Targeted Therapy for Glomerulonephritis Using Arterial Delivery of Encapsulated Etanercept
Abstract
1. Introduction
2. Results and Discussion
2.1. Conjugation of Etanercept with Cy7
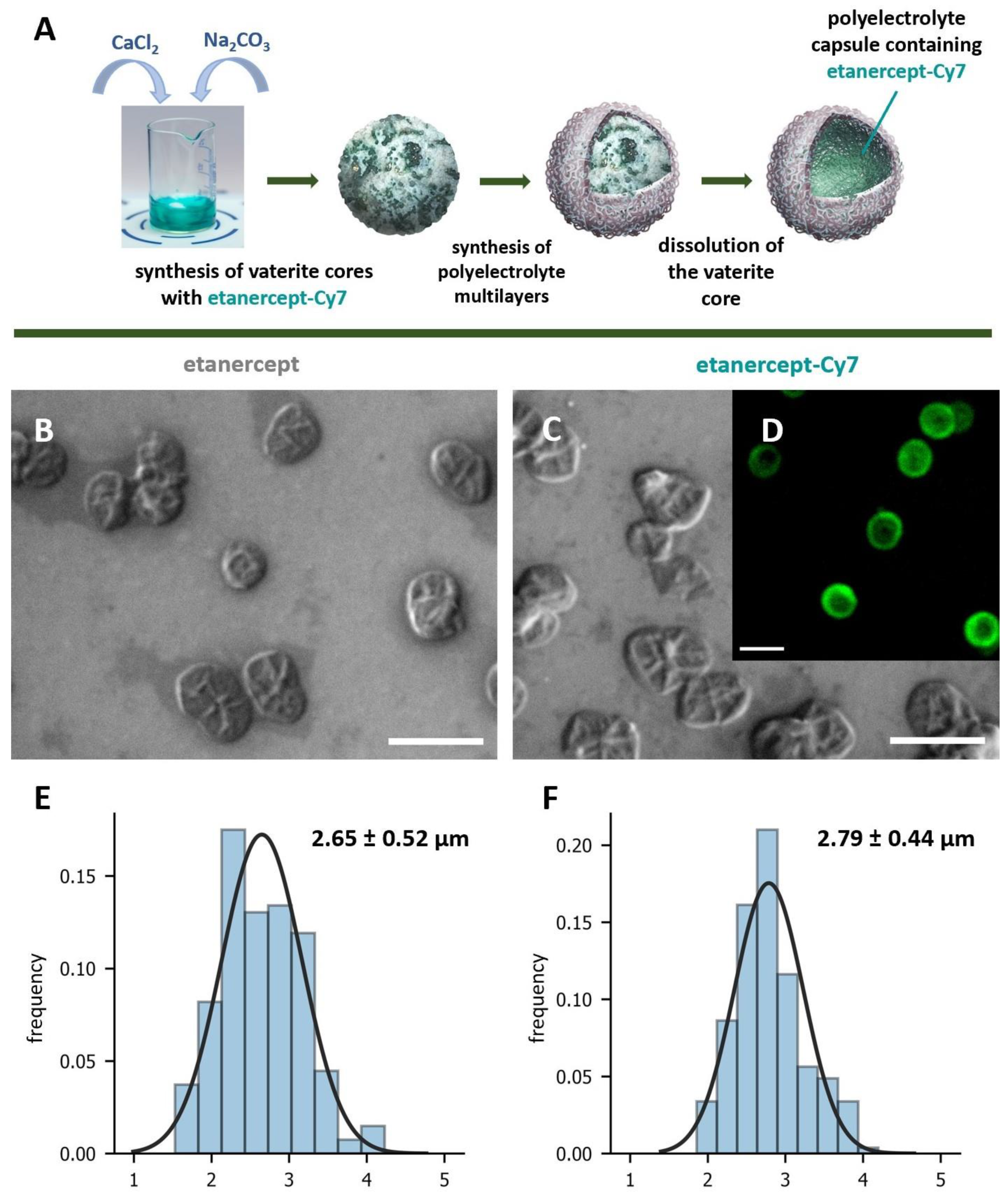
2.2. Synthesis and Characterization of Microcapsules with Etanercept and Etanercept-Cy7
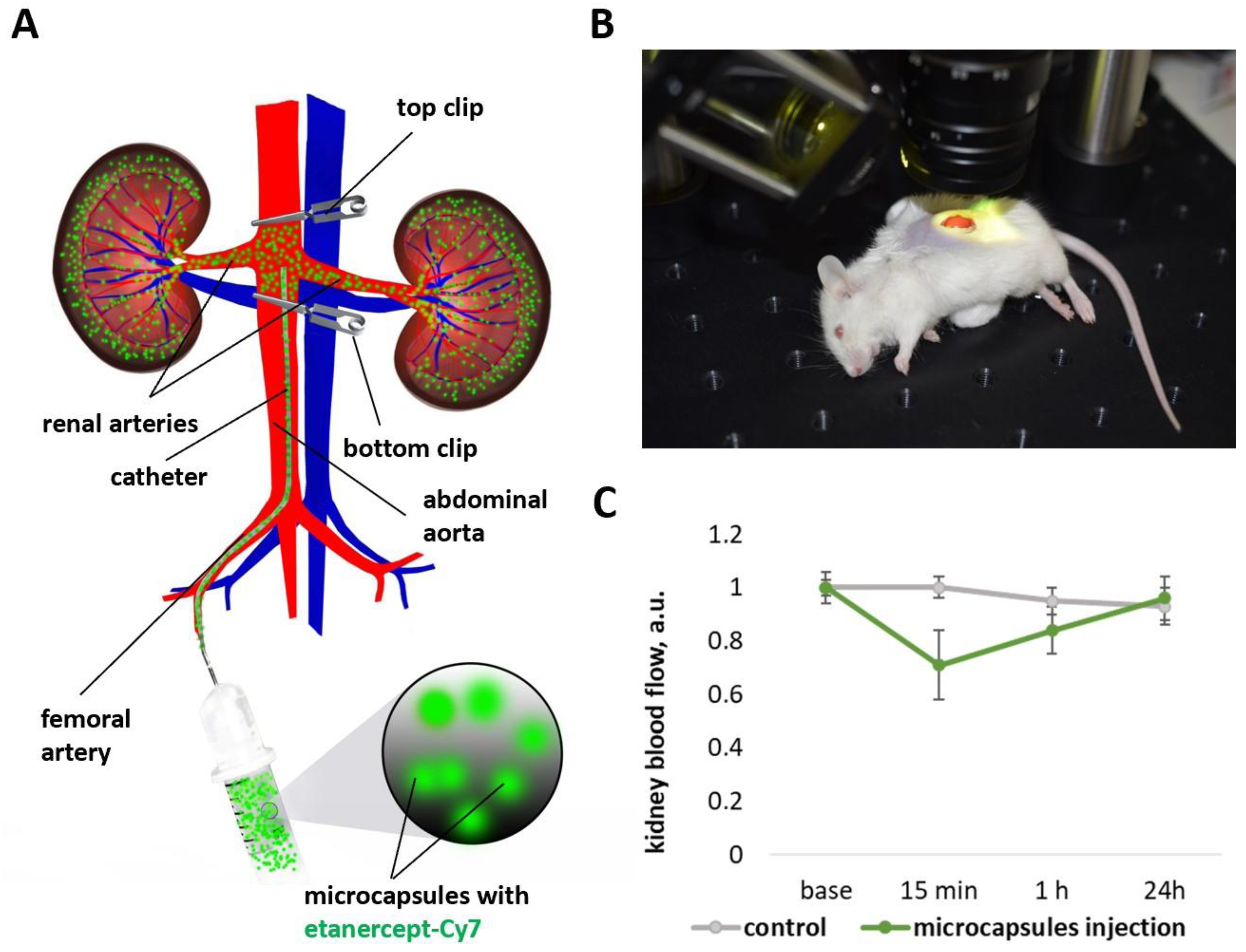
2.3. Changes of Kidney Blood Flow Associated with Microcapsules Intra-Arterial Administration
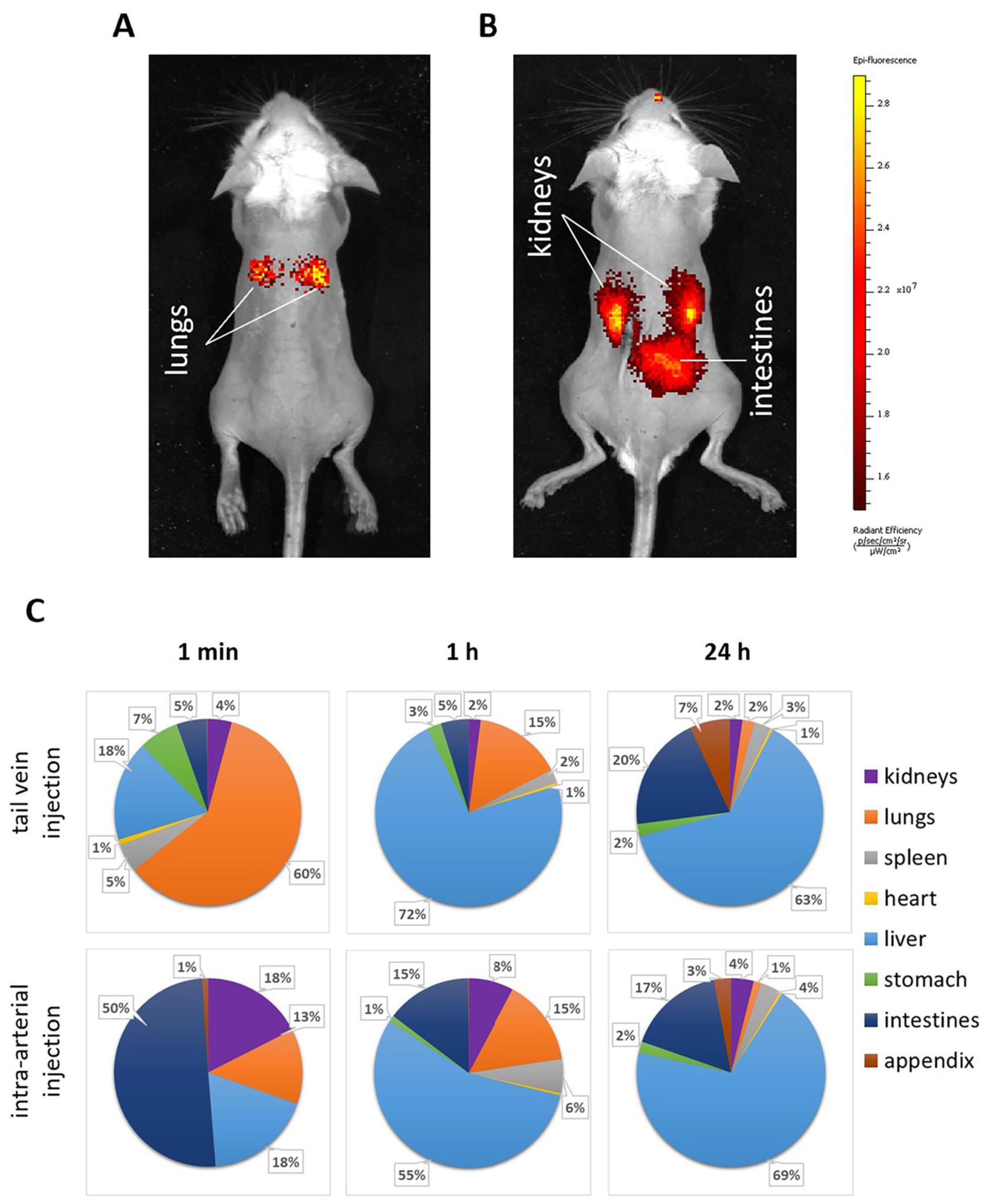
2.4. Biodistribution Kinetics of the Etanercept-Cy7 after Microcapsules Intra-Arterial or Tail Vein Administration
2.5. Capsules’ Localization and Following Elimination from Kidneys after Microcapsules Intra-Arterial or Tail Vein Administration
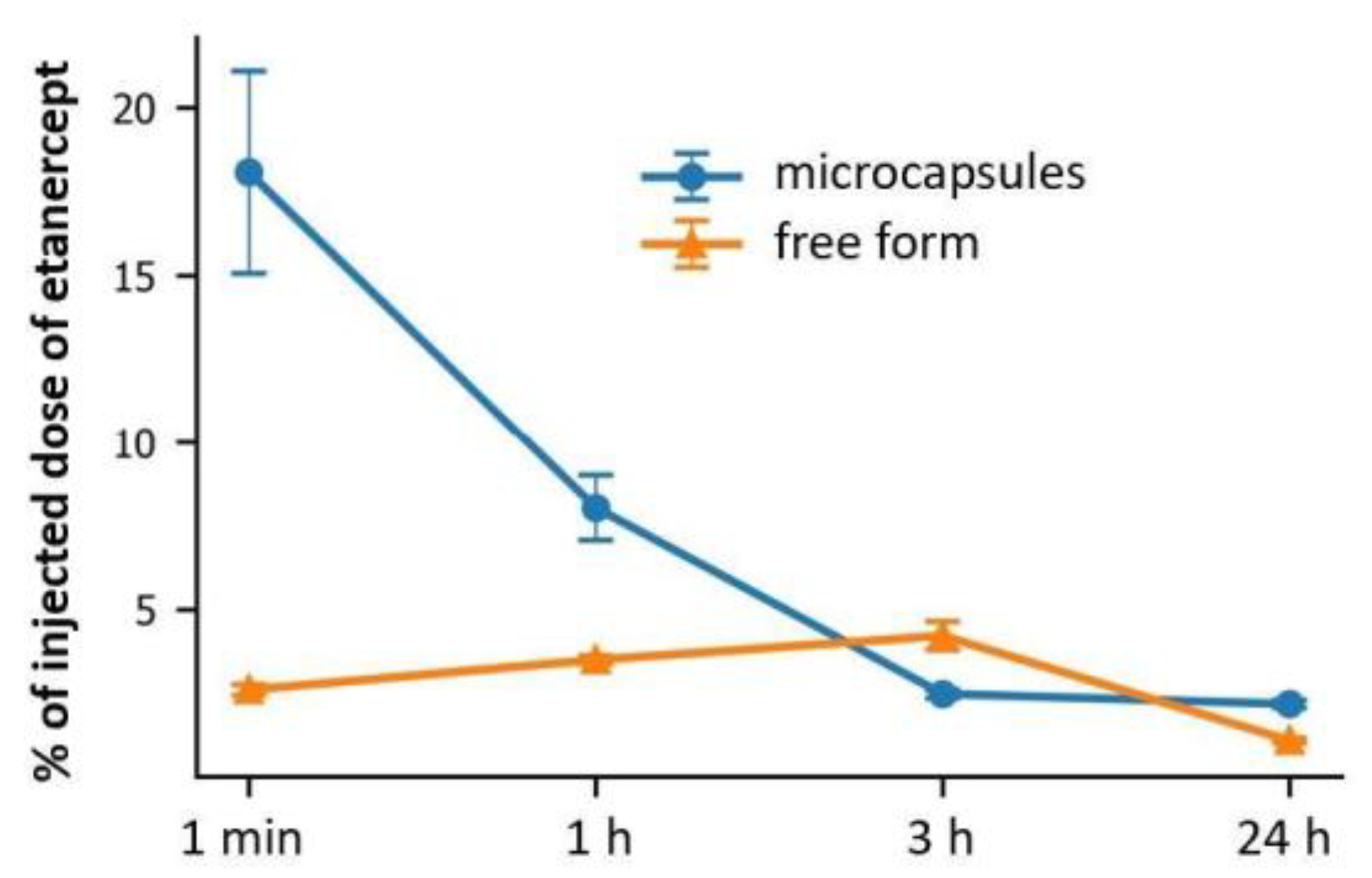
2.6. Kinetics of the Fluorescent Marker Accumulation in Kidneys after Intra-Arterial Administration of Etanercept-Cy7 in Free and Encapsulated Form
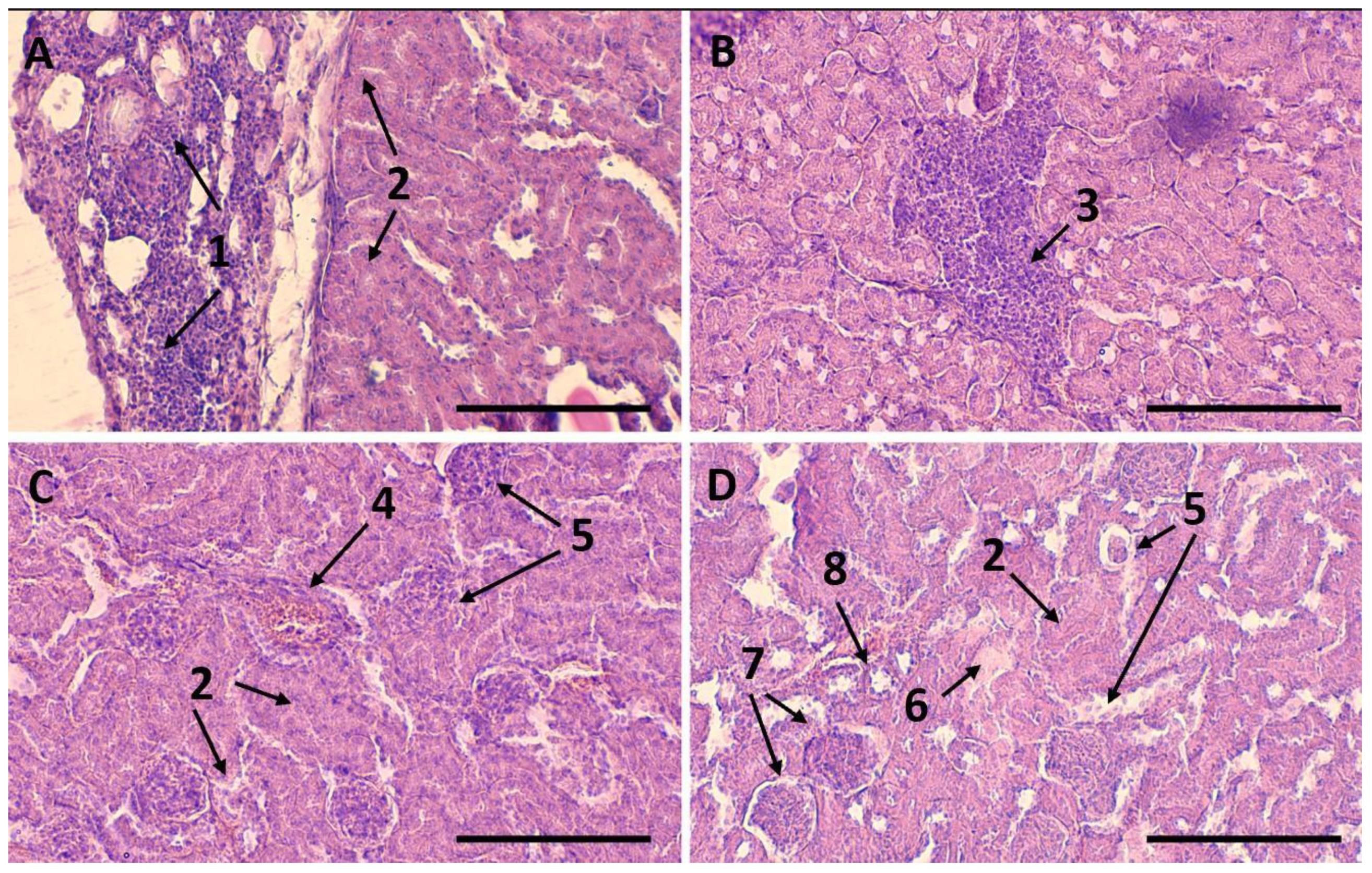
2.7. Therapeutic Effect after Intra-Arterial Administration of Etanercept-Cy7 in Free and Encapsulated Form
3. Materials and Methods
3.1. Materials
3.2. Instruments
3.3. Conjugation of Etanercept with Cy7
3.4. Synthesis and Characterization of Microcapsules with Etanercept and Etanercept-Cy7
3.5. Animal Studies
3.5.1. Capsules’ Administration
3.5.2. Blood Flow Response to Capsules’ Administration
3.5.3. Microcapsules’ Biodistribution
3.5.4. Microcapsules’ Localization in the Kidney Tissue
3.5.5. Quantification of Fluorescent Dye Content in Kidneys
3.5.6. Modeling of Acute Glomerulonephritis
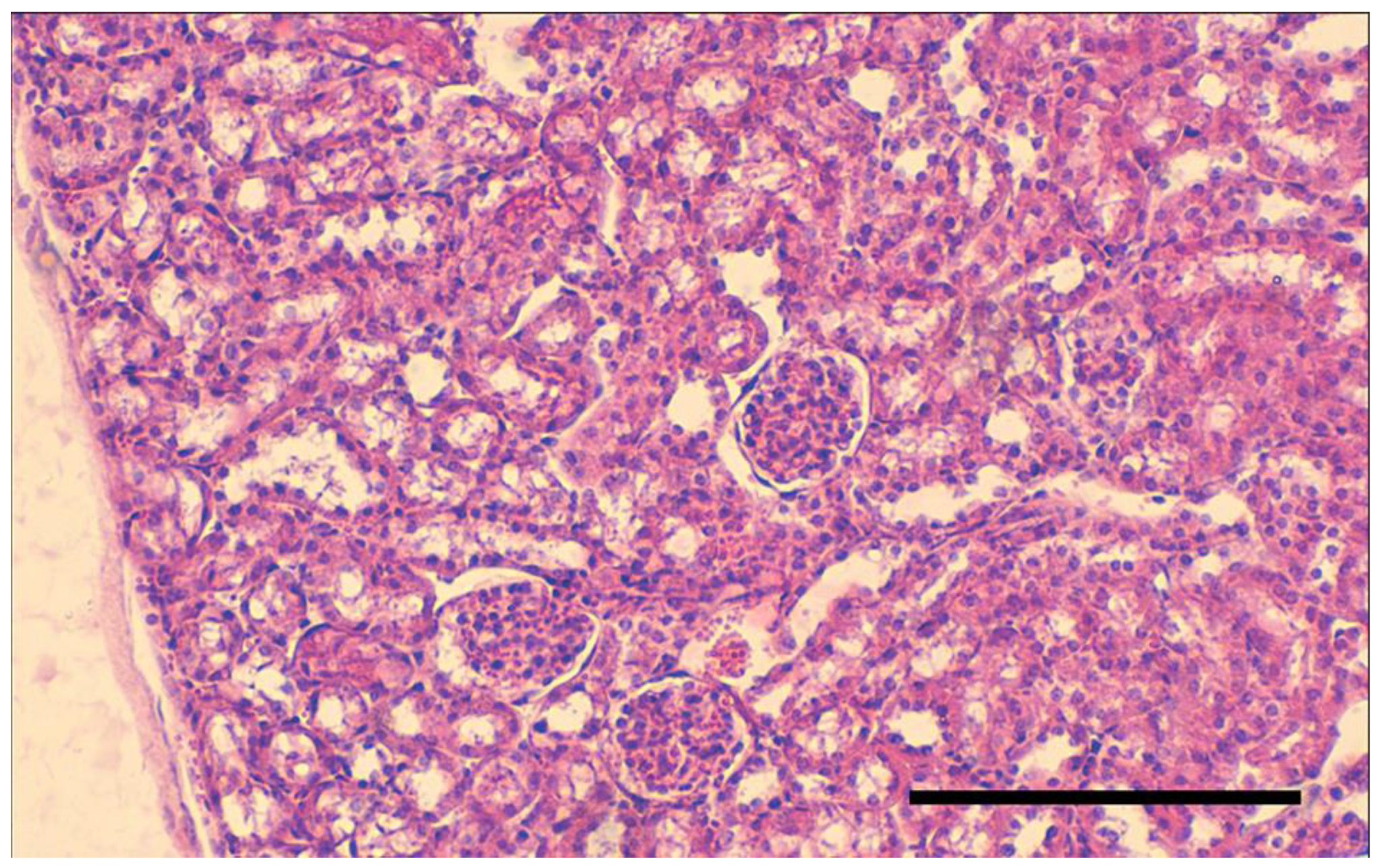
3.5.7. Histological Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
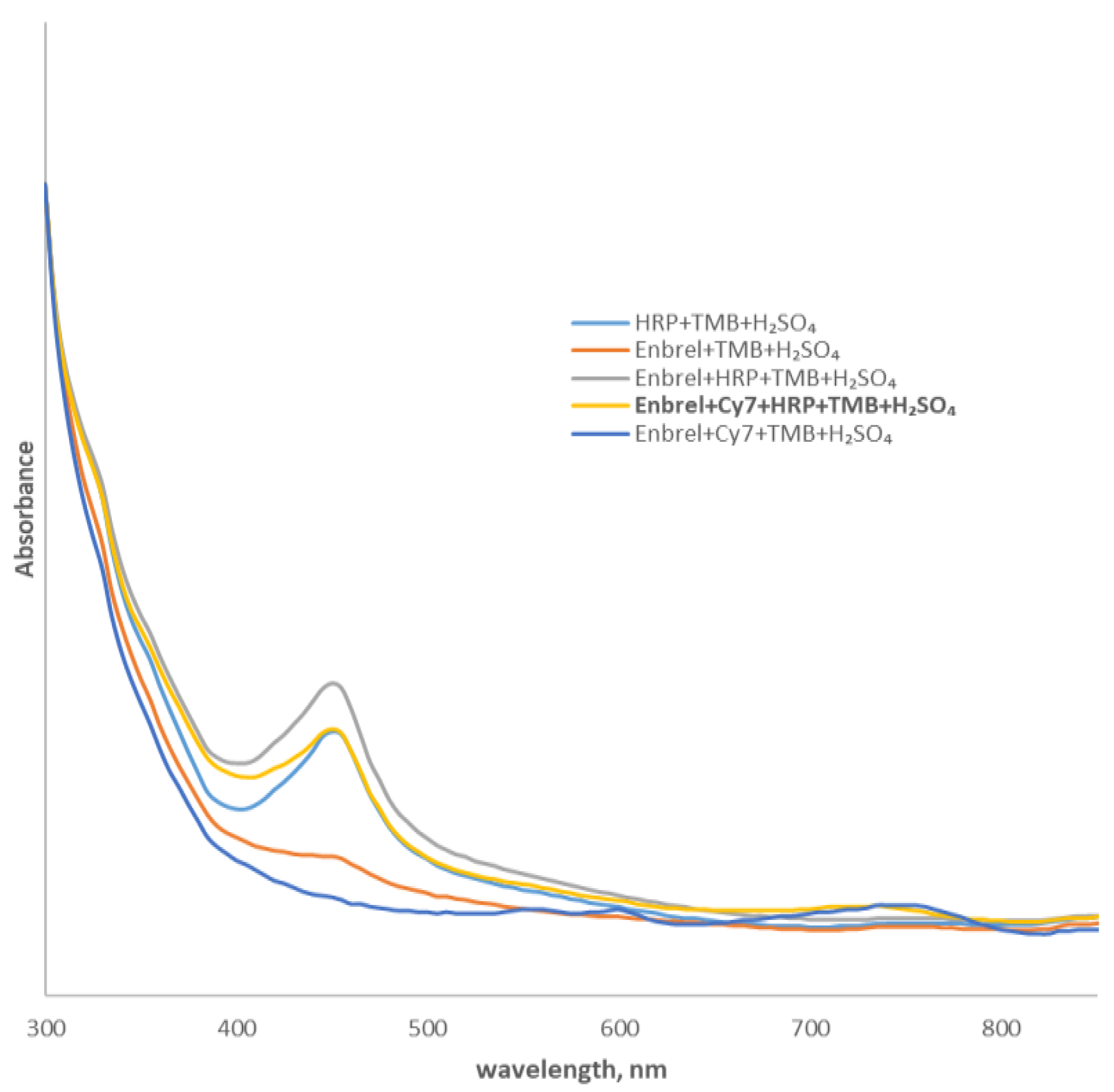
| Etanercept | Etanercept-Cy7 | |
|---|---|---|
| PBS | 8 | 7.5 |
| NaCl | 8 | 8.5 |
| Plasma | 8.5 | 9.5 |
References
- Chadban, S.; Atkins, R. Glomerulonephritis. Lancet 2005, 365, 1797–1806. [Google Scholar] [CrossRef] [PubMed]
- Hendra, H.; Salama, A.D. Steroids as treatment for glomerulonephritis: Time for a rethink. Nephrol. Dial. Transplant. 2022, 37, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Rovin, B.H.; Adler, S.G.; Barratt, J.; Bridoux, F.; Burdge, K.A.; Chan, T.M.; Cook, H.T.; Fervenza, F.C.; Gibson, K.L.; Glassock, R.J.; et al. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021, 100, S1–S276. [Google Scholar] [CrossRef]
- Liu, C.; Hu, Y.; Lin, J.; Fu, H.; Lim, L.Y.; Yuan, Z. Targeting strategies for drug delivery to the kidney: From renal glomeruli to tubules. Med. Res. Rev. 2019, 39, 561–578. [Google Scholar] [CrossRef]
- Marcucci, F.; Lefoulon, F. Active targeting with particulate drug carriers in tumor therapy: Fundamentals and recent progress. Drug Discov. Today 2004, 9, 219–228. [Google Scholar] [CrossRef]
- Benny, O.; Kim, S.-K.; Gvili, K.; Radzishevsky, I.S.; Mor, A.; Verduzco, L.; Menon, L.G.; Black, P.M.; Machluf, M.; Carroll, R.S. In vivo fate and therapeutic efficacy of PF-4/CTF microspheres in an orthotopic human glioblastoma model. FASEB J. 2008, 22, 488–499. [Google Scholar] [CrossRef]
- Lee, S.J.; Park, K.; Oh, Y.-K.; Kwon, S.-H.; Her, S.; Kim, I.-S.; Choi, K.; Lee, S.J.; Kim, H.; Lee, S.G. Tumor specificity and therapeutic efficacy of photosensitizer-encapsulated glycol chitosan-based nanoparticles in tumor-bearing mice. Biomaterials 2009, 30, 2929–2939. [Google Scholar] [CrossRef]
- Tomita, N.; Morishita, R.; Lan, H.Y.; Yamamoto, K.; Hashizume, M.; Notake, M.; Toyosawa, K.; Fujitani, B.; Mu, W.; Nikolic-Paterson, D.J.; et al. In Vivo Administration of a Nuclear Transcription Factor-κB Decoy Suppresses Experimental Crescentic Glomerulonephritis. J. Am. Soc. Nephrol. 2000, 11, 1244–1252. [Google Scholar] [CrossRef]
- Svenskaya, Y.; Garello, F.; Lengert, E.; Kozlova, A.; Verkhovskii, R.; Bitonto, V.; Ruggiero, M.R.; German, S.; Gorin, D.; Terreno, E. Biodegradable polyelectrolyte/magnetite capsules for MR imaging and magnetic targeting of tumors. Nanotheranostics 2021, 5, 362–377. [Google Scholar] [CrossRef]
- Bukreeva, T.V.; Borodina, T.N.; Trushina, D.B. Polyelectrolyte Microcapsules: On the Formation and Possibilities of Regulating Multilayer Structures. Colloid J. 2022, 84, 621–632. [Google Scholar] [CrossRef]
- Sapach, A.Y.; Sindeeva, O.A.; Nesterchuk, M.V.; Tsitrina, A.A.; Mayorova, O.A.; Prikhozhdenko, E.S.; Verkhovskii, R.A.; Mikaelyan, A.S.; Kotelevtsev, Y.V.; Sukhorukov, G.B. Macrophage In Vitro and In Vivo Tracking via Anchored Microcapsules. ACS Appl. Mater. Interfaces 2022, 14, 51579–51592. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Parakhonskiy, B.V.; Skirtach, A.G. A decade of developing applications exploiting the properties of polyelectrolyte multilayer capsules. Chem. Commun. 2023, 59, 7. [Google Scholar] [CrossRef] [PubMed]
- Mikheev, A.V.; Burmistrov, I.A.; Zaitsev, V.B.; Artemov, V.V.; Khmelenin, D.N.; Starchikov, S.S.; Veselov, M.M.; Klyachko, N.L.; Bukreeva, T.V.; Trushina, D.B. Release of TRITC-Dextran from Composite Microcapsules under the Influence of a Low-Frequency Alternating Magnetic Field. J. Surf. Investig. X-ray Synchrotron Neutron Tech. 2022, 16, 7–12. [Google Scholar] [CrossRef]
- Mayorova, O.A.; Sindeeva, O.A.; Lomova, M.V.; Gusliakov, O.I.; Tarakanchikova, Y.V.; Tyutyaev, E.V.; Pinyaev, S.I.; Kulikov, O.A.; German, S.V.; Pyataev, N.A.; et al. Endovascular addressing improves the effectiveness of magnetic targeting of drug carrier. Comparison with the conventional administration method. Nanomed. Nanotechnol. Biol. Med. 2020, 28, 102184. [Google Scholar] [CrossRef]
- Navolokin, N.; German, S.; Bucharskaya, A.; Godage, O.; Zuev, V.; Maslyakova, G.; Pyataev, N.; Zamyshliaev, P.; Zharkov, M.; Terentyuk, G.; et al. Systemic Administration of Polyelectrolyte Microcapsules: Where Do They Accumulate and When? In Vivo and Ex Vivo Study. Nanomaterials 2018, 8, 812. [Google Scholar] [CrossRef]
- Sindeeva, O.A.; Verkhovskii, R.A.; Abdurashitov, A.S.; Voronin, D.V.; Gusliakova, O.I.; Kozlova, A.A.; Mayorova, O.A.; Ermakov, A.V.; Lengert, E.V.; Navolokin, N.A.; et al. Effect of Systemic Polyelectrolyte Microcapsule Administration on the Blood Flow Dynamics of Vital Organs. ACS Biomater. Sci. Eng. 2020, 6, 389–397. [Google Scholar] [CrossRef]
- Verkhovskii, R.; Ermakov, A.; Sindeeva, O.; Prikhozhdenko, E.; Kozlova, A.; Grishin, O.; Makarkin, M.; Gorin, D.; Bratashov, D. Effect of Size on Magnetic Polyelectrolyte Microcapsules Behavior: Biodistribution, Circulation Time, Interactions with Blood Cells and Immune System. Pharmaceutics 2021, 13, 2147. [Google Scholar] [CrossRef]
- Fery, A.; Weinkamer, R. Mechanical properties of micro- and nanocapsules: Single-capsule measurements. Polymer 2007, 48, 7221–7235. [Google Scholar] [CrossRef]
- Kolesnikova, T.A.; Gorin, D.A.; Fernandes, P.; Kessel, S.; Khomutov, G.B.; Fery, A.; Shchukin, D.G.; Möhwald, H. Nanocomposite Microcontainers with High Ultrasound Sensitivity. Adv. Funct. Mater. 2010, 20, 1189–1195. [Google Scholar] [CrossRef]
- Voronin, D.V.; Sindeeva, O.A.; Kurochkin, M.A.; Mayorova, O.; Fedosov, I.V.; Semyachkina-Glushkovskaya, O.; Gorin, D.A.; Tuchin, V.V.; Sukhorukov, G.B. In Vitro and in Vivo Visualization and Trapping of Fluorescent Magnetic Microcapsules in a Bloodstream. ACS Appl. Mater. Interfaces 2017, 9, 6885–6893. [Google Scholar] [CrossRef]
- Prikhozhdenko, E.S.; Gusliakova, O.I.; Kulikov, O.A.; Mayorova, O.A.; Shushunova, N.A.; Abdurashitov, A.S.; Bratashov, D.N.; Pyataev, N.A.; Tuchin, V.V.; Gorin, D.A.; et al. Target delivery of drug carriers in mice kidney glomeruli via renal artery. Balance between efficiency and safety. J. Control Release 2021, 329, 175–190. [Google Scholar] [CrossRef]
- Gusliakova, O.I.; Prikhozhdenko, E.S.; Plastun, V.O.; Mayorova, O.A.; Shushunova, N.A.; Abdurashitov, A.S.; Kulikov, O.A.; Abakumov, M.A.; Gorin, D.A.; Sukhorukov, G.B.; et al. Renal Artery Catheterization for Microcapsules’ Targeted Delivery to the Mouse Kidney. Pharmaceutics 2022, 14, 1056. [Google Scholar] [CrossRef]
- Kakran, M.; Muratani, M.; Tng, W.J.; Liang, H.; Trushina, D.B.; Sukhorukov, G.B.; Ng, H.H.; Antipina, M.N. Layered polymeric capsules inhibiting the activity of RNases for intracellular delivery of messenger RNA. J. Mater. Chem. B 2015, 3, 5842–5848. [Google Scholar] [CrossRef] [PubMed]
- Tarakanchikova, Y.V.; Muslimov, A.R.; Zyuzin, M.V.; Nazarenko, I.; Timin, A.S.; Sukhorukov, G.B.; Lepik, K.V. Layer-by-Layer-Assembled Capsule Size Affects the Efficiency of Packaging and Delivery of Different Genetic Cargo. Part. Part. Syst. Charact. 2021, 38, 2000228. [Google Scholar] [CrossRef]
- Petrak, K. Essential properties of drug-targeting delivery systems. Drug Discov. Today 2005, 10, 1667–1673. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Wen, N.; Xiao, D.; Yan, J.; Xiong, H.; Cai, S.; Liu, Z.; Liu, Y. Aptamer-Based Targeted Drug Delivery Systems: Current Potential and Challenges. Curr. Med. Chem. 2020, 27, 2189–2219. [Google Scholar] [CrossRef] [PubMed]
- Shipunova, V.O.; Shramova, E.I.; Schulga, A.A.; Shilova, M.V.; Deyev, S.M.; Proshkina, G.M. Delivery of Barnase to Cells in Liposomes Functionalized by Her2-Specific DARPin Module. Russ. J. Bioorganic Chem. 2020, 46, 1156–1161. [Google Scholar] [CrossRef]
- Cao, Q.; Han, X.; Li, L. Enhancement of the efficiency of magnetic targeting for drug delivery: Development and evaluation of magnet system. J. Magn. Magn. Mater. 2011, 323, 1919–1924. [Google Scholar] [CrossRef]
- Kim, N.A.; Lim, D.G.; Lim, J.Y.; Kim, K.H.; Jeong, S.H. Comprehensive evaluation of etanercept stability in various concentrations with biophysical assessment. Int. J. Pharm. 2014, 460, 108–118. [Google Scholar] [CrossRef]
- Sergeeva, A.; Sergeev, R.; Lengert, E.; Zakharevich, A.; Parakhonskiy, B.; Gorin, D.; Sergeev, S.; Volodkin, D. Composite Magnetite and Protein Containing CaCO3 Crystals. External Manipulation and Vaterite → Calcite Recrystallization-Mediated Release Performance. ACS Appl. Mater. Interfaces 2015, 7, 21315–21325. [Google Scholar] [CrossRef]
- Compton, S.J.; Jones, C.G. Mechanism of dye response and interference in the Bradford protein assay. Anal. Biochem. 1985, 151, 369–374. [Google Scholar] [CrossRef] [PubMed]
- del Mercato, L.L.; Guerra, F.; Lazzari, G.; Nobile, C.; Bucci, C.; Rinaldi, R. Biocompatible multilayer capsules engineered with a graphene oxide derivative: Synthesis, characterization and cellular uptake. Nanoscale 2016, 8, 7501–7512. [Google Scholar] [CrossRef] [PubMed]
- Parakhonskiy, B.V.; Yashchenok, A.M.; Möhwald, H.; Volodkin, D.; Skirtach, A.G. Release from Polyelectrolyte Multilayer Capsules in Solution and on Polymeric Surfaces. Adv. Mater. Interfaces 2017, 4, 1600273. [Google Scholar] [CrossRef]
- Musin, E.V.; Dubrovskii, A.V.; Kim, A.L.; Tikhonenko, S.A. A Study of the Buffer Capacity of Polyelectrolyte Microcapsules Depending on Their Concentration and the Number of Layers of the Polyelectrolyte Shell. Int. J. Mol. Sci. 2022, 23, 9917. [Google Scholar] [CrossRef] [PubMed]
- Musin, E.V.; Kim, A.L.; Tikhonenko, S.A. Destruction of Polyelectrolyte Microcapsules Formed on CaCO3 Microparticles and the Release of a Protein Included by the Adsorption Method. Polymers 2020, 12, 520. [Google Scholar] [CrossRef] [PubMed]
- Usov, D.; Sukhorukov, G.B. Dextran Coatings for Aggregation Control of Layer-by-Layer Assembled Polyelectrolyte Microcapsules. Langmuir 2010, 26, 12575–12584. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Agarwal, A.; Glushakova, O.Y.; Jorgensen, M.S.; Salgar, S.K.; Poirier, A.; Flotte, T.R.; Croker, B.P.; Madsen, K.M.; Atkinson, M.A.; et al. Gene Delivery in Renal Tubular Epithelial Cells Using Recombinant Adeno-Associated Viral Vectors. J. Am. Soc. Nephrol. 2003, 14, 947–958. [Google Scholar] [CrossRef]
- Yi, H.; Kim, J.; Jung, H.; Rim, Y.A.; Kim, Y.; Jung, S.M.; Park, S.-H.; Ju, J.H. Induced production of anti-etanercept antibody in collagen-induced arthritis. Mol. Med. Rep. 2014, 9, 2301–2308. [Google Scholar] [CrossRef]
- Koca, S.S.; Isik, A.; Ozercan, I.H.; Ustundag, B.; Evren, B.; Metin, K. Effectiveness of etanercept in bleomycin-induced experimental scleroderma. Rheumatology 2007, 47, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Abdurashitov, A.S.; Prikhozhdenko, E.S.; Mayorova, O.A.; Plastun, V.O.; Gusliakova, O.I.; Shushunova, N.A.; Kulikov, O.A.; Tuchin, V.V.; Sukhorukov, G.B.; Sindeeva, O.A. Optical coherence microangiography of the mouse kidney for diagnosis of circulatory disorders. Biomed. Opt. Express 2021, 12, 4467. [Google Scholar] [CrossRef]
- Ermakov, A.V.; Volovetsky, A.B.; Zvyagintcev, A.O.; Trushina, D.B.; Zvyagin, A.V. In vivo study of polyelectrolyte microcarriers loaded with zinc phthalocyanine for image-guided photodynamic therapy. In Proceedings of the Computational Biophysics and Nanobiophotonics; Khlebtsov, B.N., Postnov, D.E., Eds.; SPIE: Bellingham, DC, USA, 2022; p. 43. [Google Scholar]
- Pollak, M.R.; Quaggin, S.E.; Hoenig, M.P.; Dworkin, L.D. The Glomerulus: The Sphere of Influence. Clin. J. Am. Soc. Nephrol. 2014, 9, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Engbjerg, J.S.; Sardella, D.; Bordoni, L.; Trepiccione, F.; Capasso, G.; Østergaard, L.; Rhodes, G.G.; Sandoval, R.; Ring, T.; Molitoris, B.; et al. The Distribution of Blood in Renal Glomerular Capillaries Is a New Physiological Parameter, Which Is Affected by Diabetes and ACE-inhibition. FASEB J. 2019, 33, 748.11. [Google Scholar] [CrossRef]
- Vinogradova, O.I.; Andrienko, D.; Lulevich, V.V.; Nordschild, S.; Sukhorukov, G.B. Young’s Modulus of Polyelectrolyte Multilayers from Microcapsule Swelling. Macromolecules 2004, 37, 1113–1117. [Google Scholar] [CrossRef]
- She, S.; Xu, C.; Yin, X.; Tong, W.; Gao, C. Shape Deformation and Recovery of Multilayer Microcapsules after Being Squeezed through a Microchannel. Langmuir 2012, 28, 5010–5016. [Google Scholar] [CrossRef] [PubMed]
- Volodkin, D.V.; Larionova, N.I.; Sukhorukov, G.B. Protein Encapsulation via Porous CaCO3 Microparticles Templating. Biomacromolecules 2004, 5, 1962–1972. [Google Scholar] [CrossRef] [PubMed]
- Heeman, W.; Steenbergen, W.; van Dam, G.M.; Boerma, E.C. Clinical applications of laser speckle contrast imaging: A review. J. Biomed. Opt. 2019, 24, 1. [Google Scholar] [CrossRef]
- Boas, D.A.; Dunn, A.K. Laser speckle contrast imaging in biomedical optics. J. Biomed. Opt. 2010, 15, 011109. [Google Scholar] [CrossRef]
- Abdurashitov, A.; Bragina, O.; Sindeeva, O.; Sindeev, S.; Semyachkina-Glushkovskaya, O.V.; Tuchin, V.V. Off-axis holographic laser speckle contrast imaging of blood vessels in tissues. J. Biomed. Opt. 2017, 22, 091514. [Google Scholar] [CrossRef]
- Abdurashitov, A.S.; Lychagov, V.V.; Sindeeva, O.A.; Semyachkina-Glushkovskaya, O.V.; Tuchin, V.V. Histogram analysis of laser speckle contrast image for cerebral blood flow monitoring. Front. Optoelectron. 2015, 8, 187–194. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shushunova, N.A.; Mayorova, O.A.; Prikhozhdenko, E.S.; Goryacheva, O.A.; Kulikov, O.A.; Plastun, V.O.; Gusliakova, O.I.; Muslimov, A.R.; Inozemtseva, O.A.; Pyataev, N.A.; et al. Targeted Therapy for Glomerulonephritis Using Arterial Delivery of Encapsulated Etanercept. Int. J. Mol. Sci. 2023, 24, 2784. https://doi.org/10.3390/ijms24032784
Shushunova NA, Mayorova OA, Prikhozhdenko ES, Goryacheva OA, Kulikov OA, Plastun VO, Gusliakova OI, Muslimov AR, Inozemtseva OA, Pyataev NA, et al. Targeted Therapy for Glomerulonephritis Using Arterial Delivery of Encapsulated Etanercept. International Journal of Molecular Sciences. 2023; 24(3):2784. https://doi.org/10.3390/ijms24032784
Chicago/Turabian StyleShushunova, Natalia A., Oksana A. Mayorova, Ekaterina S. Prikhozhdenko, Olga A. Goryacheva, Oleg A. Kulikov, Valentina O. Plastun, Olga I. Gusliakova, Albert R. Muslimov, Olga A. Inozemtseva, Nikolay A. Pyataev, and et al. 2023. "Targeted Therapy for Glomerulonephritis Using Arterial Delivery of Encapsulated Etanercept" International Journal of Molecular Sciences 24, no. 3: 2784. https://doi.org/10.3390/ijms24032784
APA StyleShushunova, N. A., Mayorova, O. A., Prikhozhdenko, E. S., Goryacheva, O. A., Kulikov, O. A., Plastun, V. O., Gusliakova, O. I., Muslimov, A. R., Inozemtseva, O. A., Pyataev, N. A., Shirokov, A. A., Gorin, D. A., Sukhorukov, G. B., & Sindeeva, O. A. (2023). Targeted Therapy for Glomerulonephritis Using Arterial Delivery of Encapsulated Etanercept. International Journal of Molecular Sciences, 24(3), 2784. https://doi.org/10.3390/ijms24032784









