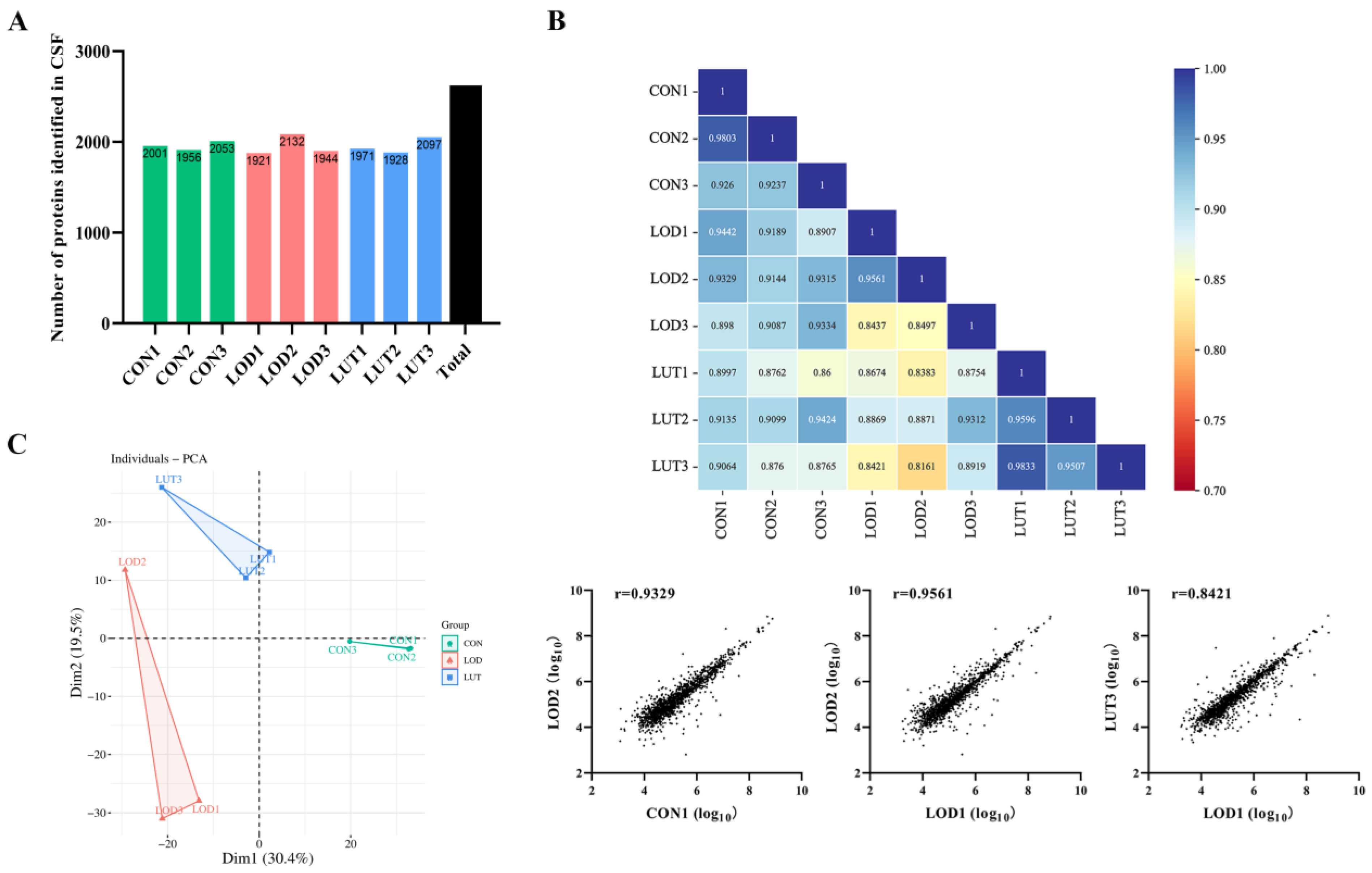
2.3.1. A General Description of CSF Proteomics Data
2.3.2. GSEA–Gene Ontology (GO) Annotation Analysis of LUT Treating LOD
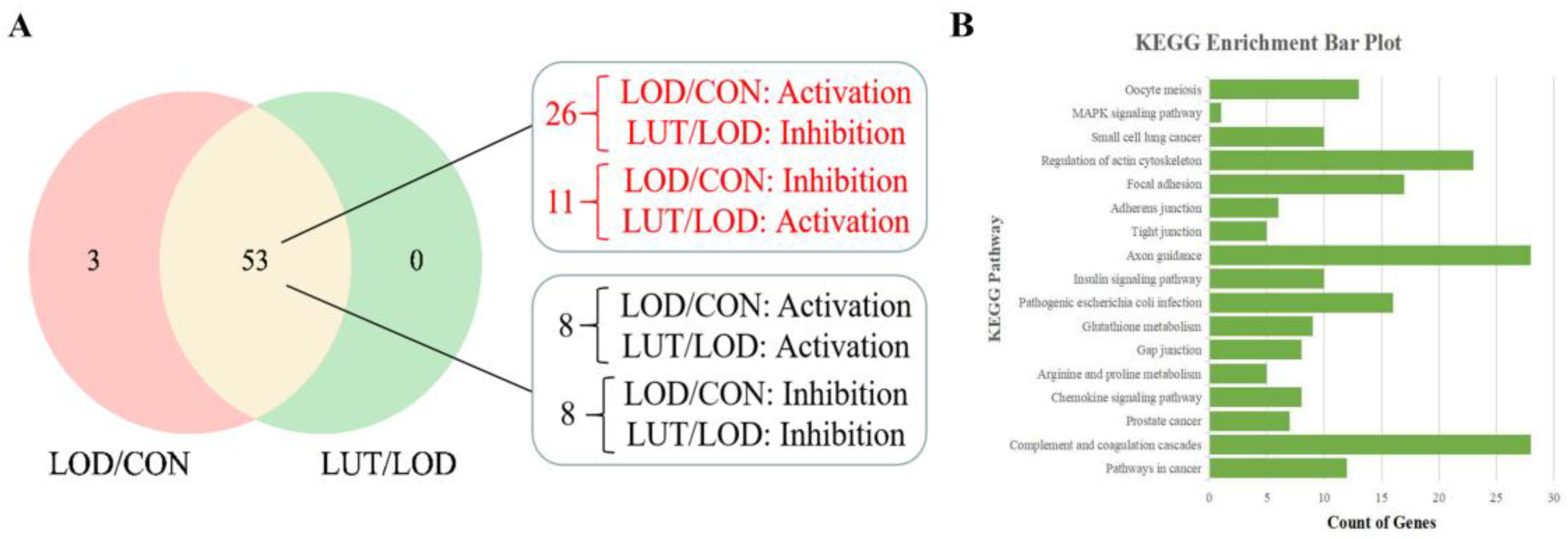
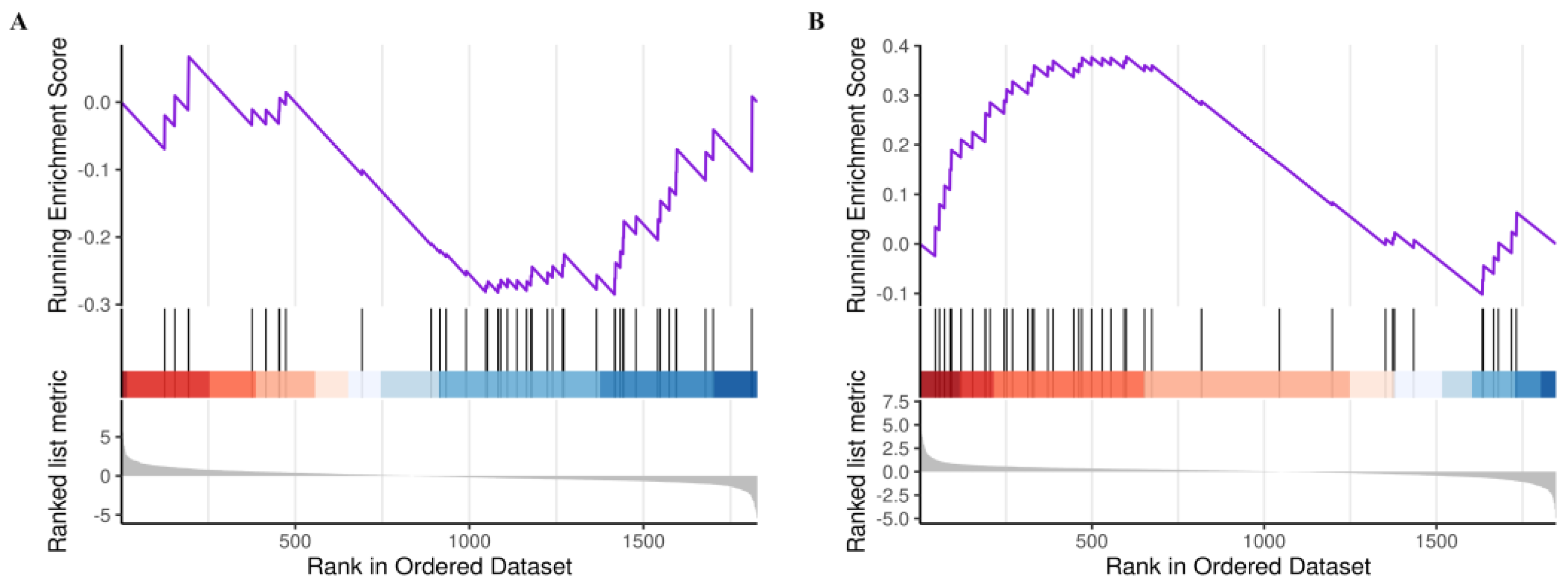
2.3.3. Analysis of the Core GSEA–KEGG Pathways for LOD Treatment by LUT
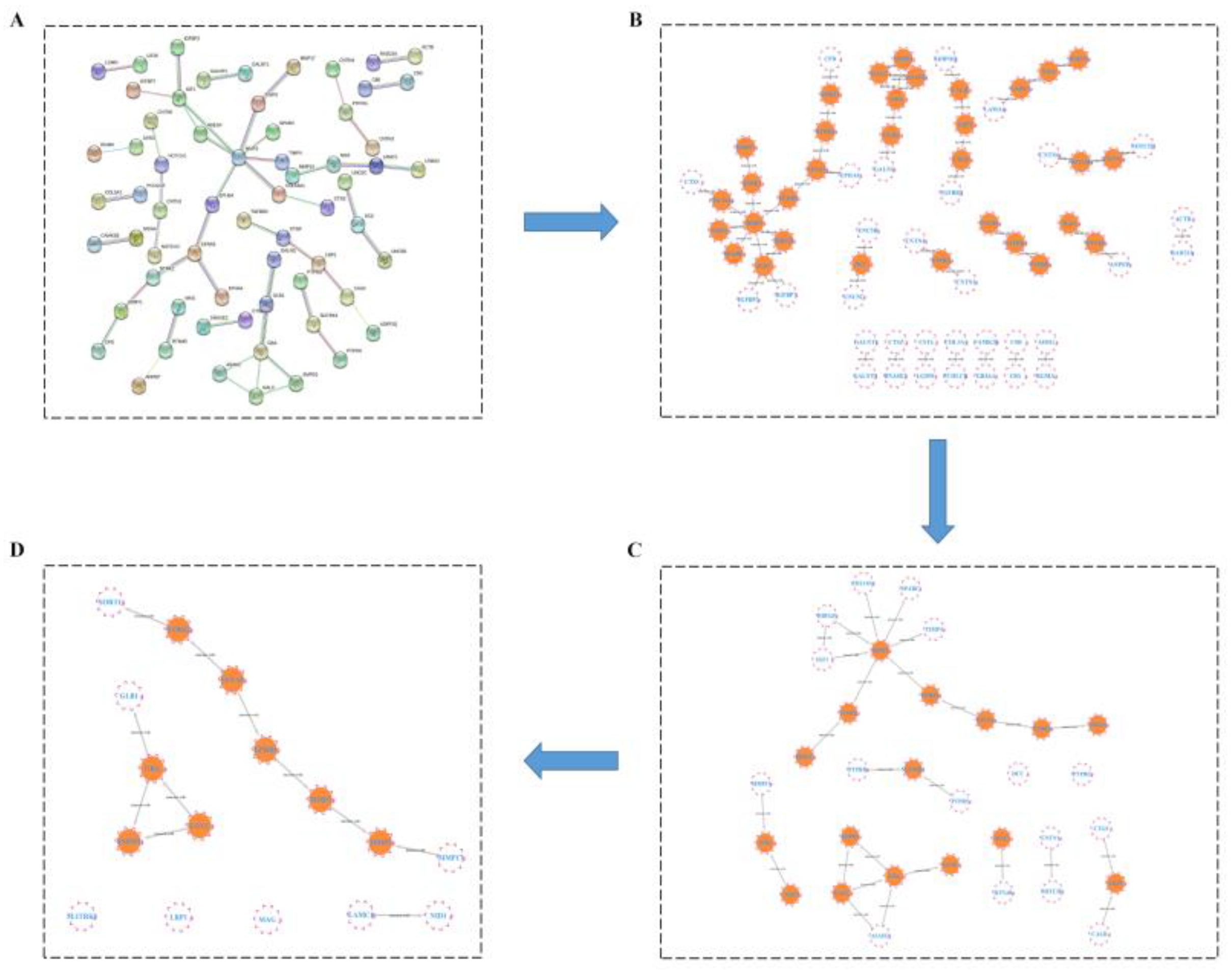
2.3.4. HUB Gene Analysis of the Treatment of LOD by LUT through a Protein–Protein Interaction (PPI) Network
2.4. Analysis of Potential Targets for LUT Treatment on LOD Based on Axon Guidance Pathway
2.4.1. GSEA–KEGG Results of Axon Guidance Pathway Based on CSF Proteomics
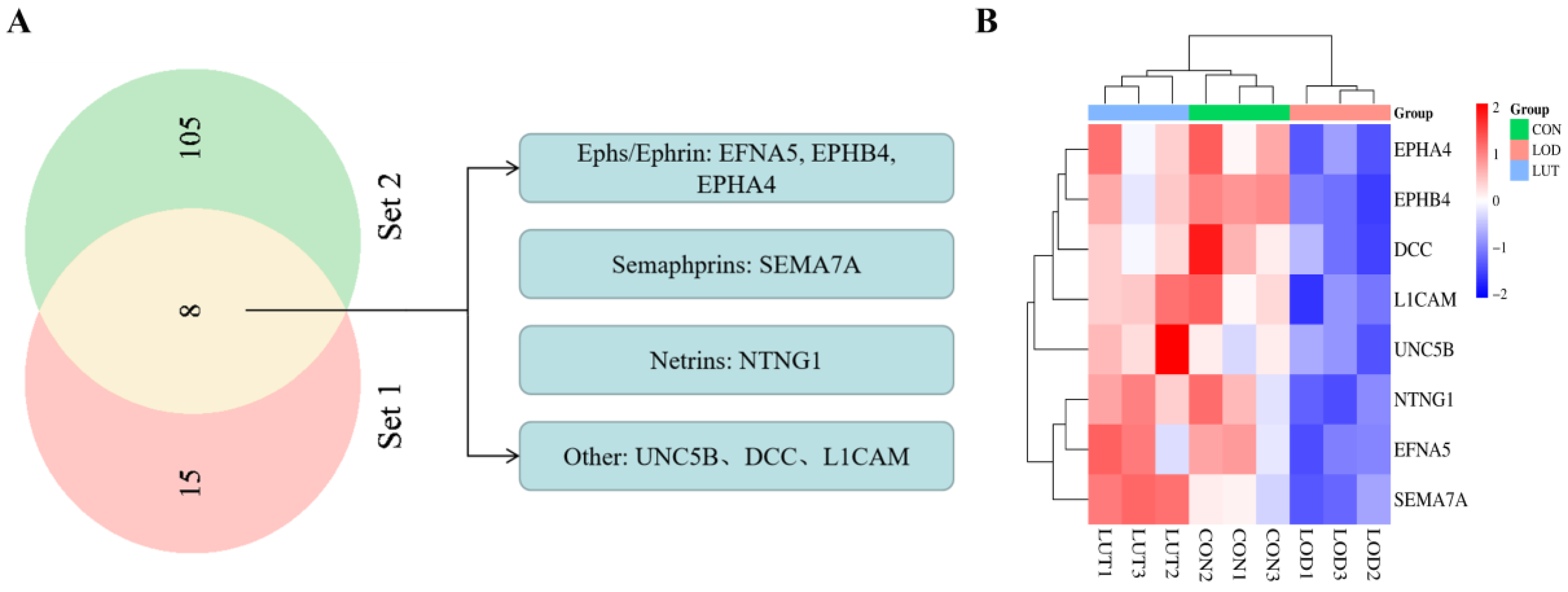
2.4.2. Analysis of Potential Targets of LUT for LOD Based on Axon Guidance Pathway
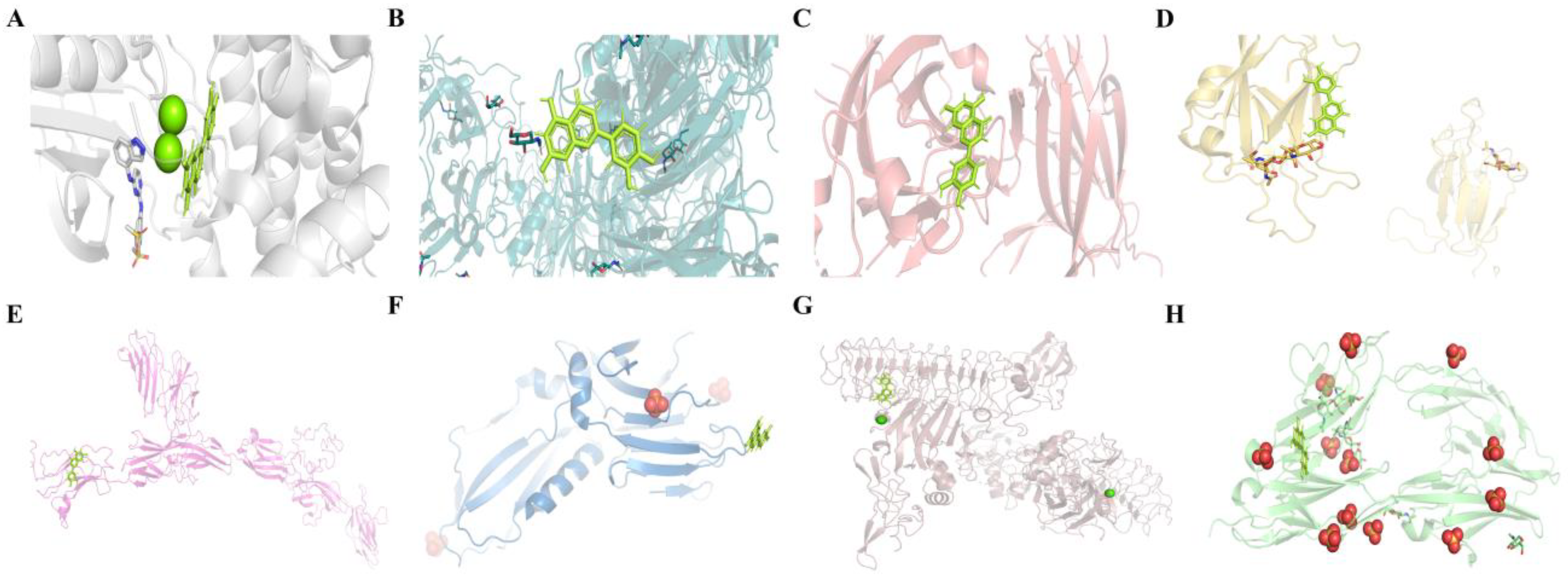
2.4.3. Molecular Docking Results of LUT with Eight Potential Targets on the Axon Guidance Pathway
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Chronic Unpredictable Mild Stress (CUMS) Modeling
4.3. Behavioral Tests
4.3.1. Sucrose Preference Test (SPT)
4.3.2. Open Field Test (OFT)
4.3.3. Forced Swimming Test (FST)
4.3.4. Morris Water Maze Test (MWM)
4.4. CSF Sample Collection
4.5. CSF Proteomics Analysis
4.5.1. Analysis of CSF Proteomics with Data Independent Acquisition (DIA) Method
4.5.2. GSEA for GO Annotation and KEGG Pathway Enrichment
- The proteins were identified commonly by the CON and LOD groups, and the LUT and LOD groups were GO-annotated employing the GSEA method;
- The GSEA method was applied for KEGG pathway enrichment from the co-identified proteins of the CON and LOD groups and the LUT and LOD groups;
- The activity of the GSEA–KEGG pathways was determined based on the enrichment score, with less than zero indicating inhibition and greater than zero indicating activation. We screened the GSEA–KEGG pathway, which is co-regulated by LOD/CON and LUT/LOD with opposite activity states, as the key pathway for the LUT treatment of LOD.
4.5.3. Protein–Protein Interaction (PPI) Network Analysis
4.5.4. Molecular Docking Experiments
4.6. Network Pharmacology Analysis
4.6.1. Target Prediction of LUT
4.6.2. Target Genes for LOD
- We searched for depression-related targets using the GeneCards, DisGenet, OMIM, TTD, PharmGKB, and DrugBank databases, with the terms “depression” or “major depressive disorder” (hereafter collectively referred to as “Depression”) used as keywords. The targets generated from the GeneCards database were filtered with a relevance score ≥ 5. The targets acquired from the DisGeNET database were screened with a score ≥ 0.3.
- Aging-related targets were investigated in the GeneCards, DisGenet, OMIM, TTD, PharmGKB, and DrugBank databases based on the keywords “aging”, “senescence”, or “anti-aging” (hereafter collectively referred to as “Aging”). Targets derived from the GeneCards database were sorted by a relevance score ≥ 5. Targets obtained from the DisGeNET database were screened by a score ≥ 0.3.
- The target genes of “Depression” and “Aging” were merged to produce the target genes of LOD.
4.6.3. Network Pharmacology Analysis of the KEGG Pathway in LUT Treatment of LOD
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Blazer, D.G. Depression in late life: Review and commentary. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, 249–265. [Google Scholar] [CrossRef] [PubMed]
- Salloway, S.; Malloy, P.; Kohn, R.; Gillard, E.; Duffy, J.; Rogg, J.; Tung, G.; Richardson, E.; Thomas, C.; Westlake, R. MRI and neuropsychological differences in early- and late-life-onset geriatric depression. Neurology 1996, 46, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, I.; Tuckwell, V.; O’Brien, J.; Ames, D. Is late onset depression a prodrome to dementia? Int. J. Geriatr. Psychiatry 2002, 17, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, L.L.; Goodwin, G.M.; Ebmeier, K.P. The cognitive neuropsychology of depression in the elderly. Psychol. Med. 2007, 37, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Naismith, S.L.; Norrie, L.M.; Mowszowski, L.; Hickie, I.B. The neurobiology of depression in later-life: Clinical, neuropsychological, neuroimaging and pathophysiological features. Prog. Neurobiol. 2012, 98, 99–143. [Google Scholar] [CrossRef]
- Yan, J.; Kuzhiumparambil, U.; Bandodkar, S.; Dale, R.C.; Fu, S. Cerebrospinal fluid metabolomics: Detection of neuroinflammation in human central nervous system disease. Clin. Transl. Immunol. 2021, 10, e1318. [Google Scholar] [CrossRef]
- Seo, J.-S.; Mantas, I.; Svenningsson, P.; Greengard, P. Ependymal cells-CSF flow regulates stress-induced depression. Mol. Psychiatry 2021, 26, 7308–7315. [Google Scholar] [CrossRef]
- Zeng, N.-X.; Li, H.-Z.; Wang, H.-Z.; Liu, K.-G.; Gong, X.-Y.; Luo, W.-L.; Yan, C.; Wu, L.-L. Exploration of the mechanism by which icariin modulates hippocampal neurogenesis in a rat model of depression. Neural. Regen. Res. 2022, 17, 632–642. [Google Scholar] [CrossRef]
- Baruch, K.; Deczkowska, A.; David, E.; Castellano, J.M.; Miller, O.; Kertser, A.; Berkutzki, T.; Barnett-Itzhaki, Z.; Bezalel, D.; Wyss-Coray, T.; et al. Aging-induced type I interferon response at the choroid plexus negatively affects brain function. Science 2014, 346, 89–93. [Google Scholar] [CrossRef]
- Sawmiller, D.; Li, S.; Shahaduzzaman, M.; Smith, A.J.; Obregon, D.; Giunta, B.; Borlongan, C.V.; Sanberg, P.R.; Tan, J. Luteolin reduces Alzheimer’s disease pathologies induced by traumatic brain injury. Int. J. Mol. Sci. 2014, 15, 895–904. [Google Scholar] [CrossRef]
- Nordeen, S.K.; Bona, B.J.; Jones, D.N.; Lambert, J.R.; Jackson, T.A. Endocrine disrupting activities of the flavonoid nutraceuticals luteolin and quercetin. Horm. Cancer 2013, 4, 293–300. [Google Scholar] [CrossRef]
- Marniemi, J.; Alanen, E.; Impivaara, O.; Seppänen, R.; Hakala, P.; Rajala, T.; Rönnemaa, T. Dietary and serum vitamins and minerals as predictors of myocardial infarction and stroke in elderly subjects. Nutr. Metab. Cardiovasc. Dis. NMCD 2005, 15, 188–197. [Google Scholar] [CrossRef]
- Liu, Y.; Gou, L.-S.; Tian, X.; Fu, X.-B.; Ling, X.; Sun, L.-Y.; Lan, N.; Li, S.; Yin, X.-X. Protective effects of luteolin on cognitive impairments induced by psychological stress in mice. Exp. Biol. Med. 2013, 238, 418–425. [Google Scholar] [CrossRef]
- Donato, F.; de Gomes, M.G.; Goes, A.T.R.; Borges, C.; Del Fabbro, L.; Antunes, M.S.; Souza, L.C.; Boeira, S.P.; Jesse, C.R. Hesperidin exerts antidepressant-like effects in acute and chronic treatments in mice: Possible role of L-arginine-NO-cGMP pathway and BDNF levels. Brain Res. Bull. 2014, 104, 19–26. [Google Scholar] [CrossRef]
- Akter, R.; Afrose, A.; Rahman, M.R.; Chowdhury, R.; Nirzhor, S.S.R.; Khan, R.I.; Kabir, M.T. A Comprehensive Analysis into the Therapeutic Application of Natural Products as SIRT6 Modulators in Alzheimer’s Disease, Aging, Cancer, Inflammation, and Diabetes. Int. J. Mol. Sci. 2021, 22, 4180. [Google Scholar] [CrossRef]
- Tassinari, M.; Mottolese, N.; Galvani, G.; Ferrara, D.; Gennaccaro, L.; Loi, M.; Medici, G.; Candini, G.; Rimondini, R.; Ciani, E.; et al. Luteolin Treatment Ameliorates Brain Development and Behavioral Performance in a Mouse Model of CDKL5 Deficiency Disorder. Int. J. Mol. Sci. 2022, 23, 8719. [Google Scholar] [CrossRef]
- Wang, J.G.; Gao, L.Q.; Lee, Y.M.; Kalesh, K.A.; Ong, Y.S.; Lim, J.H.; Jee, J.E.; Sun, H.Y.; Lee, S.S.; Hua, Z.C.; et al. Target identification of natural and traditional medicines with quantitative chemical proteomics approaches. Pharmacol. Ther. 2016, 162, 10–22. [Google Scholar] [CrossRef]
- Khalilpour, A.; Kilic, T.; Khalilpour, S.; Alvarez, M.M.; Yazdi, I.K. Proteomic-based biomarker discovery for development of next generation diagnostics. Appl. Microbiol. Biotechnol. 2017, 101, 475–491. [Google Scholar] [CrossRef]
- Wang, M.Q.; Liu, F.L.; Yao, Y.F.; Zhang, Q.Y.; Lu, Z.H.; Zhang, R.J.; Liu, C.H.; Lin, C.Z.; Zhu, C.C. Network pharmacology-based mechanism prediction and pharmacological validation of Xiaoyan Lidan formula on attenuating alpha-naphthylisothiocyanate induced cholestatic hepatic injury in rats. J. Ethnopharmacol. 2021, 270, 113816. [Google Scholar] [CrossRef]
- Hsin, K.-Y.; Ghosh, S.; Kitano, H. Combining machine learning systems and multiple docking simulation packages to improve docking prediction reliability for network pharmacology. PLoS ONE 2013, 8, e83922. [Google Scholar] [CrossRef]
- Dybedal, G.S.; Tanum, L.; Sundet, K.; Gaarden, T.L.; Bjølseth, T.M. Neuropsychological functioning in late-life depression. Front. Psychol. 2013, 4, 381. [Google Scholar] [CrossRef] [PubMed]
- Panza, F.; Frisardi, V.; Capurso, C.; D’Introno, A.; Colacicco, A.M.; Imbimbo, B.P.; Santamato, A.; Vendemiale, G.; Seripa, D.; Pilotto, A.; et al. Late-Life Depression, Mild Cognitive Impairment, and Dementia: Possible Continuum? Am. J. Geriatr. Psychiatry 2010, 18, 98–116. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, G.S.; Kelly, R.E. Research advances in geriatric depression. World Psychiatry 2009, 8, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Hu, M.M.; Hu, J.R.; Du, Z.Y.; Su, Q.; Xiang, Z.M. Luteolin Suppresses Microglia Neuroinflammatory Responses and Relieves Inflammation-Induced Cognitive Impairments. Neurotox. Res. 2021, 39, 1800–1811. [Google Scholar] [CrossRef]
- Ashaari, Z.; Hadjzadeh, M.-A.-R.; Hassanzadeh, G.; Alizamir, T.; Yousefi, B.; Keshavarzi, Z.; Mokhtari, T. The Flavone Luteolin Improves Central Nervous System Disorders by Different Mechanisms: A Review. J. Mol. Neurosci. 2018, 65, 491–506. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Cholevas, C.; Polyzoidis, K.; Politis, A. Long-COVID syndrome-associated brain fog and chemofog: Luteolin to the rescue. Biofactors 2021, 47, 232–241. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Stewart, J.M.; Hatziagelaki, E.; Kolaitis, G. Brain “fog,” inflammation and obesity: Key aspects of neuropsychiatric disorders improved by luteolin. Front. Neurosci. 2015, 9, 225. [Google Scholar] [CrossRef]
- De Luca, P.; Camaioni, A.; Marra, P.; Salzano, G.; Carriere, G.; Ricciardi, L.; Pucci, R.; Montemurro, N.; Brenner, M.J.; Di Stadio, A. Effect of Ultra-Micronized Palmitoylethanolamide and Luteolin on Olfaction and Memory in Patients with Long COVID: Results of a Longitudinal Study. Cells 2022, 11, 2552. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Braidy, N.; Gortzi, O.; Sobarzo-Sanchez, E.; Daglia, M.; Skalicka-Woźniak, K.; Nabavi, S.M. Luteolin as an anti-inflammatory and neuroprotective agent: A brief review. Brain Res. Bull. 2015, 119 Pt A, 1–11. [Google Scholar] [CrossRef]
- Kwon, Y. Luteolin as a potential preventive and therapeutic candidate for Alzheimer’s disease. Exp. Gerontol. 2017, 95, 39–43. [Google Scholar] [CrossRef]
- Tsilioni, I.; Taliou, A.; Francis, K.; Theoharides, T.C. Children with autism spectrum disorders, who improved with a luteolin-containing dietary formulation, show reduced serum levels of TNF and IL-6. Transl. Psychiatry 2015, 5, e647. [Google Scholar] [CrossRef]
- Muruganathan, N.; Dhanapal, A.R.; Baskar, V.; Muthuramalingam, P.; Selvaraj, D.; Aara, H.; Shiek Abdullah, M.Z.; Sivanesan, I. Recent Updates on Source, Biosynthesis, and Therapeutic Potential of Natural Flavonoid Luteolin: A Review. Metabolites 2022, 12, 1145. [Google Scholar] [CrossRef]
- Meissner, F.; Geddes-McAlister, J.; Mann, M.; Bantscheff, M. The emerging role of mass spectrometry-based proteomics in drug discovery. Nat. Rev. Drug Discov. 2022, 21, 637–654. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Dong, R.; Huang, R.; Shi, X.; Xu, Z.; Mang, J. Exploration of the mechanism of luteolin against ischemic stroke based on network pharmacology, molecular docking and experimental verification. Bioengineered 2021, 12, 12274–12293. [Google Scholar] [CrossRef]
- Chan, R.F.; Copeland, W.E.; Zhao, M.; Xie, L.Y.; Costello, J.; Aberg, K.A.; van den Oord, E.J.C.G. A methylation study implicates the rewiring of brain neural circuits during puberty in the emergence of sex differences in depression symptoms. J. Child Psychol. Psychiatry 2022, 63, 802–809. [Google Scholar] [CrossRef]
- Gui, S.; Liu, Y.; Pu, J.; Song, X.; Chen, X.; Chen, W.; Zhong, X.; Wang, H.; Liu, L.; Xie, P. Comparative analysis of hippocampal transcriptional features between major depressive disorder patients and animal models. J. Affect. Disord. 2021, 293, 19–28. [Google Scholar] [CrossRef]
- Kennedy, B.K.; Berger, S.L.; Brunet, A.; Campisi, J.; Cuervo, A.M.; Epel, E.S.; Franceschi, C.; Lithgow, G.J.; Morimoto, R.I.; Pessin, J.E.; et al. Geroscience: Linking Aging to Chronic Disease. Cell 2014, 159, 708–712. [Google Scholar] [CrossRef]
- Tanaka, T.; Biancotto, A.; Moaddel, R.; Moore, A.Z.; Gonzalez-Freire, M.; Aon, M.A.; Candia, J.; Zhang, P.B.; Cheung, F.; Fantoni, G.; et al. Plasma proteomic signature of age in healthy humans. Aging Cell 2018, 17, e12799. [Google Scholar] [CrossRef]
- Northcutt, R.G. Evolution of centralized nervous systems: Two schools of evolutionary thought. Proc. Natl. Acad. Sci. USA 2012, 109, 10626–10633. [Google Scholar] [CrossRef]
- Zhang, L.; Qi, Z.; Li, J.; Li, M.; Du, X.; Wang, S.; Zhou, G.; Xu, B.; Liu, W.; Xi, S.; et al. Roles and Mechanisms of Axon-Guidance Molecules in Alzheimer’s Disease. Mol. Neurobiol. 2021, 58, 3290–3307. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Lee, W.-H.; Bae, Y.C.; Suk, K. Axon Guidance Molecules Guiding Neuroinflammation. Exp. Neurobiol. 2019, 28, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Kolodkin, A.L.; Tessier-Lavigne, M. Mechanisms and molecules of neuronal wiring: A primer. Cold Spring Harb. Perspect. Biol. 2011, 3, a001727. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.-J.; Sun, S.; Gibson, J.R.; Henkemeyer, M. A dual shaping mechanism for postsynaptic ephrin-B3 as a receptor that sculpts dendrites and synapses. Nat. Neurosci. 2011, 14, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; Nomura, T.; Yoshizaki, K.; Frisén, J.; Osumi, N. Impaired hippocampal neurogenesis and vascular formation in ephrin-A5-deficient mice. Stem Cells 2010, 28, 974–983. [Google Scholar] [CrossRef]
- Ashton, R.S.; Conway, A.; Pangarkar, C.; Bergen, J.; Lim, K.-I.; Shah, P.; Bissell, M.; Schaffer, D.V. Astrocytes regulate adult hippocampal neurogenesis through ephrin-B signaling. Nat. Neurosci. 2012, 15, 1399–1406. [Google Scholar] [CrossRef]
- Liu, T.; Zeng, X.; Sun, F.; Hou, H.; Guan, Y.; Guo, D.; Ai, H.; Wang, W.; Zhang, G. EphB4 Regulates Self-Renewal, Proliferation and Neuronal Differentiation of Human Embryonic Neural Stem Cells in Vitro. Cell. Physiol. Biochem. 2017, 41, 819–834. [Google Scholar] [CrossRef]
- Khodosevich, K.; Watanabe, Y.; Monyer, H. EphA4 preserves postnatal and adult neural stem cells in an undifferentiated state in vivo. J. Cell Sci. 2011, 124 Pt 8, 1268–1279. [Google Scholar] [CrossRef]
- Bourgin, C.; Murai, K.K.; Richter, M.; Pasquale, E.B. The EphA4 receptor regulates dendritic spine remodeling by affecting beta1-integrin signaling pathways. J. Cell Biol. 2007, 178, 1295–1307. [Google Scholar] [CrossRef]
- North, H.A.; Zhao, X.; Kolk, S.M.; Clifford, M.A.; Ziskind, D.M.; Donoghue, M.J. Promotion of proliferation in the developing cerebral cortex by EphA4 forward signaling. Development 2009, 136, 2467–2476. [Google Scholar] [CrossRef]
- Fard, D.; Tamagnone, L. Semaphorins in health and disease. Cytokine Growth Factor Rev. 2021, 57, 55–63. [Google Scholar] [CrossRef]
- Pasterkamp, R.J.; Peschon, J.J.; Spriggs, M.K.; Kolodkin, A.L. Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature 2003, 424, 398–405. [Google Scholar] [CrossRef]
- Zhang, Q.; Goto, H.; Akiyoshi-Nishimura, S.; Prosselkov, P.; Sano, C.; Matsukawa, H.; Yaguchi, K.; Nakashiba, T.; Itohara, S. Diversification of behavior and postsynaptic properties by netrin-G presynaptic adhesion family proteins. Mol. Brain 2016, 9, 6. [Google Scholar] [CrossRef]
- Meltzer, S.; Boulanger, K.C.; Osei-Asante, E.; Handler, A.; Zhang, Q.; Sano, C.; Itohara, S.; Ginty, D.D. A role for axon-glial interactions and Netrin-G1 signaling in the formation of low-threshold mechanoreceptor end organs. Proc. Natl. Acad. Sci. USA 2022, 119, e2210421119. [Google Scholar] [CrossRef]
- Bhat, S.A.; Sarwar, Z.; Gillani, S.Q.; Un Nisa, M.; Reshi, I.; Nabi, N.; Xie, S.; Fazili, K.M.; Roberts, T.M.; Andrabi, S. Polyomavirus Small T Antigen Induces Apoptosis in Mammalian Cells through the UNC5B Pathway in a PP2A-Dependent Manner. J. Virol. 2020, 94, e02187-19. [Google Scholar] [CrossRef]
- Maten, M.v.d.; Reijnen, C.; Pijnenborg, J.M.A.; Zegers, M.M. L1 Cell Adhesion Molecule in Cancer, a Systematic Review on Domain-Specific Functions. Int. J. Mol. Sci. 2019, 20, 4180. [Google Scholar] [CrossRef]
- Yang, D.; Yang, H.; Luiselli, G.; Ogagan, C.; Dai, H.; Chiu, L.; Carroll, R.S.; Johnson, M.D. Increased plasmin-mediated proteolysis of L1CAM in a mouse model of idiopathic normal pressure hydrocephalus. Proc. Natl. Acad. Sci. USA 2021, 118, e2010528118. [Google Scholar] [CrossRef]
- Finci, L.; Zhang, Y.; Meijers, R.; Wang, J.H. Signaling mechanism of the netrin-1 receptor DCC in axon guidance. Prog. Biophys. Mol. Biol. 2015, 118, 153–160. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Q.; Yi, S.; Liu, Q.; Zhang, R.; Wang, P.; Qian, T.; Li, S. The microRNAs and down-regulate the axon-guidance genes and during peripheral nerve regeneration. J. Biol. Chem. 2019, 294, 3489–3500. [Google Scholar] [CrossRef]
- Li, H.-Z.; Liu, K.-G.; Zeng, N.-X.; Wu, X.-F.; Lu, W.-J.; Xu, H.-F.; Yan, C.; Wu, L.-L. Luteolin Enhances Choroid Plexus 5-MTHF Brain Transport to Promote Hippocampal Neurogenesis in LOD Rats. Front. Pharmacol. 2022, 13, 826568. [Google Scholar] [CrossRef]
- Qiao, H.; Dong, L.; Zhang, X.; Zhu, C.; Zhang, X.; Wang, L.; Liu, Z.; Chen, L.; Xing, Y.; Wang, C.; et al. Protective effect of luteolin in experimental ischemic stroke: Upregulated SOD1, CAT, Bcl-2 and claudin-5, down-regulated MDA and Bax expression. Neurochem. Res. 2012, 37, 2014–2024. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yang, W.; Ding, J.; Ji, J.; Wu, L.; Zheng, Y.; Li, Y.; Cheng, Z.; Zhang, J.; Yu, Q.; et al. Luteolin Pretreatment Attenuates Hepatic Ischemia-Reperfusion Injury in Mice by Inhibiting Inflammation, Autophagy, and Apoptosis via the ERK/PPARα Pathway. PPAR Res. 2022, 2022, 8161946. [Google Scholar] [CrossRef] [PubMed]



| Protein Name | Gene Name | Family | LOD/CON | LUT/LOD | ||
|---|---|---|---|---|---|---|
| Fold Change | p Value | Fold Change | p Value | |||
| Ephrin-A5 | EFNA5 | Ephs/Ephrin | 0.57456 | 0.01075 | 1.85970 | 0.02264 |
| EPH receptor B4 | EPHB4 | 0.56399 | 0.00026 | 1.54778 | 0.00798 | |
| Eph receptor A4 | EPHA4 | 0.69904 | 0.01011 | 1.38487 | 0.01475 | |
| Semaphorin 7A | SEMA7A | Semaphorins | 0.81877 | 0.01265 | 1.46891 | 0.00033 |
| Netrin G1 | NTNG1 | Netrins | 0.74603 | 0.01773 | 1.37943 | 0.00113 |
| Netrin receptor UNC5B | UNC5B | Other | 0.80701 | 0.02041 | 1.47754 | 0.03082 |
| Neural cell adhesion molecule L1 | L1CAM | 0.78713 | 0.01719 | 1.28610 | 0.00572 | |
| Netrin receptor DCC | DCC | 0.66085 | 0.02909 | 1.33989 | 0.01489 | |
| Compound | Target Name | Protein Name | ΔG (kJ·mol−1) | Target Name | Protein Name | ΔG (kJ·mol−1) |
|---|---|---|---|---|---|---|
| Luteolin | EPHB4 | EPH receptor B4 | −8.0970 | L1CAM | Neural cell adhesion molecule L1 | −6.9822 |
| SEMA7A | Semaphorin 7A | −7.3077 | UNC5B | Netrin receptor UNC5B | −6.9678 | |
| EPHA4 | Eph receptor A4 | −7.0616 | NTNG1 | Netrin G1 | −6.8523 | |
| EFNA5 | Ephrin-A5 | −6.9858 | DCC | Netrin receptor DCC | −6.6671 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, K.; Li, H.; Zeng, N.; Li, B.; Yao, G.; Wu, X.; Xu, H.; Yan, C.; Wu, L. Exploration of the Core Pathways and Potential Targets of Luteolin Treatment on Late-Onset Depression Based on Cerebrospinal Fluid Proteomics. Int. J. Mol. Sci. 2023, 24, 3485. https://doi.org/10.3390/ijms24043485
Liu K, Li H, Zeng N, Li B, Yao G, Wu X, Xu H, Yan C, Wu L. Exploration of the Core Pathways and Potential Targets of Luteolin Treatment on Late-Onset Depression Based on Cerebrospinal Fluid Proteomics. International Journal of Molecular Sciences. 2023; 24(4):3485. https://doi.org/10.3390/ijms24043485
Chicago/Turabian StyleLiu, Kaige, Huizhen Li, Ningxi Zeng, Bozhi Li, Gaolei Yao, Xiaofeng Wu, Hanfang Xu, Can Yan, and Lili Wu. 2023. "Exploration of the Core Pathways and Potential Targets of Luteolin Treatment on Late-Onset Depression Based on Cerebrospinal Fluid Proteomics" International Journal of Molecular Sciences 24, no. 4: 3485. https://doi.org/10.3390/ijms24043485
APA StyleLiu, K., Li, H., Zeng, N., Li, B., Yao, G., Wu, X., Xu, H., Yan, C., & Wu, L. (2023). Exploration of the Core Pathways and Potential Targets of Luteolin Treatment on Late-Onset Depression Based on Cerebrospinal Fluid Proteomics. International Journal of Molecular Sciences, 24(4), 3485. https://doi.org/10.3390/ijms24043485






