Monoclonal Antibodies: The Greatest Resource to Treat Multiple Myeloma
Abstract
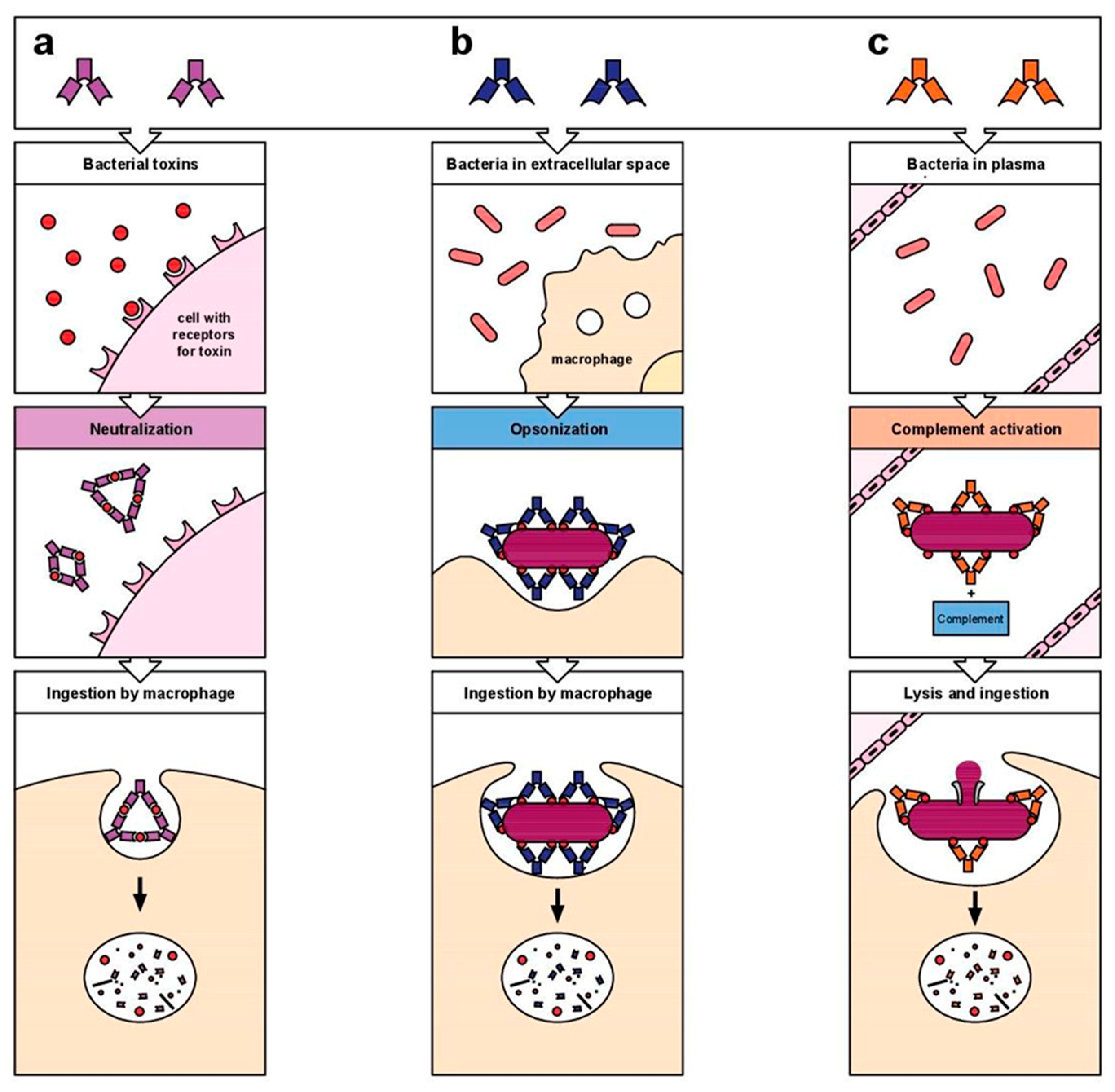
1. Introduction
- (1)
- Binding domain (varies from antibody to antibody): deputy to the antigen recognition;
- (2)
- Effector domain (common to many different antibodies), which tends to destroy the antigen.
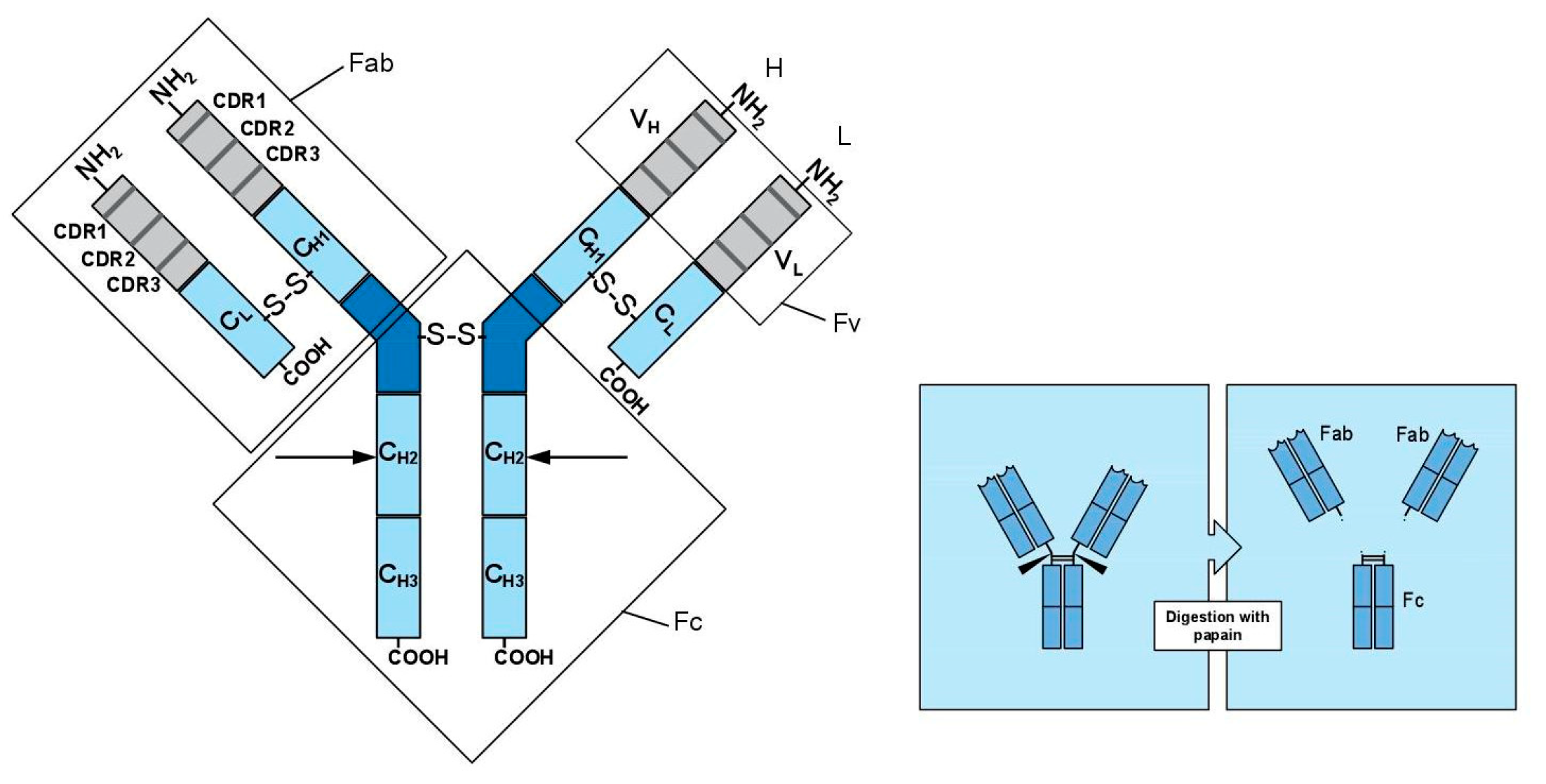
- -
- Each chain contains a variable region (V) and a constant region (C); constant regions are held together by disulfide bonds.
- -
- Both L and H chains consist of a variable NH2-terminal portion and a constant COOH-terminal.
- (1)
- Murine monoclonal antibodies (-momab) are the first to be produced, their administration encounters difficulties and inconveniences. The main limitation is their immunogenicity. In fact, from the first administration an immune response can occur in 50–80% of patients (HAMA response, Human AntiMouse Antibody) and repeated administrations significantly increase the HAMA response, which causes the immediate destruction of the subsequent doses of administered antibodies. This limits the therapeutic efficacy.
- (2)
- Chimeric monoclonal antibodies (-ximab) are characterized by a mouse portion and some segments of human origin and are obtained through genetic manipulation. The variable regions are coded by a murine antibody, and the constant regions are coded by a human antibody in the chimeric genes. The product of the constructed gene is a chimeric immunoglobulin that possesses specificity for the antigen typical of the murine monoclonal antibody (Fv mouse) with attenuated immunogenicity in humans and the effector functions of human antibodies (human Fc). Also, for chimeric antibodies the continuity of administration is limited by the HAMA response.
- (3)
- Humanized monoclonal antibodies (-zumab) are obtained by genetic manipulation, the CDR regions constitute the only segments of murine origin. The CDRs (CDR1, CDR2, and CDR3) of murine origin replace the CDRs from the human antibody. The obtained immunoglobulin has the specificity of binding for the antigen of the murine monoclonal antibody but all the other properties of the human antibody molecule.
- (4)
- Human monoclonal antibodies (-mumab) are entirely derived from human cells, thus have improved tolerability towards multiple administrations. On the other hand, they have very high production costs.
2. Daratumumab
- -
- In combination with lenalidomide and dexamethasone in patients newly diagnosed ineligible for autologous stem cell transplantation and in patients with relapsed or refractory MM who received at least one prior therapy;
- -
- In combination with bortezomib, melphalan, and prednisone in newly diagnosed patients unsuitable for autologous stem cell transplantation;
- -
- In combination with bortezomib, thalidomide, and dexamethasone in newly diagnosed patients eligible for autologous stem cell transplantation;
- -
- In combination with bortezomib and dexamethasone in patients who received at least one previous therapy;
- -
- In combination with pomalidomide and dexamethasone in patients who received at least two previous therapies including lenalidomide and a proteasome inhibitor;
- -
3. Elotuzumab
- -
- In combination with lenalidomide and dexamethasone for the treatment of MM in adult patients who received at least one prior line of therapy;
- -
- In combination with pomalidomide and dexamethasone for the treatment of adult patients with relapsed and refractory MM who received at least two lines of therapy (including lenalidomide and a proteasome inhibitor) and with disease progression [42].
4. Isatuximab
- -
- In combination with pomalidomide and dexamethasone for the treatment of adult patients with relapsed and refractory MM who received at least two previous therapies, including lenalidomide and a proteasome inhibitor, and with disease progression during the last therapy;
- -
- In combination with carfilzomib and dexamethasone for the treatment of adult patients with MM who have received at least one previous therapy.
5. ADC
6. ADCs Approved for Multiple Myeloma
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lopes, R.; Ferreira, B.V.; Caetano, J.; Barahona, F.; Carneiro, E.A.; Joao, C. Boosting immunity against multiple myeloma. Cancers 2021, 13, 1221. [Google Scholar] [CrossRef]
- D’Agostino, M.; Innorcia, S.; Boccadoro, M.; Bringhen, S. Monoclonal antibodies to treat multiple myeloma: A dream come true. Int. J. Mol. Sci. 2020, 21, 8192. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Ishibashi, M.; Sunakawa-Kii, M.; Inokuchi, K. PD-L1-PD-1 pathway in the pathophysiology of multiple myeloma. Cancers 2020, 12, 924. [Google Scholar] [CrossRef] [PubMed]
- Suyani, E.; Sucak, G.T.; Akyurek, N.; Sahin, S.; Baysal, N.A.; Yagci, M.; Haznedar, R. Tumor-associated macrophages as a prognostic parameter in multiple myeloma. Ann. Hematol. 2013, 92, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, I.R.; Martner, A.; Pisklakova, A.; Condamine, T.; Chase, T.; Vogl, T.; Roth, J.; Gabrilovich, D.; Nefedova, Y. Myeloid-derived suppressor cells regulate growth of multiple myeloma by inhibiting T cells in bone marrow. J. Immunol. 2013, 190, 3815–3823. [Google Scholar] [CrossRef]
- Brown, R.D.; Pope, B.; Murray, A.; Esdale, W.; Sze, D.M.; Gibson, J.; Ho, P.J.; Hart, D.; Joshua, D. Dendritic cells from patients with myeloma are numerically normal but functionally defective as they fail to up-regulate CD80 (B7-1) expression after huCD40LT stimulation because of inhibition by transforming growth factor-beta1 and interleukin-10. Blood 2001, 98, 2992–2998. [Google Scholar] [CrossRef] [PubMed]
- Atanackovic, D.; Luetkens, T.; Kroger, N. Coinhibitory molecule PD-1 as a potential target for the immunotherapy of multiple myeloma. Leukemia 2014, 28, 993–1000. [Google Scholar] [CrossRef]
- Guillerey, C.; Harjunpaa, H.; Carrie, N.; Kassem, S.; Teo, T.; Miles, K.; Krumeich, S.; Weulersse, M.; Cuisinier, M.; Stannard, K.; et al. TIGIT immune checkpoint blockade restores CD8+ T-cell immunity against multiple myeloma. Blood 2018, 132, 1689–1694. [Google Scholar] [CrossRef]
- Prabhala, R.H.; Neri, P.; Bae, J.E.; Tassone, P.; Shammas, M.A.; Allam, C.K.; Daley, J.F.; Chauhan, D.; Blanchard, E.; Thatte, H.S.; et al. Dysfunctional T regulatory cells in multiple myeloma. Blood 2006, 107, 301–304. [Google Scholar] [CrossRef]
- Zhang, L.; Tai, Y.T.; Ho, M.; Xing, L.; Chauhan, D.; Gang, A.; Qiu, L.; Anderson, K.C. Regulatory B cell-myeloma cell interaction confers immunosuppression and promotes their survival in the bone marrow milieu. Blood Cancer J. 2017, 7, e547. [Google Scholar] [CrossRef]
- Castella, B.; Foglietta, M.; Riganti, C.; Massaia, M. Vγ9Vδ2 T cells in the bone marrow of myeloma patients: A paradigm of microenvironment-induced immune suppression. Front. Immunol. 2018, 9, 1492. [Google Scholar] [CrossRef] [PubMed]
- Suen, H.; Brown, R.; Yang, S.; Weatherburn, C.; Ho, P.J.; Woodland, N.; Nassif, N.; Barbaro, P.; Bryant, C.; Hart, D.; et al. Multiple myeloma causes clonal T-cell immunosenescence: Identification of potential novel targets for promoting tumour immunity and implications for checkpoint blockade. Leukemia 2016, 30, 1716–1724. [Google Scholar] [CrossRef]
- Rawstron, A.C.; Davies, F.E.; Owen, R.G.; English, A.; Pratt, G.; Child, J.A.; Jack, A.S.; Morgan, G.J. B-lymphocyte suppression in multiple myeloma is a reversible phenomenon specific to normal B-cell progenitors and plasma cell precursors. Br. J. Haematol. 1998, 100, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Al Hamed, R.; Bazarbachi, A.H.; Malard, F.; Harousseau, J.L.; Mohty, M. Current status of autologous stem cell transplantation for multiple myeloma. Blood Cancer J. 2019, 9, 44. [Google Scholar] [CrossRef]
- Lu, X.; Ding, Z.C.; Cao, Y.; Liu, C.; Habtetsion, T.; Yu, M.; Lemos, H.; Salman, H.; Xu, H.; Mellor, A.L.; et al. Alkylating agent melphalan augments the efficacy of adoptive immunotherapy using tumor-specific CD4+ T cells. J. Immunol. 2015, 194, 2011–2021. [Google Scholar] [CrossRef] [PubMed]
- Bobin, A.; Liuu, E.; Moya, N.; Gruchet, C.; Sabirou, F.; Levy, A.; Gardeney, H.; Nsiala, L.; Cailly, L.; Guidez, S.; et al. Multiple myeloma: An overview of the current and novel therapeutic approaches in 2020. Cancers 2020, 12, 2855. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.G.; Lonial, S.; Jakubowiak, A.J.; Harousseau, J.L.; Anderson, K.C. Monoclonal antibodies in the treatment of multiple myeloma. Br. J. Haematol. 2011, 154, 745–754. [Google Scholar] [CrossRef]
- Tai, Y.T.; Anderson, K.C. Antibody-based therapies in multiple myeloma. Bone Marrow Res. 2011, 2011, 924058. [Google Scholar] [CrossRef]
- Laubach, J.P.; Paba Prada, C.E.; Richardson, P.G.; Longo, D.L. Daratumumab, Elotuzumab, and the development of therapeutic monoclonal antibodies in multiple myeloma. Clin. Pharmacol. Ther. 2017, 101, 81–88. [Google Scholar] [CrossRef]
- Janssen Biotech Inc. DARZALEX® (Daratumumab) Injection. Available online: http://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/DARZALEX-pi.pdf (accessed on 4 January 2023).
- Minnema, M.C.; Dispenzieri, A.; Merlini, G.; Comenzo, R.L.; Kastritis, E.; Wechalekar, A.D.; Grogan, M.; Witteles, R.; Ruberg, F.L.; Maurer, M.S.; et al. Outcomes by cardiac stage in patients with newly diagnosed AL amyloidosis: Phase 3 ANDROMEDA trial. JACC Cardio Oncol. 2022, 4, 474–487. [Google Scholar] [CrossRef]
- Krejcik, J.; Casneuf, T.; Nijhof, I.S.; Verbist, B.; Bald, J.; Plesner, T.; Syed, K.; Liu, K.; van de Donk, N.W.; Weiss, B.M.; et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood 2016, 128, 384–394. [Google Scholar] [CrossRef] [PubMed]
- de Weers, M.; Tai, Y.T.; van der Veer, M.S.; Bakker, J.M.; Vink, T.; Jacobs, D.C.; Oomen, L.A.; Peipp, M.; Valerius, T.; Slootstra, J.W.; et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J. Immunol. 2011, 186, 1840–1848. [Google Scholar] [CrossRef] [PubMed]
- Lammerts van Bueren, J.; Jakobs, D.; Kaldenhoven, N.; Roza, M.; Hiddingh, S.; Meesters, J.; Voorhorst, M.; Gresnigt, E.; Wiegman, L.; Ortiz Buijsse, A.; et al. Direct in vitro comparison of daratumumab with surrogate analogs of CD38 antibodies MOR03087, SAR650984 and Ab79. Blood 2014, 124, 3474. [Google Scholar] [CrossRef]
- Overdijk, M.B.; Verploegen, S.; Bogels, M.; van Egmond, M.; Lammerts van Bueren, J.J.; Mutis, T.; Groen, R.W.; Breij, E.; Martens, A.C.; Bleeker, W.K.; et al. Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. mAbs 2015, 7, 311–321. [Google Scholar] [CrossRef]
- Bittner, B.; Richter, W.; Schmidt, J. Subcutaneous administration of biotherapeutics: An overview of current challenges and opportunities. Biodrugs 2018, 32, 425–440. [Google Scholar] [CrossRef] [PubMed]
- Mateos, M.V.; Nahi, H.; Legiec, W.; Grosicki, S.; Vorobyev, V.; Spicka, I.; Hungria, V.; Korenkova, S.; Bahlis, N.; Flogegard, M.; et al. Subcutaneous versus intravenous daratumumab in patients with relapsed or refractory multiple myeloma (COLUMBA): A multicentre, open-label, non-inferiority, randomised, phase 3 trial. Lancet Haematol. 2020, 7, e370–e380. [Google Scholar] [CrossRef]
- Mateos, M.V.; Rigaudeau, S.; Basu, S.; Spicka, I.; Schots, R.; Wrobel, T.; Cook, G.; Beksac, M.; Gries, K.S.; Kudva, A.; et al. Switching to daratumumab SC from IV is safe and preferred by patients with multiple myeloma. J. Oncol. Pharm. Pract. 2022. [Google Scholar] [CrossRef] [PubMed]
- Usmani, S.Z.; Nahi, H.; Legiec, W.; Grosicki, S.; Vorobyev, V.; Spicka, I.; Hungria, V.; Korenkova, S.; Bahlis, N.J.; Flogegard, M.; et al. Final analysis of the phase III non-inferiority COLUMBA study of subcutaneous versus intravenous daratumumab in patients with relapsed or refractory multiple myeloma. Haematologica 2022, 107, 2408–2417. [Google Scholar] [CrossRef]
- Chapuy, C.I.; Nicholson, R.T.; Aguad, M.D.; Chapuy, B.; Laubach, J.P.; Richardson, P.G.; Doshi, P.; Kaufman, R.M. Resolving the daratumumab interference with blood compatibility testing. Transfusion 2015, 55, 1545–1554. [Google Scholar] [CrossRef] [PubMed]
- Oostendorp, M.; Lammerts van Bueren, J.J.; Doshi, P.; Khan, I.; Ahmadi, T.; Parren, P.W.; van Solinge, W.W.; De Vooght, K.M. When blood transfusion medicine becomes complicated due to interference by monoclonal antibody therapy. Transfusion 2015, 55, 1555–1562. [Google Scholar] [CrossRef]
- Song, J.; Fu, R. Review: Effects of anti-CD38 monoclonal antibodies on red blood cell transfusion and interventions. J. Clin.Lab. Anal. 2021, 35, e23832. [Google Scholar] [CrossRef] [PubMed]
- Hulin, C.; Offner, F.; Moreau, P.; Roussel, M.; Belhadj, K.; Benboubker, L.; Caillot, D.; Facon, T.; Garderet, L.; Kuhnowski, F.; et al. Stem cell yield and transplantation in transplant-eligible newly diagnosed multiple myeloma patients receiving daratumumab + bortezomib/thalidomide/dexamethasone in the phase 3 CASSIOPEIA study. Haematologica 2021, 106, 2257–2260. [Google Scholar] [CrossRef] [PubMed]
- van de Donk, N.W.C.J.; Richardson, P.G.; Malavasi, F. CD38 antibodies in multiple myeloma: Back to the future. Blood 2018, 131, 13–29. [Google Scholar] [CrossRef] [PubMed]
- van der Veer, M.S.; de Weers, M.; van Kessel, B.; Bakker, J.M.; Wittebol, S.; Parren, P.W.; Lokhorst, H.M.; Mutis, T. The therapeutic human CD38 antibody daratumumab improves the anti-myeloma effect of newly emerging multi-drug therapies. Blood Cancer J. 2011, 1, e41. [Google Scholar] [CrossRef] [PubMed]
- Nijhof, I.S.; Groen, R.W.; Lokhorst, H.M.; van Kessel, B.; Bloem, A.C.; van Velzen, J.; de Jong-Korlaar, R.; Yuan, H.; Noort, W.A.; Klein, S.K.; et al. Upregulation of CD38 expression on multiple myeloma cells by all-trans retinoic acid improves the efficacy of daratumumab. Leukemia 2015, 29, 2039–2049. [Google Scholar] [CrossRef]
- Ogiya, D.; Liu, J.; Ohguchi, H.; Kurata, K.; Samur, M.K.; Tai, Y.T.; Adamia, S.; Ando, K.; Hideshima, T.; Anderson, K.C. The JAK-STAT pathway regulates CD38 on myeloma cells in the bone marrow microenvironment: Therapeutic implications. Blood 2020, 136, 2334–2345. [Google Scholar] [CrossRef]
- Krejcik, J.; Frerichs, K.A.; Nijhof, I.S.; van Kessel, B.; van Velzen, J.F.; Bloem, A.C.; Broekmans, M.E.C.; Zweegman, S.; van Meerloo, J.; Musters, R.J.P.; et al. Monocytes and granulocytes reduce CD38 expression levels on myeloma cells in patients treated with daratumumab. Clin. Cancer Res. 2017, 23, 7498–7511. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, H.; Fang, C.; Li, C.; Xhyliu, F.; Dysert, H.; Bodo, J.; Habermehl, G.; Russell, B.E.; Li, W.; et al. Targeting of CD38 by the tumor suppressor miR-26a serves as a novel potential therapeutic agent in multiple myeloma. Cancer Res. 2020, 80, 2031–2044. [Google Scholar] [CrossRef]
- Malavasi, F.; Faini, A.C.; Morandi, F.; Castella, B.; Incarnato, D.; Oliviero, S.; Horenstein, A.L.; Massaia, M.; van de Donk, N.W.C.J.; Richardson, P.G. Molecular dynamics of targeting CD38 in multiple myeloma. Br. J. Haematol. 2021, 193, 581–591. [Google Scholar] [CrossRef]
- van de Donk, N.W.C.J.; Usmani, S.Z. CD38 Antibodies in multiple myeloma: Mechanisms of action and modes of resistance. Front. Immunol. 2018, 9, 2134. [Google Scholar] [CrossRef]
- European Medicines Agency. Empliciti, INN-elotuzumab. Available online: https://www.ema.europa.eu/en/documents/product-information/empliciti-epar-product-information_it.pdf (accessed on 6 January 2023).
- European Medicines Agency. Sarclisa, INN-isatuximab. Available online: https://www.ema.europa.eu/en/documents/product-information/sarclisa-epar-product-information_it.pdf (accessed on 6 January 2023).
- Collins, S.M.; Bakan, C.E.; Swartzel, G.D.; Hofmeister, C.C.; Efebera, Y.A.; Kwon, H.; Starling, G.C.; Ciarlariello, D.; Bhaskar, S.; Briercheck, E.L.; et al. Elotuzumab directly enhances NK cell cytotoxicity against myeloma via CS1 ligation: Evidence for augmented NK cell function complementing ADCC. Cancer Immunol. Immunother. 2013, 62, 1841–1849. [Google Scholar] [CrossRef]
- Pazina, T.; James, A.M.; MacFarlane, A.W.T.; Bezman, N.A.; Henning, K.A.; Bee, C.; Graziano, R.F.; Robbins, M.D.; Cohen, A.D.; Campbell, K.S. The anti-SLAMF7 antibody elotuzumab mediates NK cell activation through both CD16-dependent and -independent mechanisms. Oncoimmunology 2017, 6, e1339853. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Lonial, S.; Betts, K.A.; Chen, C.; Zichlin, M.L.; Brun, A.; Signorovitch, J.E.; Makenbaeva, D.; Mekan, S.; Sy, O.; et al. Elotuzumab plus lenalidomide and dexamethasone in relapsed/refractory multiple myeloma: Extended 4-year follow-up and analysis of relative progression-free survival from the randomized ELOQUENT-2 trial. Cancer 2018, 124, 4032–4043. [Google Scholar] [CrossRef] [PubMed]
- Lonial, S.; Dimopoulos, M.; Palumbo, A.; White, D.; Grosicki, S.; Spicka, I.; Walter-Croneck, A.; Moreau, P.; Mateos, M.V.; Magen, H.; et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N. Engl. J. Med. 2015, 373, 621–631. [Google Scholar] [CrossRef]
- Bristol-Myers Squibb Company Press Release Bristol Myers Squibb Reports Primary Results of ELOQUENT-1 Study Eval-uating Empliciti (elotuzumab) Plus Revlimid (lenalidomide) and Dexamethasone in Patients with Newly Diagnosed, Un-treated Multiple Myeloma Untreated Multiple Myeloma. Available online: https://news.bms.com/news/corporate-financial/2020/Bristol-Myers-Squibb-Reports-Primary-Results-of-ELOQUENT-1-Study-Evaluating-Empliciti-elotuzumab-Plus-Revlimid-lenalidomide-and-Dexamethasone-in-Patients-with-Newly-Diagnosed-Untreated-Multiple-Myeloma/default.aspx (accessed on 3 January 2023).
- Wang, H.; Shi, H.; He, X.; Liao, A. Downregulation of chemokine CCL20 involved in myeloma cells resistant to elotuzumab and lenalidomide. Onco. Targets Ther. 2021, 14, 2789–2795. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Acharya, C.; An, G.; Zhong, M.; Feng, X.; Wang, L.; Dasilva, N.; Song, Z.; Yang, G.; Adrian, F.; et al. SAR650984 directly induces multiple myeloma cell death via lysosomal-associated and apoptotic pathways, which is further enhanced by pomalidomide. Leukemia 2016, 30, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Mikhael, J.; Richardson, P.; Usmani, S.Z.; Raje, N.; Bensinger, W.; Karanes, C.; Campana, F.; Kanagavel, D.; Dubin, F.; Liu, Q.; et al. A phase 1b study of isatuximab plus pomalidomide/dexamethasone in relapsed/refractory multiple myeloma. Blood 2019, 134, 123–133. [Google Scholar] [CrossRef]
- Martin, T.; Strickland, S.; Glenn, M.; Charpentier, E.; Guillemin, H.; Hsu, K.; Mikhael, J. Phase I trial of isatuximab monotherapy in the treatment of refractory multiple myeloma. Blood Cancer J. 2019, 9, 41. [Google Scholar] [CrossRef]
- Attal, M.; Richardson, P.G.; Rajkumar, S.V.; San-Miguel, J.; Beksac, M.; Spicka, I.; Leleu, X.; Schjesvold, F.; Moreau, P.; Dimopoulos, M.A.; et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): A randomised, multicentre, open-label, phase 3 study. Lancet 2019, 394, 2096–2107. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Moreau, P.; Augustson, B.; Castro, N.; Pika, T.; Delimpasi, S.; De la Rubia, J.; Maiolino, A.; Reiman, T.; Martinez-Lopez, J.; et al. Isatuximab plus carfilzomib and dexamethasone in patients with relapsed multiple myeloma based on prior lines of treatment and refractory status: IKEMA subgroup analysis. Am. J. Hematol. 2023, 98, E15–E19. [Google Scholar] [CrossRef]
- Lonial, S.; Lee, H.C.; Badros, A.; Trudel, S.; Nooka, A.K.; Chari, A.; Abdallah, A.O.; Callander, N.; Lendvai, N.; Sborov, D.; et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): A two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2020, 21, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Nooka, A.K.; Stockerl-Goldstein, K.; Quach, H.; Forbes, A.; Mateos, M.-V.; Khot, A.; Tan, A.; Abonour, R.; Chopra, B.; Rogers, R.; et al. DREAMM-6: Safety and tolerability of belantamab mafodotin in combination with bortezomib/dexamethasone in relapsed/refractory multiple myeloma (RRMM). J. Clin. Oncol. 2020, 38, 8502. [Google Scholar] [CrossRef]
- Nejadmoghaddam, M.R.; Minai-Tehrani, A.; Ghahremanzadeh, R.; Mahmoudi, M.; Dinarvand, R.; Zarnani, A.H. Antibody-drug conjugates: Possibilities and challenges. Avicenna J. Med. Biotechnol. 2019, 11, 3–23. [Google Scholar] [PubMed]
- Yu, B.; Liu, D. Antibody-drug conjugates in clinical trials for lymphoid malignancies and multiple myeloma. J. Hematol. Oncol. 2019, 12, 94. [Google Scholar] [CrossRef]
- Abdollahpour-Alitappeh, M.; Lotfinia, M.; Gharibi, T.; Mardaneh, J.; Farhadihosseinabadi, B.; Larki, P.; Faghfourian, B.; Sepehr, K.S.; Abbaszadeh-Goudarzi, K.; Abbaszadeh-Goudarzi, G.; et al. Antibody-drug conjugates (ADCs) for cancer therapy: Strategies, challenges, and successes. J. Cell Physiol. 2019, 234, 5628–5642. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Adjei, A.A. Antibody-drug conjugates for the therapy of thoracic malignancies. J. Thorac. Oncol. 2019, 14, 358–376. [Google Scholar] [CrossRef]
- Hamblett, K.J.; Senter, P.D.; Chace, D.F.; Sun, M.M.; Lenox, J.; Cerveny, C.G.; Kissler, K.M.; Bernhardt, S.X.; Kopcha, A.K.; Zabinski, R.F.; et al. Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin. Cancer Res. 2004, 10, 7063–7070. [Google Scholar] [CrossRef]
- Gebleux, R.; Stringhini, M.; Casanova, R.; Soltermann, A.; Neri, D. Non-internalizing antibody-drug conjugates display potent anti-cancer activity upon proteolytic release of monomethyl auristatin E in the subendothelial extracellular matrix. Int. J. Cancer 2017, 140, 1670–1679. [Google Scholar] [CrossRef]
- Waight, A.B.; Bargsten, K.; Doronina, S.; Steinmetz, M.O.; Sussman, D.; Prota, A.E. Structural basis of microtubule destabilization by potent auristatin anti-mitotics. PloS ONE 2016, 11, e0160890. [Google Scholar] [CrossRef] [PubMed]
- Woitok, M.; Klose, D.; Niesen, J.; Richter, W.; Abbas, M.; Stein, C.; Fendel, R.; Bialon, M.; Puttmann, C.; Fischer, R.; et al. The efficient elimination of solid tumor cells by EGFR-specific and HER2-specific scFv-SNAP fusion proteins conjugated to benzylguanine-modified auristatin F. Cancer Lett. 2016, 381, 323–330. [Google Scholar] [CrossRef]
- Lu, J.; Jiang, F.; Lu, A.; Zhang, G. Linkers having a crucial role in antibody-drug conjugates. Int. J. Mol. Sci. 2016, 17, 561. [Google Scholar] [CrossRef]
- Bruins, W.S.C.; Zweegman, S.; Mutis, T.; van de Donk, N. Targeted therapy with immunoconjugates for multiple myeloma. Front. Immunol. 2020, 11, 1155. [Google Scholar] [CrossRef]
- Lee, L.; Bounds, D.; Paterson, J.; Herledan, G.; Sully, K.; Seestaller-Wehr, L.M.; Fieles, W.E.; Tunstead, J.; McCahon, L.; Germaschewski, F.M.; et al. Evaluation of B cell maturation antigen as a target for antibody drug conjugate mediated cytotoxicity in multiple myeloma. Br. J. Haematol. 2016, 174, 911–922. [Google Scholar] [CrossRef]
- Sanchez, E.; Li, M.; Kitto, A.; Li, J.; Wang, C.S.; Kirk, D.T.; Yellin, O.; Nichols, C.M.; Dreyer, M.P.; Ahles, C.P.; et al. Serum B-cell maturation antigen is elevated in multiple myeloma and correlates with disease status and survival. Br. J. Haematol. 2012, 158, 727–738. [Google Scholar] [CrossRef]
- European Medicines Agency. Blenrep-epar-product-information_it. Available online: https://www.ema.europa.eu/en/documents/product-information/blenrep-epar-product-information_it.pdf (accessed on 6 January 2023).
- Trudel, S.; Lendvai, N.; Popat, R.; Voorhees, P.M.; Reeves, B.; Libby, E.N.; Richardson, P.G.; Hoos, A.; Gupta, I.; Bragulat, V.; et al. Antibody-drug conjugate, GSK2857916, in relapsed/refractory multiple myeloma: An update on safety and efficacy from dose expansion phase I study. Blood Cancer J. 2019, 9, 37. [Google Scholar] [CrossRef]
- Zhao, H.; Atkinson, J.; Gulesserian, S.; Zeng, Z.; Nater, J.; Ou, J.; Yang, P.; Morrison, K.; Coleman, J.; Malik, F.; et al. Modulation of macropinocytosis-mediated internalization decreases ocular toxicity of antibody-drug conjugates. Cancer Res. 2018, 78, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.T.; Mayes, P.A.; Acharya, C.; Zhong, M.Y.; Cea, M.; Cagnetta, A.; Craigen, J.; Yates, J.; Gliddon, L.; Fieles, W.; et al. Novel anti-B-cell maturation antigen antibody-drug conjugate (GSK2857916) selectively induces killing of multiple myeloma. Blood 2014, 123, 3128–3138. [Google Scholar] [CrossRef] [PubMed]
- Trudel, S.; Lendvai, N.; Popat, R.; Voorhees, P.M.; Reeves, B.; Libby, E.N.; Richardson, P.G.; Anderson, L.D., Jr.; Sutherland, H.J.; Yong, K.; et al. Targeting B-cell maturation antigen with GSK2857916 antibody-drug conjugate in relapsed or refractory multiple myeloma (BMA117159): A dose escalation and expansion phase 1 trial. Lancet Oncol. 2018, 19, 1641–1653. [Google Scholar] [CrossRef]
- Hosoya, H.; Sidana, S. Antibody-based treatment approaches in multiple myeloma. Curr. Hematol. Malig. Rep. 2021, 16, 183–191. [Google Scholar] [CrossRef] [PubMed]
- D Souza, A.; Shah, N.; Rodriguez, C.; Voorhees, P.M.; Weisel, K.; Bueno, O.F.; Pothacamury, R.K.; Freise, K.J.; Yue, S.; Ross, J.A.; et al. A phase I first-in-human study of ABBV-383, a B-cell maturation antigen x CD3 bispecific T-cell redirecting antibody, in patients with relapsed/refractory multiple myeloma. J. Clin. Oncol. 2022, 40, 3576–3586. [Google Scholar] [CrossRef]
- Zonder, J.A.; Richter, J.; Bumma, N.; Brayer, J.; Hoffman, J.E.; Bensinger, W.I.; Wu, K.L.; Xu, L.; Chokshi, D.; Boyapati, A.; et al. MM-087 early, deep, and durable responses, and low rates of cytokine release syndrome with REGN5458, a BCMAxCD3 bispecific antibody, in a phase 1/2 first-in-human study in patients with relapsed/refractory multiple myeloma. Clin. Lymphoma Myeloma Leuk. 2022, 22, S406–S407. [Google Scholar] [CrossRef]
- DiLillo, D.J.; Olson, K.; Mohrs, K.; Meagher, T.C.; Bray, K.; Sineshchekova, O.; Startz, T.; Kuhnert, J.; Retter, M.W.; Godin, S.; et al. A BCMAxCD3 bispecific T cell-engaging antibody demonstrates robust antitumor efficacy similar to that of anti-BCMA CAR T cells. Blood Adv. 2021, 5, 1291–1304. [Google Scholar] [CrossRef] [PubMed]
- Bumma, N.; Richter, J.; Brayer, J.; Zonder, J.A.; Dhodapkar, M.; Shah, M.R.; Hoffman, J.E.; Mawad, R.; Maly, J.J.; Lentzsch, S.; et al. Updated safety and efficacy of REGN5458, a BCMAxCD3 bispecific antibody, treatment for relapsed/refractory multiple myeloma: A phase 1/2 first-in-human study. In Proceedings of the 64th ASH Annual Meeting and Exposition, New Orleans, LA, USA, 10–13 December 2022. [Google Scholar]
- Girgis, S.; Lin, S.X.W.; Pillarisetti, K.; Banerjee, A.; Stephenson, T.; Ma, X.; Shetty, S.; Yang, T.Y.; Hilder, B.W.; Jiao, Q.; et al. Translational modeling predicts efficacious therapeutic dosing range of teclistamab for multiple myeloma. Target Oncol. 2022, 17, 433–439. [Google Scholar] [CrossRef]
- Moreau, P.; Garfall, A.L.; van de Donk, N.; Nahi, H.; San-Miguel, J.F.; Oriol, A.; Nooka, A.K.; Martin, T.; Rosinol, L.; Chari, A.; et al. Teclistamab in relapsed or refractory multiple myeloma. N. Engl. J. Med. 2022, 387, 495–505. [Google Scholar] [CrossRef]
- Pillarisetti, K.; Powers, G.; Luistro, L.; Babich, A.; Baldwin, E.; Li, Y.; Zhang, X.; Mendonca, M.; Majewski, N.; Nanjunda, R.; et al. Teclistamab is an active T cell-redirecting bispecific antibody against B-cell maturation antigen for multiple myeloma. Blood Adv. 2020, 4, 4538–4549. [Google Scholar] [CrossRef]
- Berdeja, J.G.; Madduri, D.; Usmani, S.Z.; Jakubowiak, A.; Agha, M.; Cohen, A.D.; Stewart, A.K.; Hari, P.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): A phase 1b/2 open-label study. Lancet 2021, 398, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Munshi, N.C.; Anderson, L.D., Jr.; Shah, N.; Madduri, D.; Berdeja, J.; Lonial, S.; Raje, N.; Lin, Y.; Siegel, D.; Oriol, A.; et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N. Engl. J. Med. 2021, 384, 705–716. [Google Scholar] [CrossRef]
- Abrams, R.E.; Pierre, K.; El-Murr, N.; Seung, E.; Wu, L.; Luna, E.; Mehta, R.; Li, J.; Larabi, K.; Ahmed, M.; et al. Quantitative systems pharmacology modeling sheds light into the dose response relationship of a trispecific T cell engager in multiple myeloma. Sci. Rep. 2022, 12, 10976. [Google Scholar] [CrossRef]
- Hui, E.; Cheung, J.; Zhu, J.; Su, X.; Taylor, M.J.; Wallweber, H.A.; Sasmal, D.K.; Huang, J.; Kim, J.M.; Mellman, I.; et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science 2017, 355, 1428–1433. [Google Scholar] [CrossRef]
- Kamphorst, A.O.; Wieland, A.; Nasti, T.; Yang, S.; Zhang, R.; Barber, D.L.; Konieczny, B.T.; Daugherty, C.Z.; Koenig, L.; Yu, K.; et al. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science 2017, 355, 1423–1427. [Google Scholar] [CrossRef]
- Morcos, P.N.; Li, J.; Hosseini, I.; Li, C.C. Quantitative clinical pharmacology of T-cell engaging bispecifics: Current perspectives and opportunities. Clin. Transl. Sci. 2021, 14, 75–85. [Google Scholar] [CrossRef] [PubMed]

| Mab Class | Molecule (Targets) | Phase | Study | Treatment | Toxicities (>_G3) |
|---|---|---|---|---|---|
| Naked | Isatuximab (anti-CD38) | I | TCD11863 [1] NCT01749969, open label, dose escalation study | Isa (5 or 10 mg/kg [Q2W] or 10 or 20 mg/kg [QW] for 4 weeks) + R 25 mg (days 1–21) and d 40 mg (QW) | Neutropenia (60%) |
| Naked | Isatuximab (anti-CD38) | I | TCD14079 [2] NCT02283775 | Isa monotherapy ev QW or Q2W | Neutropenia (84%) |
| Naked | Isatuximab (anti-CD38) | II | NCT01084252 [1], safety and progression-free survival | Isa-d | |
| Naked | Isatuximab (anti-CD38) | II | NCT02514668 [1], safety and progression-free survival | Isa | |
| Naked | Isatuximab (anti-CD38) | III | ICARIA-MM [53] NCT029990338, randomized, multi-center open-label study | Isa-Pd (10 mg/kg + p 4 mg + d 40 mg) or Pd (p 4 mg + d 40 mg) | Neutropenia (85%) |
| Naked | Isatuximab (anti-CD38) | III | IKEMA [54] NCT02514668 prospective, randomized, open-label study | Isa-Kd vs. Kd | Respiratory infections (32.2%) |
| ADC | Belantamab mafodotin (anti-BCMA, monomethyl auristatin F payload) | I | DREAMM-1 [1] NCT02064387 | Belamaf single agent | Thrombocytopenia (35%); keratopathy (14%) |
| ADC | Belantamab mafodotin (anti-BCMA, monomethyl auristatin F payload) | II | DREAMM-2 [55] NCT03525678 | Belamaf single agent | Thrombocytopenia (20%); keratopathy (27%) |
| ADC | Belantamab mafodotin (anti-BCMA, monomethyl auristatin F payload) | I/II | DREAMM-6 [56] NCT03544281 | Belamaf-Vd | Thrombocytopenia (61%); keratopathy (56%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Luca, F.; Allegra, A.; Di Chio, C.; Previti, S.; Zappalà, M.; Ettari, R. Monoclonal Antibodies: The Greatest Resource to Treat Multiple Myeloma. Int. J. Mol. Sci. 2023, 24, 3136. https://doi.org/10.3390/ijms24043136
De Luca F, Allegra A, Di Chio C, Previti S, Zappalà M, Ettari R. Monoclonal Antibodies: The Greatest Resource to Treat Multiple Myeloma. International Journal of Molecular Sciences. 2023; 24(4):3136. https://doi.org/10.3390/ijms24043136
Chicago/Turabian StyleDe Luca, Fabiola, Alessandro Allegra, Carla Di Chio, Santo Previti, Maria Zappalà, and Roberta Ettari. 2023. "Monoclonal Antibodies: The Greatest Resource to Treat Multiple Myeloma" International Journal of Molecular Sciences 24, no. 4: 3136. https://doi.org/10.3390/ijms24043136
APA StyleDe Luca, F., Allegra, A., Di Chio, C., Previti, S., Zappalà, M., & Ettari, R. (2023). Monoclonal Antibodies: The Greatest Resource to Treat Multiple Myeloma. International Journal of Molecular Sciences, 24(4), 3136. https://doi.org/10.3390/ijms24043136







