Neurotoxic/Neuroprotective Effects of Clozapine and the Positive Allosteric Modulator of mGluR2 JNJ-46356479 in Human Neuroblastoma Cell Cultures
Abstract
1. Introduction
2. Results
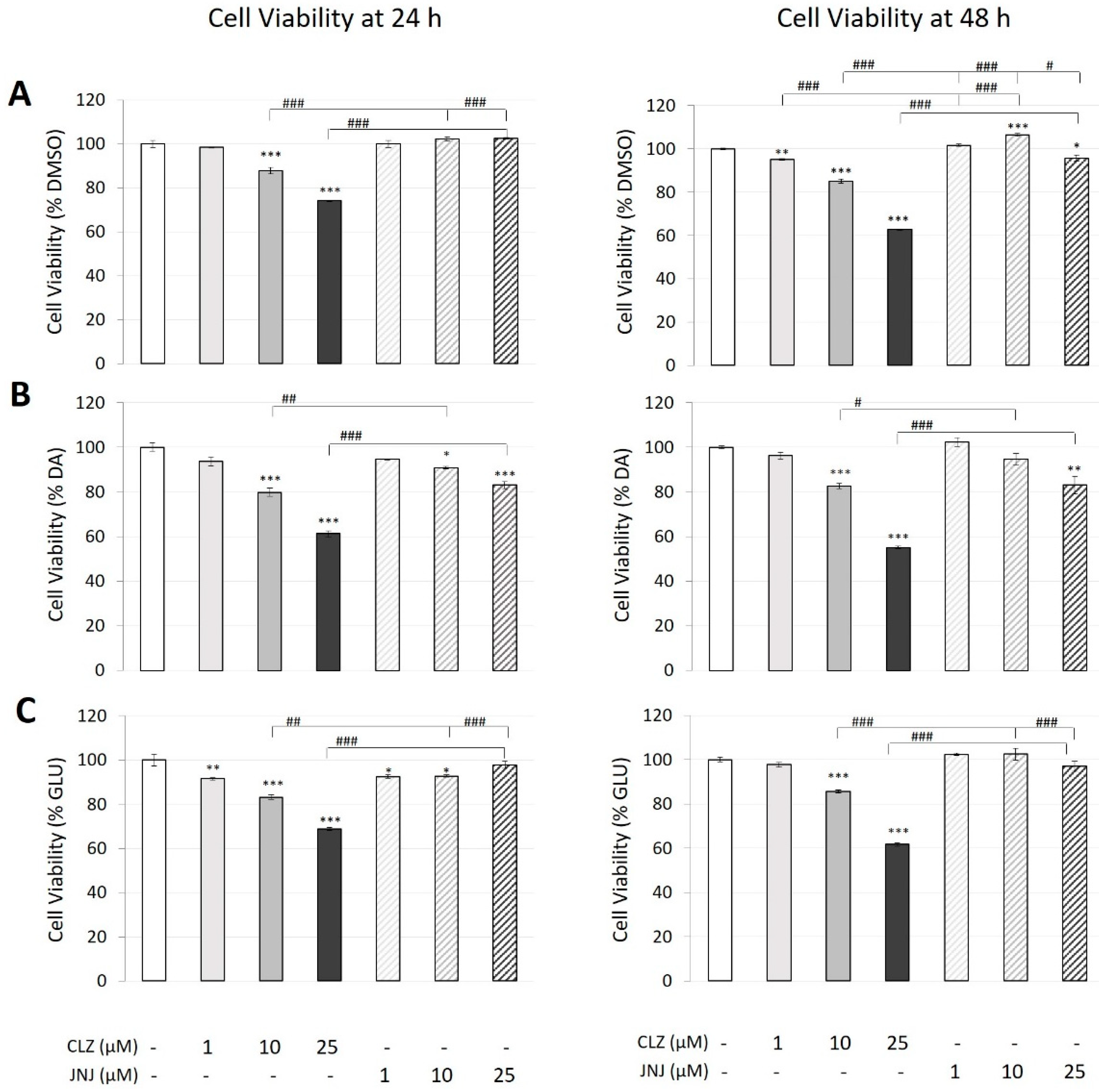
2.1. Effects of Clozapine (CLZ) and JNJ-46356479 (JNJ) Alone and in Combination with Dopamine (DA) or Glutamate (GLU) on the Viability of SK-N-SH Cells
2.2. Effects of Clozapine (CLZ) and JNJ-46356479 (JNJ) Alone and in Combination with Dopamine (DA) or Glutamate (GLU) on Caspase 3 Activity
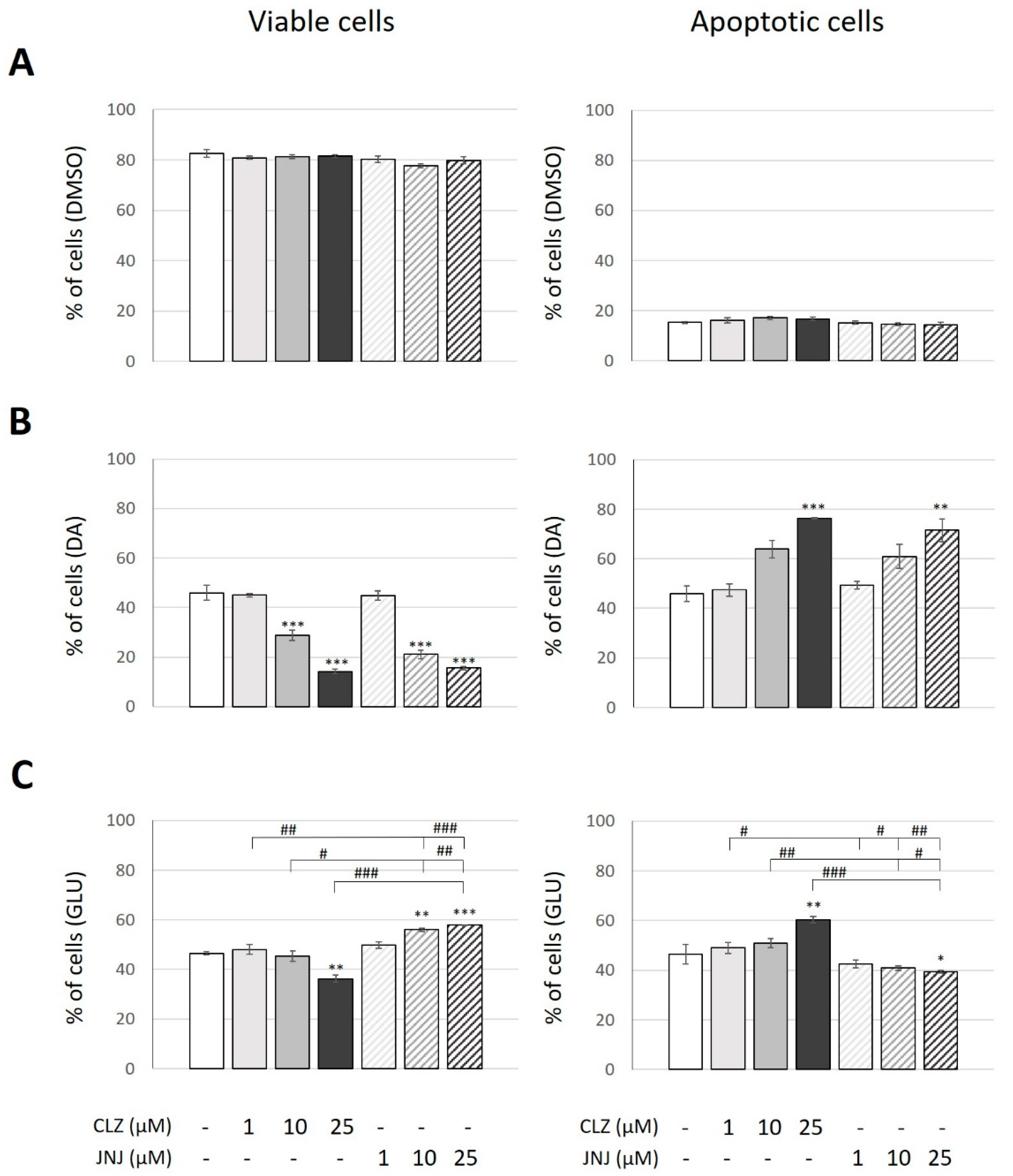
2.3. Effects of Clozapine (CLZ) and JNJ-46356479 (JNJ) Alone and in Combination with Dopamine (DA) or Glutamate (GLU) on Viable and Apoptotic Cells
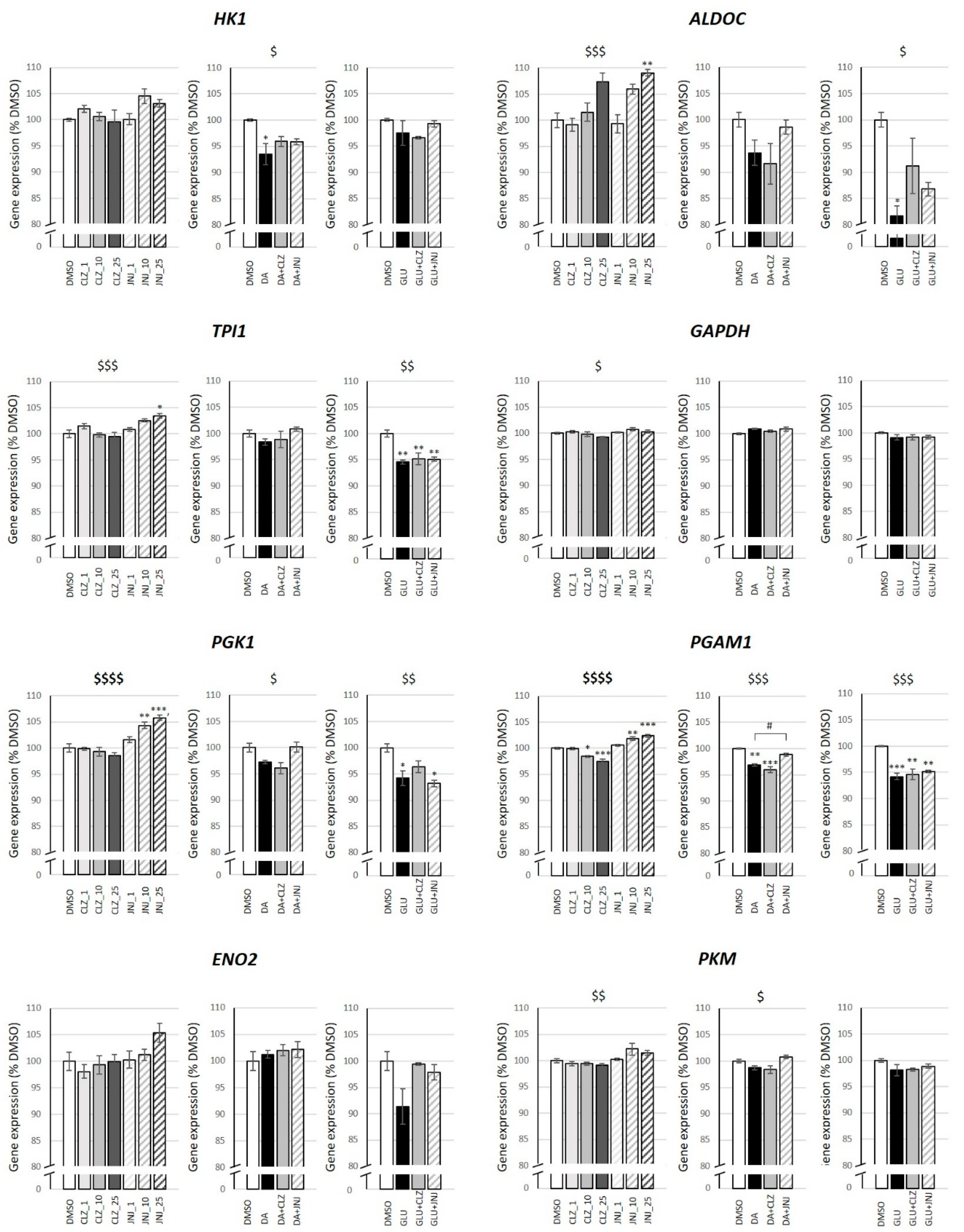
2.4. Effects of Clozapine (CLZ) and JNJ-46356479 (JNJ) Alone and in Combination with Dopamine (DA) or Glutamate (GLU) on Gene Expression
3. Discussion
4. Material and Methods
4.1. Reagents
4.2. Cell Culture
4.3. Cell Viability
4.4. Caspase 3 Activity as an Apoptotic Marker
4.5. Flow Cytometry Measurement of Apoptotic Cell Death Using Annexin-V/PI
4.6. Gene Expression Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marder, S.R.; Cannon, T.D. Schizophrenia. N. Engl. J. Med. 2019, 381, 1753–1761. [Google Scholar] [CrossRef] [PubMed]
- Coyle, J.T. Glutamate and schizophrenia: Beyond the dopamine hypothesis. Cell. Mol. Neurobiol. 2006, 26, 365–384. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, B.; Javitt, D. From revolution to evolution: The glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology 2012, 37, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Uno, Y.; Coyle, J.T. Glutamate hypothesis in schizophrenia. Psychiatry Clin. Neurosci. 2019, 73, 204–215. [Google Scholar] [CrossRef]
- Lewis, D.A.; Moghaddam, B. Cognitive dysfunction in schizophrenia: Convergence of gamma-aminobutyric acid and glutamate alterations. Arch. Neurol. 2006, 63, 1372–1376. [Google Scholar] [CrossRef]
- Snyder, M.A.; Gao, W.J. NMDA hypofunction as a convergence point for progression and symptoms of schizophrenia. Front. Cell Neurosci. 2013, 7, 31. [Google Scholar] [CrossRef]
- Glausier, J.R.; Lewis, D.A. Dendritic spine pathology in schizophrenia. Neuroscience 2013, 251, 90–107. [Google Scholar] [CrossRef]
- Parellada, E.; Gassó, P. Glutamate and microglia activation as a driver of dendritic apoptosis: A core pathophysiological mechanism to understand schizophrenia. Transl. Psychiatry 2021, 11, 271. [Google Scholar] [CrossRef]
- Yuede, C.M.; Wozniak, D.F.; Creeley, C.E.; Taylor, G.T.; Olney, J.W.; Farber, N.B. Behavioral consequences of NMDA antagonist-induced neuroapoptosis in the infant mouse brain. PLoS ONE 2010, 5, e11374. [Google Scholar] [CrossRef]
- Gassó, P.; Mas, S.; Molina, O.; Lafuente, A.; Bernardo, M.; Parellada, E. Increased susceptibility to apoptosis in cultured fibroblasts from antipsychotic-naïve first-episode schizophrenia patients. J. Psychiatr. Res. 2014, 48, 94–101. [Google Scholar] [CrossRef]
- Gassó, P.; Mas, S.; Rodríguez, N.; Boloc, D.; García-Cerro, S.; Bernardo, M.; Lafuente, A.; Parellada, E. Microarray gene-expression study in fibroblast and lymphoblastoid cell lines from antipsychotic-naïve first-episode schizophrenia patients. J. Psychiatr. Res. 2017, 95, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Batalla, A.; Bargalló, N.; Gassó, P.; Molina, O.; Pareto, D.; Mas, S.; Roca, J.M.; Bernardo, M.; Lafuente, A.; Parellada, E. Apoptotic markers in cultured fibroblasts correlate with brain metabolites and regional brain volume in antipsychotic-naive first-episode schizophrenia and healthy controls. Transl. Psychiatry 2015, 5, e626. [Google Scholar] [CrossRef] [PubMed]
- Jantas, D.; Lasoń, W. Preclinical Evidence for the Interplay between Oxidative Stress and RIP1-Dependent Cell Death in Neurodegeneration: State of the Art and Possible Therapeutic Implications. Antioxidants 2021, 10, 1518. [Google Scholar] [CrossRef]
- Goff, D.C. The Pharmacologic Treatment of Schizophrenia-2021. JAMA 2021, 325, 175–176. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.H.; Zaidi, S. Clozapine: Improvement of Negative Symptoms of Schizophrenia. Cureus 2017, 9, e1973. [Google Scholar] [CrossRef]
- Leucht, S.; Cipriani, A.; Spineli, L.; Mavridis, D.; Orey, D.; Richter, F.; Samara, M.; Barbui, C.; Engel, R.R.; Geddes, J.R.; et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: A multiple-treatments meta-analysis. Lancet 2013, 382, 951–962. [Google Scholar] [CrossRef]
- Nucifora, F.C., Jr.; Mihaljevic, M.; Lee, B.J.; Sawa, A. Clozapine as a Model for Antipsychotic Development. Neurotherapeutics 2017, 14, 750–761. [Google Scholar] [CrossRef]
- Cid, J.M.; Tresadern, G.; Vega, J.A.; de Lucas, A.I.; Del Cerro, A.; Matesanz, E.; Linares, M.L.; García, A.; Iturrino, L.; Pérez-Benito, L.; et al. Discovery of 8-Trifluoromethyl-3-cyclopropylmethyl-7-[(4-(2,4-difluorophenyl)-1-piperazinyl)methyl]-1,2,4-triazolo[4,3-a]pyridine (JNJ-46356479), a Selective and Orally Bioavailable mGlu2 Receptor Positive Allosteric Modulator (PAM). J. Med. Chem. 2016, 59, 8495–8507. [Google Scholar] [CrossRef]
- Li, M.L.; Hu, X.Q.; Li, F.; Gao, W.J. Perspectives on the mGluR2/3 agonists as a therapeutic target for schizophrenia: Still promising or a dead end? Prog. Neuropsychopharmacol. Biol. Psychiatry 2015, 60, 66–76. [Google Scholar] [CrossRef]
- Martínez-Pinteño, A.; García-Cerro, S.; Mas, S.; Torres, T.; Boloc, D.; Rodríguez, N.; Lafuente, A.; Gassó, P.; Arnaiz, J.A.; Parellada, E. The positive allosteric modulator of the mGlu2 receptor JNJ-46356479 partially improves neuropathological deficits and schizophrenia-like behaviors in a postnatal ketamine mice model. J. Psychiatr. Res. 2020, 126, 8–18. [Google Scholar] [CrossRef]
- Gassó, P.; Mas, S.; Molina, O.; Bernardo, M.; Lafuente, A.; Parellada, E. Neurotoxic/neuroprotective activity of haloperidol, risperidone and paliperidone in neuroblastoma cells. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 36, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Kingston, A.E.; O’Neill, M.J.; Lam, A.; Bales, K.R.; Monn, J.A.; Schoepp, D.D. Neuroprotection by metabotropic glutamate receptor glutamate receptor agonists: LY354740, LY379268 and LY389795. Eur. J. Pharmacol. 1999, 377, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Jantas, D.; Greda, A.; Golda, S.; Korostynski, M.; Grygier, B.; Roman, A.; Pilc, A.; Lason, W. Neuroprotective effects of metabotropic glutamate receptor group II and III activators against MPP(+)-induced cell death in human neuroblastoma SH-SY5Y cells: The impact of cell differentiation state. Neuropharmacology 2014, 83, 36–53. [Google Scholar] [CrossRef] [PubMed]
- Jantas, D.; Greda, A.; Leskiewicz, M.; Grygier, B.; Pilc, A.; Lason, W. Neuroprotective effects of mGluR II and III activators against staurosporine- and doxorubicin-induced cellular injury in SH-SY5Y cells: New evidence for a mechanism involving inhibition of AIF translocation. Neurochem. Int. 2015, 88, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Abashkin, D.A.; Kurishev, A.O.; Karpov, D.S.; Golimbet, V.E. Cellular Models in Schizophrenia Research. Int. J. Mol. Sci. 2021, 22, 8518. [Google Scholar] [CrossRef]
- Bray, N.J.; Kapur, S.; Price, J. Investigating schizophrenia in a “dish”: Possibilities, potential and limitations. World Psychiatry 2012, 11, 153–155. [Google Scholar] [CrossRef]
- Abe, C.; Nishimura, S.; Mori, A.; Niimi, Y.; Yang, Y.; Hane, M.; Kitajima, K.; Sato, C. Chlorpromazine Increases the Expression of Polysialic Acid (PolySia) in Human Neuroblastoma Cells and Mouse Prefrontal Cortex. Int. J. Mol. Sci. 2017, 18, 1123. [Google Scholar] [CrossRef]
- Du, J.; Nakachi, Y.; Kiyono, T.; Fujii, S.; Kasai, K.; Bundo, M.; Iwamoto, K. Comprehensive DNA Methylation Analysis of Human Neuroblastoma Cells Treated With Haloperidol and Risperidone. Front. Mol. Neurosci. 2021, 14, 792874. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Kim, Y.; Chung, I.W.; Kim, Y.S. Clozapine induces chloride channel-4 expression through PKA activation and modulates CDK5 expression in SH-SY5Y and U87 cells. Prog. Neuropsychopharmacol. Biol. Psychiatry 2015, 56, 168–173. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Villalpando-Rodriguez, G.E.; Gibson, S.B. Reactive Oxygen Species (ROS) Regulates Different Types of Cell Death by Acting as a Rheostat. Oxid. Med. Cell. Longev. 2021, 2021, 9912436. [Google Scholar] [CrossRef] [PubMed]
- Trabanco, A.A.; Bartolomé, J.M.; Cid, J.M. mGluR2 positive allosteric modulators: An updated patent review (2013–2018). Expert Opin. Ther. Pat. 2019, 29, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Caraci, F.; Molinaro, G.; Battaglia, G.; Giuffrida, M.L.; Riozzi, B.; Traficante, A.; Bruno, V.; Cannella, M.; Merlo, S.; Wang, X.; et al. Targeting group II Metabotropic Glutamate (mGlu) receptors for the treatment of psychosis associated with alzheimer’s disease: Selective activation of mGlu2 receptors amplifies β-amyloid toxicity in cultured neurons, whereas dual activation of mGlu2 and mGlu3 receptors is neuroprotective. Mol. Pharmacol. 2011, 79, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Kent, J.M.; Daly, E.; Kezic, I.; Lane, R.; Lim, P.; De Smedt, H.; De Boer, P.; Van Nueten, L.; Drevets, W.C.; Ceusters, M. Efficacy and safety of an adjunctive mGlu2 receptor positive allosteric modulator to a SSRI/SNRI in anxious depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 67, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Kang, U.G.; Seo, M.S.; Roh, M.S.; Kim, Y.; Yoon, S.C.; Kim, Y.S. The effects of clozapine on the GSK-3-mediated signaling pathway. FEBS Lett. 2004, 560, 115–119. [Google Scholar] [CrossRef]
- Qing, H.; Xu, H.; Wei, Z.; Gibson, K.; Li, X.M. The ability of atypical antipsychotic drugs vs. haloperidol to protect PC12 cells against MPP+-induced apoptosis. Eur. J. Neurosci. 2003, 17, 1563–1570. [Google Scholar] [CrossRef]
- Lundberg, M.; Curbo, S.; Bohman, H.; Agartz, I.; Ögren, S.O.; Patrone, C. Clozapine protects adult neural stem cells from ketamine-induced cell death in correlation with decreased apoptosis and autophagy. Biosci. Rep. 2020, 40, BSR20193156. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Chung, S.; An, H.; Kim, J.; Seo, J.; Kim, D.H.; Yoon, S.Y. Haloperidol and clozapine block formation of autophagolysosomes in rat primary neurons. Neuroscience 2012, 209, 64–73. [Google Scholar] [CrossRef]
- Chen, A.T.; Nasrallah, H.A. Neuroprotective effects of the second generation antipsychotics. Schizophr. Res. 2019, 208, 1–7. [Google Scholar] [CrossRef]
- Takaki, M.; Kodama, M.; Mizuki, Y.; Kawai, H.; Yoshimura, B.; Kishimoto, M.; Sakamoto, S.; Okahisa, Y.; Yamada, N. Effects of the antipsychotics haloperidol, clozapine, and aripiprazole on the dendritic spine. Eur. Neuropsychopharmacol. 2018, 28, 610–619. [Google Scholar] [CrossRef]
- Critchlow, H.M.; Maycox, P.R.; Skepper, J.N.; Krylova, O. Clozapine and haloperidol differentially regulate dendritic spine formation and synaptogenesis in rat hippocampal neurons. Mol. Cell. Neurosci. 2006, 32, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Bai, O.; Wei, Z.; Lu, W.; Bowen, R.; Keegan, D.; Li, X.M. Protective effects of atypical antipsychotic drugs on PC12 cells after serum withdrawal. J. Neurosci. Res. 2002, 69, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, M.A.; Marín, M.J.; Martín, M.S.; Rubio-Lopez, M.I.; López-Hoyos, M.; Miñambres, E. Effect of neuroprotective therapies (hypothermia and cyclosporine a) on dopamine-induced apoptosis in human neuronal SH-SY5Y cells. Brain Inj. 2013, 27, 354–360. [Google Scholar] [CrossRef]
- Nikolova, S.; Lee, Y.S.; Lee, Y.S.; Kim, J.A. Rac1-NADPH oxidase-regulated generation of reactive oxygen species mediates glutamate-induced apoptosis in SH-SY5Y human neuroblastoma cells. Free Radic. Res. 2005, 39, 1295–1304. [Google Scholar] [CrossRef]
- Yuksel, T.N.; Yayla, M.; Halici, Z.; Cadirci, E.; Polat, B.; Kose, D. Protective effect of 5-HT7 receptor activation against glutamate-induced neurotoxicity in human neuroblastoma SH-SY5Y cells via antioxidative and antiapoptotic pathways. Neurotoxicol. Teratol. 2019, 72, 22–28. [Google Scholar] [CrossRef]
- Kritis, A.A.; Stamoula, E.G.; Paniskaki, K.A.; Vavilis, T.D. Researching glutamate—Induced cytotoxicity in different cell lines: A comparative/collective analysis/study. Front. Cell. Neurosci. 2015, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Henkel, N.D.; Wu, X.; O’Donovan, S.M.; Devine, E.A.; Jiron, J.M.; Rowland, L.M.; Sarnyai, Z.; Ramsey, A.J.; Wen, Z.; Hahn, M.K.; et al. Schizophrenia: A disorder of broken brain bioenergetics. Mol. Psychiatry 2022, 27, 2393–2404. [Google Scholar] [CrossRef]
- Ucker, D.S.; Jain, M.R.; Pattabiraman, G.; Palasiewicz, K.; Birge, R.B.; Li, H. Externalized glycolytic enzymes are novel, conserved, and early biomarkers of apoptosis. J. Biol. Chem. 2012, 287, 10325–10343. [Google Scholar] [CrossRef]
- Martins-de-Souza, D.; Maccarrone, G.; Wobrock, T.; Zerr, I.; Gormanns, P.; Reckow, S.; Falkai, P.; Schmitt, A.; Turck, C.W. Proteome analysis of the thalamus and cerebrospinal fluid reveals glycolysis dysfunction and potential biomarkers candidates for schizophrenia. J. Psychiatr. Res. 2010, 44, 1176–1189. [Google Scholar] [CrossRef]
- Novikova, S.I.; He, F.; Cutrufello, N.J.; Lidow, M.S. Identification of protein biomarkers for schizophrenia and bipolar disorder in the postmortem prefrontal cortex using SELDI-TOF-MS ProteinChip profiling combined with MALDI-TOF-PSD-MS analysis. Neurobiol. Dis. 2006, 23, 61–76. [Google Scholar] [CrossRef]
- Martins-de-Souza, D.; Alsaif, M.; Ernst, A.; Harris, L.W.; Aerts, N.; Lenaerts, I.; Peeters, P.J.; Amess, B.; Rahmoune, H.; Bahn, S.; et al. The application of selective reaction monitoring confirms dysregulation of glycolysis in a preclinical model of schizophrenia. BMC Res. Notes 2012, 5, 146. [Google Scholar] [CrossRef] [PubMed]
- Germann, M.; Brederoo, S.G.; Sommer, I.E.C. Abnormal synaptic pruning during adolescence underlying the development of psychotic disorders. Curr. Opin. Psychiatry 2021, 34, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Millan, M.J.; Andrieux, A.; Bartzokis, G.; Cadenhead, K.; Dazzan, P.; Fusar-Poli, P.; Gallinat, J.; Giedd, J.; Grayson, D.R.; Heinrichs, M.; et al. Altering the course of schizophrenia: Progress and perspectives. Nat. Rev. Drug Discov. 2016, 15, 485–515. [Google Scholar] [CrossRef]
- Schobel, S.A.; Chaudhury, N.H.; Khan, U.A.; Paniagua, B.; Styner, M.A.; Asllani, I.; Inbar, B.P.; Corcoran, C.M.; Lieberman, J.A.; Moore, H.; et al. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron 2013, 78, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Sharp, S.I.; Hu, Y.; Weymer, J.F.; Rizig, M.; McQuillin, A.; Hunt, S.P.; Gurling, H.M.D. The effect of clozapine on mRNA expression for genes encoding G protein-coupled receptors and the protein components of clathrin-mediated endocytosis. Psychiatr. Genet. 2013, 23, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, B.S.; Irizarry, R.A. A framework for oligonucleotide microarray preprocessing. Bioinformatics 2010, 26, 2363–2367. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gassó, P.; Martínez-Pinteño, A.; Rodríguez, N.; Madero, S.; Gómez, M.; Segura, A.G.; García-Rizo, C.; Morén, C.; Mas, S.; Parellada, E. Neurotoxic/Neuroprotective Effects of Clozapine and the Positive Allosteric Modulator of mGluR2 JNJ-46356479 in Human Neuroblastoma Cell Cultures. Int. J. Mol. Sci. 2023, 24, 2054. https://doi.org/10.3390/ijms24032054
Gassó P, Martínez-Pinteño A, Rodríguez N, Madero S, Gómez M, Segura AG, García-Rizo C, Morén C, Mas S, Parellada E. Neurotoxic/Neuroprotective Effects of Clozapine and the Positive Allosteric Modulator of mGluR2 JNJ-46356479 in Human Neuroblastoma Cell Cultures. International Journal of Molecular Sciences. 2023; 24(3):2054. https://doi.org/10.3390/ijms24032054
Chicago/Turabian StyleGassó, Patricia, Albert Martínez-Pinteño, Natalia Rodríguez, Santiago Madero, Marta Gómez, Alex G. Segura, Clemente García-Rizo, Constanza Morén, Sergi Mas, and Eduard Parellada. 2023. "Neurotoxic/Neuroprotective Effects of Clozapine and the Positive Allosteric Modulator of mGluR2 JNJ-46356479 in Human Neuroblastoma Cell Cultures" International Journal of Molecular Sciences 24, no. 3: 2054. https://doi.org/10.3390/ijms24032054
APA StyleGassó, P., Martínez-Pinteño, A., Rodríguez, N., Madero, S., Gómez, M., Segura, A. G., García-Rizo, C., Morén, C., Mas, S., & Parellada, E. (2023). Neurotoxic/Neuroprotective Effects of Clozapine and the Positive Allosteric Modulator of mGluR2 JNJ-46356479 in Human Neuroblastoma Cell Cultures. International Journal of Molecular Sciences, 24(3), 2054. https://doi.org/10.3390/ijms24032054




