Sex-Inclined Piwi-Interacting RNAs in Serum Exosomes for Sex Determination in the Greater Amberjack (Seriola dumerili)
Abstract
1. Introduction
2. Results
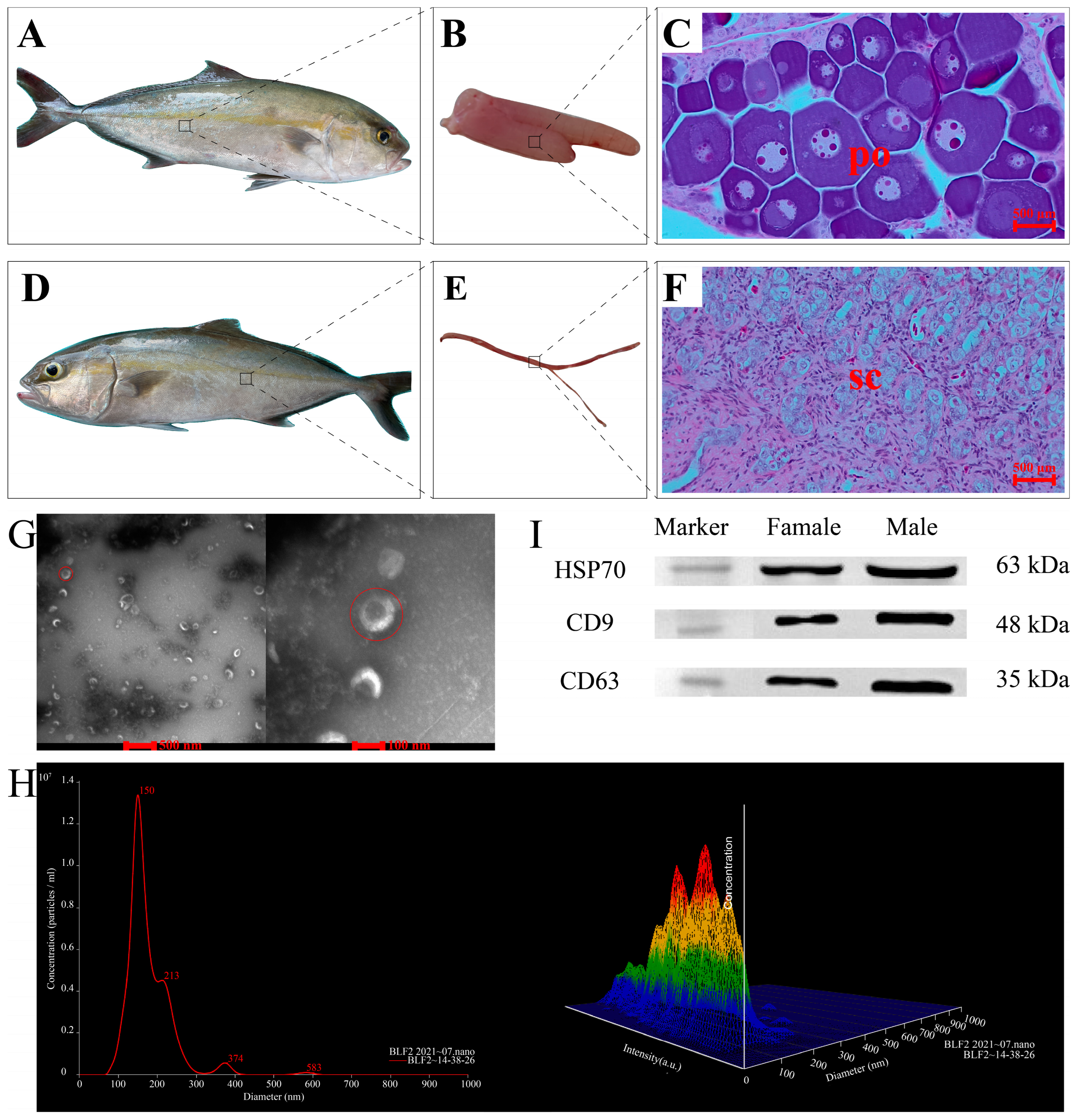
2.1. Fish Size and Gonad Histology
2.2. Identification and Characterization of Exosomes
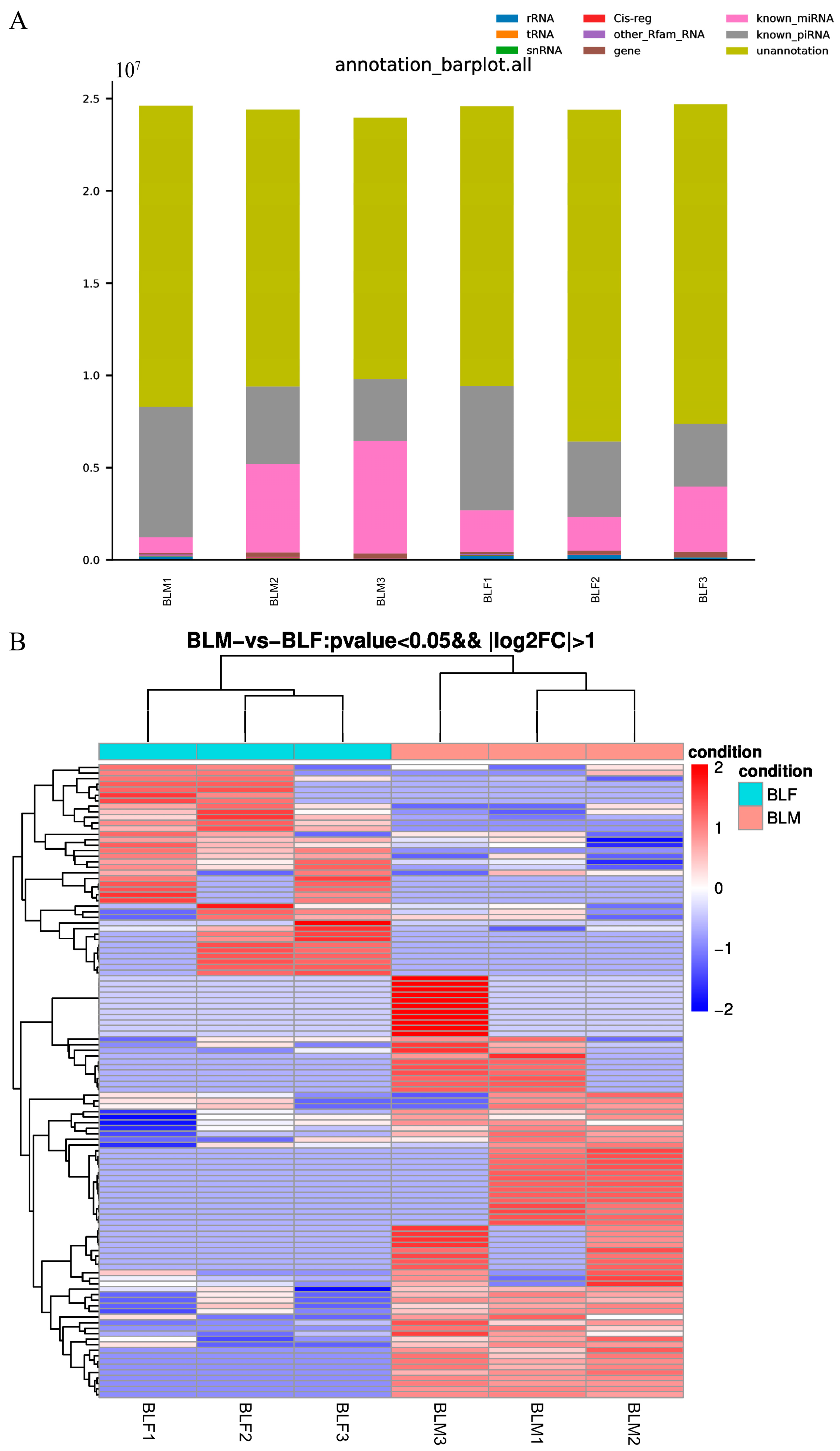
2.3. Piwi-Interacting RNAs Profiles in Male and Female Serum Exosomes
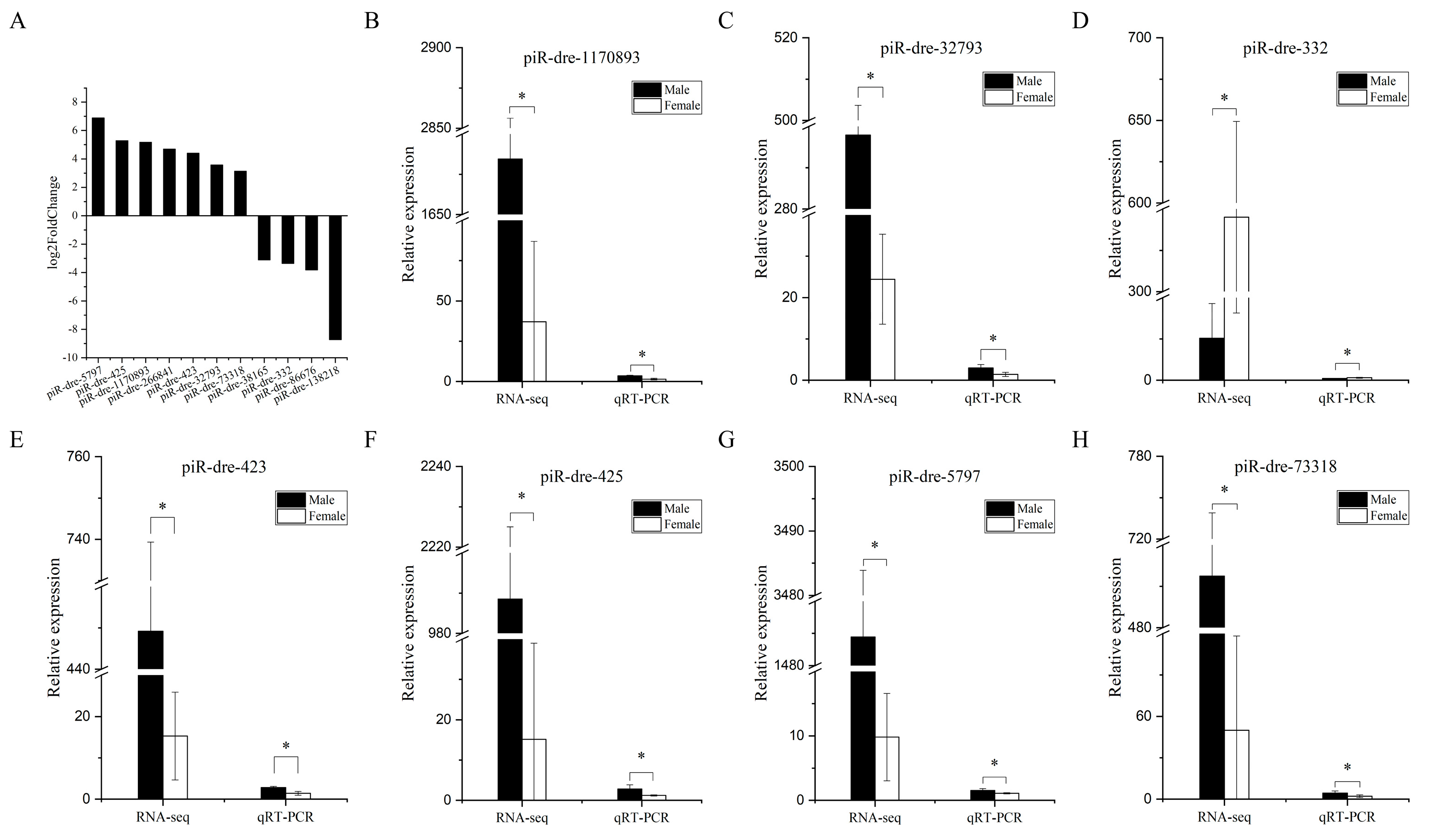
2.4. Screening Differentially Expressed Piwi-Interacting RNAs between Male and Female Greater Amberjack and Target Prediction
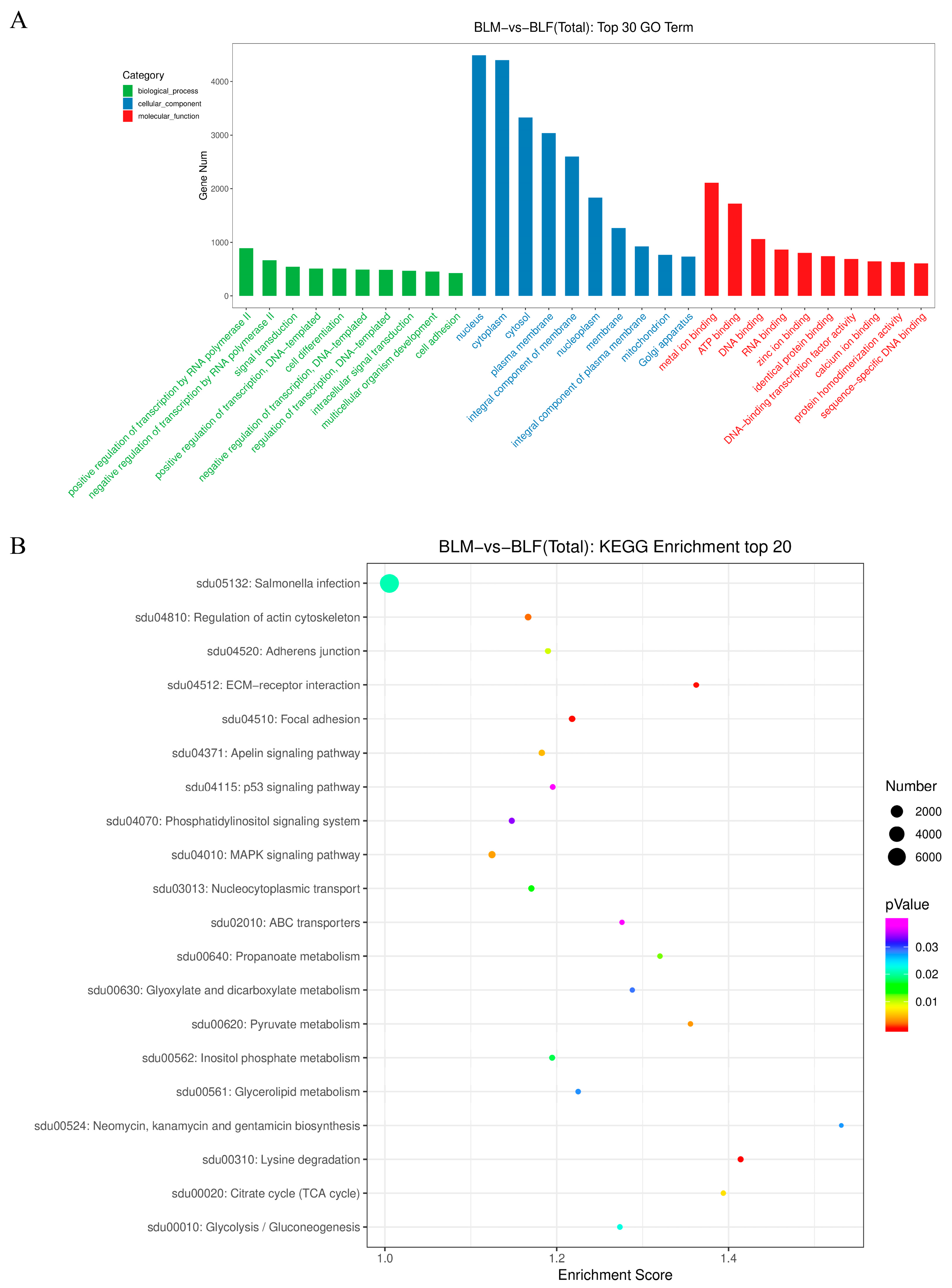
2.5. Gene Ontology and Kyoto Encyclopedia of Genes and Genomes Pathway Enrichment Analyses
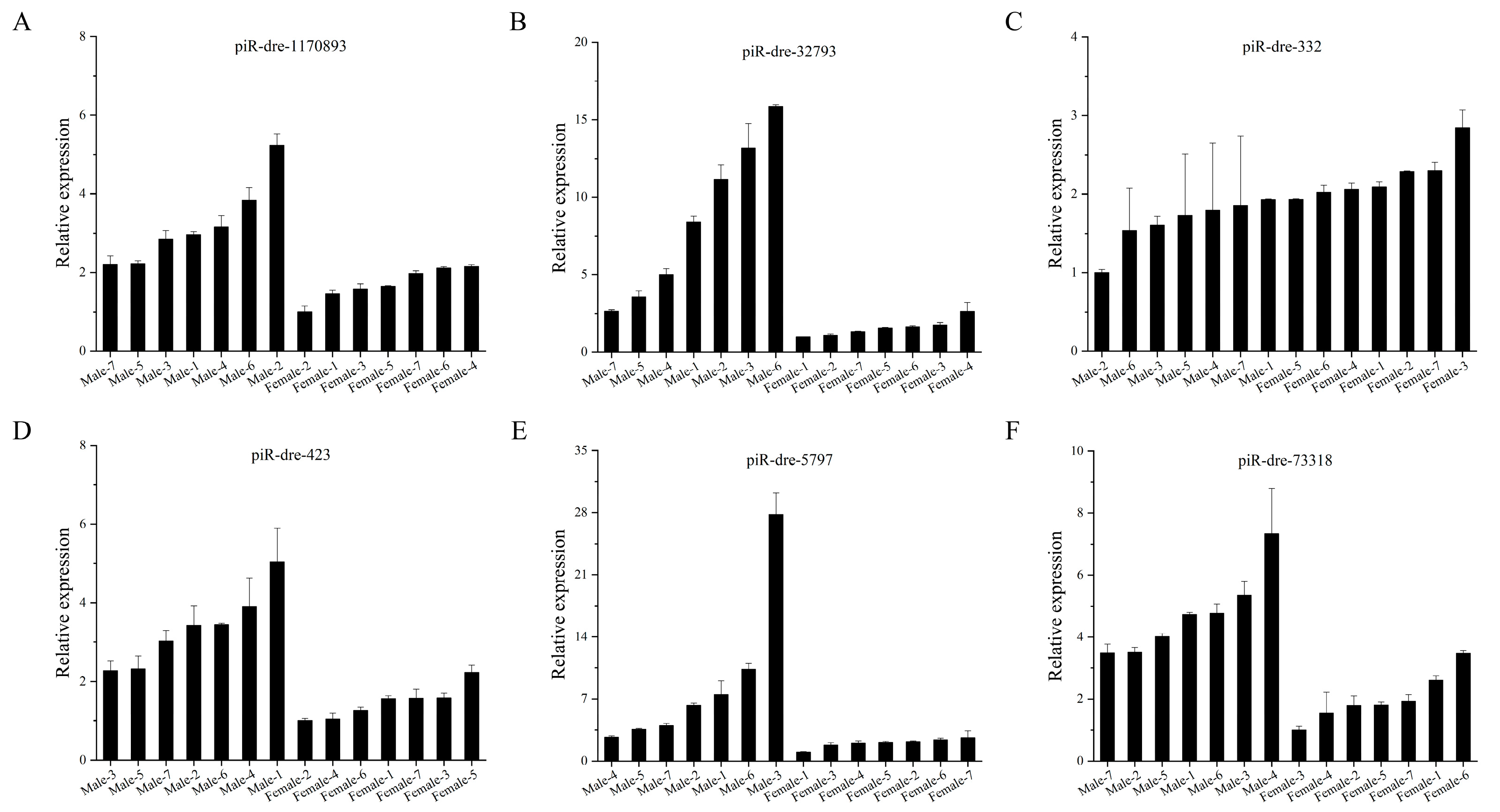
2.6. Real-Time Quantitative Polymerase Chain Reaction Verification of Signature Piwi-Interacting RNAs with Differential Expression between Male and Female Greater Amberjack
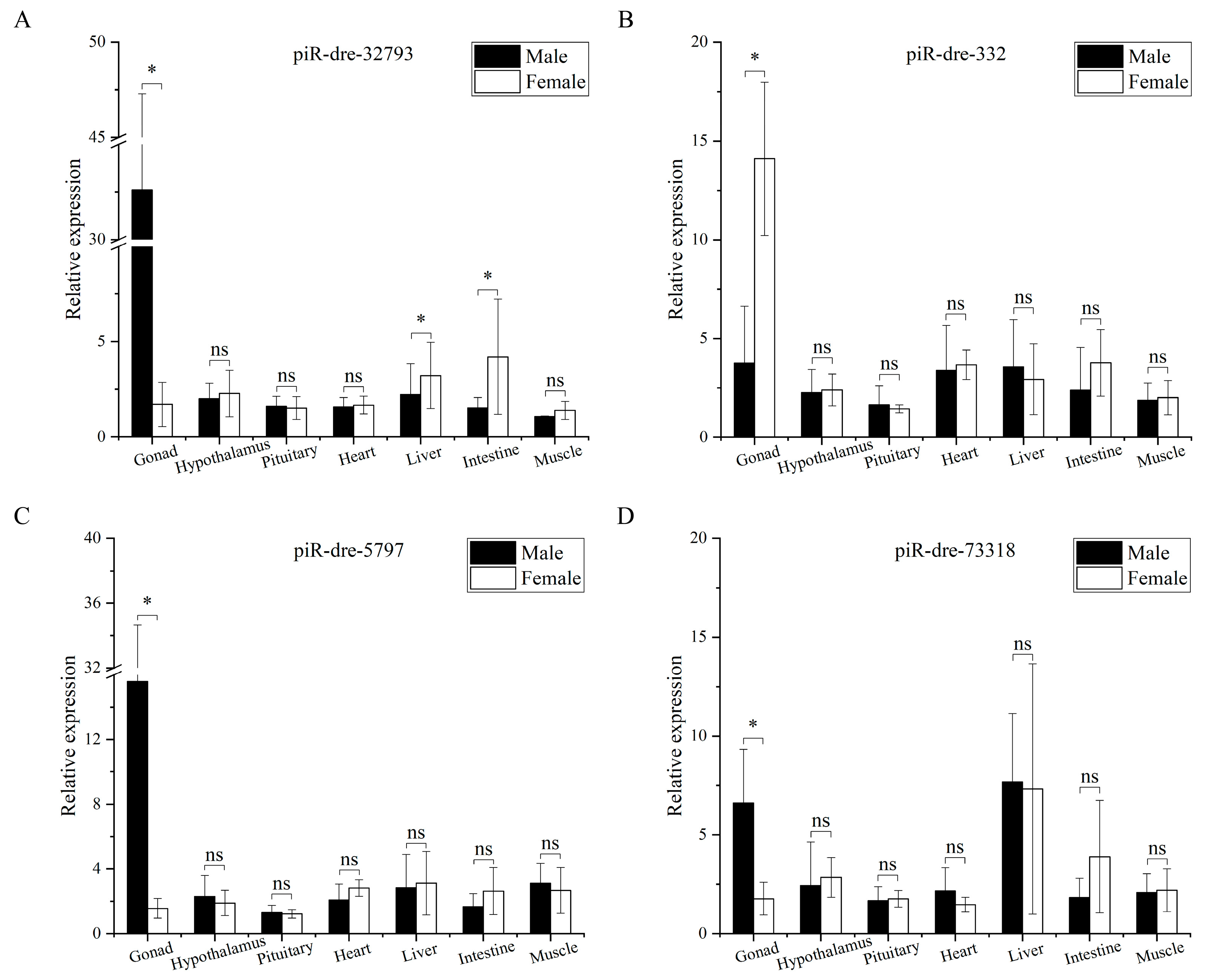
2.7. Expression of Sex-Inclined Piwi-Interacting RNAs in Other Tissues
2.8. Piwi-Interacting RNAs–Target Interaction Network Analysis
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Sampling, Histological Analysis and Isolation of Serum Exosomes from Greater Amberjack
4.3. Characterization of Serum Exosomes of Greater Amberjack
4.3.1. Transmission Electron Microscopy
4.3.2. Nanoparticle Tracking Analysis
4.3.3. Western Blotting Analysis
4.4. Small RNA Library Construction, Sequencing, and Bioinformatics Analysis
4.5. Real-Time Quantitative Polymerase Chain Reaction of Differentially Expressed Piwi-Interacting RNAs
4.6. Piwi-Interacting RNAs–Target Interaction Network Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kawase, J.; Aoki, J.Y.; Hamada, K.; Ozaki, A.; Araki, K. Identification of sex-associated SNPs of greater amberjack (Seriola dumerili). J. Genom. 2018, 6, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, M.; Mandalakis, M.; Anastasiou, T.I.; Pouli, M.; Asderis, M.; Katharios, P.; Papandroulakis, N.; Mylonas, C.C. Histological evaluation of sex differentiation and early sex identification in hatchery-produced greater amberjack (Seriola dumerili) reared in sea cages. Fish Physiol. Biochem. 2021, 47, 1777–1792. [Google Scholar] [CrossRef]
- Sarih, S.; Djellata, A.; Fernández-Palacios, H.; Ginés, R.; Fontanillas, R.; Rosenlund, G.; Izquierdo, M.; Roo, J. Adequate n-3 LC-PUFA levels in broodstock diets optimize reproductive performance in GnRH injected greater amberjack (Seriola dumerili) equaling to spontaneously spawning broodstock. Aquaculture 2020, 520, 735007. [Google Scholar] [CrossRef]
- Roo, J.; Hernández-Cruz, C.M.; Mesa-Rodriguez, A.; Fernández-Palacios, H.; Izquierdo, M.S. Effect of increasing n-3 HUFA content in enriched Artemia on growth, survival and skeleton anomalies occurrence of greater amberjack Seriola dumerili larvae. Aquaculture 2019, 500, 651–659. [Google Scholar] [CrossRef]
- Crespo, S.; Grau, A.; Padrós, F. The intensive culture of 0-group amberjack in the western Mediterranean is compromised by disease problems. Aquac. Int. 1994, 2, 262–265. [Google Scholar]
- Marino, G.; Mandich, A.; Massari, A.; Andaloro, F.; Porrello, S.; Finoia, M.G.; Cevasco, F. Aspects of reproductive biology of the Mediterranean amberjack (Seriola dumerilii Risso) during the spawning period. J. Appl. Ichthyol. 1995, 11, 9–24. [Google Scholar] [CrossRef]
- Sarropoulou, E.; Sundaram, A.Y.M.; Kaitetzidou, E.; Kotoulas, G.; Gilfillan, G.D.; Papandroulakis, N.; Mylonas, C.C.; Magoulas, A. Full genome survey and dynamics of gene expression in the greater amberjack Seriola dumerili. Gigascience 2017, 6, gix108. [Google Scholar] [CrossRef]
- Pastor, E.; Grau, A.; Riera, F.; Pou, S.; Massurti, E.; Grau, A. Experiences in the culture or new species in the Estacion de Aquiculture of the Balearic Government (1980–1998). Cah. Options Méditerr. 2000, 47, 371–379. [Google Scholar]
- Garcia, A.; Diaz, M.V.; Agulleiro, B. Inducción hormonal de la puesta y desarrollo embrionario de la seriola Mediterránea (Seriola dumerilii, Risso). Monogr. Inst. Canar. Cienc. Mar. 2001, 4, 561–566. [Google Scholar]
- Mylonas, C.C.; Papandroulakis, N.; Smboukis, A.; Papadaki, M.; Divanach, P. Induction of spawning of cultured greater amberjack (Seriola dumerili) using GnRHa implants. Aquaculture 2004, 237, 141–154. [Google Scholar] [CrossRef]
- Fakriadis, I.; Miccoli, A.; Karapanagiotis, S.; Tsele, N.; Mylonas, C.C. Optimization of a GnRHa treatment for spawning commercially reared greater amberjack Seriola dumerili: Dose response and extent of the reproductive season. Aquaculture 2020, 521, 735011. [Google Scholar] [CrossRef]
- Fakriadis, I.; Sigelaki, I.; Papadaki, M.; Papandroulakis, N.; Raftopoulos, A.; Tsakoniti, K.; Mylonas, C.C. Control of reproduction of greater amberjack Seriola dumerili reared in aquaculture facilities. Aquaculture 2020, 519, 734880. [Google Scholar] [CrossRef]
- Fakriadis, I.; Lisi, F.; Sigelaki, I.; Papadaki, M.; Mylonas, C.C. Spawning kinetics and egg/larval quality of greater amberjack (Seriola dumerili) in response to multiple GnRHa injections or implants. Gen. Comp. Endocrinol. 2019, 279, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Fakriadis, I.; Mylonas, C.C. Sperm quality of greater amberjack Seriola dumerili throughout the reproductive season and in response to GnRHa treatment with controlled release implants. Fish Physiol. Biochem. 2021, 47, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Yanlong, S.; Yinjun, J.; Ji, C.; Binbin, T.; Wen, X.; Yang, H.; Guangli, L.; Zhu, C.; Wei, H. Effects of secretoneurin and gonadotropin-releasing hormone agonist on the spawning of captive greater amberjack (Seriola dumerili). Life 2022, 12, 1457. [Google Scholar] [CrossRef]
- Nyuji, M.; Yamamoto, I.; Hamada, K.; Kazeto, Y.; Okuzawa, K. Effect of GnRHa on plasma levels of Fsh and Lh in the female greater amberjack Seriola dumerili. J. Fish Biol. 2019, 95, 1350–1354. [Google Scholar] [CrossRef]
- Jerez, S.; Samper, M.; Santamaría, F.J.; Villamandos, J.E.; Cejas, J.R.; Felipe, B.C. Natural spawning of greater amberjack (Seriola dumerili) kept in captivity in the Canary Islands. Aquaculture 2006, 252, 199–207. [Google Scholar] [CrossRef]
- Zupa, R.; Rodríguez, C.; Mylonas, C.C.; Rosenfeld, H.; Fakriadis, I.; Papadaki, M.; Pérez, J.A.; Pousis, C.; Basilone, G.; Corriero, A. Comparative study of reproductive development in wild and captive-reared greater amberjack Seriola dumerili (Risso, 1810). PLoS ONE 2017, 12, e0169645. [Google Scholar] [CrossRef]
- Araki, K.; Aokic, J.Y.; Kawase, J.; Hamada, K.; Ozaki, A.; Fujimoto, H.; Yamamoto, I.; Usuki, H. Whole genome sequencing of greater amberjack (Seriola dumerili) for SNP identification on aligned scaffolds and genome structural variation analysis using parallel resequencing. Int. J. Genom. 2018, 2018, 7984292. [Google Scholar] [CrossRef]
- Zhao, N.; Deng, Q.; Zhu, C.; Zhang, B. Application of extracellular vesicles in aquatic animals: A review of the latest decade. Rev. Fish. Sci. Aquac. 2021, 30, 447–466. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of extracellular vesicles, their origin, composition, purpose and methods for exosome isolation and analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Abels, E.R.; Breakefield, X.O. Introduction to extracellular vesicles: Biogenesis, RNA cargo selection, content, release and uptake. Cell. Mol. Neurobiol. 2016, 36, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, G.; Li, J. Recent progress on the isolation and detection methods of exosomes. Chem. Asian J. 2020, 15, 3973–3982. [Google Scholar] [CrossRef] [PubMed]
- Aravin, A.; Gaidatzis, D.; Pfeffer, S.; Lagos-Quintana, M.; Landgraf, P.; Iovino, N.; Morris, P.; Brownstein, M.J.; Kuramochi-Miyagawa, S.; Nakano, T.; et al. A novel class of small RNAs bind to MILI protein in mouse testes. Nature 2006, 442, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Girard, A.; Sachidanandam, R.; Hannon, G.J.; Carmell, M.A. A germline-specific class of small RNAs binds mammalian Piwi proteins. Nature 2006, 442, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Chen, F.; Luo, M.; Cheng, Y.; Zhao, H.; Cheng, H.; Zhou, R. Rapid evolution of piRNA pathway in the teleost fish: Implication for an adaptation to transposon diversity. Genome Biol. Evol. 2014, 6, 1393–1407. [Google Scholar] [CrossRef]
- Czech, B.; Hannon, G.J. One loop to rule them all: The ping-pong cycle and piRNA-guided silencing. Trends Biochem. Sci. 2016, 41, 324–337. [Google Scholar] [CrossRef]
- Lite, C.; Sridhar, V.V.; Sriram, S.; Juliet, M.; Arshad, A.; Arockiaraj, J. Functional role of piRNAs in animal models and its prospects in aquaculture. Rev. Aquac. 2021, 13, 2038–2052. [Google Scholar] [CrossRef]
- Zhao, C.; Zhu, W.; Yin, S.; Cao, Q.; Zhang, H.; Wen, X.; Zhang, G.; Xie, W.; Chen, S. Molecular characterization and expression of Piwil1 and Piwil2 during gonadal development and treatment with HCG and LHRH-A(2) in Odontobutis potamophila. Gene 2018, 647, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Rui, X.; Qi, L.; Hong, Y. Expression pattern of Piwi-like gene implies the potential role in germline development in the Pacific oyster Crossosrea gigas. Aquac. Rep. 2020, 18, 100486. [Google Scholar] [CrossRef]
- Tao, W.; Sun, L.; Chen, J.; Shi, H.; Wang, D. Genomic identification, rapid evolution, and expression of Argonaute genes in the tilapia, Oreochromis niloticus. Dev. Genes Evol. 2016, 226, 339–348. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, N.; Jia, L.; Che, J.; He, X.; Liu, K.; Bao, B. Identification and application of piwi-interacting RNAs from seminal plasma exosomes in Cynoglossus semilaevis. BMC Genom. 2020, 21, 302. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Jia, L.; Deng, Q.; Zhu, C.; Zhang, B. Comparative piRNAs profiles give a clue to transgenerational inheritance of sex-biased piRNAs in Cynoglossus semilaevis. Mar. Biotechnol. 2022, 24, 335–344. [Google Scholar] [CrossRef]
- Devlin, R.H.; Nagahama, Y. Sex determination and sex differentiation in fish: An overview of genetic, physiological and environmental influences. Aquaculture 2002, 208, 191–364. [Google Scholar] [CrossRef]
- Volff, J.N.; Nanda, I.; Schmid, M.; Schartl, M. Governing sex determination in fish: Regulatory putsches and ephemeral dictators. Sex Dev. 2007, 1, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zuo, Z.; Zhou, F.; Zhao, W.; Sakaguchi, Y.; Suzuki, T.; Suzuki, T.; Cheng, H.; Zhou, R. Profiling sex-specific piRNAs in zebrafish. Genetics 2010, 186, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, F.; Liu, S.; Zhong, H.; Liu, Z.; Tao, M.; Zhang, C.; Liu, Y. Human chorionic gonadotropin suppresses expression of Piwis in common carp (Cyprinus carpio) ovaries. Gen. Comp. Endocrinol. 2012, 176, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Zhou, Y.; Luo, Y.; Zhong, H.; Huang, Y.; Zhang, Y.; Luo, Z.; Ling, Z.; Zhang, M.; Gan, X. Suppression effect of LHRH-A and hCG on Piwi expression in testis of Nile tilapia Oreochromis niloticus. Gen. Comp. Endocrinol. 2013, 189, 43–50. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhong, H.; Xiao, J.; Yan, J.; Luo, Y.; Gan, X.; Yu, F. Identification and comparative analysis of piRNAs in ovary and testis of Nile tilapia (Oreochromis niloticus). Genes Genom. 2016, 38, 519–527. [Google Scholar] [CrossRef]
- Kleppe, L.; Wargelius, A.; Johnsen, H.; Andersson, E.; Edvardsen, R.B. Gonad specific genes in Atlantic salmon (Salmon salar L.): Characterization of tdrd7-2, dazl-2, piwil1 and tdrd1 genes. Gene 2015, 560, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, B.; Liu, X.; Liu, Y.; Du, X.; Zhang, Q.; Wang, X. Identification and expression of piwil2 in turbot Scophthalmus maximus, with implications of the involvement in embryonic and gonadal development. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2017, 208–209, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, B.; Liu, J.; Li, A.; Zhu, H.; Wang, X.; Zhang, Q. Piwil1 gene is regulated by hypothalamic-pituitary-gonadal axis in turbot (Scophthalmus maximus): A different effect in ovaries and testes. Gene 2018, 658, 86–95. [Google Scholar] [CrossRef]
- Papadaki, M.; Kaitetzidou, E.; Mylonas, C.C.; Sarropoulou, E. Non-coding rna expression patterns of two different teleost gonad maturation stages. Mar. Biotechnol. 2020, 22, 683–695. [Google Scholar] [CrossRef]
- Wang, C.L.; Wang, Z.P.; Wang, J.Q.; Li, M.Y.; Chen, X.W. Identification of candidate piRNAs in the gonads of Paralichthys olivaceus (Japanese flounder). Zool. Res. 2016, 37, 301–306. [Google Scholar]
- Ni, F.; Yu, H.; Liu, Y.; Meng, L.; Yan, W.; Zhang, Q.; Yu, H.; Wang, X. Roles of piwil1 gene in gonad development and gametogenesis in Japanese flounder, Paralichthys olivaceus. Gene 2019, 701, 104–112. [Google Scholar] [CrossRef]
- Ni, F.; Yu, H.; Qu, J.; Meng, L.; Liu, X.; Yan, W.; Chang, J.; Zhang, Q.; Wang, X.; Yu, H. Hypothalamic-pituitary-gonadal (HPG) axis and transcriptional regulatory elements regulate piwil2 gene expression during gametogenesis and gonadal development in Japanese flounder (Paralichthys olivaceus). J. Ocean Univ. China 2020, 19, 1378–1388. [Google Scholar] [CrossRef]
- Zhao, N.; Zhang, B.; Jia, L.; He, X.; Bao, B. Extracellular vesicles piwi-interacting RNAs from skin mucus for identification of infected Cynoglossus semilaevis with Vibrio harveyi. Fish Shellfish Immunol. 2021, 111, 170–178. [Google Scholar] [CrossRef]
- Prasad, P.; Ogawa, S.; Parhar, I.S. Role of serotonin in fish reproduction. Front. Neurosci. 2015, 9, 195. [Google Scholar] [CrossRef]
- Wang, B.; Yang, G.; Xu, Y.; Li, W.; Liu, X. Recent studies of LPXRFa receptor signaling in fish and other vertebrates. Gen. Comp. Endocrinol. 2019, 277, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.M.; Stenquist, D.S.; Calsbeek, R. Testosterone, growth and the evolution of sexual size dimorphism. J. Evol. Biol. 2009, 22, 1586–1598. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, Q.; Shi, R.; Wang, J.; Zhang, Z.; Yang, Y.; Li, W.; Chen, S.; Wang, N. Effects of long-term sex steroid hormones (estradiol and testosterone)-supplemented feeds on the growth performance of Chinese tongue sole (Cynoglossus semilaevis). Fish Physiol. Biochem. 2022, 48, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Hammond, G.L. Diverse roles for sex hormone-binding globulin in reproduction. Biol. Reprod. 2011, 85, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Marivin, E.; Yano, A.; Guérin, A.; Nguyen, T.V.; Fostier, A.; Bobe, J.; Guiguen, Y. Sex hormone-binding globulins characterization and gonadal gene expression during sex differentiation in the rainbow trout, Oncorhynchus mykiss. Mol. Reprod. Dev. 2014, 81, 757–765. [Google Scholar] [CrossRef]
- González, A.; Fernandino, J.I.; Hammond, G.L.; Somoza, G.M. Sex hormone binding globulin: Expression throughout early development and adult pejerrey fish, Odontesthes bonariensis. Gen. Comp. Endocrinol. 2017, 247, 205–214. [Google Scholar] [CrossRef]
- Hacker, B.M.; Tomlinson, J.E.; Wayman, G.A.; Sultana, R.; Chan, G.; Villacres, E.; Disteche, C.; Storm, D.R. Cloning, chromosomal mapping, and regulatory properties of the human type 9 adenylyl cyclase (ADCY9). Genomics 1998, 50, 97–104. [Google Scholar] [CrossRef]
- Toyota, T.; Hattori, E.; Meerabux, J.; Yamada, K.; Saito, K.; Shibuya, H.; Nankai, M.; Yoshikawa, T. Molecular analysis, mutation screening, and association study of adenylate cyclase type 9 gene (ADCY9) in mood disorders. Am. J. Med. Genet. 2002, 114, 84–92. [Google Scholar] [CrossRef]
- Rautureau, Y.; Deschambault, V.; Higgins, M.; Rivas, D.; Mecteau, M.; Geoffroy, P.; Miquel, G.; Uy, K.; Sanchez, R.; Lavoie, V.; et al. ADCY9 (adenylate cyclase type 9) inactivation protects from atherosclerosis only in the absence of CETP (cholesteryl ester transfer protein). Circulation 2018, 138, 1677–1692. [Google Scholar] [CrossRef]
- Yi, H.; Wang, K.; Jin, J.F.; Jin, H.; Yang, L.; Zou, Y.; Du, B.; Liu, X. Elevated adenylyl cyclase 9 expression is a potential prognostic biomarker for patients with colon cancer. Med. Sci. Monit. 2018, 24, 19–25. [Google Scholar] [CrossRef]
- Lai, P.M.R.; Du, R. Differentially expressed genes associated with the estrogen receptor pathway in cerebral aneurysms. World Neurosurg. 2019, 126, e557–e563. [Google Scholar] [CrossRef] [PubMed]
- Gamache, I.; Legault, M.A.; Grenier, J.C.; Sanchez, R.; Rhéaume, E.; Asgari, S.; Barhdadi, A.; Zada, Y.F.; Trochet, H.; Luo, Y.; et al. A sex-specific evolutionary interaction between ADCY9 and CETP. Elife 2021, 10, e69198. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Qin, Z.; Lu, Z.; Liang, R.; Zhao, L.; Pan, G.; Lin, L.; Zhang, K. Comparative transcriptomics of gonads reveals the molecular mechanisms underlying gonadal development in giant freshwater prawns (Macrobrachium rosenbergii). J. Mar. Sci. Eng. 2022, 10, 737. [Google Scholar] [CrossRef]
- Okkenhaug, K.; Vanhaesebroeck, B. PI3K in lymphocyte development, differentiation and activation. Nat. Rev. Immunol. 2003, 3, 317–330. [Google Scholar] [CrossRef]
- Porta, C.; Paglino, C.; Mosca, A. Targeting PI3K/Akt/mTOR Signaling in Cancer. Front. Oncol. 2014, 4, 64. [Google Scholar] [CrossRef] [PubMed]
- Carnagarin, R.; Dharmarajan, A.M.; Dass, C.R. Molecular aspects of glucose homeostasis in skeletal muscle—A focus on the molecular mechanisms of insulin resistance. Mol. Cell. Endocrinol. 2015, 417, 52–62. [Google Scholar] [CrossRef]
- Yu, J.S.; Cui, W. Proliferation, survival and metabolism: The role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development 2016, 143, 3050–3060. [Google Scholar] [CrossRef]
- Miricescu, D.; Totan, A.; Stanescu, S., II; Badoiu, S.C.; Stefani, C.; Greabu, M. PI3K/AKT/mTOR signaling pathway in breast cancer: From molecular landscape to clinical aspects. Int. J. Mol. Sci. 2020, 22, 173. [Google Scholar] [CrossRef]
- George, G.; Draycott, S.A.V.; Muir, R.; Clifford, B.; Elmes, M.J.; Langley-Evans, S.C. Exposure to maternal obesity during suckling outweighs in utero exposure in programming for post-weaning adiposity and insulin resistance in rats. Sci. Rep. 2019, 9, 10134. [Google Scholar] [CrossRef]
- Chen, W.; Guo, X.; Jin, Z.; Li, R.; Shen, L.; Li, W.; Cai, W.; Zhang, G. Transcriptional alterations of genes related to fertility decline in male rats induced by chronic sleep restriction. Syst. Biol. Reprod. Med. 2020, 66, 99–111. [Google Scholar] [CrossRef]
- Cao, Z.M.; Qiang, J.; Zhu, J.H.; Li, H.X.; Tao, Y.F.; He, J.; Xu, P.; Dong, Z.J. Transcriptional inhibition of steroidogenic factor 1 in vivo in Oreochromis niloticus increased weight and suppressed gonad development. Gene 2022, 809, 146023. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.C.; Wajnberg, G.; Chacko, S.; Woldemariam, N.T.; Lacroix, J.; Crapoulet, N.; Ayre, D.C.; Lewis, S.M.; Rise, M.L.; Andreassen, R.; et al. Characterization of miRNAs in Extracellular vesicles released from Atlantic salmon monocyte-like and macrophage-like cells. Front. Immunol. 2020, 11, 587931. [Google Scholar] [CrossRef] [PubMed]
- Magnadóttir, B.; Uysal-Onganer, P.; Kraev, I.; Dodds, A.W.; Guðmundsdóttir, S.; Lange, S. Extracellular vesicles, deiminated protein cargo and microRNAs are novel serum biomarkers for environmental rearing temperature in Atlantic cod (Gadus morhua L.). Aquac. Rep. 2020, 16, 100245. [Google Scholar] [CrossRef]
- Gong, Y.; Kong, T.; Ren, X.; Chen, J.; Lin, S.; Zhang, Y.; Li, S. Exosome-mediated apoptosis pathway during WSSV infection in crustacean mud crab. PLoS Pathog. 2020, 16, e1008366. [Google Scholar] [CrossRef]
- Yang, H.; Li, X.; Ji, J.; Yuan, C.; Gao, X.; Zhang, Y.; Lu, C.; Li, F.; Zhang, X. Changes of microRNAs expression profiles from red swamp crayfish (Procambarus clarkia) hemolymph exosomes in response to WSSV infection. Fish Shellfish Immunol. 2019, 84, 169–177. [Google Scholar] [CrossRef]
- Zhao, N.; Zhang, B.; Xu, Z.; Jia, L.; Li, M.; He, X.; Bao, B. Detecting Cynoglossus semilaevis infected with Vibrio harveyi using micro RNAs from mucous exosomes. Mol. Immunol. 2020, 128, 268–276. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, N.; Jia, L.; Peng, K.; Che, J.; Li, K.; He, X.; Sun, J.; Bao, B. Seminal plasma exosomes: Promising biomarkers for identification of male and pseudo-males in Cynoglossus semilaevis. Mar. Biotechnol. 2019, 21, 310–319. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Gordon, A.; Hannon, G.J. Fastx-Toolkit—FASTQ/A Short-Reads Pre-Processing Tools; Hannon Lab: Cambridge, UK, 2010. [Google Scholar]
- Patel, R.K.; Jain, M. NGS QC Toolkit: A toolkit for quality control of next generation sequencing data. PLoS ONE 2012, 7, e30619. [Google Scholar] [CrossRef]
- Griffiths-Jones, S.; Bateman, A.; Marshall, M.; Khanna, A.; Eddy, S.R. Rfam: An RNA family database. Nucleic Acids Res. 2003, 31, 439–441. [Google Scholar] [CrossRef]
- Griffiths-Jones, S.; Saini, H.K.; van Dongen, S.; Enright, A.J. miRBase: Tools for microRNA genomics. Nucleic Acids Res. 2008, 36, D154–D158. [Google Scholar] [CrossRef] [PubMed]
- Griffiths-Jones, S.; Grocock, R.J.; van Dongen, S.; Bateman, A.; Enright, A.J. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006, 34, D140–D144. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, P.; Lu, Y.; Li, Y.; Zheng, Y.; Kan, Y.; Chen, R.; He, S. piRBase: A comprehensive database of piRNA sequences. Nucleic Acids Res. 2019, 47, D175–D180. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, S.; Li, C.; Ren, Y.; Wang, J. Novel expression profiles of microRNAs suggest that specific miRNAs regulate gene expression for the sexual maturation of female Schistosoma japonicum after pairing. Parasit Vectors 2014, 7, 177. [Google Scholar] [CrossRef]
- Anders, S. Analysing RNA-Seq data with the DESeq package. Mol. Biol. 2010, 43, 1–17. [Google Scholar]
- Enright, A.J.; John, B.; Gaul, U.; Tuschl, T.; Sander, C.; Marks, D.S. MicroRNA targets in Drosophila. Genome Biol. 2003, 5, R1. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Q.; Zhao, N.; Ru, X.; Hao, R.; Zhang, B.; Zhu, C. Sex-Inclined Piwi-Interacting RNAs in Serum Exosomes for Sex Determination in the Greater Amberjack (Seriola dumerili). Int. J. Mol. Sci. 2023, 24, 3438. https://doi.org/10.3390/ijms24043438
Deng Q, Zhao N, Ru X, Hao R, Zhang B, Zhu C. Sex-Inclined Piwi-Interacting RNAs in Serum Exosomes for Sex Determination in the Greater Amberjack (Seriola dumerili). International Journal of Molecular Sciences. 2023; 24(4):3438. https://doi.org/10.3390/ijms24043438
Chicago/Turabian StyleDeng, Qiuxia, Na Zhao, Xiaoying Ru, Ruijuan Hao, Bo Zhang, and Chunhua Zhu. 2023. "Sex-Inclined Piwi-Interacting RNAs in Serum Exosomes for Sex Determination in the Greater Amberjack (Seriola dumerili)" International Journal of Molecular Sciences 24, no. 4: 3438. https://doi.org/10.3390/ijms24043438
APA StyleDeng, Q., Zhao, N., Ru, X., Hao, R., Zhang, B., & Zhu, C. (2023). Sex-Inclined Piwi-Interacting RNAs in Serum Exosomes for Sex Determination in the Greater Amberjack (Seriola dumerili). International Journal of Molecular Sciences, 24(4), 3438. https://doi.org/10.3390/ijms24043438




