Synergistic Antifungal Interactions between Antibiotic Amphotericin B and Selected 1,3,4-thiadiazole Derivatives, Determined by Microbiological, Cytochemical, and Molecular Spectroscopic Studies
Abstract
1. Introduction
2. Results
2.1. Antifungal Activity of Compounds
2.2. Interactions of the Thiadiazoles with the Antibiotic
2.3. Cell Morphology Observations
2.4. Selectivity Index of the 1,3,4-thiadiazole Derivatives
2.5. Antibiofilm Activity of the Tested Compounds
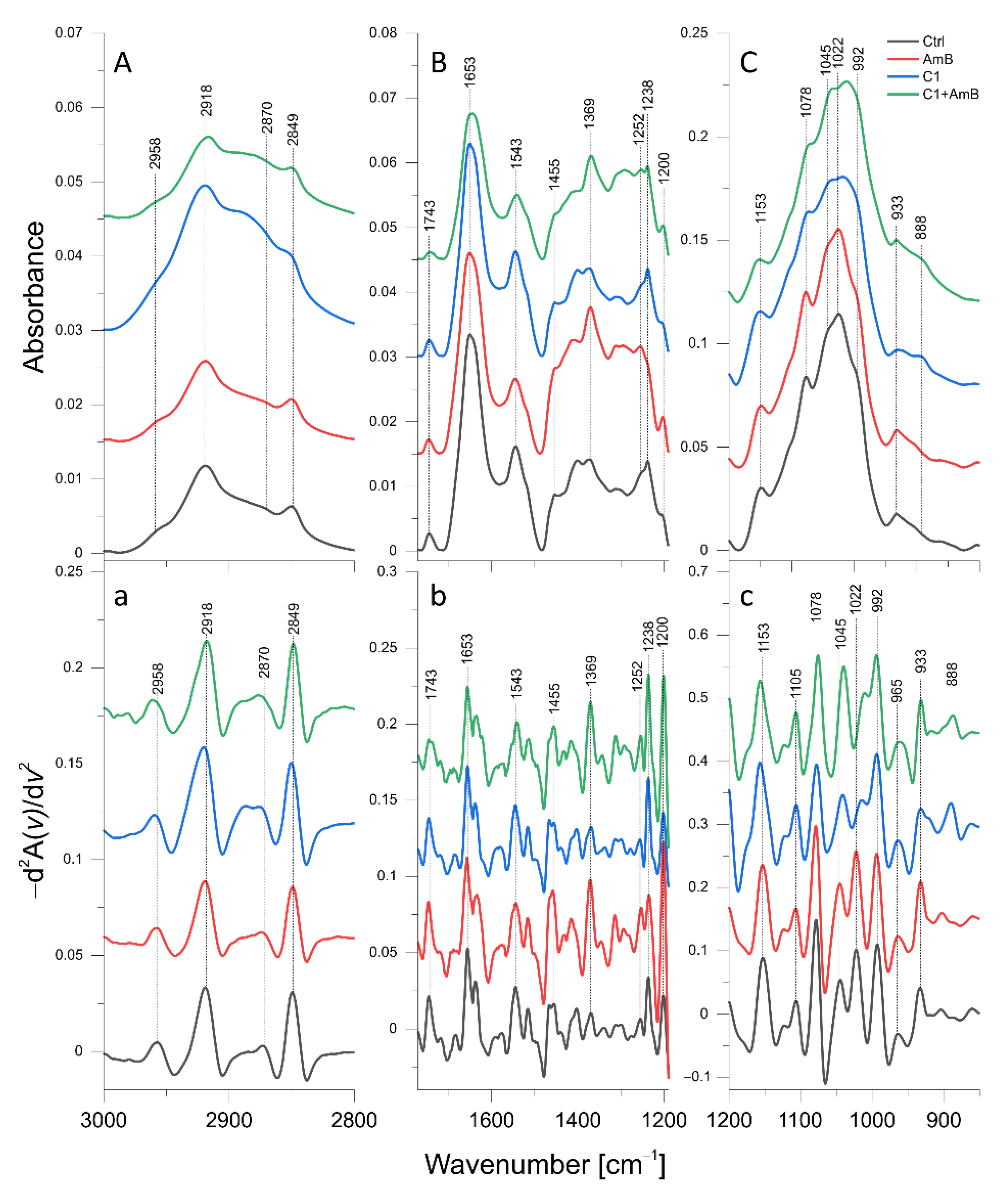
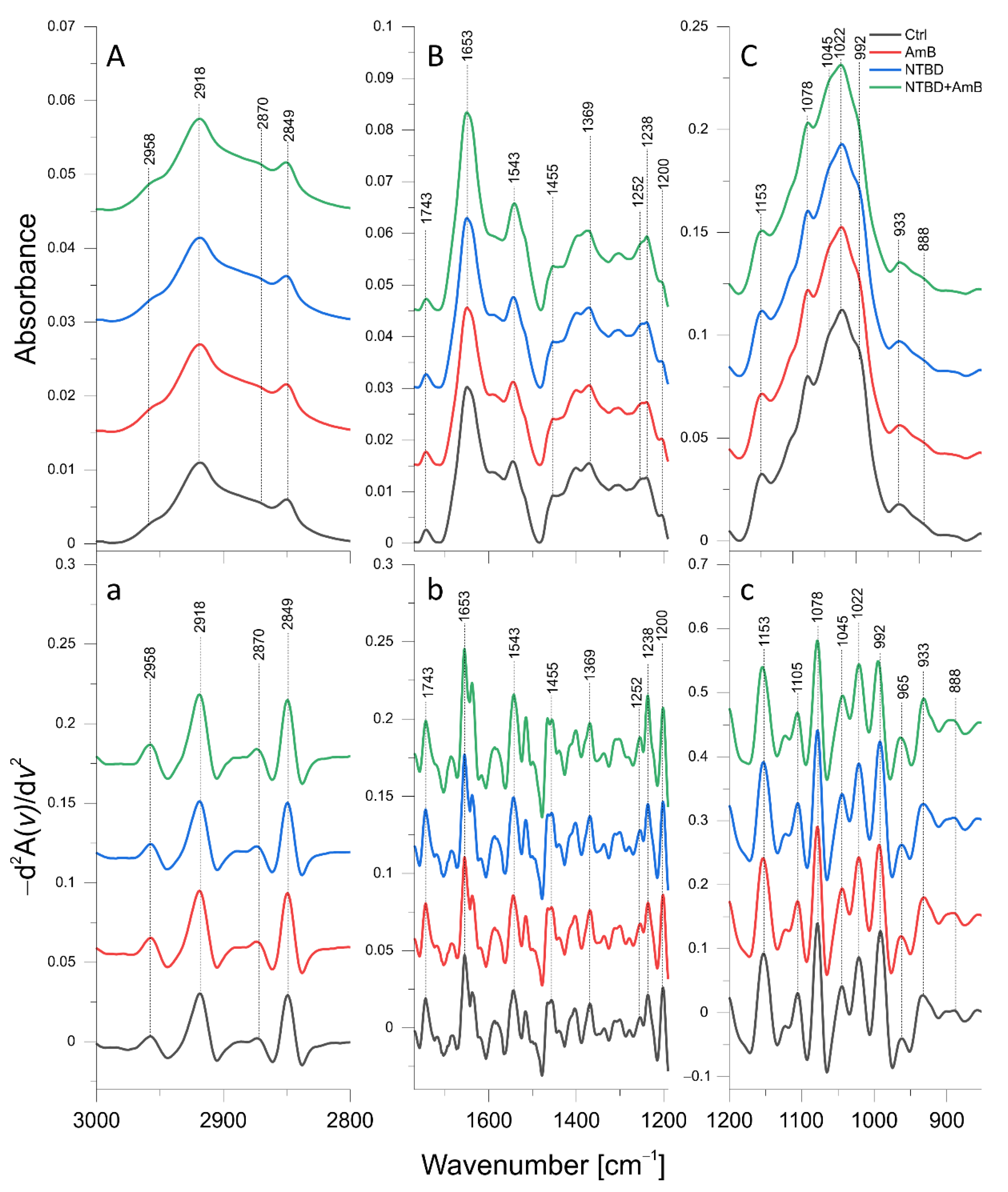
2.6. ATR-FTIR Analysis of Biomolecular Mechanisms of Activity in the Combinations of 1,3,4-thiadiazole Derivatives with AmB in C. albicans
| Band [cm−1] | Origin | Characteristic |
|---|---|---|
| 2958 | CH3 str. asym. | Lipid and polysaccharide level |
| 2918 | CH2 str. asym. | |
| 2870 | CH3 str. sym. | |
| 2849 | CH2 str. sym. | |
| 1653 | C=O str., NH bend. (Amide I) | Protein and chitin level |
| 1543 | NH bend., C-N str. (Amide II) | Protein and chitin level |
| 1455 | CH2, CH3 def. | Lipid, protein, and polysaccharide level |
| 1369 | ||
| 1252 | NH def. (Amide III) | Protein and chitin level |
| 1237 | P=O str. | Phosphomannan level |
| 1200 | PO2 str. asym. | Phosphomannan level |
| 1153 | β(1→3) glucans | β(1→3) glucan level |
| 1105 | Glycogen, β(1→3) glucans | Glycogen and β(1→3) glucan level |
| 1078 | Glycogen | Glycogen level |
| 1045 | Glycogen, mannans | Glycogen and mannan level |
| 1022 | Glycogen | Glycogen level |
| 992 | β(1→6) glucans | β(1→6) glucan level |
| 965 | Mannans | Mannan level |
| 933 | Glucans, mannans | Glucan and mannan level |
| 888 |
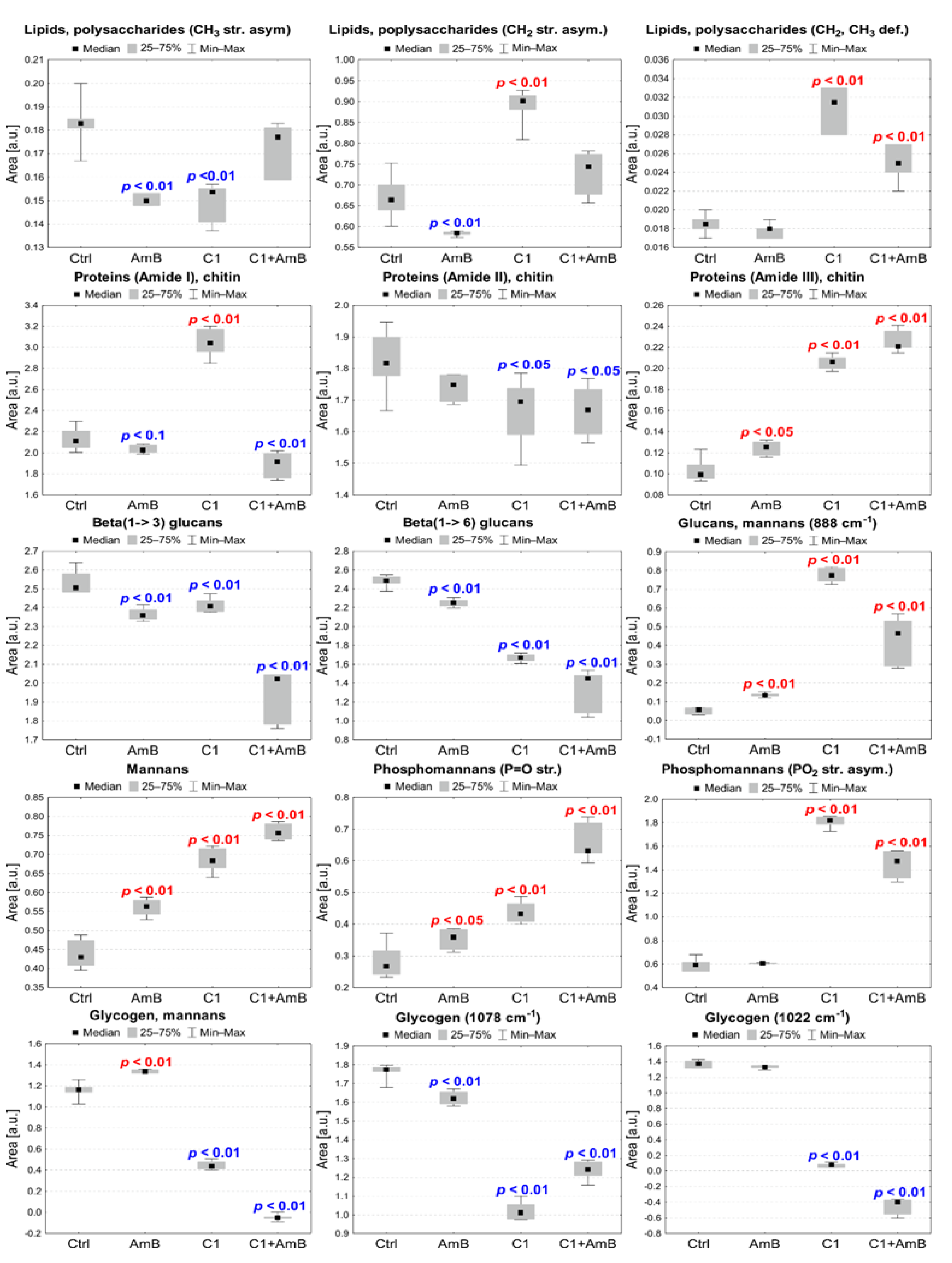
2.6.1. C1, AmB, and C1 + AmB Effect
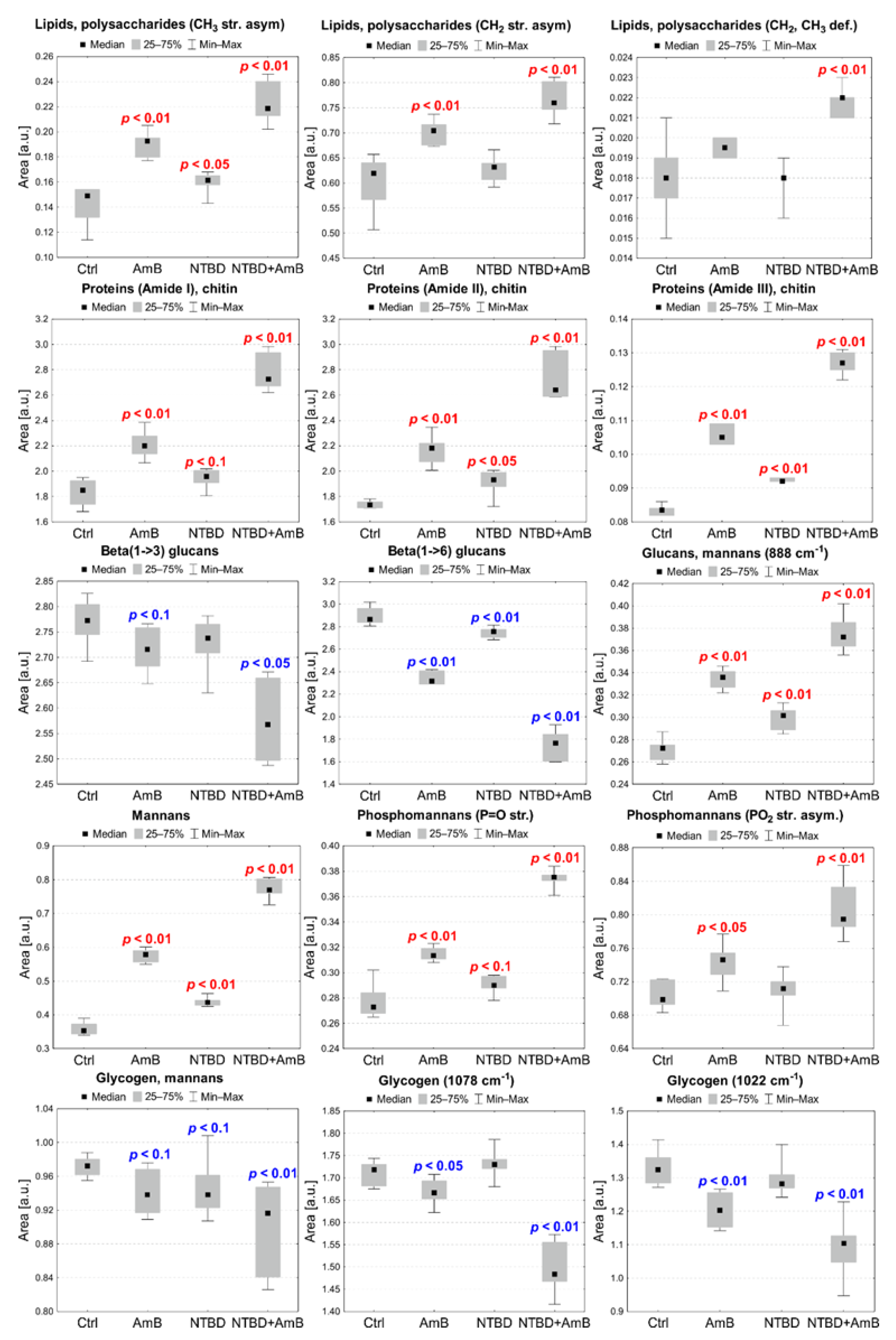
2.6.2. AmB, NTBD, and NTBD + AmB Effect
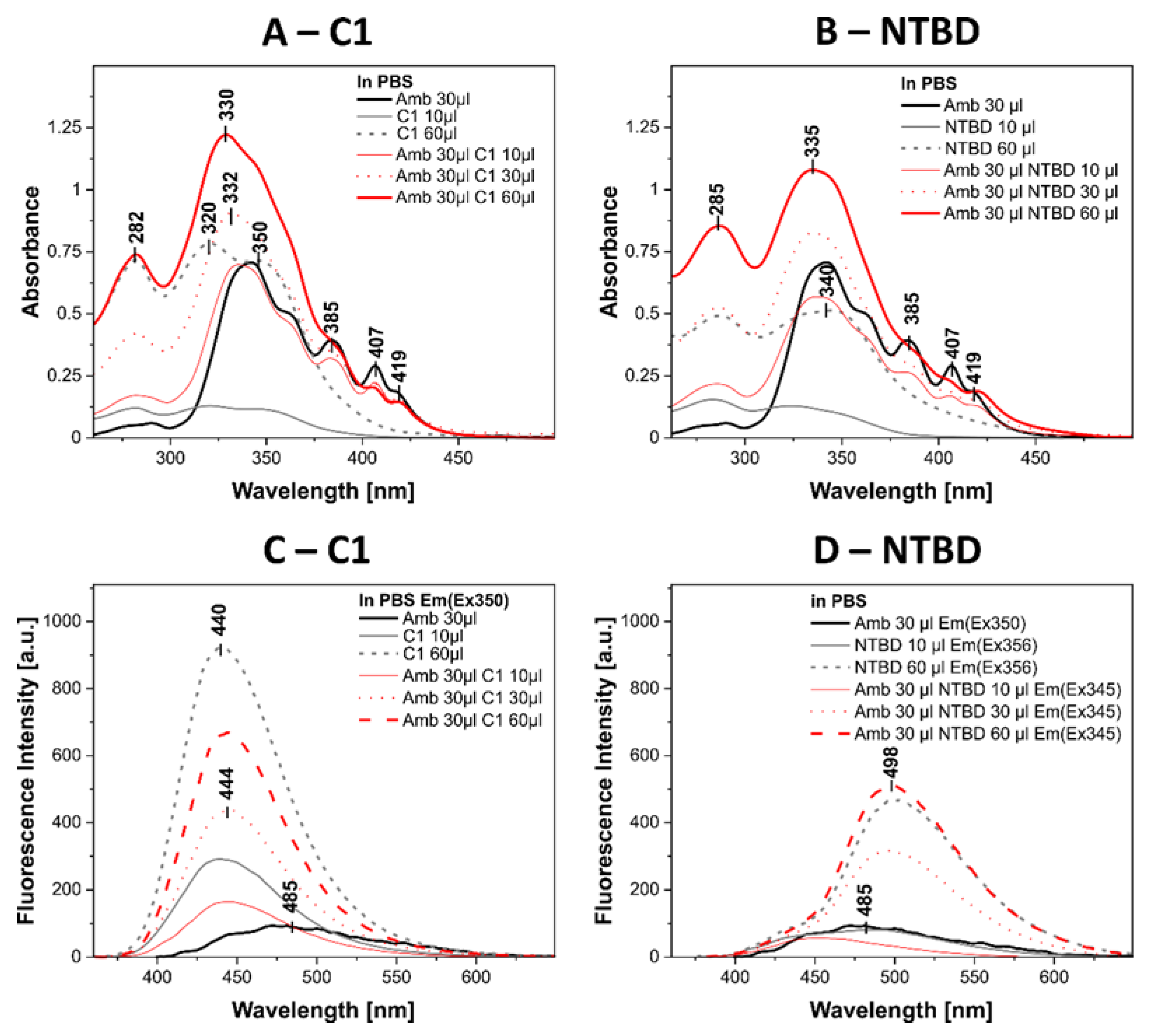
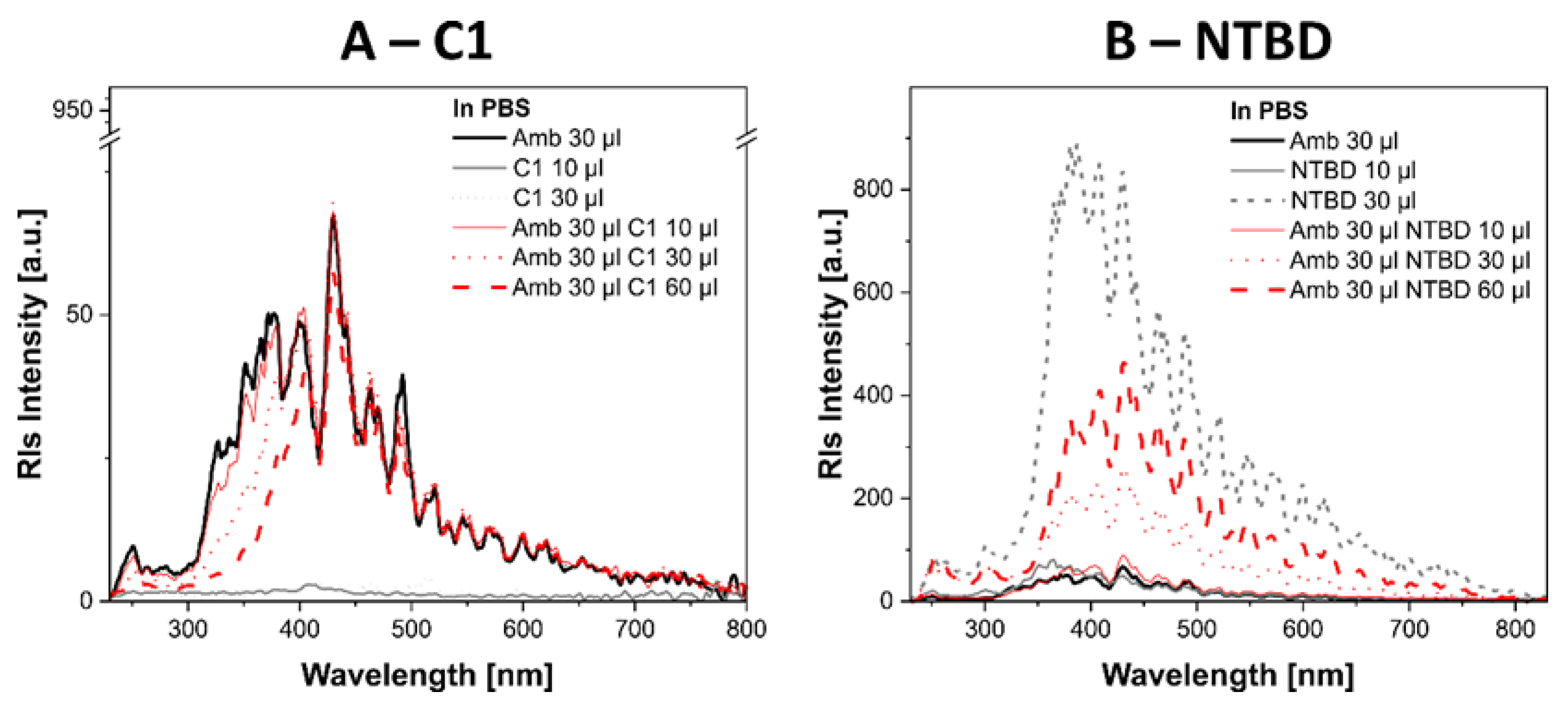
2.7. Biophysical Mechanism of Action of Systems with 1,3,4-thiadiazole Derivatives and AmB
3. Discussion
4. Materials and Methods
4.1. Strains
4.2. Antifungal Substances
4.3. Antifungal Activity of the Tested Compounds
4.4. Interactions between Thiadiazoles and Antibiotics
4.5. Morphological Observations of Fungal Cells
4.6. Selectivity Index Calculation
4.7. Antibiofilm Activity of the Tested 1,3,4-thiadiazole Derivatives
4.8. ATR-FTIR Spectroscopy
4.9. Measurements of Absorption and Electron Fluorescence Spectra
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kainz, K.; Bauer, M.A.; Madeo, F.; Carmona-Gutierrez, D. Fungal infections in humans: The silent crisis. Microb. Cell 2020, 7, 143–145. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Sae-Tia, S.; Fries, B.C. Candidiasis and Mechanisms of Antifungal Resistance. Antibiotics 2020, 9, 312. [Google Scholar] [CrossRef]
- Kontoyiannis, D.P.; Lewis, R.E. Antifungal drug resistance of pathogenic fungi. Lancet 2002, 359, 1135–1144. [Google Scholar] [CrossRef]
- Pappas, P.G. Opportunistic fungi: A view to the future. Am. J. Med. Sci. 2010, 340, 253–257. [Google Scholar] [CrossRef]
- Janniger, E.J.; Kapila, R. Public health issues with Candida auris in COVID-19 patients. World Med. Health Policy 2021, 13, 766–772. [Google Scholar] [CrossRef]
- Briano, F.; Magnasco, L.; Sepulcri, C.; Dettori, S.; Dentone, C.; Mikulska, M.; Ball, L.; Vena, A.; Robba, C.; Patroniti, N.; et al. Candida auris Candidemia in Critically Ill, Colonized Patients: Cumulative Incidence and Risk Factors. Infect. Dis. Ther. 2022, 11, 1149–1160. [Google Scholar] [CrossRef]
- Harmsen, S.; McLaren, A.C.; Pauken, C.; McLemore, R. Amphotericin B Is Cytotoxic at Locally Delivered Concentrations. Clin. Orthop. Relat. R 2011, 469, 3016–3021. [Google Scholar] [CrossRef]
- Hamill, R.J. Amphotericin B formulations: A comparative review of efficacy and toxicity. Drugs 2013, 73, 919–934. [Google Scholar] [CrossRef]
- Sterling, T.R.; Merz, W.G. Resistance to amphotericin B: Emerging clinical and microbiological patterns. Drug Resist. Updat. 1998, 1, 161–165. [Google Scholar] [CrossRef]
- Sobel, J.D. Limitations of antifungal agents in the treatment of Candida vaginitis: Future challenges. Drug Resist. Updat. 1999, 2, 148–152. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Chandra, J. Candida biofilm resistance. Drug Resist. Updat. 2004, 7, 301–309. [Google Scholar] [CrossRef]
- Li, X.Y.; Wu, X.X.; Gao, Y.; Hao, L.N. Synergistic Effects and Mechanisms of Combined Treatment With Harmine Hydrochloride and Azoles for Resistant Candida albicans. Front. Microbiol. 2019, 10, 2295. [Google Scholar] [CrossRef]
- Kane, A.; Carter, D.A. Augmenting Azoles with Drug Synergy to Expand the Antifungal Toolbox. Pharmaceuticals 2022, 15, 482. [Google Scholar] [CrossRef]
- Lewis, R.E.; Diekema, D.J.; Messer, S.A.; Pfaller, M.A.; Klepser, M.E. Comparison of Etest, chequerboard dilution and time-kill studies for the detection of synergy or antagonism between antifungal agents tested against Candida species. J. Antimicrob. Chemoth. 2002, 49, 345–351. [Google Scholar] [CrossRef]
- Chudzik, B.; Bonio, K.; Dabrowski, W.; Pietrzak, D.; Niewiadomy, A.; Olender, A.; Malodobry, K.; Gagos, M. Synergistic antifungal interactions of amphotericin B with 4-(5-methyl-1,3,4-thiadiazole-2-yl) benzene-1,3-diol. Sci. Rep. 2019, 9, 12945. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Sharma, S.; Vaidya, A.; Ravichandran, V.; Agrawal, R.K. 1,3,4-thiadiazole and its derivatives: A review on recent progress in biological activities. Chem. Biol. Drug Des. 2013, 81, 557–576. [Google Scholar] [CrossRef] [PubMed]
- Matysiak, J.; Skrzypek, A.; Niewiadomy, A. Synthesis and Antifungal Activity of Novel 5-Substituted 4-(1,3,4-Thiadiazol-2-yl) benzene-1,3-Diols. Heteroat. Chem. 2010, 21, 533–540. [Google Scholar] [CrossRef]
- Chudzik, B.; Bonio, K.; Dabrowski, W.; Pietrzak, D.; Niewiadomy, A.; Olender, A.; Pawlikowska-Pawlega, B.; Gagos, M. Antifungal effects of a 1,3,4-thiadiazole derivative determined by cytochemical and vibrational spectroscopic studies. PLoS ONE 2019, 14, e0222775. [Google Scholar] [CrossRef]
- CLSI. CLSI Document M27-A. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts Approved Standard, 3rd ed.; CLSI: Wayne, PA, USA, 2008. [Google Scholar]
- Kaminsky, R.; Schmid, C.; Brun, R. An ‘in vitro selectivity index’ for evaluation of cytotoxicity of antitrypanosomal compounds. Trop. Med. Int. Health 1996, 9, 315–324. [Google Scholar]
- Famuyide, I.M.; Aro, A.O.; Fasina, F.O.; Eloff, J.N.; McGaw, L.J. Antibacterial and antibiofilm activity of acetone leaf extracts of nine under-investigated south African Eugenia and Syzygium (Myrtaceae) species and their selectivity indices. BMC Complem. Altern. M 2019, 19, 141. [Google Scholar] [CrossRef]
- Szeghalmi, A.; Kaminskyj, S.; Gough, K.M. A synchrotron FTIR microspectroscopy investigation of fungal hyphae grown under optimal and stressed conditions. Anal. Bioanal. Chem. 2007, 387, 1779–1789. [Google Scholar] [CrossRef]
- Zimkus, A.; Misiunas, A.; Chaustova, L. Li+ effect on the cell wall of the yeast Saccharomyces cerevisiae as probed by FT-IR spectroscopy. Cent. Eur. J. Biol. 2013, 8, 724–729. [Google Scholar] [CrossRef]
- Sandula, J.; Kogan, G.; Kacurakova, M.; Machova, E. Microbial (1->3)-beta-D-glucans, their preparation, physico-chemical characterization and immunomodulatory activity. Carbohyd. Polym. 1999, 38, 247–253. [Google Scholar] [CrossRef]
- Novak, M.; Synytsya, A.; Gedeon, O.; Slepicka, P.; Prochazka, V.; Synytsya, A.; Blahovec, J.; Hejlova, A.; Copikova, J. Yeast beta(1-3),(1-6)-D-glucan films: Preparation and characterization of some structural and physical properties. Carbohyd. Polym. 2012, 87, 2496–2504. [Google Scholar] [CrossRef]
- Negrea, P.; Caunii, A.; Sarac, I.; Butnariu, M. The Study of Infrared Spectrum of Chitin and Chitosan Extract as Potential Sources of Biomass. Dig. J. Nanomater. Bios. 2015, 10, 1129–1138. [Google Scholar]
- Gao, Y.; Huo, X.; Dong, L.; Sun, X.; Sai, H.; Wei, G.; Xu, Y.; Zhang, Y.; Wu, J. Fourier transform infrared microspectroscopy monitoring of 5-fluorouracil-induced apoptosis in SW620 colon cancer cells. Mol. Med. Rep. 2015, 11, 2585–2591. [Google Scholar] [CrossRef]
- Cárdenas, G.; Cabrera, G.; Taboada, E.; Miranda, S.P. Chitin characterization by SEM, FTIR, XRD, and 13C cross polarization/mass angle spinning NMR. J. Appl. Polym. Sci. 2004, 93, 1876–1885. [Google Scholar] [CrossRef]
- Galichet, A.; Sockalingum, G.D.; Belarbi, A.; Manfait, M. FTIR spectroscopic analysis of Saccharomyces cerevisiae cell walls: Study of an anomalous strain exhibiting a pink-colored cell phenotype. FEMS Microbiol. Lett. 2001, 197, 179–186. [Google Scholar] [CrossRef]
- Adt, I.; Toubas, D.; Pinon, J.M.; Manfait, M.; Sockalingum, G. FTIR spectroscopy as a potential tool to analyse structural modifications during morphogenesis of Candida albicans. Arch. Microbiol. 2006, 185, 277–285. [Google Scholar] [CrossRef]
- Singh, B.R.; Deoliveira, D.B.; Fu, F.N.; Fuller, M.P. Fourier-Transform Infrared-Analysis of Amide-III Bands of Proteins for the Secondary Structure Estimation; SPIE: Bellingham, WA, USA, 1993; Volume 1890, pp. 47–55. [Google Scholar] [CrossRef]
- Matwijczuk, A.; Kluczyk, D.; Gorecki, A.; Niewiadomy, A.; Gagos, M. Spectroscopic Studies of Fluorescence Effects in Bioactive 4-(5-Heptyl-1,3,4-Thiadiazol-2-yl)Benzene-1,3-Diol and 4-(5-Methyl-1,3,4-Thiadiazol-2-yl)Benzene-1,3-Diol Molecules Induced by pH Changes in Aqueous Solutions. J. Fluoresc. 2017, 27, 1201–1212. [Google Scholar] [CrossRef]
- Matwijczuk, A.; Kluczyk, D.; Gorecki, A.; Niewiadomy, A.; Gagos, M. Solvent Effects on Molecular Aggregation in 4-(5-Heptyl-1,3,4-thiadiazol-2-yl)benzene-1,3-diol and 4-(5-Methyl-1,3,4-thiadiazol-2-yl)benzene-1,3-diol. J. Phys. Chem. B 2016, 120, 7958–7969. [Google Scholar] [CrossRef] [PubMed]
- Kasha, M.; Rawls, H.R.; El-Bayoumi, M.A. The Exciton Model in Molecular Spectroscopy. Pure Appl. Chem. 1965, 11, 371–392. [Google Scholar] [CrossRef]
- Gagos, M.; Gruszecki, W.I. Organization of polyene antibiotic amphotericin B at the argon-water interface. Biophys. Chem. 2008, 137, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Shervani, Z.; Etori, H.; Taga, K.; Yoshida, T.; Okabayashi, H. Aggregation of polyene antibiotics as studied by electronic absorption and circular dichroism spectroscopies. Colloids Surf. B Biointerfaces 1996, 7, 31–38. [Google Scholar] [CrossRef]
- Kluczyk, D.; Matwijczuk, A.; Gorecki, A.; Karpinska, M.M.; Szymanek, M.; Niewiadomy, A.; Gagos, M. Molecular Organization of Dipalmitoylphosphatidylcholine Bilayers Containing Bioactive Compounds 4-(5-Heptyl-1,3,4-thiadiazol-2-yl) Benzene-1,3-diol and 4-(5-Methyl-1,3,4-thiadiazol-2-yl) Benzene-1,3-diols. J. Phys. Chem. B. 2016, 120, 12047–12063. [Google Scholar] [CrossRef]
- Czernel, G.; Budziak, I.; Oniszczuk, A.; Karcz, D.; Pustula, K.; Gorecki, A.; Matwijczuk, A.; Gladyszewska, B.; Gagos, M.; Niewiadomy, A.; et al. ESIPT-Related Origin of Dual Fluorescence in the Selected Model 1,3,4-Thiadiazole Derivatives. Molecules 2020, 25, 4168. [Google Scholar] [CrossRef]
- Pasternack, R.F.; Bustamante, C.; Collings, P.J.; Giannetto, A.; Gibbs, E.J. Porphyrin assemblies on DNA as studied by a resonance light-scattering technique. J. Am. Chem. Soc. 1993, 115, 5393–5399. [Google Scholar] [CrossRef]
- Laniado-Laborin, R.; Cabrales-Vargas, M.N. Amphotericin B: Side effects and toxicity. Rev. Iberoam. Micol. 2009, 26, 223–227. [Google Scholar] [CrossRef]
- Lanza, J.S.; Pomel, S.; Loiseau, P.M.; Frezard, F. Recent advances in amphotericin B delivery strategies for the treatment of leishmaniases. Expert Opin. Drug Deliv. 2019, 16, 1063–1079. [Google Scholar] [CrossRef]
- Cavassin, F.B.; Bau-Carneiro, J.L.; Vilas-Boas, R.R.; Queiroz-Telles, F. Sixty years of Amphotericin B: An Overview of the Main Antifungal Agent Used to Treat Invasive Fungal Infections. Infect. Dis. Ther. 2021, 10, 115–147. [Google Scholar] [CrossRef]
- Orme, I.; Tuberculosis Drug Screening, P. Search for new drugs for treatment of tuberculosis. Antimicrob. Agents Chemother. 2001, 45, 1943–1946. [Google Scholar] [CrossRef]
- Bonifacio, B.V.; Ramos, M.A.D.; da Silva, P.B.; Negri, K.M.S.; Lopes, E.D.; de Souza, L.P.; Vilegas, W.; Pavan, F.R.; Chorilli, M.; Bauab, T.M. Nanostructured lipid system as a strategy to improve the anti-Candida albicans activity of Astronium sp. Int. J. Nanomed. 2015, 10, 5081–5092. [Google Scholar] [CrossRef]
- Garcia-Rubio, R.; de Oliveira, H.C.; Rivera, J.; Trevijano-Contador, N. The Fungal Cell Wall: Candida, Cryptococcus, and Aspergillus Species. Front. Microbiol. 2020, 10, 2993. [Google Scholar] [CrossRef]
- Hurtado-Guerrero, R.; Schuttelkopf, A.W.; Mouyna, I.; Ibrahim, A.F.; Shepherd, S.; Fontaine, T.; Latge, J.P.; van Aalten, D.M. Molecular mechanisms of yeast cell wall glucan remodeling. J. Biol. Chem. 2009, 284, 8461–8469. [Google Scholar] [CrossRef]
- Aimanianda, V.; Clavaud, C.; Simenel, C.; Fontaine, T.; Delepierre, M.; Latge, J.P. Cell wall beta-(1,6)-glucan of Saccharomyces cerevisiae: Structural characterization and in situ synthesis. J. Biol. Chem. 2009, 284, 13401–13412. [Google Scholar] [CrossRef]
- Lesage, G.; Bussey, H. Cell wall assembly in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2006, 70, 317–343. [Google Scholar] [CrossRef]
- Okada, H.; Ohnuki, S.; Roncero, C.; Konopka, J.B.; Ohya, Y. Distinct roles of cell wall biogenesis in yeast morphogenesis as revealed by multivariate analysis of high-dimensional morphometric data. Mol. Biol. Cell 2014, 25, 222–233. [Google Scholar] [CrossRef]
- Arvindekar, A.U.; Patil, N.B. Glycogen--a covalently linked component of the cell wall in Saccharomyces cerevisiae. Yeast 2002, 19, 131–139. [Google Scholar] [CrossRef]
- Lowman, D.W.; Sameer Al-Abdul-Wahid, M.; Ma, Z.; Kruppa, M.D.; Rustchenko, E.; Williams, D.L. Glucan and glycogen exist as a covalently linked macromolecular complex in the cell wall of Candida albicans and other Candida species. Cell Surf. 2021, 7, 100061. [Google Scholar] [CrossRef]
- Francois, J.; Parrou, J.L. Reserve carbohydrates metabolism in the yeast Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2001, 25, 125–145. [Google Scholar] [CrossRef]
- Hernandez-Chavez, M.J.; Franco, B.; Clavijo-Giraldo, D.M.; Hernandez, N.V.; Estrada-Mata, E.; Mora-Montes, H.M. Role of protein phosphomannosylation in the Candida tropicalis-macrophage interaction. FEMS Yeast Res. 2018, 18, foy053. [Google Scholar] [CrossRef] [PubMed]
- Krizkova, L.; Durackova, Z.; Sandula, J.; Sasinkova, V.; Krajcovic, J. Antioxidative and antimutagenic activity of yeast cell wall mannans in vitro. Mutat. Res. Gen. Tox. En. 2001, 497, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Lewis, L.E.; Bain, J.M.; Lowes, C.; Gillespie, C.; Rudkin, F.M.; Gow, N.A.; Erwig, L.P. Stage specific assessment of Candida albicans phagocytosis by macrophages identifies cell wall composition and morphogenesis as key determinants. PLoS Pathog. 2012, 8, e1002578. [Google Scholar] [CrossRef] [PubMed]
- Pérez-García, L.A.; Csonka, K.; Flores-Carreón, A.; Estrada-Mata, E.; Mellado-Mojica, E.; Németh, T.; López-Ramírez, L.A.; Toth, R.; López, M.G.; Vizler, C.; et al. Role of Protein Glycosylation in Candida parapsilosis Cell Wall Integrity and Host Interaction. Front. Microbiol. 2016, 7, 306. [Google Scholar] [CrossRef] [PubMed]
- de Nobel, J.G.; Klis, F.M.; Priem, J.; Munnik, T.; van den Ende, H. The glucanase-soluble mannoproteins limit cell wall porosity in Saccharomyces cerevisiae. Yeast 1990, 6, 491–499. [Google Scholar] [CrossRef]
- Harris, M.; Mora-Montes, H.M.; Gow, N.A.R.; Coote, P.J. Loss of mannosylphosphate from Candida albicans cell wall proteins results in enhanced resistance to the inhibitory effect of a cationic antimicrobial peptide via reduced peptide binding to the cell surface. Microbiol. Read. 2009, 155, 1058–1070. [Google Scholar] [CrossRef]
- Gonzalez-Hernandez, R.J.; Jin, K.; Hernandez-Chavez, M.J.; Diaz-Jimenez, D.F.; Trujillo-Esquivel, E.; Clavijo-Giraldo, D.M.; Tamez-Castrellon, A.K.; Franco, B.; Gow, N.A.R.; Mora-Montes, H.M. Phosphomannosylation and the Functional Analysis of the Extended Candida albicans MNN4-Like Gene Family. Front. Microbiol. 2017, 8, 2156. [Google Scholar] [CrossRef]
- Gagos, M.; Czernel, G. Oxidized forms of polyene antibiotic amphotericin B. Chem. Phys. Lett. 2014, 598, 5–9. [Google Scholar] [CrossRef]
- Snyder, R.; Arvidson, E.; Foote, C.; Harrigan, L.; Christensen, R.L. Electronic energy levels in long polyenes: S2.fwdarw. S0 emission in all-trans-1,3,5,7,9,11,13-tetradecaheptaene. J. Am. Chem. Soc. 1985, 107, 4117–4122. [Google Scholar] [CrossRef]
- Gagos, M.; Gabrielska, J.; Dalla Serra, M.; Gruszecki, W.I. Binding of antibiotic amphotericin B to lipid membranes: Monomolecular layer technique and linear dichroism-FTIR studies. Mol. Membr. Biol. 2005, 22, 433–442. [Google Scholar] [CrossRef]
- Bolard, J.; Legrand, P.; Heitz, F.; Cybulska, B. One-sided action of amphotericin B on cholesterol-containing membranes is determined by its self-association in the medium. Biochemistry 1991, 30, 5707–5715. [Google Scholar] [CrossRef]
- Barwicz, J.; Tancrède, P. The effect of aggregation state of amphotericin-B on its interactions with cholesterol- or ergosterol-containing phosphatidylcholine monolayers. Chem. Phys. Lipids 1997, 85, 145–155. [Google Scholar] [CrossRef]
- Bolard, J.; Cleary, J.D.; Kramer, R.E. Evidence that impurities contribute to the fluorescence of the polyene antibiotic amphotericin B. J. Antimicrob. Chemother. 2009, 63, 921–927. [Google Scholar] [CrossRef]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemoth. 2003, 52, 1. [Google Scholar] [CrossRef]
- Drozdz, A.; Slawinska-Brych, A.; Kubera, D.; Kimsa-Dudek, M.; Gola, J.M.; Adamska, J.; Kruszniewska-Rajs, C.; Matwijczuk, A.; Karcz, D.; Dabrowski, W.; et al. Effect of Antibiotic Amphotericin B Combinations with Selected 1,3,4-Thiadiazole Derivatives on RPTECs in an In Vitro Model. Int. J. Mol. Sci. 2022, 23, 15260. [Google Scholar] [CrossRef]
- Parolin, C.; Croatti, V.; Giordani, B.; Vitali, B. Vaginal Lactobacillus Impair Candida Dimorphic Switching and Biofilm Formation. Microorganisms 2022, 10, 2091. [Google Scholar] [CrossRef]
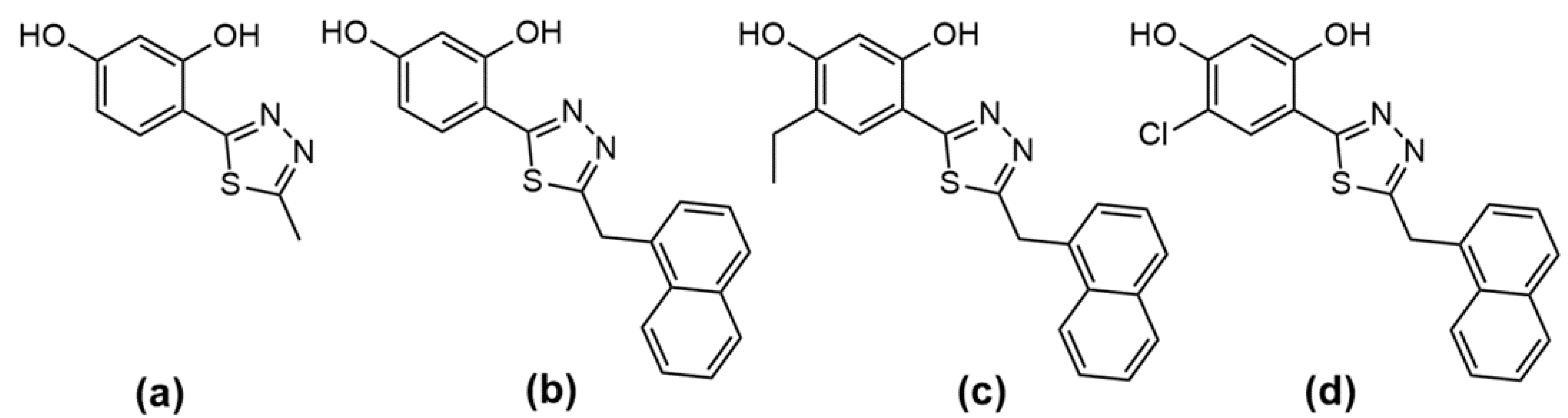
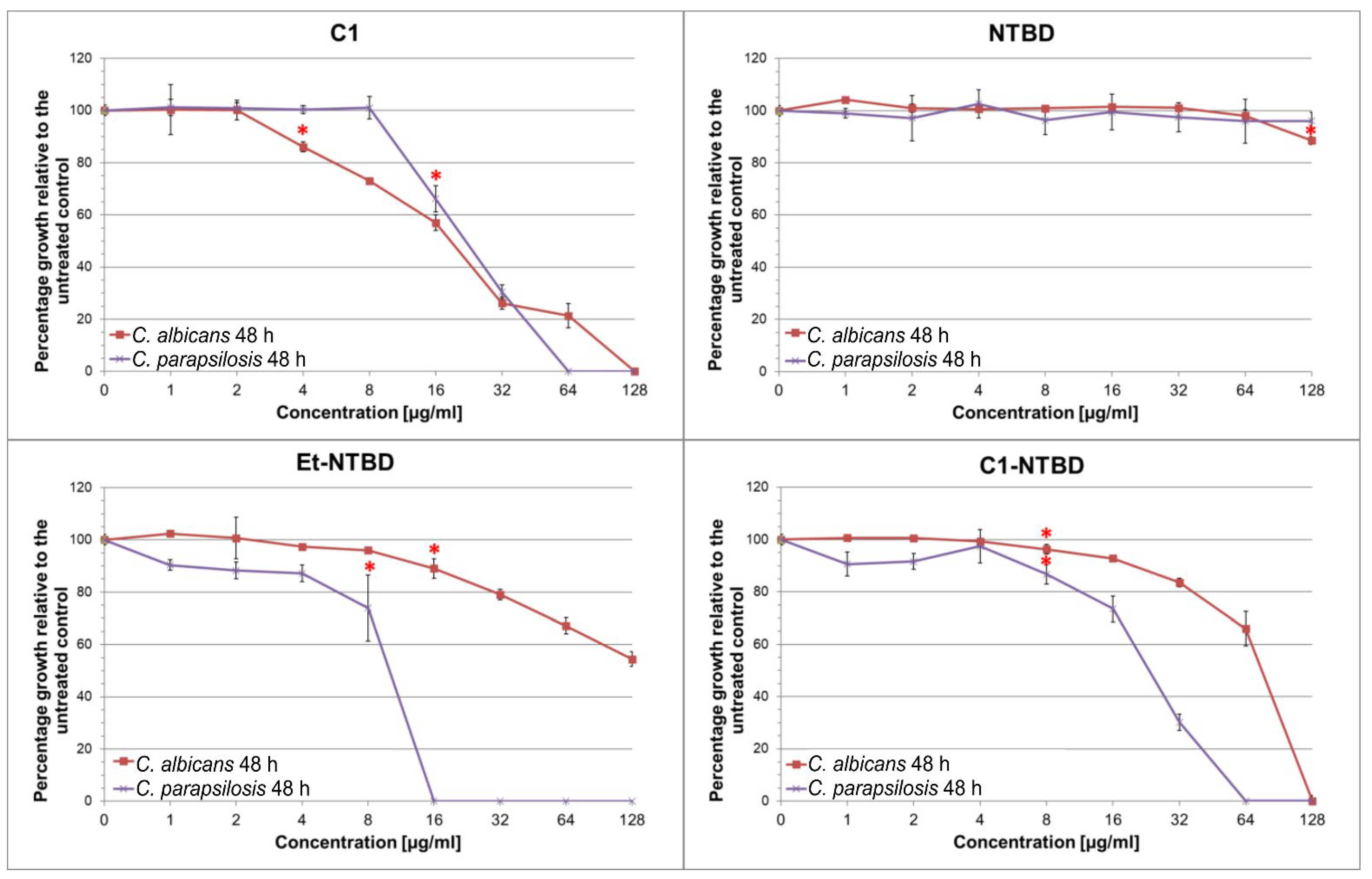
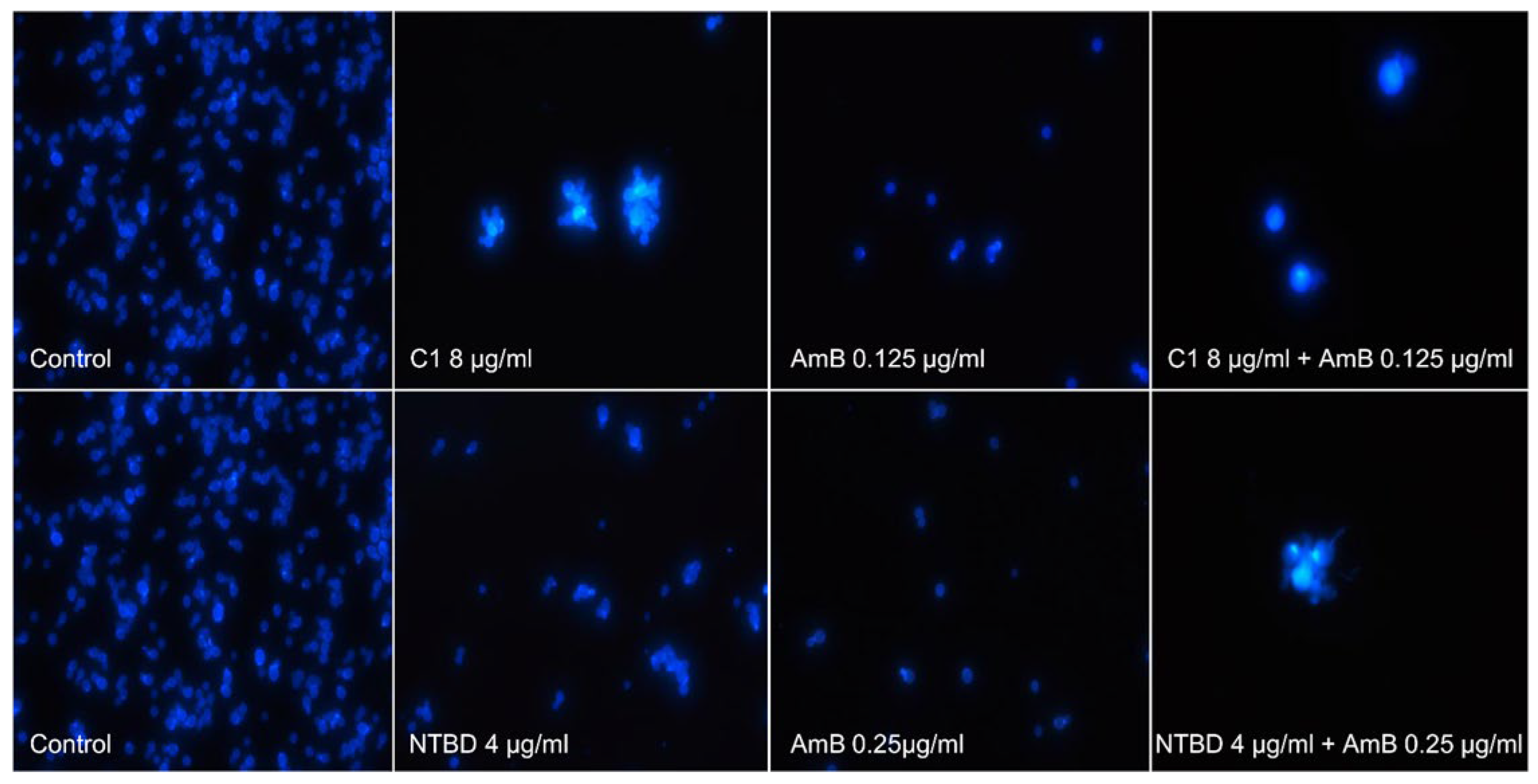
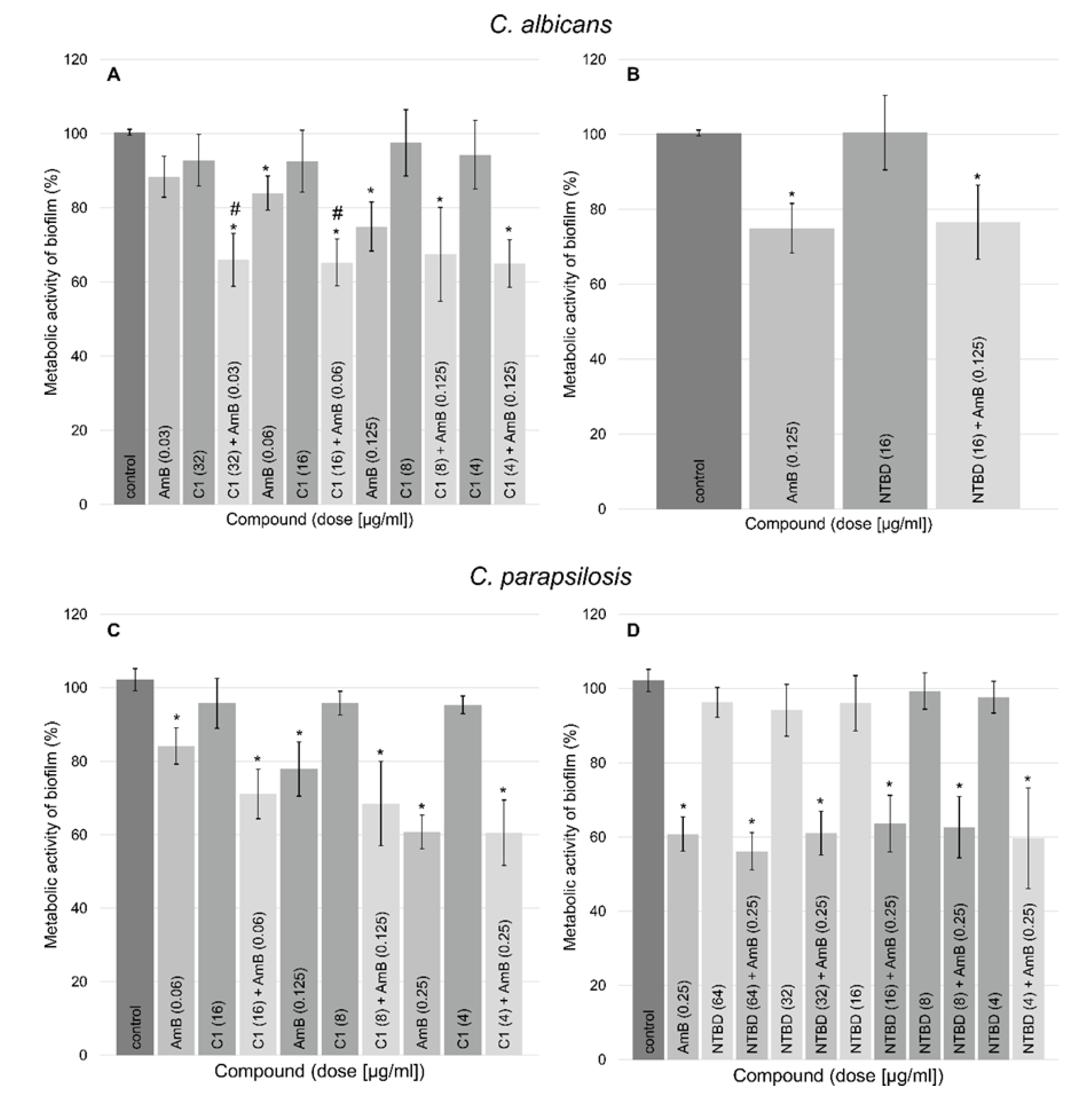

Strain | 1,3,4-thiadiazole | Separate Treatment | Combinatory Treatment 1,3,4-thiadiazole +AmB | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 (µg/mL) of 1,3,4-thiadiazole | 4 (µg/mL) of 1,3,4-thiadiazole | 8 (µg/mL) of 1,3,4-thiadiazole | 16 (µg/mL) of 1,3,4-thiadiazole | 32 (µg/mL) of 1,3,4-thiadiazole | 64 (µg/mL) of 1,3,4-thiadiazole | ||||||||||
| MIC 1,3,4-thiadiazole (µg/mL) | MIC AmB (µg/mL) | MIC AmB (µg/mL) | ƩFIC | MIC AmB (µg/mL) | ƩFIC | MIC AmB (µg/mL) | ƩFIC | MIC AmB (µg/mL) | ƩFIC | MIC AmB (µg/mL) | ƩFIC | MIC AmB (µg/mL) | ƩFIC | ||
| C. albicans NCPF 3153 | C1 | 128 | 0.5 | 0.25 | 0.52 | 0.125 | 0.29 ** | 0.125 | 0.33 ** | 0.0625 | 0.29 ** | 0.0312 | 0.40 ** | 0.0312 | 0.73 |
| NTBD | >128 | 0.5 | 0.25 | 0.51 | 0.25 | 0.52 | 0.25 | 0.53 | 0.125 | 0.31 ** | 0.25 | 0.62 ** | 0.25 | 0.75 | |
| Et-NTBD | >128 | 0.5 | 0.5 | 1.01 | 0.5 | 1.02 | 0.5 | 1.03 | 0.5 | 1.06 | 0.5 | 1.13 | 0.25 | 0.625 | |
| C1-NTBD | 128 | 0.5 | 0.5 | 1.01 | 0.5 | 1.03 | 0.5 | 1.06 | 0.5 | 1.13 | 0.5 | 1.25 | 0.25 | 1 | |
| C. parapsilosis ATCC 22019 | C1 | 64 | 1 | 0.5 | 0.53 | 0.25 | 0.31 ** | 0.125 | 0.25 ** | 0.0625 | 0.31 ** | 0.0312 | 0.53 | - | - |
| NTBD | >128 | 1 | 0.5 | 0.51 | 0.25 | 0.26 ** | 0.25 | 0.28 ** | 0.25 | 0.31 ** | 0.25 | 0.38 ** | 0.25 | 0.5 ** | |
| Et-NTBD | 16 | 1 | 1 | 1.125 | 0.5 | 0.75 | 0.5 | 1 | - | - | - | - | - | - | |
| C1-NTBD | 64 | 1 | 1 | 1.03 | 1 | 1.06 | 1 | 1.13 | 1 | 1.25 | 0.5 | 1 | - | - | |
| Strain | Separate Treatment | Combinatory Treatment C1 + AmB | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 (µg/mL) of C1 | 4 (µg/mL) of C1 | 8 (µg/mL) of C1 | 16 (µg/mL) of C1 | 32 (µg/mL) of C1 | 64 (µg/mL) of C1 | |||||||||
| MIC C1 (µg/mL) | MIC AmB (µg/mL) | MIC AmB (µg/mL) | ƩFIC | MIC AmB (µg/mL) | ƩFIC | MIC AmB (µg/mL) | ƩFIC | MIC AmB (µg/mL) | ƩFIC | MIC AmB (µg/mL) | ƩFIC | MIC AmB (µg/mL) | ƩFIC | |
| C. albicans isolate 40 | >128 | 0.125 | 0.125 | 1.01 | 0.0625 | 0.52 | 0.0312 | 0.28 ** | 0.0625 | 0.56 | 0.0625 | 0.63 | 0.125 | 1.25 |
| C. parapsilosis isolate 73 | 128 | 0.25 | 0.0312 | 0.14 ** | 0.0312 | 0.16 ** | 0.0312 | 0.19 ** | 0.0312 | 0.25 ** | 0.0625 | 0.5 ** | 0.0625 | 0.75 |
| C. krusei isolate 39 | >128 | 0.25 | 0.5 | 2.01 | 0.5 | 2.02 | 0.5 | 2.03 | 0.5 | 2.06 | 1 | 4.125 | 1 | 4.25 |
| C. glabrata isolate 5 | >128 | 0.125 | 0.125 | 1.01 | 0.125 | 1.01 | 0.25 | 2.03 | 0.25 | 2.06 | 0.25 | 2.125 | 0.25 | 2.25 |
| Strain | Separate Treatment | Combinatory Treatment NTBD + AmB | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 (µg/mL) of NTBD | 4 (µg/mL) of NTBD | 8 (µg/mL) of NTBD | 16 (µg/mL) of NTBD | 32 (µg/mL) of NTBD | 64 (µg/mL) of NTBD | |||||||||
| MIC NTBD (µg/mL) | MIC AmB (µg/mL) | MIC AmB (µg/mL) | ƩFIC | MIC AmB (µg/mL) | ƩFIC | MIC AmB (µg/mL) | ƩFIC | MIC AmB (µg/mL) | ƩFIC | MIC AmB (µg/mL) | ƩFIC | MIC AmB (µg/mL) | ƩFIC | |
| C. albicans isolate 40 | >128 | 0.125 | 0.125 | 1.01 | 0.125 | 1.02 | 0.125 | 1.03 | 0.125 | 1.06 | 0.125 | 1.125 | 0.125 | 1.25 |
| C. parapsilosis isolate 73 | >128 | 0.25 | 0.25 | 1.01 | 0.25 | 1.02 | 0.25 | 1.03 | 0.25 | 1.06 | 0.25 | 1.25 | 0.5 | 2.25 |
| C. krusei isolate 39 | >128 | 0.25 | 0.5 | 2.01 | 0.5 | 2.02 | 0.5 | 2.03 | 0.5 | 2.06 | 0.5 | 1.125 | 0.5 | 2.25 |
| C. glabrata isolate 5 | >128 | 0.125 | 0.125 | 1.01 | 0.125 | 1.02 | 0.125 | 1.03 | 0.125 | 1.06 | 0.125 | 1.125 | 0.125 | 1.25 |
| Drug | IC50 RPTECs (μg/mL) | Strain | Drug Concentration (μg/mL) | Selectivity Index (μg/mL) |
|---|---|---|---|---|
| C1 | 161.4 ± 4.0 | C. albicans | 128 * | 1.26 |
| 4–32 ** | 40.35–5.04 | |||
| C. parasitosis | 64 * | 2.51 | ||
| 4–16 ** | 40.35–10.09 | |||
| NTBD | 18.82 ± 5.0 | C. albicans | 256 * | 0.07 |
| 16 ** | 1.18 | |||
| C. parapsilosis | 256 * | 0.07 | ||
| 4–64 ** | 4.71–0.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dróżdż, A.; Kubera, D.; Sławińska-Brych, A.; Matwijczuk, A.; Ślusarczyk, L.; Czernel, G.; Karcz, D.; Olender, A.; Bogut, A.; Pietrzak, D.; et al. Synergistic Antifungal Interactions between Antibiotic Amphotericin B and Selected 1,3,4-thiadiazole Derivatives, Determined by Microbiological, Cytochemical, and Molecular Spectroscopic Studies. Int. J. Mol. Sci. 2023, 24, 3430. https://doi.org/10.3390/ijms24043430
Dróżdż A, Kubera D, Sławińska-Brych A, Matwijczuk A, Ślusarczyk L, Czernel G, Karcz D, Olender A, Bogut A, Pietrzak D, et al. Synergistic Antifungal Interactions between Antibiotic Amphotericin B and Selected 1,3,4-thiadiazole Derivatives, Determined by Microbiological, Cytochemical, and Molecular Spectroscopic Studies. International Journal of Molecular Sciences. 2023; 24(4):3430. https://doi.org/10.3390/ijms24043430
Chicago/Turabian StyleDróżdż, Agnieszka, Dominika Kubera, Adrianna Sławińska-Brych, Arkadiusz Matwijczuk, Lidia Ślusarczyk, Grzegorz Czernel, Dariusz Karcz, Alina Olender, Agnieszka Bogut, Daniel Pietrzak, and et al. 2023. "Synergistic Antifungal Interactions between Antibiotic Amphotericin B and Selected 1,3,4-thiadiazole Derivatives, Determined by Microbiological, Cytochemical, and Molecular Spectroscopic Studies" International Journal of Molecular Sciences 24, no. 4: 3430. https://doi.org/10.3390/ijms24043430
APA StyleDróżdż, A., Kubera, D., Sławińska-Brych, A., Matwijczuk, A., Ślusarczyk, L., Czernel, G., Karcz, D., Olender, A., Bogut, A., Pietrzak, D., Dąbrowski, W., Stepulak, A., Wójcik-Załuska, A., & Gagoś, M. (2023). Synergistic Antifungal Interactions between Antibiotic Amphotericin B and Selected 1,3,4-thiadiazole Derivatives, Determined by Microbiological, Cytochemical, and Molecular Spectroscopic Studies. International Journal of Molecular Sciences, 24(4), 3430. https://doi.org/10.3390/ijms24043430











