Phosphorylation of Thymidylate Synthase and Dihydrofolate Reductase in Cancer Cells and the Effect of CK2α Silencing
Abstract
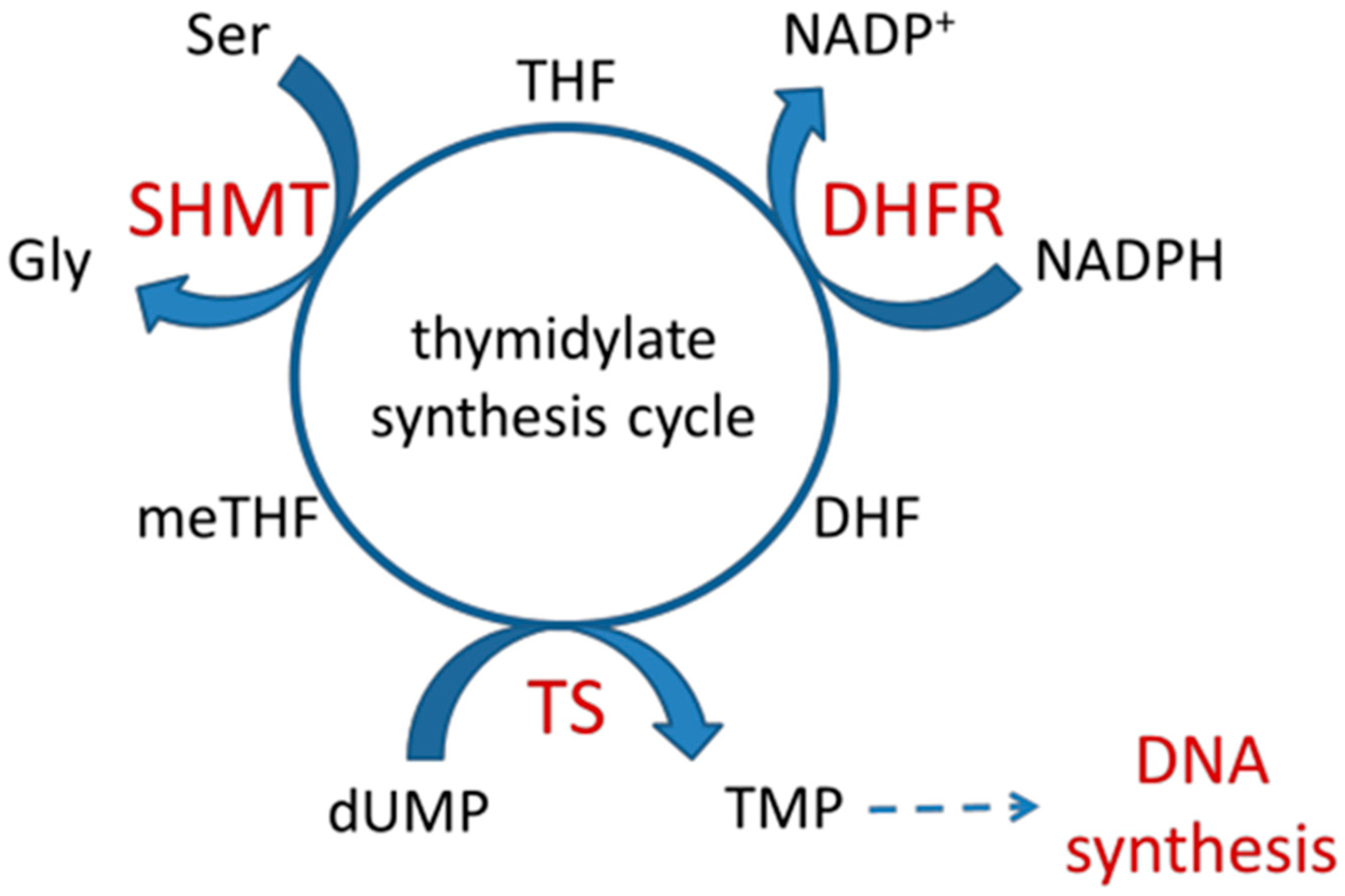
1. Introduction
2. Results
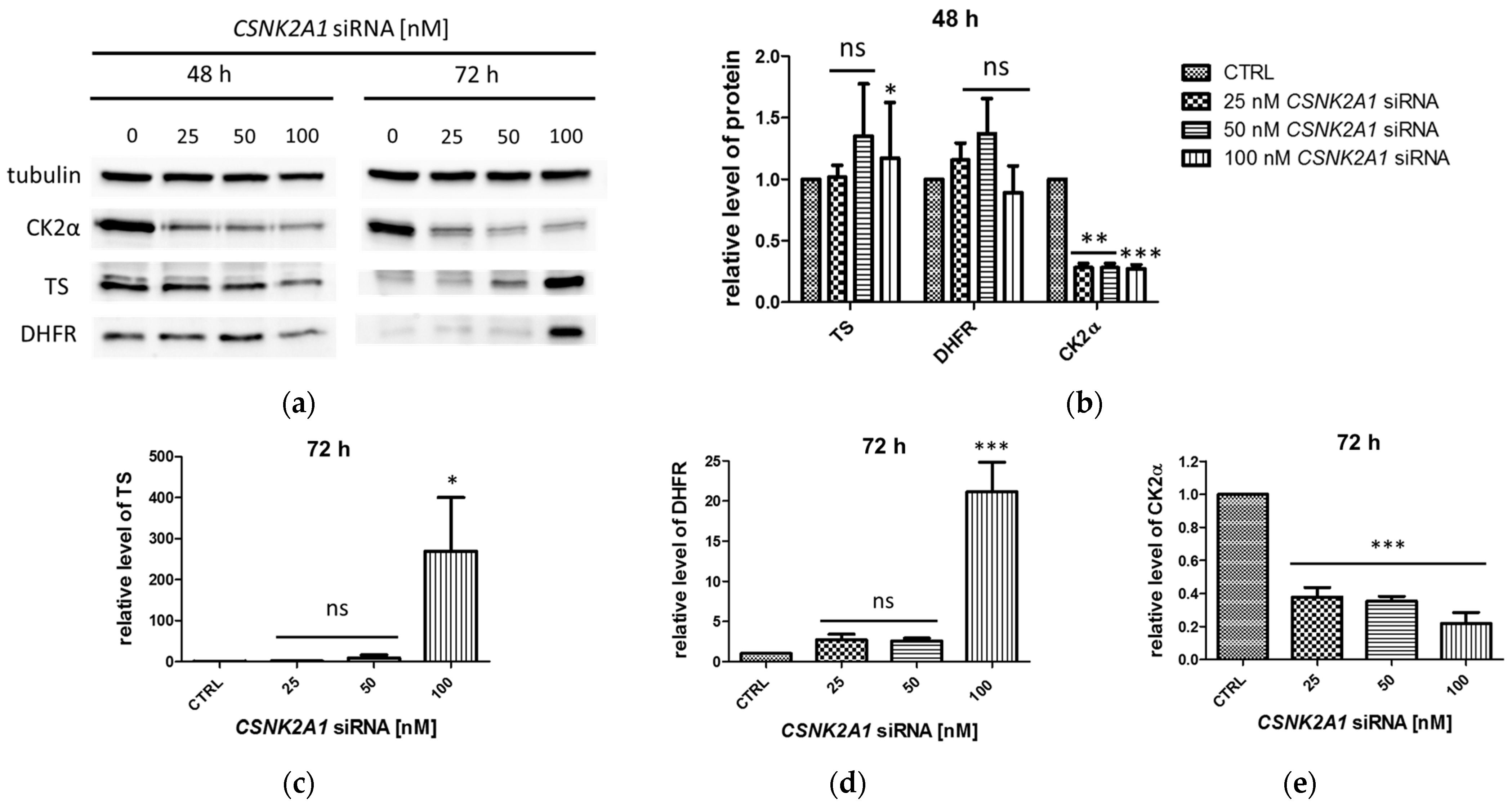
2.1. Protein Levels of TS and DHFR in A-549 with the Silenced CSNK2A1 Gene
2.2. TS and DHFR mRNA Levels in A-549 with the Silenced CSNK2A1
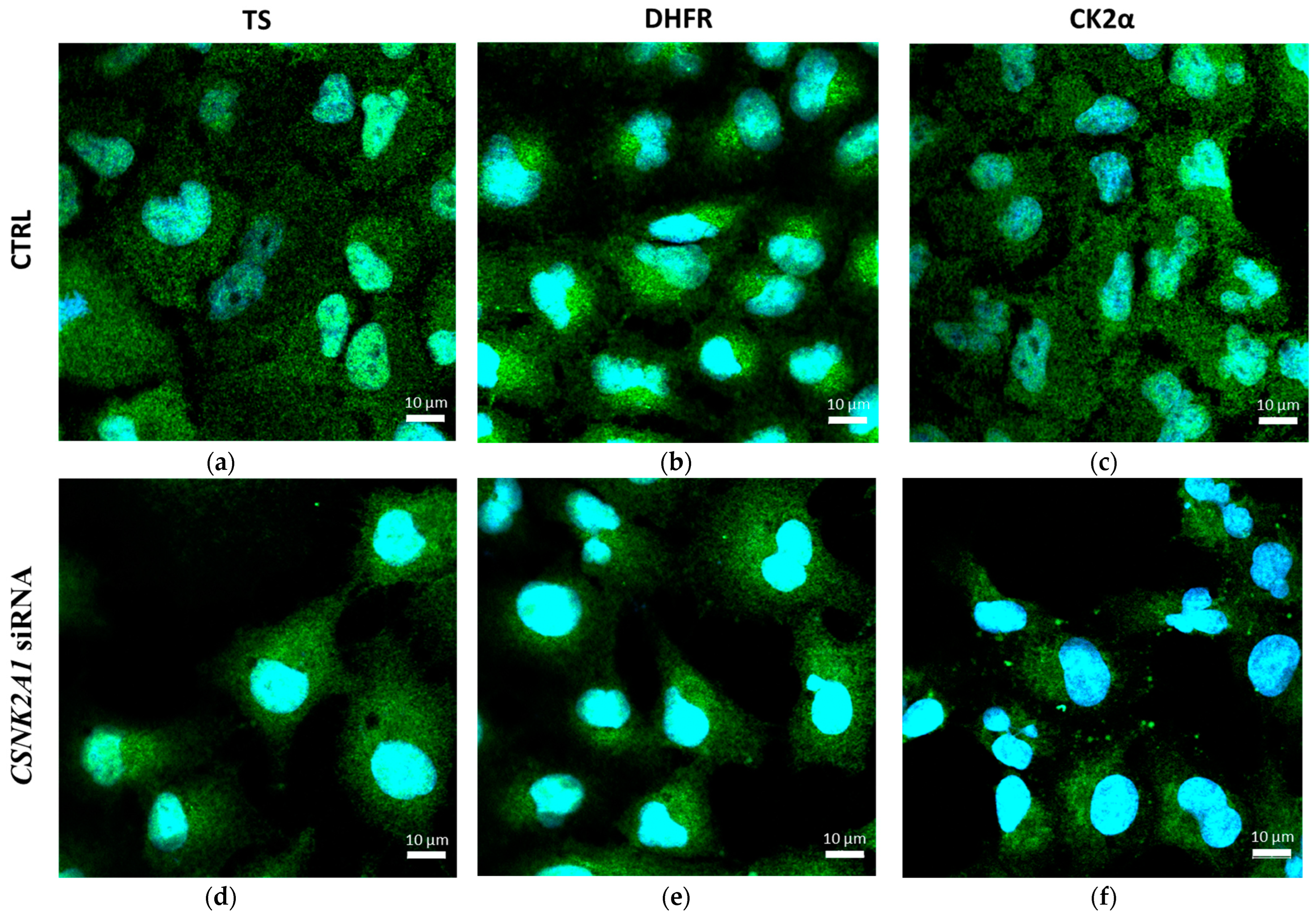
2.3. The Effect of Silencing of CSNK2A1 on Localisation of DHFR and TS in A-549 Cells
2.4. Phosphorylation of TS and DHFR by Endogenous Protein Kinases from CCRF-CEM and A-549 Cells
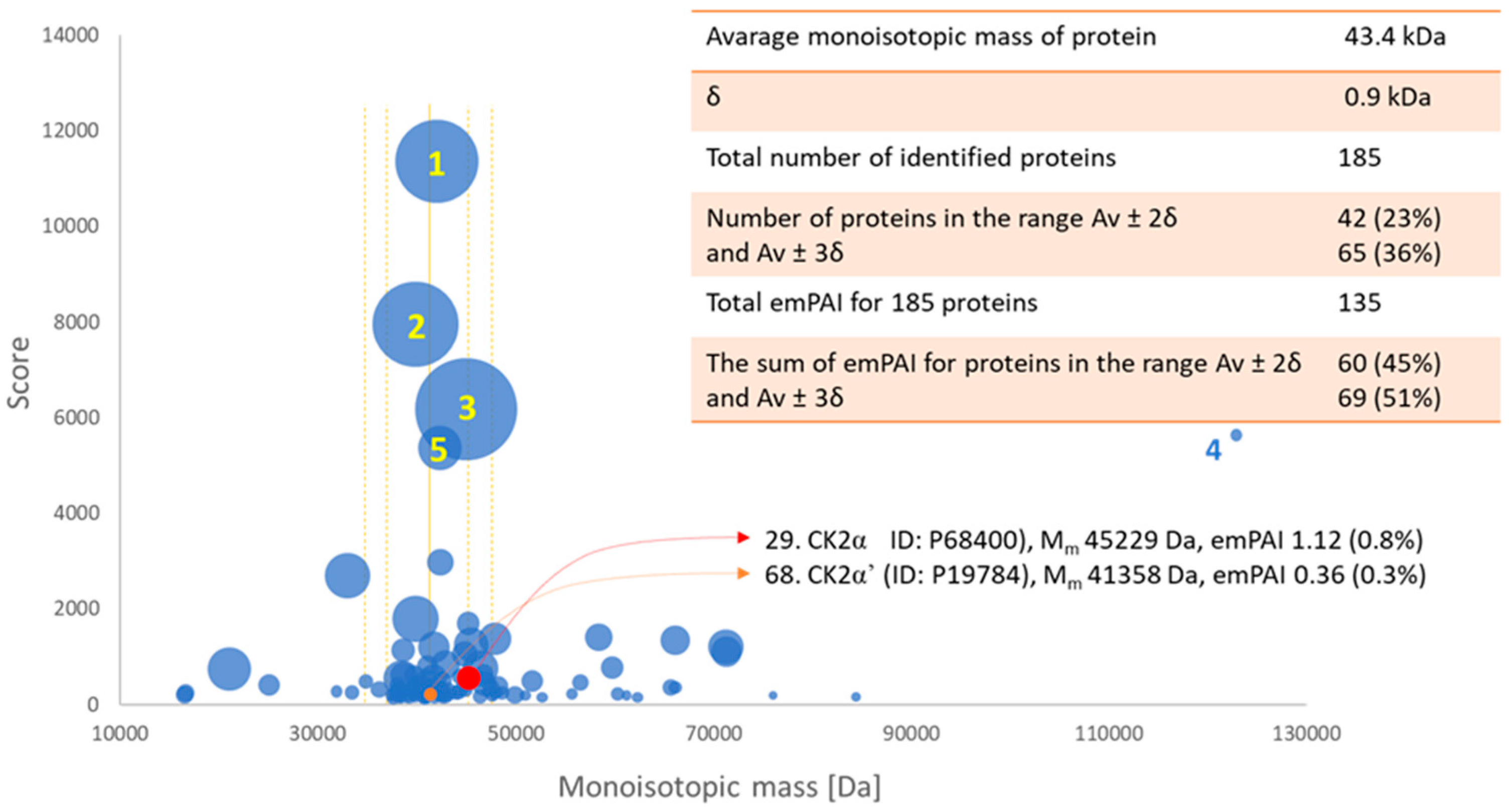
2.5. Confirmation of the Identity of Casein Kinases 2 Subunit α and α
2.6. Identification of Other Protein Kinases
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Cell Culture
4.3. Protein Preparations
4.4. In-Gel Kinase Assay
4.5. Mass Spectrometry Analysis
4.5.1. Large-Scale Mass Spectrometry Analysis
4.5.2. Evaluation of Mass Spectrometry Platform
4.5.3. Size Distribution of Proteins in Excised Gel Band and Their Relative Concentration
4.6. Obtaining A-549 Cell Line with the Silenced CSNK2A1 Gene
4.7. Western Blotting
4.8. Immunocytochemical Staining and Microscopy Analysis
4.9. RNA Isolation Followed by the Reverse Transcription–Quantitative Polymerase Chain Reaction (RT-qPCR)
4.10. Densitometry
4.11. Statistical Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pang, K.; Wang, W.; Qin, J.; Shi, Z.; Hao, L.; Ma, Y.; Xu, H.; Wu, Z.; Pan, D.; Chen, Z.; et al. Role of protein phosphorylation in cell signaling, disease, and the intervention therapy. Medcomm 2022, 3, 175. [Google Scholar] [CrossRef]
- Mori, Y.; Kawamura, H.; Sato, T.; Fujita, T.; Nagata, R.; Fujihashi, M.; Miki, K.; Atomi, H. Identification and enzymatic analysis of an archaeal ATP-dependent serine kinase from the hyperthermophilic archaeon Staphylothermus marinus. J. Bacteriol. 2021, 203, e00025-21. [Google Scholar] [CrossRef]
- Tavernier, N.; Sicheri, F.; Pintard, L. Aurora A kinase activation: Different means to different ends. J. Cell Biol. 2021, 220, e202106128. [Google Scholar] [CrossRef]
- Boulos, J.C.; Idres, M.R.Y.; Efferth, T. Investigation of cancer drug resistance mechanisms by phosphoproteomics. Pharmacol. Res. 2020, 160, 105091. [Google Scholar] [CrossRef]
- Nuñez de Villavicencio-Diaz, T.; Rabalski, A.J.; Litchfield, D.W. Protein Kinase CK2: Intricate Relationships within Regulatory Cellular Networks. Pharmaceuticals 2017, 10, 27. [Google Scholar] [CrossRef]
- Trembley, J.H.; Wang, G.; Unger, G.; Slaton, J.; Ahmed, K. Protein Kinase CK2 in Health and Disease: CK2: A key player in cancer biology. Cell. Mol. Life Sci. 2009, 66, 1858–1867. [Google Scholar] [CrossRef]
- Duncan, J.S.; Litchfield, D.W. Too much of a good thing: The role of protein kinase CK2 in tumorigenesis and prospects for therapeutic inhibition of CK2. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2008, 1784, 33–47. [Google Scholar] [CrossRef]
- Trembley, J.H.; Kren, B.T.; Afzal, M.; Scaria, G.A.; Klein, M.A.; Ahmed, K. Protein kinase CK2—Diverse roles in cancer cell biology and therapeutic promise. Mol. Cell. Biochem. 2022, 1–28, Online Ahead of Print. [Google Scholar] [CrossRef]
- Lian, H.; Su, M.; Zhu, Y.; Zhou, Y.; Soomro, S.H.; Fu, H. Protein Kinase CK2, a Potential Therapeutic Target in Carcinoma Management. Asian Pac. J. Cancer Prev. 2019, 20, 23–32. [Google Scholar] [CrossRef]
- Frączyk, T.; Kubiński, K.; Masłyk, M.; Cieśla, J.; Hellman, U.; Shugar, D.; Rode, W. Phosphorylation of thymidylate synthase from various sources by human protein kinase CK2 and its catalytic subunits. Bioorganic Chem. 2010, 38, 124–131. [Google Scholar] [CrossRef]
- Jarmuła, A.; Frączyk, T.; Cieplak, P.; Rode, W. Mechanism of influence of phosphorylation on serine 124 on a decrease of catalytic activity of human thymidylate synthase. Bioorganic Med. Chem. 2010, 18, 3361–3370. [Google Scholar] [CrossRef]
- Lovelace, L.L.; Johnson, S.R.; Gibson, L.M.; Bell, B.J.; Berger, S.H.; Lebioda, L. Variants of human thymidylate synthase with loop 181-197 stabilized in the inactive conformation. Protein Sci. 2009, 18, 1628–1636. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Repalli, J.; Yousef, A.-M.; Johnson, S.R.; Lebioda, L.; Berger, S.H. Human thymidylate synthase with loop 181-197 stabilized in an inactive conformation: Ligand interactions, phosphorylation, and inhibition profiles. Protein Sci. 2011, 20, 87–94. [Google Scholar] [CrossRef]
- Skierka, K.; Wilamowski, P.; Wielechowska, M.; Cysewski, D.; Senkara, E.; Wińska, P.; Bretner, M.; Cieśla, J. Human dihydrofolate reductase is a substrate of protein kinase CK2α. Biochem. Biophys. Res. Commun. 2019, 513, 368–373. [Google Scholar] [CrossRef]
- Wińska, P.; Widło, Ł.; Skierka, K.; Krzyśko, A.; Koronkiewicz, M.; Cieśla, J.M.; Cieśla, J.; Bretner, M. Simultaneous Inhibition of Protein Kinase CK2 and Dihydrofolate Reductase Results in Synergistic Effect on Acute Lymphoblastic Leukemia Cells. Anticancer Res. 2019, 39, 3531–3542. [Google Scholar] [CrossRef]
- Wińska, P.; Widło, Ł.; Senkara, E.; Koronkiewicz, M.; Cieśla, J.M.; Krzyśko, A.; Skierka, K.; Cieśla, J. Inhibition of Protein Kinase CK2 Affects Thymidylate Synthesis Cycle Enzyme Level and Distribution in Human Cancer Cells. Front. Mol. Biosci. 2022, 9, 847829. [Google Scholar] [CrossRef]
- Wińska, P.; Skierka, K.; Łukowska-Chojnacka, E.; Koronkiewicz, M.; Cieśla, J.; Bretner, M. Effect of Simultaneous Inhibition of Protein Kinase CK2 and Thymidylate Synthase in Leukemia and Breast Cancer Cells. Anticancer Res. 2018, 38, 4617–4627. [Google Scholar] [CrossRef]
- Wińska, P.; Karatsai, O.; Staniszewska, M.; Koronkiewicz, M.; Chojnacki, K.; Rędowicz, M.J. Synergistic Interactions of 5-Fluorouracil with Inhibitors of Protein Kinase CK2 Correlate with p38 MAPK Activation and FAK Inhibition in the Triple-Negative Breast Cancer Cell Line. Int. J. Mol. Sci. 2020, 21, 6234. [Google Scholar] [CrossRef]
- Bondestam, J.; Huotari, M.-A.; Morén, A.; Ustinov, J.; Kaivo-Oja, N.; Kallio, J.; Horelli-Kuitunen, N.; Aaltonen, J.; Fujii, M.; Moustakas, A.; et al. cDNA cloning, expression studies and chromosome mapping of human type I serine/threonine kinase receptor ALK7 (ACVR1C). Cytogenet. Genome Res. 2001, 95, 157–162. [Google Scholar] [CrossRef]
- Navalgund, L.; Rossana, C.; Muench, A.; Johnson, L. Cell cycle regulation of thymidylate synthetase gene expression in cultured mouse fibroblasts. J. Biol. Chem. 1980, 255, 7386–7390. [Google Scholar] [CrossRef]
- DeGregori, J.; Kowalik, T.; Nevins, J.R. Cellular targets for activation by the E2F1 transcription factor include DNA synthesis- and G1/S-regulatory genes. Mol. Cell. Biol. 1995, 15, 4215–4224. [Google Scholar] [CrossRef] [PubMed]
- Abali, E.E.; Skacel, N.E.; Celikkaya, H.; Hsieh, Y. Chapter 9 Regulation of Human Dihydrofolate Reductase Activity and Expression. Vitam. Horm. 2008, 79, 267–292. [Google Scholar] [CrossRef] [PubMed]
- Gowda, C.; Song, C.; Ding, Y.; Iyer, S.; Dhanyamraju, P.K.; McGrath, M.; Bamme, Y.; Soliman, M.; Kane, S.; Payne, J.; et al. Cellular signaling and epigenetic regulation of gene expression in leukemia. Adv. Biol. Regul. 2019, 75, 100665. [Google Scholar] [CrossRef] [PubMed]
- Frączyk, T.; Ruman, T.; Rut, D.; Dąbrowska-Maś, E.; Cieśla, J.; Zieliński, Z.; Sieczka, K.; Dębski, J.; Gołos, B.; Wińska, P.; et al. Histidine Phosphorylation, or Tyrosine Nitra-tion, Affect Thymidylate Synthase Properties. Pteridines 2009, 20, 137–142. [Google Scholar]
- Anderson, D.D.; Eom, J.Y.; Stover, P.J. Competition between Sumoylation and Ubiquitination of Serine Hydroxymethyltransferase 1 Determines Its Nuclear Localization and Its Accumulation in the Nucleus. J. Biol. Chem. 2012, 287, 4790–4799. [Google Scholar] [CrossRef]
- Anderson, D.D.; Woeller, C.F.; Chiang, E.-P.; Shane, B.; Stover, P.J. Serine Hydroxymethyltransferase Anchors de Novo Thymidylate Synthesis Pathway to Nuclear Lamina for DNA Synthesis. J. Biol. Chem. 2012, 287, 7051–7062. [Google Scholar] [CrossRef]
- Woeller, C.F.; Anderson, D.D.; Szebenyi, D.M.; Stover, P.J. Evidence for Small Ubiquitin-like Modifier-dependent Nuclear Import of the Thymidylate Biosynthesis Pathway. J. Biol. Chem. 2007, 282, 17623–17631. [Google Scholar] [CrossRef]
- Hietakangas, V.; Anckar, J.; Blomster, H.A.; Fujimoto, M.; Palvimo, J.J.; Nakai, A.; Sistonen, L. PDSM, a motif for phosphoryla-tion-dependent SUMO modification. Proc. Natl. Acad. Sci. USA 2006, 103, 45–50. [Google Scholar] [CrossRef]
- Klein, S.; Meng, R.; Montenarh, M.; Götz, C. The Phosphorylation of PDX-1 by Protein Kinase CK2 Is Crucial for Its Stability. Pharmaceuticals 2016, 10, 2. [Google Scholar] [CrossRef]
- Filipčík, P.; Curry, J.R.; Mace, P.D. When Worlds Collide—Mechanisms at the Interface between Phosphorylation and Ubiquitination. J. Mol. Biol. 2017, 429, 1097–1113. [Google Scholar] [CrossRef]
- Stehmeier, P.; Muller, S. Phospho-Regulated SUMO Interaction Modules Connect the SUMO System to CK2 Signaling. Mol. Cell 2009, 33, 400–409. [Google Scholar] [CrossRef]
- Tripathi, V.; Chatterjee, K.S.; Das, R. Casein kinase-2–mediated phosphorylation increases the SUMO-dependent activity of the cytomegalovirus transactivator IE2. J. Biol. Chem. 2019, 294, 14546–14561. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Tan, M.; Pamarthy, D.; Wang, G.; Ahmed, K.; Sun, Y. CK2 phosphorylation of SAG at Thr10 regulates SAG stability, but not its E3 ligase activity. Mol. Cell. Biochem. 2006, 295, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Pei, H.Z.; Chang, H.-W.; Baek, S.-H. The E3 ubiquitin ligase Trim13 regulates Nur77 stability via casein kinase 2α. Sci. Rep. 2018, 8, 13895. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.H.; Berger, F.G.; Lebioda, L. Effects of ligand binding and conformational switching on intracellular stability of human thymidylate synthase. Biochim. et Biophys. Acta (BBA)—Proteins Proteom. 2003, 1696, 15–22. [Google Scholar] [CrossRef]
- Wooten, M.W. In-Gel Kinase Assay as a Method to Identify Kinase Substrates. Sci. STKE 2002, 2002, l15. [Google Scholar] [CrossRef]
- Roffey, S.E.; Litchfield, D.W. CK2 Regulation: Perspectives in 2021. Biomedicines 2021, 9, 1361. [Google Scholar] [CrossRef]
- Donella-Deana, A.; Cesaro, L.; Sarno, S.; Brunati, A.M.; Ruzzene, M.; Pinna, L.A. Autocatalytic tyrosine-phosphorylation of protein kinase CK2 α and α′ subunits: Implication of Tyr182. Biochem. J. 2001, 357, 563–567. [Google Scholar] [CrossRef]
- Rusin, S.F.; Adamo, M.E.; Kettenbach, A.N. Identification of Candidate Casein Kinase 2 Substrates in Mitosis by Quantitative Phosphoproteomics. Front. Cell Dev. Biol. 2017, 5, 97. [Google Scholar] [CrossRef]
- Antosiewicz, A.; Senkara, E.; Cieśla, J. Quartz crystal microbalance with dissipation and microscale thermophoresis as tools for investigation of protein complex formation between thymidylate synthesis cycle enzymes. Biosens. Bioelectron. 2015, 64, 36–42. [Google Scholar] [CrossRef]
- Chojnacki, K.; Wińska, P.; Karatsai, O.; Koronkiewicz, M.; Milner-Krawczyk, M.; Wielechowska, M.; Rędowicz, M.; Bretner, M.; Borowiecki, P. Synthesis of Novel Acyl Derivatives of 3-(4,5,6,7-Tetrabromo-1H-benzimidazol-1-yl)propan-1-ols—Intracellular TBBi-Based CK2 Inhibitors with Proapoptotic Properties. Int. J. Mol. Sci. 2021, 22, 6261. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhu, J.-K. Assessing Kinase Activity in Plants with In-Gel Kinase Assays. Methods Mol. Biol. 2016, 1363, 189–197. [Google Scholar] [CrossRef]
- Zhao, Y.; Lin, Y.-H. Whole-Cell Protein Identification Using the Concept of Unique Peptides. Genom. Proteom. Bioinform. 2010, 8, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Elias, J.E.; Thoreen, C.C.; Licklider, L.J.; Gygi, S.P. Evaluation of Multidimensional Chromatography Coupled with Tandem Mass Spectrometry (LC/LC−MS/MS) for Large-Scale Protein Analysis: The Yeast Proteome. J. Proteome Res. 2002, 2, 43–50. [Google Scholar] [CrossRef] [PubMed]
- E Elias, J.; Gibbons, F.D.; King, O.D.; Roth, F.; Gygi, S.P. Intensity-based protein identification by machine learning from a library of tandem mass spectra. Nat. Biotechnol. 2004, 22, 214–219. [Google Scholar] [CrossRef]
- Ishihama, Y.; Oda, Y.; Tabata, T.; Sato, T.; Nagasu, T.; Rappsilber, J.; Mann, M. Exponentially modified protein abundance index (emPAI) for estimation of absolute protein amount in proteomics by the number of sequenced peptides per protein. Mol. Cell. Proteom. 2005, 4, 1265–1272. [Google Scholar] [CrossRef]
- Guerra, B.; Iwabuchi, K.; Issinger, O.-G. Protein kinase CK2 is required for the recruitment of 53BP1 to sites of DNA double-strand break induced by radiomimetic drugs. Cancer Lett. 2014, 345, 115–123. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wińska, P.; Sobiepanek, A.; Pawlak, K.; Staniszewska, M.; Cieśla, J. Phosphorylation of Thymidylate Synthase and Dihydrofolate Reductase in Cancer Cells and the Effect of CK2α Silencing. Int. J. Mol. Sci. 2023, 24, 3023. https://doi.org/10.3390/ijms24033023
Wińska P, Sobiepanek A, Pawlak K, Staniszewska M, Cieśla J. Phosphorylation of Thymidylate Synthase and Dihydrofolate Reductase in Cancer Cells and the Effect of CK2α Silencing. International Journal of Molecular Sciences. 2023; 24(3):3023. https://doi.org/10.3390/ijms24033023
Chicago/Turabian StyleWińska, Patrycja, Anna Sobiepanek, Katarzyna Pawlak, Monika Staniszewska, and Joanna Cieśla. 2023. "Phosphorylation of Thymidylate Synthase and Dihydrofolate Reductase in Cancer Cells and the Effect of CK2α Silencing" International Journal of Molecular Sciences 24, no. 3: 3023. https://doi.org/10.3390/ijms24033023
APA StyleWińska, P., Sobiepanek, A., Pawlak, K., Staniszewska, M., & Cieśla, J. (2023). Phosphorylation of Thymidylate Synthase and Dihydrofolate Reductase in Cancer Cells and the Effect of CK2α Silencing. International Journal of Molecular Sciences, 24(3), 3023. https://doi.org/10.3390/ijms24033023






