SOCS7-Derived BC-Box Motif Peptide Mediated Cholinergic Differentiation of Human Adipose-Derived Mesenchymal Stem Cells
Abstract
1. Introduction
2. Results
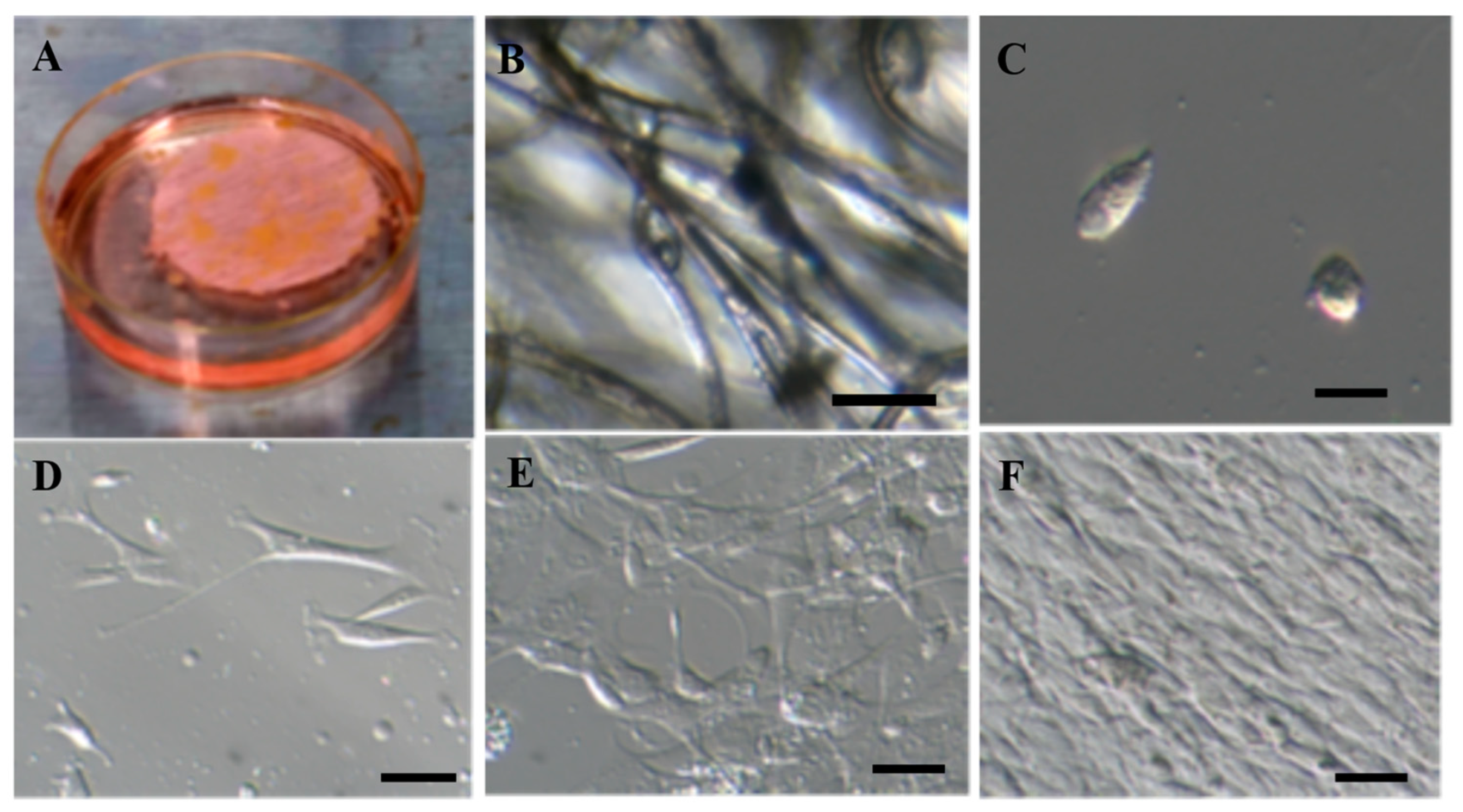
2.1. Isolation and Proliferation of Adipose-Derived Mesenchymal Stem Cells
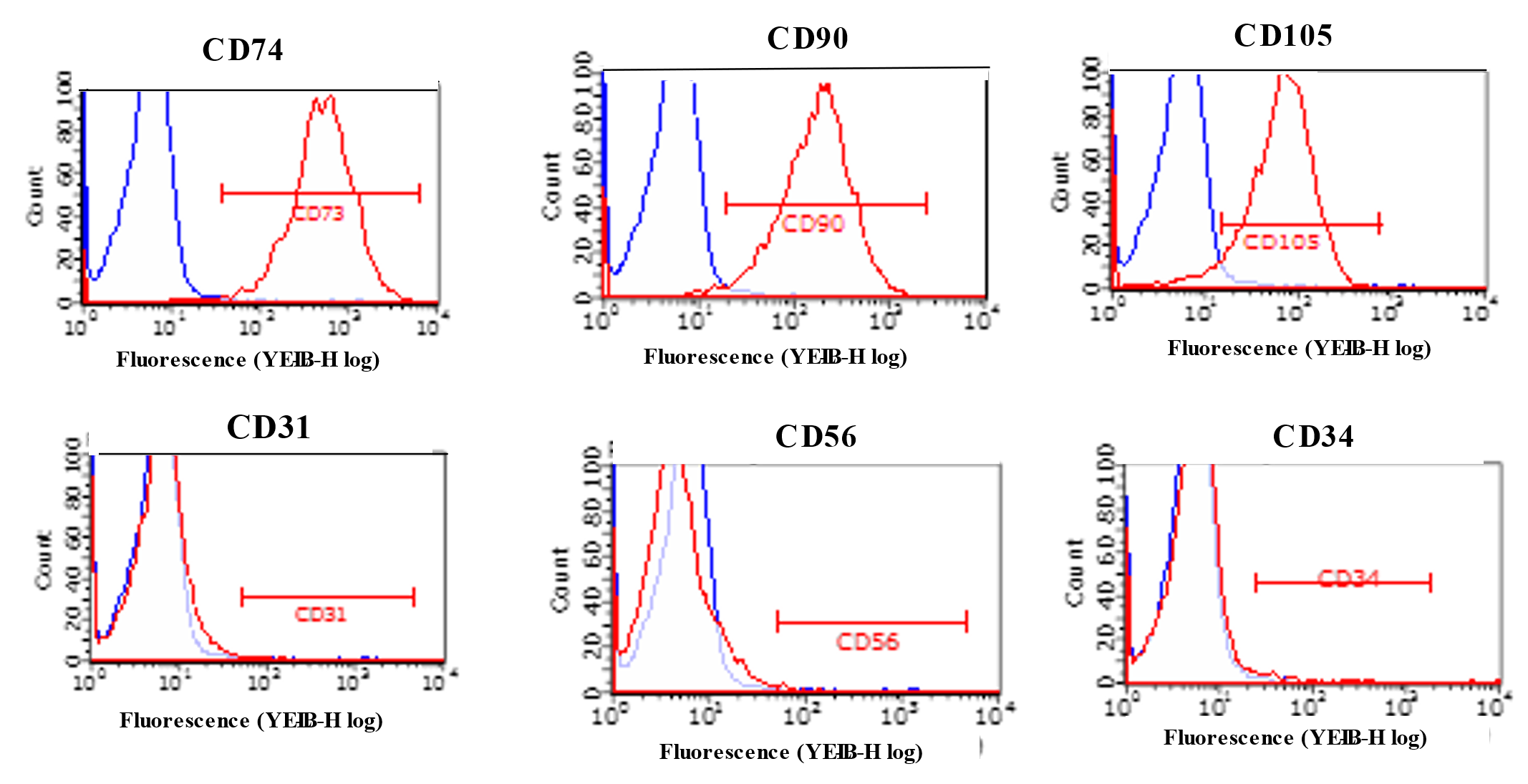
2.2. Characterization of ADMSCs with Flowcytometry and Immunocytochemistry
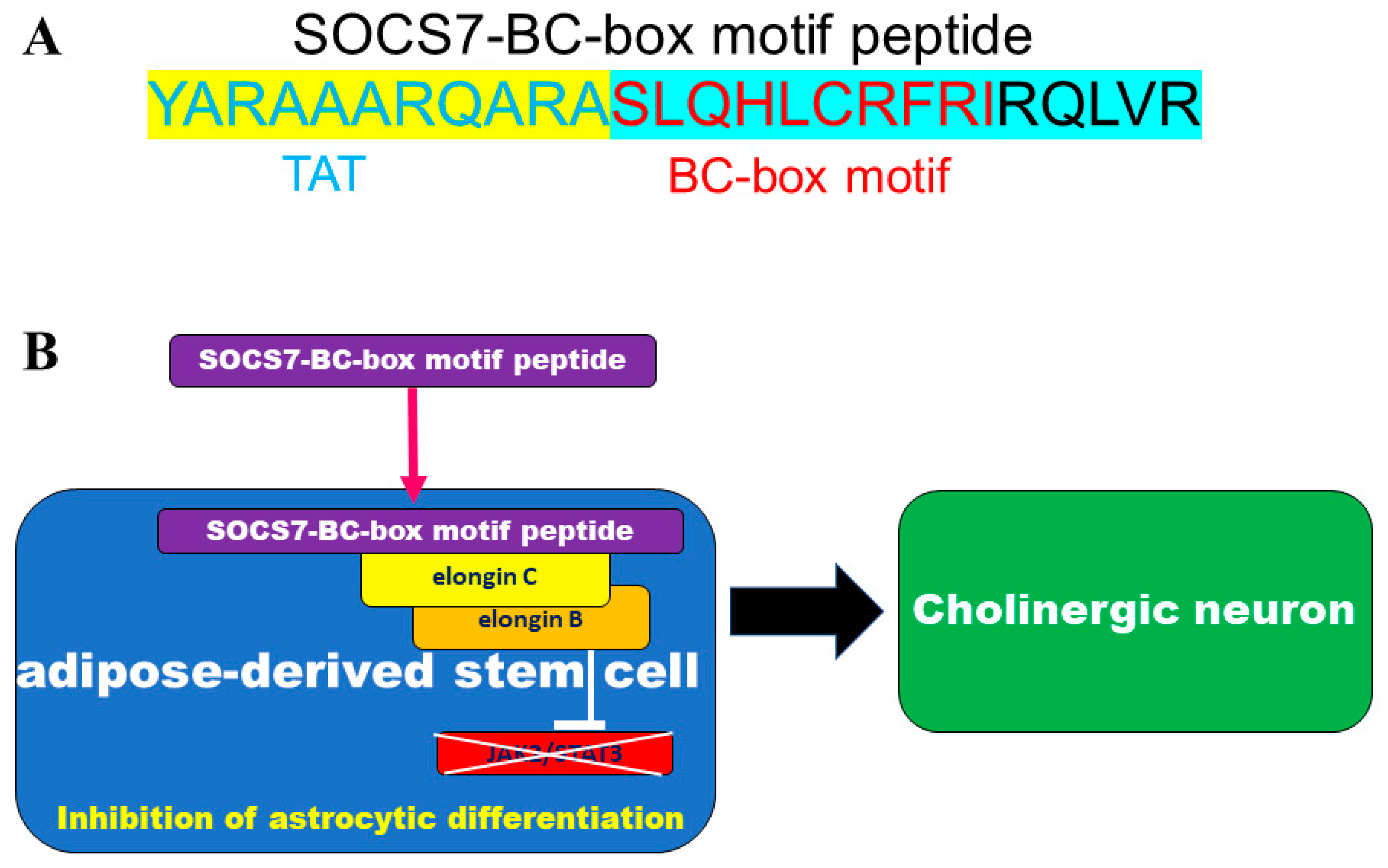
2.3. Neuronal Differentiation with SOCS7-Derived BC-Box Motif Peptide
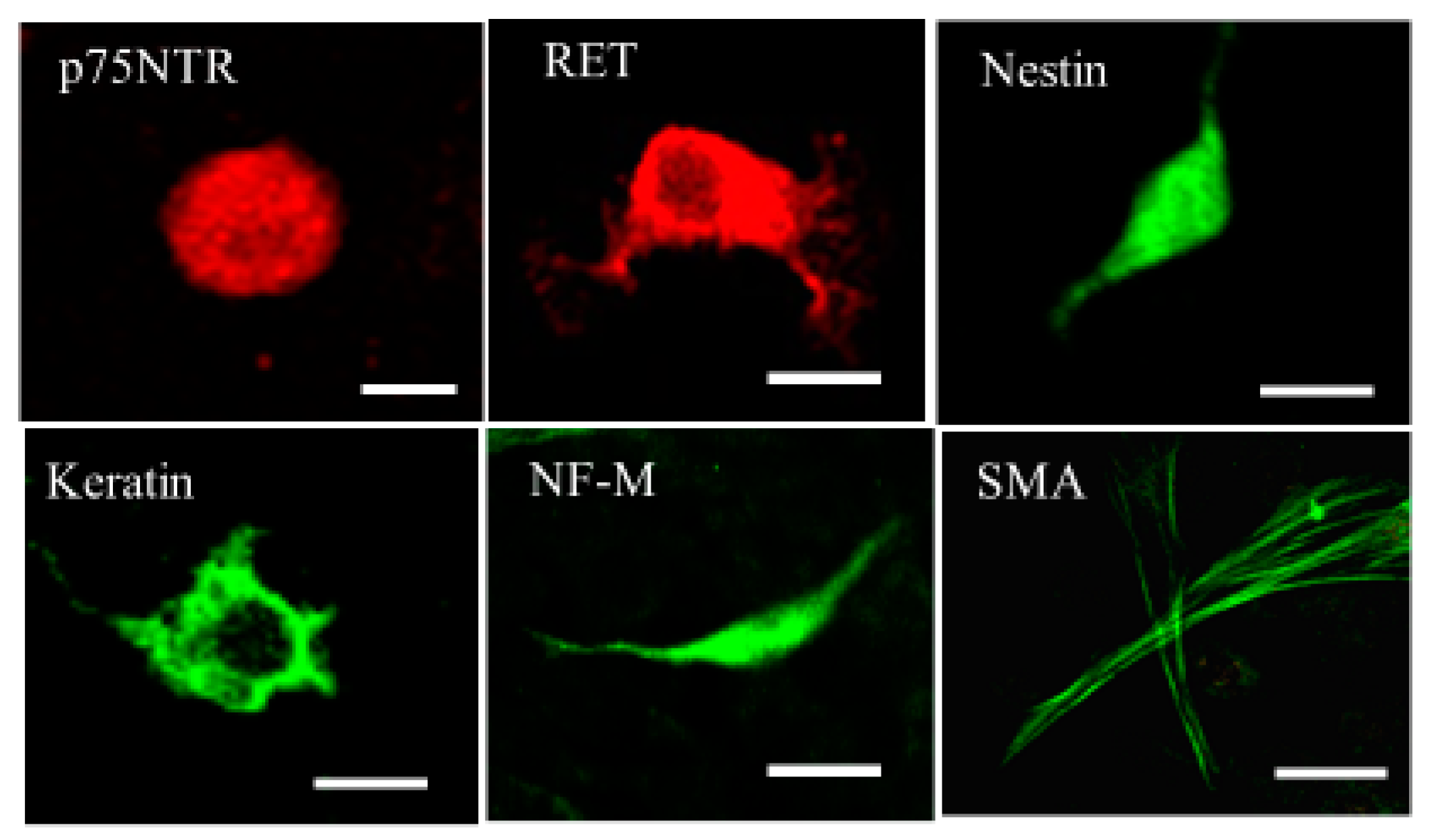
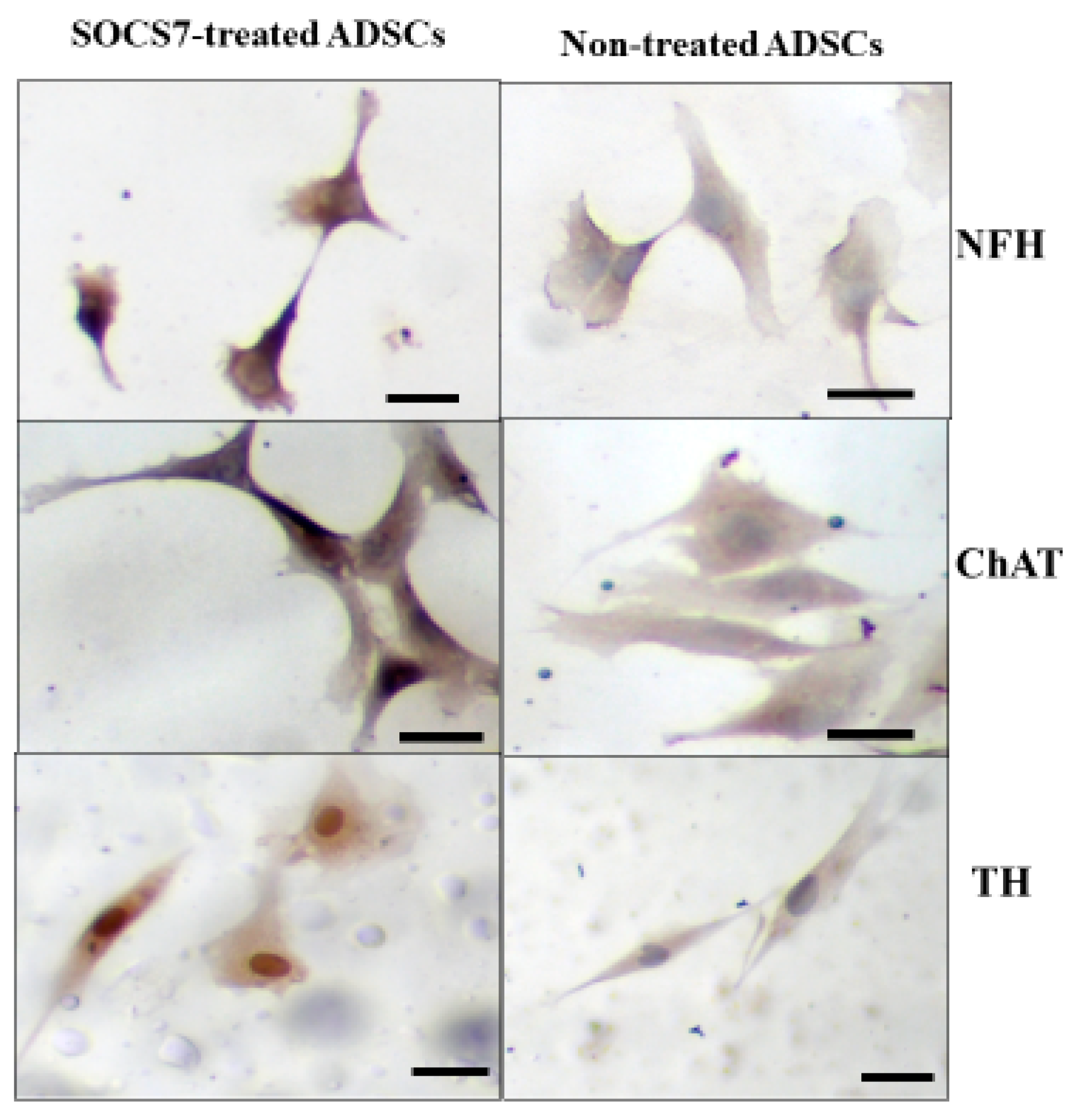
2.4. Immunocytochemistry for ADMSCs with or without the SOCS7 Peptide Treatment
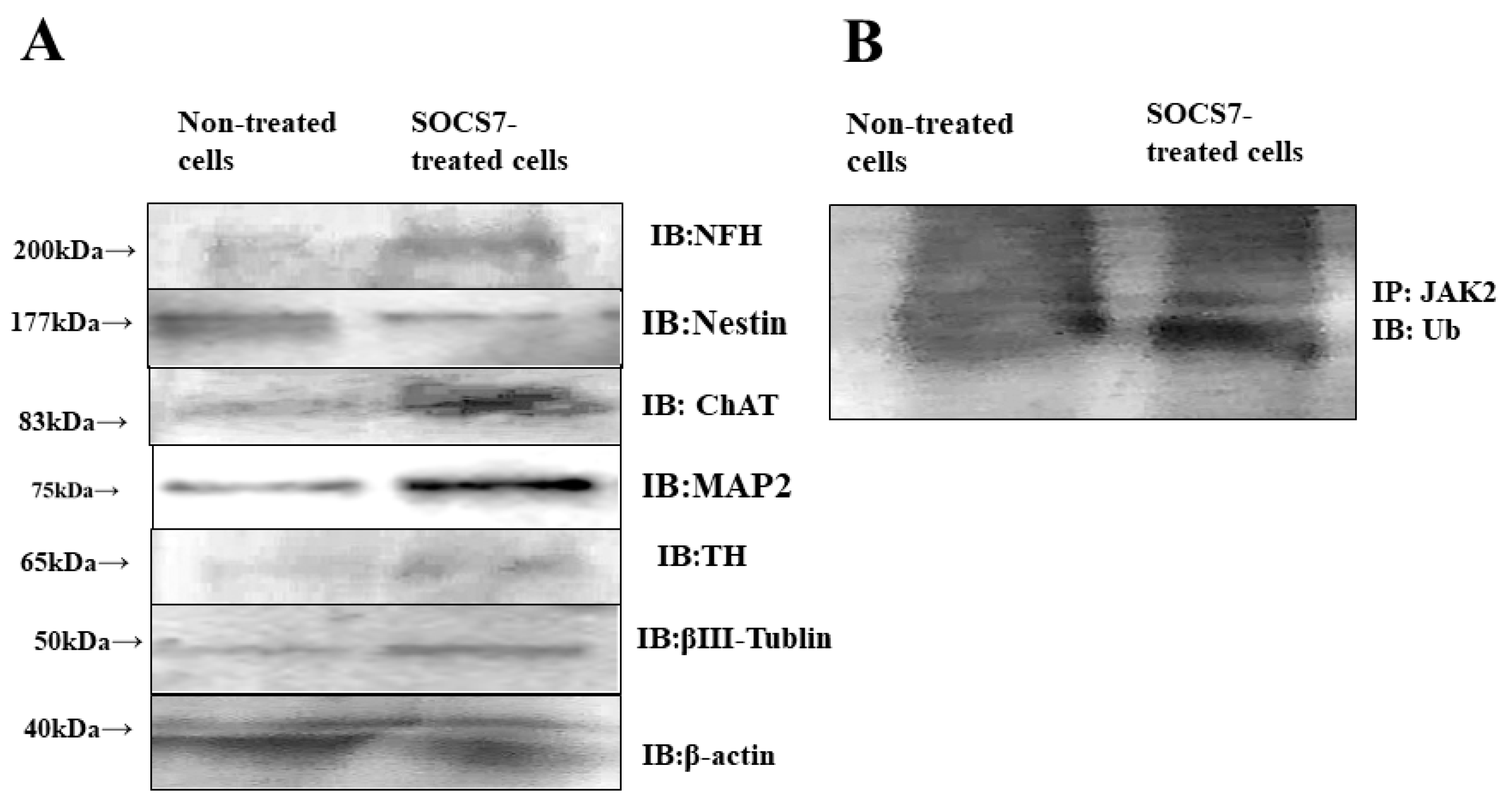
2.5. Western Blotting
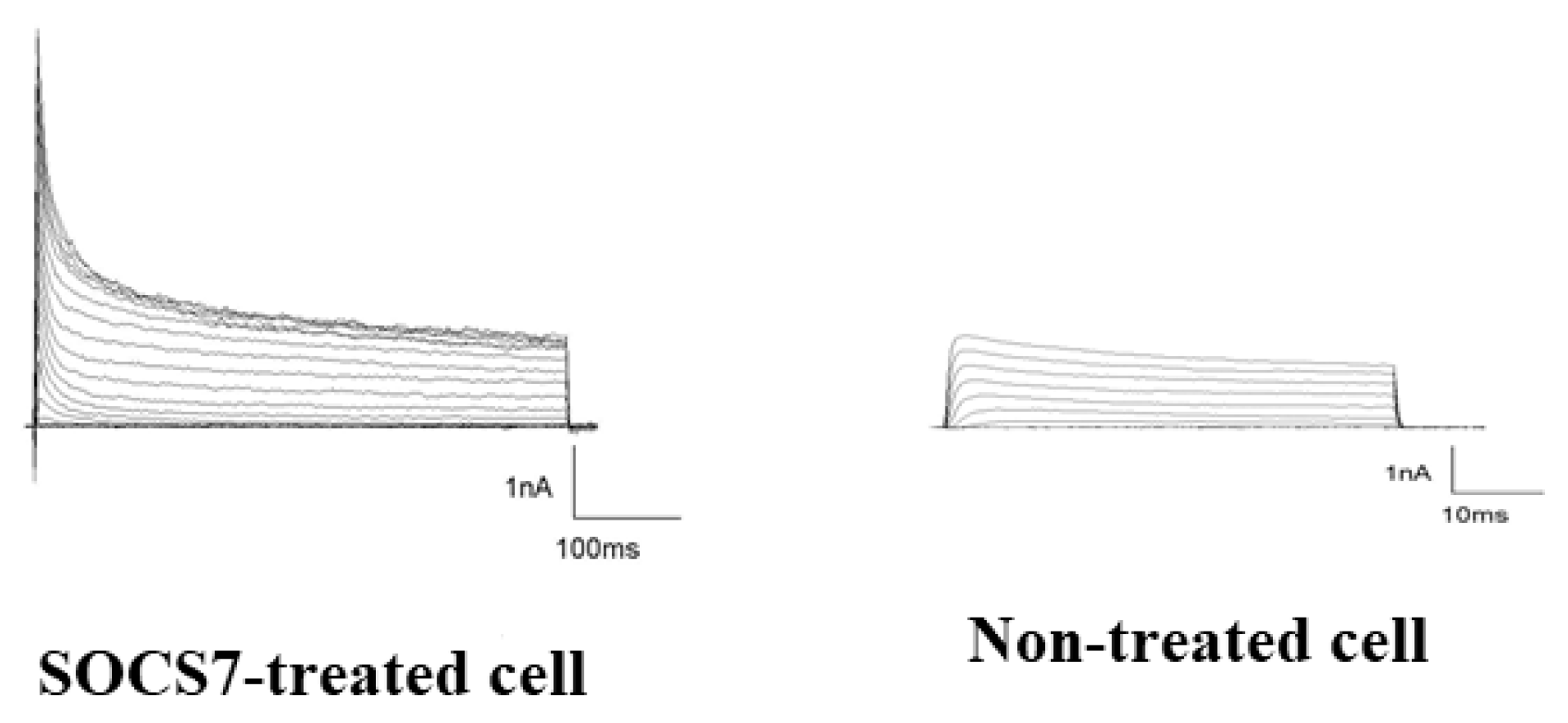
2.6. Electrophysiological Analysis by Use of the Patch-Cramp Configuration
3. Discussion
4. Experimental Section
4.1. Isolation of Adipose-Tissue Derived Mesenchymal Stem Cells from Fat Tissue and Cell Culture
4.2. Flow Cytometry Analysis for Characterization of Isolated Adipose-Derived Mesenchymal Stem Cells
4.3. BC-Box Motif Peptide Design and Synthesis
4.4. Neuronal Induction with BC-Box Motif in SOCS7 Peptide
4.5. Immunocytochemistry
4.6. Western Blotting
4.7. Ubiquitination Assay
4.8. Electrophysiology with Patch-Cramp Configuration
4.9. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- de Oliveira, N.B.; Irioda, A.C.; Stricker, P.E.F.; Mogharbel, B.F.; da Rosa, N.N.; Dziedzic, D.S.M.; de Carvalho, K.A.T. Natural Membrane Differentiates Human Adipose-Derived Mesenchymal Stem Cells to Neurospheres by Mechanotransduction Related to YAP and AMOT Proteins. Membranes 2021, 11, 687. [Google Scholar] [CrossRef] [PubMed]
- Freitag, J.; Bates, D.; Wickham, J.; Shah, K.; Huguenin, L.; Tenen, A.; Paterson, K.; Boyd, R. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: A randomized controlled trial. Regen. Med. 2019, 14, 213–230. [Google Scholar] [CrossRef] [PubMed]
- de Celis-Ruiz, E.; Fuentes, B.; Moniche, F.; Montaner, J.; Borobia, A.M.; Gutiérrez-Fernández, M.; Díez-Tejedor, E. Allogeneic adipose tissue-derived mesenchymal stem cells in ischaemic stroke (AMASCIS-02): A phase IIb, multicentre, double-blind, placebo-controlled clinical trial protocol. BMJ Open 2021, 11, e051790. [Google Scholar] [CrossRef]
- Bydon, M.; Dietz, A.B.; Goncalves, S.; Moinuddin, F.M.; Alvi, M.A.; Goyal, A.; Yolcu, Y.; Hunt, C.L.; Garlanger, K.L.; Del Fabro, A.S.; et al. CELLTOP clinical trial: First report from a phase 1 trial of autologous adipose tissue-derived mesenchymal stem cells in the treatment of paralysis due to traumatic spinal cord injury. Mayo Clin. Proc. 2020, 95, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.M.; Karimi, S.H.; Arab, L.; Sanjari, L.; Mardpour, S.; Azimian, V.; Jarughi, N.; Ghaheri, A.; Hosseini, S.E.; Aghdami, N.; et al. Safety and efficacy of allogeneic adipose tissue mesenchymal stromal cells in amyotrophic lateral sclerosis patients, Single-center, Prospective, Open-label, Single-arm clinical trial, long-term follow-up. Cell J. 2021, 23, 772–778. [Google Scholar]
- Fernández, O.; Izquierdo, G.; Fernández, V.; Leyva, L.; Reyes, V.; Guerrero, M.; León, A.; Arnaiz, C.; Navarro, G.; Páramo, M.D.; et al. Adipose-Research Group Study Eudra CT 2008-004015-35. Adipose-derived mesenchymal stem cells (AdMSC) for the treatment of secondary-progressive multiple sclerosis: A triple blinded, placebo controlled, randomized phase I/II safety and feasibility study. PLoS ONE 2018, 13, e0195891. [Google Scholar] [CrossRef]
- Kokai, L.E.; Marra, K.; Rubin, J.P. Adipose stem cells: Biology and clinical applications for tissue repair and regeneration. Transl. Res. 2014, 163, 399–408. [Google Scholar] [CrossRef]
- Bacakova, L.; Zarubova, J.; Travnickova, M.; Musilkova, J.; Pajorova, J.; Slepicka, P.; Kasalkova, N.S.; Svorcik, V.; Kolska, Z.; Motarjemi, H.; et al. Stem cells: Their source, potency and use in regenerative therapies with focus on adipose-derived stem cells—A review. Biotechnol. Adv. 2018, 36, 1111–1126. [Google Scholar] [CrossRef]
- Jo, C.H.; Lee, Y.G.; Shin, W.H.; Kim, H.; Chai, J.W.; Jeong, E.C.; Kim, J.E.; Shin, J.S.; Shin, I.S.; Ra, J.C.; et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: A proof-of-concept clinical trial. Stem. Cells 2014, 32, 1254–1266. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Fernández, M.; Otero-Ortega, L.; Ramos-Cejudo, J.; Rodríguez-Frutos, B.; Fuentes, B.; Díez-Tejedor, E. Adipose tissue-derived mesenchymal stem cells as a strategy to improve recovery after stroke. Expert. Opin. Biol. Ther. 2015, 15, 873–881. [Google Scholar] [CrossRef]
- Hilton, D.J.; Richardson, R.T.; Alexander, W.S.; Viney, E.M.; Willson, T.A.; Sprigg, N.S.; Starr, R.; Nicholson, S.E.; Metcalf, D.; Nicola, N.A. Twenty proteins containing a C-terminal SOCS box form five structural classes. Proc. Natl. Acad. Sci. USA 1998, 95, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Kamura, T.; Sato, S.; Haque, D.; Liu, L.; Kaelin, W.G., Jr.; Conaway, R.; Conaway, J.W. The Elongin BC complex interacts wth the conserved SOCS-box motif present in members of the SOCS, ras, WD-40 repeat, and ankyrin repeat families. Genes Dev. 1998, 12, 3872–3881. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.G.; Farley, A.; Nicholson, S.E.; Willson, T.A.; Zugaro, L.M.; Simpson, R.J.; Moritz, R.L.; Cary, D.; Richardson, R.; Hausmann, G.; et al. The conserved SOCS box motif in suppressors of cytokine signaling binds to elongins B and C and may couple bound proteins to proteasomal degradation. Proc. Natl. Acad. Sci. USA 1999, 96, 2071–2076. [Google Scholar] [CrossRef]
- Polizzotto, M.N.; Bartlett, P.F.; Turnley, A.M.; Polizzotto, M.N. Expression of suppressor of cytokine signaling (SOCS) genes in the developing and adult mouse nervous system. J. Comp. Neurol. 2000, 423, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Turnley, A.M.; Starr, R.; Bartlett, P.F. SOCS1 regulates interferon-gamma mediated sensory neuron survival. Neuroreport 2001, 12, 3443–3445. [Google Scholar] [CrossRef]
- Cui, M.; Dai, B.; Xin, J.; He, J.; Feng, S. Overexpression of suppressors of cytokine signaling 1 promotes the neuronal differentiation of C17.2. Neural Stem Cells. Cell Physiol. Biochem. 2014, 33, 528–538. [Google Scholar] [CrossRef]
- Cui, M.; Ma, X.L.; Sun, J.; He, J.Q.; Shen, L.; Li, F.G. Overexpression of suppressors of cytokine signaling 1 regulate the proliferation and differentiation of rat-derived neural stem cells. Acta Histochem. 2017, 119, 680–688. [Google Scholar] [CrossRef]
- Goldshmit, Y.; Greenhalgh, C.J.; Turnley, A.M. Suppressor of cytokine signalling-2 and epidermal growth factor regulate neurite outgrowth of cortical neurons. Eur. J. Neurosci. 2004, 20, 2260–2266. [Google Scholar] [CrossRef]
- Scott, H.J.; Stebbing, M.J.; Walters, C.E.; McLenachan, S.; Ransome, M.I.; Nichols, N.R.; Turnley, A.M. Differential effects of SOCS2 on neuronal differentiation and morphology. Brain Res. 2006, 1067, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.K.; Gupta, S.; Banerjee, K. SOCS3 induces neurite differentiation and promotes neuronal cell survival. IUBMB Life 2016, 68, 468–476. [Google Scholar] [CrossRef]
- Gupta, S.; Mishra, K.; Surolia, A.; Banerjee, K. Suppressor of cytokine signalling-6 promotes neurite outgrowth via JAK2/STAT5-mediated signaling pathway, involving negative feedback inhibition. PLoS ONE 2011, 6, e26674. [Google Scholar] [CrossRef]
- Yoshizumi, T.; Kubo, A.; Murata, H.; Shinonaga, M.; Kanno, H. BC-box motif in SOCS6 induces differentiation of epidermal stem cells into GABAnergic neurons. Int. J. Mol. Sci. 2020, 21, 4947. [Google Scholar] [CrossRef] [PubMed]
- Cianciulli, A.; Calvello, R.; Porro, C.; Trotta, T.; Panaro, M.A. Understanding the role of SOCS signaling in neurodegenerative diseases: Current and emerging concepts. Cytokine Growth Factor Rev. 2017, 37, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; IJou, I.; Joe, E.H. Suppression of miR-155 expression in IFN-gamma- Treated astrocytes and microglia by DJ-1: A possible mechanism for maintaining SOCS1 expression. Exp. Neurobiol. 2014, 23, 148–154. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, B.; Deng, B.; Zhang, F.; Wu, J.; Wang, Y.; Le, Y.; Wang, Q. Amyloid-induces hepatic insulin resistance in vivo via JAK2. Diabetes 2013, 62, 1159–1166. [Google Scholar] [CrossRef]
- Rasmussen, S.; Wang, Y.; Kivisäkk, P.; Bronson, R.T.; Meyer, M.; Imitola, J.; Khoury, S.J. Persistent activation of microglia is associated with neuronal dysfunction of callosal projecting pathways and multiple sclerosis-like lesions in relapsing-remitting experimental autoimmune encephalomyelitis. Brain 2007, 130, 2816–2829. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroeck, K.; Alvarez, J.; Swaminathan, B.; Alloza, I.; Matesanz, F.; Urcelay, E.; Comabella, M.; Alcina, A.; Fedetz, M.; Ortiz, M.A.; et al. A cytokine gene screen uncovers SOCS1 as genetic risk factor for multiple sclerosis. Genes Immun. 2012, 13, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra Rao, V.L.; Bowen, K.K.; Dhodda, V.K.; Song, G.; Franklin, J.L.; Gavva, N.R.; Dempsey, R.J. Gene expression analysis of spontaneously hypertensive rat cerebral cortex following transient focal cerebral ischemia. J. Neurochem. 2002, 83, 1072–1086. [Google Scholar] [CrossRef]
- Jakkula, E.; Leppä, V.; Sulonen, A.M.; Varilo, T.; Kallio, S.; Kemppinen, A.; Purcell, S.; Koivisto, K.; Tienari, P.; Sumelahti, M.L.; et al. Genome-wide association study in a high-risk isolate for multiple sclerosis reveals associated variants in STAT3 gene. Am. J. Hum. Genet. 2010, 86, 285–291. [Google Scholar] [CrossRef]
- Frisullo, G.; Angelucci, F.; Caggiula, M.; Nociti, V.; Iorio, R.; Patanella, A.K.; Sancricca, C.; Mirabella, M.; Tonali, P.A.; Batocchi, A.P. pSTAT1, pSTAT3, and T-bet expression in peripheral blood mononuclear cells from relapsing-remitting multiple sclerosis patients correlates with disease activity. J. Neurosci. Res. 2006, 84, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Planas, A.M.; Gorina, R.; Chamorro, A. Signaling pathways mediating inflammatory responses in brain ischaemia. Biochem. Soc. Trans. 2006, 34, 1267–1270. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, S.T. Gene expression changes after focal stroke, traumatic brain and spinal cord injuries. Curr. Opin. Neurol. 2003, 16, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.G.; Whetzel, A.M.; Lue, L.F. Expression of suppressor of cytokine signaling genes in human elderly and Alzheimer’s disease brains and human microglia. Neuroscience 2015, 302, 121–137. [Google Scholar] [CrossRef]
- Lawrenson, I.D.; Krebs, D.L.; Linossi, E.M.; Zhang, J.G.; McLennan, T.J.; Collin, C.; McRae, H.M.; Kolesnik, T.B.; Koh, K.; Britto, J.M.; et al. Cortical Layer Inversion and Deregulation of Reelin Signaling in the Absence of SOCS6 and SOCS7. Cereb. Cortex. 2017, 27, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Sekine, K.; Kubo, K.; Nakajima, K. How does Reelin control neuronal migration and layer formation in the developing mammalian neocortex? Neurosci. Res. 2014, 86, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Fairchild, C.L.; Hino, K.; Han, J.S.; Miltner, A.M.; Peinado Allina, G.; Brown, C.E.; Burns, M.E.; La Torre, A.; Simó, S. RBX2 maintains final retinal cell position in a DAB1-dependent and -independent fashion. Development 2018, 145, dev155283. [Google Scholar] [CrossRef] [PubMed]
- Kubo, A.; Yoshida, T.; Kobayashi, N.; Yokoyama, T.; Mimura, T.; Nishiguchi, T.; Higashida, T.; Yamamoto, I.; Kanno, H. Efficient generation of dopamine neuron-like cells from skin-derived precursors with a synthetic peptide derived from von Hippel-Lindau protein. Stem. Cells Dev. 2009, 18, 1523–1532. [Google Scholar] [CrossRef]
- Ntege, E.H.; Sunami, H.; Denda, J.; Futenma, N.; Shimizu, Y. Effects of hydroxyapatite-coated nonwoven polyethylene/polypropylene fabric on non-mesodermal lineage-specific differentiation of human adipose-derived stem cells. BMC Res. Notes 2020, 13, 471. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar]
- Kanno, H.; Xu, Y.; Miyakawa, T.; Kubo, A.; Higashida, T.; Kobayashi, N.; Yoshida, T.; Tanokura, M. BC box motif-mediated Neuronal differentiation of somatic stem cells. Int. J. Mol. Sci. 2018, 19, 466. [Google Scholar] [CrossRef] [PubMed]
- Kanno, H.; Nakano, S.; Kubo, A.; Mimura, T.; Tajima, N.; Sugimoto, N. Neuronal differentiation of neural progenitor cells by intracellular delivery of synthetic oligopeptide derived from von Hippel-Lindau protein. Protein Pept. Lett. 2009, 16, 1291–1296. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Fernández, M.; Rodríguez-Frutos, B.; Ramos-Cejudo, J.; Teresa Vallejo-Cremades, M.; Fuentes, B.; Cerdán, S.; Díez-Tejedor, E. Effects of intravenous administration of allogenic bone marrow- and adipose tissue-derived mesenchymal stem cells on functional recovery and brain repair markers in experimental ischemic stroke. Stem. Cell Res. Ther. 2013, 4, 11. [Google Scholar] [CrossRef]
- Chen, K.H.; Chen, C.H.; Wallace, C.G.; Yuen, C.M.; Kao, G.S.; Chen, Y.L.; Shao, P.L.; Chen, Y.L.; Chai, H.T.; Lin, K.C.; et al. Intravenous administration of xenogenic adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes markedly reduced brain infarct volume and preserved neurological function in rat after acute ischemic stroke. Oncotarget 2016, 7, 74537–74556. [Google Scholar] [CrossRef]
- Marei, H.E.S.; El-Gamal, A.; Althani, A.; Afifi, N.; Abd-Elmaksoud, A.; Farag, A.; Cenciarelli, C.; Thomas, C.; Anwarul, H. Cholinergic and dopaminergic neuronal differentiation of human adipose tissue derived mesenchymal stem cells. J. Cell Physiol. 2018, 233, 936–945. [Google Scholar] [CrossRef]
- Jang, S.; Kang, Y.H.; Ullah, I.; Shivakumar, S.B.; Rho, G.J.; Cho, Y.C.; Sung, I.Y.; Park, B.W. Cholinergic nerve differentiation of mesenchymal stem cells derived from long-term cryopreserved human dental pulp in vitro and analysis of their motor nerve regeneration potential in vivo. Int. J. Mol. Sci. 2018, 19, 2434. [Google Scholar] [CrossRef] [PubMed]
- Snyder, E.L.; Dowdy, S.F. Cell penetrating peptides in drug delivery. Pharm. Res. 2004, 21, 389–393. [Google Scholar] [CrossRef]
- Kang, Y.H.; Shivakumar, S.B.; Son, Y.B.; Bharti, D.; Jang, S.J.; Heo, K.S.; Park, W.U.; Byun, J.H.; Park, B.W.; Rho, G.J. Comparative analysis of three different protocols for cholinergic neuron differentiation in vitro using mesenchymal stem cells from human dental pulp. Anim. Cells Syst. 2019, 23, 275–287. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanno, H.; Matsumoto, S.; Yoshizumi, T.; Nakahara, K.; Shinonaga, M.; Kubo, A.; Fujii, S.; Ishizuka, Y.; Tanaka, M.; Ichihashi, M.; et al. SOCS7-Derived BC-Box Motif Peptide Mediated Cholinergic Differentiation of Human Adipose-Derived Mesenchymal Stem Cells. Int. J. Mol. Sci. 2023, 24, 2786. https://doi.org/10.3390/ijms24032786
Kanno H, Matsumoto S, Yoshizumi T, Nakahara K, Shinonaga M, Kubo A, Fujii S, Ishizuka Y, Tanaka M, Ichihashi M, et al. SOCS7-Derived BC-Box Motif Peptide Mediated Cholinergic Differentiation of Human Adipose-Derived Mesenchymal Stem Cells. International Journal of Molecular Sciences. 2023; 24(3):2786. https://doi.org/10.3390/ijms24032786
Chicago/Turabian StyleKanno, Hiroshi, Shutaro Matsumoto, Tetsuya Yoshizumi, Kimihiro Nakahara, Masamichi Shinonaga, Atsuhiko Kubo, Satoshi Fujii, Yasuyuki Ishizuka, Masaki Tanaka, Masamitsu Ichihashi, and et al. 2023. "SOCS7-Derived BC-Box Motif Peptide Mediated Cholinergic Differentiation of Human Adipose-Derived Mesenchymal Stem Cells" International Journal of Molecular Sciences 24, no. 3: 2786. https://doi.org/10.3390/ijms24032786
APA StyleKanno, H., Matsumoto, S., Yoshizumi, T., Nakahara, K., Shinonaga, M., Kubo, A., Fujii, S., Ishizuka, Y., Tanaka, M., Ichihashi, M., & Murata, H. (2023). SOCS7-Derived BC-Box Motif Peptide Mediated Cholinergic Differentiation of Human Adipose-Derived Mesenchymal Stem Cells. International Journal of Molecular Sciences, 24(3), 2786. https://doi.org/10.3390/ijms24032786





