A Receptor Tyrosine Kinase Inhibitor Sensitivity Prediction Model Identifies AXL Dependency in Leukemia
Abstract
1. Introduction
2. Results
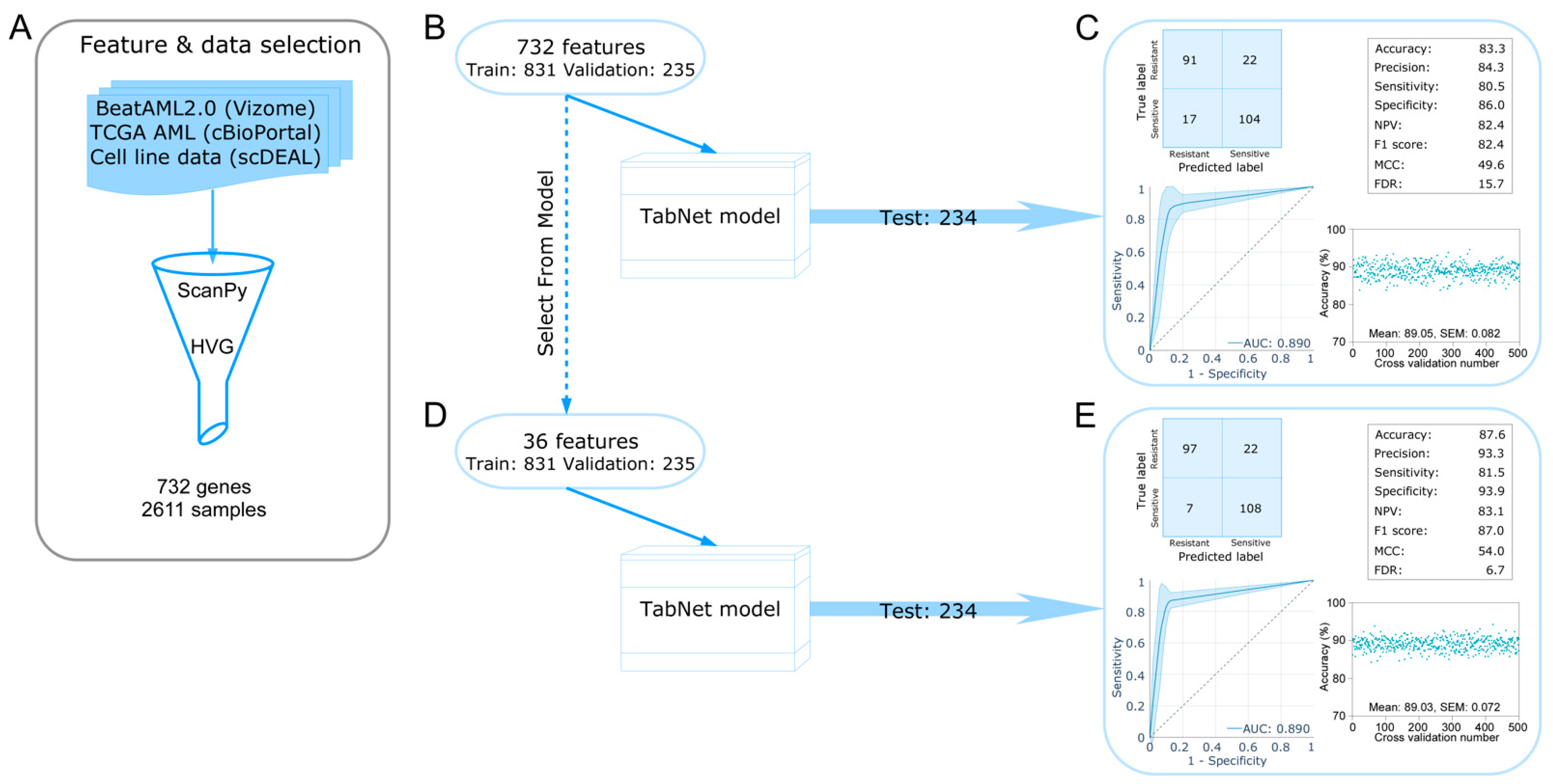
2.1. Development of a TabNet Model to Predict Sorafenib Sensitivity in Leukemia
2.2. Shapley Additive Explanations (SHAP) Identify AXL and HTRA4 as Important Features
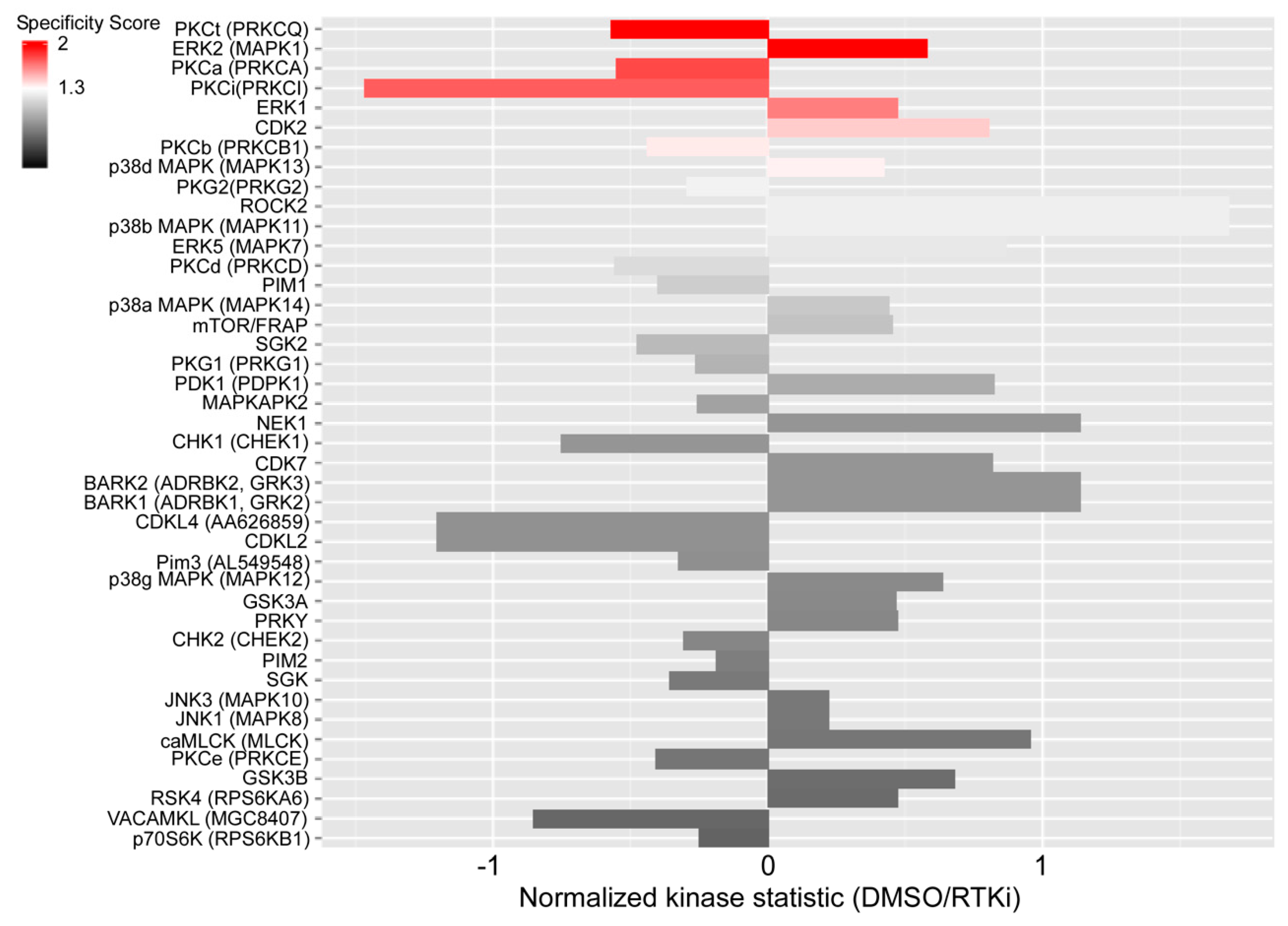
2.3. Sorafenib Activates Protein Kinase C Signaling
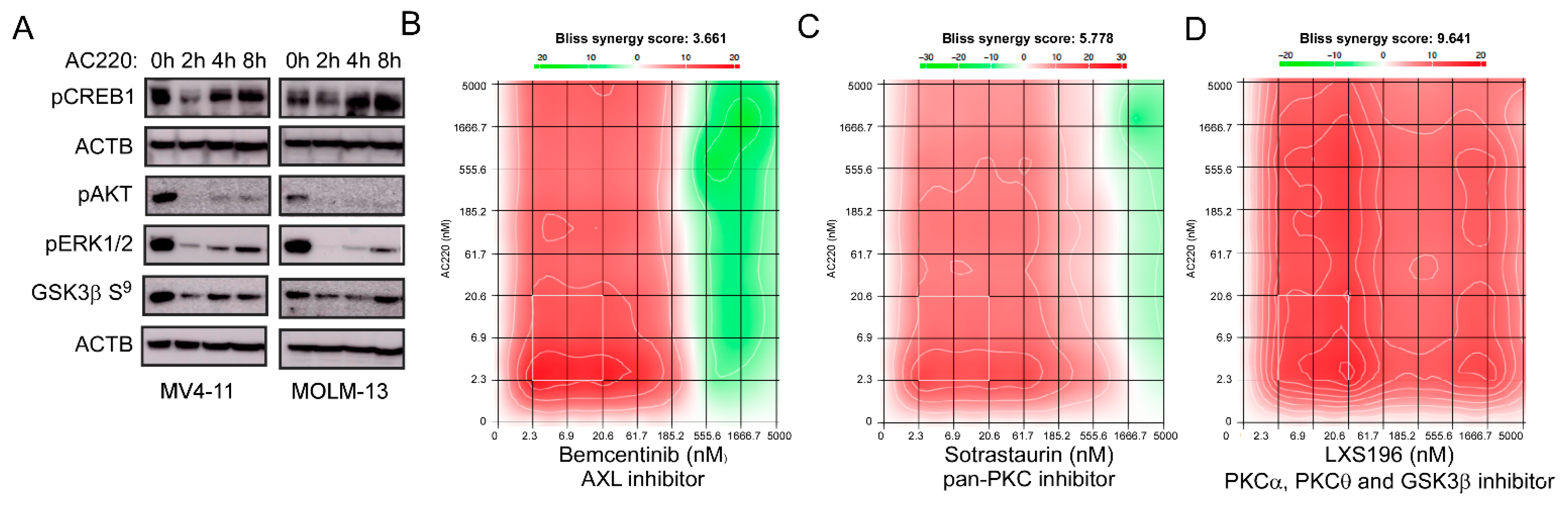
2.4. Inhibition of the Receptor Tyrosine Kinase FLT3 Results in Transcriptional Upregulation of AXL
2.5. An Inhibitor Targeting FLT3 Displays Synergy with AXL and PKC Inhibitors
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Esteban-Villarrubia, J.; Soto-Castillo, J.J.; Pozas, J.; San Roman-Gil, M.; Orejana-Martin, I.; Torres-Jimenez, J.; Carrato, A.; NAlonso-Gordoa, T.; Molina-Cerrillo, J. Tyrosine Kinase Receptors in Oncology. Int. J. Mol. Sci. 2020, 21, 8529. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, T.; Kusumoto, S.; Ando, K.; Ohba, M.; Ohmori, T. Receptor Tyrosine Kinase-Targeted Cancer Therapy. Int. J. Mol. Sci. 2018, 19, 3491. [Google Scholar] [CrossRef] [PubMed]
- Labbozzetta, M.; Barreca, M.; Spano, V.; Raimondi, M.V.; Poma, P.; Notarbartolo, M.; Barraja, P.; Montalbano, A. Novel insights on [1,2]oxazolo[5,4-e]isoindoles on multidrug resistant acute myeloid leukemia cell line. Drug Dev. Res. 2022, 83, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Qiu, R.Z.; Sun, S.L.; Zhao, C.; Fan, T.Y.; Chen, M.; Li, N.G.; Shi, Z.H. Small-Molecule Fms-like Tyrosine Kinase 3 Inhibitors: An Attractive and Efficient Method for the Treatment of Acute Myeloid Leukemia. J. Med. Chem. 2020, 63, 12403–12428. [Google Scholar] [CrossRef]
- Wang, Z.; Cai, J.; Cheng, J.; Yang, W.; Zhu, Y.; Li, H.; Lu, T.; Chen, Y.; Lu, S. FLT3 Inhibitors in Acute Myeloid Leukemia: Challenges and Recent Developments in Overcoming Resistance. J. Med. Chem. 2021, 64, 2878–2900. [Google Scholar] [CrossRef]
- Juchum, M.; Gunther, M.; Laufer, S.A. Fighting cancer drug resistance: Opportunities and challenges for mutation-specific EGFR inhibitors. Drug Resist. Updates 2015, 20, 12–28. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Chen, X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019, 2, 141–160. [Google Scholar] [CrossRef]
- Boumahdi, S.; de Sauvage, F.J. The great escape: Tumour cell plasticity in resistance to targeted therapy. Nat. Rev. Drug Discov. 2020, 19, 39–56. [Google Scholar] [CrossRef]
- Kazi, J.U. Mechanisms of Anticancer Therapy Resistance: The Role of Cancer Stem Cells. Int. J. Mol. Sci. 2020, 21, 9006. [Google Scholar] [CrossRef]
- Shaffer, S.M.; Dunagin, M.C.; Torborg, S.R.; Torre, E.A.; Emert, B.; Krepler, C.; Beqiri, M.; Sproesser, K.; Brafford, P.A.; Xiao, M.; et al. Rare cell variability and drug-induced reprogramming as a mode of cancer drug resistance. Nature 2017, 546, 431–435. [Google Scholar] [CrossRef]
- Shah, K.; Ahmed, M.; Kazi, J.U. The Aurora kinase/beta-catenin axis contributes to dexamethasone resistance in leukemia. NPJ Precis. Oncol. 2021, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Kazi, J.U.; Rönnstrand, L. FMS-like Tyrosine Kinase 3/FLT3: From Basic Science to Clinical Implications. Physiol. Rev. 2019, 99, 1433–1466. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Jones, D.; Medeiros, L.J.; Chen, W.; Vega-Vazquez, F.; Luthra, R. Activating FLT3 mutations are detectable in chronic and blast phase of chronic myeloproliferative disorders other than chronic myeloid leukemia. Am. J. Clin. Pathol. 2006, 126, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Kabir, N.N.; Rönnstrand, L.; Kazi, J.U. FLT3 mutations in patients with childhood acute lymphoblastic leukemia (ALL). Med. Oncol. 2013, 30, 462. [Google Scholar] [CrossRef]
- Bains, A.; Luthra, R.; Medeiros, L.J.; Zuo, Z. FLT3 and NPM1 mutations in myelodysplastic syndromes: Frequency and potential value for predicting progression to acute myeloid leukemia. Am. J. Clin. Pathol. 2011, 135, 62–69. [Google Scholar] [CrossRef]
- Wander, S.A.; Levis, M.J.; Fathi, A.T. The evolving role of FLT3 inhibitors in acute myeloid leukemia: Quizartinib and beyond. Ther. Adv. Hematol. 2014, 5, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, E.; Roesel, J.; Furet, P.; Bold, G.; Imbach, P.; Florsheimer, A.; Caravatti, G.; Jiang, J.; Manley, P.; Ray, A.; et al. Antileukemic Effects of Novel First- and Second-Generation FLT3 Inhibitors: Structure-Affinity Comparison. Genes Cancer 2010, 1, 1021–1032. [Google Scholar] [CrossRef]
- Antar, A.I.; Otrock, Z.K.; Jabbour, E.; Mohty, M.; Bazarbachi, A. FLT3 inhibitors in acute myeloid leukemia: Ten frequently asked questions. Leukemia 2020, 34, 682–696. [Google Scholar] [CrossRef]
- Staudt, D.; Murray, H.C.; McLachlan, T.; Alvaro, F.; Enjeti, A.K.; Verrills, N.M.; Dun, M.D. Targeting Oncogenic Signaling in Mutant FLT3 Acute Myeloid Leukemia: The Path to Least Resistance. Int. J. Mol. Sci. 2018, 19, 3198. [Google Scholar] [CrossRef]
- Lindblad, O.; Cordero, E.; Puissant, A.; Macaulay, L.; Ramos, A.; Kabir, N.N.; Sun, J.; Vallon-Christersson, J.; Haraldsson, K.; Hemann, M.T.; et al. Aberrant activation of the PI3K/mTOR pathway promotes resistance to sorafenib in AML. Oncogene 2016, 35, 5119–5131. [Google Scholar] [CrossRef] [PubMed]
- Burchert, A.; Bug, G.; Fritz, L.V.; Finke, J.; Stelljes, M.; Röllig, C.; Wollmer, E.; Wäsch, R.; Bornhäuser, M.; Berg, T.; et al. Sorafenib Maintenance After Allogeneic Hematopoietic Stem Cell Transplantation for Acute Myeloid Leukemia With FLT3-Internal Tandem Duplication Mutation (SORMAIN). J. Clin. Oncol. 2020, 38, 2993–3002. [Google Scholar] [CrossRef]
- Pollard, J.A.; Alonzo, T.A.; Gerbing, R.; Brown, P.; Fox, E.; Choi, J.; Fisher, B.; Hirsch, B.; Kahwash, S.; Getz, K.; et al. Sorafenib in Combination With Standard Chemotherapy for Children With High Allelic Ratio FLT3/ITD+ Acute Myeloid Leukemia: A Report From the Children’s Oncology Group Protocol AAML1031. J. Clin. Oncol. 2022, 40, 2023–2035. [Google Scholar] [CrossRef] [PubMed]
- Borthakur, G.; Zeng, Z.; Cortes, J.E.; Chen, H.C.; Huang, X.; Konopleva, M.; Ravandi, F.; Kadia, T.; Patel, K.P.; Daver, N.; et al. Phase 1 study of combinatorial sorafenib, G-CSF, and plerixafor treatment in relapsed/refractory, FLT3-ITD-mutated acute myelogenous leukemia patients. Am. J. Hematol. 2020, 95, 1296–1303. [Google Scholar] [CrossRef]
- Scholl, S.; Spies-Weisshart, B.; Klink, A.; Muegge, L.O.; Fricke, H.J.; Hochhaus, A. Secondary resistance to sorafenib in two patients with acute myeloid leukemia (AML) harboring FLT3-ITD mutations. Ann. Hematol. 2011, 90, 473–475. [Google Scholar] [CrossRef] [PubMed]
- Damnernsawad, A.; Bottomly, D.; Kurtz, S.E.; Eide, C.A.; McWeeney, S.K.; Tyner, J.W.; Nechiporuk, T. Genome-wide CRISPR screen identifies regulators of MAPK and MTOR pathways mediating sorafenib resistance in acute myeloid leukemia. Haematologica 2022, 107, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Bottomly, D.; Long, N.; Schultz, A.R.; Kurtz, S.E.; Tognon, C.E.; Johnson, K.; Abel, M.; Agarwal, A.; Avaylon, S.; Benton, E.; et al. Integrative analysis of drug response and clinical outcome in acute myeloid leukemia. Cancer Cell 2022, 40, 850–864. [Google Scholar] [CrossRef]
- Tyner, J.W.; Tognon, C.E.; Bottomly, D.; Wilmot, B.; Kurtz, S.E.; Savage, S.L.; Long, N.; Schultz, A.R.; Traer, E.; Abel, M.; et al. Functional genomic landscape of acute myeloid leukaemia. Nature 2018, 562, 526–531. [Google Scholar] [CrossRef]
- Wolf, F.A.; Angerer, P.; Theis, F.J. SCANPY: Large-scale single-cell gene expression data analysis. Genome Biol. 2018, 19, 15. [Google Scholar] [CrossRef]
- Arik, S.O.; Pfister, T. TabNet: Attentive Interpretable Tabular Learning. arXiv 2019, arXiv:1908.07442. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Chen, T.; Guestrin, C. XGBoost: A Scalable Tree Boosting System. arXiv 2016, arXiv:1603.02754. [Google Scholar]
- Lundberg, S.M.; Erion, G.; Chen, H.; DeGrave, A.; Prutkin, J.M.; Nair, B.; Katz, R.; Himmelfarb, J.; Bansal, N.; Lee, S.I. From Local Explanations to Global Understanding with Explainable AI for Trees. Nat. Mach. Intell. 2020, 2, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nie, G. Overview of Human HtrA Family Proteases and Their Distinctive Physiological Roles and Unique Involvement in Diseases, Especially Cancer and Pregnancy Complications. Int. J. Mol. Sci. 2021, 22, 10756. [Google Scholar] [CrossRef] [PubMed]
- Wenta, T.; Rychlowski, M.; Jarzab, M.; Lipinskka, B. HtrA4 Protease Promotes Chemotherapeutic-Dependent Cancer Cell Death. Cells 2019, 8, 1112. [Google Scholar] [CrossRef]
- Wium, M.; Ajayi-Smith, A.F.; Paccez, J.D.; Zerbini, L.F. The Role of the Receptor Tyrosine Kinase Axl in Carcinogenesis and Development of Therapeutic Resistance: An Overview of Molecular Mechanisms and Future Applications. Cancers 2021, 13, 1521. [Google Scholar] [CrossRef]
- Goyette, M.A.; Cote, J.F. AXL Receptor Tyrosine Kinase as a Promising Therapeutic Target Directing Multiple Aspects of Cancer Progression and Metastasis. Cancers 2022, 14, 466. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Ishiguro-Watanabe, M.; Tanabe, M. KEGG: I ntegrating viruses and cellular organisms. Nucleic Acids Res. 2021, 49, D545–D551. [Google Scholar] [CrossRef]
- Ge, S.X.; Son, E.W.; Yao, R. iDEP: An integrated web application for differential expression and pathway analysis of RNA-Seq data. BMC Bioinform. 2018, 19, 534. [Google Scholar] [CrossRef]
- Luo, W.; Pant, G.; Bhavnasi, Y.K.; Blanchard, S.G., Jr.; Brouwer, C. Pathview Web: User friendly pathway visualization and data integration. Nucleic Acids Res. 2017, 45, W501–W508. [Google Scholar] [CrossRef]
- Kazi, J.U.; Kabir, N.N.; Rönnstrand, L. Protein kinase C (PKC) as a drug target in chronic lymphocytic leukemia. Med. Oncol. 2013, 30, 757. [Google Scholar] [CrossRef]
- Kabir, N.N.; Kazi, J.U. Selective mutation in ATP-binding site reduces affinity of drug to the kinase: A possible mechanism of chemo-resistance. Med. Oncol. 2013, 30, 448. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, R.; Menezes, A.C.; Azevedo, A.; Leckenby, A.; Davies, S.; Seedhouse, C.; Gilkes, A.; Knapper, S.; Tonks, A.; Darley, R.L. Protein Kinase C Epsilon Overexpression Is Associated With Poor Patient Outcomes in AML and Promotes Daunorubicin Resistance Through p-Glycoprotein-Mediated Drug Efflux. Front. Oncol. 2022, 12, 840046. [Google Scholar] [CrossRef] [PubMed]
- Kazi, J.U.; Soh, J.W. Induction of the nuclear proto-oncogene c-fos by the phorbol ester TPA and v-H-Ras. Mol. Cells 2008, 26, 462–467. [Google Scholar] [PubMed]
- Chougule, R.A.; Shah, K.; Moharram, S.A.; Vallon-Christersson, J.; Kazi, J.U. Glucocorticoid-resistant B cell acute lymphoblastic leukemia displays receptor tyrosine kinase activation. NPJ Genom. Med. 2019, 4, 7. [Google Scholar] [CrossRef]
- Park, I.K.; Mishra, A.; Chandler, J.; Whitman, S.P.; Marcucci, G.; Caligiuri, M.A. Inhibition of the receptor tyrosine kinase Axl impedes activation of the FLT3 internal tandem duplication in human acute myeloid leukemia: Implications for Axl as a potential therapeutic target. Blood 2013, 121, 2064–2073. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, J.; Liu, J.; Ma, W.; Duan, Y.; Liu, D. Novel AXL-targeted agents overcome FLT3 inhibitor resistance in FLT3-ITD(+) acute myeloid leukemia cells. Oncol. Lett. 2021, 21, 397. [Google Scholar] [CrossRef]
- Lin, D.C.; Yin, T.; Koren-Michowitz, M.; Ding, L.W.; Gueller, S.; Gery, S.; Tabayashi, T.; Bergholz, U.; Kazi, J.U.; Rönnstrand, L.; et al. Adaptor protein Lnk binds to and inhibits normal and leukemic FLT3. Blood 2012, 120, 3310–3317. [Google Scholar] [CrossRef]
- Kazi, J.U.; Sun, J.; Phung, B.; Zadjali, F.; Flores-Morales, A.; Rönnstrand, L. Suppressor of cytokine signaling 6 (SOCS6) negatively regulates Flt3 signal transduction through direct binding to phosphorylated tyrosines 591 and 919 of Flt3. J. Biol. Chem. 2012, 287, 36509–36517. [Google Scholar] [CrossRef]
- Chougule, R.A.; Kazi, J.U.; Rönnstrand, L. FYN expression potentiates FLT3-ITD induced STAT5 signaling in acute myeloid leukemia. Oncotarget 2016, 7, 9964–9974. [Google Scholar] [CrossRef]
- Kazi, J.U.; Rönnstrand, L. The role of SRC family kinases in FLT3 signaling. Int. J. Biochem. Cell Biol. 2019, 107, 32–37. [Google Scholar] [CrossRef]
- Puissant, A.; Fenouille, N.; Alexe, G.; Pikman, Y.; Bassil, C.F.; Mehta, S.; Du, J.; Kazi, J.U.; Luciano, F.; Rönnstrand, L.; et al. SYK is a critical regulator of FLT3 in acute myeloid leukemia. Cancer Cell 2014, 25, 226–242. [Google Scholar] [CrossRef] [PubMed]
- Dosil, M.; Wang, S.; Lemischka, I.R. Mitogenic signalling and substrate specificity of the Flk2/Flt3 receptor tyrosine kinase in fibroblasts and interleukin 3-dependent hematopoietic cells. Mol. Cell Biol. 1993, 13, 6572–6585. [Google Scholar] [PubMed]
- Arora, D.; Stopp, S.; Böhmer, S.A.; Schons, J.; Godfrey, R.; Masson, K.; Razumovskaya, E.; Rönnstrand, L.; Tänzer, S.; Bauer, R.; et al. Protein-tyrosine phosphatase DEP-1 controls receptor tyrosine kinase FLT3 signaling. J. Biol. Chem. 2011, 286, 10918–10929. [Google Scholar] [CrossRef]
- Takami, M.; Katayama, K.; Noguchi, K.; Sugimoto, Y. Protein kinase C alpha-mediated phosphorylation of PIM-1L promotes the survival and proliferation of acute myeloid leukemia cells. Biochem. Biophys. Res. Commun. 2018, 503, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.S.; Faisal, A.; Mak, G.W.Y.; Miraki-Moud, F.; Bavetsias, V.; Valenti, M.; Box, G.; Hallsworth, A.; de Haven Brandon, A.; Xavier, C.P.R.; et al. Quizartinib-resistant FLT3-ITD acute myeloid leukemia cells are sensitive to the FLT3-Aurora kinase inhibitor CCT241736. Blood Adv. 2020, 4, 1478–1491. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.S.; Blagg, J.; Linardopoulos, S.; Pearson, A.D. Aurora kinase inhibitors: Novel small molecules with promising activity in acute myeloid and Philadelphia-positive leukemias. Leukemia 2010, 24, 671–678. [Google Scholar] [CrossRef]
- Xie, H.; Rothstein, T.L. Protein kinase C mediates activation of nuclear cAMP response element-binding protein (CREB) in B lymphocytes stimulated through surface Ig. J. Immunol. 1995, 154, 1717–1723. [Google Scholar] [CrossRef]
- Steven, A.; Seliger, B. Control of CREB expression in tumors: From molecular mechanisms and signal transduction pathways to therapeutic target. Oncotarget 2016, 7, 35454–35465. [Google Scholar] [CrossRef]
- Du, K.; Montminy, M. CREB is a regulatory target for the protein kinase Akt/PKB. J. Biol. Chem. 1998, 273, 32377–32379. [Google Scholar] [CrossRef]
- Koga, Y.; Tsurumaki, H.; Aoki-Saito, H.; Sato, M.; Yatomi, M.; Takehara, K.; Hisada, T. Roles of Cyclic AMP Response Element Binding Activation in the ERK1/2 and p38 MAPK Signalling Pathway in Central Nervous System, Cardiovascular System, Osteoclast Differentiation and Mucin and Cytokine Production. Int. J. Mol. Sci. 2019, 20, 1346. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Lazarovici, P.; Quirion, R.; Zheng, W. cAMP Response Element-Binding Protein (CREB): A Possible Signaling Molecule Link in the Pathophysiology of Schizophrenia. Front. Mol. Neurosci. 2018, 11, 255. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.H.; Liu, L.C.; Kao, E.S.; Lin, H.; Hsu, L.S.; Hsu, C.W.; Lin, Y.Y.; Lin, Y.S.; Liu, J.Y.; Lee, C.J. Protein kinase C alpha is involved in the regulation of AXL receptor tyrosine kinase expression in triple-negative breast cancer cells. Mol. Med. Rep. 2016, 14, 1636–1642. [Google Scholar] [CrossRef]
- Huey, M.G.; Minson, K.A.; Earp, H.S.; DeRyckere, D.; Graham, D.K. Targeting the TAM Receptors in Leukemia. Cancers 2016, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Rankin, E.B.; Giaccia, A.J. The Receptor Tyrosine Kinase AXL in Cancer Progression. Cancers 2016, 8, 103. [Google Scholar] [CrossRef]
- Hong, C.C.; Lay, J.D.; Huang, J.S.; Cheng, A.L.; Tang, J.L.; Lin, M.T.; Lai, G.M.; Chuang, S.E. Receptor tyrosine kinase AXL is induced by chemotherapy drugs and overexpression of AXL confers drug resistance in acute myeloid leukemia. Cancer Lett. 2008, 268, 314–324. [Google Scholar] [CrossRef]
- Kim, K.C.; Lee, C. Reversal of Cisplatin resistance by epigallocatechin gallate is mediated by downregulation of axl and tyro 3 expression in human lung cancer cells. Korean J. Physiol. Pharmacol. 2014, 18, 61–66. [Google Scholar] [CrossRef]
- Lin, J.Z.; Wang, Z.J.; De, W.; Zheng, M.; Xu, W.Z.; Wu, H.F.; Armstrong, A.; Zhu, J.G. Targeting AXL overcomes resistance to docetaxel therapy in advanced prostate cancer. Oncotarget 2017, 8, 41064–41077. [Google Scholar] [CrossRef]
- Fang, S.; Luo, Y.; Zhang, Y.; Wang, H.; Liu, Q.; Li, X.; Yu, T. NTNG1 Modulates Cisplatin Resistance in Epithelial Ovarian Cancer Cells via the GAS6/AXL/Akt Pathway. Front. Cell Dev. Biol. 2021, 9, 652325. [Google Scholar] [CrossRef]
- Bruce, S.F.; Cho, K.; Noia, H.; Lomonosova, E.; Stock, E.C.; Oplt, A.; Blachut, B.; Mullen, M.M.; Kuroki, L.M.; Hagemann, A.R.; et al. GAS6-AXL Inhibition by AVB-500 Overcomes Resistance to Paclitaxel in Endometrial Cancer by Decreasing Tumor Cell Glycolysis. Mol. Cancer Ther. 2022, 21, 1348–1359. [Google Scholar] [CrossRef]
- Murakami, Y.; Kusakabe, D.; Watari, K.; Kawahara, A.; Azuma, K.; Akiba, J.; Taniguchi, M.; Kuwano, M.; Ono, M. AXL/CDCP1/SRC axis confers acquired resistance to osimertinib in lung cancer. Sci. Rep. 2022, 12, 8983. [Google Scholar] [CrossRef]
- Iida, M.; McDaniel, N.K.; Kostecki, K.L.; Welke, N.B.; Kranjac, C.A.; Liu, P.; Longhurst, C.; Bruce, J.Y.; Hong, S.; Salgia, R.; et al. AXL regulates neuregulin1 expression leading to cetuximab resistance in head and neck cancer. BMC Cancer 2022, 22, 447. [Google Scholar] [CrossRef]
- Cardone, C.; Blauensteiner, B.; Moreno-Viedma, V.; Martini, G.; Simeon, V.; Vitiello, P.P.; Ciardiello, D.; Belli, V.; Matrone, N.; Troiani, T.; et al. AXL is a predictor of poor survival and of resistance to anti-EGFR therapy in RAS wild-type metastatic colorectal cancer. Eur. J. Cancer 2020, 138, 1–10. [Google Scholar] [CrossRef]
- Adam-Artigues, A.; Arenas, E.J.; Martinez-Sabadell, A.; Braso-Maristany, F.; Cervera, R.; Tormo, E.; Hernando, C.; Martinez, M.T.; Carbonell-Asins, J.; Simon, S.; et al. Targeting HER2-AXL heterodimerization to overcome resistance to HER2 blockade in breast cancer. Sci. Adv. 2022, 8, eabk2746. [Google Scholar] [CrossRef]
- Dumas, P.Y.; Naudin, C.; Martin-Lannerée, S.; Izac, B.; Casetti, L.; Mansier, O.; Rousseau, B.; Artus, A.; Dufossée, M.; Giese, A.; et al. Hematopoietic niche drives FLT3-ITD acute myeloid leukemia resistance to quizartinib via STAT5-and hypoxia-dependent upregulation of AXL. Haematologica 2019, 104, 2017–2027. [Google Scholar] [CrossRef]
- Suh, Y.A.; Jo, S.Y.; Lee, H.Y.; Lee, C. Inhibition of IL-6/STAT3 axis and targeting Axl and Tyro3 receptor tyrosine kinases by apigenin circumvent taxol resistance in ovarian cancer cells. Int. J. Oncol. 2015, 46, 1405–1411. [Google Scholar] [CrossRef]
- Elkabets, M.; Pazarentzos, E.; Juric, D.; Sheng, Q.; Pelossof, R.A.; Brook, S.; Benzaken, A.O.; Rodon, J.; Morse, N.; Yan, J.J.; et al. AXL mediates resistance to PI3Kalpha inhibition by activating the EGFR/PKC/mTOR axis in head and neck and esophageal squamous cell carcinomas. Cancer Cell 2015, 27, 533–546. [Google Scholar] [CrossRef]
- Rafique, R.; Islam, S.M.R.; Kazi, J.U. Machine learning in the prediction of cancer therapy. Comput. Struct. Biotechnol. J. 2021, 19, 4003–4017. [Google Scholar] [CrossRef]
- Nasimian, A.; Ahmed, M.; Hedenfalk, I.; Kazi, J.U. A deep tabular data learning model predicting cisplatin sensitivity identifies BCL2L1 dependency in cancer. Comput. Struct. Biotechnol. J. 2023, 21, 956–964. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasimian, A.; Al Ashiri, L.; Ahmed, M.; Duan, H.; Zhang, X.; Rönnstrand, L.; Kazi, J.U. A Receptor Tyrosine Kinase Inhibitor Sensitivity Prediction Model Identifies AXL Dependency in Leukemia. Int. J. Mol. Sci. 2023, 24, 3830. https://doi.org/10.3390/ijms24043830
Nasimian A, Al Ashiri L, Ahmed M, Duan H, Zhang X, Rönnstrand L, Kazi JU. A Receptor Tyrosine Kinase Inhibitor Sensitivity Prediction Model Identifies AXL Dependency in Leukemia. International Journal of Molecular Sciences. 2023; 24(4):3830. https://doi.org/10.3390/ijms24043830
Chicago/Turabian StyleNasimian, Ahmad, Lina Al Ashiri, Mehreen Ahmed, Hongzhi Duan, Xiaoyue Zhang, Lars Rönnstrand, and Julhash U. Kazi. 2023. "A Receptor Tyrosine Kinase Inhibitor Sensitivity Prediction Model Identifies AXL Dependency in Leukemia" International Journal of Molecular Sciences 24, no. 4: 3830. https://doi.org/10.3390/ijms24043830
APA StyleNasimian, A., Al Ashiri, L., Ahmed, M., Duan, H., Zhang, X., Rönnstrand, L., & Kazi, J. U. (2023). A Receptor Tyrosine Kinase Inhibitor Sensitivity Prediction Model Identifies AXL Dependency in Leukemia. International Journal of Molecular Sciences, 24(4), 3830. https://doi.org/10.3390/ijms24043830







