Comparative Analysis of Retinal Organotypic Cultures and In Vivo Axotomized Retinas
Abstract
1. Introduction
2. Results
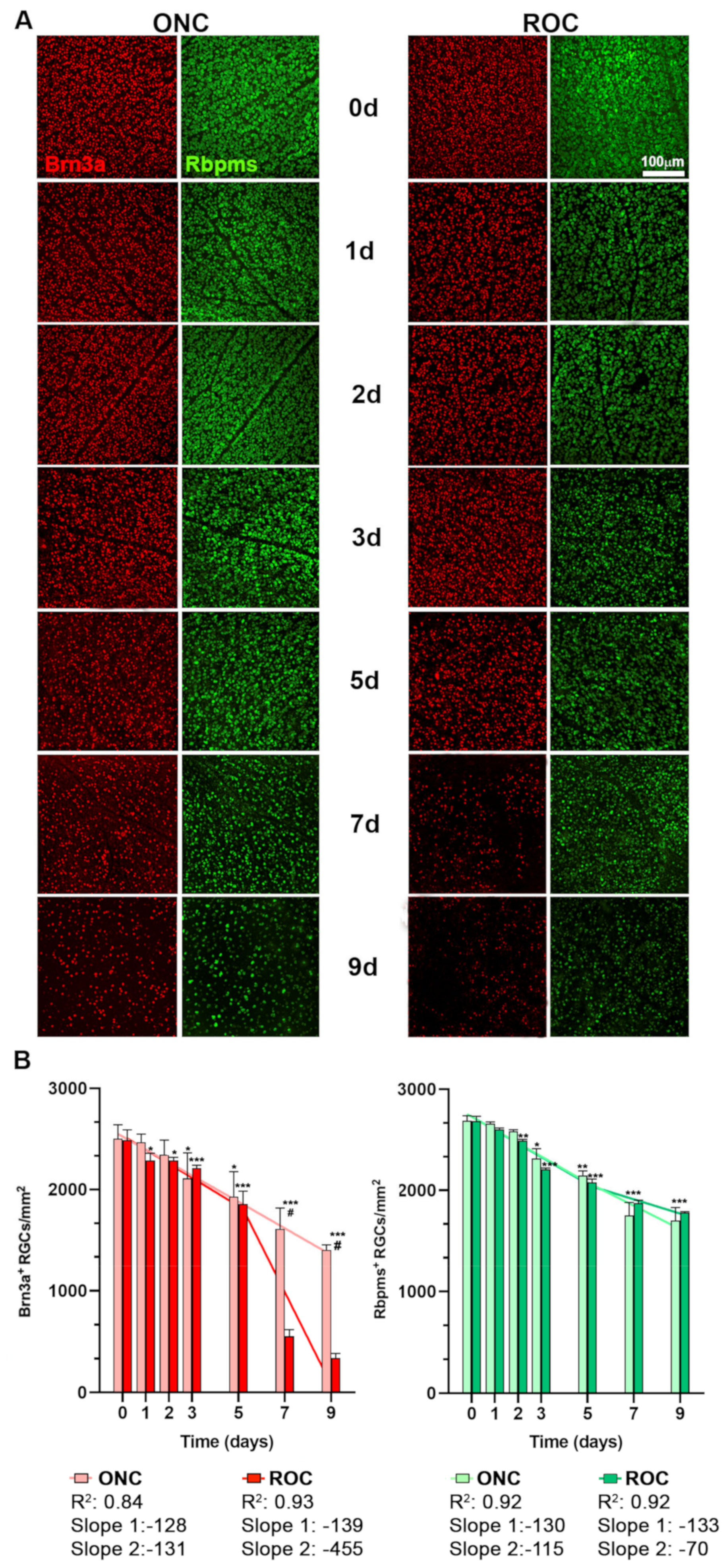
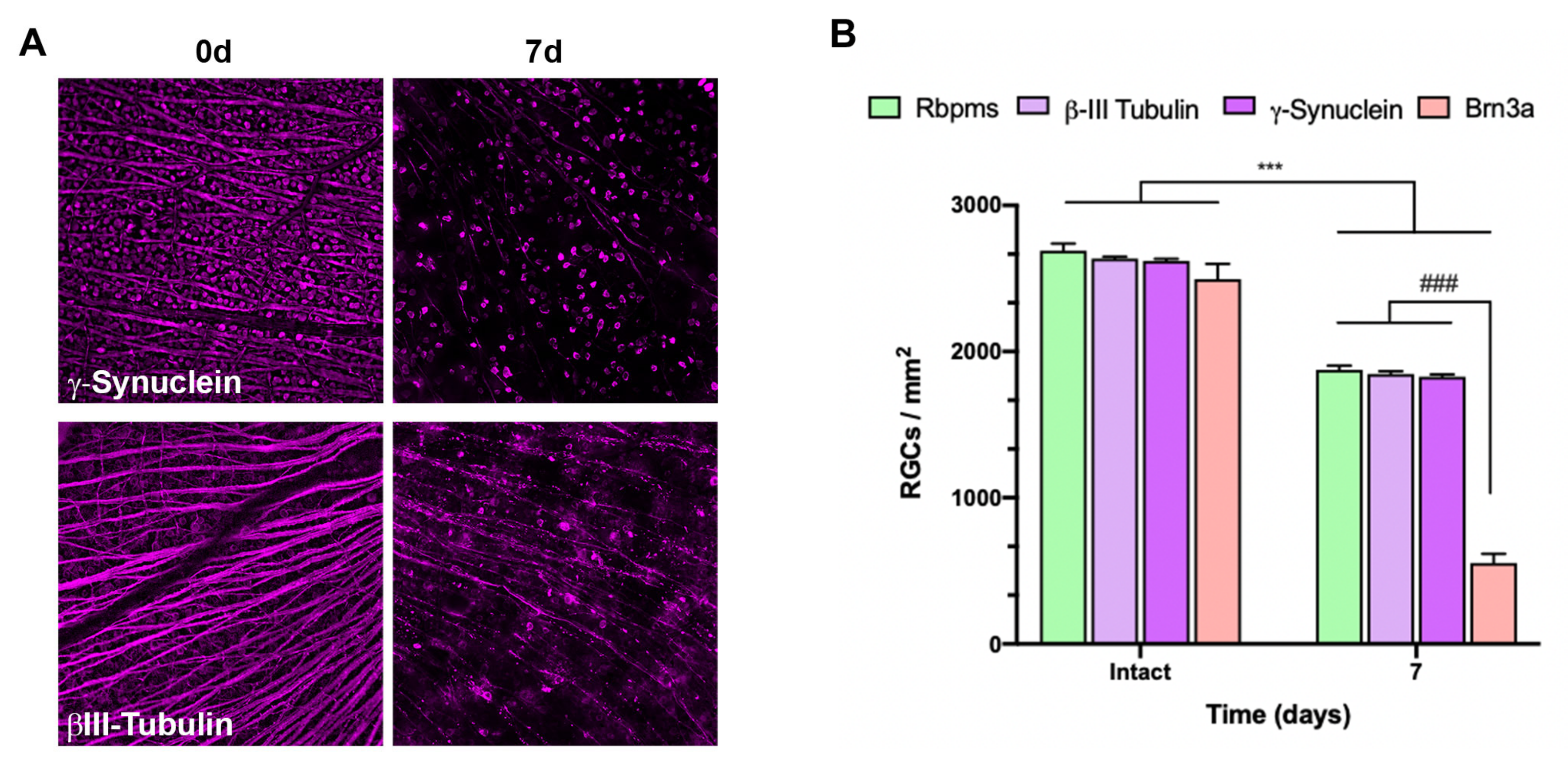
2.1. Course of RGC Death: ROCs vs. ONC
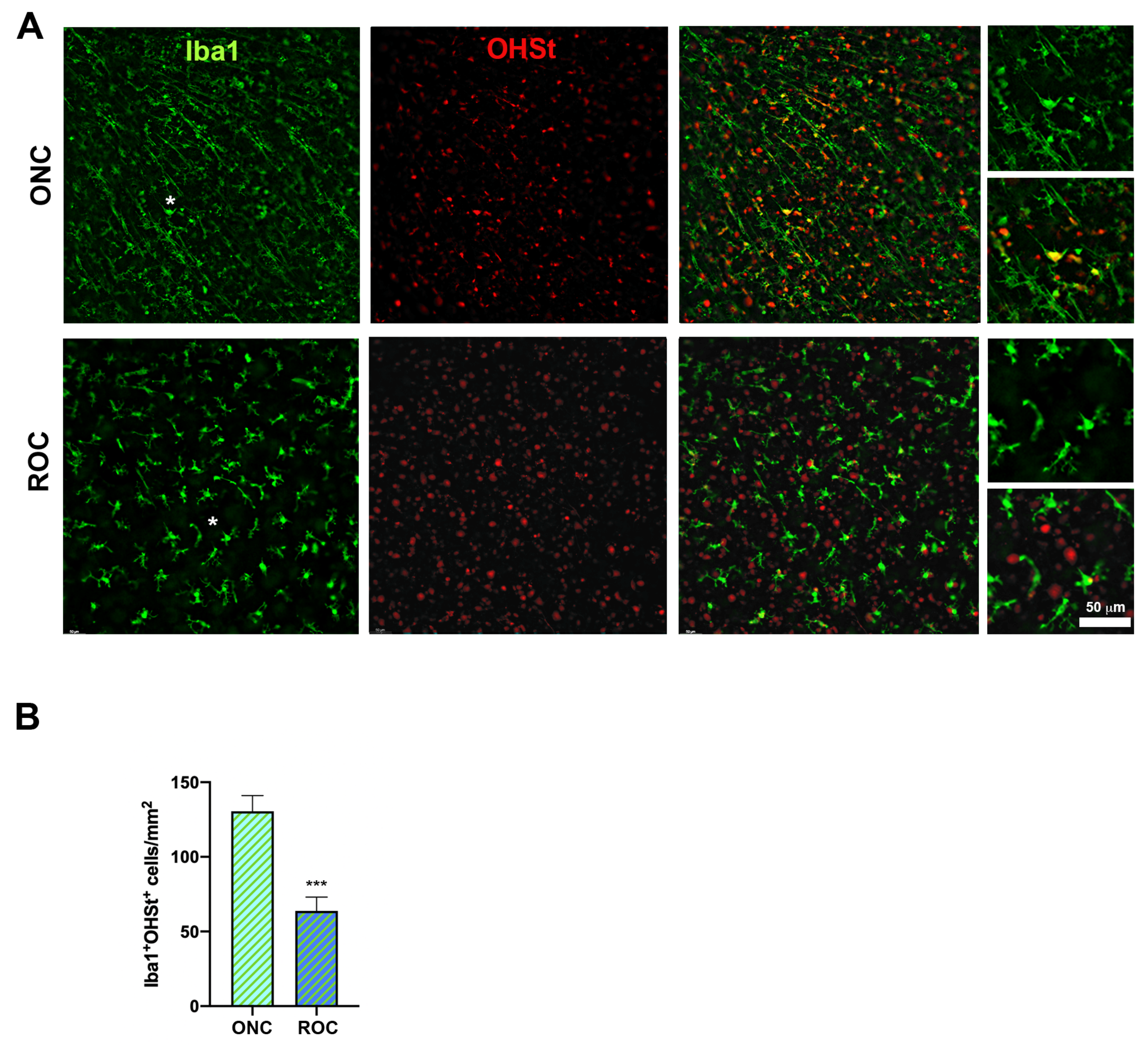
2.2. Microglial Behavior in ROCs
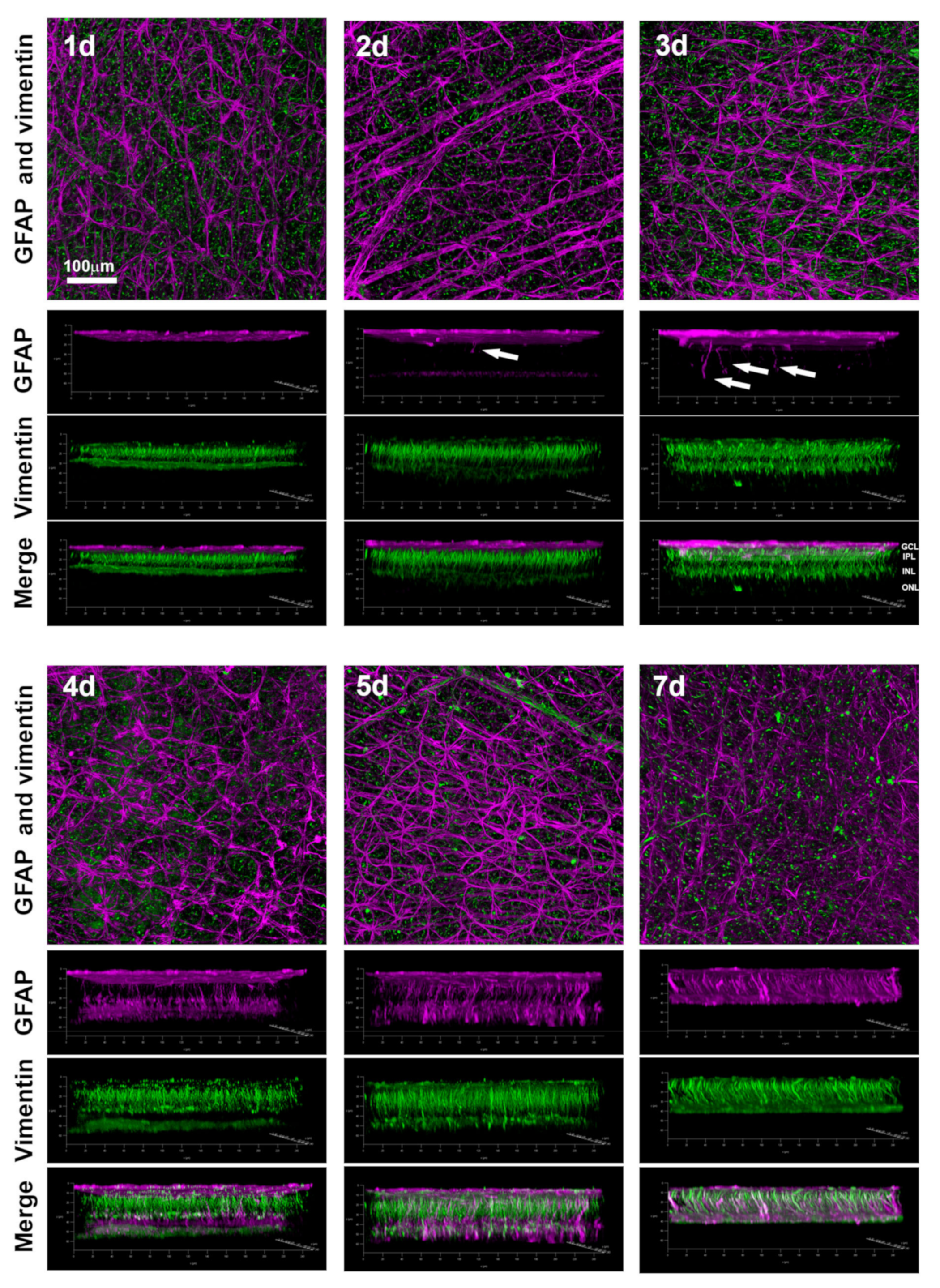
2.3. Macroglial Activation
3. Discussion
4. Materials and Methods
4.1. Animals
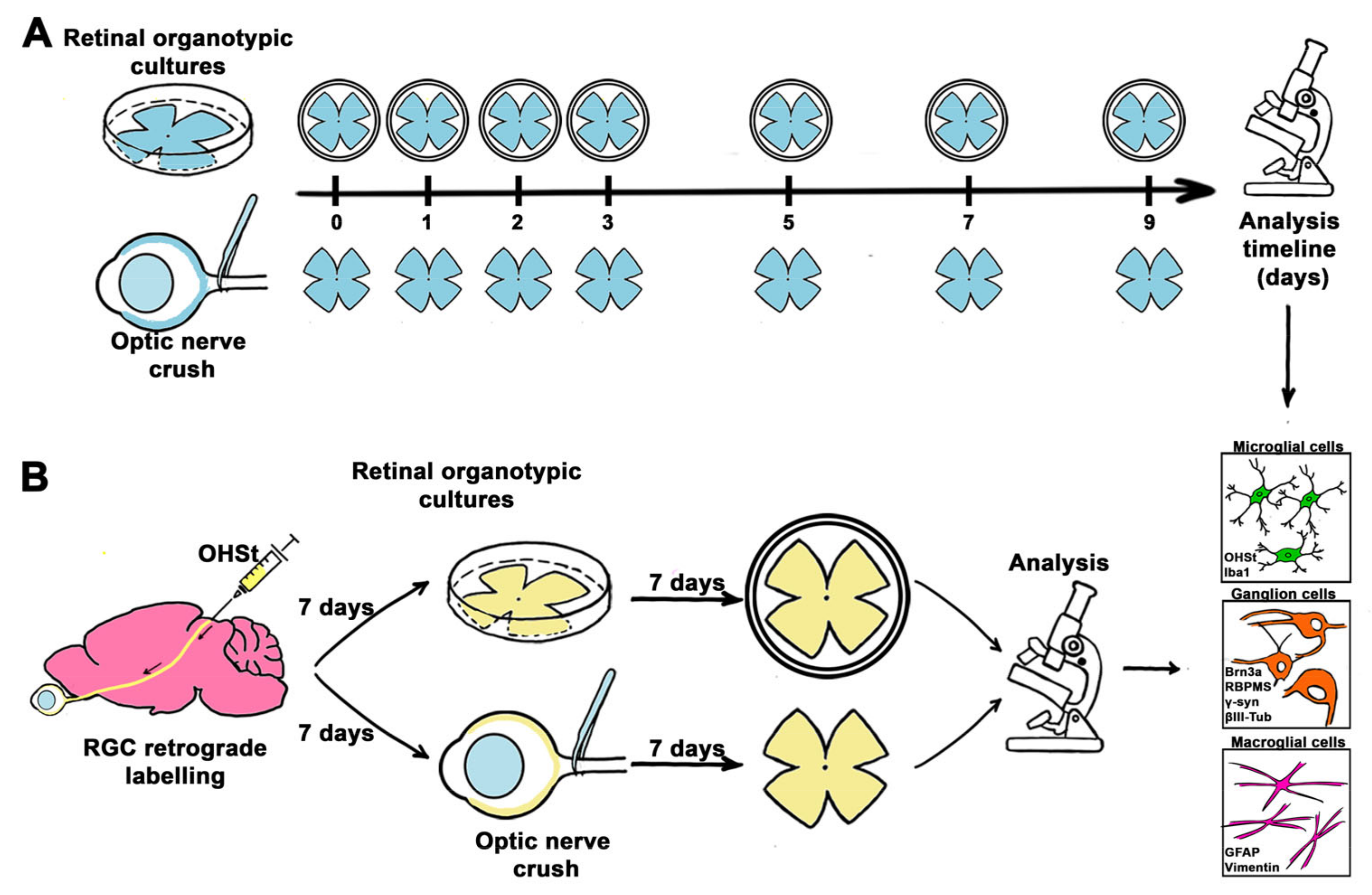
4.2. Experimental Design
4.3. Retinal Organotypic Cultures (ROCs)
4.4. Optic Nerve Crush (ONC)
4.5. RGC Retrograde Tracing
4.6. Tissue Preparation
4.7. Immunohistofluorescence
4.7.1. Whole Mount Retinas
4.7.2. Retinal Organotypic Cultures
4.8. Image Acquisition, Quantification, and Analysis
4.9. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boia, R.; Dias, P.A.; Galindo-Romero, C.; Ferreira, H.; Aires, I.D.; Vidal-Sanz, M.; Agudo-Barriuso, M.; Bernardes, R.; Santos, P.F.; de Sousa, H.C.; et al. Intraocular implants loaded with A3R agonist rescue retinal ganglion cells from ischemic damage. J. Control. Release 2022, 343, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Romero, C.; Valiente-Soriano, F.J.; Jiménez-López, M.; García-Ayuso, D.; Villegas-Pérez, M.P.; Vidal-Sanz, M.; Agudo-Barriuso, M. Effect of Brain-Derived Neurotrophic Factor on Mouse Axotomized Retinal Ganglion Cells and Phagocytic Microglia. Investig. Opthalmology Vis. Sci. 2013, 54, 974–985. [Google Scholar] [CrossRef] [PubMed]
- Lucas-Ruiz, F.; Galindo-Romero, C.; Albaladejo-García, V.; Vidal-Sanz, M.; Agudo-Barriuso, M. Mechanisms implicated in the contralateral effect in the central nervous system after unilateral injury: Focus on the visual system. Neural Regen. Res. 2021, 16, 2125–2131. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Migallón, M.C.; Valiente-Soriano, F.J.; Nadal-Nicolás, F.M.; Vidal-Sanz, M.; Agudo-Barriuso, M. Apoptotic Retinal Ganglion Cell Death after Optic Nerve Transection or Crush in Mice: Delayed RGC Loss With BDNF or a Caspase 3 Inhibitor. Investig. Opthalmology Vis. Sci. 2016, 57, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Valiente-Soriano, F.J.; Ortín-Martínez, A.; Di Pierdomenico, J.; García-Ayuso, D.; Gallego-Ortega, A.; de Imperial-Ollero, J.A.M.; Jiménez-López, M.; Villegas-Pérez, M.P.; Wheeler, L.A.; Vidal-Sanz, M. Topical Brimonidine or Intravitreal BDNF, CNTF, or bFGF Protect Cones Against Phototoxicity. Transl. Vis. Sci. Technol. 2019, 8, 36. [Google Scholar] [CrossRef]
- García-Ayuso, D.; Di Pierdomenico, J.; Valiente-Soriano, F.J.; Martínez-Vacas, A.; Agudo-Barriuso, M.; Vidal-Sanz, M.; Picaud, S.; Villegas-Pérez, M.P. β-alanine supplementation induces taurine depletion and causes alterations of the retinal nerve fiber layer and axonal transport by retinal ganglion cells. Exp. Eye Res. 2019, 188, 107781. [Google Scholar] [CrossRef]
- Norte-Muñoz, M.; Lucas-Ruiz, F.; Gallego-Ortega, A.; García-Bernal, D.; Valiente-Soriano, F.J.; de la Villa, P.; Vidal-Sanz, M.; Agudo-Barriuso, M. Neuroprotection and Axonal Regeneration Induced by Bone Marrow Mesenchymal Stromal Cells Depend on the Type of Transplant. Front. Cell Dev. Biol. 2021, 9, 772223. [Google Scholar] [CrossRef]
- Sánchez-Migallón, M.C.; Valiente-Soriano, F.J.; Salinas-Navarro, M.; Nadal-Nicolás, F.M.; Jiménez-López, M.; Vidal-Sanz, M.; Agudo-Barriuso, M. Nerve fibre layer degeneration and retinal ganglion cell loss long term after optic nerve crush or transection in adult mice. Exp. Eye Res. 2018, 170, 40–50. [Google Scholar] [CrossRef]
- Vidal-Sanz, M.; Galindo-Romero, C.; Valiente-Soriano, F.J.; Nadal-Nicolas, F.M.; Ortin-Martinez, A.; Rovere, G.; Salinas-Navarro, M.; Lucas-Ruiz, F.; Sanchez-Migallon, M.C.; Sobrado-Calvo, P.; et al. Shared and Differential Retinal Responses against Optic Nerve Injury and Ocular Hypertension. Front. Neurosci. 2017, 11, 235. [Google Scholar] [CrossRef]
- Galindo-Romero, C.; Avilés-Trigueros, M.; Jiménez-López, M.; Valiente-Soriano, F.; Salinas-Navarro, M.; Nadal-Nicolás, F.; Villegas-Pérez, M.; Vidal-Sanz, M.; Agudo-Barriuso, M. Axotomy-induced retinal ganglion cell death in adult mice: Quantitative and topographic time course analyses. Exp. Eye Res. 2011, 92, 377–387. [Google Scholar] [CrossRef]
- De Imperial-Ollero, J.A.M.; Gallego-Ortega, A.; Ortín-Martínez, A.; Villegas-Pérez, M.P.; Valiente-Soriano, F.J.; Vidal-Sanz, M. Animal Models of LED-Induced Phototoxicity. Short- and Long-Term In Vivo and Ex Vivo Retinal Alterations. Life 2021, 11, 1137. [Google Scholar] [CrossRef]
- Nadal-Nicolás, F.M.; López, M.J.; Sobrado-Calvo, P.; Nieto-Lo’pez, L.; Ca´novas-Marti´nez, I.; Salinas-Navarro, M.; Vidal-Sanz, M.; Agudo-Barriuso, M. Brn3a as a Marker of Retinal Ganglion Cells: Qualitative and Quantitative Time Course Studies in Naïve and Optic Nerve–Injured Retinas. Investig. Opthalmology Vis. Sci. 2009, 50, 3860–3868. [Google Scholar] [CrossRef]
- Salinas-Navarro, M.; Alarcón-Martínez, L.; Valiente-Soriano, F.J.; Jiménez-López, M.; Mayor-Torroglosa, S.; Avilés-Trigueros, M.; Villegas-Pérez, M.P.; Vidal-Sanz, M. Ocular hypertension impairs optic nerve axonal transport leading to progressive retinal ganglion cell degeneration. Exp. Eye Res. 2010, 90, 168–183. [Google Scholar] [CrossRef]
- De Hoz, R.; Gallego, B.I.; Ramírez, A.I.; Rojas, B.; Salazar, J.J.; Valiente-Soriano, F.J.; Avilés-Trigueros, M.; Villegas-Perez, M.P.; Vidal-Sanz, M.; Triviño, A.; et al. Rod-Like Microglia Are Restricted to Eyes with Laser-Induced Ocular Hypertension but Absent from the Microglial Changes in the Contralateral Untreated Eye. PLoS ONE 2013, 8, e83733. [Google Scholar] [CrossRef]
- Ramírez, A.I.; Salazar, J.J.; de Hoz, R.; Rojas, B.; Gallego, B.I.; Salinas-Navarro, M.; Alarcón-Martínez, L.; Ortín-Martínez, A.; Avilés-Trigueros, M.; Vidal-Sanz, M.; et al. Quantification of the Effect of Different Levels of IOP in the Astroglia of the Rat Retina Ipsilateral and Contralateral to Experimental Glaucoma. Investig. Opthalmology Vis. Sci. 2010, 51, 5690–5696. [Google Scholar] [CrossRef]
- Ramírez, A.I.; Salazar, J.J.; de Hoz, R.; Rojas, B.; Gallego, B.I.; Salobrar-García, E.; Valiente-Soriano, F.J.; Triviño, A.; Ramirez, J.M. Macro- and microglial responses in the fellow eyes contralateral to glaucomatous eyes. Prog. Brain Res. 2015, 220, 155–172. [Google Scholar] [CrossRef]
- Ramírez, A.I.; de Hoz, R.; Fernández-Albarral, J.A.; Salobrar-Garcia, E.; Rojas, B.; Valiente-Soriano, F.J.; Avilés-Trigueros, M.; Villegas-Pérez, M.P.; Vidal-Sanz, M.; Triviño, A.; et al. Time course of bilateral microglial activation in a mouse model of laser-induced glaucoma. Sci. Rep. 2020, 10, 4890. [Google Scholar] [CrossRef]
- Xue, Y.; Browne, A.W.; Tang, W.C.; Delgado, J.; McLelland, B.T.; Nistor, G.; Chen, J.T.; Chew, K.; Lee, N.; Keirstead, H.S.; et al. Retinal Organoids Long-Term Functional Characterization Using Two-Photon Fluorescence Lifetime and Hyperspectral Microscopy. Front. Cell. Neurosci. 2021, 15, 796903. [Google Scholar] [CrossRef]
- Tanaka, Y.; Park, I.-H. Regional specification and complementation with non-neuroectodermal cells in human brain organoids. J. Mol. Med. 2021, 99, 489–500. [Google Scholar] [CrossRef]
- Tansley, K. The Formation of Rosettes in the Rat Retina. Br. J. Ophthalmol. 1933, 17, 321–336. [Google Scholar] [CrossRef]
- Aires, I.D.; Boia, R.; Rodrigues-Neves, A.C.; Madeira, M.H.; Marques, C.; Ambrósio, A.F.; Santiago, A.R. Blockade of microglial adenosine A 2A receptor suppresses elevated pressure-induced inflammation, oxidative stress, and cell death in retinal cells. Glia 2019, 67, 896–914. [Google Scholar] [CrossRef] [PubMed]
- Madeira, M.H.; Elvas, F.; Boia, R.; Goncalves, F.Q.; Cunha, R.A.; Ambrosio, A.F.; Santiago, A.R. Adenosine A2AR blockade prevents neuroinflammation-induced death of retinal ganglion cells caused by elevated pressure. J. Neuroinflamm. 2015, 12, 115. [Google Scholar] [CrossRef] [PubMed]
- Müller, B. Organotypic culture of adult mouse retina. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2019; Volume 1940, pp. 181–191. [Google Scholar] [CrossRef]
- Kwong, J.M.K.; Caprioli, J.; Piri, N. RNA Binding Protein with Multiple Splicing: A New Marker for Retinal Ganglion Cells. Investig. Opthalmology Vis. Sci. 2010, 51, 1052–1058. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.R.; Müller, L.P.D.S.; Brecha, N.C. The RNA binding protein RBPMS is a selective marker of ganglion cells in the mammalian retina. J. Comp. Neurol. 2014, 522, 1411–1443. [Google Scholar] [CrossRef]
- Nadal-Nicolás, F.M.; Galindo-Romero, C.; Lucas-Ruiz, F.; Marsh-Amstrong, N.; Li, W.; Vidal-Sanz, M.; Agudo-Barriuso, M. Pan-retinal ganglion cell markers in mice, rats, and rhesus macaques. Zool. Res. 2022, 44, 226–248. [Google Scholar] [CrossRef]
- Chen, C.; Kiyama, T.; Weber, N.; Whitaker, C.M.; Pan, P.; Badea, T.C.; Massey, S.C.; Mao, C. Characterization of Tbr2-expressing retinal ganglion cells. J. Comp. Neurol. 2021, 529, 3513–3532. [Google Scholar] [CrossRef]
- Buckingham, B.P.; Inman, D.; Lambert, W.; Oglesby, E.; Calkins, D.J.; Steele, M.R.; Vetter, M.L.; Marsh-Armstrong, N.; Horner, P.J. Progressive Ganglion Cell Degeneration Precedes Neuronal Loss in a Mouse Model of Glaucoma. J. Neurosci. 2008, 28, 2735–2744. [Google Scholar] [CrossRef]
- Miller, D.A.; Grannonico, M.; Liu, M.; Kuranov, R.V.; Netland, P.A.; Liu, X.; Zhang, H.F. Visible-Light Optical Coherence Tomography Fibergraphy for Quantitative Imaging of Retinal Ganglion Cell Axon Bundles. Transl. Vis. Sci. Technol. 2020, 9, 11. [Google Scholar] [CrossRef]
- González-Riquelme, M.J.; Galindo-Romero, C.; Lucas-Ruiz, F.; Martínez-Carmona, M.; Rodríguez-Ramírez, K.T.; Cabrera-Maqueda, J.M.; Norte-Muñoz, M.; Vidal-Sanz, M.; Agudo-Barriuso, M. Axonal Injuries Cast Long Shadows: Long Term Glial Activation in Injured and Contralateral Retinas after Unilateral Axotomy. Int. J. Mol. Sci. 2021, 22, 8517. [Google Scholar] [CrossRef]
- Nadal-Nicolás, F.M.; Jiménez-López, M.; Salinas-Navarro, M.; Sobrado-Calvo, P.; Vidal-Sanz, M.; Agudo-Barriuso, M. Microglial dynamics after axotomy-induced retinal ganglion cell death. J. Neuroinflammation 2017, 14, 218. [Google Scholar] [CrossRef]
- Bringmann, A.; Pannicke, T.; Grosche, J.; Francke, M.; Wiedemann, P.; Skatchkov, S.N.; Osborne, N.N.; Reichenbach, A. Müller cells in the healthy and diseased retina. Prog. Retin. Eye Res. 2006, 25, 397–424. [Google Scholar] [CrossRef]
- Bull, N.D.; Johnson, T.V.; Welsapar, G.; DeKorver, N.W.; Tomarev, S.I.; Martin, K.R. Use of an Adult Rat Retinal Explant Model for Screening of Potential Retinal Ganglion Cell Neuroprotective Therapies. Investig. Opthalmology Vis. Sci. 2011, 52, 3309–3320. [Google Scholar] [CrossRef]
- Pattamatta, U.; McPherson, Z.; White, A. A mouse retinal explant model for use in studying neuroprotection in glaucoma. Exp. Eye Res. 2016, 151, 38–44. [Google Scholar] [CrossRef]
- Johnson, T.V.; Martin, K.R. Development and Characterization of an Adult Retinal Explant Organotypic Tissue Culture System as an In Vitro Intraocular Stem Cell Transplantation Model. Investig. Opthalmology Vis. Sci. 2008, 49, 3503–3512. [Google Scholar] [CrossRef]
- Kuhrt, H.; Wurm, A.; Karl, A.; Iandiev, I.; Wiedemann, P.; Reichenbach, A.; Bringmann, A.; Pannicke, T. Müller cell gliosis in retinal organ culture mimics gliotic alterations after ischemia in vivo. Int. J. Dev. Neurosci. 2008, 26, 745–751. [Google Scholar] [CrossRef]
- Lee, T.-I.; Yang, C.-S.; Fang, K.-M.; Tzeng, S.-F. Role of Ciliary Neurotrophic Factor in Microglial Phagocytosis. Neurochem. Res. 2009, 34, 109–117. [Google Scholar] [CrossRef]
- Van Adel, B.; Arnold, J.; Phipps, J.; Doering, L.; Ball, A. Ciliary neurotrophic factor protects retinal ganglion cells from axotomy-induced apoptosis via modulation of retinal gliain vivo. J. Neurobiol. 2005, 63, 215–234. [Google Scholar] [CrossRef]
- Lindborg, J.A.; Tran, N.M.; Chenette, D.M.; DeLuca, K.; Foli, Y.; Kannan, R.; Sekine, Y.; Wang, X.; Wollan, M.; Kim, I.-J.; et al. Optic nerve regeneration screen identifies multiple genes restricting adult neural repair. Cell Rep. 2021, 34, 108777. [Google Scholar] [CrossRef]
- Tran, N.M.; Shekhar, K.; Whitney, I.E.; Jacobi, A.; Benhar, I.; Hong, G.; Yan, W.; Adiconis, X.; Arnold, M.E.; Lee, J.M.; et al. Single-Cell Profiles of Retinal Ganglion Cells Differing in Resilience to Injury Reveal Neuroprotective Genes. Neuron 2019, 104, 1039–1055.e12. [Google Scholar] [CrossRef]
- Nadal-Nicolás, F.M.; Sobrado-Calvo, P.; Jiménez-López, M.; Vidal-Sanz, M.; Agudo-Barriuso, M. Long-Term Effect of Optic Nerve Axotomy on the Retinal Ganglion Cell Layer. Investig. Opthalmology Vis. Sci. 2015, 56, 6095–6112. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, Y.; Gao, C.; Fariss, R.N.; Tam, J.; Wong, W.T. Monocyte infiltration and proliferation reestablish myeloid cell homeostasis in the mouse retina following retinal pigment epithelial cell injury. Sci. Rep. 2017, 7, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Rathnasamy, G.; Foulds, W.S.; Ling, E.-A.; Kaur, C. Retinal microglia—A key player in healthy and diseased retina. Prog. Neurobiol. 2019, 173, 18–40. [Google Scholar] [CrossRef] [PubMed]
- Di Pierdomenico, J.; Martínez-Vacas, A.; Hernández-Muñoz, D.; Gómez-Ramírez, A.M.; Valiente-Soriano, F.J.; Agudo-Barriuso, M.; Vidal-Sanz, M.; Villegas-Pérez, M.P.; García-Ayuso, D. Coordinated Intervention of Microglial and Müller Cells in Light-Induced Retinal Degeneration. Investig. Opthalmology Vis. Sci. 2020, 61, 47. [Google Scholar] [CrossRef] [PubMed]
- Müller, B.; Wagner, F.; Lorenz, B.; Stieger, K. Organotypic Cultures of Adult Mouse Retina: Morphologic Changes and Gene Expression. Investig. Opthalmology Vis. Sci. 2017, 58, 1930–1940. [Google Scholar] [CrossRef]
- Tassoni, A.; Gutteridge, A.; Barber, A.C.; Osborne, A.; Martin, K.R. Molecular Mechanisms Mediating Retinal Reactive Gliosis Following Bone Marrow Mesenchymal Stem Cell Transplantation. Stem Cells 2015, 33, 3006–3016. [Google Scholar] [CrossRef]
- Luna, G.; Keeley, P.W.; Reese, B.E.; Linberg, K.A.; Lewis, G.P.; Fisher, S.K. Astrocyte structural reactivity and plasticity in models of retinal detachment. Exp. Eye Res. 2016, 150, 4–21. [Google Scholar] [CrossRef]
- Lucas-Ruiz, F.; Galindo-Romero, C.; Salinas-Navarro, M.; González-Riquelme, M.J.; Vidal-Sanz, M.; Barriuso, M.A. Systemic and Intravitreal Antagonism of the TNFR1 Signaling Pathway Delays Axotomy-Induced Retinal Ganglion Cell Loss. Front. Neurosci. 2019, 13, 1096. [Google Scholar] [CrossRef]
- Palmhof, M.; Frank, V.; Rappard, P.; Kortenhorn, E.; DeMuth, J.; Biert, N.; Stute, G.; Dick, H.B.; Joachim, S.C. From Ganglion Cell to Photoreceptor Layer: Timeline of Deterioration in a Rat Ischemia/Reperfusion Model. Front. Cell. Neurosci. 2019, 13, 174. [Google Scholar] [CrossRef]

| Antigen | Expressed in | Host | Dilution | Company | Cat. Number |
|---|---|---|---|---|---|
| Brn3a | RGCs | Mouse | 1:300 | Millipore | MAB1585 |
| RBPMS | RGCs | Rabbit | 1:750 | Genetex | GTX118619 |
| β-III Tubulin | RGCs | Rabbit | 1:1000 | Santa Cruz Biotechnologies | Sc-5274 |
| γ-synuclein | RGCs | Mouse | 1:1000 | Abnova | H00006623-M01 |
| Iba1 | Microglial cells | Rabbit | 1:500 | Abcam | Ab178846 |
| GFAP | Astrocytes and activated Müller cells | Rabbit | 1:500 | Merck | G9269 |
| Goat | 1:500 | Abcam | Ab53554 | ||
| Vimentin | Müller cells | Goat | 1:250 | Santa Cruz Biotechnologies | Sc-7557 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Riquelme, M.J.; Lucas-Ruiz, F.; Galindo-Romero, C.; Boia, R.; Ambrósio, A.F.; Vidal-Sanz, M.; Raquel Santiago, A.; Agudo-Barriuso, M. Comparative Analysis of Retinal Organotypic Cultures and In Vivo Axotomized Retinas. Int. J. Mol. Sci. 2023, 24, 3481. https://doi.org/10.3390/ijms24043481
González-Riquelme MJ, Lucas-Ruiz F, Galindo-Romero C, Boia R, Ambrósio AF, Vidal-Sanz M, Raquel Santiago A, Agudo-Barriuso M. Comparative Analysis of Retinal Organotypic Cultures and In Vivo Axotomized Retinas. International Journal of Molecular Sciences. 2023; 24(4):3481. https://doi.org/10.3390/ijms24043481
Chicago/Turabian StyleGonzález-Riquelme, María José, Fernando Lucas-Ruiz, Caridad Galindo-Romero, Raquel Boia, António Francisco Ambrósio, Manuel Vidal-Sanz, Ana Raquel Santiago, and Marta Agudo-Barriuso. 2023. "Comparative Analysis of Retinal Organotypic Cultures and In Vivo Axotomized Retinas" International Journal of Molecular Sciences 24, no. 4: 3481. https://doi.org/10.3390/ijms24043481
APA StyleGonzález-Riquelme, M. J., Lucas-Ruiz, F., Galindo-Romero, C., Boia, R., Ambrósio, A. F., Vidal-Sanz, M., Raquel Santiago, A., & Agudo-Barriuso, M. (2023). Comparative Analysis of Retinal Organotypic Cultures and In Vivo Axotomized Retinas. International Journal of Molecular Sciences, 24(4), 3481. https://doi.org/10.3390/ijms24043481








