Machine Learning Quantified Tumor-Stroma Ratio Is an Independent Prognosticator in Muscle-Invasive Bladder Cancer
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
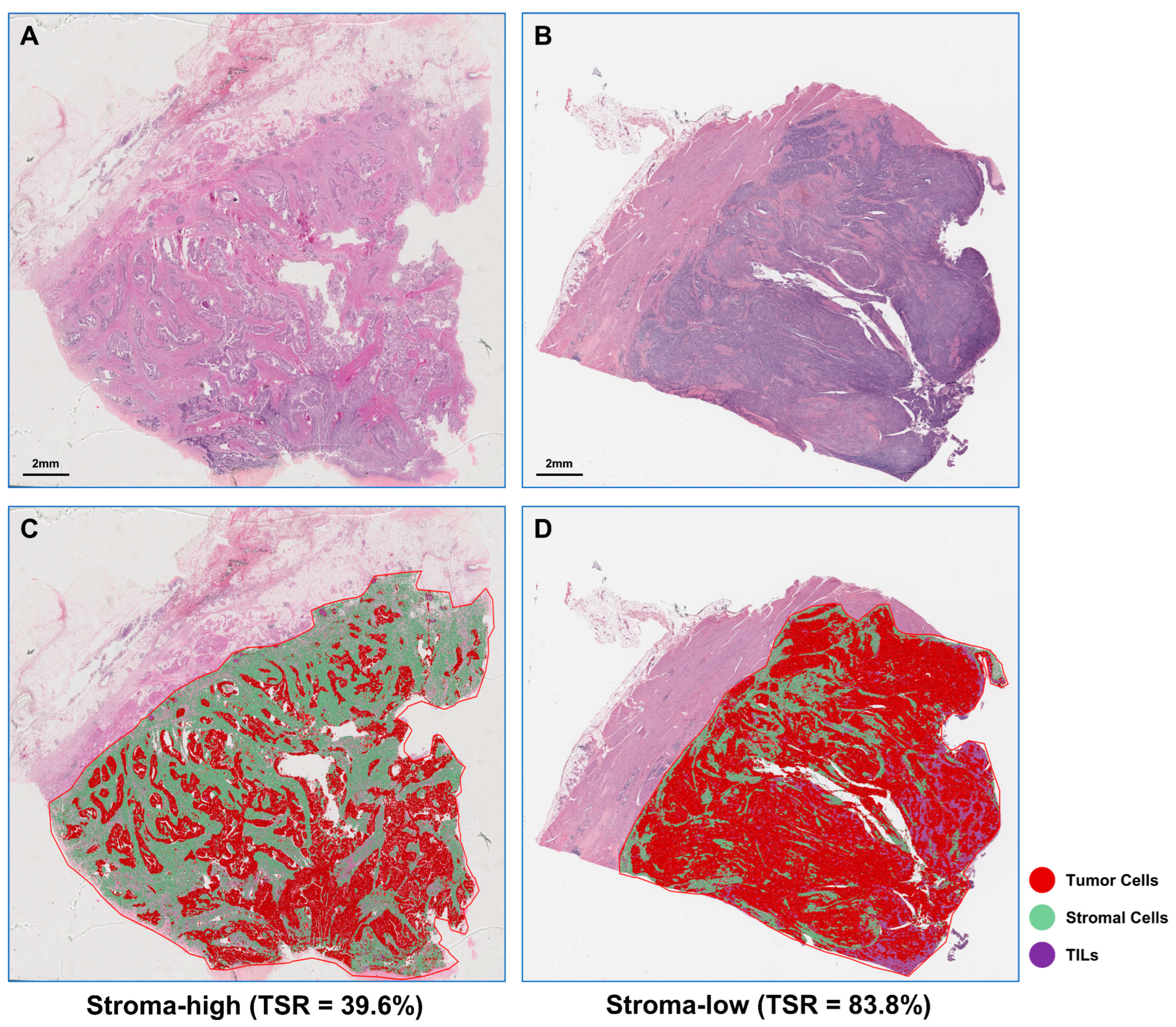
2.2. TSR Automated Assessment
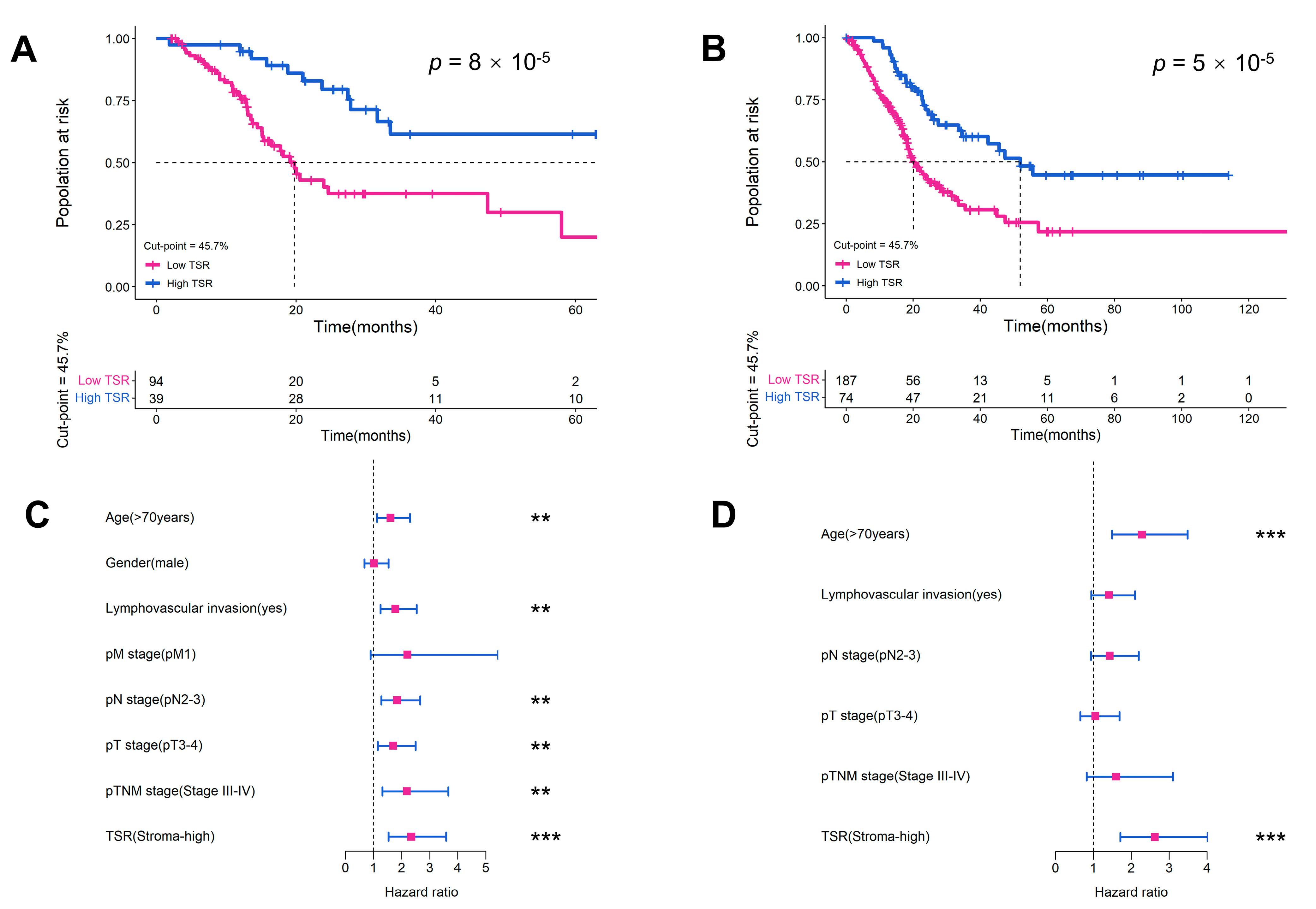
2.3. Evaluation of TSR as a Prognostic Variable in Two Cohorts
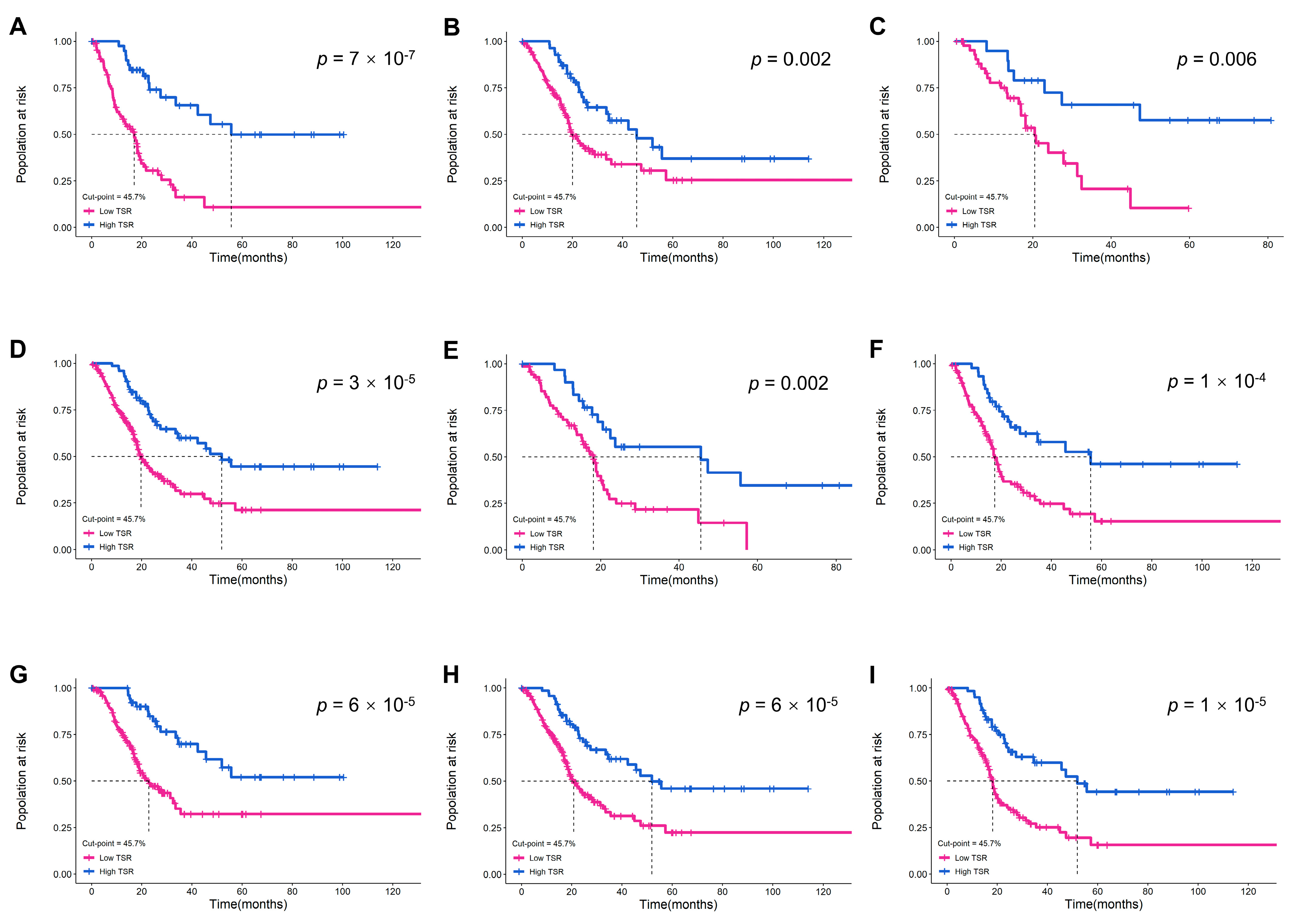
2.4. Validation of the Prognostic Value of TSR in Different Subgroups
2.5. Gene Expression Correlation with TSR
3. Discussion
4. Materials and Methods
4.1. Patient Cohorts
4.2. Ethics
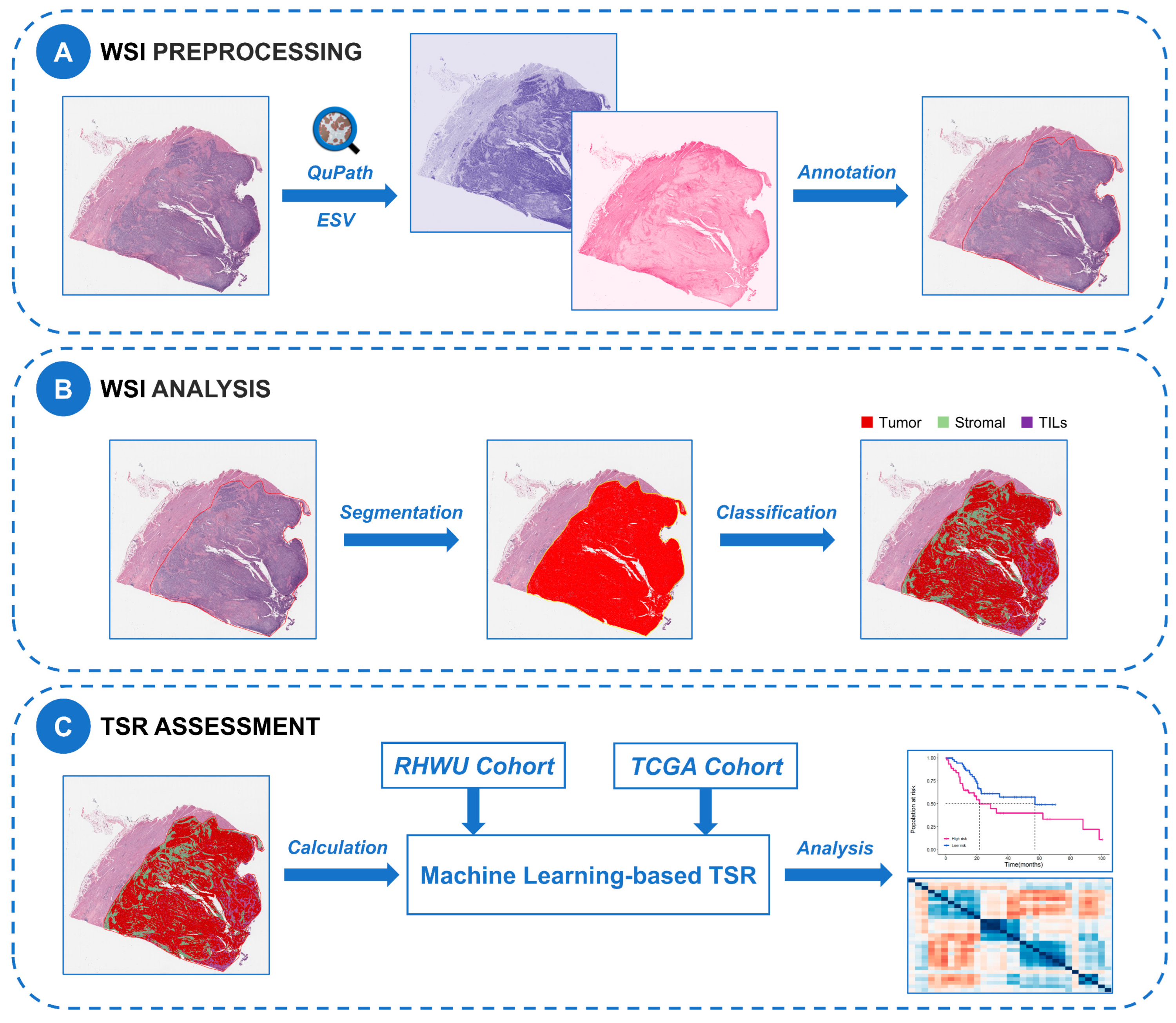
4.3. WSI Preprocessing
4.4. WSI Image Analysis
4.5. TSR Assessment
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ploussard, G.; Shariat, S.F.; Dragomir, A.; Kluth, L.A.; Xylinas, E.; Masson-Lecomte, A.; Rieken, M.; Rink, M.; Matsumoto, K.; Kikuchi, E.; et al. Conditional survival after radical cystectomy for bladder cancer: Evidence for a patient changing risk profile over time. Eur. Urol. 2014, 66, 361–370. [Google Scholar]
- Hautmann, R.E.; de Petriconi, R.C.; Pfeiffer, C.; Volkmer, B.G. Radical cystectomy for urothelial carcinoma of the bladder without neoadjuvant or adjuvant therapy: Long-term results in 1100 patients. Eur. Urol. 2012, 61, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Nuhn, P.; May, M.; Sun, M.; Fritsche, H.M.; Brookman-May, S.; Buchner, A.; Bolenz, C.; Moritz, R.; Herrmann, E.; Burger, M.; et al. External validation of postoperative nomograms for prediction of all-cause mortality, cancer-specific mortality, and recurrence in patients with urothelial carcinoma of the bladder. Eur. Urol. 2012, 61, 58–64. [Google Scholar]
- Rouprêt, M.; Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Cowan, N.C.; Dominguez-Escrig, J.L.; Gontero, P.; Hugh, M.A.; et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2020 Update. Eur. Urol. 2021, 79, 62–79. [Google Scholar] [CrossRef]
- Warrick, J.I.; Sjödahl, G.; Kaag, M.; Raman, J.D.; Merrill, S.; Shuman, L.; Chen, G.; Walter, V.; Degraff, D.J. Intratumoral heterogeneity of bladder cancer by molecular subtypes and histologic variants. Eur. Urol. 2019, 75, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Advani, D.; Sharma, S.; Ambasta, R.K.; Kumar, P. Combinatorial therapy in tumor microenvironment: Where do we stand? Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188585. [Google Scholar] [CrossRef]
- Hui, L.; Chen, Y. Tumor microenvironment: Sanctuary of the devil. Cancer Lett. 2015, 368, 7–13. [Google Scholar]
- Vangangelt, K.; Tollenaar, L.; van Pelt, G.W.; de Kruijf, E.M.; Dekker, T.; Kuppen, P.; Tollenaar, R.; Mesker, W.E. The prognostic value of tumor-stroma ratio in tumor-positive axillary lymph nodes of breast cancer patients. Int. J. Cancer 2018, 143, 3194–3200. [Google Scholar] [CrossRef]
- Zong, L.; Zhang, Q.; Kong, Y.; Yang, F.; Zhou, Y.; Yu, S.; Wu, M.; Chen, J.; Zhang, Y.; Xiang, Y. The tumor-stroma ratio is an independent predictor of survival in patients with 2018 FIGO stage IIIC squamous cell carcinoma of the cervix following primary radical surgery. Gynecol. Oncol. 2020, 156, 676–681. [Google Scholar]
- Huijbers, A.; Tollenaar, R.A.; V, P.G.; Zeestraten, E.C.; Dutton, S.; Mcconkey, C.C.; Domingo, E.; Smit, V.T.; Midgley, R.; Warren, B.F.; et al. The proportion of tumor-stroma as a strong prognosticator for stage II and III colon cancer patients: Validation in the VICTOR trial. Ann. Oncol. 2013, 24, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Karpathiou, G.; Vieville, M.; Gavid, M.; Camy, F.; Dumollard, J.M.; Magné, N.; Froudarakis, M.; Prades, J.M.; Peoc’H, M. Prognostic significance of tumor budding, tumor-stroma ratio, cell nests size, and stroma type in laryngeal and pharyngeal squamous cell carcinomas. Head Neck 2019, 41, 1918–1927. [Google Scholar] [CrossRef] [PubMed]
- Efstathiou, J.A.; Mouw, K.W.; Gibb, E.A.; Liu, Y.; Wu, C.L.; Drumm, M.R.; Da, C.J.; du Plessis, M.; Wang, N.Q.; Davicioni, E.; et al. Impact of immune and stromal infiltration on outcomes following Bladder-Sparing trimodality therapy for Muscle-Invasive bladder cancer. Eur. Urol. 2019, 76, 59–68. [Google Scholar] [CrossRef] [PubMed]
- van Pelt, G.W.; Sandberg, T.P.; Morreau, H.; Gelderblom, H.; van Krieken, J.; Tollenaar, R.; Mesker, W.E. The tumour-stroma ratio in colon cancer: The biological role and its prognostic impact. Histopathology 2018, 73, 197–206. [Google Scholar] [CrossRef]
- Liu, J.; Li, C.; Huang, K.; Aldanakh, A.; Yang, D.; Wang, J.; Sun, X.; Song, X. A classification based on tumor-stroma ratio and tumor budding for patients with muscle-invasive bladder cancer. Expert Rev. Anticancer. Ther. 2022, 22, 323–330. [Google Scholar] [CrossRef]
- van Pelt, G.W.; Kjær-Frifeldt, S.; van Krieken, J.; Al, D.R.; Morreau, H.; Tollenaar, R.; Sørensen, F.B.; Mesker, W.E. Scoring the tumor-stroma ratio in colon cancer: Procedure and recommendations. Virchows Arch. 2018, 473, 405–412. [Google Scholar] [CrossRef]
- Niazi, M.; Parwani, A.V.; Gurcan, M.N. Digital pathology and artificial intelligence. Lancet Oncol. 2019, 20, e253–e261. [Google Scholar] [CrossRef]
- Xu, H.; Cong, F.; Hwang, T.H. Machine learning and artificial intelligence-driven spatial analysis of the tumor immune microenvironment in pathology slides. Eur. Urol. Focus 2021, 7, 706–709. [Google Scholar] [CrossRef]
- Liang, N.; Li, B.; Jia, Z.; Wang, C.; Wu, P.; Zheng, T.; Wang, Y.; Qiu, F.; Wu, Y.; Su, J.; et al. Ultrasensitive detection of circulating tumour DNA via deep methylation sequencing aided by machine learning. Nat. Biomed. Eng. 2021, 5, 586–599. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, N.; Jiang, L.; Gao, F.; Shao, J.; Wang, T.; Zhang, E.; Yu, H.; Wang, X.; Zheng, J. Clinical use of a machine learning histopathological image signature in diagnosis and survival prediction of clear cell renal cell carcinoma. Int. J. Cancer 2021, 148, 780–790. [Google Scholar] [CrossRef]
- Acs, B.; Ahmed, F.S.; Gupta, S.; Wong, P.F.; Gartrell, R.D.; Sarin, P.J.; Rizk, E.M.; Gould, R.B.; Saenger, Y.M.; Rimm, D.L. An open source automated tumor infiltrating lymphocyte algorithm for prognosis in melanoma. Nat. Commun. 2019, 10, 5440. [Google Scholar] [CrossRef]
- Ding, W.; Chen, G.; Shi, T. Integrative analysis identifies potential DNA methylation biomarkers for pan-cancer diagnosis and prognosis. Epigenetics 2019, 14, 67–80. [Google Scholar] [CrossRef]
- Yan, D.; Ju, X.; Luo, B.; Guan, F.; He, H.; Yan, H.; Yuan, J. Tumour stroma ratio is a potential predictor for 5-year disease-free survival in breast cancer. BMC Cancer 2022, 22, 1082. [Google Scholar] [CrossRef]
- Millar, E.K.; Browne, L.H.; Beretov, J.; Lee, K.; Lynch, J.; Swarbrick, A.; Graham, P.H. Tumour stroma ratio assessment using digital image analysis predicts survival in triple negative and luminal breast cancer. Cancers 2020, 12, 3749. [Google Scholar] [CrossRef]
- Jakab, A.; Patai, Á.V.; Micsik, T. Digital image analysis provides robust tissue microenvironment-based prognosticators in patients with stage I-IV colorectal cancer. Hum. Pathol. 2022, 128, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Geessink, O.; Baidoshvili, A.; Klaase, J.M.; Ehteshami, B.B.; Litjens, G.; van Pelt, G.W.; Mesker, W.E.; Nagtegaal, I.D.; Ciompi, F.; van der Laak, J. Computer aided quantification of intratumoral stroma yields an independent prognosticator in rectal cancer. Cell Oncol. 2019, 42, 331–341. [Google Scholar] [CrossRef]
- Zheng, Q.; Yang, R.; Ni, X.; Yang, S.; Jiao, P.; Wu, J.; Xiong, L.; Wang, J.; Jian, J.; Jiang, Z. Quantitative assessment of Tumor-Infiltrating lymphocytes using machine learning predicts survival in Muscle-Invasive bladder cancer. J. Clin. Med. 2022, 11, 7081. [Google Scholar] [CrossRef] [PubMed]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; Mcart, D.G.; Dunne, P.D.; Mcquaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Ghandour, R.; Singla, N.; Lotan, Y. Treatment options and outcomes in nonmetastatic muscle invasive bladder cancer. Trends Cancer 2019, 5, 426–439. [Google Scholar] [CrossRef]
- Lenis, A.T.; Lec, P.M.; Chamie, K.; Mshs, M.D. Bladder cancer: A review. JAMA 2020, 324, 1980–1991. [Google Scholar] [CrossRef] [PubMed]
- Grossman, H.B.; Natale, R.B.; Tangen, C.M.; Speights, V.O.; Vogelzang, N.J.; Trump, D.L.; Devere, W.R.; Sarosdy, M.F.; Wood, D.J.; Raghavan, D.; et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N. Engl. J. Med. 2003, 349, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, P.A.; Moch, H.; Cubilla, A.L.; Ulbright, T.M.; Reuter, V.E. The 2016 WHO classification of tumours of the urinary system and male genital Organs-Part b: Prostate and bladder tumours. Eur. Urol. 2016, 70, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Yuan, L.; Ma, B.; Wang, G.; Tian, Y. Tumour microenvironment (TME) characterization identified prognosis and immunotherapy response in muscle-invasive bladder cancer (MIBC). Cancer Immunol. Immunother. 2021, 70, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Shen, Z.; Deng, Z.; Mei, L. Impact of Tumor-Stroma ratio on the prognosis of colorectal cancer: A systematic review. Front. Oncol. 2021, 11, 738080. [Google Scholar] [CrossRef]
- Xi, K.X.; Wen, Y.S.; Zhu, C.M.; Yu, X.Y.; Qin, R.Q.; Zhang, X.W.; Lin, Y.B.; Rong, T.H.; Wang, W.D.; Chen, Y.Q.; et al. Tumor-stroma ratio (TSR) in non-small cell lung cancer (NSCLC) patients after lung resection is a prognostic factor for survival. J. Thorac. Dis. 2017, 9, 4017–4026. [Google Scholar] [CrossRef]
- Zhang, R.; Song, W.; Wang, K.; Zou, S. Tumor-stroma ratio(TSR) as a potential novel predictor of prognosis in digestive system cancers: A meta-analysis. Clin. Chim. Acta 2017, 472, 64–68. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, R.; Zargari, A.; Thai, T.C.; Gunderson, C.C.; Moxley, K.M.; Liu, H.; Zheng, B.; Qiu, Y. Classification of tumor epithelium and stroma by exploiting image features learned by deep convolutional neural networks. Ann. Biomed. Eng. 2018, 46, 1988–1999. [Google Scholar] [CrossRef]
- Fancello, L.; Gandini, S.; Pelicci, P.G.; Mazzarella, L. Tumor mutational burden quantification from targeted gene panels: Major advancements and challenges. J. Immunother. Cancer 2019, 7, 183. [Google Scholar] [CrossRef]
- Chan, T.A.; Yarchoan, M.; Jaffee, E.; Swanton, C.; Quezada, S.A.; Stenzinger, A.; Peters, S. Development of tumor mutation burden as an immunotherapy biomarker: Utility for the oncology clinic. Ann. Oncol. 2019, 30, 44–56. [Google Scholar] [CrossRef]
- Patel, S.P.; Kurzrock, R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol. Cancer Ther. 2015, 14, 847–856. [Google Scholar] [CrossRef]
- Chu, Q.; Han, N.; Yuan, X.; Nie, X.; Wu, H.; Chen, Y.; Guo, M.; Yu, S.; Wu, K. DACH1 inhibits cyclin D1 expression, cellular proliferation and tumor growth of renal cancer cells. J. Hematol. Oncol. 2014, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Aman, S.; Li, Y.; Cheng, Y.; Yang, Y.; Lv, L.; Li, B.; Xia, K.; Li, S.; Wu, H. DACH1 inhibits breast cancer cell invasion and metastasis by down-regulating the transcription of matrix metalloproteinase 9. Cell Death Discov. 2021, 7, 351. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, L.; Li, Y.; Ma, X.; Dai, W.; Gao, X.; Rao, X.; Fu, G.; Wang, R.; Pan, M.; et al. Organoid modelling identifies that DACH1 functions as a tumour promoter in colorectal cancer by modulating BMP signalling. EBioMedicine 2020, 56, 102800. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Wang, D.; Yang, X.; Wang, A.; Lin, Y.; Zheng, M.; Zhang, H.; Sang, X.; Wang, H.; Hu, K.; et al. Identification of NOTCH4 mutation as a response biomarker for immune checkpoint inhibitor therapy. BMC Med. 2021, 19, 154. [Google Scholar] [CrossRef]
- Seruggia, D.; Oti, M.; Tripathi, P.; Canver, M.C.; Leblanc, L.; Di Giammartino, D.C.; Bullen, M.J.; Nefzger, C.M.; Sun, Y.; Farouni, R.; et al. TAF5L and TAF6L maintain Self-Renewal of embryonic stem cells via the MYC regulatory network. Mol. Cell 2019, 74, 1148–1163. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yue, Y.; Pan, M.; Sun, J.; Chu, J.; Lin, X.; Xu, W.; Feng, L.; Chen, Y.; Chen, D.; et al. Histone deacetylase 3 inhibits new tumor suppressor gene DTWD1 in gastric cancer. Am. J. Cancer Res. 2015, 5, 663–673. [Google Scholar]
- Son, H.J.; Choi, E.J.; Yoo, N.J.; Lee, S.H. Inactivating mutations of tumor suppressor genes KLOTHO and DTWD1 in colorectal cancers. Pathol. Res. Pract. 2020, 216, 152816. [Google Scholar] [CrossRef]
- Nakamura, N.; Kimura, Y.; Tokuda, M.; Honda, S.; Hirose, S. MARCH-V is a novel mitofusin 2- and Drp1-binding protein able to change mitochondrial morphology. EMBO Rep. 2006, 7, 1019–1022. [Google Scholar] [CrossRef]
- Hu, J.; Meng, Y.; Zhang, Z.; Yan, Q.; Jiang, X.; Lv, Z.; Hu, L. MARCH5 RNA promotes autophagy, migration, and invasion of ovarian cancer cells. Autophagy 2017, 13, 333–344. [Google Scholar] [CrossRef]
- Paner, G.P.; Stadler, W.M.; Hansel, D.E.; Montironi, R.; Lin, D.W.; Amin, M.B. Updates in the eighth edition of the tumor-node-metastasis staging classification for urologic cancers. Eur. Urol. 2018, 73, 560–569. [Google Scholar] [CrossRef]
- Camp, R.L.; Dolled-Filhart, M.; Rimm, D.L. X-tile: A new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin. Cancer Res. 2004, 10, 7252–7259. [Google Scholar] [CrossRef] [PubMed]


| RHWU (N = 133) | TCGA (N = 261) | |
|---|---|---|
| Age (years) | 66 (26, 87) | 69 (37, 90) |
| Sex | ||
| female | 20 (15.04%) | 64 (24.52%) |
| male | 113 (84.96%) | 197 (75.48%) |
| pT stage | ||
| pT2 | 52 (39.10%) | 75 (28.74%) |
| pT3 | 63 (47.37%) | 141 (54.02%) |
| pT4 | 18 (13.53%) | 40 (15.33%) |
| pTx | 0 (0%) | 5 (1.92%) |
| pN stage | ||
| pN0 | 66 (49.62%) | 151 (57.85%) |
| pN1 | 34 (25.56%) | 34 (13.03%) |
| pN2 | 18 (13.53%) | 58 (22.22%) |
| pN3 | 15 (11.28%) | 5 (1.92%) |
| pNx | 0 (0%) | 13 (4.98%) |
| pM stage | ||
| pM0 | 129 (96.99%) | 112 (42.91%) |
| pM1 | 4 (3.01%) | 7 (2.68%) |
| pMx | 0 (0%) | 142 (54.41%) |
| pTNM stage | ||
| Stage II | 38 (28.57%) | 65 (24.90%) |
| Stage III | 74 (55.64%) | 95 (36.40%) |
| Stage IV | 21 (15.79%) | 101 (38.70%) |
| Lymphovascular invasion | ||
| No | 83 (62.41%) | 80 (30.65%) |
| Yes | 50 (37.59%) | 102 (39.08%) |
| Missing | 0 (0%) | 79 (30.27%) |
| Survival status | ||
| Alive | 77 (57.89%) | 137 (52.49%) |
| Dead | 56 (42.11%) | 124 (47.51%) |
| OS time (months) | 15.3 (1.9, 66.0) | 17.4 (0, 132.7) |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age | ||||
| <70 | Ref. | Ref. | ||
| ≥70 | 1.610 (1.127, 2.298) | 0.009 | 2.281 (1.493, 3.487) | <0.001 |
| Gender | ||||
| female | Ref. | |||
| male | 1.020 (0.679, 1.533) | 0.923 | ||
| pT stage | ||||
| pT1-2 | Ref. | Ref. | ||
| pT3-4 | 1.700 (1.157, 2.499) | 0.007 | 1.052 (0.654, 1.692) | 0.552 |
| pN stage | ||||
| pN0-1 | Ref. | Ref. | ||
| pN2-3 | 1.842 (1.275, 2.662) | 0.001 | 1.433 (0.934, 2.197) | 0.142 |
| pM stage | ||||
| pM0 | Ref. | |||
| pM1 | 2.211 (0.899, 5.439) | 0.084 | ||
| pTNM stage | ||||
| Stage II | Ref. | Ref. | ||
| Stage III-IV | 2.193 (1.314, 3.660) | 0.003 | 1.598 (0.826, 3.094) | 0.138 |
| Lymphovascular invasion | ||||
| No | Ref. | Ref. | ||
| Yes | 1.779 (1.249, 2.533) | 0.001 | 1.410 (0.947, 2.100) | 0.070 |
| TSR | ||||
| Stroma-low | Ref. | Ref. | ||
| Stroma-high | 2.346 (1.537, 3.581) | <0.001 | 2.622 (1.712, 4.015) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Q.; Jiang, Z.; Ni, X.; Yang, S.; Jiao, P.; Wu, J.; Xiong, L.; Yuan, J.; Wang, J.; Jian, J.; et al. Machine Learning Quantified Tumor-Stroma Ratio Is an Independent Prognosticator in Muscle-Invasive Bladder Cancer. Int. J. Mol. Sci. 2023, 24, 2746. https://doi.org/10.3390/ijms24032746
Zheng Q, Jiang Z, Ni X, Yang S, Jiao P, Wu J, Xiong L, Yuan J, Wang J, Jian J, et al. Machine Learning Quantified Tumor-Stroma Ratio Is an Independent Prognosticator in Muscle-Invasive Bladder Cancer. International Journal of Molecular Sciences. 2023; 24(3):2746. https://doi.org/10.3390/ijms24032746
Chicago/Turabian StyleZheng, Qingyuan, Zhengyu Jiang, Xinmiao Ni, Song Yang, Panpan Jiao, Jiejun Wu, Lin Xiong, Jingping Yuan, Jingsong Wang, Jun Jian, and et al. 2023. "Machine Learning Quantified Tumor-Stroma Ratio Is an Independent Prognosticator in Muscle-Invasive Bladder Cancer" International Journal of Molecular Sciences 24, no. 3: 2746. https://doi.org/10.3390/ijms24032746
APA StyleZheng, Q., Jiang, Z., Ni, X., Yang, S., Jiao, P., Wu, J., Xiong, L., Yuan, J., Wang, J., Jian, J., Wang, L., Yang, R., Chen, Z., & Liu, X. (2023). Machine Learning Quantified Tumor-Stroma Ratio Is an Independent Prognosticator in Muscle-Invasive Bladder Cancer. International Journal of Molecular Sciences, 24(3), 2746. https://doi.org/10.3390/ijms24032746







