Abstract
Intranasal (IN) drug delivery is a non-invasive and effective route for the administration of drugs to the brain at pharmacologically relevant concentrations, bypassing the blood–brain barrier (BBB) and minimizing adverse side effects. IN drug delivery can be particularly promising for the treatment of neurodegenerative diseases. The drug delivery mechanism involves the initial drug penetration through the nasal epithelial barrier, followed by drug diffusion in the perivascular or perineural spaces along the olfactory or trigeminal nerves, and final extracellular diffusion throughout the brain. A part of the drug may be lost by drainage through the lymphatic system, while a part may even enter the systemic circulation and reach the brain by crossing the BBB. Alternatively, drugs can be directly transported to the brain by axons of the olfactory nerve. To improve the effectiveness of drug delivery to the brain by the IN route, various types of nanocarriers and hydrogels and their combinations have been proposed. This review paper analyzes the main biomaterials-based strategies to enhance IN drug delivery to the brain, outlining unsolved challenges and proposing ways to address them.
1. Introduction
Pathologies affecting the brain tissue, such as neurodegenerative diseases, brain tumors and stroke, need the local administration of drugs for treatment. However, the effectiveness of drug administration to the brain is hampered by the presence of the blood–brain barrier (BBB), which is the biological barrier that regulates the transport of molecules between the blood and the brain. The BBB is constituted by the brain microvascular endothelium (formed by a monolayer of endothelial cells) with its basement membrane, pericytes surrounding the endothelium and astrocytes mediating the interaction between neuronal cells and oligodendrocytes with the brain capillaries. The BBB has a selective permeability, which is fundamental for normal brain physiology [1,2]. The interplay among endothelial cells, pericytes and astrocytes regulates BBB integrity in vivo as well as in vitro [3,4]. Hence, the BBB protects the brain from the entry of potentially toxic substances, however, it also prevents the delivery of therapeutics into the central nervous system (CNS) for disease treatment. The BBB shows a low level of pinocytosis and possesses tight junctions, which form a seal between opposing endothelial membranes. The presence of tight junctions causes a high transendothelial electrical resistance of 1500–2000 Ω·cm2 compared to 3–30 Ω·cm2 in the peripheral microvasculature [5]. For these reasons, the BBB highly restricts paracellular diffusion of solutes from the blood into the brain. Typically, only small lipophilic molecules may cross the BBB via transcellular passive diffusion, although some limited transport of certain peptides and peptide analogs has been reported [6]. Transcellular active diffusion occurs through specific transporters or receptors, such as glucose transporter 1 for glucose and transferrin receptor for iron [2,5]. In addition, receptors and transporters for gastrointestinal hormones involved in regulating metabolism are expressed at the BBB in order to convey information between CNS and peripheral parts of the body [5]. Besides the low paracellular diffusion and low rate of pinocytosis, the endothelial layer of the BBB is also provided with efflux pumps (such as P-glycoprotein) which further restrict the entry of substances that would be otherwise predicted to cross the BBB based on their chemical characteristics and molecular weight [7].
Figure 1 reports the main transport mechanisms across the endothelial layer of the BBB.
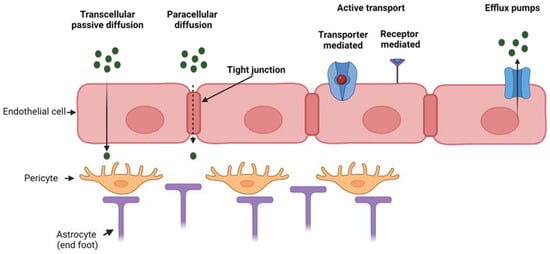
Figure 1.
Mechanism of transport through the BBB which is composed of three main cell types (endothelial cells, pericytes and astrocytes). Passive transport through transcellular or paracellular diffusion, active transport (either transporter- or receptor-mediated) and efflux active pumps. Created with BioRender.com.
Although a few low molecular weight drugs can cross the BBB, high molecular weight hydrophilic substances are severely restricted from crossing the BBB under normal conditions. Intraparenchymal, intracerebroventricular and intrathecal injections/infusions can be used to directly deliver therapeutics into the CNS [8], but these routes of administration are invasive and likely not practical for drugs which need to be given frequently for the treatment of chronic diseases.
Additional invasive methods include the temporary disruption of BBB integrity, e.g., by osmotic shock to the endothelial layer by mannitol administration or by an ultrasound disruption technique [8]. Such methods are costly, require long-term hospitalization and are associated with several drawbacks related to enhanced BBB permeability, such as neuron damage by cytotoxic molecules crossing the BBB.
Alternative strategies include a chemical modification of drugs to enhance their lipophilicity without affecting their activity or the introduction of hydrophilic molecules into nanomicelles with a hydrophobic shell, e.g., based on polaxamer-type copolymers [8]. Further “physiological approaches” are possible exploiting the mechanisms involved in the transport of metabolites and catabolites across the BBB, i.e., transcytosis mechanisms [8]. For example, it is possible to incorporate drugs within nanocarriers surface functionalized with ligands interacting with insulin or transferrin receptors on the endothelial cell layer of the BBB. One main disadvantage is that such transcytosis mechanisms are not specific of the BBB, therefore part of the drug-loaded nanocarriers may reach different tissues than the CNS with possible side effects. Moreover, systemic delivery of drug-loaded nanocarriers to reach the brain by intravenous or oral administration is challenging due to the hepatic first pass metabolism effect reducing drug half-life [9].
Hence, intranasal (IN) delivery has emerged as a non-invasive and direct route for drug administration to the brain bypassing the BBB and allowing rapid brain localization [10]. The benefits afforded by IN delivery, especially the removal of systemic side effects and the possibility to deliver biologics to the brain (peptides, proteins, oligonucleotides and even cells), places it as a potentially powerful route for brain disorder treatment [10]. However, the main limitations of IN delivery of drugs include: (i) loss of non-absorbed drugs in the respiratory and digestive tracts with potential side effects; (ii) rapid muco-ciliary clearance increasing drug loss; (iii) low nasal epithelium permeability for macromolecular and hydrophilic drugs; (iv) nasal mucosa metabolism of drugs by proteolytic enzymes. To bypass these limitations, new advanced IN drug delivery systems are required with the following characteristics: (i) high encapsulation efficiency, (ii) ability to protect the drugs from degradation/denaturation, (iii) ability to favor drug retention at the nasal mucosa and (iv) ability to promote drug transport through the nasal epithelium to reach the brain tissue. Several types of nanoparticles and hydrogels have been developed to achieve these aims in order to increase the effectiveness of the drug transport by the IN route. This review is intended to explain the general mechanism for drug delivery to the brain by the IN route, and how this has been enhanced by the design and use of nanoparticles and hydrogels as drug carriers. Research efforts aimed at improving efficiency of IN drug carrier systems are discussed.
2. Overview of Nasal Anatomy
Since ancient time, drugs have been delivered though the nasal cavity for local and systemic drug delivery. For instance, in Ayurveda, the traditional Indian medicine, Nasya therapy, one of the Panchakarmas, is the process by which a medicine (in form of decoctions, oils and fumes) is intranasally administered [11].
The nose allows the entrance of air into the body during respiration: its main function is to filter, warm and humidify air. Moreover, the nose provides an immunological barrier to protect the nasal cavity from irritations and infections and it is the seat of the olfactory sense. The nasal cavity has a total volume of 16–19 mL [12] and it is composed of five main regions (Figure 2): the vestibule, the atrium, the olfactory region, the respiratory zone and the nasopharynx.
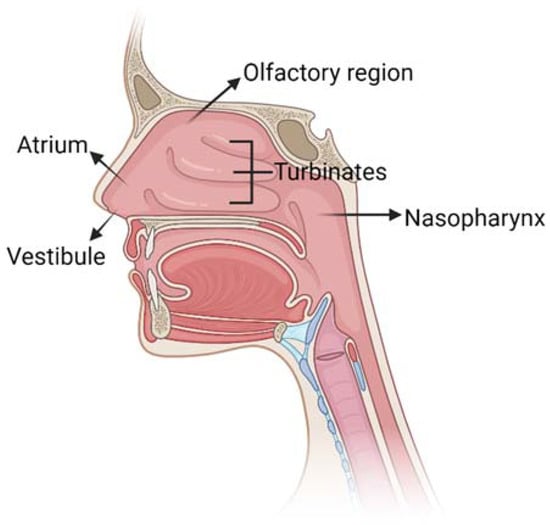
Figure 2.
Schematic representation of nose anatomy with its five main regions: vestibule, atrium, turbinates in the respiratory region, olfactory region and nasopharynx. Created with BioRender.com.
The respiratory and olfactory regions are the ones involved in IN drug delivery due to their superior permeability and vascularization compared to the other nasal sites. The respiratory and olfactory regions have an extent of around 160 cm2 and 15 cm2, respectively [5]. The olfactory epithelium is composed of different cells (Figure 3): (i) the olfactory receptor cells provided with non-motile cilia; (ii) the supporting cells and (iii) the basal cells [13]. Moreover, it is coated with a mucus layer produced by the Bowman’s glands. The olfactory receptor cell is a bipolar neuron, forming an amyelinated axon at its basal surface, conveying olfactory information to brain. In its apical surface, each olfactory receptor develops a unique dendritic process that expands into a protuberance with several microvilli called olfactory cilia. Olfactory cilia are coated with a mucus layer: when, during a cold, the mucus layer thickens, olfactory sensibility decreases [14]. Amyelinic fibers and blood vessels run along the basal part of the mucosa, called lamina propria.
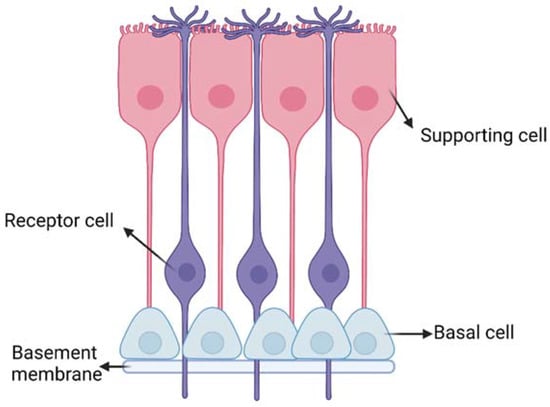
Figure 3.
Schematic representation of the olfactory epithelium with the three main cells: (i) the olfactory receptor cells provided with non-motile cilia; (ii) the supporting cells and (iii) the basal cells. Created with BioRender.com.
The respiratory epithelium (Figure 4) is composed of: (i) ciliated pseudostratified columnar epithelial cells (with around 100 cilia per cell); (ii) non-ciliated cells; (iii) goblet cells and (iv) basal cells. Each ciliated and non-ciliated cell possesses around 300 microvilli [15]. The respiratory epithelium is coated with a mucus layer and its cilia are responsible for muco-ciliary clearance, the protective mechanism of the respiratory system.
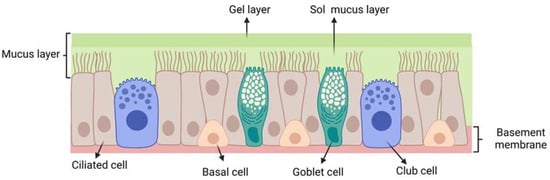
Figure 4.
Schematic representation of the respiratory epithelium: ciliated pseudostratified columnar epithelial cells; club cells (non-ciliated cells); goblet cells; mucus layer (gel layer); sol mucus layer; basal cells; basement membrane. Created with BioRender.com.
3. Mechanism for Drug Delivery to the Brain through the Intranasal Route
3.1. Mucus Layer: A First Barrier to Intranasal Drug Delivery
A first barrier for intranasal drug transport is represented by the mucus layer on the nasal epithelium. Mucus, mainly composed by mucin polysaccharide, is a hydrogel layer with intrinsic porosity and negative charges. Such features may slow down the drug diffusion rate through the mucus layer. When the drug diffusion rate across the mucus layer is lower compared to the muco-ciliary clearance rate, drug bioavailability decreases. It has been estimated that the muco-ciliary clearance half-time is around 15–30 min [16,17]. Clearance may be counteracted by the use of muco-adhesive drug carriers, as described in Section 5.
Furthermore, the nasal cavity has a slightly acidic pH (5.5–6-5) and contains enzymes that may catalyze the degradation of drugs, such as peptides and proteins, thus reducing drug bioavailability [18]. Typical approaches to address such issues are the use of enzyme inhibitors or drug doses above the saturation concentration of enzymes [19].
3.2. Transport of Drugs from Nasal Epithelium to the Brain
The precise pathways and mechanisms by which a drug travels from the nasal epithelium to various regions of the brain have not been fully elucidated. Lochhead et al. [5] and Thorne et al. [20] have described such mechanisms. Studies on [125I]-labeled proteins following IN administration in rats and monkeys have shown that delivery occurs along the olfactory and trigeminal nerve components in the nasal epithelium to the olfactory bulb and brainstem, respectively, with further dispersion to other brain areas [20]. At least three sequential transport steps are necessary for drugs to be delivered to the brain following IN administration [5].
3.2.1. Transport across the Olfactory and Respiratory Epithelial Barriers
Transport across the olfactory or respiratory epithelia may occur either by intracellular or extracellular mechanisms.
Intracellular pathways are activated by the olfactory sensory neurons, the trigeminal nerve and the nasal epithelial cells and include two different mechanisms depending on the involved cells: (i) endocytosis by the neural cells on the nasal epithelial surface and subsequent intraneuronal transport; (ii) transcytosis (i.e., transcellular transport) across the cells of the respiratory and olfactory epithelium to the lamina propria.
Extracellular transport pathways include paracellular diffusion across the olfactory and the respiratory epithelia to the underlying lamina propria. Paracellular transport depends on the presence of tight junctions in the olfactory and respiratory epithelia: tight junction tightness and continuity determine the permeability to paracellular transport. It has been suggested that the regular turnover of cells in the nasal epithelium may lead to continuous rearrangement and loosening of tight junctions which favors paracellular transport of drugs [5].
3.2.2. Transport from the Nasal Mucosa to the Sites at Brain Entry
This transport may occur via intracellular pathways (intraneuronal transport through endocytosis within olfactory sensory neurons or trigeminal ganglion cells) or extracellular pathways (diffusion or convection within perineural, perivascular or lymphatic channels, associated with olfactory or trigeminal nerve bundles extending from the lamina propria to the brain). In the case of substances reaching the extracellular space, different fates are possible: (i) absorption into blood vessels, entering the systemic circulation; (ii) absorption into lymphatic vessels reaching neck cervical lymph nodes; (iii) extracellular diffusion or convection in perineural or perivascular nerve bundles spaces, leading to access to the cranial site. Drugs absorbed into the systemic circulation should cross the BBB or blood–cerebrospinal fluid (CSF) barrier to reach the brain. Hence, the nasal vasculature may act as a sink hindering some molecules from reaching the brain. Drugs that have reached the lamina propria and escaped the local absorption into the blood stream and drainage within nasal lymphatics may enter the brain tissue. Studies have shown that drugs can be transported in the spaces of the perineural sheath surrounding the olfactory nerve [5]. Moreover, although tight junctions are present, olfactory ensheathing cells maintain open spaces for the regrowth of olfactory nerve fibers, creating an additional extracellular path that substances may take to reach the brain, along with entering the olfactory nerve bundles. Finally, the perineural spaces of cranial nerves, such as the olfactory and trigeminal nerves, appear to allow communication with CSF of the subarachnoid space for some substances, providing an additional route for molecules to reach the brain [5].
3.2.3. Transport from the Initial Brain Entry Sites to Other Brain Areas
Once the drug has reached the olfactory bulb and brainstem, it is distributed to other brain areas by two distinct possible mechanisms: (i) intracellular transport, by drug transfer to neurons forming a synapsis with peripheral olfactory sensory neurons or trigeminal ganglion cells; (ii) extracellular transport, through distribution within the cerebral perivascular spaces into the parenchyma. For instance, in the case of [125I]-labeled proteins such as insulin-like growth factor 1 (IGF-1) and interferon-β1b (INF-β1b), a convective mechanism within perivascular spaces of the cerebral blood vessels caused their distribution to different brain sites [20]. Expansion and contraction of perivascular spaces with the cardiac cycle may generate a pronounced fluid flow within them under normal conditions. Although modeling studies have been performed to understand the direction and characteristics of this flow, different results have been obtained and predictions of drug distribution in the brain are still a challenge [21,22]. However, increased blood pressure and heart rate have been demonstrated to improve the intraparenchymal distribution of large substances via the perivascular spaces [23]. Another study has also found that the rostral migratory stream, the pathway used by neuronal progenitors to migrate from perivascular regions to the olfactory bulb, may also play a role in the delivery of molecules from the nasal cavity into the brain [24].
Among the possible transport mechanisms to the brain, the direct axonal transport has been found to be incompatible with the short measured time taken by intranasally administered drugs to reach the brain [25]. Such findings have demonstrated that direct axonal transport is not the main route for IN drug delivery to the brain.
3.3. Synthesis of the Main Features of Intranasal Drug Transport to the Brain
Figure 5 is a schematic representation of the overall mechanism for IN drug delivery to the brain.
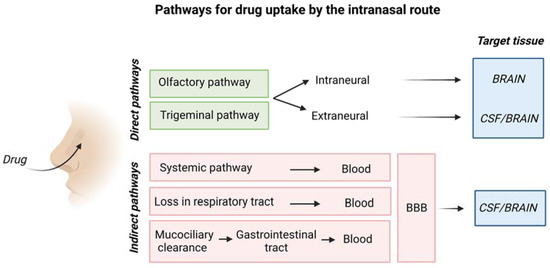
Figure 5.
Schematic representation of drug uptake by the intranasal route. Created with BioRender.com.
The olfactory pathway conveys drugs directly to the olfactory bulb, whereas the trigeminal nerve enters the CNS in the pons transporting drugs to cerebrum and cerebellum [26].
Table 1 describes advantages and disadvantages of the IN drug delivery route.

Table 1.
Summary of main advantages and disadvantages of the IN route for drug delivery to the brain.
The degree of drug targeting to the brain after intranasal (IN) administration with respect to intravenous (IV) administration can be assessed by the drug targeting efficiency (DTE %) and the direct transport percentage (DTP %) [26]:
AUC is the area under the drug concentration–time curve.
BIN is the AUC0–24h (brain) following intranasal administration.
BX is the brain AUC fraction from systemic circulation after intranasal administration.
BIV is the AUC0–24h (brain) following intravenous administration.
PIN is the AUC0–24h (blood) following intranasal administration.
PIV is the AUC0–24h (blood) following intravenous administration.
DTE% is used to indicate the tendency of the drug to accumulate in the brain when administered via the IN route compared to IV administration. Values above 100% evidence a higher efficacy of the IN compared to IV route. DTP% refers to the quantity of drug entering the brain through direct pathways (i.e., trigeminal and olfactory pathways). When DTP% is higher than zero, drug brain targeting is reached via the direct pathways, while for values below 0 the IV route is more effective compared to IN administration. Finally, a DTP% of 100 can only be achieved if the drug is not adsorbed by the blood circulation after IN administration (i.e., PIV: AUC0–24h (blood) is zero) or if the drug is not able to cross the BBB (i.e., BIV: AUC0–24h (brain) is zero) [27,28].
Alternatively, the percentage of drug accumulation in the brain (bioavailability, B%) can be evaluated considering only the AUC data for the brain areas, without including AUC for blood:
As for the DTE, a higher accumulation of the drug in the brain is represented by B% values above 100.
When nanocarriers are employed, the relative bioavailability (RB%) can be evaluated, comparing the IN drug delivery through nanoparticles with respect to the delivery of the drug in solution form.
Values of RB% above 100 indicate a higher accumulation of drug in the brain with the use of the nanocarriers compared to the “naked” drug.
Moreover, RDTE% and RDTP% can also be utilized to evaluate the effect of the nanocarriers in drug administration through the IN route.
It has been found that an efficient delivery to the brain by the intranasal route also depends on the head position, type of used formulation and delivery device [10].
3.4. Prevalent Transport Mechanisms for Hydrophilic Drugs by the Intranasal Route
Intranasally administered drugs mainly cross the nasal epithelium barrier by a transcellular or paracellular mechanism (rather than by direct axonal transport) and, then, a portion of them is successfully transported to the brain, by diffusion in the perivascular or perineural spaces along the olfactory or trigeminal nerves. However, transport mechanisms depend on drug chemistry. A paracellular mechanism is possible for polar drugs with molecular weight lower than 1000 Da, allowing the diffusion thorough the 10 Å sized channels of transiently opened tight junctions in the nasal epithelium [29]. A polar drug with molecular weight higher than 1000 Da, such as polypeptides and proteins, can pass the nasal membrane by endocytic transport, although in low amounts [29]. For high molecular weight hydrophilic drugs, absorption enhancers can be used, including surfactants, bile salts and their derivatives, fatty acids and their derivatives, phospholipids, various cyclodextrins and cationic molecules (e.g., poly(lysine) or chitosan and its derivatives) [29], as explained in detail in the next section. Briefly, absorption enhancers increase the permeability of the epithelial cell membrane by different mechanisms depending on the type of enhancer, for example, increasing transcellular transport [29]. Moreover, they can also improve paracellular transport by promoting the transient opening of tight junctions. However, due to their interaction with the nasal epithelium layer, absorption enhancers can elicit cytotoxic effects towards the nasal mucosa, especially in the case of repeated drug administration for the treatment of chronic diseases [30,31].
4. Penetration Enhancers in IN Drug Delivery
Penetration enhancers have been proposed for IN drug delivery formulations: they can be components of the drug carriers or additives of the drug formulations, and can be used alone or in combination to exploit synergistic effects. A range of surfactants have been investigated using both synthetic (e.g., sodium lauryl-sulfate) and naturally derived (e.g., BC9-BS biosurfactant isolated from Lactobacillus gasseri BC9) materials to improve drug penetration by inducing membrane rupture or improving the fluidity of the membrane, then favoring transcytosis [32,33]. Non-ionic alkyl saccharide surfactants, including dodecyl maltoside (Intravail®), have also been associated with the possibility to enhance drug penetration by transcellular transport involving cellular internalization of drugs into vesicles, although the mechanism of action is still under investigation [34]. Nonetheless, dodecyl maltoside is commercially available in US FDA-approved nasal sprays for migraine (Tosymra®) and seizure clusters (Valtoco®).
Cationic polymers (e.g., chitosan or poly-L-arginine) have been shown to interact with the negatively charged cellular residues, reducing transepithelial resistance and promoting tight junction opening, which affects paracellular transport [35,36]. Tight junctions opening can also be favored by a reduction of endogenous calcium ions through the use of calcium chelators (e.g., ethylenediaminetetraacetic acid, EDTA) or anionic poly(acrylic acid) polymers (e.g., Carbapol®) able to bind cations [33,37]. Finally, the use of peptide-mimicking toxins has also been investigated to modulate the function of tight junctions. The C-terminal fragment of an enterotoxin derived from the Gram-positive bacterium Clostridium perfringens (C-CPE) has been shown to increase paracellular transport of nasal pneumococcal vaccine by binding with claudins, proteins involved in tight junction formation [38,39]. The peptide AT1002 is an analog of Vibrio cholera toxin, acting on zonula occludens in tight junctions. Peptide AT1002 enhances IN permeation by binding its receptor reversibly and opening tight junctions [40].
Mucolytic agents constitute another class of penetration enhancers for IN delivery able to decrease the viscosity of mucus. N-acetyl-l-cysteine is a widely used mucolytic agent able to cleave the disulfide bonds responsible for mucin fiber crosslinking, increasing mucus mesh size and permeability [18].
5. Biomaterials-Based Vehicles for IN Drug Delivery
Biomaterials-based delivery systems, consisting of microparticles, nanoparticles and hydrogels, offer the possibility to improve the efficiency of intranasal drug delivery to the brain, as they may protect drugs from degradation, increase their absorption into and transport across the nasal epithelium (while decreasing drug loss in the respiratory and digestive tracts during administration) and—in the case of tailor-designed nanoparticles—favor brain targeting.
As mucus represents the first physical barrier for drug absorption by the nasal mucosa, specific chemical and physical features of the mucus layer have to be taken into account for the design of efficient IN drug delivery systems. A thin mucus layer of approximately 10–15 µm in thickness covers the nasal epithelium, consisting of a highly hydrated polymeric network secreted by the goblet cells in the mucosa, mainly composed by water (around 95 wt.%) and mucins (2–5 wt.%), glycoproteins containing sialic acid units. Previous studies have reported different values of mucus hydrogel mesh size (50–1800 nm), however, the average size of mucus intrinsic pores is considered to be 20–200 nm [41]. Besides water and mucins, mucus also contains various amounts of DNA, plasma proteins, immunoglobulins (particularly secretory IgA), lysozyme, lactoferrin, lipids and polysaccharides [41]. The pH of mucus is slightly acidic (pH 5.5–6.5) [42]. Furthermore, mucus is continuously propelled towards the pharynx by the cilia present in the respiratory mucosa, at a rate of 5 mm/min [43] with an average clearance time of around 15–30 min [16,17]. Muco-adhesivity, i.e., the ability to attach to the mucus, has been frequently proposed as a key feature for efficient polymeric drug carriers in the IN route. Different theories have been exploited to explain muco-adhesivity, as reviewed by Khutoryanskiy et al. [42]. In brief, muco-adhesion can arise from electrostatic interactions between positively charged materials in contact with negatively charged mucins (electronic theory). Additionally, hydrogen and van der Waals bonding or hydrophobic interactions can be established contributing to muco-adhesion (absorption theory). Based on the wetting theory, biomaterial adhesion to mucus layer is promoted by the ability of the drug formulation to wet and spread over the mucus layer. According to the diffusion theory, an interpenetration between macromolecules and the mucin network is responsible for muco-adhesion. Furthermore, the mechanical theory predicts that muco-adhesion depends on the contact area between the biomaterial and the mucus layer, which in turns depends on the carrier surface roughness. Finally, the fracture theory predicts that muco-adhesion is due to chemical compatibility between mucus and the biomaterial, leading to an interface resistant to relative detachment. Based on that, muco-adhesivity is typically a property of polymers with positively charged chains, such as chitosan, able to interact with negatively charged mucins or able to form hydrogen bonds or other secondary interactions (e.g., hydrophobic interactions) with mucins. On the other hand, non-adhesive materials include antifouling polymers, such as poly(ethylene glycol) (PEG). A review article by Sosnik et al. has discussed the main muco-adhesive polymers for drug delivery, including chitosan, cellulose derivates and poly(acrylic acid) and poly(methacrylic acid) derivatives [41].
5.1. Microparticles and Nanoparticles for IN Drug Delivery
Drug-loaded polymer microparticles, having sizes larger than 500 nm, are a suboptimal choice for IN drug delivery, as they are unable to diffuse through the smaller nanometric pores of the mucus layer (Figure 6). However, muco-adhesive microparticle with sizes in the 500 nm–10 µm range, escaping the filtration by the nasal vestibule, have been proposed for IN drug delivery, exploiting their ability to bind to the mucus layer [44,45]. Their drug delivery efficiency depends on the ability of loaded drugs to diffuse through the microparticles and the nasal mucus layer, reaching the nasal epithelium in a shorter time than that required for muco-ciliary clearance. Furthermore, in this application, drugs should be resistant to degradation and able to cross the nasal epithelium, without the help of biomaterial carriers.
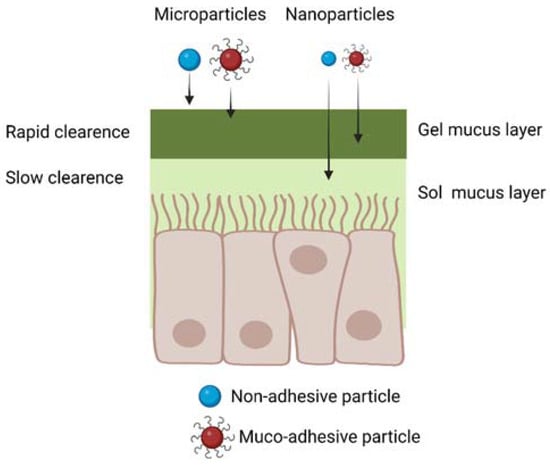
Figure 6.
Schematic representation of micro- and nanoparticle diffusion through the nasal mucus considering both muco-adhesive and non-muco-adhesive particles. Created with BioRender.com.
On the other hand, drug-loaded nanoparticles are more advantageous than microparticles, due to their ability to penetrate and diffuse through the nasal mucus (Figure 6), and to support all the subsequent phases of drug transport to the brain, previously described in Section 3. Muco-adhesive nanoparticles have been frequently proposed for IN drug delivery, as they attach to the nasal mucus after administration, decreasing the possibility for their loss in the respiratory and digestive tracts [45,46]. On the other hand, muco-adhesive nanoparticles show limited drug delivery efficiency: as they slowly diffuse across the mucus layer, they are partially lost during the muco-ciliary clearance mechanism. As a solution, antiadhesive nanoparticles weakly interact with the mucus layer: although this property increases the quantity of nanoparticles lost in the digestive and respiratory systems post-administration, it increases nanoparticle diffusion rate across mucus. For this reason, antiadhesive nanoparticles have also been proposed for IN drug delivery [47,48,49,50].
Table 2 collects exemplary types of nanocarriers exploited for IN drug delivery.

Table 2.
Main nanocarriers exploited in IN drug delivery to treat brain pathologies.
Table 2 evidences that nanocarriers for IN drug delivery have been prepared from both natural materials and synthetic polymers. Among natural polymers, gelatin nanoparticles have been widely employed due to their biocompatibility, biodegradability, low immunogenicity and possibility for surface functionalization [70]. Frequently, gelatin nanoparticles have been prepared in combination with Poloxamer 188 to reduce mucus viscosity and elasticity, and modulate tight junction opening [58,71]. Chitosan nanoparticles have also been widely exploited for IN drug delivery [36,72]. Chitosan is a cationic polysaccharide able to induce tight junction opening, favoring paracellular transport of drugs when used in the form of nanoparticles, hydrogel or nasal solution [36,72].
Among synthetic polymers, poly(lactic-co-glycolic acid) (PLGA) copolymer has been widely investigated as a biocompatible and biodegradable material for the production of numerous drug delivery formulations, enabling encapsulation and controlled release of both hydrophobic and hydrophilic drugs [73]. Chitosan coating of PLGA-based nanoparticles has been mainly investigated to introduce muco-adhesive properties [55,56].
Lipid-based nanocarriers have also been investigated for IN drug delivery thanks to their low toxicity, biocompatibility, biodegradability and flexibility. In particular, second-generation lipid carriers, solid lipid nanocarriers (SLNs) and nanostructured lipid carriers (NLPs) have been mainly studied [56,62,63,69]. SLNs are composed of a solid lipid core containing the drug stabilized by surfactants, while NLPs possess a solid lipid core with a surfactant outer shell. SLNs and NLPs have shown higher drug loading, better drug stability and prolonged release profile compared to liposomes [74,75]. Finally, exosomes, naturally derived nanocarriers secreted by cells, have recently attracted interest as drug delivery systems for the treatment of brain disorders through IN administration thanks to their low immunogenicity, good biocompatibility, possibility of functionalization for targeted action and broad spectrum of endogenous and exogenous bioactive cargo molecules, including protein, nucleic acids, growth factors and therapeutic agents [65,67,76]. Interestingly, the nanocarrier surface has been frequently functionalized with:
- Muco-adhesive polymers such as chitosan or polymer containing thiol groups (thiomers);
- Molecules for adsorption endocytosis by the epithelial layer (e.g., lectins, cell-penetrating peptides, such as penetratin, Tat peptide, etc.);
- Molecules for ligand-mediated endocytosis (e.g., lactoferrin);
- Mucus-penetrating (non-adhesive) polymers (e.g., PEG).
Muco-adhesive functionalities have been used to decrease the muco-ciliary clearance time, increasing IN drug release efficiency. In some cases, the muco-adhesive molecules have additional functionalities, e.g., chitosan is able to enhance drug passage through the nasal mucosa by transiently opening the tight junctions connecting the epithelial cells as previously underlined. Thiomers have also been investigated for their muco-adhesive properties thanks to their interactions with cysteine residues of mucus glycoproteins [60,77].
Lectins are proteins able to reversibly bind mono-sugars or oligosaccharides, promoting both muco-adhesion and endocytosis [78,79]. Different types of lectins have been tested for surface functionalization of nanoparticles for IN drug delivery. Gao et al. reported the conjugation of WGA onto PEG-PLA particles [52,80], showing their enhanced brain targeting ability with respect to unfunctionalized particles, or intranasally or intravenously administered drug solutions. Gao et al. have also conjugated wheat germ agglutinin to PEG-PLA particles as it binds to L-fucose in the olfactory epithelium, enhancing drug release to the brain [52]. Chen et al. coupled STL to PLGA nanoparticles with a 1.89–2.45-fold increase in brain targeting [81]. STL-conjugated PEG-PLGA nanoparticles loaded with basic fibroblast growth factor (bFGF) enhanced brain delivery compared to intravenous injection of bFGF, and IN administration of bFGF solution and bFGF-loaded PEG-PLGA nanoparticles. The superior DTP % values of STL-conjugated PEG-PLGA nanoparticles indicated that more than 70% of the drug was directly delivered to the brain by the IN route [26].
Cell penetrating peptides have shown enhanced nose-to-brain transport [82,83]. Wan et al. have identified specific peptides for nose-to-brain drug delivery by the phage display method [82]. Yang et al. prepared liposomes loaded with rivastigmine and surface functionalized with a cell-penetrating peptide [64]. Zhai et al. modified the surface of exosomes with rabies virus glycoprotein (RVG) peptide able to specifically bind to neuronal acetylcholine receptor for the delivery of brain-derived neurotrophic factor for multiple sclerosis applications [67]. Finally, Peng et al. proposed a novel nanocarrier system, characterized by a polymer micellar core composed of PPS−PEG, encapsulated in an exosome outer shell modified with penetratin and RVG peptides for the delivery of curcumin for Parkinson’s disease treatment [66].
Lactoferrin is a natural protein binding iron, that has the ability to interact with the lactoferrin receptors that are abundant on respiratory epithelial cells. The functionalization of drug-loaded nanoparticles with lactoferrin allows their absorption by epithelial cells through a transcytosis mechanism. Liu et al. prepared PEG-co-poly(ε-caprolactone) nanoparticles surface functionalized with lactoferrin and incorporating NAP (NAPVSIPQ) a neuroprotective peptide [51].
As mentioned above, non-muco-adhesive properties have also been investigated to develop systems able to penetrate through the mucus barrier without adhering to it [49,50]. PEGylation is the main modification investigated to develop mucus-penetrating systems. However, the molecular weight and density of charge are two main parameters that affect the adhesion ability of PEG, as recently reviewed by Lai et al. [84]. Low molecular weight PEG combined with a high PEG density favors penetration, due to the generation of an almost neutral surface able to minimize mucus interactions. On the contrary, high molecular weight PEG and a low density of coatings have been shown to induce muco-adhesive properties [46,84]. Porfiryeva et al. showed the higher penetration of PEGylated derivatives of polyelectrolyte complexes formed by oppositely charged Eudragits (i.e., anionic Eudragit® L100-55 and cationic Eudragit® EPO) compared to non-modified complexes [54]. Ways et al. investigated the possibility to create mucus-inert chitosan nanoparticles through grafting with PEG, PHEA, POZ and PVP. All the modified nanocarriers showed superior mucus penetration compared to unmodified chitosan with PVP showing the highest penetration depth in ex vivo sheep nasal mucosa [48].
5.2. Hydrogels for IN Drug Delivery
Hydrogels are three-dimensional crosslinked networks of hydrophilic polymers able to absorb and retain a considerable amount of water without dissolving, preserving their shape [85]. Hydrogels for IN drug delivery have been generally designed to be muco-adhesive in order to bind to the nasal mucosa. Hydrogels may also undergo some mixing with the mucus layer, facilitating drug penetration [86]. Moreover, IN administration of hydrogels affects the viscosity of the mucus–hydrogel systems, increasing the muco-ciliary clearance time and enhancing the effectiveness of IN drug uptake [87]. Finally, hydrogels can shield drugs from undergoing chemical and enzymatic degradation in the nasal cavity, prolonging their activity.
Hydrogels are generally sprayed into the nasal cavity or administered as solution drops, converting into a hydrogel upon contact with the nasal mucosa. Stimuli-responsive hydrogels have been generally used for IN drug delivery exploiting the following physical features of the nasal cavity: (1) pH 5.5–6.5; (2) temperature of 32 °C; (3) presence of sodium, calcium and potassium ions [86]. Hence, thermosensitive and pH- and ion-responsive hydrogels have been proposed for IN drug delivery as reviewed by Chonkar et al. and Protopapa et al. [86,88].
Table 3 collects exemplary types of promising hydrogel formulations for drug delivery to the brain by the IN route developed to date.

Table 3.
Main hydrogels exploited in IN drug delivery to treat brain pathologies.
5.3. Nanocarrier-Loaded Hydrogels for IN Delivery
As discussed in Section 5.1, both antiadhesive and muco-adhesive nanoparticles have been proposed for IN drug delivery. However, the potential advantages of muco-adhesive nanoparticles have not been confirmed by in vivo biodistribution trials. Indeed, studies in mouse models have shown that muco-adhesive chitosan-coated nanostructured lipid nanocarriers, loaded with protein drugs, although effective for drug delivery to the brain, mainly accumulated in the lungs, followed by the liver, the kidneys, the spleen and, finally, the brain [68]. These findings have prompted criticisms on the real benefits of muco-adhesive nanoparticles for IN drug delivery to the brain, as such nanoparticles could more easily reach different tissues with potential side effects.
To overcome these drawbacks, more complex pharmaceutical formulations could be exploited, based on hydrogels releasing drug-loaded nanoparticles. Both weakly muco-adhesive hydrogels, such as Poloxamer [98,99], and highly muco-adhesive hydrogels, such as chitosan, Poloxamer/gellan and Poloxamer/chitosan [100,101,102,103,104], have been proposed. Such formulations are collected and described in Table 4. The hydrogel should improve local nanoparticle retention, decreasing losses in the digestive and respiratory tracts. Furthermore, hydrogels may increase the muco-ciliary clearance time and improve nanoparticle uptake by the nasal epithelium.

Table 4.
Exemplary nanoparticle-loaded hydrogel formulations investigated for IN drug delivery to reach the brain.
Although different types of nanoparticles have been administered in combination with hydrogels, nanoparticles based on lipids and/or natural polymers are affected by limited stability and can be more easily disassembled/degraded in vivo with respect to nanoparticles based on synthetic polymers [105,106]. Hence, the type of hydrogel embedding nanoparticles should be carefully selected to avoid unfavorable interactions with lipid- or natural polymer-based nanoparticles, leading to their disaggregation in contact with the hydrogel.
Figure 7 schematically shows the routes for drug transport to the brain of intranasally administered drugs, mediated by the use of the above-described biomaterials (i.e., various types of NPs, hydrogels and NP/hydrogel systems). The olfactory and trigeminal pathways are highlighted as being the two main direct routes for drug delivery to the brain.
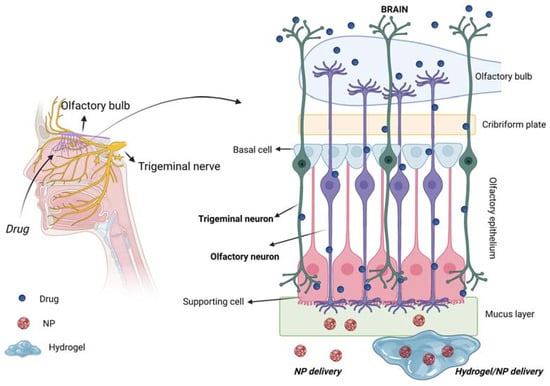
Figure 7.
Scheme of IN drug delivery to the brain following the two direct olfactory and trigeminal pathways for drug uptake by the brain. Created with BioRender.com.
6. Discussion
IN delivery represents a direct route for effective drug administration to the brain, particularly suitable for hydrophilic high molecular weight drugs, such as growth factors, which are not able to cross the BBB and can induce side effects in the peripheral tissues when systemically administered.
Kozlovskaya et al. analysed 73 publications between 1970 and 2014 concerning the quantitative analysis of the delivery of drugs or model agents to the brain via IN and parenteral routes. According to this literature investigation, the use of nanoparticles or hydrogel carriers for IN drug delivery has offered limited advantages with respect to drug solutions. On the other hand, compounds able to favor drug delivery through the IN route, such as absorption enhancers muco-adhesive compounds and targeting ligands have shown a higher impact on drug delivery effectiveness of IN therapy [107].
Particularly, drug-loaded nanoparticles have a key role in protecting drugs from enzymatic degradation and in efficiently transporting them across the nasal barrier to the brain through proper surface functionalities. Drugs crossing the nasal barrier are partially absorbed by the systemic circulation and hence not able to reach the brain (Figure 5). Their encapsulation into intranasally administered nanoparticles, provided with the additional ability to cross the BBB, could increase the efficiency of drug release to the brain. Additionally, nanoparticles could be functionalized with specific ligands potentially favoring drug release to specific brain cells. Among nanoparticles prepared from natural polymers or their derivatives (Table 2), chitosan-based nanoparticles have been widely used for IN delivery due to their muco-adhesive properties [36,72]. However, they do not have the intrinsic ability to cross the BBB or to reach target brain cells, unless properly functionalized [108]. Due to their hydrophilicity and consequent limited stability in physiological media, chitosan nanoparticles cannot be easily surface functionalized after their preparation. On the other hand, surface functionalization could be achieved through complex and laborious methods involving chemical derivatization of chitosan molecules before nanoparticle preparation [109]. One additional drawback of chitosan nanoparticles for protein or polypeptide drug encapsulation is the use of acidic solution for their preparation, with the risk of drug denaturation/degradation [110]. Similarly, nanocarriers provided with a lipid shell and a gelatin core are among the most widely exploited for IN drug delivery: they can be prepared in mild conditions and the lipid shell favors cell internalization (Table 3) [57,58]. However, their stability could be limited by rapid in vivo disassembly and gelatin degradation by proteolytic enzymes [75,111,112]. Unfunctionalized lipid–gelatin nanoparticles have been generally used for IN drug delivery, however, their surface functionalization with targeting ligands could be achieved by the use of previously synthesized ligand-functionalized lipids [113]. PEG-polyester nanocarriers (Table 2) represent another class of nanoparticles widely used for IN drug delivery, due to their biocompatibility, biodegradability and antifouling properties [51,52]. However, they also show a few limitations when applied for the release of protein drugs: (i) proteins can be loaded into PEG-polyester nanoparticles by emulsion methods, using organic solvents, which may affect protein bioactivity; (ii) surface functionalization of PEG-polyester nanoparticles with targeting ligands generally requires their incubation into functionalizing solutions, potentially causing partial protein drug release and/or denaturation/degradation; (iii) degradation of PEG-polyester nanoparticles by bulk hydrolysis may cause denaturation/degradation of loaded proteins before they reach their therapeutic target [114].
Overall, state-of-the-art analysis carried out in this review paper has shown that optimal nanocarriers for IN delivery of drugs (particularly proteins and polypeptides) to the brain have not yet been designed. Requirements for optimal nanoparticles for IN drug delivery to the brain include: high encapsulation efficiency, preparation using mild conditions, suitable size for diffusion through the intrinsic pores of the mucus layer, stability in contact with mucus and blood, antifouling surface functionalities (to facilitate diffusion through the mucus) coupled with specific ligands favoring transport through the nasal epithelium and subsequent brain targeting. One additional requirement is nanoparticle stability in the presence of an additional hydrogel exploited to increase IN drug delivery efficiency. Indeed, the combination of drug-loaded nanocarriers and hydrogels could enhance drug absorption by the nasal mucosa, decreasing the amount of nanocarriers lost in the gastrointestinal and respiratory systems. However, previous research studies have suggested that hydrogels need to be properly designed to avoid the risk of slowing down the diffusion ability of polymer nanocarriers to reach the nasal epithelium, with a consequent decrease in nanocarrier uptake [115]. Intermolecular interactions between the nanocarrier surface and the hydrogel should be weak while hydrogel (as well as hydrogel/mucus) mesh size should be larger compared to nanocarrier diameter.
Based on our recent research work (unpublished), we have found that polymer nanocarriers with a neutral surface are generally stable and undergo sustained release from hydrogels at a rate depending on nanocarrier–hydrogel reciprocal interactions and mesh size. Conversely, positively charged nanocarriers should be embedded in hydrogels with neutral charge to avoid their collapse within the hydrogel with consequent premature cargo release. Hydrogel composition should also be selected considering its possible positive effect as an enhancer for nanocarrier uptake by the nasal epithelium.
More broadly, the design of efficient systems for IN drug delivery to the brain should ensure:
- Therapeutic concentrations of drugs to the brain within a short time from the administration (~30 min), with prompt therapeutic benefits for the patients.
- Improved drug bioavailability avoiding hepatic first pass metabolism.
- Reduced side effects due to the lack of accumulation into non-target tissues, such as the liver, or the possibility to avoid gastroprotective drugs (needed for orally administered drugs in the treatment of chronic diseases).
- Patient-compliant treatment exploiting a minimally invasive route.
- Reduction of therapy costs due to enhanced effectiveness including brain-targeting ability.
- Avoidance of local and systemic toxicity, which is frequently associated with long-term treatment of chronic diseases.
- Improvement of patient’s quality of life, by an effective drug administration route.
IN therapy could be applied for the treatment of several brain pathologies, including neurodegenerative diseases, stroke and brain tumors. To date, IN drug formulations for specific treatment of brain pathologies are missing on the market or still at the stage of clinical trials. One major example is represented by the IN drug delivery of insulin, investigated in the SNIFF clinical trials as a possible treatment for cognitive defects in patients affected by Alzheimer’s disease (clinicaltrials.gov: NCT03857321, NCT00438568, NCT01767909). Clinical trials have shown side effects of IN delivery of insulin formulations, such as nosebleeds and rhinitis [116]. Indeed, all the tested insulin formulations (Detemir, Humalog, Apidra, etc.) made use of excipients, such as cresol, meta-cresol and phenol, responsible for rhinitis, nosebleeds and allergic reactions [117]. Additionally, insulin formulations have shown effectiveness in improving the cognition of adults with mild cognitive impairment or early-stage Alzheimer’s disease [118]. On the contrary, they have failed to impact on cognition in individuals with mild–moderate Alzheimer’s disease carrying the ε4 allele of apolipoprotein E, an established risk factor for late-onset Alzheimer’s disease [119,120]. These findings suggest that although insulin may have some therapeutic effect in the Alzheimer’s disease treatment, improved IN insulin delivery formulations are needed. Phase 2 and 3 controlled trials have been recently completed, investigating the efficacy of two IN insulin devices (ViaNase by Kurve Technology and I109 Precision Olfactory Delivery by Impel NeuroPharma) on patients with mild cognitive impairment or Alzheimer’s disease over 18 months. Preliminary results suggested a better performance of the ViaNase device, showing higher cognitive performance scores and Alzheimer’s disease biomarker production. However, possible differences in the dosage delivered by the two systems might have led to the discrepancies in the results [121,122], leading to the set-up of a new clinical trial, currently ongoing, to evaluate the specific dosage of insulin delivered by the devices (SNIFF Device, clinicaltrials.gov: NCT01767909).
One main problem in the design of intranasal drug delivery formulations is their preclinical validation through poorly predictive in vivo mouse and rat models [123,124]. Recently, physiologically relevant in vitro models of the human nasal epithelium have been developed and are commercially available for preclinical investigations. For example, EpiNasal™ by MatTek Life Sciences mimics the nasal epithelium structure by an air–liquid interface coculture of epithelial cells and mucus-producing goblet cells with functional tight junctions and beating cilia. Moreover, Gholizadeh et al. developed a first nasal epithelial mucosa (NEM)-on-a-chip model able to measure in real time the barrier integrity (obtained through transepithelial electrical resistance, TEER, measurements) and the drug transport (e.g., ibuprofen transport, chosen as a model agent) across a human nasal cell layer cultured at the ALI interface in both static and dynamic (via pulsatile systemic circulation in the basolateral compartment) conditions [125]. Capuana et al. developed a perfusion bioreactor system mimicking the nasal mucosa, characterized by a scaffold with an inner channel allowing cells to be in contact with both cell culture medium and air under dynamic conditions [126]. However, micro- or milli-fluidic devices recapitulating drug transport through the nose to reach the brain would be required to improve the design of optimal drug formulations for IN drug delivery to the brain, allowing better preclinical validation studies and reduction in the use of animal experimentation, according to the principles of replacement, reduction and refinement (the 3Rs).
7. Conclusions
This review paper highlighted the advantages of IN drug delivery systems, for their potential ability to provide a direct, rapid and effective route for drug delivery to the brain for the treatment of cancer, neurodegenerative diseases, stroke and other pathologies (e.g., migraine). The knowledge of the routes taken by intranasally administered drugs, together with the main biological barriers involved in the transport, is fundamental for the design of biomaterials-based carriers able to efficiently deliver drugs to the brain. Although different delivery vehicles have been proposed, more research is needed to improve drug delivery efficiency, decreasing side effects, both locally and systemically. Multifunctional formulations based on hydrogels encapsulating drug-loaded nanocarriers could represent the solution and their composition should be optimized based on the physicochemical properties of drugs, nanocarrier release kinetics and optimal adsorption by the nasal epithelium. These efforts require the parallel development of improved in vitro models for accurate preclinical evaluation before clinical trials.
The increasing incidence of brain pathologies as a consequence of progressive aging makes urgent the need for effective IN drug delivery nanoformulations. IN drug delivery systems targeting the brain tissue have the potentiality to address societal challenges, providing effective treatments for age-related diseases, including neurodegenerative diseases and brain cancers.
Author Contributions
V.C. and E.M. wrote the manuscript. V.C. and E.M. conceived the manuscript. V.C. supervised, reviewed the manuscript and acquired funding. All authors have read and agreed to the published version of the manuscript.
Funding
The project has received funding from the “Smart Injectable Drug-Delivery systems for Parkinson’s and. Alzheimer’s Disease Treatment—PAD-INJ” project (Call for Joint Projects for the Internationalization of Research, Compagnia di San Paolo). Support from the European Research Council (ERC) under the European Union’s Horizon 2020 Research and Innovation Program (Grant Agreement No. 772168) is also acknowledged.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviation
| ADNP | activity-dependent neuroprotective protein |
| AUC | area under the drug |
| B% | Bioavailability |
| BBB | blood brain barrier |
| bFGF | basic Fibroblast growth factor |
| C-CPE | Clostridium perfringens |
| CNS | central nervous system |
| CPP | Cell penetrating peptide |
| CS | Chitosan |
| CSF | blood-cerebrospinal fluid |
| DTE % | drug targeting efficiency |
| DTP % | direct transport percentage |
| EDTA | Ethylenediaminetetraacetic acid |
| GCS | glycol chitosan |
| GMS | Glyceryl monostearate |
| GNLs | Gelatin nanostructured lipid carriers |
| IGF-1 | insulin-like growth factor 1 |
| IGF-I | Insulin-like Growth Factor-I |
| IN | Intranasal |
| INF-β1b | interferon-β1b |
| Lf | lactoferrin |
| LPS | lipopolysaccharide |
| MAG | Magnolia officinalis |
| Mal-PEG-PCL | maleimide poly(ethylene glycol)-co-poly(ε-caprolactone) copolymer |
| Me-PEG-PCL | Methoxy poly(ethylene glycol)-co-poly(ε-caprolactone) copolymer |
| NP | nanoparticle |
| PEG | poly(ethylene glycol) |
| PHEA | poly(2-hydroxyethyl acrylate) |
| PLGA | poly(D,L-lactic acid-co-glycolic acid) copolymer |
| POZ | poly(2-ethyl-2-oxazoline) |
| PPS−PEG | poly(propylene sulphide)-polyethylene glycol |
| PVP | poly(N-vinyl pyrrolidone) |
| RB% | relative bioavailability (RB%) |
| RDTE% | relative drug targeting efficiency |
| RDTP% | relative direct transport percentage |
| SBE-β-CD | sulfobutyl-ether-β-cyclodextrin |
| SP | Neuropeptide substance P |
| STL | Soranum tuberosum lectin |
| TEER | transepithelial electrical resistance |
| TPP | tripolyphosphate |
| VIP | Vasoactive intestinal peptide |
| WGA | Wheat germ agglutinin |
References
- Stamatovic, S.M.; Keep, R.F.; Andjelkovic, A. V Brain Endothelial Cell-Cell Junctions: How to “Open” the Blood Brain Barrier. Curr. Neuropharmacol. 2008, 6, 179. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Drug transport across the blood–brain barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1959. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, B.T.; Davis, T.P. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 2005, 57, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Cecchelli, R.; Berezowski, V.; Lundquist, S.; Culot, M.; Renftel, M.; Dehouck, M.P.; Fenart, L. Modelling of the blood-brain barrier in drug discovery and development. Nat. Rev. Drug Discov. 2007, 6, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Lochhead, J.J.; Thorne, R.G. Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev. 2012, 64, 614–628. [Google Scholar] [CrossRef]
- Banks, W.A. Characteristics of compounds that cross the blood-brain barrier. BMC Neurol. 2009, 9, S3. [Google Scholar] [CrossRef]
- Van Tellingen, O.; Yetkin-Arik, B.; De Gooijer, M.C.; Wesseling, P.; Wurdinger, T.; De Vries, H.E. Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist. Updates 2015, 19, 1–12. [Google Scholar] [CrossRef]
- Gabathuler, R. Approaches to transport therapeutic drugs across the blood-brain barrier to treat brain diseases. Neurobiol. Dis. 2010, 37, 48–57. [Google Scholar] [CrossRef]
- Pond, S.M.; Tozer, T.N. First-pass elimination Basic concepts and clinical consequences. Clin. Pharmacokinet. 1984, 9, 1–25. [Google Scholar] [CrossRef]
- Mittal, D.; Ali, A.; Md, S.; Baboota, S.; Sahni, J.K.; Ali, J. Insights into direct nose to brain delivery: Current status and future perspective. Drug Deliv. 2014, 21, 75–86. [Google Scholar] [CrossRef]
- Jaiswal, Y.S.; Williams, L.L. A glimpse of Ayurveda—The forgotten history and principles of Indian traditional medicine. J. Tradit. Complement. Med. 2017, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Gizurarson, S. Anatomical and Histological Factors Affecting Intranasal Drug and Vaccine Delivery. Curr. Drug Deliv. 2012, 9, 566. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.; Goldstein, B.J. Olfactory epithelium: Cells, clinical disorders, and insights from an adult stem cell niche. Laryngoscope Investig. Otolaryngol. 2018, 3, 35. [Google Scholar] [CrossRef] [PubMed]
- Beule, A.G. Physiology and pathophysiology of respiratory mucosa of the nose and the paranasal sinuses. GMS Curr. Top. Otorhinolaryngol. Head Neck Surg. 2010, 9, Doc07. [Google Scholar] [CrossRef] [PubMed]
- Morgenroth, K.; Ebsen, M. Anatomy. Mech. Vent. Clin. Appl. Pathophysiol. 2008, 8, 69–85. [Google Scholar] [CrossRef]
- Chari, S.; Sridhar, K.; Walenga, R.; Kleinstreuer, C. Computational analysis of a 3D mucociliary clearance model predicting nasal drug uptake. J. Aerosol Sci. 2021, 155, 105757. [Google Scholar] [CrossRef]
- Ramvikas, M.; Arumugam, M.; Chakrabarti, S.R.; Jaganathan, K.S. Nasal Vaccine Delivery. In Micro and Nanotechnology in Vaccine Development; Skwarczynski, M., Toth, I., Eds.; William Andrew Publishing: Oxford, UK, 2017; pp. 279–301. [Google Scholar] [CrossRef]
- Duan, X.; Mao, S. New strategies to improve the intranasal absorption of insulin. Drug Discov. Today 2010, 15, 416–427. [Google Scholar] [CrossRef]
- Ghadiri, M.; Young, P.M.; Traini, D. Strategies to Enhance Drug Absorption via Nasal and Pulmonary Routes. Pharmaceutics 2019, 11, 113. [Google Scholar] [CrossRef]
- Thorne, R.G.; Pronk, G.J.; Padmanabhan, V.; Frey, W.H. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience 2004, 127, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Bilston, L.E.; Fletcher, D.F.; Brodbelt, A.R.; Stoodley, M.A. Arterial Pulsation-driven Cerebrospinal Fluid Flow in the Perivascular Space: A Computational Model. Comput. Methods Biomech. Biomed. Eng. 2010, 6, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Schley, D.; Carare-Nnadi, R.; Please, C.P.; Perry, V.H.; Weller, R.O. Mechanisms to explain the reverse perivascular transport of solutes out of the brain. J. Theor. Biol. 2006, 238, 962–974. [Google Scholar] [CrossRef] [PubMed]
- Hadaczek, P.; Yamashita, Y.; Mirek, H.; Tamas, L.; Bohn, M.C.; Noble, C.; Park, J.W.; Bankiewicz, K. The “perivascular pump” driven by arterial pulsation is a powerful mechanism for the distribution of therapeutic molecules within the brain. Mol. Ther. 2006, 14, 69–78. [Google Scholar] [CrossRef]
- Scranton, R.A.; Fletcher, L.; Sprague, S.; Jimenez, D.F.; Digicaylioglu, M. The Rostral Migratory Stream Plays a Key Role in Intranasal Delivery of Drugs into the CNS. PLoS ONE 2011, 6, e18711. [Google Scholar] [CrossRef]
- Selvaraj, K.; Gowthamarajan, K.; Venkata Satyanarayana Reddy Karri, V. Nose to brain transport pathways an overview: Potential of nanostructured lipid carriers in nose to brain targeting. Artif. Cells Nanomed. Biotechnol. 2018, 46, 2088–2095. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, J.; Feng, C.; Shao, X.; Liu, Q.; Zhang, Q.; Pang, Z.; Jiang, X. Intranasal nanoparticles of basic fibroblast growth factor for brain delivery to treat Alzheimer’s disease. Int. J. Pharm. 2014, 461, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Pires, P.C.; Santos, A.O. Nanosystems in nose-to-brain drug delivery: A review of non-clinical brain targeting studies. J. Control. Release 2018, 270, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Formica, M.L.; Real, D.A.; Picchio, M.L.; Catlin, E.; Donnelly, R.F.; Paredes, A.J. On a highway to the brain: A review on nose-to-brain drug delivery using nanoparticles. Appl. Mater. Today 2022, 29, 101631. [Google Scholar] [CrossRef]
- Illum, L. Transport of drugs from the nasal cavity to the central nervous system. Eur. J. Pharm. Sci. 2000, 11, 1–18. [Google Scholar] [CrossRef]
- Miyamoto, M.; Natsume, H.; Iwata, S.; Ohtake, K.; Yamaguchi, M.; Kobayashi, D.; Sugibayashi, K.; Yamashina, M.; Morimoto, Y. Improved nasal absorption of drugs using poly-l-arginine: Effects of concentration and molecular weight of poly-l-arginine on the nasal absorption of fluorescein isothiocyanate–dextran in rats. Eur. J. Pharm. Biopharm. 2001, 52, 21–30. [Google Scholar] [CrossRef]
- Merkus, F.W.H.M.; Schipper, N.G.M.; Hermens, W.A.J.J.; Romeijn, S.G.; Verhoef, J.C. Absorption enhancers in nasal drug delivery: Efficacy and safety. J. Control. Release 1993, 24, 201–208. [Google Scholar] [CrossRef]
- Corazza, E.; Abruzzo, A.; Giordani, B.; Cerchiara, T.; Bigucci, F.; Vitali, B.; Pio Di Cagno, M.; Luppi, B. Human Lactobacillus Biosurfactants as Natural Excipients for Nasal Drug Delivery of Hydrocortisone. Pharmaceutics 2022, 14, 524. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.H.; Quay, S.C. Advances in nasal drug delivery through tight junction technology. Expert Opin. Drug Deliv. 2005, 2, 281–298. [Google Scholar] [CrossRef] [PubMed]
- Rabinowicz, A.L.; Carrazana, E.; Maggio, E.T. Improvement of Intranasal Drug Delivery with Intravail® Alkylsaccharide Excipient as a Mucosal Absorption Enhancer Aiding in the Treatment of Conditions of the Central Nervous System. Drugs R D 2021, 21, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, M.; Natsume, H.; Satoh, I.; Ohtake, K.; Yamaguchi, M.; Kobayashi, D.; Sugibayashi, K.; Morimoto, Y. Effect of poly-L-arginine on the nasal absorption of FITC-dextran of different molecular weights and recombinant human granulocyte colony-stimulating factor (rhG-CSF) in rats. Int. J. Pharm. 2001, 226, 127–138. [Google Scholar] [CrossRef]
- Illum, L.; Jabbal-Gill, I.; Hinchcliffe, M.; Fisher, A.N.; Davis, S.S. Chitosan as a novel nasal delivery system for vaccines. Adv. Drug Deliv. Rev. 2001, 51, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Coucke, D.; Schotsaert, M.; Libert, C.; Pringels, E.; Vervaet, C.; Foreman, P.; Saelens, X.; Remon, J.P. Spray-dried powders of starch and crosslinked poly(acrylic acid) as carriers for nasal delivery of inactivated influenza vaccine. Vaccine 2009, 27, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Watari, A.; Hashimoto, E.; Yonemitsu, M.; Kiyono, H.; Yagi, K.; Kondoh, M.; Kunisawa, J. C-Terminal Clostridium perfringens Enterotoxin-Mediated Antigen Delivery for Nasal Pneumococcal Vaccine. PLoS ONE 2015, 10, e0126352. [Google Scholar] [CrossRef]
- Brunner, J.; Ragupathy, S.; Borchard, G. Target specific tight junction modulators. Adv. Drug Deliv. Rev. 2021, 171, 266–288. [Google Scholar] [CrossRef]
- Song, K.H.; Kim, S.B.; Shim, C.K.; Chung, S.J.; Kim, D.D.; Rhee, S.K.; Choi, G.J.; Kim, C.H.; Kim, K. Paracellular permeation-enhancing effect of AT1002 C-terminal amidation in nasal delivery. Drug Des. Devel. Ther. 2015, 9, 1815–1823. [Google Scholar] [CrossRef]
- Sosnik, A.; Das Neves, J.; Sarmento, B. Mucoadhesive polymers in the design of nano-drug delivery systems for administration by non-parenteral routes: A review. Prog. Polym. Sci. 2014, 12, 2030–2075. [Google Scholar] [CrossRef]
- Khutoryanskiy, V.V. Advances in mucoadhesion and mucoadhesive polymers. Macromol. Biosci. 2011, 11, 748–764. [Google Scholar] [CrossRef] [PubMed]
- Schipper, N.G.M.; Verhoef, J.C.; Merkus, F.W.H.M. The nasal mucociliary clearance: Relevance to nasal drug delivery. Pharm. Res. 1991, 8, 807–814. [Google Scholar] [CrossRef]
- Garcia, G.J.M.; Tewksbury, E.W.; Wong, B.A.; Kimbell, J.S. Interindividual variability in nasal filtration as a function of nasal cavity geometry. J. Aerosol Med. Pulm. Drug Deliv. 2009, 22, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Xie, Y.; Lin, X.; Xu, W. The Mucoadhesive Nanoparticle-Based Delivery System in the Development of Mucosal Vaccines. Int. J. Nanomed. 2022, 17, 4579–4598. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Lai, S.K.; Suk, J.S.; Pace, A.; Cone, R.; Hanes, J. Addressing the PEG mucoadhesivity paradox to engineer nanoparticles that “slip” through the human mucus barrier. Angew. Chem. Int. Ed. 2008, 47, 9726–9729. [Google Scholar] [CrossRef]
- Köllner, S.; Dünnhaupt, S.; Waldner, C.; Hauptstein, S.; Pereira De Sousa, I.; Bernkop-Schnürch, A. Mucus permeating thiomer nanoparticles. Eur. J. Pharm. Biopharm. 2015, 97, 265–272. [Google Scholar] [CrossRef]
- Ways, T.M.M.; Filippov, S.K.; Maji, S.; Glassner, M.; Cegłowski, M.; Hoogenboom, R.; King, S.; Lau, W.M.; Khutoryanskiy, V.V. Mucus-penetrating nanoparticles based on chitosan grafted with various non-ionic polymers: Synthesis, structural characterisation and diffusion studies. J. Colloid Interface Sci. 2022, 626, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.S.; Xu, Q.; Boylan, N.J.; Chisholm, J.; Tang, B.C.; Schuster, B.S.; Henning, A.; Ensign, L.M.; Lee, E.; Adstamongkonkul, P.; et al. Nanoparticles that do not adhere to mucus provide uniform and long-lasting drug delivery to airways following inhalation. Sci. Adv. 2017, 3, e1601556. [Google Scholar] [CrossRef]
- Zierden, H.C.; Josyula, A.; Shapiro, R.L.; Hsueh, H.T.; Hanes, J.; Ensign, L.M. Avoiding a Sticky Situation: Bypassing the Mucus Barrier for Improved Local Drug Delivery. Trends Mol. Med. 2021, 27, 436–450. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, M.; Kang, T.; Miao, D.; Gu, G.; Song, Q.; Yao, L.; Hu, Q.; Tu, Y.; Pang, Z.; et al. Lactoferrin-modified PEG-co-PCL nanoparticles for enhanced brain delivery of NAP peptide following intranasal administration. Biomaterials 2013, 34, 3870–3881. [Google Scholar] [CrossRef]
- Gao, X.; Wu, B.; Zhang, Q.; Chen, J.; Zhu, J.; Zhang, W.; Rong, Z.; Chen, H.; Jiang, X. Brain delivery of vasoactive intestinal peptide enhanced with the nanoparticles conjugated with wheat germ agglutinin following intranasal administration. J. Control. Release 2007, 121, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Sharma, R.K.; Sharma, N.; Gabrani, R.; Sharma, S.K.; Ali, J.; Dang, S. Nose-To-Brain Delivery of PLGA-Diazepam Nanoparticles. AAPS PharmSciTech 2015, 16, 1108–1121. [Google Scholar] [CrossRef] [PubMed]
- Porfiryeva, N.N.; Semina, I.I.; Salakhov, I.A.; Moustafine, R.I.; Khutoryanskiy, V.V. Mucoadhesive and mucus-penetrating interpolyelectrolyte complexes for nose-to-brain drug delivery. Nanomed. Nanotechnol. Biol. Med. 2021, 37, 102432. [Google Scholar] [CrossRef]
- Ahmad, S.; Khan, I.; Pandit, J.; Emad, N.A.; Bano, S.; Dar, K.I.; Rizvi, M.M.A.; Ansari, M.D.; Aqil, M.; Sultana, Y. Brain targeted delivery of carmustine using chitosan coated nanoparticles via nasal route for glioblastoma treatment. Int. J. Biol. Macromol. 2022, 221, 435–445. [Google Scholar] [CrossRef]
- Akel, H.; Ismail, R.; Katona, G.; Sabir, F.; Ambrus, R.; Csóka, I. A comparison study of lipid and polymeric nanoparticles in the nasal delivery of meloxicam: Formulation, characterization, and in vitro evaluation. Int. J. Pharm. 2021, 604, 120724. [Google Scholar] [CrossRef]
- Zhao, Y.Z.; Jin, R.R.; Yang, W.; Xiang, Q.; Yu, W.Z.; Lin, Q.; Tian, F.R.; Mao, K.L.; Lv, C.Z.; Wáng, Y.X.J.; et al. Using Gelatin Nanoparticle Mediated Intranasal Delivery of Neuropeptide Substance P to Enhance Neuro-Recovery in Hemiparkinsonian Rats. PLoS ONE 2016, 11, 148848. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Z.; Li, X.; Lu, C.T.; Lin, M.; Chen, L.J.; Xiang, Q.; Zhang, M.; Jin, R.R.; Jiang, X.; Shen, X.T.; et al. Gelatin nanostructured lipid carriers-mediated intranasal delivery of basic fibroblast growth factor enhances functional recovery in hemiparkinsonian rats. Nanomedicine 2013, 10, 755–764. [Google Scholar] [CrossRef]
- Gulati, N.; Nagaich, U.; Saraf, S.A. Intranasal Delivery of Chitosan Nanoparticles for Migraine Therapy. Sci. Pharm. 2013, 81, 843–854. [Google Scholar] [CrossRef]
- Di Gioia, S.; Trapani, A.; Mandracchia, D.; De Giglio, E.; Cometa, S.; Mangini, V.; Arnesano, F.; Belgiovine, G.; Castellani, S.; Pace, L.; et al. Intranasal delivery of dopamine to the striatum using glycol chitosan/sulfobutylether-β-cyclodextrin based nanoparticles. Eur. J. Pharm. Biopharm. 2015, 94, 180–193. [Google Scholar] [CrossRef]
- Annu; Baboota, S.; Ali, J. In vitro appraisals and ex vivo permeation prospect of chitosan nanoparticles designed for schizophrenia to intensify nasal delivery. Polym. Bull. 2022, 79, 2263–2285. [Google Scholar] [CrossRef]
- Hasan, N.; Imran, M.; Kesharwani, P.; Khanna, K.; Karwasra, R.; Sharma, N.; Rawat, S.; Sharma, D.; Ahmad, F.J.; Jain, G.K.; et al. Intranasal delivery of Naloxone-loaded solid lipid nanoparticles as a promising simple and non-invasive approach for the management of opioid overdose. Int. J. Pharm. 2021, 599, 120428. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M.; Chauhan, I.; Zafar, A.; Verma, M.; Noorulla, K.M.; Tura, A.J.; Alruwaili, N.K.; Haji, M.J.; Puri, D.; Gobena, W.G.; et al. Buspirone loaded solid lipid nanoparticles for amplification of nose to brain efficacy: Formulation development, optimization by Box-Behnken design, in-vitro characterization and in-vivo biological evaluation. J. Drug Deliv. Sci. Technol. 2021, 61, 102164. [Google Scholar] [CrossRef]
- Yang, Z.Z.; Zhang, Y.Q.; Wang, Z.Z.; Wu, K.; Lou, J.N.; Qi, X.R. Enhanced brain distribution and pharmacodynamics of rivastigmine by liposomes following intranasal administration. Int. J. Pharm. 2013, 452, 344–354. [Google Scholar] [CrossRef]
- Zhuang, X.; Xiang, X.; Grizzle, W.; Sun, D.; Zhang, S.; Axtell, R.C.; Ju, S.; Mu, J.; Zhang, L.; Steinman, L.; et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol. Ther. 2011, 19, 1769–1779. [Google Scholar] [CrossRef]
- Peng, H.; Li, Y.; Ji, W.; Zhao, R.; Lu, Z.; Shen, J.; Wu, Y.; Wang, J.; Hao, Q.; Wang, J.; et al. Intranasal Administration of Self-Oriented Nanocarriers Based on Therapeutic Exosomes for Synergistic Treatment of Parkinson’s Disease. ACS Nano 2022, 16, 869–884. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Wang, Q.; Zhu, Z.; Hao, Y.; Han, F.; Hong, J.; Zheng, W.; Ma, S.; Yang, L.; Cheng, G. High-efficiency brain-targeted intranasal delivery of BDNF mediated by engineered exosomes to promote remyelination. Biomater. Sci. 2022, 10, 5707–5718. [Google Scholar] [CrossRef] [PubMed]
- Gartziandia, O.; Herran, E.; Pedraz, J.L.; Carro, E.; Igartua, M.; Hernandez, R.M. Chitosan coated nanostructured lipid carriers for brain delivery of proteins by intranasal administration. Colloids Surf. B Biointerfaces 2015, 134, 304–313. [Google Scholar] [CrossRef]
- Noorulla, K.M.; Yasir, M.; Muzaffar, F.; Roshan, S.; Ghoneim, M.M.; Almurshedi, A.S.; Tura, A.J.; Alshehri, S.; Gebissa, T.; Mekit, S.; et al. Intranasal delivery of chitosan decorated nanostructured lipid carriers of Buspirone for brain targeting: Formulation development, optimization and In-Vivo preclinical evaluation. J. Drug Deliv. Sci. Technol. 2022, 67, 102939. [Google Scholar] [CrossRef]
- Sahoo, N.; Sahoo, R.K.; Biswas, N.; Guha, A.; Kuotsu, K. Recent advancement of gelatin nanoparticles in drug and vaccine delivery. Int. J. Biol. Macromol. 2015, 81, 317–331. [Google Scholar] [CrossRef]
- Zaki, N.M.; Mortada, N.D.; Awad, G.A.S.; ElHady, S.S.A. Rapid-onset intranasal delivery of metoclopramide hydrochloride Part II: Safety of various absorption enhancers and pharmacokinetic evaluation. Int. J. Pharm. 2006, 327, 97–103. [Google Scholar] [CrossRef]
- Casettari, L.; Illum, L. Chitosan in nasal delivery systems for therapeutic drugs. J. Control. Release 2014, 190, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Naskar, S.; Das, S.K.; Sharma, S.; Kuotsu, K. A Review on Designing Poly (Lactic-co-glycolic Acid) Nanoparticles as Drug Delivery Systems. Pharm. Nanotechnol. 2021, 9, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Structure, Preparation and Application. Adv. Pharm. Bull. 2015, 5, 305. [Google Scholar] [CrossRef]
- Shirodkar, R.K.; Kumar, L.; Mutalik, S.; Lewis, S. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Emerging Lipid Based Drug Delivery Systems. Pharm. Chem. J. 2019, 53, 440–453. [Google Scholar] [CrossRef]
- Herman, S.; Fishel, I.; Offen, D. Intranasal delivery of mesenchymal stem cells-derived extracellular vesicles for the treatment of neurological diseases. Stem Cells 2021, 39, 1589–1600. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Hurkat, P.; Jain, A.; Jain, A.; Jain, A.; Jain, S.K. Thiolated Polymers: Pharmaceutical Tool in Nasal Drug Delivery of Proteins and Peptides. Int. J. Pept. Res. Ther. 2019, 25, 15–26. [Google Scholar] [CrossRef]
- Wu, A.M.; Lisowska, E.; Duk, M.; Yang, Z. Lectins as tools in glycoconjugate research. Glycoconj. J. 2009, 26, 899–913. [Google Scholar] [CrossRef]
- Gabor, F.; Bogner, E.; Weissenboeck, A.; Wirth, M. The lectin-cell interaction and its implications to intestinal lectin-mediated drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 459–480. [Google Scholar] [CrossRef]
- Gao, X.; Tao, W.; Lu, W.; Zhang, Q.; Zhang, Y.; Jiang, X.; Fu, S. Lectin-conjugated PEG-PLA nanoparticles: Preparation and brain delivery after intranasal administration. Biomaterials 2006, 27, 3482–3490. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, C.; Liu, Q.; Shao, X.; Feng, C.; Shen, Y.; Zhang, Q.; Jiang, X. Solanum tuberosum lectin-conjugated PLGA nanoparticles for nose-to-brain delivery: In vivo and in vitro evaluations. J. Drug Target. 2012, 20, 174–184. [Google Scholar] [CrossRef]
- Wan, X.M.; Chen, Y.P.; Xu, W.R.; Yang, W.J.; Wen, L.P. Identification of nose-to-brain homing peptide through phage display. Peptides 2009, 30, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, J.; Xu, D. Cell-penetrating peptides as noninvasive transmembrane vectors for the development of novel multifunctional drug-delivery systems. J. Control. Release 2016, 229, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.K.; Wang, Y.Y.; Hanes, J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev. 2009, 61, 158. [Google Scholar] [CrossRef] [PubMed]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Chonkar, A.; Nayak, U.; Udupa, N. Smart Polymers in Nasal Drug Delivery. Indian J. Pharm. Sci. 2015, 77, 367. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhi, F.; Jia, X.; Zhang, X.; Ambardekar, R.; Meng, Z.; Paradkar, A.R.; Hu, Y.; Yang, Y. Enhanced brain targeting of curcumin by intranasal administration of a thermosensitive poloxamer hydrogel. J. Pharm. Pharmacol. 2013, 65, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Protopapa, C.; Siamidi, A.; Pavlou, P.; Vlachou, M. Excipients Used for Modified Nasal Drug Delivery: A Mini-Review of the Recent Advances. Materials 2022, 15, 6547. [Google Scholar] [CrossRef] [PubMed]
- Ravi, P.R.; Aditya, N.; Patil, S.; Cherian, L. Nasal in-situ gels for delivery of rasagiline mesylate: Improvement in bioavailability and brain localization. Drug Deliv. 2015, 22, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Liu, Y.; Liu, Y.; Ma, R.; Luo, J.; Hong, H.; Chen, X.; Wang, S.; Liu, C.; Zhang, Y.; et al. Rational Design of Thermosensitive Hydrogel to Deliver Nanocrystals with Intranasal Administration for Brain Targeting in Parkinson’s Disease. Research 2021, 2021, 9812523. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yang, Z.; Liu, M.; Tao, Y.; Li, Z.; Wu, Z.; Gui, S. Facile nose-to-brain delivery of rotigotine-loaded polymer micelles thermosensitive hydrogels: In vitro characterization and in vivo behavior study. Int. J. Pharm. 2020, 577, 119046. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Hu, J.; Wang, C.; Wan, D.; Li, Q.; Jiang, Q.; Du, L.; Jin, Y. Nasal Delivery of Cinnarizine Thermo- and Ion-Sensitive In Situ Hydrogels for Treatment of Microwave-Induced Brain Injury. Gels 2022, 8, 108. [Google Scholar] [CrossRef]
- Qi, X.J.; Xu, D.; Tian, M.L.; Zhou, J.F.; Wang, Q.S.; Cui, Y.L. Thermosensitive hydrogel designed for improving the antidepressant activities of genipin via intranasal delivery. Mater. Des. 2021, 206, 109816. [Google Scholar] [CrossRef]
- Zhong, M.; Kou, H.; Zhao, P.; Zheng, W.; Xu, H.; Zhang, X.; Lan, W.; Guo, C.; Wang, T.; Guo, F.; et al. Nasal Delivery of D-Penicillamine Hydrogel Upregulates a Disintegrin and Metalloprotease 10 Expression via Melatonin Receptor 1 in Alzheimer’s Disease Models. Front. Aging Neurosci. 2021, 13, 191. [Google Scholar] [CrossRef]
- Li, Y.; He, J.; Lyu, X.; Yuan, Y.; Wang, G.; Zhao, B. Chitosan-based thermosensitive hydrogel for nasal delivery of exenatide: Effect of magnesium chloride. Int. J. Pharm. 2018, 553, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Shaghlil, L.; Alshishani, A.; Sa’aleek, A.A.; Abdalkader, H.; Al-ebini, Y. Formulation and evaluation of nasal insert for nose-to-brain drug delivery of rivastigmine tartrate. J. Drug Deliv. Sci. Technol. 2022, 76, 103736. [Google Scholar] [CrossRef]
- Jelkmann, M.; Leichner, C.; Zaichik, S.; Laffleur, F.; Bernkop-Schnürch, A. A gellan gum derivative as in-situ gelling cationic polymer for nasal drug delivery. Int. J. Biol. Macromol. 2020, 158, 1037–1046. [Google Scholar] [CrossRef]
- Li, J.C.; Zhang, W.J.; Zhu, J.X.; Zhu, N.; Zhang, H.M.; Wang, X.; Zhang, J.; Wang, Q.Q. Preparation and brain delivery of nasal solid lipid nanoparticles of quetiapine fumarate in situ gel in rat model of schizophrenia. Int. J. Clin. Exp. Med. 2015, 8, 17590. [Google Scholar]
- Jose, S.; Ansa, C.R.; Cinu, T.A.; Chacko, A.J.; Aleykutty, N.A.; Ferreira, S.V.; Souto, E.B. Thermo-sensitive gels containing lorazepam microspheres for intranasal brain targeting. Int. J. Pharm. 2013, 441, 516–526. [Google Scholar] [CrossRef]
- Perez, A.P.; Mundiña-Weilenmann, C.; Romero, E.L.; Morilla, M.J. Increased brain radioactivity by intranasal 32P-labeled siRNA dendriplexes within in situ-forming mucoadhesive gels. Int. J. Nanomed. 2012, 7, 1373. [Google Scholar] [CrossRef]
- Cunha, S.; Swedrowska, M.; Bellahnid, Y.; Xu, Z.; Sousa Lobo, J.M.; Forbes, B.; Silva, A.C. Thermosensitive in situ hydrogels of rivastigmine-loaded lipid-based nanosystems for nose-to-brain delivery: Characterisation, biocompatibility, and drug deposition studies. Int. J. Pharm. 2022, 620, 121720. [Google Scholar] [CrossRef]
- Ribeiro, T.d.C.; Sábio, R.M.; Luiz, M.T.; de Souza, L.C.; Fonseca-Santos, B.; Cides da Silva, L.C.; Fantini, M.C.d.A.; Planeta, C.d.S.; Chorilli, M. Curcumin-Loaded Mesoporous Silica Nanoparticles Dispersed in Thermo-Responsive Hydrogel as Potential Alzheimer Disease Therapy. Pharmaceutics 2022, 14, 1976. [Google Scholar] [CrossRef] [PubMed]
- Kashif, M.u.R.; Sohail, M.; Khan, S.A.; Minhas, M.U.; Mahmood, A.; Shah, S.A.; Mohsin, S. Chitosan/guar gum-based thermoreversible hydrogels loaded with pullulan nanoparticles for enhanced nose-to-brain drug delivery. Int. J. Biol. Macromol. 2022, 215, 579–595. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Aqil, M.; Imam, S.S.; Ahad, A.; Sultana, Y.; Ali, A.; Khan, K. Temozolomide loaded nano lipid based chitosan hydrogel for nose to brain delivery: Characterization, nasal absorption, histopathology and cell line study. Int. J. Biol. Macromol. 2018, 116, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Famta, P.; Raghuvanshi, R.S.; Singh, S.B.; Srivastava, S. Lipid polymer hybrid nanocarriers: Insights into synthesis aspects, characterization, release mechanisms, surface functionalization and potential implications. Colloid Interface Sci. Commun. 2022, 46, 100570. [Google Scholar] [CrossRef]
- Vasile, C. Polymeric Nanomaterials: Recent Developments, Properties and Medical Applications. In Polymeric Nanomaterials in Nanotherapeutics; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–66. [Google Scholar] [CrossRef]
- Kozlovskaya, L.; Abou-Kaoud, M.; Stepensky, D. Quantitative analysis of drug delivery to the brain via nasal route. J. Control. Release 2014, 189, 133–140. [Google Scholar] [CrossRef]
- Caprifico, A.E.; Foot, P.J.S.; Polycarpou, E.; Calabrese, G. Overcoming the Blood-Brain Barrier: Functionalised Chitosan Nanocarriers. Pharmaceutics 2020, 12, 1013. [Google Scholar] [CrossRef] [PubMed]
- Nicolle, L.; Journot, C.M.A.; Gerber-Lemaire, S. Chitosan Functionalization: Covalent and Non-Covalent Interactions and Their Characterization. Polymers 2021, 13, 4118. [Google Scholar] [CrossRef]
- Divya, K.; Jisha, M.S. Chitosan nanoparticles preparation and applications. Environ. Chem. Lett. 2017, 16, 101–112. [Google Scholar] [CrossRef]
- Yasmin, R.; Shah, M.; Khan, S.A.; Ali, R. Gelatin nanoparticles: A potential candidate for medical applications. Nanotechnol. Rev. 2017, 6, 191–207. [Google Scholar] [CrossRef]
- Andrée, L.; Oude Egberink, R.; Dodemont, J.; Hassani Besheli, N.; Yang, F.; Brock, R.; Leeuwenburgh, S.C.G. Gelatin Nanoparticles for Complexation and Enhanced Cellular Delivery of mRNA. Nanomaterials 2022, 12, 3423. [Google Scholar] [CrossRef]
- Menon, I.; Zaroudi, M.; Zhang, Y.; Aisenbrey, E.; Hui, L. Fabrication of active targeting lipid nanoparticles: Challenges and perspectives. Mater. Today Adv. 2022, 16, 100299. [Google Scholar] [CrossRef]
- Pai, S.S.; Tilton, R.D.; Przybycien, T.M. Poly(ethylene glycol)-Modified Proteins: Implications for Poly(lactide-co-glycolide)-Based Microsphere Delivery. AAPS J. 2009, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Garg, T.; Rath, G.; Goyal, A.K. In situ nasal gel drug delivery: A novel approach for brain targeting through the mucosal membrane. Artif. Cells Nanomed. Biotechnol. 2015, 44, 1167–1176. [Google Scholar] [CrossRef]
- Craft, S.; Baker, L.D.; Montine, T.J.; Minoshima, S.; Watson, G.S.; Claxton, A.; Arbuckle, M.; Callaghan, M.; Tsai, E.; Plymate, S.R.; et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: A pilot clinical trial. Arch. Neurol. 2012, 69, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Rajpar, S.F.; Foulds, I.S.; Abdullah, A.; Maheshwari, M. Severe adverse cutaneous reaction to insulin due to cresol sensitivity. Contact Dermat. 2006, 55, 119–120. [Google Scholar] [CrossRef]
- Claxton, A.; Baker, L.D.; Hanson, A.; Trittschuh, E.H.; Cholerton, B.; Morgan, A.; Callaghan, M.; Arbuckle, M.; Behl, C.; Craft, S. Long Acting Intranasal Insulin Detemir Improves Cognition for Adults with Mild Cognitive Impairment or Early-Stage Alzheimer’s Disease Dementia. J. Alzheimers Dis. 2015, 45, 1269–1270. [Google Scholar] [CrossRef]
- Rosenbloom, M.H.; Barclay, T.R.; Pyle, M.; Owens, B.L.; Cagan, A.B.; Anderson, C.P.; Frey, W.H.; Hanson, L.R. A single-dose pilot trial of intranasal rapid-acting insulin in apolipoprotein E4 carriers with mild-moderate Alzheimer’s disease. CNS Drugs 2014, 28, 1185–1189. [Google Scholar] [CrossRef]
- Reger, M.A.; Watson, G.S.; Frey, W.H.; Baker, L.D.; Cholerton, B.; Keeling, M.L.; Belongia, D.A.; Fishel, M.A.; Plymate, S.R.; Schellenberg, G.D.; et al. Effects of intranasal insulin on cognition in memory-impaired older adults: Modulation by APOE genotype. Neurobiol. Aging 2006, 27, 451–458. [Google Scholar] [CrossRef]
- Craft, S.; Raman, R.; Chow, T.W.; Rafii, M.S.; Sun, C.K.; Rissman, R.A.; Donohue, M.C.; Brewer, J.B.; Jenkins, C.; Harless, K.; et al. Safety, Efficacy, and Feasibility of Intranasal Insulin for the Treatment of Mild Cognitive Impairment and Alzheimer Disease Dementia: A Randomized Clinical Trial. JAMA Neurol. 2020, 77, 1099–1109. [Google Scholar] [CrossRef]
- Kellar, D.; Craft, S. Brain insulin resistance in Alzheimer’s disease and related disorders: Mechanisms and therapeutic approaches. Lancet Neurol. 2020, 19, 758–766. [Google Scholar] [CrossRef]
- Chamanza, R.; Wright, J.A. A Review of the Comparative Anatomy, Histology, Physiology and Pathology of the Nasal Cavity of Rats, Mice, Dogs and Non-human Primates. Relevance to Inhalation Toxicology and Human Health Risk Assessment. J. Comp. Pathol. 2015, 153, 287–314. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.A.; Vanbever, R. Preclinical models for pulmonary drug delivery. Expert Opin. Drug Deliv. 2009, 6, 1231–1245. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, H.; Ong, H.X.; Bradbury, P.; Kourmatzis, A.; Traini, D.; Young, P.; Li, M.; Cheng, S. Real-time quantitative monitoring of in vitro nasal drug delivery by a nasal epithelial mucosa-on-a-chip model. Expert Opin. Drug Deliv. 2021, 18, 803–818. [Google Scholar] [CrossRef] [PubMed]
- Capuana, E.; Fucarino, A.; Burgio, S.; Intili, G.; Manna, O.M.; Pitruzzella, A.; Brucato, V.; La Carrubba, V.; Carfì Pavia, F. A dynamic air–liquid interface system for in vitro mimicking of the nasal mucosa. Biotechnol. Bioeng. 2022, 119, 2004–2009. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).