Effects of Temperature and Salt Stress on the Expression of delta-12 Fatty Acid Desaturase Genes and Fatty Acid Compositions in Safflower
Abstract
1. Introduction
2. Results
2.1. Cloning and Functional Analysis of the Safflower FAD6 Gene in Cyanobacterium
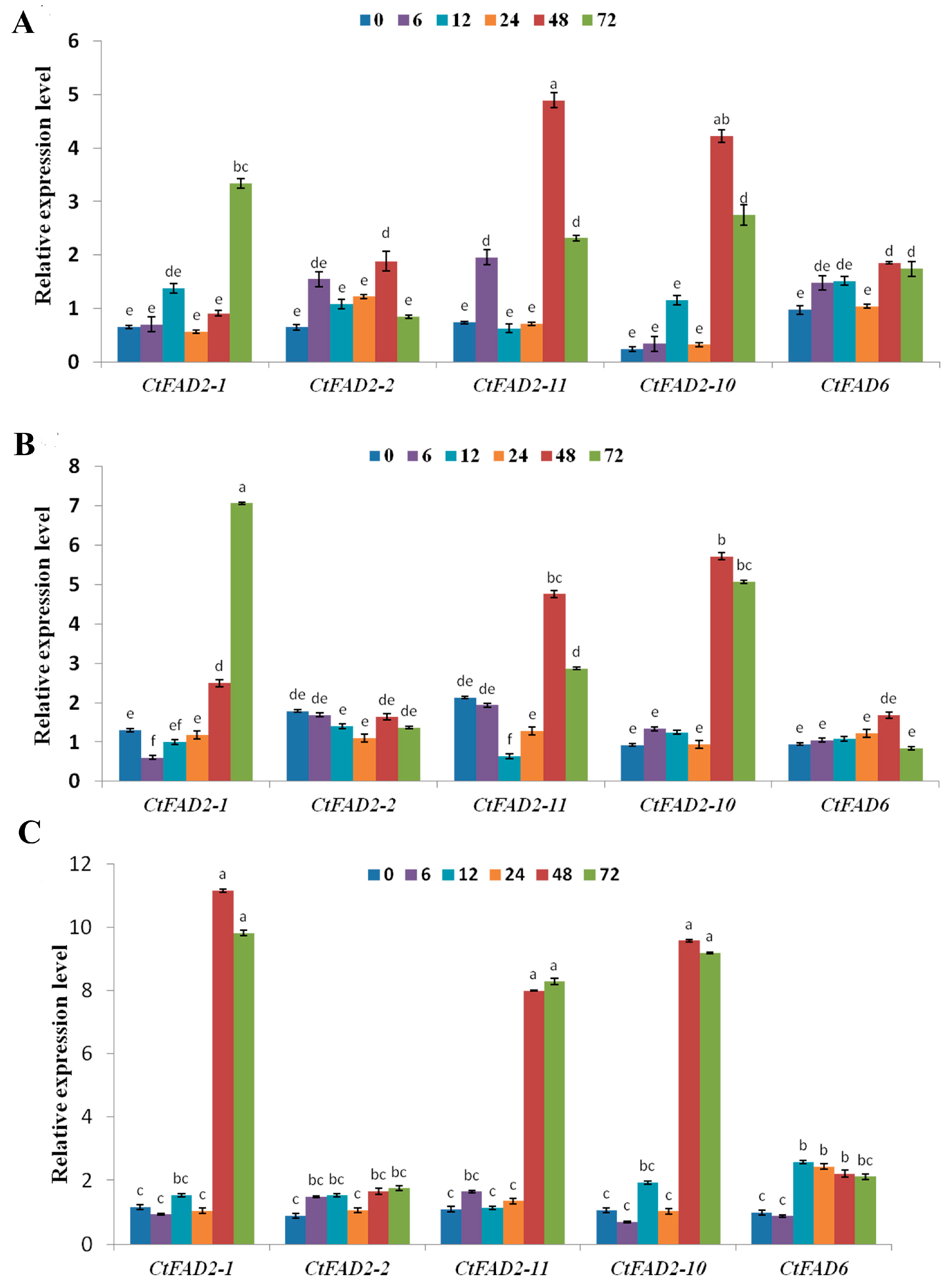
2.2. Safflower Oleate Desaturase Genes Are Transcriptionally Regulated by Temperature
2.3. Safflower Oleate Desaturase Genes Are Regulated by Salt
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Plant Material and Stress Treatments
5.2. Total RNA Extraction and cDNA Synthesis
5.3. Isolation of a Full-Length Plastidial Oleate Desaturase cDNA Clone from Safflower
5.4. Quantitative Real-Time PCR (qRT-PCR)
5.5. Expression and Sequence Analysis of the Safflower CtFAD6 Gene in Cyanobacterium
5.6. Fatty Acid Analysis
5.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cooke, D.T.; Burden, R.S. Lipid modulation of plasma membrane-bound ATPase. Physiol. Plantarum. 2010, 78, 153–159. [Google Scholar] [CrossRef]
- Cao, S.J.; Zhou, X.R.; Wood, C.C.; Green, A.G.; Singh, S.P.; Liu, L.; Liu, Q. A large and functionally diverse family of FAD2 genes in safflower (Carthamus tinctorius L.). BMC Plant Biol. 2013, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Dar, A.A.; Choudhury, A.R.; Kancharla, P.K.; Arumugam, N. The FAD2 Gene in Plants: Occurrence, Regulation, and Role. Front. Plant Sci. 2017, 8, 1789. [Google Scholar] [CrossRef] [PubMed]
- Deuticke, B.; Haest, C.W. Lipid modulation of transport proteins in vertebrate cell membranes. Annu. Rev. Physiol. 1987, 49, 221. [Google Scholar] [CrossRef]
- Guan, L.L. Accumulation pattern and regulatory mechanisms of fatty acid in different safflower (Carthamus tinctorius L.) tissues. Dissertation Thesis, Sichuan Agriculture University, Yaan, China, 2011. [Google Scholar]
- Golden, S.S.; Brusslan, J.; Haselkorn, R. Genetic engineering of the cyanobacterial chromosome. Methods Enzymoll. 1987, 53, 215–231. [Google Scholar]
- Guan, L.L.; Wu, W.; Zheng, Y.L. Seed oil contents and fatty acid compositions of seed oil from different safflower accessions introduced into Yaan and the correlation analysis with the agronomic traits and photosynthetic parameters. Philipp. Agric. Sci. 2008, 91, 383–388. [Google Scholar]
- Guan, L.L.; Xu, Y.W.; Wang, Y.B.; Chen, L.; Shao, J.F.; Wu, W. Isolation and Characterization of a Temperature-Regulated Microsomal Oleate Desaturase Gene (CtFAD2-1) from Safflower (Carthamus tinctorius L.). Plant Mol. Biol. Rep. 2012, 30, 391–402. [Google Scholar] [CrossRef]
- Harwood, J.L. Fatty acid biosynthesis. In Plant Lipids; Murphy, D.J., Ed.; Blackwell Publishing: Oxford, UK, 2005; pp. 27–101. [Google Scholar]
- Hussain, M.I.; Lyra, D.-A.; Farooq, M.; Nikoloudakis, N.; Khalid, N. Salt and drought stresses in safflower: A review. Agron. Sustain. Dev. 2016, 36, 1–31. [Google Scholar] [CrossRef]
- Herńndez, M.L.; Padilla, M.N.; Mancha, M.; Martínez-Rivas, J.M. Expression Analysis Identifies FAD2-2 as the Olive Oleate Desaturase Gene Mainly Responsible for the Linoleic Acid Content in Virgin Olive Oil. J. Agric. Food Chem. 2009, 57, 6199–6206. [Google Scholar] [CrossRef]
- Hernández, M.L.; Padilla, M.N.; Sicardo, M.D.; Mancha, M.; Martínez-Rivas, J.M. Effect of different environmental stresses on the expression of oleate desaturase genes and fatty acid composition in olive fruit. Phytochemistry 2011, 72, 178–187. [Google Scholar] [CrossRef]
- Kargiotidou, A.; Deli, D.; Galanopoulou, D.; Tsaftaris, A.; Farmaki, T. Low temperature and light regulate delta 12 fatty acid desaturases (FAD2) at a transcriptional level in cotton (Gossypium hirsutum). J. Exp. Bot. 2008, 59, 2043. [Google Scholar] [CrossRef]
- Li, D.J. Progress of safflower (Carthamus tinctorius L.) research and production in China. In Proceedings of the Third International Safflower Conference, Beijing, China, 14–18 June 1993; pp. 35–46. [Google Scholar]
- Li, D.; Hu, B.; Wang, Q.; Liu, H.; Pan, F.; Wu, W. Identification and evaluation of reference genes for accurate transcription normalization in safflower under different Experimental Conditions. PLoS ONE 2015, 10, e0140218. [Google Scholar] [CrossRef]
- Los, D.A.; Murata, N. Structure and expression of fatty acid desaturases. Biochim. Biophys. Acta 1998, 1394, 3–15. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Li, D.J.; Zhou, M.D.; Ramanatha, R.V. Characterization and Evaluation of Safflower Germplasm; Geological Publishing House: Beijing, China, 1993; 260p. [Google Scholar]
- Mirzajani, F.; Bernard, F.; Zeinali, S.M.; Goodarzi, R. Identification of hydroxy-safflor yellow A, safflor yellow B, and precarthaminin safflower using LC/ESI–MSMS. J. Food Meas. Charact. 2015, 9, 332–336. [Google Scholar] [CrossRef]
- Okuley, J.; Lightner, J.; Feldmann, K.; Yadav, N.; Lark, E.; Browse, J. Arabidopsis FAD2 gene encodes the enzyme that is essential for polyunsaturated lipid synthesis. Plant Cell 1994, 6, 147–158. [Google Scholar]
- Rodríguez-Vargas, S.; Sánchez-García, A.; Martínez-Rivas, J.M.; Prieto, J.A.; Randez-Gil, F. Fluidization of membrane lipids enhances the tolerance of Saccharomyces cerevisiae to freezing and salt stress. Appl. Environ. Microbiol. 2007, 73, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Somerville, C.; Browse, J. Dissecting desaturation: Plants prove advantageous. Trends Cell Biol. 1996, 6, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Shanklin, J.; Cahoon, E.B. Desaturation and related modifications of fatty acids. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 611–641. [Google Scholar] [CrossRef] [PubMed]
- Weber, H. Fatty acid-derived signals in plants. Trends Plant Sci. 2002, 7, 217–224. [Google Scholar] [CrossRef]
- Wang, X.; Benomoualem, D.; Kobiler, I.; Leikin-Frenkel, A.; Lichter, A.; Prusky, D. Expression of delta 12 fatty acid desaturase during the induced accumulation of the antifungal diene in avocado fruits. Mol. Plant Pathol. 2004, 5, 575. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cao, F.; Zhang, W.; Wang, G.; Yu, W. Cloning and expression of stearoyl-ACP desaturase and two oleate desaturases genes from Ginkgo biloba L. Plant Mol. Biol. Rep. 2013, 31, 633–648. [Google Scholar] [CrossRef]
- Wada, H.; Schmidt, H.; Heinz, E.; Murata, N. In vitro ferredoxin-dependent desaturation of fatty acids in cyanobacterial thylakoid membranes. J. Bacteriol. 1993, 175, 544. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Yin, N.; Chen, B.; Liao, F.; Win, A.N.; Jiang, J.; Wang, R.; Jin, X.; Lin, N.; Chai, Y. Molecular cloning and expression analysis of two FAD2 genes from chia (Salvia hispanica). Acta. Physiol. Plant 2017, 39, 95. [Google Scholar] [CrossRef]
- Xue, Y.; Zhang, X.; Wang, R.; Chen, B.; Jiang, J.; Win, A.N.; Chai, Y. Cloning and expression of Perilla frutescens FAD2 gene and polymorphism analysis among cultivars. Acta. Physiol. Plant 2017, 39, 84. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, H.; Sun, J.; Li, B.; Zhu, Q.; Chen, S.; Zhang, H. Arabidopsis fatty acid desaturase FAD2 is required for salt tolerance during seed germination and early seedling growth. PLoS ONE 2012, 7, e30355. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Wang, C.C.; Hu, H.H.; Yang, L. Cloning and expression of three fatty acid desaturase genes from cold-sensitive lima bean (Phaseolus lunatus L.). Biotechnol Lett. 2011, 33, 395–401. [Google Scholar] [CrossRef]
- Zhang, J.T.; Zhu, J.Q.; Zhu, Q.; Liu, H.; Gao, X.S.; Zhang, H.X. Fatty acid desaturase-6 (FAD6) is required for plant salt tolerance in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2009, 390, 469–474. [Google Scholar] [CrossRef]


| Roots (%) | ||||||||||
| Fatty acids | NT (16 °C) | 4 °C—1 | 4 °C—6 | 4 °C—12 | 10 °C—1 | 10 °C—6 | 10 °C—12 | 24 °C—1 | 24 °C—6 | 24 °C—12 |
| Linoleic acid (18:2) | 4.306 ± 0.144 a | 4.510 ± 0.190 a | 4.514 ± 0.121 a | 4.676 ± 0.184 a | 4.353 ± 0.193 a | 4.321 ± 0.113 a | 4.597 ± 0.177 a | 4.467 ± 0.158 a | 4.090 ± 0.193 a | 4.282 ± 0.875 a |
| Linolenic acid (18:3) | 2.969 ± 0.321 b | 3.337 ± 0.154 ab | 3.614 ± 0.231 ab | 4.368 ± 0.120 a | 3.007 ± 0.156 b | 2.684 ± 0.032 b | 3.162 ± 0.188 b | 3.181 ± 0.056 b | 2.567 ± 0.012 b | 2.951 ± 0.024 b |
| palmitic acid (16:0) | 53.953 ± 0.022 b | 61.275 ± 0.026 a | 60.066 ± 0.009 a | 60.658 ± 0.005 a | 61.212 ± 0.165 a | 61.746 ± 0.165 a | 61.087 ± 0.199 a | 61.549 ± 0.078 a | 61.258 ± 0.482 a | 61.297 ± 0.320 a |
| stearic acid (18:0) | 38.773 ± 0.175 a | 30.879 ± 0.018 b | 31.807 ± 0.568 b | 30.109 ± 0.062 b | 30.934 ± 0.224 b | 31.588 ± 0.226 b | 31.072 ± 0.210 b | 30.804 ± 0.261 b | 31.086 ± 0.889 b | 31.471 ± 0.124 b |
| Stems (%) | ||||||||||
| Fatty acids | NT (16 °C) | 4 °C—1 | 4 °C—6 | 4 °C—12 | 10 °C—1 | 10 °C—6 | 10 °C—12 | 24 °C—1 | 24 °C—6 | 24 °C—12 |
| Linoleic acid (18:2) | 4.284 ± 0.185 ab | 4.897 ± 0.097 a | 4.690 ± 0.253 a | 4.988 ± 0.227 a | 3.79 ± 0.281 b | 4.229 ± 0.237 ab | 4.572 ± 0.193 a | 4.433 ± 0.164 a | 4.88 ± 0.056 a | 4.919 ± 0.187 a |
| Linolenic acid (18:3) | 2.474 ± 0.077 b | 3.414 ± 0.077 a | 3.258 ± 0.262 a | 3.549 ± 0.056 a | 2.926 ± 0.281 ab | 2.810 ± 0.195 ab | 2.707 ± 0.322 ab | 2.664 ± 0.617 ab | 4.038 ± 0.356 a | 3.984 ± 0.170 a |
| palmitic acid (16:0) | 61.869 ± 0.056 a | 60.805 ± 0.232 b | 60.997 ± 0.256 b | 60.809 ± 0.003 b | 62.237 ± 0.233 a | 62.286 ± 0.002 a | 61.961 ± 0.310 a | 62.585 ± 0.352 a | 60.777 ± 0.338 b | 60.870 ± 0.586 b |
| stearic acid (18:0) | 30.727 ± 0.354 a | 30.885 ± 0.321 a | 31.055 ± 0.322 a | 30.655 ± 0.008 a | 31.097 ± 0.552 a | 30.576 ± 0.114 a | 30.761 ± 0.284 a | 30.619 ± 0.214 a | 30.443 ± 0.258 a | 31.030 ± 0.362 a |
| Leaves (%) | ||||||||||
| Fatty acids | NT (16 °C) | 4 °C—1 | 4 °C—6 | 4 °C—12 | 10 °C—1 | 10 °C—6 | 10 °C—12 | 24 °C—1 | 24 °C—6 | 24 °C—12 |
| Linoleic acid (18:2) | 5.009 ± 0.079 c | 7.602 ± 0.058 a | 5.980 ± 0.237 b | 7.363 ± 0.346 a | 4.678 ± 0.232 c | 5.558 ± 0.213 bc | 5.597 ± 0.177 bc | 6.011 ± 0.071 b | 5.836 ± 0.075 b | 6.862 ± 0.224 ab |
| Linolenic acid (18:3) | 20.575 ± 0.321 c | 23.044 ± 0.612 a | 23.434 ± 0.234 a | 22.217 ± 0.131 ab | 17.669 ± 0.543 d | 20.859 ± 0.320 c | 17.618 ± 0.156 d | 22.961 ± 0.231 a | 23.009 ± 0.304 a | 23.558 ± 0.125 a |
| palmitic acid (16:0) | 45.722 ± 0.889 d | 45.856 ± 0.907 d | 47.649 ± 0.256 c | 47.215 ± 0.078 c | 52.384 ± 0.127 a | 48.996 ± 0.482 c | 50.716 ± 1.26 b | 48.161 ± 0.226 c | 48.106 ± 0.178 c | 47.739 ± 0.056 c |
| stearic acid (18:0) | 26.514 ± 0.299 a | 23.500 ± 0.470 c | 22.938 ± 0.455 c | 23.206 ± 0.019 c | 25.570 ± 0.119 ab | 26.388 ± 0.889 ab | 26.071 ± 0.262 a | 22.869 ± 0.084 c | 22.864 ± 0.300 c | 22.842 ± 0.124 c |
| Roots (%) | ||||||
| Fatty acids | CK | Salt—6 h | Salt—12 h | Salt—24 h | Salt—48 h | Salt—72 h |
| Linoleic acid (18:2) | 2.259 ± 0.088 b | 2.250 ± 0.025 b | 2.212 ± 0.002 b | 2.738 ± 0.047 ab | 3.573 ± 0.332 a | 3.602 ± 0.049 a |
| Linolenic acid (18:3) | 1.038 ± 0.024b | 1.012 ± 0.009 b | 1.038 ± 0.007 b | 1.445 ± 0.008 ab | 1.937 ± 0.019 a | 1.241 ± 0.002 b |
| palmitic acid (16:0) | 58.407 ± 0.013 a | 58.056 ± 0.012 a | 58.010 ± 0.137 a | 57.972 ± 0.010 a | 58.942 ± 0.007 a | 58.515 ± 0.029 a |
| stearic acid (18:0) | 38.296 ± 0.006 a | 38.682 ± 0.177 a | 38.74 ± 0.021 a | 37.415 ± 0.002 ab | 37.078 ± 0.006 b | 37.642 ± 0.004 b |
| Stems (%) | ||||||
| Fatty acids | CK | Salt—6 h | Salt—12 h | Salt—24 h | Salt—48 h | Salt—72 h |
| Linoleic acid (18:2) | 3.056 ± 0.200 b | 3.122 ± 0.087 b | 3.800 ± 0.061 ab | 3.707 ± 0.185 ab | 4.033 ± 0.065 a | 4.205 ± 0.046 a |
| Linolenic acid (18:3) | 1.297 ± 0.332 b | 1.255 ± 0.133 b | 1.219 ± 0.081 b | 2.254 ± 0.066 a | 2.450 ± 0.016 a | 2.576 ± 0.018 a |
| palmitic acid (16:0) | 57.529 ± 0.000 a | 57. 004 ± 0.009 a | 57.027 ± 0.008 a | 57.890 ± 0.001 a | 57.370 ± 0.013 a | 56.776 ± 0.003 ab |
| stearic acid (18:0) | 38.118 ± 0.008 a | 38.619 ± 0.007 a | 37.954 ± 0.173 a | 36.149 ± 0.058 b | 36.147 ± 0.010 b | 36.443 ± 0.002 b |
| Leaves (%) | ||||||
| Fatty acids | CK | Salt—6 h | Salt—12 h | Salt—24 h | Salt—48 h | Salt—72 h |
| Linoleic acid (18:2) | 5.263 ± 0.173 a | 5.168 ± 0.055 a | 5.566 ± 0.250 a | 5.325 ± 0.606 a | 5.503 ± 0.183 a | 5.038 ± 0.166 a |
| Linolenic acid (18:3) | 16.262 ± 0.107 c | 16.986 ± 0.157 c | 16.563 ± 0.065 c | 22.794 ± 0.359 a | 20.703 ± 0.369 b | 20.750 ± 0.262 b |
| palmitic acid (16:0) | 50.259 ± 0.008 a | 47.663 ± 0.004 ab | 49.556 ± 0.002 a | 43.544 ± 0. 008 c | 49.336 ± 0.025 a | 50.716 ± 0.009 a |
| stearic acid (18:0) | 29.063 ± 0.012 ab | 30.183 ± 0.002 a | 28.315 ± 0. 008 b | 28.337 ± 0.001 b | 22.458 ± 0.024 c | 23.496 ± 0.889 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Li, K.; Zhou, G.; He, S. Effects of Temperature and Salt Stress on the Expression of delta-12 Fatty Acid Desaturase Genes and Fatty Acid Compositions in Safflower. Int. J. Mol. Sci. 2023, 24, 2765. https://doi.org/10.3390/ijms24032765
Li D, Li K, Zhou G, He S. Effects of Temperature and Salt Stress on the Expression of delta-12 Fatty Acid Desaturase Genes and Fatty Acid Compositions in Safflower. International Journal of Molecular Sciences. 2023; 24(3):2765. https://doi.org/10.3390/ijms24032765
Chicago/Turabian StyleLi, Dandan, Kaijie Li, Guangchong Zhou, and Songtao He. 2023. "Effects of Temperature and Salt Stress on the Expression of delta-12 Fatty Acid Desaturase Genes and Fatty Acid Compositions in Safflower" International Journal of Molecular Sciences 24, no. 3: 2765. https://doi.org/10.3390/ijms24032765
APA StyleLi, D., Li, K., Zhou, G., & He, S. (2023). Effects of Temperature and Salt Stress on the Expression of delta-12 Fatty Acid Desaturase Genes and Fatty Acid Compositions in Safflower. International Journal of Molecular Sciences, 24(3), 2765. https://doi.org/10.3390/ijms24032765





