Cyclophilin A Inhibitors Suppress Proliferation and Induce Apoptosis of MKN45 Gastric Cancer Stem-like Cells by Regulating CypA/CD147-Mediated Signaling Pathway
Abstract
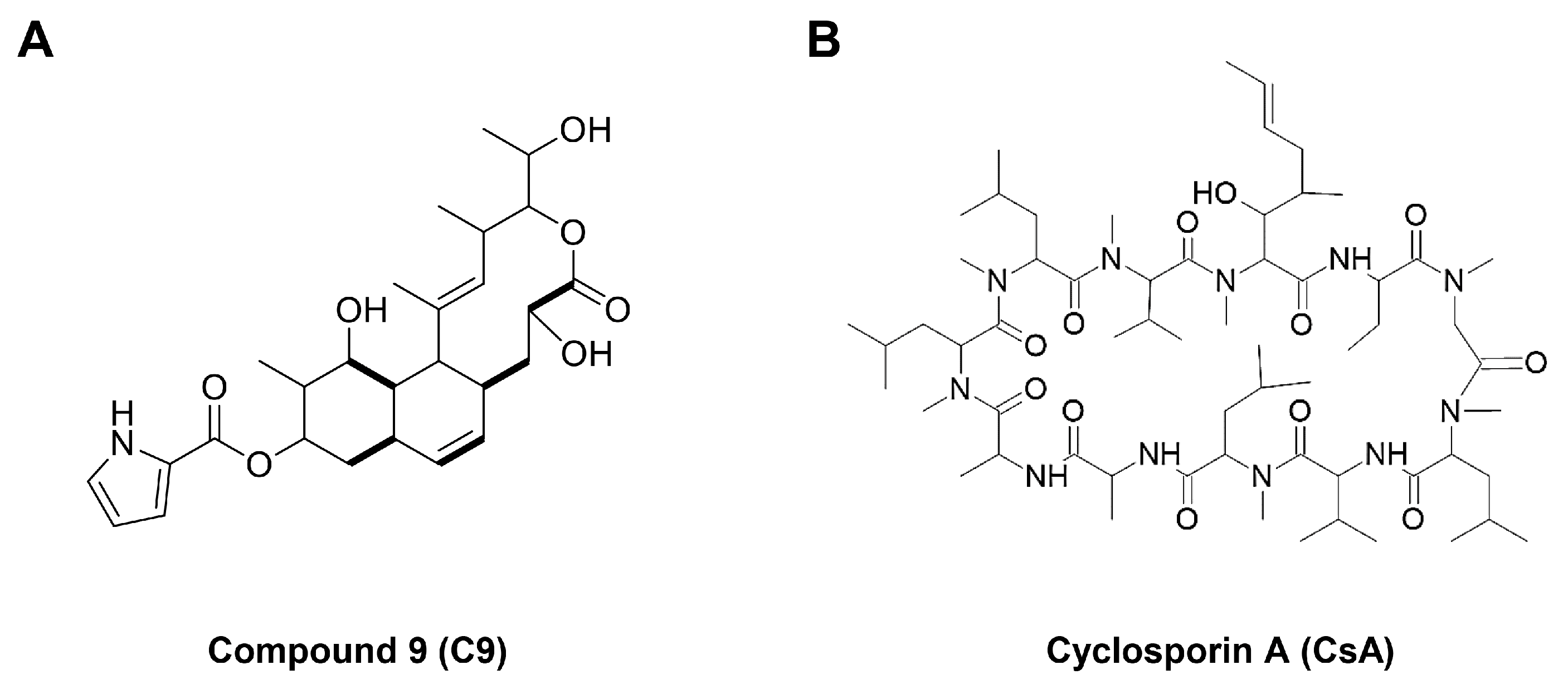
1. Introduction
2. Results
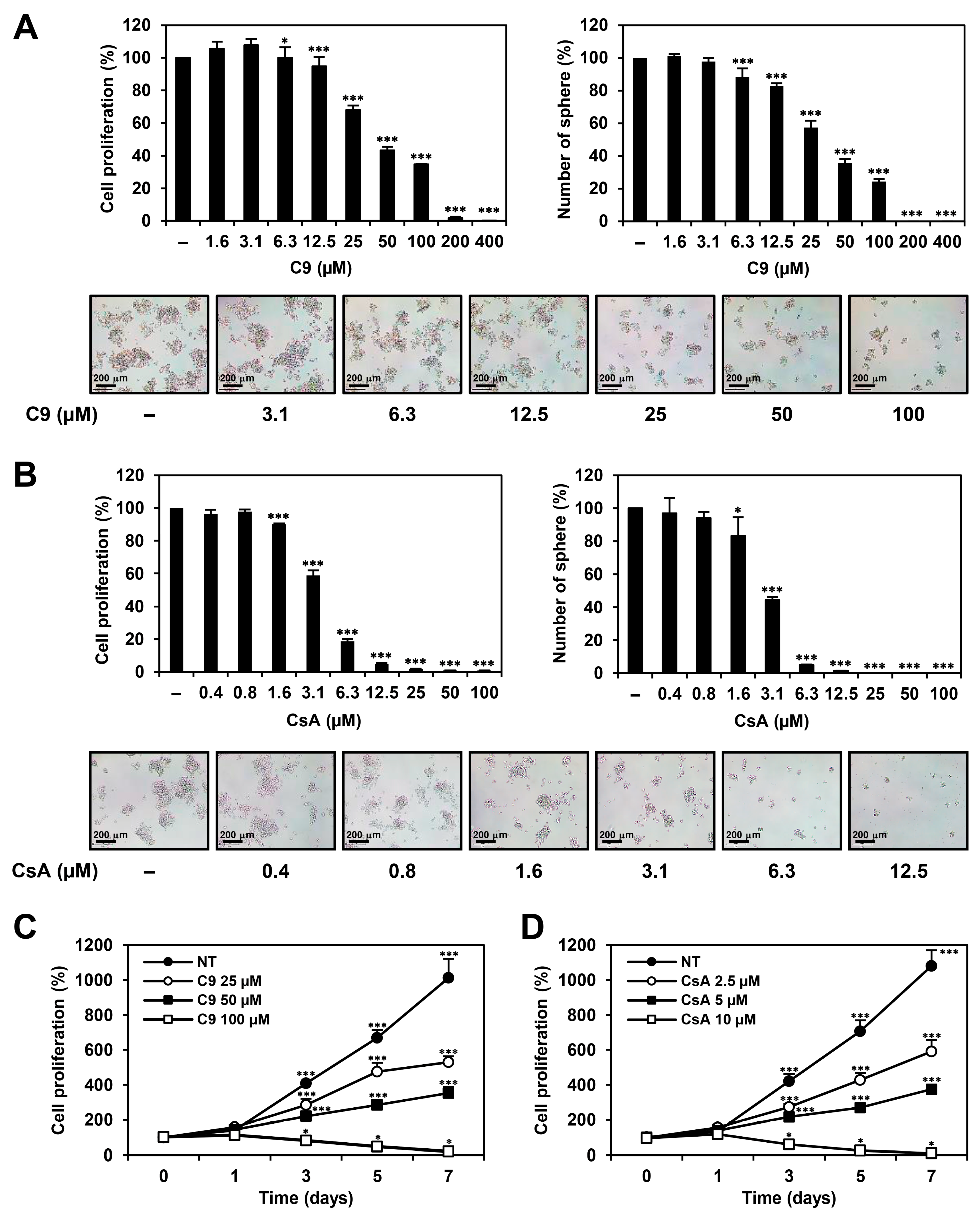
2.1. C9 and CsA Inhibit Proliferation of MKN45-Derived GCSCs Both In Vitro and In Vivo
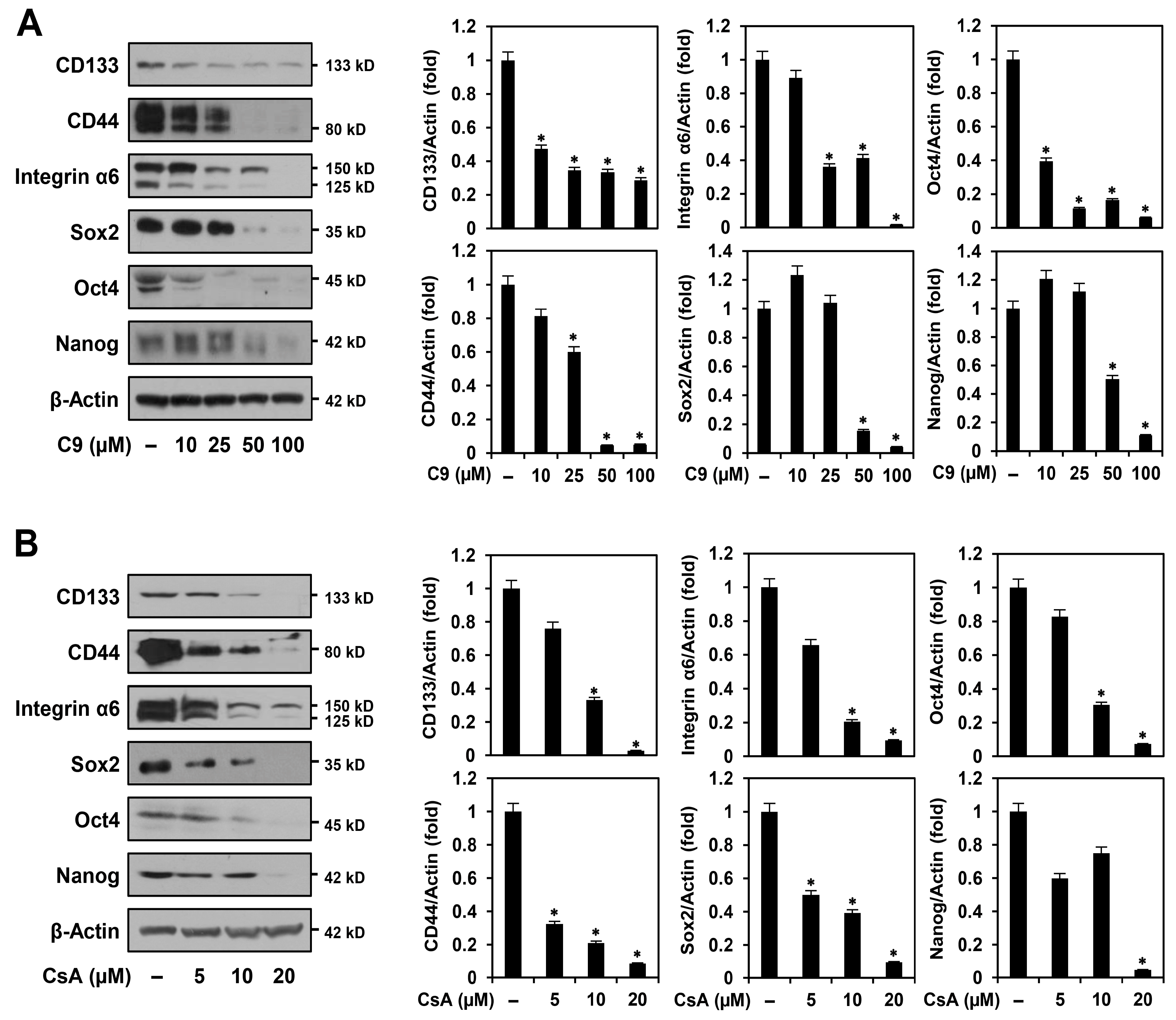
2.2. C9 and CsA Suppress Expression of Key Cancer Stemness Markers in MKN45-Derived GCSCs
2.3. C9 and CsA Induce Cell Cycle Arrest and Apoptosis in MKN45-Derived GCSCs
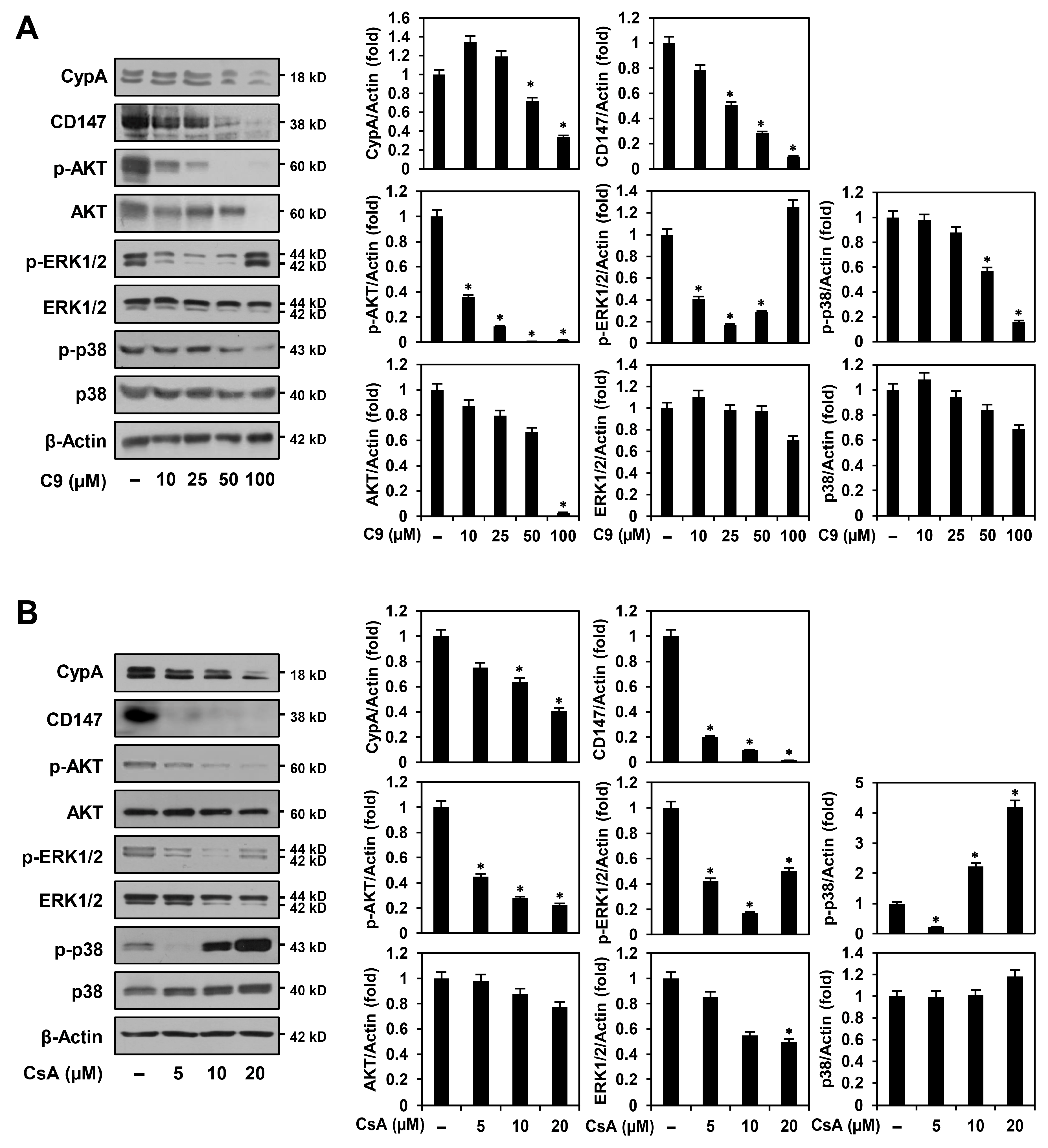
2.4. C9 and CsA Affect CypA/CD147-Mediated Signaling Pathways in MKN45-Derived GCSCs
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Cell Proliferation Assay
4.4. Tumorsphere Formation Assay
4.5. Chick Embryo Chorioallantoic Membrane (CAM) Assay
4.6. Cell Cycle Analysis
4.7. Apoptosis Analysis
4.8. DAPI Staining
4.9. ROS Measurement
4.10. Western Blot Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Chao, J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.C.; Enzler, T.; Fanta, P.; et al. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 167–192. [Google Scholar] [CrossRef]
- Sexton, R.E.; Al Hallak, M.N.; Diab, M.; Azmi, A.S. Gastric cancer: A comprehensive review of current and future treatment strategies. Cancer Metastasis Rev. 2020, 39, 1179–1203. [Google Scholar] [CrossRef] [PubMed]
- Quadri, H.S.; Smaglo, B.G.; Morales, S.J.; Phillips, A.C.; Martin, A.D.; Chalhoub, W.M.; Haddad, N.G.; Unger, K.R.; Levy, A.D.; Al-Refaie, W.B. Gastric adenocarcinoma: A multimodal approach. Front. Surg. 2017, 4, 42. [Google Scholar] [CrossRef] [PubMed]
- Bilici, A. Treatment options in patients with metastatic gastric cancer: Current status and future perspectives. World J. Gastroenterol. 2014, 20, 3905–3915. [Google Scholar] [CrossRef]
- Yang, Y.; Meng, W.J.; Wang, Z.Q. The origin of gastric cancer stem cells and their effects on gastric cancer: Novel therapeutic targets for gastric cancer. Front. Oncol. 2022, 12, 960539. [Google Scholar] [CrossRef]
- Hsieh, H.L.; Yu, M.C.; Cheng, L.C.; Yeh, T.S.; Tsai, M.M. Molecular mechanism of therapeutic approaches for human gastric cancer stem cells. World J. Stem Cells 2022, 14, 76–91. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Sowden, M.P.; Berk, B.C. Extracellular and intracellular Cyclophilin A, native and post-translationally modified, show diverse and specific pathological roles in diseases. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Nigro, P.; Pompilio, G.; Capogrossi, M.C. Cyclophilin A: A key player for human disease. Cell Death Dis. 2013, 4, e888. [Google Scholar] [CrossRef] [PubMed]
- Yurchenko, V.; Constant, S.; Eisenmesser, E.; Bukrinsky, M. Cyclophilin-CD147 interactions: A new target for anti-inflammatory therapeutics. Clin. Exp. Immunol. 2010, 160, 305–317. [Google Scholar] [CrossRef]
- Han, J.M.; Jung, H.J. Cyclophilin A/CD147 interaction: A promising target for anticancer therapy. Int. J. Mol. Sci. 2022, 23, 9341. [Google Scholar] [CrossRef]
- Wang, G.; Shen, J.; Sun, J.; Jiang, Z.; Fan, J.; Wang, H.; Yu, S.; Long, Y.; Liu, Y.; Bao, H.; et al. Cyclophilin A maintains glioma-initiating cell stemness by regulating Wnt/β-catenin signaling. Clin. Cancer Res. 2017, 23, 6640–6649. [Google Scholar] [CrossRef] [PubMed]
- Lucchetti, D.; Colella, F.; Perelli, L.; Ricciardi-Tenore, C.; Calapà, F.; Fiori, M.E.; Carbone, F.; De Maria, R.; Sgambato, A. CD147 promotes cell small extracellular vesicles release during colon cancer stem cells differentiation and triggers cellular changes in recipient cells. Cancers 2020, 12, 260. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.Y.; He, D.; Sheng, C.B.; Wang, B.; Wang, L.J.; Wu, X.Q.; Xu, L.; Jiang, J.L.; Li, L.; Chen, Z.N. Therapeutic anti-CD147 antibody sensitizes cells to chemoradiotherapy via targeting pancreatic cancer stem cells. Am. J. Transl. Res. 2019, 11, 3543–3554. [Google Scholar] [PubMed]
- Chen, J.; Pan, Y.; He, B.; Ying, H.; Wang, F.; Sun, H.; Deng, Q.; Liu, X.; Lin, K.; Peng, H.; et al. Inhibition of CD147 expression by RNA interference reduces proliferation, invasion and increases chemosensitivity in cancer stem cell-like HT-29 cells. Int. J. Oncol. 2015, 47, 1476–1484. [Google Scholar] [CrossRef]
- Meng, Y.; Fan, X.Y.; Yang, L.J.; Xu, B.Q.; He, D.; Xu, Z.; Wu, D.; Wang, B.; Cui, H.Y.; Wang, S.J.; et al. Detachment activated CypA and CD147 induces cancer stem cell potential in non-stem breast cancer cells. Front. Cell Dev. Biol. 2020, 8, 543856. [Google Scholar] [CrossRef]
- Han, J.M.; Sohng, J.K.; Lee, W.H.; Oh, T.J.; Jung, H.J. Identification of Cyclophilin A as a potential anticancer target of novel Nargenicin A1 analog in AGS gastric cancer cells. Int. J. Mol. Sci. 2021, 22, 2473. [Google Scholar] [CrossRef]
- Dhakal, D.; Han, J.M.; Mishra, R.; Pandey, R.P.; Kim, T.S.; Rayamajhi, V.; Jung, H.J.; Yamaguchi, T.; Sohng, J.K. Characterization of tailoring steps of Nargenicin A1 biosynthesis reveals a novel analogue with anticancer activities. ACS Chem. Biol. 2020, 15, 1370–1380. [Google Scholar] [CrossRef]
- Han, J.M.; Choi, Y.S.; Dhakal, D.; Sohng, J.K.; Jung, H.J. Novel Nargenicin A1 analog inhibits angiogenesis by downregulating the endothelial VEGF/VEGFR2 signaling and tumoral HIF-1α/VEGF pathway. Biomedicines 2020, 8, 252. [Google Scholar] [CrossRef]
- Werneck, M.B.; Hottz, E.; Bozza, P.T.; Viola, J.P. Cyclosporin A inhibits colon cancer cell growth independently of the calcineurin pathway. Cell Cycle 2012, 11, 3997–4008. [Google Scholar] [CrossRef]
- Xing, X.L.; Lu, Y.; Qiu, H.L. Effect of cyclosporin A particles of varying diameters on gastric cancer cell apoptosis. Genet. Mol. Res. 2016, 15, 1–8. [Google Scholar] [CrossRef]
- Nakahara, C.; Nakamura, K.; Yamanaka, N.; Baba, E.; Wada, M.; Matsunaga, H.; Noshiro, H.; Tanaka, M.; Morisaki, T.; Katano, M. Cyclosporin-A enhances docetaxel-induced apoptosis through inhibition of nuclear factor-kappaB activation in human gastric carcinoma cells. Clin. Cancer Res. 2003, 9, 5409–5416. [Google Scholar]
- Choi, Y.S.; Cho, H.J.; Jung, H.J. Atorvastatin inhibits the proliferation of MKN45-derived gastric cancer stem cells in a mevalonate pathway-independent manner. Korean J. Physiol. Pharmacol. 2022, 26, 367–375. [Google Scholar] [CrossRef]
- Li, K.; Dan, Z.; Nie, Y.Q. Gastric cancer stem cells in gastric carcinogenesis, progression, prevention and treatment. World J. Gastroenterol. 2014, 20, 5420–5426. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ma, L.; Xu, J.; Liu, C.; Zhang, J.; Liu, J.; Chen, R.; Zhou, Y. Spheroid body-forming cells in the human gastric cancer cell line MKN-45 possess cancer stem cell properties. Int. J. Oncol. 2013, 42, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Chaitanya, G.V.; Steven, A.J.; Babu, P.P. PARP-1 cleavage fragments: Signatures of cell-death proteases in neurodegeneration. Cell Commun. Signal. 2010, 8, 31. [Google Scholar] [CrossRef]
- Dubrovska, A.; Kim, S.; Salamone, R.J.; Walker, J.R.; Maira, S.M.; García-Echeverría, C.; Schultz, P.G.; Reddy, V.A. The role of PTEN/Akt/PI3K signaling in the maintenance and viability of prostate cancer stem-like cell populations. Proc. Natl. Acad. Sci. USA 2009, 106, 268–273. [Google Scholar] [CrossRef]
- Yue, J.; López, J.M. Understanding MAPK signaling pathways in apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef]
- Phi, L.T.H.; Sari, I.N.; Yang, Y.G.; Lee, S.H.; Jun, N.; Kim, K.S.; Lee, Y.K.; Kwon, H.Y. Cancer stem cells (CSCs) in drug resistance and their therapeutic implications in cancer treatment. Stem Cells Int. 2018, 2018, 5416923. [Google Scholar] [CrossRef]
- Addeo, M.; Di Paola, G.; Verma, H.K.; Laurino, S.; Russi, S.; Zoppoli, P.; Falco, G.; Mazzone, P. Gastric cancer stem cells: A glimpse on metabolic reprogramming. Front. Oncol. 2021, 11, 698394. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Wang, T.; Fan, J.; Zhang, Z.; Zhang, J.; Xu, C.; Li, Y.; Zhao, G.; He, C.; Meng, H.; et al. The effects and mechanisms of SLC34A2 on maintaining stem cell-like phenotypes in CD147+ breast cancer stem cells. Tumour Biol. 2017, 39, 1010428317695927. [Google Scholar] [CrossRef] [PubMed]
- Rustighi, A.; Zannini, A.; Tiberi, L.; Sommaggio, R.; Piazza, S.; Sorrentino, G.; Nuzzo, S.; Tuscano, A.; Eterno, V.; Benvenuti, F.; et al. Prolyl-isomerase Pin1 controls normal and cancer stem cells of the breast. EMBO Mol. Med. 2014, 6, 99–119. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.; Zhu, S.; Li, J.; Ji, G.; Wang, W.; Wu, G.; Zheng, J. CD147 expression in human gastric cancer is associated with tumor recurrence and prognosis. PLoS ONE 2014, 9, e101027. [Google Scholar] [CrossRef] [PubMed]
- Han, J.M.; Kim, Y.J.; Jung, H.J. Discovery of a new CaMKII-targeted synthetic lethal therapy against glioblastoma stem-like cells. Cancers 2022, 14, 1315. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, H.J.; Jung, H.J. Cyclophilin A Inhibitors Suppress Proliferation and Induce Apoptosis of MKN45 Gastric Cancer Stem-like Cells by Regulating CypA/CD147-Mediated Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 4734. https://doi.org/10.3390/ijms24054734
Cho HJ, Jung HJ. Cyclophilin A Inhibitors Suppress Proliferation and Induce Apoptosis of MKN45 Gastric Cancer Stem-like Cells by Regulating CypA/CD147-Mediated Signaling Pathway. International Journal of Molecular Sciences. 2023; 24(5):4734. https://doi.org/10.3390/ijms24054734
Chicago/Turabian StyleCho, Hee Jeong, and Hye Jin Jung. 2023. "Cyclophilin A Inhibitors Suppress Proliferation and Induce Apoptosis of MKN45 Gastric Cancer Stem-like Cells by Regulating CypA/CD147-Mediated Signaling Pathway" International Journal of Molecular Sciences 24, no. 5: 4734. https://doi.org/10.3390/ijms24054734
APA StyleCho, H. J., & Jung, H. J. (2023). Cyclophilin A Inhibitors Suppress Proliferation and Induce Apoptosis of MKN45 Gastric Cancer Stem-like Cells by Regulating CypA/CD147-Mediated Signaling Pathway. International Journal of Molecular Sciences, 24(5), 4734. https://doi.org/10.3390/ijms24054734




