PEGylation Prolongs the Half-Life of Equine Anti-SARS-CoV-2 Specific F(ab’)2
Abstract
1. Introduction
2. Result
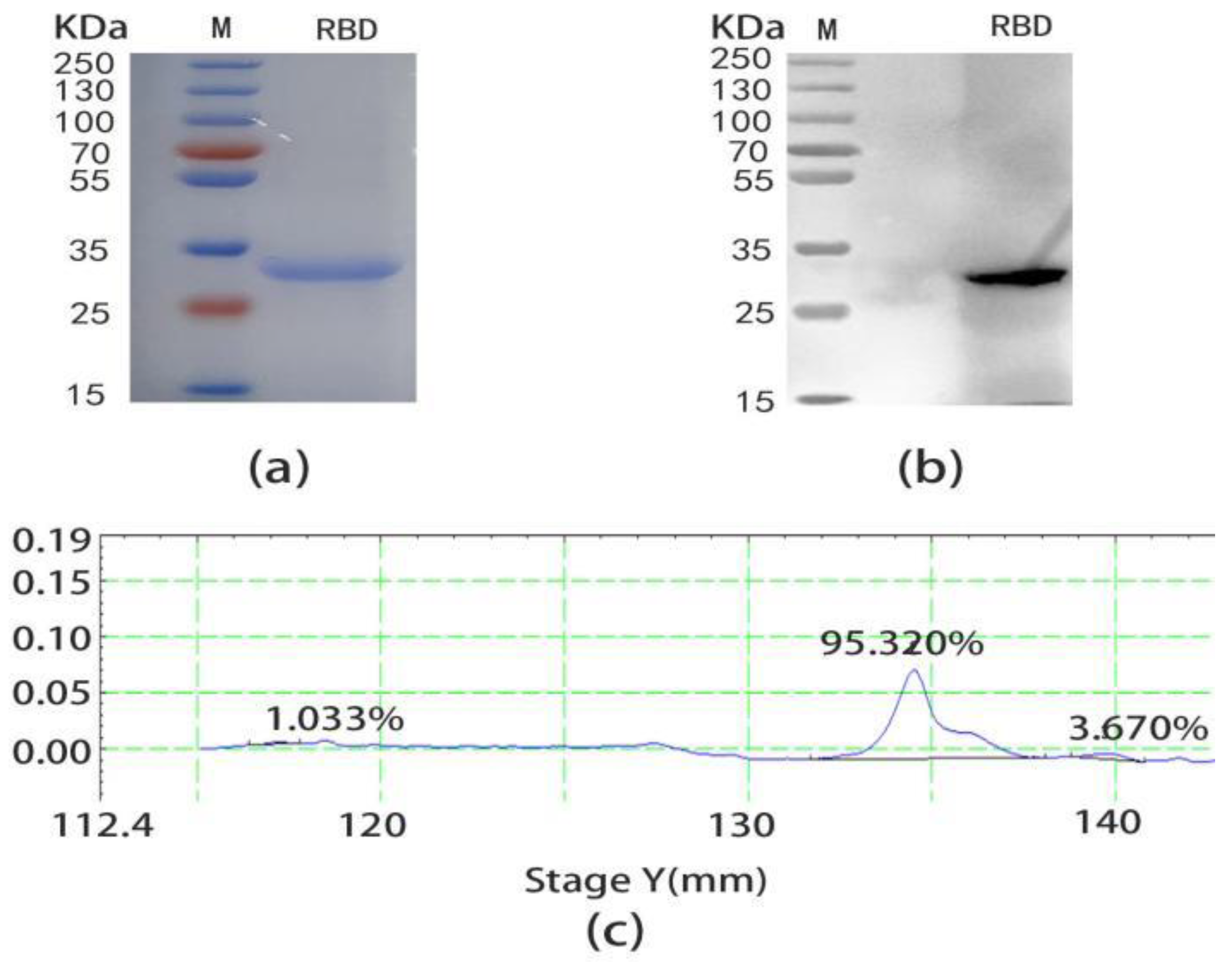
2.1. Expression and Purification of SARS-CoV-2 RBD
2.2. Preparation of Specific F(ab’)2
2.3. Comparison of the Affinity and Activity for Specific F(ab’)2 and Total F(ab’)2
2.4. The Recovery of Specific F(ab’)2 and Total F(ab’)2
2.5. Preparation and Purification of PEGylated F(ab’)2
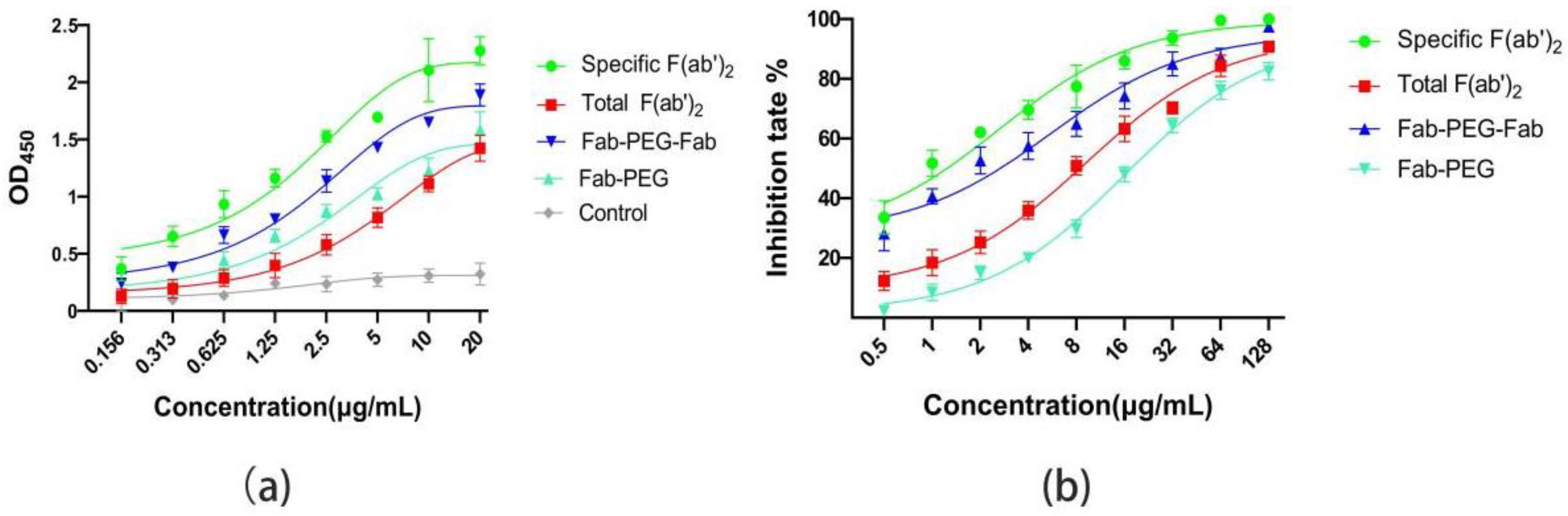
2.6. Compared the Affinity and Activity of Specific F(ab’)2, PEGylated F(ab’)2, and Total F(ab’)2
2.7. Pharmacokinetic Properties
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals, Cells, and Virus Strains
4.3. Preparation and Purification of SARS-CoV-2 RBD
4.4. Preparation of SARS-CoV-2 RBD Immunoaffinity Chromatography Column
4.5. Purification of Total IgG
4.6. Preparation of the Total F(ab’)2
4.7. Purification of Total F(ab’)2
4.8. Preparation of SARS-CoV-2 Specific F(ab’)2
4.9. Comparison of the Affinity and Activity for Specific F(ab’)2 and Total F(ab’)2
4.10. The Preparation of PEGylation F(ab’)2
4.11. Purification of PEGylated F(ab’)2
4.12. Comparison of the Affinity and Activity for Specific F(ab’)2 and PEGylation F(ab’)2
4.13. Pharmacokinetics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ciotti, M.; Angeletti, S.; Minieri, M.; Giovannetti, M.; Benvenuto, D.; Pascarella, S.; Sagnelli, C.; Bianchi, M.; Bernardini, S.; Ciccozzi, M. COVID-19 Outbreak: An Overview. Chemotherapy 2019, 64, 215–223. [Google Scholar] [CrossRef]
- Sharma, A.; Ahmad Farouk, I.; Lal, S.K. COVID-19: A Review on the Novel Coronavirus Disease Evolution, Transmission, Detection, Control and Prevention. Viruses 2021, 13, 202. [Google Scholar] [CrossRef]
- Madjunkov, M.; Dviri, M.; Librach, C. A comprehensive review of the impact of COVID-19 on human reproductive biology, assisted reproduction care and pregnancy: A Canadian perspective. J. Ovarian Res. 2020, 13, 140. [Google Scholar] [CrossRef]
- Tregoning, J.S.; Flight, K.E.; Higham, S.L.; Wang, Z.; Pierce, B.F. Progress of the COVID-19 vaccine effort: Viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat. Rev. Immunol. 2021, 21, 626–636. [Google Scholar] [CrossRef]
- Leeder, S. Re: Public health. Aust. N. Z. J. Public Health 1997, 21, 675–676. [Google Scholar] [CrossRef]
- Fiolet, T.; Kherabi, Y.; MacDonald, C.J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin. Microbiol. Infect. 2022, 28, 202–221. [Google Scholar] [CrossRef]
- Focosi, D.; Maggi, F. Neutralising antibody escape of SARS-CoV-2 spike protein: Risk assessment for antibody-based Covid-19 therapeutics and vaccines. Rev. Med. Virol. 2021, 31, e2231. [Google Scholar] [CrossRef]
- Jahanshahlu, L.; Rezaei, N. Monoclonal antibody as a potential anti-COVID-19. Biomed. Pharmacother. 2020, 129, 110337. [Google Scholar] [CrossRef]
- Focosi, D.; Anderson, A.O.; Tang, J.W.; Tuccori, M. Convalescent Plasma Therapy for COVID-19: State of the Art. Clin. Microbiol. Rev. 2020, 33, e00072-20. [Google Scholar] [CrossRef]
- Nelson, P.N.; Reynolds, G.M.; Waldron, E.E.; Ward, E.; Giannopoulos, K.; Murray, P.G. Monoclonal antibodies. Mol. Pathol. 2000, 53, 111–117. [Google Scholar] [CrossRef]
- Wootla, B.; Denic, A.; Rodriguez, M. Polyclonal and monoclonal antibodies in clinic. Methods Mol. Biol. 2014, 1060, 79–110. [Google Scholar]
- Cui, Z.; Li, D.; Yi, S.; Guo, Y.; Dong, G.; Niu, J.; Zhao, H.; Zhang, Y.; Zhang, S.; Cao, L.; et al. Equine immunoglobulin F(ab’)2 fragments protect cats against feline calicivirus infection. Int. Immunopharmacol. 2019, 75, 105714. [Google Scholar] [CrossRef]
- Herbreteau, C.H.; Jacquot, F.; Rith, S.; Vacher, L.; Nguyen, L.; Carbonnelle, C.; Lotteau, V.; Jolivet, M.; Raoul, H.; Buchy, P.; et al. Specific polyclonal F(ab’)2 neutralize a large panel of highly pathogenic avian influenza A viruses (H5N1) and control infection in mice. Immunotherapy 2014, 6, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Lopardo, G.; Belloso, W.H.; Nannini, E.; Colonna, M.; Sanguineti, S.; Zylberman, V.; Munoz, L.; Dobarro, M.; Lebersztein, G.; Farina, J.; et al. RBD-specific polyclonal F(ab)2 fragments of equine antibodies in patients with moderate to severe COVID-19 disease: A randomized, multicenter, double-blind, placebo-controlled, adaptive phase 2/3 clinical trial. eClinicalMedicine 2021, 34, 100843. [Google Scholar] [CrossRef] [PubMed]
- Cunha, L.E.R.; Stolet, A.A.; Strauch, M.A.; Pereira, V.A.R.; Dumard, C.H.; Gomes, A.M.O.; Monteiro, F.L.; Higa, L.M.; Souza, P.N.C.; Fonseca, J.G.; et al. Polyclonal F(ab’)2 fragments of equine antibodies raised against the spike protein neutralize SARS-CoV-2 variants with high potency. iScience 2021, 24, 103315. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Zhou, P.; Fan, T.; Wu, Y.; Zhang, J.; Shi, X.; Shang, W.; Fang, L.; Jiang, X.; Shi, J.; et al. Immunoglobulin fragment F(ab’)2 against RBD potently neutralizes SARS-CoV-2 in vitro. Antiviral. Res. 2020, 182, 104868. [Google Scholar] [CrossRef]
- Vazquez, H.; Chavez-Haro, A.; Garcia-Ubbelohde, W.; Paniagua-Solis, J.; Alagon, A.; Sevcik, C. Pharmacokinetics of a F(ab’)2 scorpion antivenom administered intramuscularly in healthy human volunteers. Int. Immunopharmacol. 2010, 10, 1318–1324. [Google Scholar] [CrossRef]
- Wilson, B.Z.; Bahadir, A.; Andrews, M.; Karpen, J.; Winkler, G.; Smelski, G.; Dudley, S.; Walter, F.G.; Shirazi, F.M. Initial Experience with F(ab’)2 Antivenom Compared with Fab Antivenom for Rattlesnake Envenomations Reported to a single poison center during 2019. Toxicon 2022, 209, 10–17. [Google Scholar] [CrossRef]
- Harris, J.M.; Chess, R.B. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2003, 2, 214–221. [Google Scholar] [CrossRef]
- Caliceti, P. Pharmacokinetic and biodistribution properties of poly(ethylene glycol)–protein conjugates. Adv. Drug Deliv. Rev. 2003, 55, 1261–1277. [Google Scholar] [CrossRef]
- Dozier, J.K.; Distefano, M.D. Site-Specific PEGylation of Therapeutic Proteins. Int. J. Mol. Sci. 2015, 16, 25831–25864. [Google Scholar] [CrossRef]
- Pasut, G.; Veronese, F.M. State of the art in PEGylation: The great versatility achieved after forty years of research. J. Control. Release 2012, 161, 461–472. [Google Scholar] [CrossRef]
- Zheng, Z.; Okada, R.; Kobayashi, H.; Nagaya, T.; Wei, J.; Zhou, Q.; Lee, F.; Bera, T.K.; Gao, Y.; Kuhlman, W.; et al. Site-Specific PEGylation of Anti-Mesothelin Recombinant Immunotoxins Increases Half-life and Antitumor Activity. Mol. Cancer Ther. 2020, 19, 812–821. [Google Scholar] [CrossRef]
- Ginn, C.; Choi, J.W.; Brocchini, S. Disulfide-bridging PEGylation during refolding for the more efficient production of modified proteins. Biotechnol. J. 2016, 11, 1088–1099. [Google Scholar] [CrossRef]
- Khalili, H.; Godwin, A.; Choi, J.W.; Lever, R.; Brocchini, S. Comparative binding of disulfide-bridged PEG-Fabs. Bioconjug. Chem. 2012, 23, 2262–2277. [Google Scholar] [CrossRef]
- Nie, Q.; Jia, D.; Yang, H.; Feng, Y.; Fan, Q.; Shi, Q.; Wan, L.; Lu, X. Conjugation to 10 kDa Linear PEG Extends Serum Half-Life and Preserves the Receptor-Binding Ability of mmTRAIL with Minimal Stimulation of PEG-Specific Antibodies. Mol. Pharm. 2017, 14, 502–512. [Google Scholar] [CrossRef]
- Holtsberg, F.W.; Ensor, C.M.; Steiner, M.R.; Bomalaski, J.S.; Clark, M.A. Poly(ethylene glycol) (PEG) conjugated arginine deiminase: Effects of PEG formulations on its pharmacological properties. J. Control. Release 2002, 80, 259–271. [Google Scholar] [CrossRef]
- Salian, V.S.; Wright, J.A.; Vedell, P.T.; Nair, S.; Li, C.; Kandimalla, M.; Tang, X.; Carmona Porquera, E.M.; Kalari, K.R.; Kandimalla, K.K. COVID-19 Transmission, Current Treatment, and Future Therapeutic Strategies. Mol. Pharm. 2021, 18, 754–771. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.; Qiu, B.; Li, C.; Wang, H.; Jin, H.; Gai, W.; Zheng, X.; Wang, T.; Sun, W.; et al. Passive immunotherapy for Middle East Respiratory Syndrome coronavirus infection with equine immunoglobulin or immunoglobulin fragments in a mouse model. Antivir. Res. 2017, 137, 125–130. [Google Scholar] [CrossRef]
- Wilson, B.Z.; Larsen, J.; Smelski, G.; Dudley, S.; Shirazi, F.M. Use of Crotalidae equine immune F(ab’)2 antivenom for treatment of an Agkistrodon envenomation. Clin. Toxicol. 2021, 59, 1023–1026. [Google Scholar] [CrossRef]
- Khalili, H.; Godwin, A.; Choi, J.W.; Lever, R.; Khaw, P.T.; Brocchini, S. Fab-PEG-Fab as a potential antibody mimetic. Bioconjug. Chem. 2013, 24, 1870–1882. [Google Scholar] [CrossRef]
- Zarzar, J.; Shatz, W.; Peer, N.; Taing, R.; McGarry, B.; Liu, Y.; Greene, D.G.; Zarraga, I.E. Impact of polymer geometry on the interactions of protein-PEG conjugates. Biophys. Chem. 2018, 236, 22–30. [Google Scholar] [CrossRef]





| Product | Manufacturer | Drug | Year |
|---|---|---|---|
| Adagen | Enzon | Adenosine Deaminase (ADA) | 1990 |
| Oncasper | Enzon | Asparagine Synthase (ASNS) | 1994 |
| Doxil | Schering | Liposomes | 1995 |
| PEG-intron | Schering | IFN-α-2B | 2000 |
| PEGASYS | Roche | IFN-α-2α | 2001 |
| Oncasper | Enzon | Asparagine Synthase (ASNS) | 1994 |
| Doxil | Schering | Liposomes | 1995 |
| PEG-intron | Schering | IFN-α-2B | 2000 |
| PEGASYS | Roche | IFN-α-2α | 2001 |
| Neusalta | Amgen | G-CSF | 2002 |
| Somavert | Pfizer | Growth Hormone Antagonist (GHA) | 2003 |
| Mircera | Roche | Erythropoietin (EPO) | 2007 |
| Sylatron | Merck | IFN-α-2B | 2011 |
| Plegridy | Biogen | IFN-β-1α | 2014 |
| Adynovate | Baxalta | antihemophiliac globulin (AHG) | 2015 |
| Rebinyn | Novo Nordisk | Coagulation Factor IX | 2017 |
| Jivi | Bayer Healthcare | antihemophiliac globulin (AHG) | 2018 |
| Fulphila | Mylan GmbH | G-CSF | 2018 |
| Udenyca | Coherus Bioscience | G-CSF | 2018 |
| Ziextenzo | Sandoz | G-CSF | 2019 |
| Sample | Concentration (mg/mL) | Recover Rates (%) |
|---|---|---|
| Total IgG | 8.58 | 100 |
| Total F(ab’)2 | 6.77 | 78.9 |
| Special F(ab’)2 | 1.05 | 4.89 |
| Product | Dose (mg/kg) | Half-Life (h) |
|---|---|---|
| Specific F(ab’)2 | 1.0 | 38.32 |
| Fab-PEG-Fab | 1.0 | 71.41 |
| Fab-PEG | 1.0 | 26.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Bi, J.; Liang, B.; Wang, X.; Mo, R.; Feng, N.; Yan, F.; Wang, T.; Yang, S.; Zhao, Y.; et al. PEGylation Prolongs the Half-Life of Equine Anti-SARS-CoV-2 Specific F(ab’)2. Int. J. Mol. Sci. 2023, 24, 3387. https://doi.org/10.3390/ijms24043387
Xu M, Bi J, Liang B, Wang X, Mo R, Feng N, Yan F, Wang T, Yang S, Zhao Y, et al. PEGylation Prolongs the Half-Life of Equine Anti-SARS-CoV-2 Specific F(ab’)2. International Journal of Molecular Sciences. 2023; 24(4):3387. https://doi.org/10.3390/ijms24043387
Chicago/Turabian StyleXu, Mengyuan, Jinhao Bi, Bo Liang, Xinyue Wang, Ruo Mo, Na Feng, Feihu Yan, Tiecheng Wang, Songtao Yang, Yongkun Zhao, and et al. 2023. "PEGylation Prolongs the Half-Life of Equine Anti-SARS-CoV-2 Specific F(ab’)2" International Journal of Molecular Sciences 24, no. 4: 3387. https://doi.org/10.3390/ijms24043387
APA StyleXu, M., Bi, J., Liang, B., Wang, X., Mo, R., Feng, N., Yan, F., Wang, T., Yang, S., Zhao, Y., & Xia, X. (2023). PEGylation Prolongs the Half-Life of Equine Anti-SARS-CoV-2 Specific F(ab’)2. International Journal of Molecular Sciences, 24(4), 3387. https://doi.org/10.3390/ijms24043387







