Melatonin Delays Postharvest Senescence through Suppressing the Inhibition of BrERF2/BrERF109 on Flavonoid Biosynthesis in Flowering Chinese Cabbage
Abstract
1. Introduction
2. Results
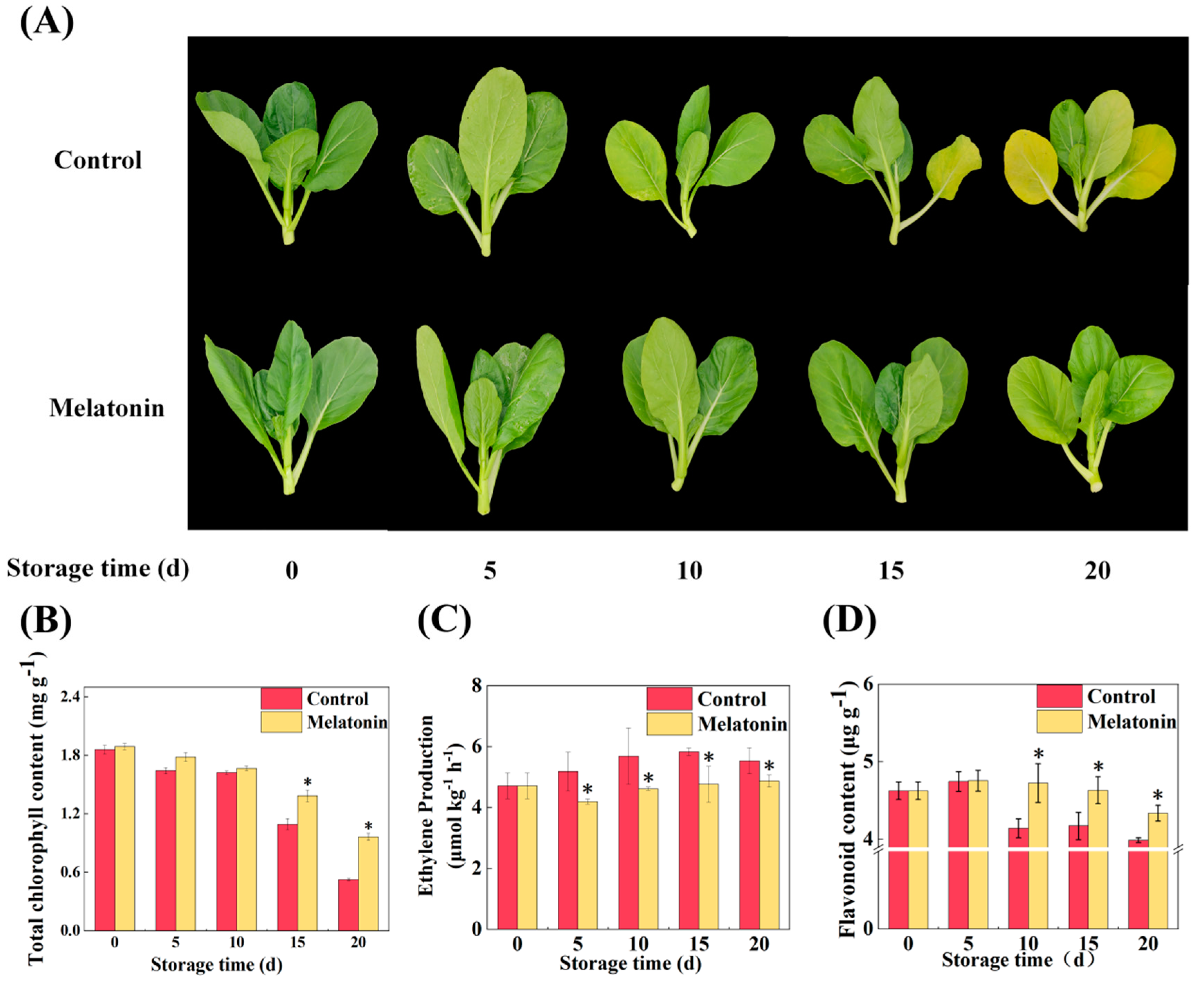
2.1. Melatonin Delaying Leaf Senescence was Associated with Chlorophyll Degradation, Ethylene Biosynthesis, and Flavonoid Accumulation
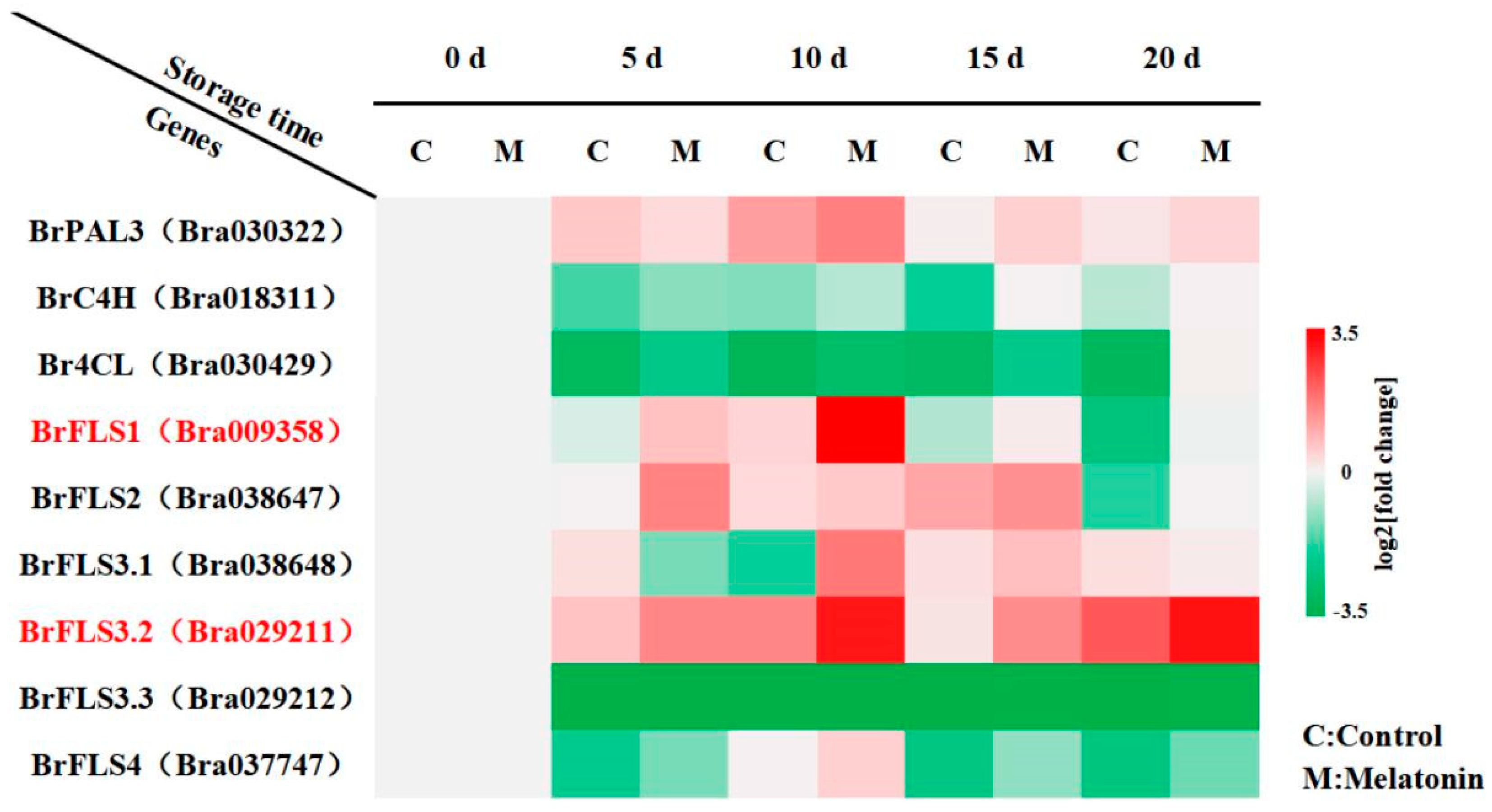
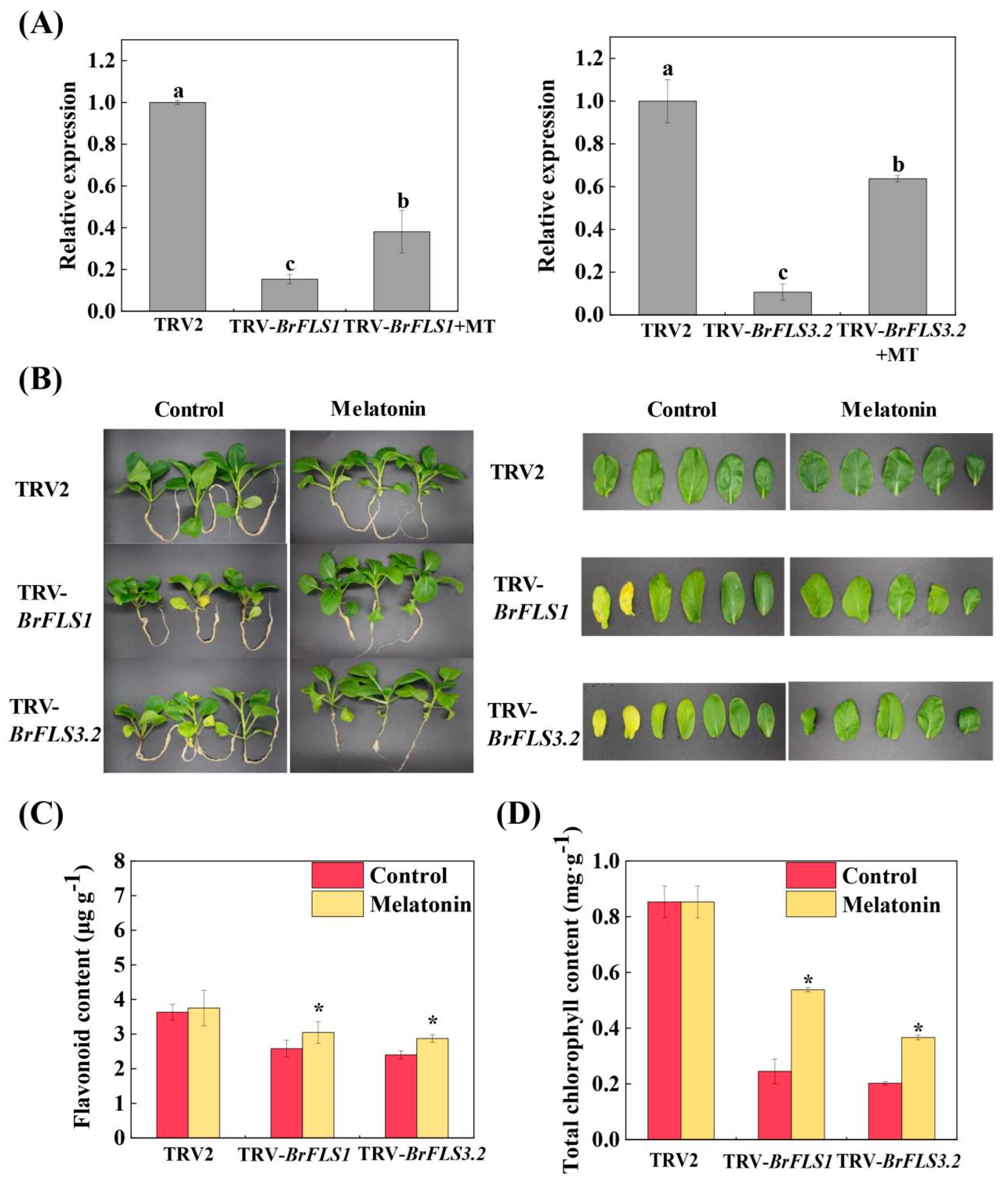
2.2. BrFLS1 and BrFLS3.2, Key Genes for Flavonoid Biosynthesis, Played a Positive Regulatory Role in Melatonin-Delayed Leaf Senescence
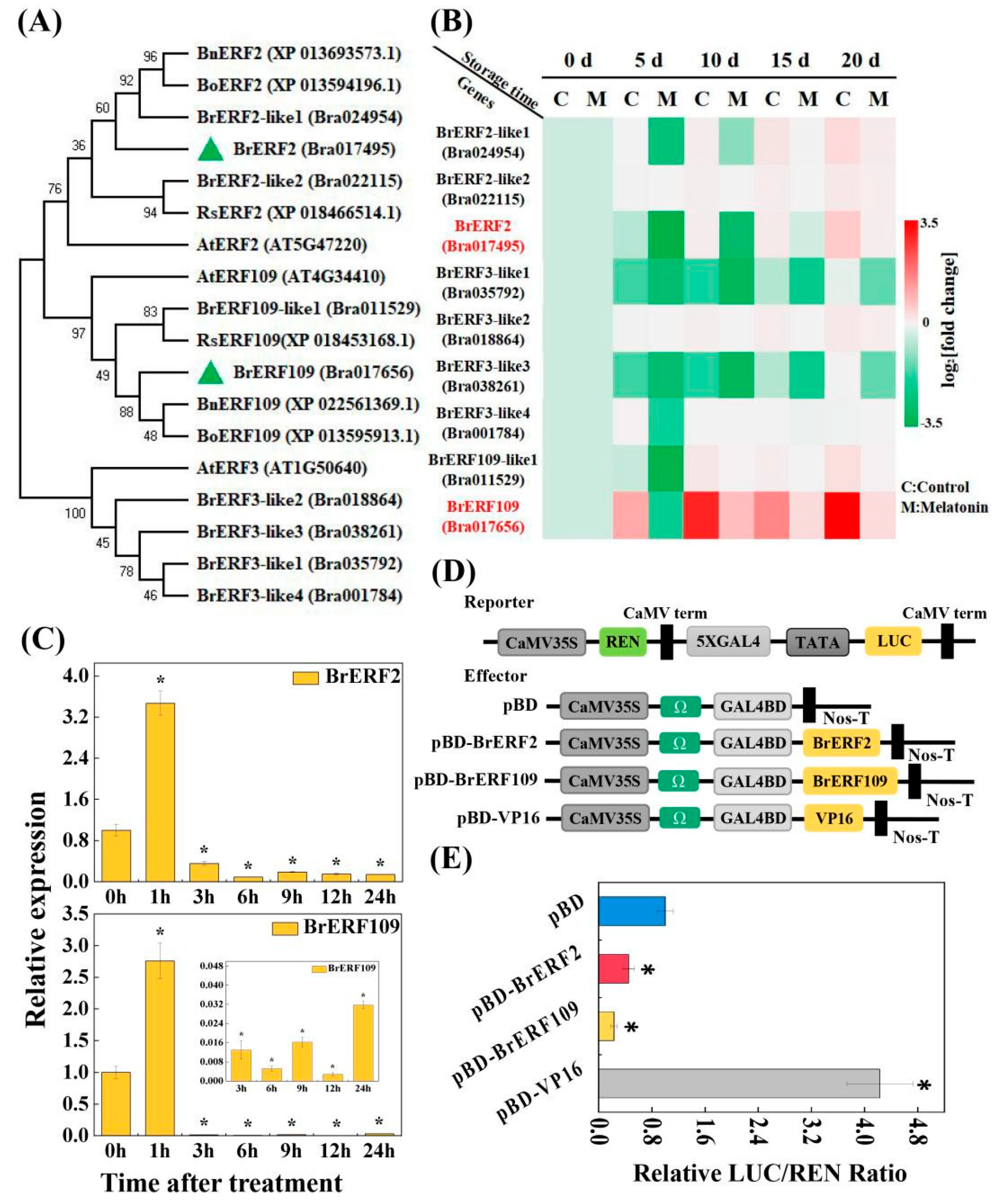
2.3. BrERF2 and BrERF109, the Transcriptional Suppressors, Were Involved in Leaf Senescence and Repressed by Melatonin
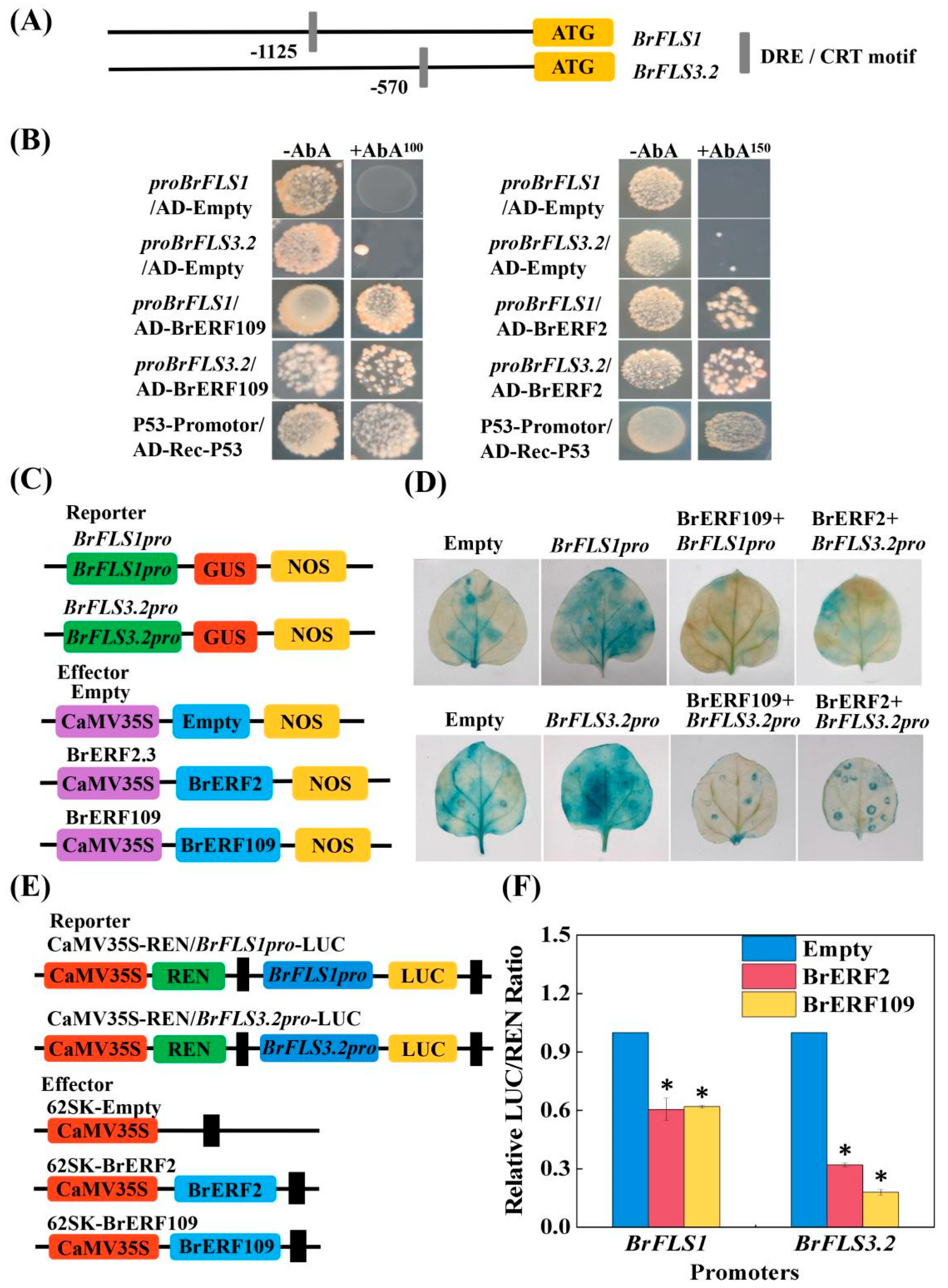
2.4. BrERF2 and BrERF109 Directly Bound to the Promoters of BrFLSs and Suppressed Their Expression
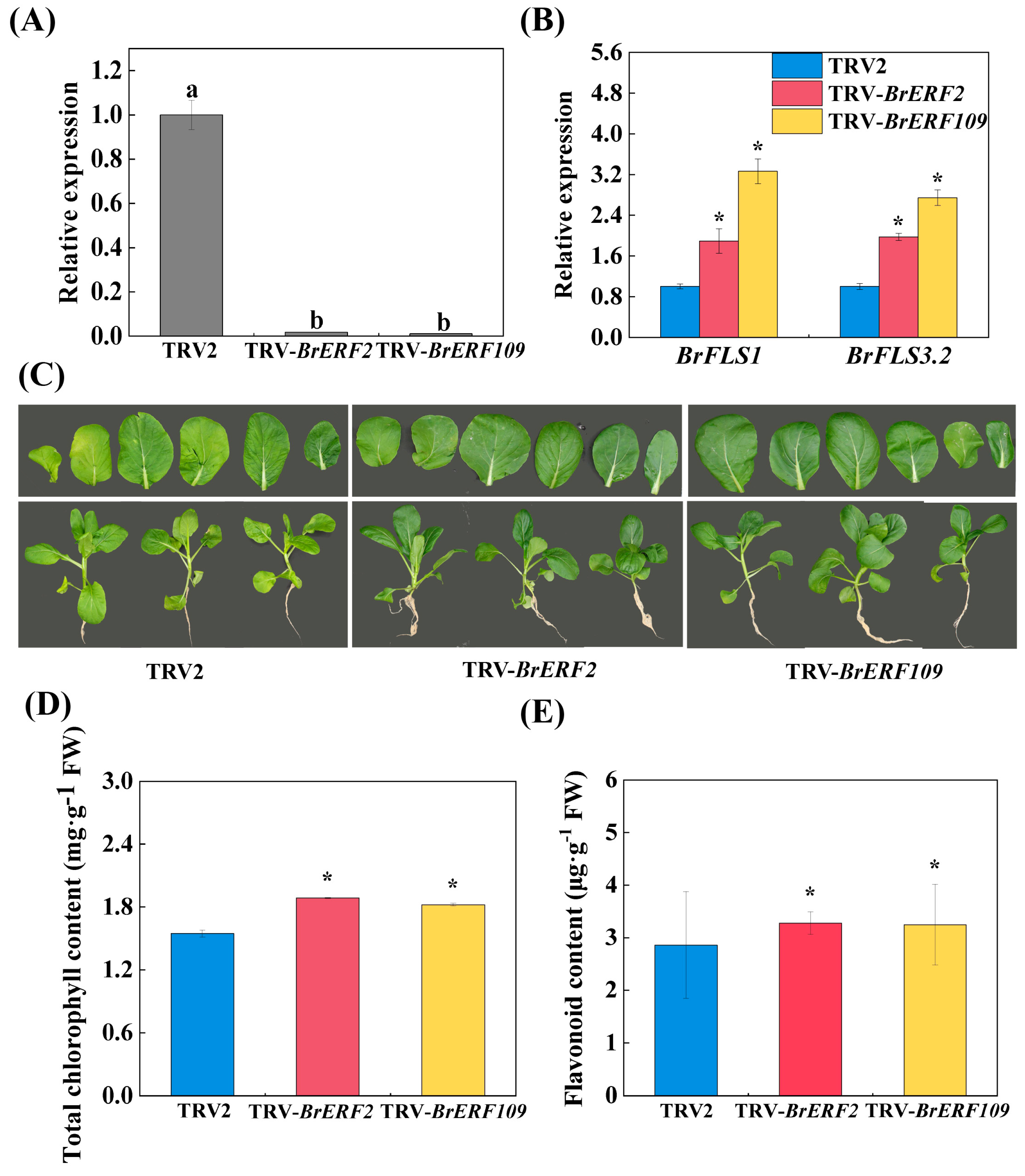
2.5. Silencing BrERF2 and BrERF109 Promoted Flavonoid Biosynthesis and Delayed Aging
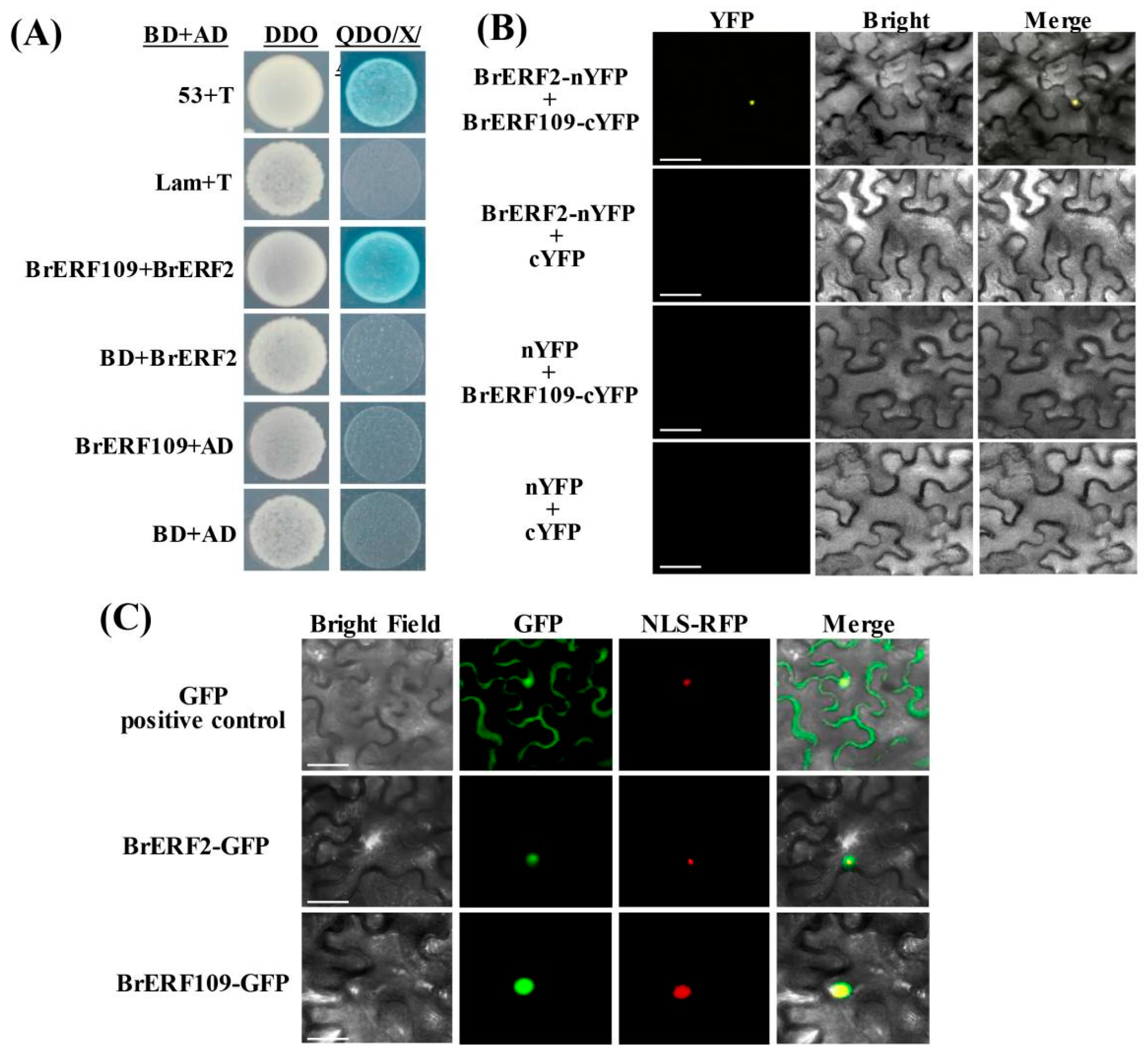
2.6. BrERF2 Interacted with BrERF109, Which Formed a BrERF2-BrERF109 Complex
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Determination of Ethylene Content
4.3. Determination of Chlorophyll and Total Flavonoid Content
4.4. Total RNA Extraction, Quantitative Real-Time PCR (qRT-PCR) and Sequence Analysis
4.5. Virus-Induced Gene Silencing (VIGS)
4.6. Subcellular Localization Analysis
4.7. Transcriptional Assay
4.8. Yeast One-Hybrid (Y1H) Assay
4.9. β-Glucuronidase (GUS) Analysis
4.10. Dual Luciferase (LUC) Assay
4.11. Y2H Screening
4.12. Bimolecular Fluorescence Complementation (BiFC) Assay
4.13. Statistical Analysis
4.14. Primers
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Woo, H.R.; Kim, H.J.; Nam, H.G.; Lim, P.O. Plant leaf senescence and death-regulation by multiple layers of control and implications for aging in general. J. Cell Sci. 2013, 126, 4823–4833. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Ryu, H.; Hong, S.H.; Woo, H.R.; Lim, P.O.; Lee, I.C.; Sheen, J.; Nam, H.G.; Hwang, I. Cytokinin-mediated control of leaf longevity by AHK3 through phosphorylation of ARR2 in Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.C.; Schippers, J.H.; Hille, J.; Dijkwel, P.P. Ethylene-induced leaf senescence depends on age-related changes and OLD genes in Arabidopsis. J. Exp. Bot. 2005, 56, 2915–2923. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.N.; Han, Z.Y.; Sun, Y.Q.; Wang, S.; Wang, T.; Wang, Y.; Xu, K.N.; Zhang, X.Z.; Xu, X.F.; Han, Z.H.; et al. ERF4 affects fruit firmness through TPL4 by reducing ethylene production. Plant J. 2020, 103, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.T.; Ling, J.; Zhou, H.S.; Tian, M.Y.; Huang, W.; Luo, S.F.; Hu, H.L.; Li, P.X. 1-methylcyclopropene counteracts ethylene inhibition of anthocyanin accumulation in peach skin after harvest. Postharvest Biol. Technol. 2022, 183, 111737. [Google Scholar] [CrossRef]
- Hao, D.Y.; Yamasaki, K.; Sarai, A.; Ohme-Takagi, M. Determinants in the sequence specific binding of two plant transcription factors, CBF1 and NtERF2, to the DRE and GCC motifs. Biochemistry 2002, 41, 4202–4208. [Google Scholar] [CrossRef]
- Sakuma, Y.; Liu, Q.; Dubouzet, J.G.; Abe, H.; Shinozaki, K.; Yamaguchi-Shin-ozaki, K. DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration and cold-inducible gene expression. Biochem. Biophys. Res. Commun. 2002, 290, 998–1009. [Google Scholar] [CrossRef]
- Luo, J.; Chen, S.; Cao, S.; Zhang, T.; Li, R.; Chan, Z.L.; Wang, C. Rose (Rosa hybrida) ethylene responsive factor 3 promotes rose flower senescence via direct activation of the abscisic acid synthesis-related 9-cis-epoxycarotenoid dioxygenase gene. Plant Cell Physiol. 2021, 62, 1030–1043. [Google Scholar] [CrossRef]
- Park, S.J.; Park, S.; Kim, Y.M.; Hyeon, D.Y.; Park, H.; Jeong, J.; Jeong, U.; Yoon, Y.S.; You, D.; Kwak, J.; et al. Ethylene responsive factor 34 mediates stress-induced leaf senescence by regulating salt stress-responsive genes. Plant Cell Environ. 2022, 45, 1719–1733. [Google Scholar] [CrossRef]
- Lim, C.; Kang, K.; Shim, Y.; Sakuraba, Y.; An, G.; Paek, N.C. Rice ETHYLENE RESPONSE FACTOR 101 promotes leaf senescence through jasmonic acid-mediated regulation of OsNAP and OsMYC2. Front. Plant Sci. 2020, 11, 1096. [Google Scholar] [CrossRef]
- Chen, Y.N.; Feng, P.P.; Tang, B.Y.; Hu, Z.L.; Xie, Q.L.; Zhou, S.; Chen, G.P. The AP2/ERF transcription factor SlERF.F5 functions in leaf senescence in tomato. Plant Cell Rep. 2022, 41, 1181–1195. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.L.; Huang, Y.T.; Schmidt, W.; Klein, P.; Chan, M.T.; Pan, I.C. Ethylene response factor 109 attunes immunity, photosynthesis, and iron homeostasis in Arabidopsis leaves. Front. Plant Sci. 2022, 69, 4968–4980. [Google Scholar]
- Bhardwaj, R.; Pareek, S.; Domínguez-Avila, J.A.; Gonzalez-Aguilar, G.A.; Valero, D.; Serrano, M. An exogenous pre-storage melatonin alleviates chilling injury in some mango fruit cultivars, by acting on the enzymatic and non-enzymatic antioxidant system. Antioxidants 2022, 11, 384. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.X.; Cai, S.Y.; Xing, Q.F.; Qi, Z.Y.; Fotopoulos, V.; Yu, J.Q.; Zhou, J. Melatonin delays dark-induced leaf senescence by inducing mir171b expression in tomato. J. Pineal Res. 2022, 72, e12792. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, D.D.; Wang, J.C.; Tian, B.; Li, Y.Y.; Sun, G.Y.; Zhang, H.H. Exogenous melatonin alleviates NO2 damage in tobacco leaves by promoting antioxidant defense, modulating redox homeostasis, and signal transduction. J. Hazard. Mater. 2022, 424, 127265. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Protective effect of melatonin against chlorophyll degradation during the senescence of barley leaves. J. Pineal Res. 2010, 46, 58–63. [Google Scholar] [CrossRef]
- Khan, M.; Ali, S.; Manghwar, H.; Saqib, S.; Ullah, F.; Ayaz, A.; Zaman, W. Melatonin function and crosstalk with other phytohormones under normal and stressful conditions. Genes 2022, 13, 1699. [Google Scholar] [CrossRef]
- Ze, Y.; Gao, H.J.; Li, T.T.; Yang, B.; Jiang, Y.M. Insights into the roles of melatonin in maintaining quality and extending shelf life of postharvest fruits. Trends Food Sci. Technol. 2021, 109, 569–578. [Google Scholar] [CrossRef]
- Zhu, L.L.; Hu, H.L.; Luo, S.F.; Wu, Z.X.; Li, P.X. Melatonin delaying senescence of postharvest broccoli by regulating respiratory metabolism and antioxidant activity. Trans. Chin. Soc. Agric. Eng. 2018, 34, 300–308. [Google Scholar]
- Tan, X.L.; Fan, Z.Q.; Kuang, J.F.; Lu, W.J.; Reiter, R.J.; Lakshmanan, P.; Su, X.G.; Zhou, J.; Chen, J.Y.; Shan, W. Melatonin delays leaf senescence of Chinese flowering cabbage by suppressing ABFs-mediated abscisic acid biosynthesis and chlorophyll degradation. J. Pineal Res. 2019, 67, e12570. [Google Scholar] [CrossRef]
- Tan, X.L.; Fan, Z.Q.; Zeng, Z.X.; Wei, S.; Kuang, J.F.; Lu, W.J.; Su, X.G.; Tao, N.G.; Lakshmanan, P.; Chen, J.Y.; et al. Exogenous melatonin maintains leaf quality of postharvest Chinese flowering cabbage by modulating respiratory metabolism and energy status. Postharvest Biol. Technol. 2021, 177, 111524. [Google Scholar] [CrossRef]
- Zhai, R.; Liu, J.L.; Liu, F.X.; Zhao, Y.X.; Liu, L.L.; Chen, F.; Wang, H.B.; Li, X.Y.; Wang, Z.G.; Ma, F.W.; et al. Melatonin limited ethylene production, softening and reduced physiology disorder in pear (Pyrus communis L.) fruit during senescence. Postharvest Biol. Technol. 2018, 139, 38–46. [Google Scholar] [CrossRef]
- Cheng, J.; Zheng, A.; Li, H.H.; Chen, H.; Jiang, T.J.; Shen, S.L.; Zheng, X.L. Effects of melatonin treatment on ethanol fermenation and ERF expression in kiwifruit cv. Bruno during postharvest. Sci. Hortic. 2022, 293, 110696. [Google Scholar] [CrossRef]
- Gilbert, E.R.; Liu, D. Flavonoids influence epigenetic-modifying enzyme activity: Structure-function relationships and the therapeutic potential for cancer. Curr. Med. Chem. 2010, 17, 1756–1768. [Google Scholar] [CrossRef]
- Bertolini, A.; Petrussa, E.; Patui, S.; Zancani, M.; Peresson, C.; Casolo, V.; Vianello, A.; Braidot, E. Flavonoids and darkness lower PCD in senescing Vitis vinifera suspension cell cultures. BMC Plant Biol. 2016, 16, 233. [Google Scholar] [CrossRef]
- Reyes Jara, A.M.; Gómez Lobato, M.E.; Civello, P.M.; Martínez, G.A. Phenylalanine ammonia lyase is more relevant than Chalcone synthase and Chalcone isomerase in the biosynthesis of flavonoids during postharvest senescence of broccoli. J. Food Biochem. 2022, 46, e14054. [Google Scholar] [CrossRef]
- Zhang, Y.; Butelli, E.; De Stefano, R.D.; Schoonbeek, H.J.; Magusin, A.; Pagliarani, C.; Wellner, N.; Hill, L.; Orzaez, D.; Granell, A.; et al. Anthocyanins double the shelf life of tomatoes by delaying overripening and reducing susceptibility to gray mold. Curr. Biol. 2013, 23, 1094–1100. [Google Scholar] [CrossRef]
- Xue, J.; Lu, D.B.; Wang, S.G.; Lu, Z.H.; Liu, W.; Wang, X.F.; Fang, Z.Q.; He, X.Y. Integrated transcriptomic and metabolomic analysis provides insight into the regulation of leaf senescence in rice. Sci. Rep. 2021, 11, 14083. [Google Scholar] [CrossRef]
- Liang, D.; Shen, Y.Q.; Ni, Z.Y.; Wang, Q.; Lei, Z.; Xu, N.Q.; Deng, Q.X.; Lin, L.J.; Wang, J.; Lv, X.L.; et al. Exogenous melatonin application delays senescence of kiwifruit leaves by regulating the antioxidant capacity and biosynthesis of flavonoids. Front. Plant Sci. 2018, 9, 426. [Google Scholar] [CrossRef]
- Horbowicz, M.; Wiczkowski, W.; Góraj-Koniarska, J.; Miyamoto, K.; Ueda, J.; Saniewski, M. Effect of methyl jasmonate on the terpene trilactones, flavonoids, and phenolic acids in Ginkgo biloba L. Leaves: Relevance to leaf senescence. Molecules 2021, 26, 4682. [Google Scholar] [CrossRef]
- Liu, C.H.; Zheng, H.H.; Sheng, K.L.; Liu, W.; Zheng, L. Effects of melatonin treatment on the postharvest quality of strawberry fruit. Postharvest Biol. Technol. 2018, 139, 47–55. [Google Scholar] [CrossRef]
- Liu, H.L.; Su, B.B.; Zhang, H.; Gong, J.X.; Zhang, B.X.; Liu, Y.L.; Du, L.J. Identification and functional analysis of a flavonol synthase gene from grape hyacinth. Molecules 2019, 24, 1579. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Kim, D.H.; Park, B.R.; Lee, J.Y.; Lim, S.H. Molecular and functional characterization of Oryza sativa flavonol synthase (OsFLS), a bifunctional dioxygenase. J. Agric. Food Chem. 2019, 67, 7399–7409. [Google Scholar] [CrossRef]
- Yue, L.Q.; Li, Y.S.; Zhong, M.; Chai, X.R.; Zhao, P.Y.; Huang, R.M.; Kang, Y.Y.; Yang, X. Benzoic acid, chlorine dioxide, and 1-methylcyclopropene induce flavonoid metabolic shifts in postharvest flowering Chinese cabbage revealed by high-dimensional analytical data. Int. J. Mol. Sci. 2022, 23, 6011. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Zhang, L.J.; Zhu, S.J. 1-methylcyclopropene (1-MCP)-induced protein expression associated with changes in Tsai Tai (Brassica chinensis) leaves during low temperature storage. Postharvest Biol. Technol. 2014, 87, 120–125. [Google Scholar] [CrossRef]
- Kuang, L.F.; Kang, Y.Y.; Wang, H.; Huang, R.M.; Lei, B.F.; Zhong, M.; Yang, X. The roles of Salvia miltiorrhiza-derived carbon dots involving in maintaining quality by delaying senescence of postharvest flowering Chinese cabbage. Food Chem. 2022, 404, 134704. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, H.X.; Zeng, H.L.; Cai, Q.S.; Zhou, X.; Yin, C.X. Exogenous Jasmonic Acid and Cytokinin Antagonistically Regulate Rice Flag Leaf Senescence by Mediating Chlorophyll Degradation, Membrane Deterioration, and Senescence Associated Genes Expression. J. Plant Growth Regul. 2016, 35, 366–376. [Google Scholar] [CrossRef]
- Liu, K.; Jing, T.T.; Wang, Y.N.; Ai, X.Z.; Bi, H.A. Melatonin delays leaf senescence and improves cucumber yield by modulating chlorophyll degradation and photoinhibition of PSII and PSI. Environ. Exp. Bot. 2022, 200, 104915. [Google Scholar] [CrossRef]
- Zhou, Y.S.; Zhang, X.M.; Chen, J.; Guo, X.P.; Wang, H.Y.; Zhen, W.B.; Zhang, J.L.; Hu, Z.B.; Zhang, X.B.; Botella, J.R.; et al. Overexpression of AHL9 accelerates leaf senescence in Arabidopsis thaliana. BMC Plant Biol. 2022, 22, 248. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Pirrello, J.; Jaimes-Miranda, F.; Sanchez-Ballesta, M.T.; Tournier, B.; Khalil-Ahmad, Q.; Regad, F.; Latché, A.; Pech, J.C.; Bouzayen, M. SlERF2, a tomato ethylene response factor involved in ethylene response and seed germination. Plant Cell Physiol. 2006, 47, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.Y.; Zhang, W.W.; Qu, H.Y.; Gou, S.S.; Li, L.X.; Song, H.H.; Yang, H.Q.; Li, W.J.; Zhang, H.; Hu, K.D.; et al. Transcriptomics reveals the ERF2-bHLH2-CML5 module responses to H2S and ROS in postharvest calcium deficiency apples. Int. J. Mol. Sci. 2021, 22, 13013. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.T.; Xu, P.; Zhao, P.X.; Liu, R.; Yu, L.H.; Xiang, C.B. Arabidopsis ERF109 mediates cross-talk between jasmonic acid and auxin biosynthesis during lateral root formation. Nat. Commun. 2014, 5, 5833. [Google Scholar] [CrossRef]
- Wang, M.; Dai, W.S.; Du, J.; Ming, R.H.; Dahro, B.; Liu, J.H. ERF 109 of trifoliate orange (Poncirus trifoliata (L.) Raf.) contributes to cold tolerance by directly regulating expression of Prx1 involved in antioxidative process. Plant Biotechnol. J. 2019, 17, 1316–1332. [Google Scholar] [CrossRef]
- Ma, H.Y.; Yang, T.; Li, Y.; Zhang, J.; Wu, T.; Song, T.T.; Yao, Y.C.; Tian, J. The long noncoding RNA MdLNC499 bridges MdWRKY1 and MdERF109 function to regulate early-stage light-induced anthocyanin accumulation in apple fruit. Plant Cell 2021, 33, 3309–3330. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Jiang, Z.Y.; Zhang, L.C.; Tan, D.M.; Wei, Y.; Yuan, H.; Li, T.L.; Wang, A.D. Apple (Malus domestica) MdERF2 negatively affects ethylene biosynthesis during fruit ripening by suppressing MdACS1 transcription. Plant J. 2016, 88, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Xu, Y.X.; Zhang, L.C.; Ji, Y.L.; Tan, D.M.; Yuan, H.; Wang, A.D. The jasmonate-activated transcription factor MdMYC2 regulates ETHYLENE RESPONSE FACTOR and ethylene biosynthetic genes to promote ethylene biosynthesis during apple fruit ripening. Plant Cell 2017, 29, 1316–1334. [Google Scholar] [CrossRef]
- Chen, K.; Tang, W.S.; Zhou, Y.B.; Chen, J.; Xu, Z.S.; Ma, R.; Dong, Y.S.; Ma, Y.Z.; Chen, M. AP2/ERF transcription factor GmDREB1 confers drought tolerance in transgenic soybean by interacting with GmERFs. Plant Physiol. Biochem. 2022, 170, 287–295. [Google Scholar] [CrossRef]
- Zhou, X.; Dong, L.; Li, R.; Zhou, Q.; Wang, J.W.; Ji, S.J. Low temperature conditioning prevents loss of aroma-related esters from ‘Nanguo’ pears during ripening at room temperature. Postharvest Biol. Technol. 2015, 100, 23–32. [Google Scholar] [CrossRef]
- Powell, A.L.; Nguyen, C.V.; Hill, T.; Cheng, K.L.; Figueroa-Balderas, R.; Aktas, H.; Ashrafi, H.; Pons, C.; Fernandez-Munoz, R.; Vicente, A.; et al. Uniform ripening encodes a Golden 2-like transcription factor regulating tomato fruit chloroplast development. Science 2012, 336, 1711–1715. [Google Scholar] [CrossRef]
- Rao, S.; Yu, T.; Cong, X.; Zhang, W.W.; Zhu, Z.Z.; Liao, Y.L.; Ye, J.B.; Cheng, S.Y.; Xu, F. Effects of selenate applied at two growth stages on the nutrient quality of Cardamine violifolia. Sci. Hortic. 2021, 288, 110352. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Li, D.H.; Zaman, W.; Lu, J.J.; Niu, Q.Q.; Zhang, X.H.; Ayaz, A.; Saqib, S.; Yang, B.M.; Zhang, J.X.; Zhao, H.Y.; et al. Natural lupeol level variation among castor accessions and the upregulation of lupeol synthesis in response to light. Ind. Crops Prod. 2023, 192, 116090. [Google Scholar] [CrossRef]
- Cui, X.F.; Fan, B.F.; Scholz, J.; Chen, Z.X. Roles of cyclin-dependent kinasec complexes in cauliflower mosaic virus infection, plant growth, and development. Plant Cell 2007, 19, 1388–1402. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, L.; Kang, Y.; Zhong, M.; Kang, D.; Zhao, P.; Chai, X.; Yang, X. Melatonin Delays Postharvest Senescence through Suppressing the Inhibition of BrERF2/BrERF109 on Flavonoid Biosynthesis in Flowering Chinese Cabbage. Int. J. Mol. Sci. 2023, 24, 2933. https://doi.org/10.3390/ijms24032933
Yue L, Kang Y, Zhong M, Kang D, Zhao P, Chai X, Yang X. Melatonin Delays Postharvest Senescence through Suppressing the Inhibition of BrERF2/BrERF109 on Flavonoid Biosynthesis in Flowering Chinese Cabbage. International Journal of Molecular Sciences. 2023; 24(3):2933. https://doi.org/10.3390/ijms24032933
Chicago/Turabian StyleYue, Lingqi, Yunyan Kang, Min Zhong, Dengjin Kang, Puyan Zhao, Xirong Chai, and Xian Yang. 2023. "Melatonin Delays Postharvest Senescence through Suppressing the Inhibition of BrERF2/BrERF109 on Flavonoid Biosynthesis in Flowering Chinese Cabbage" International Journal of Molecular Sciences 24, no. 3: 2933. https://doi.org/10.3390/ijms24032933
APA StyleYue, L., Kang, Y., Zhong, M., Kang, D., Zhao, P., Chai, X., & Yang, X. (2023). Melatonin Delays Postharvest Senescence through Suppressing the Inhibition of BrERF2/BrERF109 on Flavonoid Biosynthesis in Flowering Chinese Cabbage. International Journal of Molecular Sciences, 24(3), 2933. https://doi.org/10.3390/ijms24032933





