The Effects of Sirolimus and Magnesium on Primary Human Coronary Endothelial Cells: An In Vitro Study
Abstract
1. Introduction
2. Results
2.1. The Effects of Mg and SIR on hCAEC Viability
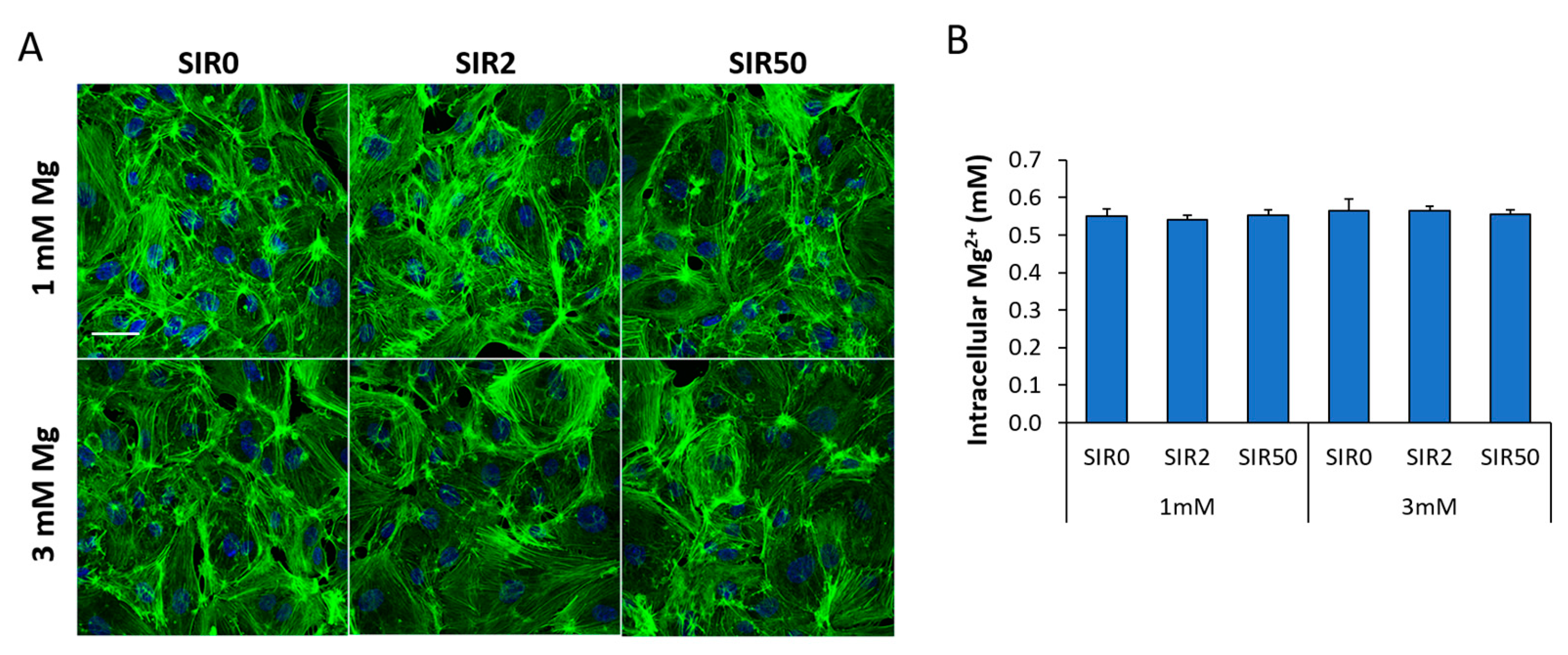
2.2. The Effects of Mg and SIR on hCAEC Cytoskeletal Organization and Mg2+ Intracellular Content
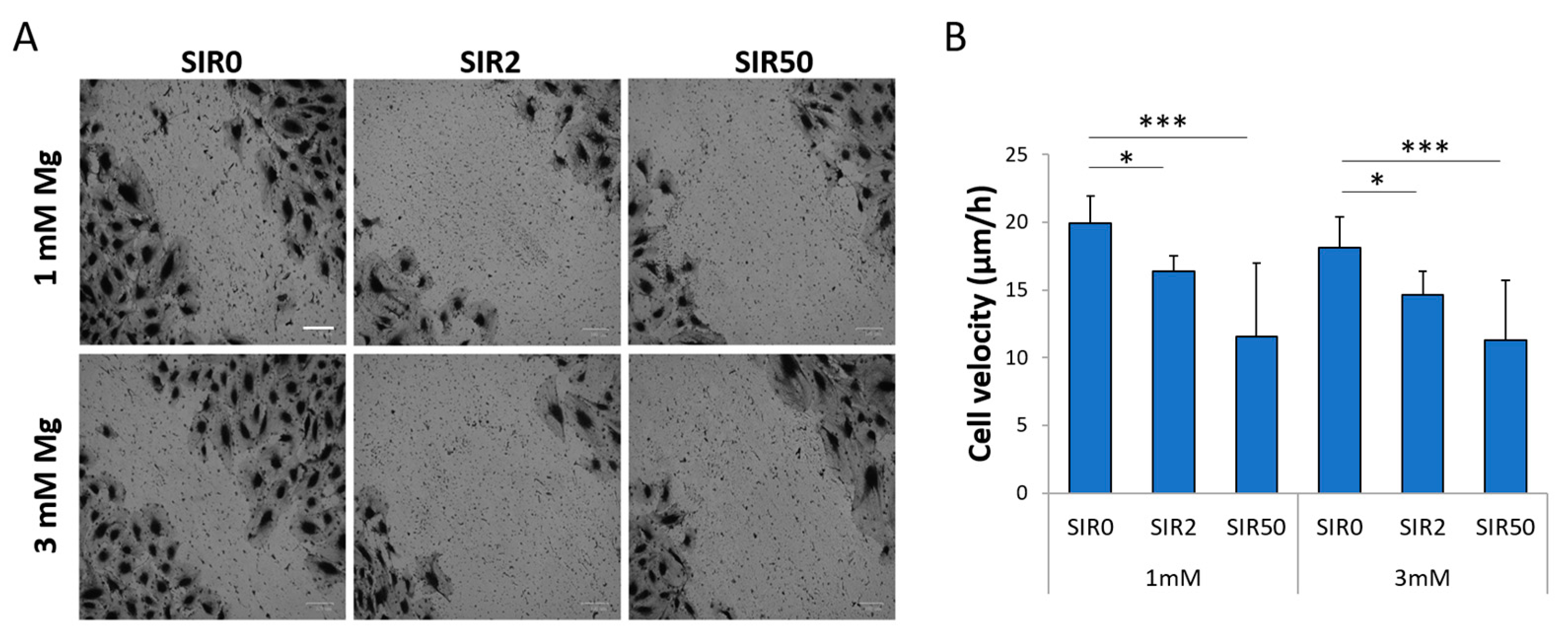
2.3. The Effects of Mg and SIR on hCAEC Migration
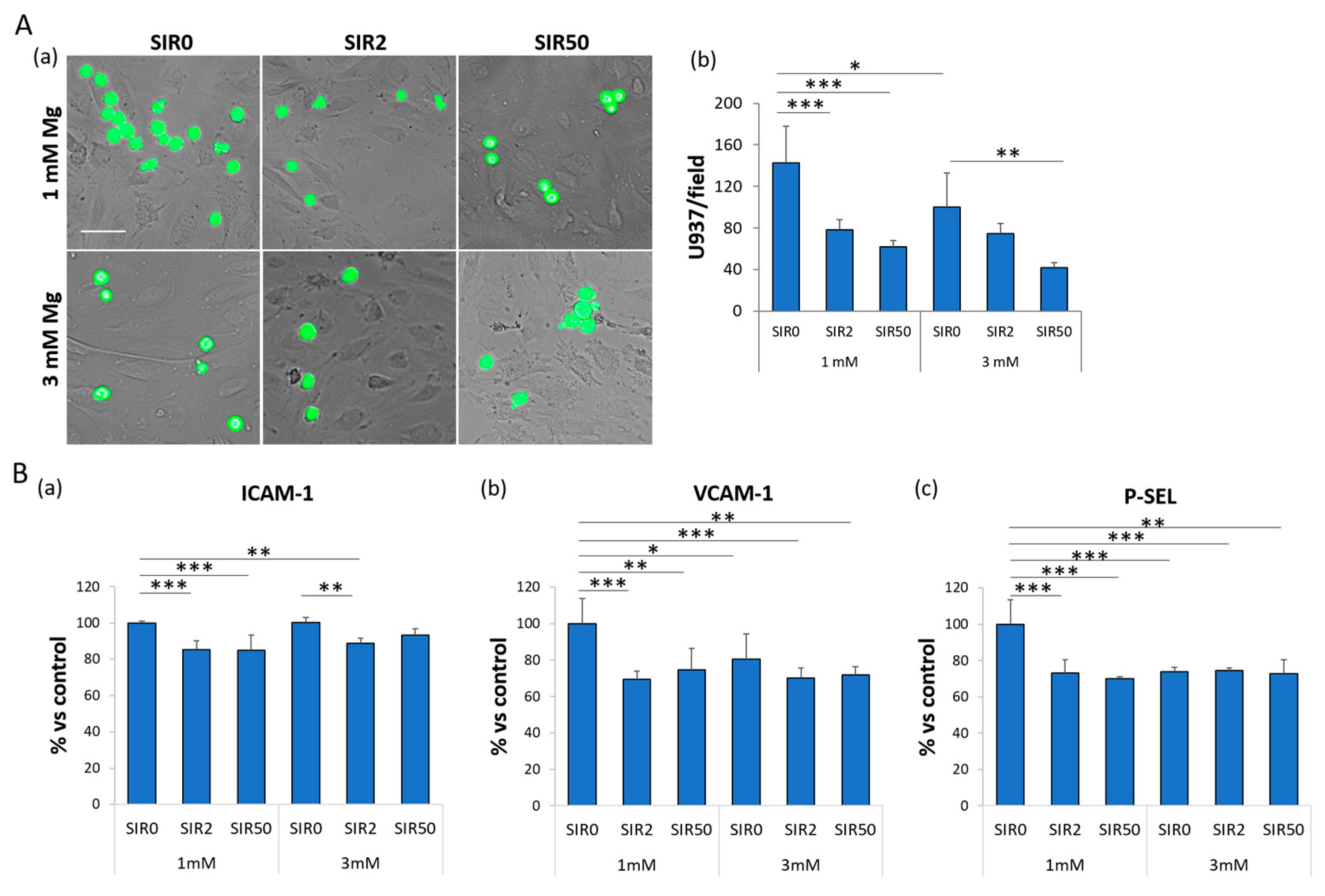
2.4. The Effects of Mg and SIR on the Interaction between hCAEC and Monocyte-Like Cells
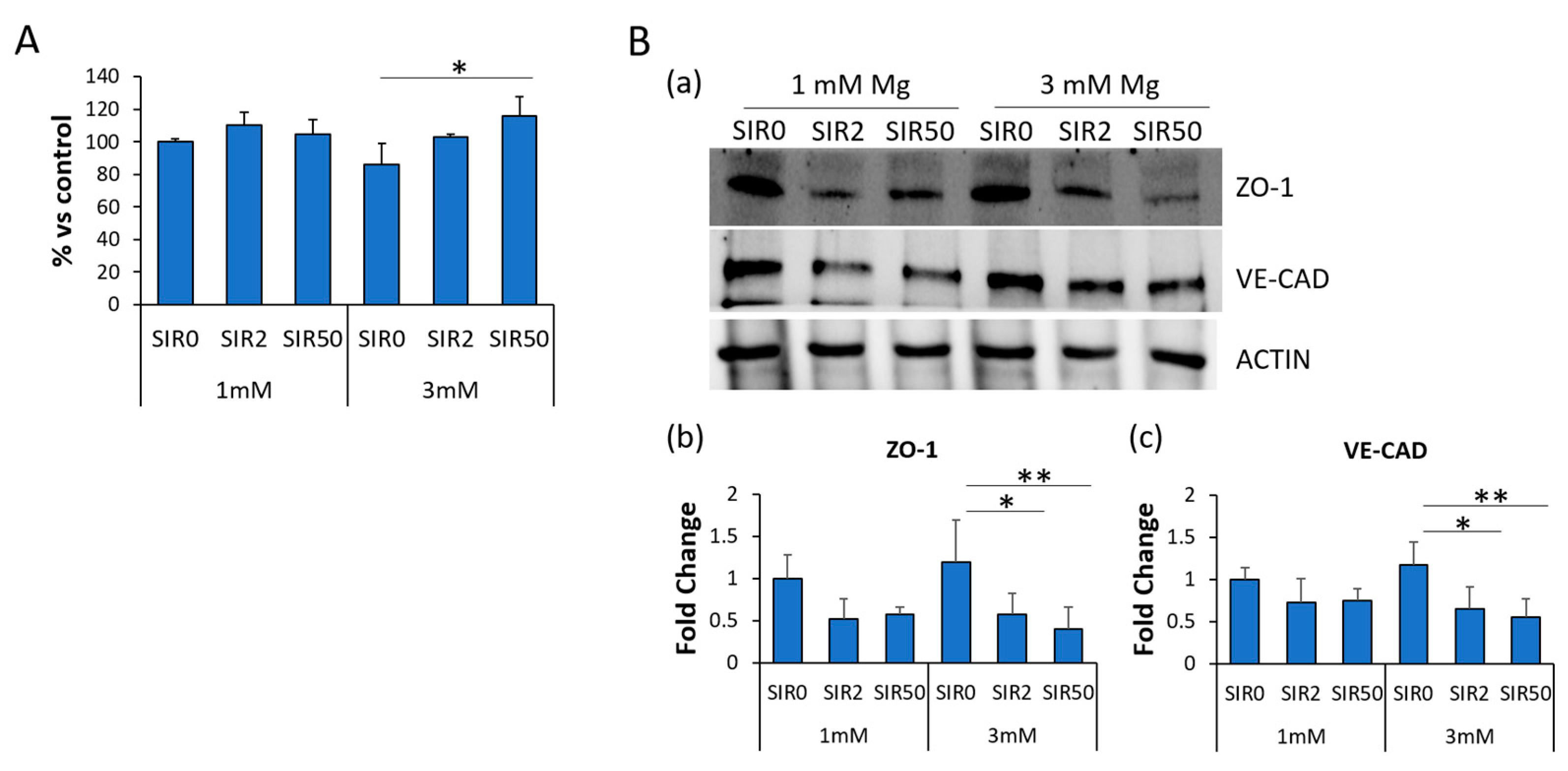
2.5. The Effects of Mg and SIR on Endothelial Permeability
2.6. The Effects of Mg and SIR on the Localization of ZO-1 and VE-CAD
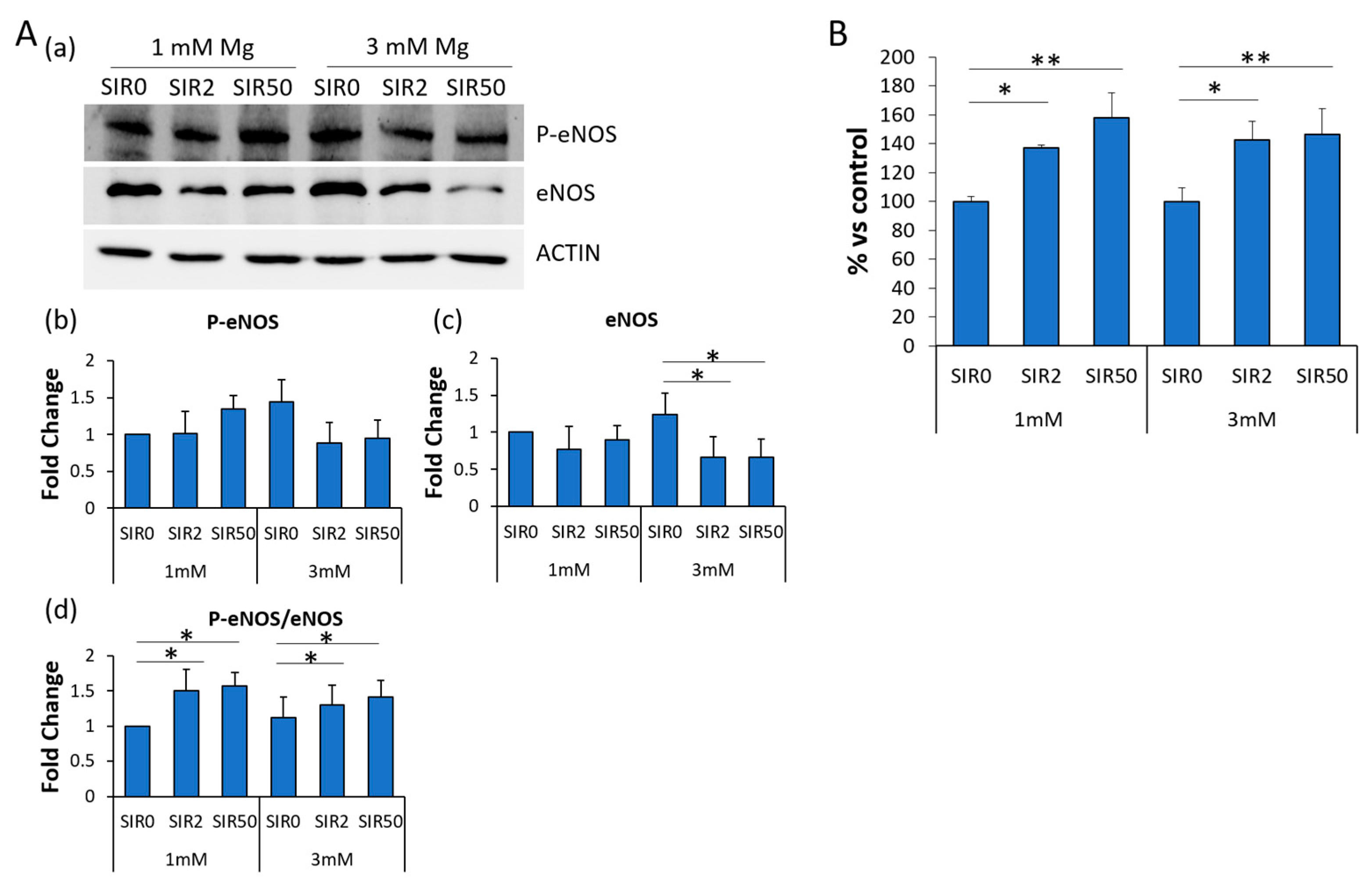
2.7. The Effects of Mg and SIR on Nitric Oxide Production
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Viability Assay
4.3. Intracellular Mg2+ Quantification
4.4. In Vitro Wound Assay
4.5. Adhesion Assay
4.6. Confocal Imaging
4.7. Enzyme-Linked Immunosorbent Assay–ELISA
4.8. Western Blot
4.9. X-PerT Assay
4.10. Measurement of Released NO
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ralapanawa, U.; Sivakanesan, R. Epidemiology and the magnitude of coronary artery disease and acute coronary syndrome: A narrative review. J. Epidemiol. Glob. Health 2021, 11, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Han, Y.; Lu, J. Structural design of vascular stents: A review. Micromachines 2021, 12, 770. [Google Scholar] [CrossRef] [PubMed]
- Borhani, S.; Hassanajili, S.; Ahmadi Tafti, S.H.; Rabbani, S. Cardiovascular stents: Overview, evolution, and next generation. Prog. Biomater. 2018, 7, 175–205. [Google Scholar] [CrossRef]
- Zong, J.; He, Q.; Liu, Y.; Qiu, M.; Wu, J.; Hu, B. Advances in the development of biodegradable coronary stents: A translational perspective. Mater. Today Bio 2022, 16, 100368. [Google Scholar] [CrossRef]
- Poon, M.; Marx, S.O.; Gallo, R.; Badimon, J.J.; Taubman, M.B.; Marks, A.R. Rapamycin inhibits vascular smooth muscle cell migration. J. Clin. Invest. 1996, 98, 2277–2283. [Google Scholar] [CrossRef]
- Hong, S.-J.; Hong, M.-K. Drug-eluting stents for the treatment of coronary artery disease: A review of recent advances. Expert Opin. Drug Deliv. 2022, 19, 269–280. [Google Scholar] [CrossRef]
- Onuma, Y.; Serruys, P.W. Bioresorbable scaffold: The advent of a new era in percutaneous coronary and peripheral revascularization? Circulation 2011, 123, 779–797. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xiao, D.; Wu, Y.; Li, M.; Liu, J.; Zhuang, R.; Ma, L.; Li, J.; Zhang, L. Bioresorbable scaffolds vs. drug-eluting stents for patients with myocardial infarction: A systematic review and meta-analysis of randomized clinical trials. Front. Cardiovasc. Med. 2022, 9, 974957. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, W.; Liu, J.; Wang, L.; Tang, Y.; Wang, K. A review on magnesium alloys for biomedical applications. Front. Bioeng. Biotechnol. 2022, 10, 953344. [Google Scholar] [CrossRef]
- Uddin, M.S.; Hall, C.; Murphy, P. Surface treatments for controlling corrosion rate of biodegradable Mg and Mg-based alloy implants. Sci. Technol. Adv. Mater. 2015, 16, 53501. [Google Scholar] [CrossRef]
- Qiu, T.; Zhao, L. Research into biodegradable polymeric stents: A review of experimental and modelling work. Vessel Plus 2018, 2, 12. [Google Scholar] [CrossRef]
- Fedele, G.; Castiglioni, S.; Maier, J.A.; Locatelli, L. High magnesium and sirolimus on rabbit vascular cells—An in vitro proof of concept. Materials 2021, 14, 1970. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.M.; Locatelli, L.; Fedele, G.; Cazzaniga, A.; Mazur, A. Magnesium and the brain: A focus on neuroinflammation and neurodegeneration. Int. J. Mol. Sci. 2023, 24, 223. [Google Scholar] [CrossRef]
- Maier, J.A.; Castiglioni, S.; Locatelli, L.; Zocchi, M.; Mazur, A. Magnesium and inflammation: Advances and perspectives. Semin. Cell Dev. Biol. 2020, 115, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, L.; Fedele, G.; Castiglioni, S.; Maier, J.A. Magnesium deficiency induces lipid accumulation in vascular endothelial cells via oxidative stress—The potential contribution of EDF-1 and PPARγ. Int. J. Mol. Sci. 2021, 22, 1050. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.M. Endothelial cells and magnesium: Implications in atherosclerosis. Clin. Sci. 2012, 122, 397–407. [Google Scholar] [CrossRef]
- Zhao, Y.; Vanhoutte, P.M.; Leung, S.W.S. Vascular nitric oxide: Beyond eNOS. J. Pharmacol. Sci. 2015, 129, 83–94. [Google Scholar] [CrossRef]
- Castiglioni, S.; Maier, J.A.M. Magnesium alloys for vascular stents: The biological bases: A focus on the effects of magnesium on vascular cells. BioNanoMaterials 2015, 16, 23–29. [Google Scholar] [CrossRef]
- Ozaki, Y.; Garcia-Garcia, H.M.; Shlofmitz, E.; Hideo-Kajita, A.; Waksman, R. Second-generation drug-eluting resorbable magnesium scaffold: Review of the clinical evidence. Cardiovasc. Revasc. Med. 2020, 21, 127–136. [Google Scholar] [CrossRef]
- Verheye, S.; Wlodarczak, A.; Montorsi, P.; Torzewski, J.; Bennett, J.; Haude, M.; Starmer, G.; Buck, T.; Wiemer, M.; Nuruddin, A.A.B.; et al. BIOSOLVE-IV-registry: Safety and performance of the Magmaris scaffold: 12-month outcomes of the first cohort of 1075 patients. Catheter. Cardiovasc. Interv. 2021, 98, E1–E8. [Google Scholar] [CrossRef]
- Seguchi, M.; Aytekin, A.; Lenz, T.; Nicol, P.; Alvarez-Covarrubias, H.A.; Xhepa, E.; Klosterman, G.R.; Beele, A.; Sabic, E.; Utsch, L.; et al. Challenges of the newer generation of resorbable magnesium scaffolds: Lessons from failure mechanisms of the past generation. J. Cardiol. 2023, 81, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, K.; Gratz, M.; Koeck, K.; Mostertz, J.; Begunk, R.; Loebler, M.; Semmling, B.; Seidlitz, A.; Hildebrandt, P.; Homuth, G.; et al. Magnesium used in bioabsorbable stents controls smooth muscle cell proliferation and stimulates endothelial cells in vitro. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100B, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Habib, A.; Karmali, V.; Polavarapu, R.; Akahori, H.; Cheng, Q.; Pachura, K.; Kolodgie, F.D.; Finn, A.V. Sirolimus-FKBP12.6 impairs endothelial barrier function through protein kinase C-α activation and disruption of the p120-vascular endothelial cadherin interaction. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2425–2431. [Google Scholar] [CrossRef] [PubMed]
- Finn, A.V.; Kolodgie, F.D.; Harnek, J.; Guerrero, L.J.; Acampado, E.; Tefera, K.; Skorija, K.; Weber, D.K.; Gold, H.K.; Virmani, R. Differential response of delayed healing and persistent inflammation at sites of overlapping sirolimus- or paclitaxel-eluting stents. Circulation 2005, 112, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.M.; Wagner, D.D. Leukocyte-endothelium adhesion molecules in atherosclerosis. J. Lab. Clin. Med. 1998, 132, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Krüger-Genge, A.; Blocki, A.; Franke, R.-P.; Jung, F. Vascular endothelial cell biology: An update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef] [PubMed]
- Vestweber, D. Adhesion and signaling molecules controlling the transmigration of leukocytes through endothelium. Immunol. Rev. 2007, 218, 178–196. [Google Scholar] [CrossRef]
- Vestweber, D. How leukocytes cross the vascular endothelium. Nat. Rev. Immunol. 2015, 15, 692–704. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Endothelial barrier and its abnormalities in cardiovascular disease. Front. Physiol. 2015, 6, 365. [Google Scholar] [CrossRef]
- Medina-Leyte, D.J.; Zepeda-García, O.; Domínguez-Pérez, M.; González-Garrido, A.; Villarreal-Molina, T.; Jacobo-Albavera, L. Endothelial dysfunction, inflammation and coronary artery disease: Potential biomarkers and promising therapeutical approaches. Int. J. Mol. Sci. 2021, 22, 3850. [Google Scholar] [CrossRef]
- Mundi, S.; Massaro, M.; Scoditti, E.; Carluccio, M.A.; van Hinsbergh, V.W.M.; Iruela-Arispe, M.L.; De Caterina, R. Endothelial permeability, LDL deposition, and cardiovascular risk factors—A review. Cardiovasc. Res. 2018, 114, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Nicol, P.; Bulin, A.; Castellanos, M.I.; Stöger, M.; Obermeier, S.; Lewerich, J.; Lenz, T.; Hoppmann, P.; Baumgartner, C.; Fischer, J.; et al. Preclinical investigation of neoatherosclerosis in magnesium-based bioresorbable scaffolds versus thick-strut drug-eluting stents. EuroIntervention 2020, 16, e922–e929. [Google Scholar] [CrossRef] [PubMed]
- Daniel, J.-M.; Dutzmann, J.; Brunsch, H.; Bauersachs, J.; Braun-Dullaeus, R.; Sedding, D.G. Systemic application of sirolimus prevents neointima formation not via a direct anti-proliferative effect but via its anti-inflammatory properties. Int. J. Cardiol. 2017, 238, 79–91. [Google Scholar] [CrossRef]
- Zhou, Y.-D.; Cao, X.-Q.; Liu, Z.-H.; Cao, Y.-J.; Liu, C.-F.; Zhang, Y.-L.; Xie, Y. Rapamycin inhibits oxidized low density lipoprotein uptake in human umbilical vein endothelial cells via mTOR/NF-κB/LOX-1 pathway. PLoS ONE 2016, 11, e0146777. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E. Nitric oxide signaling in health and disease. Cell 2022, 185, 2853–2878. [Google Scholar] [CrossRef]
- Emdin, C.A.; Khera, A.V.; Klarin, D.; Natarajan, P.; Zekavat, S.M.; Nomura, A.; Haas, M.; Aragam, K.; Ardissino, D.; Wilson, J.G.; et al. Phenotypic consequences of a genetic predisposition to enhanced nitric oxide signaling. Circulation 2018, 137, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Q.; Zhang, Z.; Peng, K.; Zhang, D.-M.; Yang, Q.; Passerini, A.G.; Simon, S.I.; Sun, C. mTOR contributes to endothelium-dependent vasorelaxation by promoting eNOS expression and preventing eNOS uncoupling. Commun. Biol. 2022, 5, 726. [Google Scholar] [CrossRef]
- Jambusaria, A.; Hong, Z.; Zhang, L.; Srivastava, S.; Jana, A.; Toth, P.T.; Dai, Y.; Malik, A.B.; Rehman, J. Endothelial heterogeneity across distinct vascular beds during homeostasis and inflammation. Elife 2020, 9, e51413. [Google Scholar] [CrossRef]
- Giannotta, M.; Trani, M.; Dejana, E. VE-cadherin and endothelial adherens junctions: Active guardians of vascular integrity. Dev. Cell 2013, 26, 441–454. [Google Scholar] [CrossRef]
- Dejana, E.; Orsenigo, F.; Lampugnani, M.G. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J. Cell Sci. 2008, 121, 2115–2122. [Google Scholar] [CrossRef]
- Vestweber, D. VE-cadherin: The major endothelial adhesion molecule controlling cellular junctions and blood vessel formation. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 223–232. [Google Scholar] [CrossRef]
- Tornavaca, O.; Chia, M.; Dufton, N.; Almagro, L.O.; Conway, D.E.; Randi, A.M.; Schwartz, M.A.; Matter, K.; Balda, M.S. ZO-1 controls endothelial adherens junctions, cell-cell tension, angiogenesis, and barrier formation. J. Cell Biol. 2015, 208, 821–838. [Google Scholar] [CrossRef]
- Komarova, Y.A.; Kruse, K.; Mehta, D.; Malik, A.B. Protein interactions at endothelial junctions and signaling mechanisms regulating endothelial permeability. Circ. Res. 2017, 120, 179–206. [Google Scholar] [CrossRef] [PubMed]
- Hautefort, A.; Pfenniger, A.; Kwak, B.R. Endothelial connexins in vascular function. Vasc. Biol. 2019, 1, H117–H124. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Steward, R.L.J. Probing endothelial cell mechanics through connexin 43 disruption. Exp. Mech. 2019, 59, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Moss, S.C.; Lightell, D.J.J.; Marx, S.O.; Marks, A.R.; Woods, T.C. Rapamycin regulates endothelial cell migration through regulation of the cyclin-dependent kinase inhibitor p27Kip1. J. Biol. Chem. 2010, 285, 11991–11997. [Google Scholar] [CrossRef]
- Mu, L.; Liu, X.; Liu, M.; Long, L.; Chi, Q.; He, Y.; Pan, Y.; Ji, C.; Gao, G.; Li, X. In Vitro study of endothelial cell morphology and gene expression in response to wall shear stress induced by arterial stenosis. Front. Bioeng. Biotechnol. 2022, 10, 854109. [Google Scholar] [CrossRef]
- Maier, J.A.M.; Malpuech-Brugère, C.; Zimowska, W.; Rayssiguier, Y.; Mazur, A. Low magnesium promotes endothelial cell dysfunction: Implications for atherosclerosis, inflammation and thrombosis. Biochim. Biophys. Acta 2004, 1689, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Cazzaniga, A.; Locatelli, L.; Castiglioni, S.; Maier, J. The contribution of EDF1 to PPARγ transcriptional activation in VEGF-treated human endothelial cells. Int. J. Mol. Sci. 2018, 19, 1830. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedele, G.; Castiglioni, S.; Maier, J.A.M.; Locatelli, L. The Effects of Sirolimus and Magnesium on Primary Human Coronary Endothelial Cells: An In Vitro Study. Int. J. Mol. Sci. 2023, 24, 2930. https://doi.org/10.3390/ijms24032930
Fedele G, Castiglioni S, Maier JAM, Locatelli L. The Effects of Sirolimus and Magnesium on Primary Human Coronary Endothelial Cells: An In Vitro Study. International Journal of Molecular Sciences. 2023; 24(3):2930. https://doi.org/10.3390/ijms24032930
Chicago/Turabian StyleFedele, Giorgia, Sara Castiglioni, Jeanette A. M. Maier, and Laura Locatelli. 2023. "The Effects of Sirolimus and Magnesium on Primary Human Coronary Endothelial Cells: An In Vitro Study" International Journal of Molecular Sciences 24, no. 3: 2930. https://doi.org/10.3390/ijms24032930
APA StyleFedele, G., Castiglioni, S., Maier, J. A. M., & Locatelli, L. (2023). The Effects of Sirolimus and Magnesium on Primary Human Coronary Endothelial Cells: An In Vitro Study. International Journal of Molecular Sciences, 24(3), 2930. https://doi.org/10.3390/ijms24032930




