Multifunctional Scaffolds Based on Emulsion and Coaxial Electrospinning Incorporation of Hydroxyapatite for Bone Tissue Regeneration
Abstract
1. Introduction
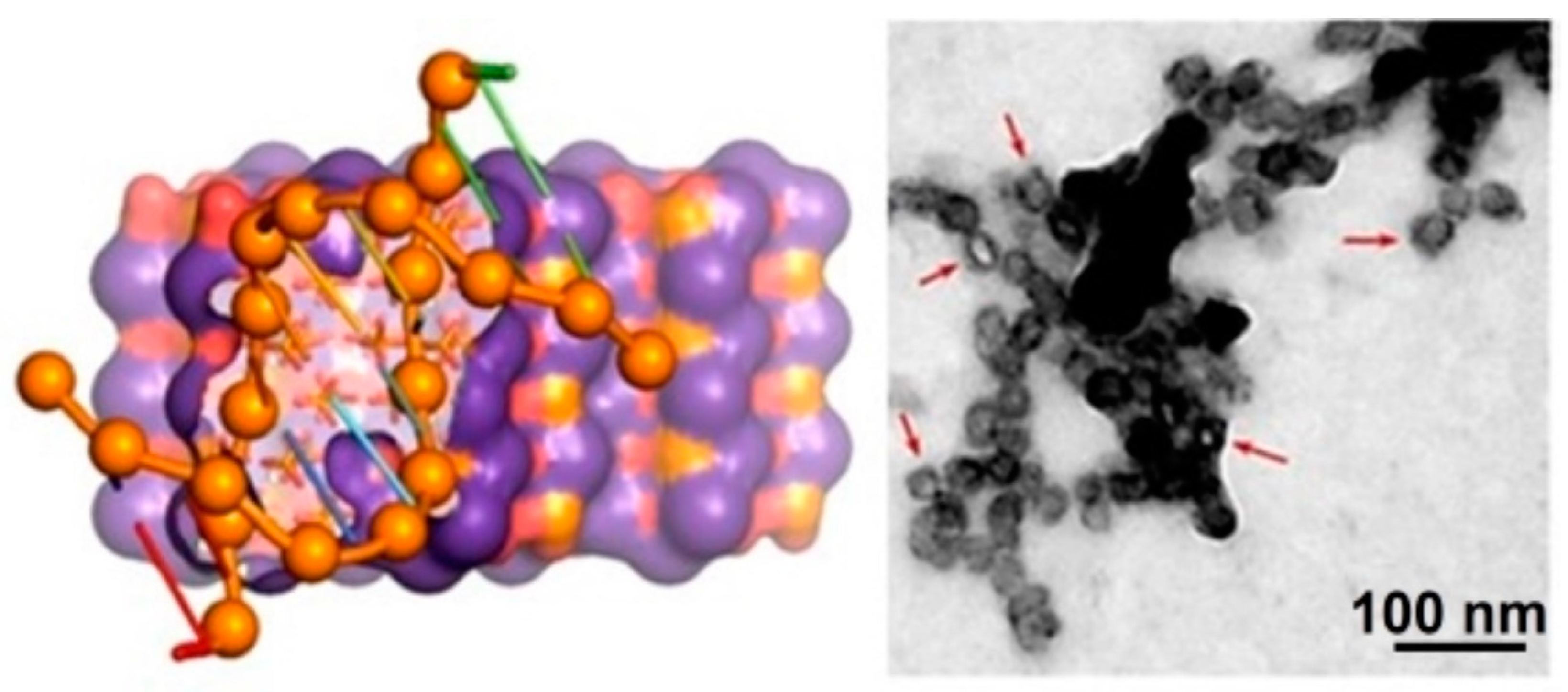
2. Hydroxyapatite for Tissue Regeneration and Drug Delivery
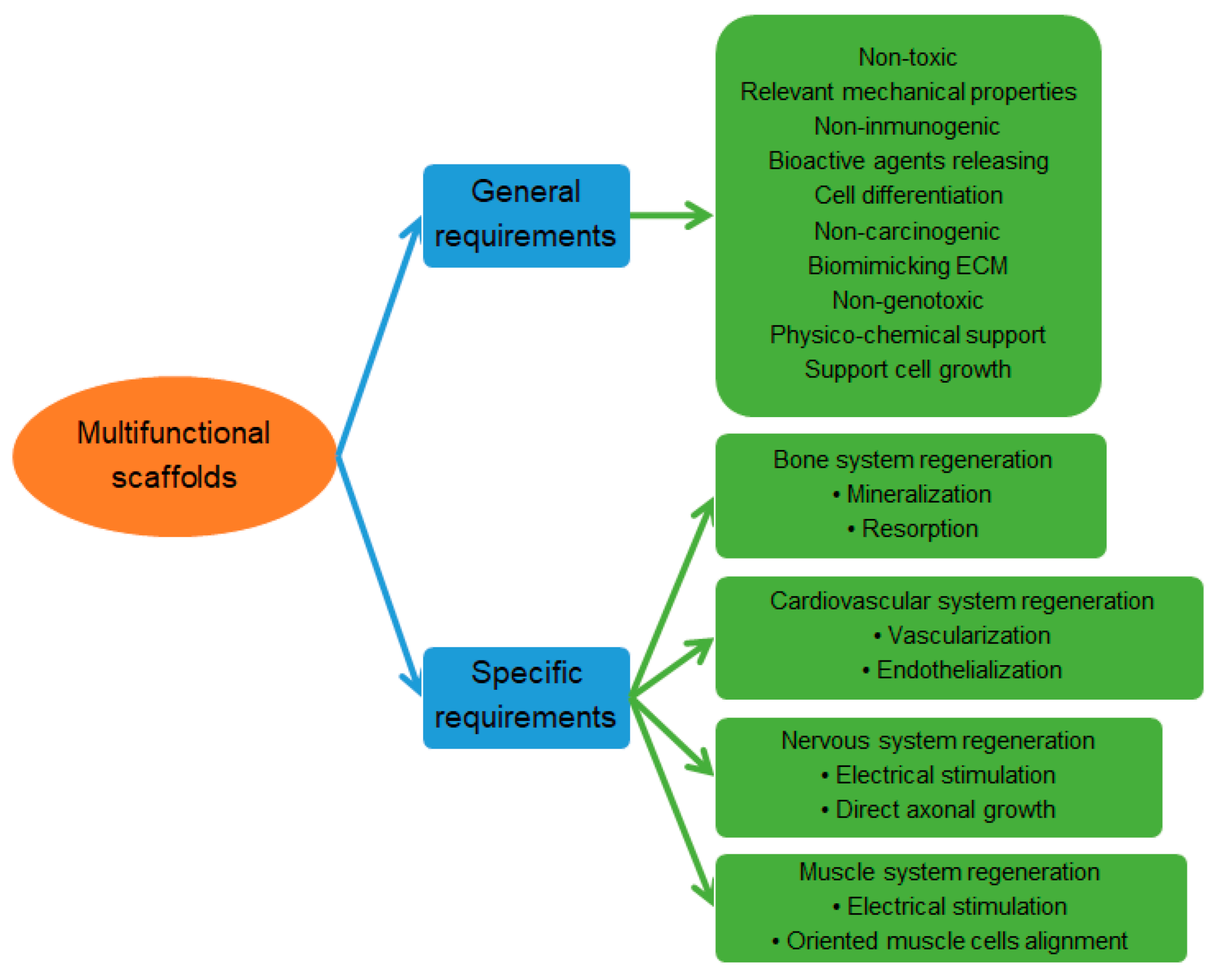
3. Multifunctional Scaffolds
3.1. Biopolymers and Synthetic Polymers
3.2. Surface Functionalization
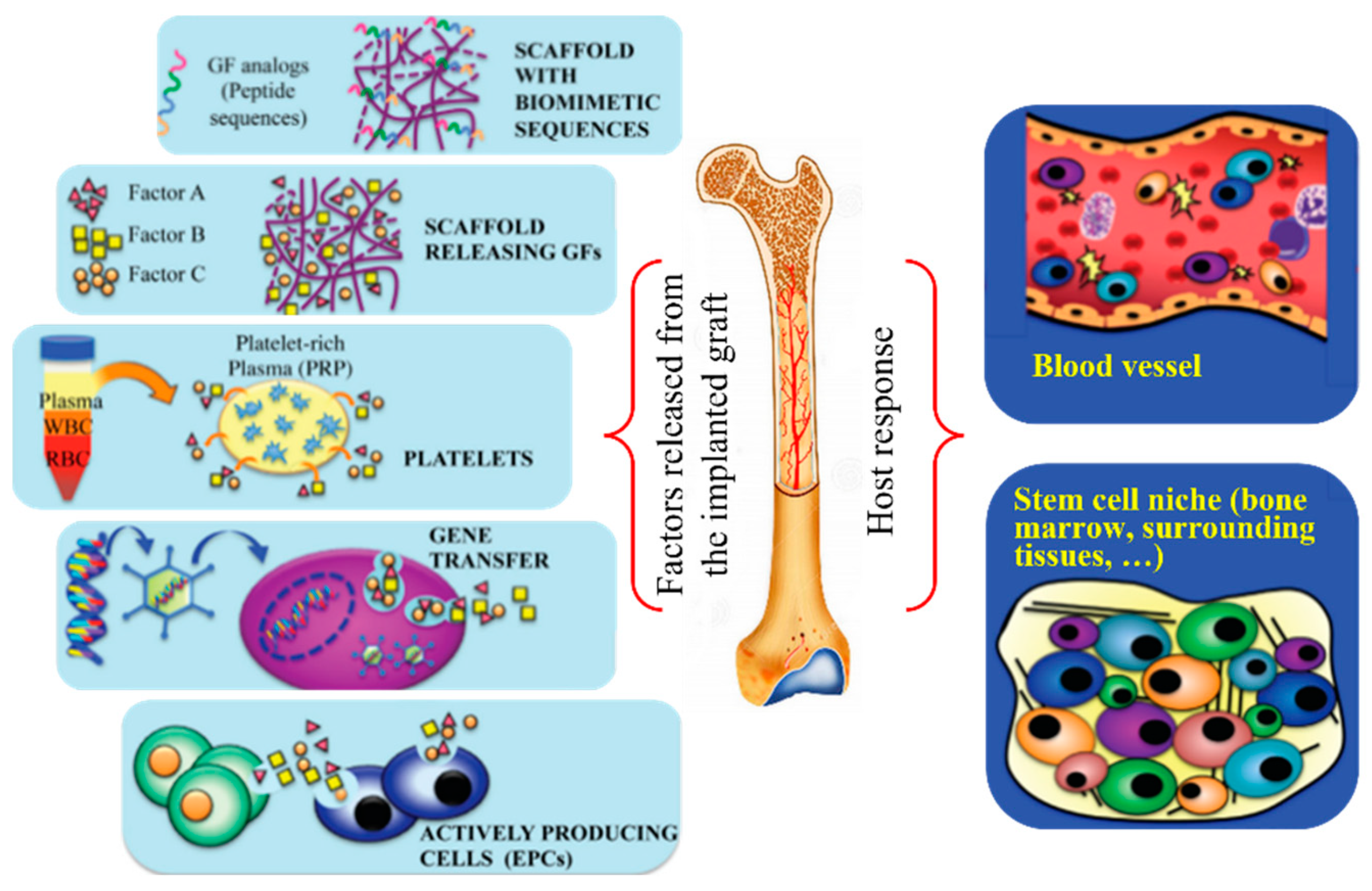
3.3. Incorporation of Functional Compounds
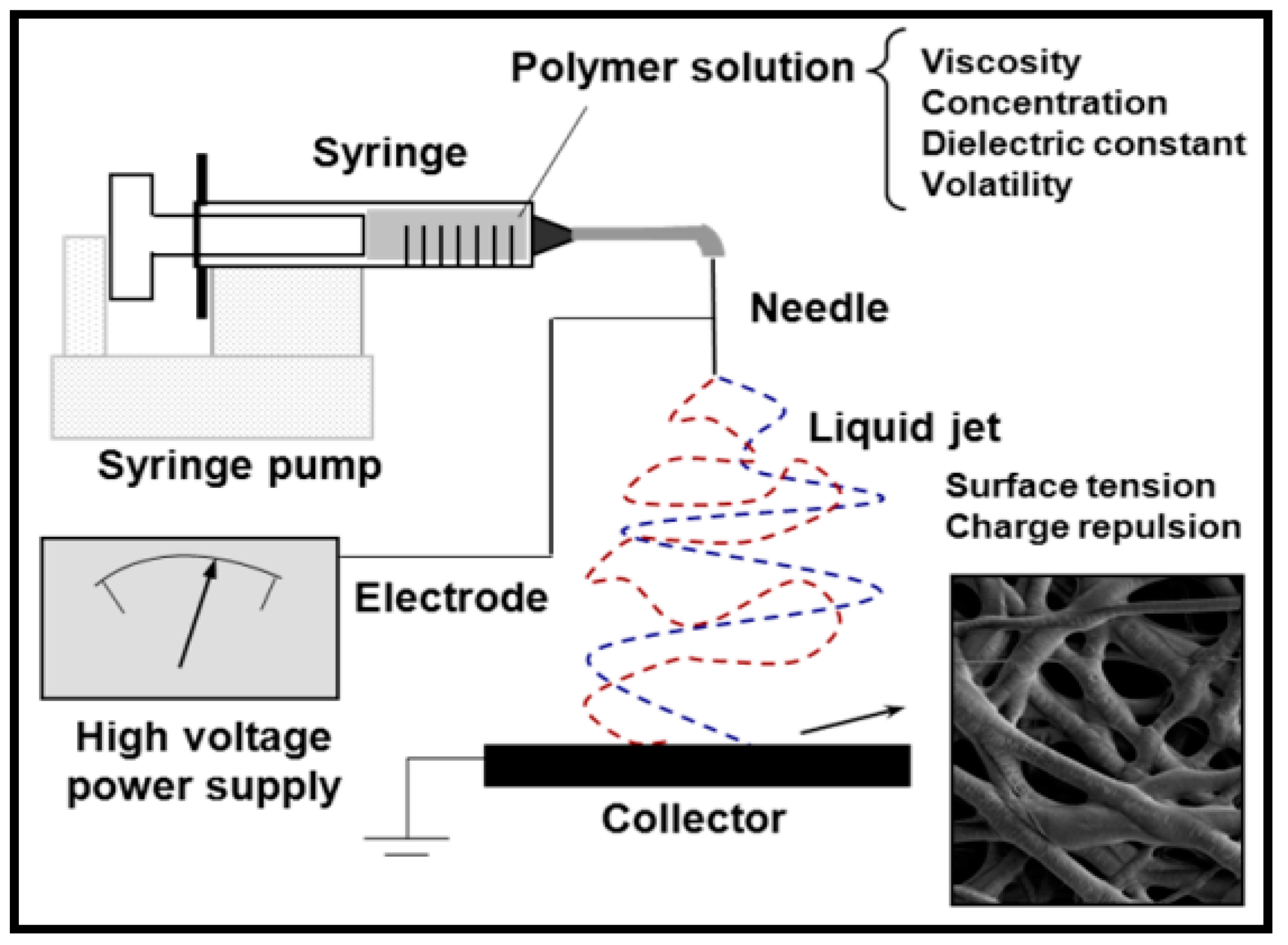
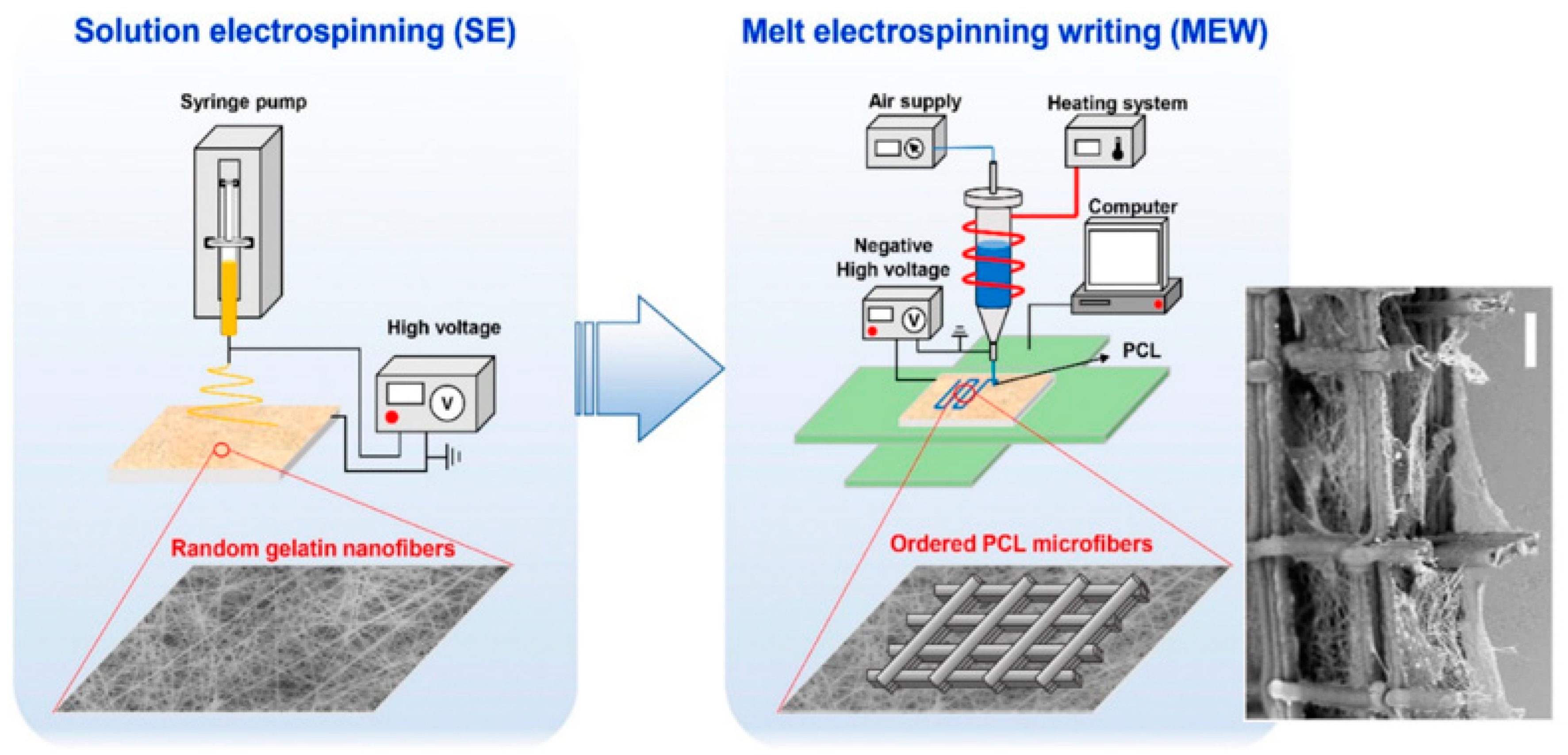
4. Electrospinning
4.1. Coaxial Electrospinning
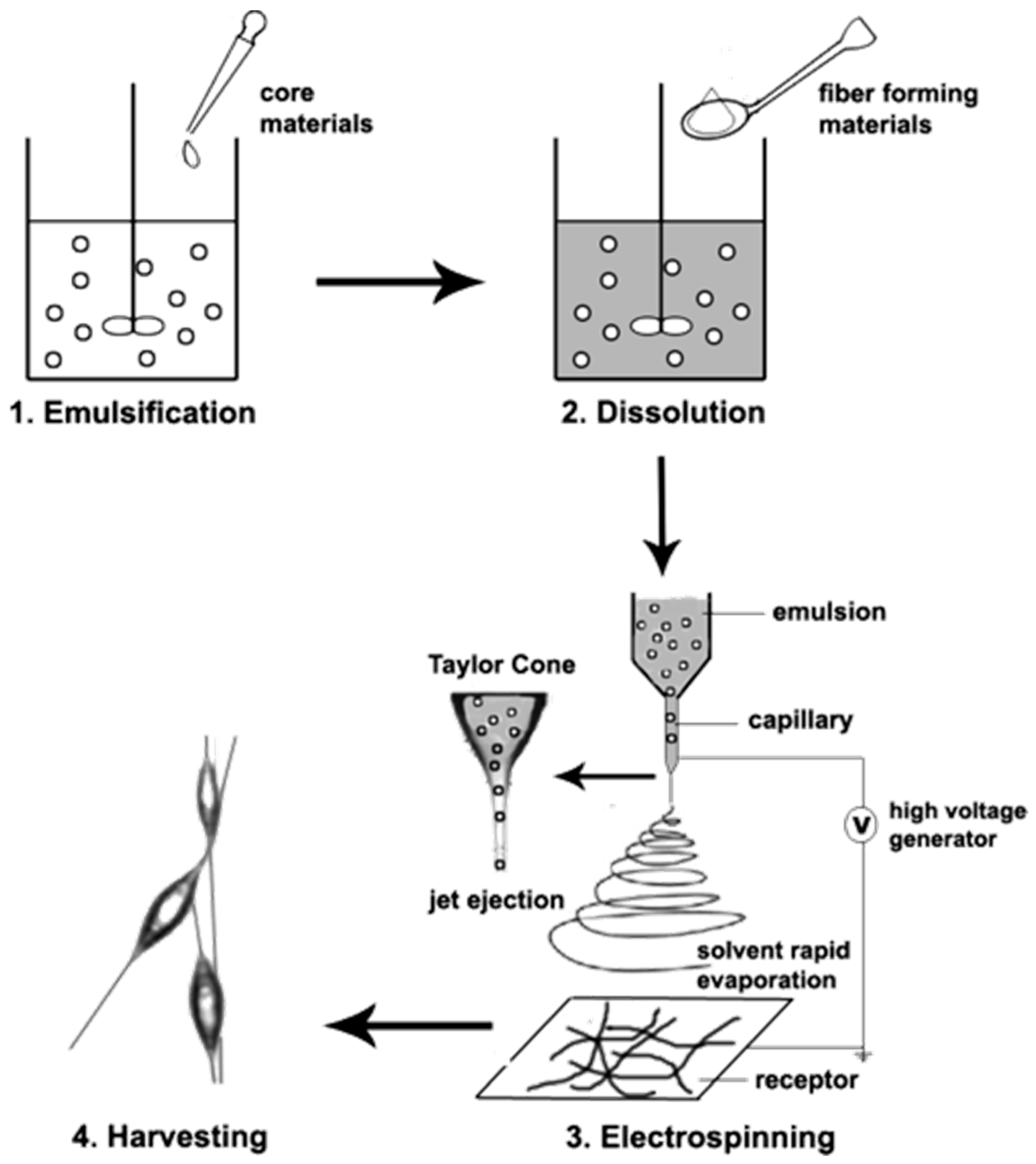
4.2. Emulsion Electrospinning
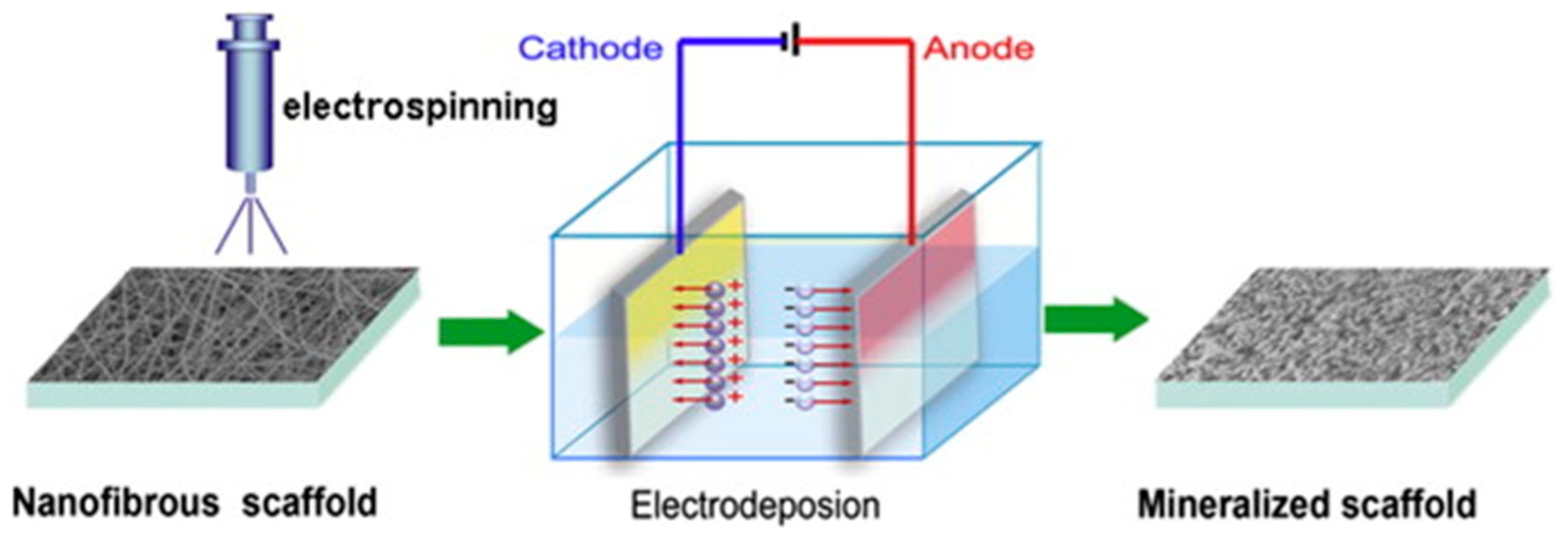
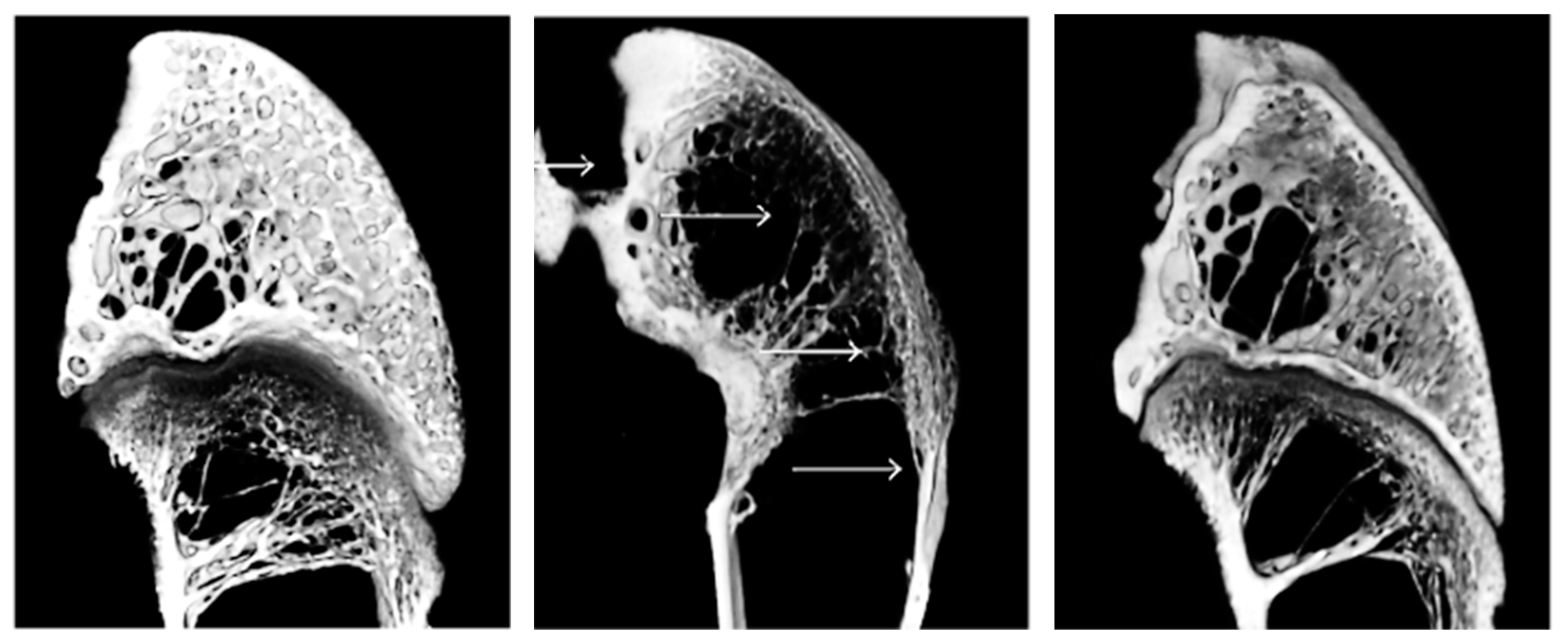
5. Recent Developments on Multifunctional Electrospun Scaffolds Incorporating HAp
| Multifunctional Composite | Applications/Effects | Ref. |
|---|---|---|
| PLA, osteogenonTM (ossein, HAp), osteocalcin, Col I | Hydrogel for cartilage reparation. The mineralization and cell adhesion enhanced. | [231] |
| PLLA, Col, HAp | Reparation of bone periosteum. Improve tensile properties and mimic the nanoscale structure of the extracellular matrix. | [233] |
| PCL, polyurethane, nanoHAp | Promotion of bone regeneration. Controlled degradation and cell ingrowth. | [237] |
| PLLA, HAp, dopamine, PPy, Ag | This showed long-term antibacterial, bioactivity, and osteoconductivity properties. | [238] |
| PLA, HAp, PCL, BMP-2 | Bone tissue engineering by slower degradation, acid neutralization, and the enhancement of the mesenquimal cell attachment and osteogenic differentiation. | [239] |
| PCL, Gel, poloxamer 188, β-lactoglobulin, vitamin K12 | The scaffold provides anti-thrombotic, anti-inflammatory, and cytoprotective activities | [241] |
| Silk fibroin, HAp, | Biocompatibility and good mechanical performance. Bone-like architecture. | [243] |
| Silk fibroin, CA | Employed for bone implants. | [247] |
| CA, silk fibroin, PEO, Hap, BMF-2 | Improve mechanical properties. Promotion of osteogenic differentiation. | [249] |
| PVP, ethylcellulose, Au NPs | Bone tissue regeneration. The porosity, mechanical performance, biocompatibility, and osteogenic activity was enhanced. | [250] |
| PCL, Gel, heparin, VEGF | Hydrogel with angioactive molecules and hemocompatible surface. | [253] |
| PVA, PCL, oregano oil, silica, HAp | Provide antioxidant, anti-inflammatory, and antibacterial properties. | [254] |
| PLGA, HAp, Col, amoxicillin | Promote fibroblast, bone growth, and wound healing. | [260] |
6. Perspective and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920. [Google Scholar] [CrossRef] [PubMed]
- Callister, W.D.; Rethwisch, D.G. Materials Science and Engineering: An Introduction, 8th ed.; Wiley: Hoboken, NJ, USA, 2009; p. 975. [Google Scholar]
- Baroli, B. From natural bone grafts to tissue engineering therapeutics: Brainstorming on pharmaceutical formulative requirements and challenges. J. Pharm. Sci. 2009, 98, 1317–1375. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone Tissue Engineering: Recent Advances and Challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed]
- Okike, K.; Bhattacharyya, T. Trends in the Management of Open Fractures. J. Bone Jt. Surg. 2006, 88, 2739–2748. [Google Scholar] [CrossRef]
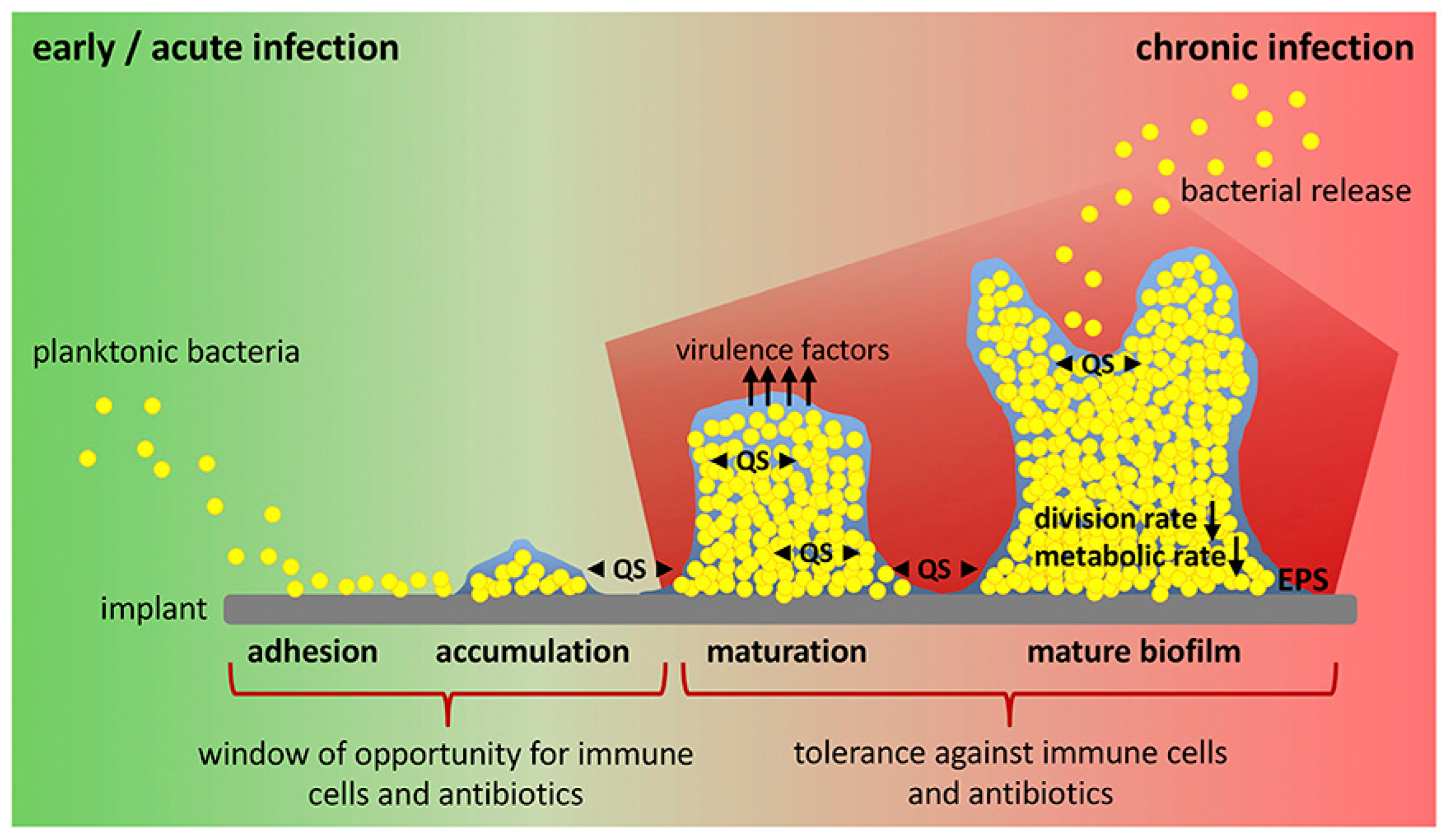
- Seebach, E.; Kubatzky, K.F. Chronic Implant-Related Bone Infections—Can Immune Modulation be a Therapeutic Strategy? Front. Immunol. 2019, 10, 1724. [Google Scholar] [CrossRef]
- Kohane, D.S.; Langer, R. Polymeric Biomaterials in Tissue Engineering. Pediatr. Res. 2008, 63, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, R.K.; Thompson, W.R.; Warden, S.J. Bone Repair Biomaterials, 2nd ed.; Woodhead Publishing Series in Biomaterials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 15–52. [Google Scholar]
- Wang, H.; Zeng, X.; Pang, L.; Wang, H.; Lin, B.; Deng, Z.; Qi, E.L.X.; Miao, N.; Wang, D.; Huang, P.; et al. Integrative treatment of anti-tumor/bone repair by combination of MoS2 nanosheets with 3D printed bioactive borosilicate glass scaffolds. Chem. Eng. J. 2020, 396, 125081. [Google Scholar] [CrossRef]
- Chen, M.; Li, Y.-F.; Besenbacher, F. Electrospun Nanofibers-Mediated On-Demand Drug Release. Adv. Health Mater. 2014, 3, 1721–1732. [Google Scholar] [CrossRef]
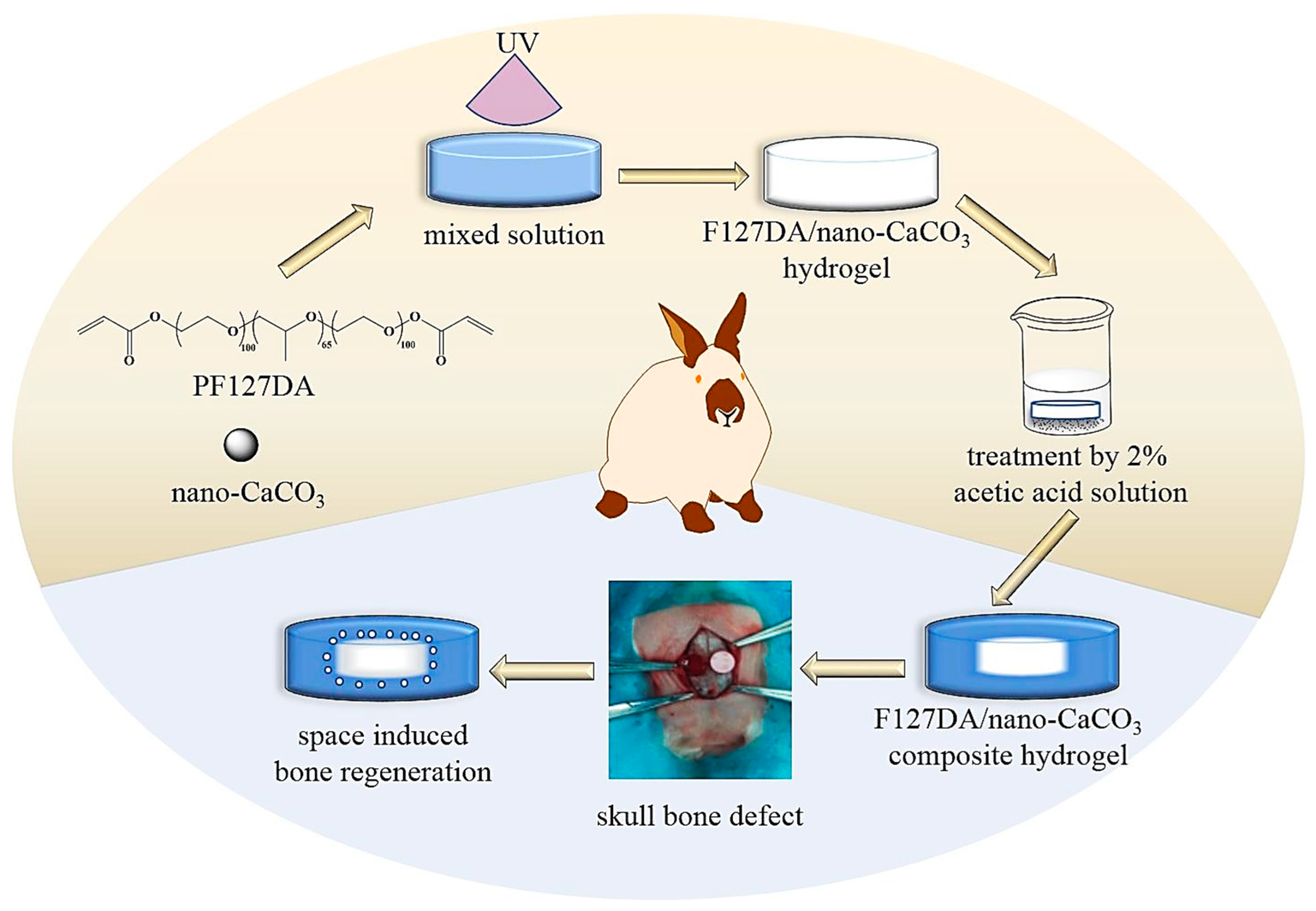
- Bao, Z.; Gu, Z.; Xu, J.; Zhao, M.; Liu, G.; Wu, J. Acid-responsive composite hydrogel platform with space-controllable stiffness and calcium supply for enhanced bone regeneration. Chem. Eng. J. 2020, 396, 125353. [Google Scholar] [CrossRef]
- Huang, K.; Liu, G.; Gu, Z.; Wu, J. Tofu as excellent scaffolds for potential bone regeneration. Chin. Chem. Lett. 2020, 31, 3190–3194. [Google Scholar] [CrossRef]
- Stevens, M.M. Biomaterials for bone tissue engineering. Mater. Today 2008, 11, 18–25. [Google Scholar] [CrossRef]
- Bharadwaz, A.; Jayasuriya, A.C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater. Sci. Eng. C 2020, 110, 110698. [Google Scholar] [CrossRef] [PubMed]
- Roveri, N.; Iafisco, M. Evolving application of biomimetic nanostructured hydroxyapatite. Nanotechnol. Sci. Appl. 2010, 3, 107–125. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Bao, X. Influence of temperature, ripening time and calcination on the morphology and crystallinity of hydroxyapatite nanoparticles. J. Eur. Ceram. Soc. 2003, 23, 1697–1704. [Google Scholar] [CrossRef]
- Ye, F.; Guo, H.; Zhang, H.; He, X. Polymeric micelle-templated synthesis of hydroxyapatite hollow nanoparticles for a drug delivery system. Acta Biomater. 2010, 6, 2212–2218. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Liao, L.; Zhao, S. Synthesis of mesoporous hydroxyapatite using a modified hard-templating route. Mater. Res. Bull. 2009, 44, 1626–1629. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, J.; Quan, Z.; Yang, P.; Li, C.; Hou, Z.; Lin, J. Hydroxyapatite Nano- and Microcrystals with Multiform Morphologies: Controllable Synthesis and Luminescence Properties. Cryst. Growth Des. 2009, 9, 2725–2733. [Google Scholar] [CrossRef]
- Ren, F.; Leng, Y.; Ding, Y.; Wang, K. Hydrothermal growth of biomimetic carbonated apatite nanoparticles with tunable size, morphology and ultrastructure. CrystEngComm 2013, 15, 2137–2146. [Google Scholar] [CrossRef]
- Sun, R.; Chen, K.; Lu, Y. Fabrication and dissolution behavior of hollow hydroxyapatite microspheres intended for controlled drug release. Mater. Res. Bull. 2009, 44, 1939–1942. [Google Scholar] [CrossRef]
- Shum, H.C.; Bandyopadhyay, A.; Bose, S.; Weitz, D.A. Double Emulsion Droplets as Microreactors for Synthesis of Mesoporous Hydroxyapatite. Chem. Mater. 2009, 21, 5548–5555. [Google Scholar] [CrossRef]
- Dou, Y.; Cai, S.; Ye, X.; Xu, G.; Hu, H.; Ye, X. Preparation of mesoporous hydroxyapatite films used as biomaterials via sol–gel technology. J. Sol-Gel Sci. Technol. 2011, 61, 126–132. [Google Scholar] [CrossRef]
- Lee, W.-H.; Loo, C.-Y.; Rohanizadeh, R. A review of chemical surface modification of bioceramics: Effects on protein adsorption and cellular response. Colloids Surfaces B Biointerfaces 2014, 122, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Rechendorff, K.; Hovgaard, M.B.; Foss, M.; Zhdanov, V.P.; Besenbacher, F. Enhancement of Protein Adsorption Induced by Surface Roughness. Langmuir 2006, 22, 10885–10888. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-H.; Loo, C.-Y.; Zavgorodniy, A.V.; Ghadiri, M.; Rohanizadeh, R. A novel approach to enhance protein adsorption and cell proliferation on hydroxyapatite: Citric acid treatment. RSC Adv. 2013, 3, 4040–4051. [Google Scholar] [CrossRef]
- Holt, K.B.; Bard, A.J. Interaction of Silver(I) Ions with the Respiratory Chain of Escherichia coli: An Electrochemical and Scanning Electrochemical Microscopy Study of the Antimicrobial Mechanism of Micromolar Ag+. Biochemistry 2005, 44, 13214–13223. [Google Scholar] [CrossRef] [PubMed]
- Thangavelu, M.; Narasimha, R.R.; Adithan, A.A.C.; Jong-Hoon, K.; Parvathaleswara, S.T. Reengineered graft copolymers as a potential alternative for the bone tissue engineering application by inducing osteogenic markers expression and biocompatibility. Colloids Surfaces B Biointerfaces 2016, 143, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Liu, A.; Chen, L.; Zhang, P.; Chen, X.; Jing, X. The Surface Modification of Hydroxyapatite Nanoparticles by the Ring Opening Polymerization of γ -Benzyl-L-glutamate N -carboxyanhydride. Macromol. Biosci. 2009, 9, 631–638. [Google Scholar] [CrossRef]
- Tang, Y.-F.; Liu, J.-G.; Wang, Z.-L.; Wang, Y.; Cui, L.-G.; Zhang, P.-B.; Chen, X.-S. In vivo degradation behavior of porous composite scaffolds of poly(lactide-co-glycolide) and nano-hydroxyapatite surface grafted with poly(L-lactide). Chin. J. Polym. Sci. 2014, 32, 805–816. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, H.; Zhuang, X.; Zhang, P.; Cui, Y.; Wang, X.; Wei, Y.; Chen, X. Nano-hydroxyapatite Surfaces Grafted with Electroactive Aniline Tetramers for Bone-Tissue Engineering. Macromol. Biosci. 2013, 13, 356–365. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, Y.; Tian, X.; Cui, G.; Zhao, Y.; Yang, Q.; Yu, S.; Xing, G.; Zhang, B. Segmental bone regeneration using an rhBMP-2-loaded gelatin/nanohydroxyapatite/fibrin scaffold in a rabbit model. Biomaterials 2009, 30, 6276–6285. [Google Scholar] [CrossRef]
- Sotome, S.; Uemura, T.; Kikuchi, M.; Chen, J.; Itoh, S.; Tanaka, J.; Tateishi, T.; Shinomiya, K. Synthesis and in vivo evaluation of a novel hydroxyapatite/collagen–alginate as a bone filler and a drug delivery carrier of bone morphogenetic protein. Mater. Sci. Eng. C 2004, 24, 341–347. [Google Scholar] [CrossRef]
- Maehara, H.; Sotome, S.; Yoshii, T.; Torigoe, I.; Kawasaki, Y.; Sugata, Y.; Yuasa, M.; Hirano, M.; Mochizuki, N.; Kikuchi, M.; et al. Repair of large osteochondral defects in rabbits using porous hydroxyapatite/collagen (HAp/Col) and fibroblast growth factor-2 (FGF-2). J. Orthop. Res. 2010, 28, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Phipps, M.C.; Xu, Y.; Bellis, S.L. Delivery of Platelet-Derived Growth Factor as a Chemotactic Factor for Mesenchymal Stem Cells by Bone-Mimetic Electrospun Scaffolds. PLoS ONE 2012, 7, e40831. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.-J.; Lee, J.-S.; Ryu, T.-K.; Kang, R.-H.; Jeong, K.-Y.; Jun, D.-R.; Koh, J.-M.; Kim, S.-E.; Choi, S.-W. Alendronate-modified hydroxyapatite nanoparticles for bone-specific dual delivery of drug and bone mineral. Macromol. Res. 2016, 24, 623–628. [Google Scholar] [CrossRef]
- Dou, X.-C.; Zhu, X.-P.; Zhou, J.; Cai, H.-Q.; Tang, J.; Li, Q.-L. Minocycline-released hydroxyapatite–gelatin nanocomposite and its cytocompatibility. Vitro Biomed. Mater. 2011, 6, 025002. [Google Scholar] [CrossRef]
- Song, W.; Yu, X.; Markel, D.C.; Shi, T.; Ren, W. Coaxial PCL/PVA electrospun nanofibers: Osseointegration enhancer and controlled drug release device. Biofabrication 2013, 5, 035006. [Google Scholar] [CrossRef]
- McNally, M.A.; Ferguson, J.Y.; Lau, A.C.K.; Diefenbeck, M.; Scarborough, M.; Ramsden, A.J.; Atkins, B.L. Single-stage treatment of chronic osteomyelitis with a new absorbable, gentamicin-loaded, calcium sulphate/hydroxyapatite biocomposite. Bone Jt. J. 2016, 98, 1289–1296. [Google Scholar] [CrossRef]
- Ferraz, M.; Mateus, A.; Sousa, J.; Monteiro, F. Nanohydroxyapatite microspheres as delivery system for antibiotics: Release kinetics, antimicrobial activity, and interaction with osteoblasts. J. Biomed. Mater. Res. Part A 2007, 81, 994–1004. [Google Scholar] [CrossRef]
- Liu, T.-Y.; Chen, S.-Y.; Li, J.-H.; Liu, D.-M. Study on drug release behaviour of CDHA/chitosan nanocomposites—Effect of CDHA nanoparticles. J. Control. Release 2006, 112, 88–95. [Google Scholar] [CrossRef]
- Rivas, M.; del Valle, L.J.; Rodríguez-Rivero, A.M.; Turon, P.; Puiggalí, J.; Alemán, C. Loading of Antibiotic into Biocoated Hydroxyapatite Nanoparticles: Smart Antitumor Platforms with Regulated Release. ACS Biomater. Sci. Eng. 2018, 4, 3234–3245. [Google Scholar] [CrossRef]
- Rivas, M.; Pelechà, M.; Franco, L.; Turon, P.; Alemán, C.; del Valle, L.J.; Puiggalí, J. Incorporation of Chloramphenicol Loaded Hydroxyapatite Nanoparticles into Polylactide. Int. J. Mol. Sci. 2019, 20, 5056. [Google Scholar] [CrossRef]
- Kadkhodaie-Elyaderani, A.; de Lama-Odría, M.D.C.; Rivas, M.; Martínez-Rovira, I.; Yousef, I.; Puiggalí, J.; del Valle, L.J. Medicated Scaffolds Prepared with Hydroxyapatite/Streptomycin Nanoparticles Encapsulated into Polylactide Microfibers. Int. J. Mol. Sci. 2022, 23, 1282. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, V.V.; Radtke, I.; Heumann, R.; Epple, M. Effective transfection of cells with multi-shell calcium phosphate-DNA nanoparticles. Biomaterials 2006, 27, 3147–3153. [Google Scholar] [CrossRef] [PubMed]
- Welzel, T.; Radtke, I.; Meyer-Zaika, W.; Heumann, R.; Epple, M. Transfection of cells with custom-made calcium phosphate nanoparticles coated with DNA. J. Mater. Chem. 2004, 14, 2213–2217. [Google Scholar] [CrossRef]
- Zuo, G.; Wan, Y.; Meng, X.; Zhao, Q.; Ren, K.; Jia, S.; Wang, J. Synthesis and characterization of a lamellar hydroxyapatite/DNA nanohybrid. Mater. Chem. Phys. 2011, 126, 470–475. [Google Scholar] [CrossRef]
- Revilla-López, G.; Casanovas, J.; Bertran, O.; Turon, P.; Puiggalí, J.; Alemán, C. Modeling biominerals formed by apatites and DNA. Biointerphases 2013, 8, 10. [Google Scholar] [CrossRef]
- Bertran, O.; del Valle, L.J.; Revilla-López, G.; Chaves, G.; Cardús, L.; Casas, M.T.; Casanovas, J.; Turon, P.; Puiggalí, J.; Alemán, C. Mineralization of DNA into nanoparticles of hydroxyapatite. Dalton Trans. 2014, 43, 317–327. [Google Scholar] [CrossRef]
- Litowczenko, J.; Woźniak-Budych, M.J.; Staszak, K.; Wieszczycka, K.; Jurga, S.; Tylkowski, B. Milestones and current achievements in development of multifunctional bioscaffolds for medical application. Bioact. Mater. 2021, 6, 2412–2438. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Lozano, D.; González, B.; Izquierdo-Barba, I. Biomaterials against Bone Infection. Adv. Healthc. Mater. 2020, 9, 2000310. [Google Scholar] [CrossRef]
- Bigham, A.; Hassanzadeh-Tabrizi, S.; Rafienia, M.; Salehi, H. Ordered mesoporous magnesium silicate with uniform nanochannels as a drug delivery system: The effect of calcination temperature on drug delivery rate. Ceram. Int. 2016, 42, 17185–17191. [Google Scholar] [CrossRef]
- Ficai, A.; Marques, C.; Ferreira, J.M.; Andronescu, E.; Ficai, D.; Sonmez, M. Multifunctional materials for bone cancer treatment. Int. J. Nanomed. 2014, 9, 2713–2725. [Google Scholar] [CrossRef] [PubMed]
- Bigham, A.; Foroughi, F.; Rezvani Ghomi, E.; Rafienia, M.; Neisiany, R.E.; Ramakrishna, S. The journey of multifunctional bone scaffolds fabricated from traditional toward modern techniques. Bio-Des. Manuf. 2020, 3, 281–306. [Google Scholar] [CrossRef]
- Eliaz, N.; Metoki, N. Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [PubMed]
- Koester, K.J.; Ager, J.W.; Ritchie, R.O. The true toughness of human cortical bone measured with realistically short cracks. Nat. Mater. 2008, 7, 672–677. [Google Scholar] [CrossRef]
- Chen, F.-M.; Zhang, M.; Wu, Z.-F. Toward delivery of multiple growth factors in tissue engineering. Biomaterials 2010, 31, 6279–6308. [Google Scholar] [CrossRef]
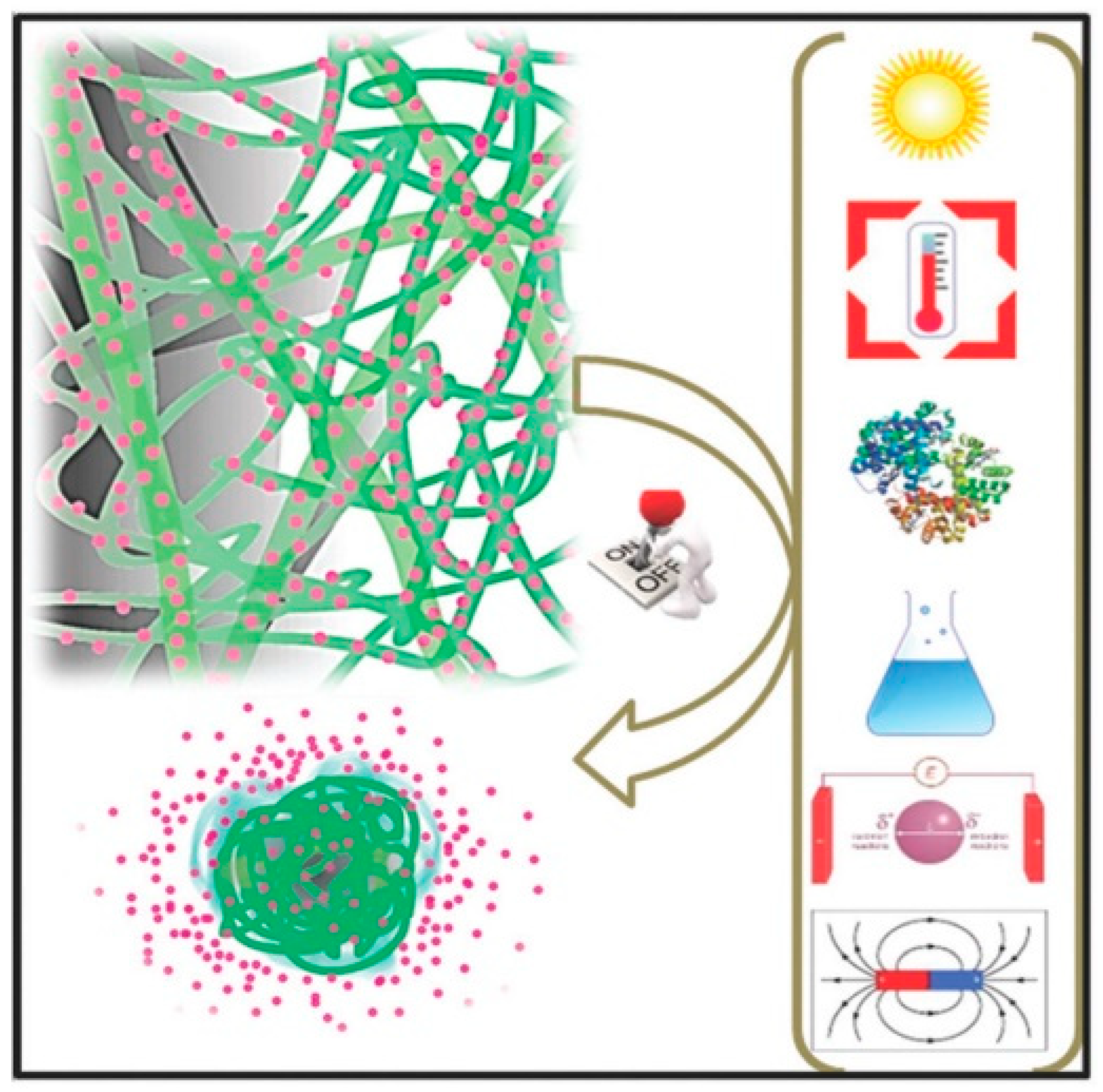
- Tai, Y.; Banerjee, A.; Goodrich, R.; Jin, L.; Nam, J. Development and Utilization of Multifunctional Polymeric Scaffolds for the Regulation of Physical Cellular Microenvironments. Polymers 2021, 13, 3880. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, L.; Yang, L.; Zhu, F.; Ding, M.; Lin, F.; Wang, Z.; Li, Y. “Click” chemistry in polymeric scaffolds: Bioactive materials for tissue engineering. J. Control. Release 2018, 273, 160–179. [Google Scholar] [CrossRef]
- Hassan, M.; Dave, K.; Chandrawati, R.; Dehghani, F.; Gomes, V.G. 3D printing of biopolymer nanocomposites for tissue engineering: Nanomaterials, processing and structure-function relation. Eur. Polym. J. 2019, 121, 109340. [Google Scholar] [CrossRef]
- Viguet-Carrin, S.; Garnero, P.; Delmas, P.D. The role of collagen in bone strength. Osteoporos. Int. 2005, 17, 319–336. [Google Scholar] [CrossRef]
- Kołodziejska, B.; Kaflak, A.; Kolmas, J. Biologically Inspired Collagen/Apatite Composite Biomaterials for Potential Use in Bone Tissue Regeneration—A Review. Materials 2020, 13, 1748. [Google Scholar] [CrossRef]
- Cui, F.-Z.; Li, Y.; Ge, J. Self-assembly of mineralized collagen composites. Mater. Sci. Eng. R Rep. 2007, 57, 1–27. [Google Scholar] [CrossRef]
- Cunniffe, G.M.; Dickson, G.R.; Partap, S.; Stanton, K.T.; O’Brien, F.J. Development and characterisation of a collagen nano-hydroxyapatite composite scaffold for bone tissue engineering. J. Mater. Sci. Mater. Med. 2010, 21, 2293–2298. [Google Scholar] [CrossRef] [PubMed]
- Fukui, N.; Sato, T.; Kuboki, Y.; Aoki, H. Bone tissue reaction of nano-hydroxyapatite/collagen composite at the early stage of implantation. Bio-Medical. Mater. Eng. 2008, 18, 25–33. [Google Scholar]
- Nishikawa, T.; Masuno, K.; Tominaga, K.; Koyama, Y.; Yamada, T.; Takakuda, K.; Kikuchi, M.; Tanaka, J.; Tanaka, A. Bone Repair Analysis in a Novel Biodegradable Hydroxyapatite/Collagen Composite Implanted in Bone. Implant. Dent. 2005, 14, 252–260. [Google Scholar] [CrossRef]
- Kikuchi, M. Hydroxyapatite/Collagen Bone-Like Nanocomposite. Biol. Pharm. Bull. 2013, 36, 1666–1669. [Google Scholar] [CrossRef]
- Díaz, A.; Puiggalí, J. Hydrogels for Biomedical Applications: Cellulose, Chitosan, and Protein/Peptide Derivatives. Gels 2017, 3, 27. [Google Scholar]
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef]
- Kawano, M.; Ariyoshi, W.; Iwanaga, K.; Okinaga, T.; Habu, M.; Yoshioka, I.; Tominaga, K.; Nishihara, T. Mechanism involved in enhancement of osteoblast differentiation by hyaluronic acid. Biochem. Biophys. Res. Commun. 2011, 405, 575–580. [Google Scholar] [CrossRef]
- Sall, I.; Férard, G. Comparison of the sensitivity of 11 crosslinked hyaluronic acid gels to bovine testis hyaluronidase. Polym. Degrad. Stab. 2007, 92, 915–919. [Google Scholar] [CrossRef]
- Chang, Y.-L.; Hsieh, C.-Y.; Yeh, C.-Y.; Lin, F.-H. The Development of Gelatin/Hyaluronate Copolymer Mixed with Calcium Sulfate, Hydroxyapatite, and Stromal-Cell-Derived Factor-1 for Bone Regeneration Enhancement. Polymers 2019, 11, 1454. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Lee, J.S.; Shin, J.; Lee, M.S.; Kang, D.; Hwang, N.S.; Lee, H.; Yang, H.S.; Cho, S.-W. Osteoconductive hybrid hyaluronic acid hydrogel patch for effective bone formation. J. Control. Release 2020, 327, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Wenz, A.; Borchers, K.; Tovar, G.E.M.; Kluger, P.J. Bone matrix production in hydroxyapatite-modified hydrogels suitable for bone bioprinting. Biofabrication 2017, 9, 044103. [Google Scholar] [CrossRef] [PubMed]
- Khor, E. Chitin: Fulfilling a Biomaterials Promise, 2nd ed.; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2014; pp. 1–155. [Google Scholar]
- Yamaguchi, I.; Tokuchi, K.; Fukuzaki, H.; Koyama, Y.; Takakuda, K.; Monma, H.; Tanaka, J. Preparation and microstructure analysis of chitosan/hydroxyapatite nanocomposites. J. Biomed. Mater. Res. 2001, 55, 20–27. [Google Scholar] [CrossRef]
- Ito, M. In vitro properties of a chitosan-bonded hydroxyapatite bone-filling paste. Biomaterials 1991, 12, 41–45. [Google Scholar] [CrossRef]
- Ramesh, N.; Moratti, S.C.; Dias, G.J. Hydroxyapatite-polymer biocomposites for bone regeneration: A review of current trends. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2046–2057. [Google Scholar] [CrossRef]
- Dhivya, S.; Saravanan, S.; Sastry, T.P.; Selvamurugan, N. Nanohydroxyapatite-reinforced chitosan composite hydrogel for bone tissue repair in vitro and in vivo. J. Nanobiotechnol. 2015, 13, 40. [Google Scholar] [CrossRef]
- Biazar, E.; Heidari Keshel, S.; Tavirani, M.R.; Jahandideh, R. Bone reconstruction in rat calvarial defects by chitosan/hydroxyapatite nanoparticles scaffold loaded with unrestricted somatic stem cells. Artif. Cells Nanomed. Biotechnol. 2015, 43, 112–116. [Google Scholar] [CrossRef]
- Ma, X.-Y.; Feng, Y.-F.; Ma, Z.-S.; Li, X.; Wang, J.; Wang, L.; Lei, W. The promotion of osteointegration under diabetic conditions using chitosan/hydroxyapatite composite coating on porous titanium surfaces. Biomaterials 2014, 35, 7259–7270. [Google Scholar] [CrossRef]
- Stanford, E.C.C. Improvements in the Manufacture of Useful Products from Seaweeds. British Patent 142, 1881. [Google Scholar]
- Tampieri, A.; Sandri, M.; Landi, E.; Celotti, G.; Roveri, N.; Mattioli-Belmonte, M.; Virgili, L.; Gabbanelli, F.; Biagini, G. HA/alginate hybrid composites prepared through bio-inspired nucleation. Acta Biomater. 2005, 1, 343–351. [Google Scholar] [CrossRef]
- Rajkumar, M.; Meenakshisundaram, N.; Rajendran, V. Development of nanocomposites based on hydroxyapatite/sodium alginate: Synthesis and characterisation. Mater. Charact. 2011, 62, 469–479. [Google Scholar] [CrossRef]
- Jorfi, M.; Foster, E.J. Recent advances in nanocellulose for biomedical applications. J. Appl. Polym. Sci. 2014, 132, 41719. [Google Scholar] [CrossRef]
- Fu, L.; Zhang, J.; Yang, G. Present status and applications of bacterial cellulose-based materials for skin tissue repair. Carbohydr. Polym. 2013, 92, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Petersen, N.; Gatenholm, P. Bacterial cellulose-based materials and medical devices: Current state and perspectives. Appl. Microbiol. Biotechnol. 2011, 91, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Aravamudhan, A.; Ramos, D.M.; Nip, J.; Harmon, M.D.; James, R.; Deng, M.; Laurencin, C.T.; Yu, X.; Kumbar, S.G. Cellulose and Collagen Derived Micro-Nano Structured Scaffolds for Bone Tissue Engineering. J. Biomed. Nanotechnol. 2013, 9, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, K.A.; LeBlanc, J.M.; Sheets, K.T.; Fox, R.W.; Gatenholm, P. Biomimetic design of a bacterial cellulose/hydroxyapatite nanocomposite for bone healing applications. Mater. Sci. Eng. C 2011, 31, 43–49. [Google Scholar] [CrossRef]
- Balla, E.; Daniilidis, V.; Karlioti, G.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Vlachopoulos, A.; Koumentakou, I.; Bikiaris, D.N. Poly(lactic Acid): A Versatile Biobased Polymer for the Future with Multifunctional Properties—From Monomer Synthesis, Polymerization Techniques and Molecular Weight Increase to PLA Applications. Polymers 2021, 13, 1822. [Google Scholar] [CrossRef]
- Elmowafy, E.M.; Tiboni, M.; Soliman, M.E. Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles. J. Pharm. Investig. 2019, 49, 347–380. [Google Scholar] [CrossRef]
- Wiegand, T.; Karr, J.; Steinkruger, J.D.; Hiebner, K.; Simetich, B.; Beatty, M.; Redepenning, J. Reconstruction of Anorganic Mammalian Bone by Surface-Initiated Polymerization of L-Lactide. Chem. Mater. 2008, 20, 5016–5022. [Google Scholar] [CrossRef]
- Wiegand, T.; Hiebner, K.; Gauza, L.; Schwartz, C.; Song, Z.; Miller, S.; Zacharias, N.; Wooley, P.H.; Redepenning, J. Biomimetic composites by surface-initiated polymerization of cyclic lactones at anorganic bone: Preparation and in vitro evaluation of osteoblast and osteoclast competence. J. Biomed. Mater. Res. Part A 2014, 102, 1755–1766. [Google Scholar] [CrossRef]
- Hong, Z.; Zhang, P.; He, C.; Qiu, X.; Liu, A.; Chen, L.; Zhu, X.; Jing, X. Nano-composite of poly(L-lactide) and surface grafted hydroxyapatite: Mechanical properties and biocompatibility. Biomaterials 2005, 26, 6296–6304. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Hong, Z.; Hu, J.; Chen, L.; Chen, X.; Jing, X. Hydroxyapatite Surface Modified by L-Lactic Acid and Its Subsequent Grafting Polymerization of L-Lactide. Biomacromolecules 2005, 6, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Hong, Z.; Yu, T.; Chen, X.; Jing, X. In vivo mineralization and osteogenesis of nanocomposite scaffold of poly(lactide-co-glycolide) and hydroxyapatite surface-grafted with poly(l-lactide). Biomaterials 2009, 30, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Ma, P.X. Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering. Biomaterials 2004, 25, 4749–4757. [Google Scholar] [CrossRef]
- Wei, G.; Ma, P.X. Macroporous and nanofibrous polymer scaffolds and polymer/bone-like apatite composite scaffolds generated by sugar spheres. J. Biomed. Mater. Res. Part A 2006, 78, 306–315. [Google Scholar] [CrossRef]
- He, C.; Jin, X.; Ma, P.X. Calcium phosphate deposition rate, structure and osteoconductivity on electrospun poly(l-lactic acid) matrix using electrodeposition or simulated body fluid incubation. Acta Biomater. 2014, 10, 419–427. [Google Scholar] [CrossRef]
- Huang, Y.X.; Ren, J.; Chen, C.; Ren, T.B.; Zhou, X.Y. Preparation and Properties of Poly(lactide-co-glycolide) (PLGA)/ Nano-Hydroxyapatite (NHA) Scaffolds by Thermally Induced Phase Separation and Rabbit MSCs Culture on Scaffolds. J. Biomater. Appl. 2008, 22, 409–432. [Google Scholar] [CrossRef]
- Johnson, R.; Ding, Y.; Nagiah, N.; Monnet, E.; Tan, W. Coaxially-structured fibres with tailored material properties for vascular graft implant. Mater. Sci. Eng. C 2018, 97, 1–11. [Google Scholar] [CrossRef]
- Chopra, V.; Thomas, J.; Sharma, A.; Panwar, V.; Kaushik, S.; Sharma, S.; Porwal, K.; Kulkarni, C.; Rajput, S.; Singh, H.; et al. Synthesis and Evaluation of a Zinc Eluting rGO/Hydroxyapatite Nanocomposite Optimized for Bone Augmentation. ACS Biomater. Sci. Eng. 2020, 6, 6710–6725. [Google Scholar] [CrossRef]
- Visakh, P.M. Polyhydroxyalkanoate (PHA) Based Blends, Composites and Nanocomposites, 1st ed.; Royal Society of Chemistry: London, UK, 2015; pp. 1–17. [Google Scholar]
- Chen, G.-Q.; Wu, Q. The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials 2005, 26, 6565–6578. [Google Scholar] [CrossRef]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Cheng, S.; Chen, G.; Leski, M.; Zou, B.; Wang, Y.; Wu, Q. The effect of D,L-β-hydroxybutyric acid on cell death and proliferation in L929 cells. Biomaterials 2006, 27, 3758–3765. [Google Scholar] [CrossRef] [PubMed]
- Kushwah, B.S.; Kushwah, A.V.S.; Singh, V. RETRACTED ARTICLE: Towards understanding polyhydroxyalkanoates and their use. J. Polym. Res. 2016, 23, 153. [Google Scholar] [CrossRef]
- Brigham, C.J.; Sinskey, A.J. Applications of Polyhydroxyalkanoates in the Medical Industry. Int. J. Biotechnol. Wellness Ind. 2012, 1, 52–60. [Google Scholar] [CrossRef]
- Hong, S.-G.; Hsu, H.-W.; Ye, M.-T. Thermal properties and applications of low molecular weight polyhydroxybutyrate. J. Therm. Anal. Calorim. 2013, 111, 1243–1250. [Google Scholar] [CrossRef]
- Lopes, P.P.; Garcia, M.P.; Fernandes, M.H.; Fernandes, M.H.V. Acrylic formulations containing bioactive and biodegradable fillers to be used as bone cements: Properties and biocompatibility assessment. Mater. Sci. Eng. C 2013, 33, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Sadat-Shojai, M.; Khorasani, M.-T.; Jamshidi, A.; Irani, S. Nano-hydroxyapatite reinforced polyhydroxybutyrate composites: A comprehensive study on the structural and in vitro biological properties. Mater. Sci. Eng. C 2013, 33, 2776–2787. [Google Scholar] [CrossRef]
- Shishatskaya, E.I.; Khlusov, I.A.; Volova, T.G. A hybrid PHB–hydroxyapatite composite for biomedical application: Production, in vitro and in vivo investigation. J. Biomater. Sci. Polym. Ed. 2006, 17, 481–498. [Google Scholar] [CrossRef]
- Ramier, J.; Grande, D.; Bouderlique, T.; Stoilova, O.; Manolova, N.; Rashkov, I.; Langlois, V.; Albanese, P.; Renard, E. From design of bio-based biocomposite electrospun scaffolds to osteogenic differentiation of human mesenchymal stromal cells. J. Mater. Sci. Mater. Med. 2014, 25, 1563–1575. [Google Scholar] [CrossRef]
- Bernd, H.E.; Kunze, C.; Freier, T.; Sternberg, K.; Kramer, S.; Behrend, D.; Prall, F.; Donat, M.; Kramp, B. Poly(3-hydroxybutyrate) (PHB) patches for covering anterior skull base defects—An animal study with minipigs. Acta Oto-Laryngol. 2009, 129, 1010–1017. [Google Scholar] [CrossRef]
- Gredes, T.; Gedrange, T.; Hinüber, C.; Gelinsky, M.; Kunert-Keil, C. Histological and molecular-biological analyses of poly(3-hydroxybutyrate) (PHB) patches for enhancement of bone regeneration. Ann. Anat.—Anat. Anz. 2015, 199, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.G.L.; Rezende, C.M.D.F.; Serakides, R.; Pereira, M.D.M.; Rosado, I.R. Orthopedic implant of a polyhydroxybutyrate (PHB) and hydroxyapatite composite in cats. J. Feline Med. Surg. 2011, 13, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Celarek, A.; Kraus, T.; Tschegg, E.K.; Fischerauer, S.F.; Stanzl-Tschegg, S.; Uggowitzer, P.J.; Weinberg, A.M. PHB, crystalline and amorphous magnesium alloys: Promising candidates for bioresorbable osteosynthesis implants? Mater. Sci. Eng. C 2012, 32, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Doyle, C.; Tanner, E.T.; Bonfield, W. In vitro and in vivo evaluation of polyhydroxybutyrate and of polyhydroxybutyrate reinforced with hydroxyapatite. Biomaterials 1991, 12, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Wang, M. In vitro evaluation of hydroxyapatite reinforced polyhydroxybutyrate composite. Mater. Sci. Eng. C 2002, 20, 101–109. [Google Scholar] [CrossRef]
- Galego, N.; Rozsa, C.; Sánchez, R.; Fung, J.; Vázquez, A.; Santo Tomás, J. Characterization and application of poly(β-hydroxyalkanoates) family as composite biomaterials. Polym. Test. 2000, 19, 485–492. [Google Scholar] [CrossRef]
- Guo, B.; Ma, P.X. Conducting Polymers for Tissue Engineering. Biomacromolecules 2018, 19, 1764–1782. [Google Scholar] [CrossRef]
- Fujii, E.; Ohkubo, M.; Tsuru, K.; Hayakawa, S.; Osaka, A.; Kawabata, K.; Bonhomme, C.; Babonneau, F. Selective protein adsorption property and characterization of nano-crystalline zinc-containing hydroxyapatite. Acta Biomater. 2006, 2, 69–74. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Zhang, S.; Gangal, G.; Uludağ, H. ‘Magic bullets’ for bone diseases: Progress in rational design of bone-seeking medicinal agents. Chem. Soc. Rev. 2007, 36, 507–531. [Google Scholar] [CrossRef]
- Iafisco, M.; Palazzo, B.; Falini, G.; di Foggia, M.; Bonora, S.; Nicolis, S.; Casella, L.; Roveri, N. Adsorption and Conformational Change of Myoglobin on Biomimetic Hydroxyapatite Nanocrystals Functionalized with Alendronate. Langmuir 2008, 24, 4924–4930. [Google Scholar] [CrossRef] [PubMed]
- Schuessele, A.; Mayr, H.; Tessmar, J.; Goepferich, A. Enhanced bone morphogenetic protein-2 performance on hydroxyapatite ceramic surfaces. J. Biomed. Mater. Res. Part A 2009, 90, 959–971. [Google Scholar] [CrossRef]
- Kandori, K.; Oda, S.; Tsuyama, S. Effects of Pyrophosphate Ions on Protein Adsorption onto Calcium Hydroxyapatite. J. Phys. Chem. B 2008, 112, 2542–2547. [Google Scholar] [CrossRef] [PubMed]
- Szurkowska, K.; Laskus, A.; Kolmas, J. Hydroxyapatite—Advances in Composite Nanomaterials, Biomedical Applications and Its Technological Facets; Intechopen: London, UK, 2018; p. 186. [Google Scholar]
- Kolmas, J.; Krukowski, S.; Laskus, A.; Jurkitewicz, M. Synthetic hydroxyapatite in pharmaceutical applications. Ceram. Int. 2016, 42, 2472–2487. [Google Scholar] [CrossRef]
- Turon, P.; del Valle, L.J.; Alemán, C.; Puiggalí, J. Biopolymer Grafting Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 45–80. [Google Scholar]
- Lian, X.; Mao, K.; Liu, X.; Wang, X.; Cui, F. In Vivo Osteogenesis of Vancomycin Loaded Nanohydroxyapatite/Collagen/Calcium Sulfate Composite for Treating Infectious Bone Defect Induced by Chronic Osteomyelitis. J. Nanomater. 2015, 2015, 13. [Google Scholar] [CrossRef]
- Suvannapruk, W.; Thammarakcharoen, F.; Phanpiriya, P.; Suwanprateeb, J. Development of Antibiotics Impregnated Nanosized Silver Phosphate-Doped Hydroxyapatite Bone Graft. J. Nanomater. 2013, 2013, 4. [Google Scholar] [CrossRef]
- Tesema, Y.; Raghavan, D.; Stubbs, J. Bone cell viability on collagen immobilized poly(3-hydroxybutrate-co-3-hydroxyvalerate) membrane: Effect of surface chemistry. J. Appl. Polym. Sci. 2004, 93, 2445–2453. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, X.; Zhu, Y.; Zhang, J.; Hu, P. Surface Modification of Polyhydroxyalkanoates by Ion Implantation. Characterization and Cytocompatibility Improvement. Polym. J. 2003, 35, 148–154. [Google Scholar] [CrossRef]
- Zuo, C.; Huang, Y.; Bajis, R.; Sahih, M.; Li, Y.-P.; Dai, K.; Zhang, X. Osteoblastogenesis regulation signals in bone remodeling. Osteoporos. Int. 2012, 23, 1653–1663. [Google Scholar] [CrossRef]
- Tafazoli Moghadam, E.; Yazdanian, M.; Alam, M.; Tebyanian, H.; Tafazoli, A.; Tahmasebi, E.; Ranjbar, R.; Yazdanian, A.; Seifalian, A. Current natural bioactive materials in bone and tooth regeneration in dentistry: A comprehensive overview. J. Mater. Res. Technol. 2021, 13, 2078–2114. [Google Scholar] [CrossRef]
- Kaida, K.; Honda, Y.; Hashimoto, Y.; Tanaka, M.; Baba, S. Application of Green Tea Catechin for Inducing the Osteogenic Differentiation of Human Dedifferentiated Fat Cells In Vitro. Int. J. Mol. Sci. 2015, 16, 27988–28000. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Chandra, S.; Chatterjee, P.; Dey, P. Evaluation of anti-inflammatory effects of green tea and black tea: A comparative in vitro study. J. Adv. Pharm. Technol. Res. 2012, 3, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Hengge, R. Targeting Bacterial Biofilms by the Green Tea Polyphenol EGCG. Molecules 2019, 24, 2403. [Google Scholar] [CrossRef] [PubMed]
- Boonyagul, S.; Banlunara, W.; Sangvanich, P.; Thunyakitpisal, P. Effect of acemannan, an extracted polysaccharide from Aloe vera, on BMSCs proliferation, differentiation, extracellular matrix synthesis, mineralization, and bone formation in a tooth extraction model. Odontology 2013, 102, 310–317. [Google Scholar] [CrossRef]
- Le Van, C.; Thu, H.P.T.; Sangvanich, P.; Chuenchompoonut, V.; Thunyakitpisal, P. Acemannan induces rapid early osseous defect healing after apical surgery: A 12-month follow-up of a randomized controlled trial. J. Dent. Sci. 2020, 15, 302–309. [Google Scholar] [CrossRef]
- Xie, Y.; Sun, W.; Yan, F.; Liu, H.; Deng, Z.; Cai, L. Icariin-loaded porous scaffolds for bone regeneration through the regulation of the coupling process of osteogenesis and osteoclastic activity. Int. J. Nanomed. Ume 2019, 14, 6019–6033. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Cao, H.; Wang, X.; Chen, S.; Zhang, M.; Wang, N.; Yao, Z.; Dai, Y.; Xie, X.; Zhang, P.; et al. Porous composite scaffold incorporating osteogenic phytomolecule icariin for promoting skeletal regeneration in challenging osteonecrotic bone in rabbits. Biomaterials 2018, 153, 1–13. [Google Scholar] [CrossRef]
- Ahangari, N.; Kargozar, S.; Ghayour-Mobarhan, M.; Baino, F.; Pasdar, A.; Sahebkar, A.; Ferns, G.A.A.; Kim, H.; Mozafari, M. Curcumin in tissue engineering: A traditional remedy for modern medicine. BioFactors 2019, 45, 135–151. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.-Z. Sustained curcumin release from PLGA microspheres improves bone formation under diabetic conditions by inhibiting the reactive oxygen species production. Drug Des. Dev. Ther. Ume. 2018, 12, 1453–1466. [Google Scholar] [CrossRef]
- Sarkar, N.; Bose, S. Liposome-Encapsulated Curcumin-Loaded 3D Printed Scaffold for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2019, 11, 17184–17192. [Google Scholar] [CrossRef]
- Zhai, J.-L.; Weng, X.-S.; Wu, Z.-H.; Guo, S.-G. Effect of Resveratrol on Preventing Steroid-induced Osteonecrosis in a Rabbit Model. Chin. Med. J. 2016, 129, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Casarin, R.; Casati, M.; Pimentel, S.; Cirano, F.; Algayer, M.; Pires, P.; Ghiraldini, B.; Duarte, P.; Ribeiro, F. Resveratrol improves bone repair by modulation of bone morphogenetic proteins and osteopontin gene expression in rats. Int. J. Oral Maxillofac. Surg. 2014, 43, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-C.; Wang, C.-H.; Chen, H.-C.; Cherng, J.-H.; Chang, S.-J.; Wang, Y.-W.; Chang, A.; Yeh, J.-Z.; Huang, Y.-H.; Liu, C.-C. Combination of resveratrol-containing collagen with adipose stem cells for craniofacial tissue-engineering applications. Int. Wound J. 2018, 15, 660–672. [Google Scholar] [CrossRef] [PubMed]
- Poornima, B.; Korrapati, P.S. Fabrication of chitosan-polycaprolactone composite nanofibrous scaffold for simultaneous delivery of ferulic acid and resveratrol. Carbohydr. Polym. 2017, 157, 1741–1749. [Google Scholar] [CrossRef] [PubMed]
- Notodihardjo, F.Z.; Kakudo, N.; Kushida, S.; Suzuki, K.; Kusumoto, K. Bone regeneration with BMP-2 and hydroxyapatite in critical-size calvarial defects in rats. J. Cranio-Maxillofac. Surg. 2012, 40, 287–291. [Google Scholar] [CrossRef]
- Feito, M.J.; Serrano, M.C.; Oñaderra, M.; Matesanz, M.C.; Sánchez-Salcedo, S.; Arcos, D.; Vallet-Regí, M.; Portolés, M.T. Effects of immobilized VEGF on endothelial progenitor cells cultured on silicon substituted and nanocrystalline hydroxyapatites. RSC Adv. 2016, 6, 92586–92595. [Google Scholar] [CrossRef][Green Version]
- Rozen, N.; Bick, T.; Bajayo, A.; Shamian, B.; Schrift-Tzadok, M.; Gabet, Y.; Yayon, A.; Bab, I.; Soudry, M.; Lewinson, D. Transplanted blood-derived endothelial progenitor cells (EPC) enhance bridging of sheep tibia critical size defects. Bone 2009, 45, 918–924. [Google Scholar] [CrossRef]
- Tsurushima, H.; Marushima, A.; Suzuki, K.; Oyane, A.; Sogo, Y.; Nakamura, K.; Matsumura, A.; Ito, A. Enhanced bone formation using hydroxyapatite ceramic coated with fibroblast growth factor-2. Acta Biomater. 2010, 6, 2751–2759. [Google Scholar] [CrossRef]
- Sun, T.; Qu, Y.; Cui, W.; Yang, L.; Ji, Y.; Yu, W.; Navinduth, R.; Shao, Z.; Yang, H.; Guo, X. Evaluation of osteogenic inductivity of a novel BMP2-mimicking peptide P28 and P28-containing bone composite. J. Biomed. Mater. Res. Part A 2017, 106, 210–220. [Google Scholar] [CrossRef]
- Huang, Y.; Ren, J.; Ren, T.; Gu, S.; Tan, Q.; Zhang, L.; Lv, K.; Pan, K.; Jiang, X. Bone marrow stromal cells cultured on poly (lactide-co-glycolide)/nano-hydroxyapatite composites with chemical immobilization of Arg-Gly-Asp peptide and preliminary bone regeneration of mandibular defect thereof. J. Biomed. Mater. Res. Part A 2010, 95, 993–1003. [Google Scholar] [CrossRef]
- Kim, H.K.; Kim, J.H.; Park, D.S.; Park, K.S.; Kang, S.S.; Lee, J.S.; Jeong, M.H.; Yoon, T.R. Osteogenesis induced by a bone forming peptide from the prodomain region of BMP-7. Biomaterials 2012, 33, 7057–7063. [Google Scholar] [CrossRef] [PubMed]
- Reneker, D.H.; Chun, I. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology 1996, 7, 216–223. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L.; Fong, H.; Koombhongse, S. Bending instability of electrically charged liquid jets of polymer solutions in electrospinning. J. Appl. Phys. 2000, 87, 4531–4547. [Google Scholar] [CrossRef]
- Frenot, A.; Chronakis, I.S. Polymer nanofibers assembled by electrospinning. Curr. Opin. Colloid Interface Sci. 2003, 8, 64–75. [Google Scholar] [CrossRef]
- Dzenis, Y. Spinning Continuous Fibers for Nanotechnology. Science 2004, 304, 1917–1919. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Electrospinning of Nanofibers: Reinventing the Wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Jayaraman, K.; Kotaki, M.; Zhang, Y.; Mo, X.; Ramakrishna, S. Recent advances in polymer nanofibers. J. Nanosci. Nanotechnol. 2004, 4, 52–65. [Google Scholar]
- Dhakate, S.R.; Singla, B.; Uppal, M.; Mathur, R.B. Effect of processing parameters on morphology and thermal properties of electrospun polycarbonate nanofibers. Adv. Mater. Lett. 2010, 1, 200–204. [Google Scholar]
- Sharma, S. Ferrolectric Nanofibers: Principle, Processing and Applications. Adv. Mater. Lett. 2013, 4, 522–533. [Google Scholar] [CrossRef]
- Dersch, R.; Steinhart, M.; Boudriot, U.; Greiner, A.; Wendorff, J.H. Nanoprocessing of polymers: Applications in medicine, sensors, catalysis, photonics. Polym. Adv. Technol. 2005, 16, 276–282. [Google Scholar] [CrossRef]
- Chronakis, I.S. Novel nanocomposites and nanoceramics based on polymer nanofibers using electrospinning process—A review. J. Mater. Process. Technol. 2005, 167, 283–293. [Google Scholar] [CrossRef]
- Deitzel, J.M.; Kleinmeyer, J.; Harris, D.; Beck Tan, N.C. The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer 2001, 42, 261–272. [Google Scholar] [CrossRef]
- Llorens, E.; Armelin, E.; del Mar Pérez-Madrigal, M.; del Valle, L.J.; Alemán, C.; Puiggalí, J. Nanomembranes and Nanofibers from Biodegradable Conducting Polymers. Polymers 2013, 5, 1115–1157. [Google Scholar] [CrossRef]
- Sankar, S.; Sharma, C.S.; Rath, S.N.; Ramakrishna, S. Electrospun Fibers for Recruitment and Differentiation of Stem Cells in Regenerative Medicine. Biotechnol. J. 2017, 12, 1700263. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, L.; Xie, J.; Shen, L.; Tao, J.; Zhu, J. Facile Strategy to Generate Aligned Polymer Nanofibers: Effects on Cell Adhesion. ACS Appl. Mater. Interfaces 2018, 10, 1566–1574. [Google Scholar] [CrossRef] [PubMed]
- Denchai, A.; Tartarini, D.; Mele, E. Cellular Response to Surface Morphology: Electrospinning and Computational Modeling. Front. Bioeng. Biotechnol. 2018, 6, 155. [Google Scholar] [CrossRef]
- Badami, A.S.; Kreke, M.R.; Thompson, M.S.; Riffle, J.S.; Goldstein, A.S. Effect of fiber diameter on spreading, proliferation, and differentiation of osteoblastic cells on electrospun poly(lactic acid) substrates. Biomaterials 2006, 27, 596–606. [Google Scholar] [CrossRef]
- Chen, Y.; Shafiq, M.; Liu, M.; Morsi, Y.; Mo, X. Advanced fabrication for electrospun three-dimensional nanofiber aerogels and scaffolds. Bioact. Mater. 2020, 5, 963–979. [Google Scholar] [CrossRef]
- Blakeney, B.A.; Tambralli, A.; Anderson, J.M.; Andukuri, A.; Lim, D.-J.; Dean, D.R.; Jun, H.-W. Cell infiltration and growth in a low density, uncompressed three-dimensional electrospun nanofibrous scaffold. Biomaterials 2011, 32, 1583–1590. [Google Scholar] [CrossRef]
- Chainani, A.; Hippensteel, K.J.; Kishan, A.; Garrigues, N.W.; Ruch, D.S.; Guilak, F.; Little, D. Multilayered Electrospun Scaffolds for Tendon Tissue Engineering. Tissue Eng. Part A 2013, 19, 2594–2604. [Google Scholar] [CrossRef]
- Sun, B.; Li, J.; Liu, W.; Aqeel, B.M.; El-Hamshary, H.; Al-Deyab, S.S.; Mo, X. Fabrication and characterization of mineralized P(LLA-CL)/SF three-dimensional nanoyarn scaffolds. Iran. Polym. J. 2015, 24, 29–40. [Google Scholar] [CrossRef]
- Kim, T.G.; Chung, H.J.; Park, T.G. Macroporous and nanofibrous hyaluronic acid/collagen hybrid scaffold fabricated by concurrent electrospinning and deposition/leaching of salt particles. Acta Biomater. 2008, 4, 1611–1619. [Google Scholar] [CrossRef] [PubMed]
- Shim, I.K.; Suh, W.H.; Lee, S.Y.; Lee, S.H.; Heo, S.J.; Lee, M.C.; Lee, S.J. Chitosan nano-/microfibrous double-layered membrane with rolled-up three-dimensional structures for chondrocyte cultivation. J. Biomed. Mater. Res. Part A 2009, 90, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Sampath Kumar, T.S.; Yogeshwar Chakrapani, V. Electrospun 3D Scaffolds for Tissue Regeneration. In Cutting-Edge Enabling Technologies for Regenerative Medicine. Advances in Experimental Medicine and Biology; Springer: Singapore, 2018; Volume 1078, pp. 29–47. [Google Scholar]
- Chen, Y.; Yu, Z.; Meng, X.; Li, H.; Sun, X.; He, D.; Zhang, Y.; Zhang, Z. Localized surface plasmon resonance improves transdermal photodynamic therapy of hypertrophic scars. Nano Res. 2022, 15, 4258–4265. [Google Scholar] [CrossRef]
- Vyas, C.; Ates, G.; Aslan, E.; Hart, J.; Huang, B.; Bartolo, P. Three-Dimensional Printing and Electrospinning Dual-Scale Polycaprolactone Scaffolds with Low-Density and Oriented Fibers to Promote Cell Alignment. 3D Print. Addit. Manuf. 2020, 7, 105–113. [Google Scholar] [CrossRef]
- Guo, Y.; Huang, J.; Fang, Y.; Huang, H.; Wu, J. 1D, 2D, and 3D scaffolds promoting angiogenesis for enhanced wound healing. Chem. Eng. J. 2022, 437, 134690. [Google Scholar] [CrossRef]
- Shafiq, M.; Zhang, Q.; Zhi, D.; Wang, K.; Kong, D.; Kim, D.-H.; Kim, S.H. In Situ Blood Vessel Regeneration Using SP (Substance P) and SDF (Stromal Cell–Derived Factor)-1α Peptide Eluting Vascular Grafts. Arter. Thromb. Vasc. Biol. 2018, 38, e117–e134. [Google Scholar] [CrossRef]
- Gao, X.; Han, S.; Zhang, R.; Liu, G.; Wu, J. Progress in electrospun composite nanofibers: Composition, performance and applications for tissue engineering. J. Mater. Chem. B 2019, 7, 7075–7089. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Adhikary, P.; Jana, S.; Biswas, A.; Sencadas, V.; Gupta, S.D.; Tudu, B.; Mandal, D. Electrospun gelatin nanofiber based self-powered bio-e-skin for health care monitoring. Nano Energy 2017, 36, 166–175. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.-T.; Hu, P.-Y.; Liu, J.-J.; Liu, X.-F.; Hu, M.; Cui, Z.; Wang, N.; Niu, Z.; Xiang, H.-F.; et al. Laparoscopic electrospinning for in situ hemostasis in minimally invasive operation. Chem. Eng. J. 2020, 395, 125089. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Dalton, P.D. Melt Electrospinning. Chem. Asian J. 2011, 6, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Bachs-Herrera, A.; Yousefzade, O.; del Valle, L.; Puiggali, J. Melt Electrospinning of Polymers: Blends, Nanocomposites, Additives and Applications. Appl. Sci. 2021, 11, 1808. [Google Scholar] [CrossRef]
- Brown, T.D.; Dalton, P.D.; Hutmacher, D.W. Melt electrospinning today: An opportune time for an emerging polymer process. Prog. Polym. Sci. 2016, 56, 116–166. [Google Scholar] [CrossRef]
- Zhang, L.-H.; Duan, X.-P.; Yan, X.; Yu, M.; Ning, X.; Zhao, Y.; Long, Y.-Z. Recent advances in melt electrospinning. RSC Adv. 2016, 6, 53400–53414. [Google Scholar] [CrossRef]
- Góra, A.; Sahay, R.; Thavasi, V.; Ramakrishna, S. Melt-Electrospun Fibers for Advances in Biomedical Engineering, Clean Energy, Filtration, and Separation. Polym. Rev. 2011, 51, 265–287. [Google Scholar] [CrossRef]
- Dalton, P.D. Melt electrowriting with additive manufacturing principles. Curr. Opin. Biomed. Eng. 2017, 2, 49–57. [Google Scholar] [CrossRef]
- Castilho, M.; Feyen, D.; Flandes-Iparraguirre, M.; Hochleitner, G.; Groll, J.; Doevendans, P.A.F.; Vermonden, T.; Ito, K.; Sluijter, J.P.G.; Malda, J. Melt Electrospinning Writing of Poly-Hydroxymethylglycolide-co-ε-Caprolactone-Based Scaffolds for Cardiac Tissue Engineering. Adv. Healthc. Mater. 2017, 6, 1700311. [Google Scholar] [CrossRef]
- Sun, Z.; Zussman, E.; Yarin, A.L.; Wendorff, J.H.; Greiner, A. Compound Core–Shell Polymer Nanofibers by Co-Electrospinning. Adv. Mater. 2003, 15, 1929–1932. [Google Scholar] [CrossRef]
- Huang, J.; Cao, Y.; Huang, Z.; Imbraguglio, S.A.; Wang, Z.; Peng, X.; Guo, Z. Comparatively Thermal and Crystalline Study of Poly(methyl-methacrylate)/Polyacrylonitrile Hybrids: Core-Shell Hollow Fibers, Porous Fibers, and Thin Films. Macromol. Mater. Eng. 2016, 301, 1327–1336. [Google Scholar] [CrossRef]
- Elahi, M.F.; Lu, W.; Guoping, G.; Khan, F. Core-shell fibers for biomedical applications—A review. J. Bioeng. Biomed. Sci. 2013, 3, 121. [Google Scholar] [CrossRef]
- Han, D.; Steckl, A.J. Triaxial Electrospun Nanofiber Membranes for Controlled Dual Release of Functional Molecules. ACS Appl. Mater. Interfaces 2013, 5, 8241–8245. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-S.; Lee, B.-S.; You, B.-C.; Sohn, H.-J.; Yu, W.-R. Fabrication of carbon nanofibers with Si nanoparticle-stuffed cylindrical multi-channels via coaxial electrospinning and their anodic performance. RSC Adv. 2014, 4, 47389–47395. [Google Scholar] [CrossRef]
- Rahimi, M.; Mokhtari, J. Fabrication of thermo-regulating hexadecane-polyurethane core-shell composite nanofibrous mat as advanced technical layer: Effect of coaxial nozzle geometry. J. Ind. Text. 2018, 47, 1134–1151. [Google Scholar] [CrossRef]
- Yu, D.-G.; Branford-White, C.; Bligh, S.W.A.; White, K.; Chatterton, N.P.; Zhu, L.-M. Improving Polymer Nanofiber Quality Using a Modified Co-axial Electrospinning Process. Macromol. Rapid Commun. 2011, 32, 744–750. [Google Scholar] [CrossRef]
- Xu, Y.; Li, J.-J.; Yu, D.-G.; Williams, G.R.; Yang, J.-H.; Wang, X. Influence of the drug distribution in electrospun gliadin fibers on drug-release behavior. Eur. J. Pharm. Sci. 2017, 106, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yu, D.-G.; Zhang, L.-L.; Liu, X.-K.; Deng, Y.-C.; Zhao, M. Electrospun hypromellose-based hydrophilic composites for rapid dissolution of poorly water-soluble drug. Carbohydr. Polym. 2017, 174, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.T.; Chung, O.H.; Park, J.S. Coaxial electrospun poly(lactic acid)/chitosan (core/shell) composite nanofibers and their antibacterial activity. Carbohydr. Polym. 2011, 86, 1799–1806. [Google Scholar] [CrossRef]
- Yi, F.; LaVan, D.A. Poly(glycerol sebacate) Nanofiber Scaffolds by Core/Shell Electrospinning. Macromol. Biosci. 2008, 8, 803–806. [Google Scholar] [CrossRef]
- Huang, Z.-M.; Zhang, Y.; Ramakrishna, S. Double-layered composite nanofibers and their mechanical performance. J. Polym. Sci. Part B Polym. Phys. 2005, 43, 2852–2861. [Google Scholar] [CrossRef]
- He, C.-L.; Huang, Z.-M.; Han, X.-J. Fabrication of drug-loaded electrospun aligned fibrous threads for suture applications. J. Biomed. Mater. Res. Part A 2009, 89, 80–95. [Google Scholar] [CrossRef]
- Sill, T.J.; von Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef] [PubMed]
- Xiaoqiang, L.; Yan, S.; Rui, C.; Chuanglong, H.; Hongsheng, W.; Xiumei, M. Fabrication and properties of core-shell structure P(LLA-CL) nanofibers by coaxial electrospinning. J. Appl. Polym. Sci. 2008, 111, 1564–1570. [Google Scholar] [CrossRef]
- Llorens, E.; Ibañez, H.; del Valle, L.J.; Puiggalí, J. Biocompatibility and drug release behavior of scaffolds prepared by coaxial electrospinning of poly(butylene succinate) and polyethylene glycol. Mater. Sci. Eng. C 2015, 49, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Khalf, A.; Singarapu, K.; Madihally, S.V. Cellulose acetate core–shell structured electrospun fiber: Fabrication and characterization. Cellulose 2015, 22, 1389–1400. [Google Scholar] [CrossRef]
- Lallave, M.; Bedia, J.; Ruiz-Rosas, R.; Rodríguez-Mirasol, J.; Cordero, T.; Otero, J.C.; Marquez, M.; Barrero, A.; Loscertales, I.G. Filled and Hollow Carbon Nanofibers by Coaxial Electrospinning of Alcell Lignin without Binder Polymers. Adv. Mater. 2007, 19, 4292–4296. [Google Scholar] [CrossRef]
- Ou, K.-L.; Chen, C.-S.; Lin, L.-H.; Lu, J.-C.; Shu, Y.-C.; Tseng, W.-C.; Yang, J.-C.; Lee, S.-Y.; Chen, C.-C. Membranes of epitaxial-like packed, super aligned electrospun micron hollow poly(l-lactic acid) (PLLA) fibers. Eur. Polym. J. 2011, 47, 882–892. [Google Scholar] [CrossRef]
- Pakravan, M.; Heuzey, M.-C.; Ajji, A. Core–Shell Structured PEO-Chitosan Nanofibers by Coaxial Electrospinning. Biomacromolecules 2012, 13, 412–421. [Google Scholar] [CrossRef]
- Zhou, F.-L.; Hubbard, P.L.; Eichhorn, S.J.; Parker, G.J.M. Coaxially Electrospun Axon-Mimicking Fibers for Diffusion Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces 2012, 4, 6311–6316. [Google Scholar] [CrossRef]
- Ji, X.; Su, Z.; Wang, P.; Ma, G.; Zhang, S. Polyelectrolyte Doped Hollow Nanofibers for Positional Assembly of Bienzyme System for Cascade Reaction at O/W Interface. ACS Catal. 2014, 4, 4548–4559. [Google Scholar] [CrossRef]
- Esmaeili, A.; Haseli, M. Electrospinning of thermoplastic carboxymethyl cellulose/poly(ethylene oxide) nanofibers for use in drug-release systems. Mater. Sci. Eng. C 2017, 77, 1117–1127. [Google Scholar] [CrossRef]
- Zhu, J.; Huang, W.; Zhang, Q.; Ling, S.; Chen, Y.; Kaplan, D.L. Aqueous-Based Coaxial Electrospinning of Genetically Engineered Silk Elastin Core-Shell Nanofibers. Materials 2016, 9, 221. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-S.; Yang, H.-S.; Yu, W.-R. Fabrication of double-tubular carbon nanofibers using quadruple coaxial electrospinning. Nanotechnology 2014, 25, 465602. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Fu, J.; Li, Y.; Li, F. Nanomechanical mapping of glass fiber reinforced polymer composites using atomic force acoustic microscopy. J. Appl. Polym. Sci. 2013, 131, 39800. [Google Scholar] [CrossRef]
- Forward, K.M.; Flores, A.; Rutledge, G.C. Production of core/shell fibers by electrospinning from a free surface. Chem. Eng. Sci. 2013, 104, 250–259. [Google Scholar] [CrossRef]
- Qi, H.; Hu, P.; Xu, J.; Wang, A. Encapsulation of Drug Reservoirs in Fibers by Emulsion Electrospinning: Morphology Characterization and Preliminary Release Assessment. Biomacromolecules 2006, 7, 2327–2330. [Google Scholar] [CrossRef]
- Nikmaram, N.; Roohinejad, S.; Hashemi, S.; Koubaa, M.; Barba, F.J.; Abbaspourrad, A.; Greiner, R. Emulsion-based systems for fabrication of electrospun nanofibers: Food, pharmaceutical and biomedical applications. RSC Adv. 2017, 7, 28951–28964. [Google Scholar] [CrossRef]
- Agarwal, S.; Greiner, A. On the way to clean and safe electrospinning-green electrospinning: Emulsion and suspension electrospinning. Polym. Adv. Technol. 2011, 22, 372–378. [Google Scholar] [CrossRef]
- Xu, X.; Yang, L.; Xu, X.; Wang, X.; Chen, X.; Liang, Q.; Zeng, J.; Jing, X. Ultrafine medicated fibers electrospun from W/O emulsions. J. Control. Release 2005, 108, 33–42. [Google Scholar] [CrossRef]
- Bazilevsky, A.V.; Yarin, A.L.; Megaridis, C.M. Co-electrospinning of Core−Shell Fibers Using a Single-Nozzle Technique. Langmuir 2007, 23, 2311–2314. [Google Scholar] [CrossRef]
- Yarin, A. Coaxial electrospinning and emulsion electrospinning of core-shell fibers. Polym. Adv. Technol. 2010, 22, 310–317. [Google Scholar] [CrossRef]
- Xu, X.; Zhuang, X.; Chen, X.; Wang, X.; Yang, L.; Jing, X. Preparation of Core-Sheath Composite Nanofibers by Emulsion Electrospinning. Macromol. Rapid Commun. 2006, 27, 1637–1642. [Google Scholar] [CrossRef]
- Crespy, D.; Friedemann, K.; Popa, A.-M. Colloid-Electrospinning: Fabrication of Multicompartment Nanofibers by the Electrospinning of Organic or/and Inorganic Dispersions and Emulsions. Macromol. Rapid Commun. 2012, 33, 1978–1995. [Google Scholar] [CrossRef]
- Rajzer, I.; Kurowska, A.; Jabłoński, A.; Jatteau, S.; Śliwka, M.; Ziąbka, M.; Menaszek, E. Layered gelatin/PLLA scaffolds fabricated by electrospinning and 3D printing- for nasal cartilages and subchondral bone reconstruction. Mater. Des. 2018, 155, 297–306. [Google Scholar] [CrossRef]
- Chiara, G.; Letizia, F.; Lorenzo, F.; Edoardo, S.; Diego, S.; Stefano, S.; Eriberto, B.; Barbara, Z. Nanostructured Biomaterials for Tissue Engineered Bone Tissue Reconstruction. Int. J. Mol. Sci. 2012, 13, 737–757. [Google Scholar] [CrossRef]
- Cao, D.; Wu, Y.-P.; Fu, Z.-F.; Tian, Y.; Li, C.-J.; Gao, C.-Y.; Chen, Z.-L.; Feng, X.-Z. Cell adhesive and growth behavior on electrospun nanofibrous scaffolds by designed multifunctional composites. Colloids Surfaces B Biointerfaces 2011, 84, 26–34. [Google Scholar] [CrossRef]
- Yu, N.Y.C.; O’Brien, C.A.; Slapetova, I.; Whan, R.M.; Knothe Tate, M.L. Live Tissue Imaging to Elucidate Mechanical Modulation of Stem Cell Niche Quiescence. Stem Cells Transl. Med. 2017, 6, 285–292. [Google Scholar] [CrossRef]
- Lin, Z.; Fateh, A.; Salem, D.M.; Intini, G. Periosteum: Biology and Applications in Craniofacial Bone Regeneration. J. Dent. Res. 2014, 93, 109–116. [Google Scholar] [CrossRef]
- Squier, C.A.; Ghoneim, S.; Kremenak, C.R. Ultrastructure of the periosteum from membrane bone. J. Anat. 1990, 171, 233–239. [Google Scholar]
- Sun, F.; Chen, J.; Jin, S.; Wang, J.; Man, Y.; Li, J.; Zou, Q.; Li, Y.; Zuo, Y. Development of biomimetic trilayer fibrous membranes for guided bone regeneration. J. Mater. Chem. B 2018, 7, 665–675. [Google Scholar] [CrossRef]
- Liu, F.; Wang, X.; Chen, T.; Zhang, N.; Wei, Q.; Tian, J.; Wang, Y.; Ma, C.; Lu, Y. Hydroxyapatite/silver electrospun fibers for anti-infection and osteoinduction. J. Adv. Res. 2020, 21, 91–102. [Google Scholar] [CrossRef]
- Kareem, M.M.; Hodgkinson, T.; Sanchez, M.S.; Dalby, M.J.; Tanner, K.E. Hybrid core–shell scaffolds for bone tissue engineering. Biomed. Mater. 2019, 14, 025008. [Google Scholar] [CrossRef] [PubMed]
- Tanner, K.E. Bioactive composites for bone tissue engineering. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2010, 224, 1359–1372. [Google Scholar] [CrossRef] [PubMed]
- Alam, H.A.; Dalgıç, A.D.; Tezcaner, A.; Ozen, C.; Keskin, D. A comparative study of monoaxial and coaxial PCL/gelatin/Poloxamer 188 scaffolds for bone tissue engineering. Int. J. Polym. Mater. Polym. Biomater. 2019, 69, 339–350. [Google Scholar] [CrossRef]
- Hunter, R.L.; Luo, A.Z.; Zhang, R.; Kozar, R.A.; Moore, F.A. Poloxamer 188 inhibition of ischemia/reperfusion injury: Evidence for a novel anti-adhesive mechanism. Ann. Clin. Lab. Sci. 2010, 40, 115–125. [Google Scholar]
- Panda, N.; Bissoyi, A.; Pramanik, K.; Biswas, A. Development of novel electrospun nanofibrous scaffold from P. ricini and A. mylitta silk fibroin blend with improved surface and biological properties. Mater. Sci. Eng. C 2015, 48, 521–532. [Google Scholar] [CrossRef]
- Zhang, F.; Zuo, B.Q.; Zhang, H.X.; Bai, L. Studies of electrospun regenerated SF/TSF nanofibers. Polymer 2009, 50, 279–285. [Google Scholar] [CrossRef]
- Altman, G.H.; Horan, R.L.; Lu, H.H.; Moreau, J.; Martin, I.; Richmond, J.C.; Kaplan, D.L. Silk matrix for tissue engineered anterior cruciate ligaments. Biomaterials 2002, 23, 4131–4141. [Google Scholar] [CrossRef]
- Shao, W.; He, J.; Sang, F.; Ding, B.; Chen, L.; Cui, S.; Li, K.; Han, Q.; Tan, W. Coaxial electrospun aligned tussah silk fibroin nanostructured fiber scaffolds embedded with hydroxyapatite–tussah silk fibroin nanoparticles for bone tissue engineering. Mater. Sci. Eng. C 2016, 58, 342–351. [Google Scholar] [CrossRef]
- Choi, Y.; Cho, S.Y.; Heo, S.; Jin, H.-J. Enhanced mechanical properties of silk fibroin-based composite plates for fractured bone healing. Fibers Polym. 2013, 14, 266–270. [Google Scholar] [CrossRef]
- Nosar, M.N.; Salehi, M.; Ghorbani, S.; Pour Beiranvand, S.; Goodarzi, A.; Azami, M. Characterization of wet-electrospun cellulose acetate based 3-dimensional scaffolds for skin tissue engineering applications: Influence of cellulose acetate concentration. Cellulose 2016, 23, 3239–3248. [Google Scholar] [CrossRef]
- Tao, C.; Zhang, Y.; Li, B.; Chen, L. Hierarchical micro/submicrometer-scale structured scaffolds prepared via coaxial electrospinning for bone regeneration. J. Mater. Chem. B 2017, 5, 9219–9228. [Google Scholar] [CrossRef]
- Huang, C.; Dong, J.; Zhang, Y.; Chai, S.; Wang, X.; Kang, S.; Yu, D.; Wang, P.; Jiang, Q. Gold Nanoparticles-Loaded Polyvinylpyrrolidone/Ethylcellulose Coaxial Electrospun Nanofibers with Enhanced Osteogenic Capability for Bone Tissue Regeneration. Mater. Des. 2021, 212, 110240. [Google Scholar] [CrossRef]
- Liang, H.; Xu, X.; Feng, X.; Ma, L.; Deng, X.; Wu, S.; Liu, X.; Yang, C. Gold nanoparticles-loaded hydroxyapatite composites guide osteogenic differentiation of human mesenchymal stem cells through Wnt/β-catenin signaling pathway. Int. J. Nanomed. 2019, 2019, 6151–6163. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, P.; Mao, H.; Zhang, Y.; Zheng, L.; Yu, P.; Guo, Z.; Li, L.; Jiang, Q. PEGylated gold nanoparticles promote osteogenic differentiation in in vitro and in vivo systems. Mater. Des. 2020, 197, 109231. [Google Scholar] [CrossRef]
- Pathmanapan, S.; Sekar, M.; Pandurangan, A.K.; Anandasadagopan, S.K. Fabrication of Mesoporous Silica Nanoparticle–Incorporated Coaxial Nanofiber for Evaluating the In Vitro Osteogenic Potential. Appl. Biochem. 2022, 194, 302–322. [Google Scholar] [CrossRef]
- Zhou, X.; Feng, W.; Qiu, K.; Chen, L.; Wang, W.; Nie, W.; Mo, X.; He, C. BMP-2 Derived Peptide and Dexamethasone Incorporated Mesoporous Silica Nanoparticles for Enhanced Osteogenic Differentiation of Bone Mesenchymal Stem Cells. ACS Appl. Mater. Interfaces 2015, 7, 15777–15789. [Google Scholar] [CrossRef]
- Lu, Y.; Jiang, H.; Tu, K.; Wang, L. Mild immobilization of diverse macromolecular bioactive agents onto multifunctional fibrous membranes prepared by coaxial electrospinning. Acta Biomater. 2009, 5, 1562–1574. [Google Scholar] [CrossRef]
- Kuo, S.M.; Chang, S.J.; Niu, G.C.-C.; Lan, C.-W.; Cheng, W.T.; Yang, C.Z. Guided tissue regeneration with use of β-TCP/chitosan composite membrane. J. Appl. Polym. Sci. 2009, 112, 3127–3134. [Google Scholar] [CrossRef]
- Kharaziha, M.; Fathi, M.H.; Edris, H.; Nourbakhsh, N.; Talebi, A.; Salmanizadeh, S. PCL-forsterite nanocomposite fibrous membranes for controlled release of dexamethasone. J. Mater. Sci. Mater. Electron. 2015, 26, 36. [Google Scholar] [CrossRef]
- He, M.; Xue, J.; Geng, H.; Gu, H.; Chen, D.; Shi, R.; Zhang, L. Fibrous guided tissue regeneration membrane loaded with anti-inflammatory agent prepared by coaxial electrospinning for the purpose of controlled release. Appl. Surf. Sci. 2015, 335, 121–129. [Google Scholar] [CrossRef]
- Jose, M.V.; Thomas, V.; Johnson, K.T.; Dean, D.R.; Nyairo, E. Aligned PLGA/HA nanofibrous nanocomposite scaffolds for bone tissue engineering. Acta Biomater. 2009, 5, 305–315. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, L.; Zhao, K.; Wu, Z.; Wang, Y.; Tan, Q. Fabrication of PLGA/HA (core)-collagen/amoxicillin (shell) nanofiber membranes through coaxial electrospinning for guided tissue regeneration. Compos. Sci. Technol. 2016, 125, 100–107. [Google Scholar] [CrossRef]
- Mahalingam, S.; Bayram, C.; Gultekinoglu, M.; Ulubayram, K.; Homer-Vanniasinkam, S.; Edirisinghe, M. Co-Axial Gyro-Spinning of PCL/PVA/HA Core-Sheath Fibrous Scaffolds for Bone Tissue Engineering. Macromol. Biosci. 2021, 21, 2100177. [Google Scholar] [CrossRef]
- Mahalingam, S.; Homer-Vanniasinkam, S.; Edirisinghe, M. Novel pressurised gyration device for making core-sheath polymer fibres. Mater. Des. 2019, 178, 107846. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, M.; Wang, Y.; Zhao, H.; Sun, D. Using co-axial electrospray deposition to eliminate burst release of simvastatin from microparticles and to enhance induced osteogenesis. J. Biomater. Sci. Polym. Ed. 2019, 30, 355–375. [Google Scholar] [CrossRef]
- Wu, S.; Weng, Z.; Liu, X.; Yeung, K.; Chu, P.K. Functionalized TiO2 Based Nanomaterials for Biomedical Applications. Adv. Funct. Mater. 2014, 24, 5464–5481. [Google Scholar] [CrossRef]
- Kailasanathan, C.; Selvakumar, N.; Naidu, V. Structure and properties of titania reinforced nano-hydroxyapatite/gelatin bio-composites for bone graft materials. Ceram. Int. 2012, 38, 571–579. [Google Scholar] [CrossRef]
- Brammer, K.S.; Frandsen, C.J.; Jin, S. TiO2 nanotubes for bone regeneration. Trends Biotechnol. 2012, 30, 315–322. [Google Scholar] [CrossRef]
- Adhikari, S.P.; Pant, H.R.; Mousa, H.M.; Lee, J.; Kim, H.J.; Park, C.H.; Kim, C.S. Synthesis of high porous electrospun hollow TiO2 nanofibers for bone tissue engineering application. J. Ind. Eng. Chem. 2016, 35, 75–82. [Google Scholar] [CrossRef]
- Song, P.; Wang, W.; Li, J.; Cao, S.; Shi, J. Self-assembly of hydroxyapatite around Ti3C2 MXene/gold nanorods for efficient remotely triggered drug delivery. Ceram. Int. 2022, 48, 27957–27966. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, J.; Lin, H.; Mo, A. 2D titanium carbide(MXene) nanosheets and 1D hydroxyapatite nanowires into free standing nanocomposite membrane: In vitro and in vivo evaluations for bone regeneration. Mater. Sci. Eng. C 2020, 118, 111367. [Google Scholar] [CrossRef]
- Pogorielov, M.; Smyrnova, K.; Kyrylenko, S.; Gogotsi, O.; Zahorodna, V.; Pogrebnjak, A. MXenes—A New Class of Two-Dimensional Materials: Structure, Properties and Potential Applications. Nanomaterials 2021, 11, 3412. [Google Scholar] [CrossRef]
- Kyrylenko, S.; Gogotsi, O.; Baginskiy, I.; Balitskyi, V.; Zahorodna, V.; Husak, Y.; Yanko, I.; Pernakov, M.; Roshchupkin, A.; Lyndin, M.; et al. MXene-Assisted Ablation of Cells with a Pulsed Near-Infrared Laser. ACS Appl. Mater. Interfaces 2022, 14, 28683–28696. [Google Scholar] [CrossRef]
- Sadat-Shojai, M. Electrospun Polyhydroxybutyrate/Hydroxyapatite Nanohybrids: Microstructure and Bone Cell Response. J. Mater. Sci. Technol. 2016, 32, 1013–1020. [Google Scholar] [CrossRef]
- Pal, J.; Sharma, S.; Sanwaria, S.; Kulshreshtha, R.; Nandan, B.; Srivastava, R.K. Conducive 3D porous mesh of poly(ε-caprolactone) made via emulsion electrospinning. Polymer 2014, 55, 3970–3979. [Google Scholar] [CrossRef]
- Pal, J.; Singh, S.; Sharma, S.; Kulshreshtha, R.; Nandan, B.; Srivastava, R.K. Emulsion electrospun composite matrices of poly(ε-caprolactone)-hydroxyapatite: Strategy for hydroxyapatite confinement and retention on fiber surface. Mater. Lett. 2016, 167, 288–296. [Google Scholar] [CrossRef]
- Pal, J.; Wu, D.; Hakkarainen, M.; Srivastava, R.K. The viscoelastic interaction between dispersed and continuous phase of PCL/HA-PVA oil-in-water emulsion uncovers the theoretical and experimental basis for fiber formation during emulsion electrospinning. Eur. Polym. J. 2017, 96, 44–54. [Google Scholar] [CrossRef]
- Dai, Y.; Niu, J.; Liu, J.; Yin, L.; Xu, J. In situ encapsulation of laccase in microfibers by emulsion electrospinning: Preparation, characterization, and application. Bioresour. Technol. 2010, 101, 8942–8947. [Google Scholar] [CrossRef]
- Li, X.; Su, Y.; Liu, S.; Tan, L.; Mo, X.; Ramakrishna, S. Encapsulation of proteins in poly(l-lactide-co-caprolactone) fibers by emulsion electrospinning. Colloids Surfaces B Biointerfaces 2010, 75, 418–424. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Cui, W.; Zhou, S.; Tan, R.; Wang, C. Structural stability and release profiles of proteins from core-shell poly (DL-lactide) ultrafine fibers prepared by emulsion electrospinning. J. Biomed. Mater. Res. Part A 2007, 86, 374–385. [Google Scholar] [CrossRef]
- Srikar, R.; Yarin, A.L.; Megaridis, C.; Bazilevsky, A.A.V.; Kelley, E. Desorption-Limited Mechanism of Release from Polymer Nanofibers. Langmuir 2007, 24, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Prabhakaran, M.P.; Ding, X.; Ramakrishna, S. Biocompatibility evaluation of emulsion electrospun nanofibers using osteoblasts for bone tissue engineering. J. Biomater. Sci. Polym. Ed. 2013, 24, 1952–1968. [Google Scholar] [CrossRef] [PubMed]
- Briggs, T.; Matos, J.; Collins, G.; Arinzeh, T.L. Evaluating protein incorporation and release in electrospun composite scaffolds for bone tissue engineering applications. J. Biomed. Mater. Res. Part A 2015, 103, 3117–3127. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Maeda, H.; Fujii, S.; Nakamura, Y.; Furuzono, T. Formation of Pickering Emulsions Stabilized via Interaction between Nanoparticles Dispersed in Aqueous Phase and Polymer End Groups Dissolved in Oil Phase. Langmuir 2012, 28, 9405–9412. [Google Scholar] [CrossRef]
- Samanta, A.; Takkar, S.; Kulshreshtha, R.; Nandan, B.; Srivastava, R.K. Hydroxyapatite stabilized pickering emulsions of poly(ε-caprolactone) and their composite electrospun scaffolds. Colloids Surf. A Physicochem. Eng. Asp. 2017, 533, 224–230. [Google Scholar] [CrossRef]
- Pal, J.; Skrifvars, M.; Nandan, B.; Srivastava, R.K. Electrospun composite matrices from tenside-free poly(ε-caprolactone)-grafted acrylic acid/hydroxyapatite oil-in-water emulsions. J. Mater. Sci. 2016, 52, 2254–2262. [Google Scholar] [CrossRef]
- Abdal-hay, A.; Abbasi, N.; Gwiazda, M.; Hamlet, S.; Ivanovski, S. Novel polycaprolactone/hydroxyapatite nanocomposite fibrous scaffolds by direct melt-electrospinning writing. Eur. Polym. J. 2018, 105, 257–264. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; Wang, J.; Li, C. Preparation and characterization of PLLA/nHA nonwoven mats via laser melt electrospinning. Mater. Lett. 2012, 73, 103–106. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Xiong, J.; Li, J.; Miao, X.; Lan, X.; Liu, X.; Wang, W.; Cai, N.; Tang, Y. Fabrication and in vitro evaluation of PCL/gelatin hierarchical scaffolds based on melt electrospinning writing and solution electrospinning for bone regeneration. Mater. Sci. Eng. C 2021, 128, 112287. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elyaderani, A.K.; De Lama-Odría, M.d.C.; Valle, L.J.d.; Puiggalí, J. Multifunctional Scaffolds Based on Emulsion and Coaxial Electrospinning Incorporation of Hydroxyapatite for Bone Tissue Regeneration. Int. J. Mol. Sci. 2022, 23, 15016. https://doi.org/10.3390/ijms232315016
Elyaderani AK, De Lama-Odría MdC, Valle LJd, Puiggalí J. Multifunctional Scaffolds Based on Emulsion and Coaxial Electrospinning Incorporation of Hydroxyapatite for Bone Tissue Regeneration. International Journal of Molecular Sciences. 2022; 23(23):15016. https://doi.org/10.3390/ijms232315016
Chicago/Turabian StyleElyaderani, Amirmajid Kadkhodaie, María del Carmen De Lama-Odría, Luis J. del Valle, and Jordi Puiggalí. 2022. "Multifunctional Scaffolds Based on Emulsion and Coaxial Electrospinning Incorporation of Hydroxyapatite for Bone Tissue Regeneration" International Journal of Molecular Sciences 23, no. 23: 15016. https://doi.org/10.3390/ijms232315016
APA StyleElyaderani, A. K., De Lama-Odría, M. d. C., Valle, L. J. d., & Puiggalí, J. (2022). Multifunctional Scaffolds Based on Emulsion and Coaxial Electrospinning Incorporation of Hydroxyapatite for Bone Tissue Regeneration. International Journal of Molecular Sciences, 23(23), 15016. https://doi.org/10.3390/ijms232315016







