Abstract
Reactive oxygen species, including singlet oxygen, play an important role in the onset and progression of disease, as well as in aging. Singlet oxygen can be formed non-enzymatically by chemical, photochemical, and electron transfer reactions, or as a byproduct of endogenous enzymatic reactions in phagocytosis during inflammation. The imbalance of antioxidant enzymes and antioxidant networks with the generation of singlet oxygen increases oxidative stress, resulting in the undesirable oxidation and modification of biomolecules, such as proteins, DNA, and lipids. This review describes the molecular mechanisms of singlet oxygen production in vivo and methods for the evaluation of damage induced by singlet oxygen. The involvement of singlet oxygen in the pathogenesis of skin and eye diseases is also discussed from the biomolecular perspective. We also present our findings on lipid oxidation products derived from singlet oxygen-mediated oxidation in glaucoma, early diabetes patients, and a mouse model of bronchial asthma. Even in these diseases, oxidation products due to singlet oxygen have not been measured clinically. This review discusses their potential as biomarkers for diagnosis. Recent developments in singlet oxygen scavengers such as carotenoids, which can be utilized to prevent the onset and progression of disease, are also described.
1. Introduction
Oxygen can be recognized as a “double-edged sword” because aerobic organisms cannot live without it, even though the oxidative metabolism produces toxic by-products. Most deleterious phenomena arise because of free radicals. Hence, for survival, the elimination of highly reactive oxygen species (ROS) is important for living organisms. The human body is also constantly threatened by oxidative injury and radicals. Over the course of evolution, humans have developed defense systems to counteract oxidative damage, including the transformation and/or elimination of ROS, as a part of normal physiological homeostasis. When this homeostatic balance is disturbed by stress caused by environmental or radiation exposure, it is termed oxidative stress, that is, a state in which the homeostatic balance between oxidation reactions and antioxidant defenses is lost, leading to the oxidation of vital biomolecules and, finally, the onset of disease.
Thousands of studies have been reported over the past decade on the role of ROS in cell and tissue injury. ROS are generally accepted to be involved in various pathologies, including inflammation, asthma, muscular dystrophy, dementia, anaphylaxis, rheumatoid arthritis, reperfusion injury following ischemic stroke or heart attacks, cardiac toxicity of anti-cancer drugs, carcinogenicity of various chemicals, and smoking [1,2,3,4,5].
In this review, we focus on singlet oxygen (1O2) in ROS. However, we exclude photodynamic therapy (PDT), in which 1O2 is stimulated by the light irradiation of a photosensitizing agent to induce a therapeutic effect on cancer [6], because many interesting review articles on PDT have already been published in recent years. The molecular aspects of (1) the mechanism of 1O2 production in vivo, (2) methods for detecting 1O2 and its oxidation-modification products, (3) implications in the pathogenesis of skin and eye diseases, and (4) compounds that scavenge 1O2 are presented. Furthermore, we present our findings on the measurement of 1O2-mediated oxidation products in diabetes, bronchial asthma, and ocular diseases. Although 1O2 has been known to potentially be involved in the pathogenesis of certain diseases, lipid peroxidation products and other products produced by 1O2 have not been actively measured clinically. In this review, we examine the usefulness of lipid peroxidation products produced by 1O2 in diagnosing and understanding the pathophysiology of diseases.
2. Chemical Properties and Possible Production of Singlet Oxygen In Vivo
ROS are redox-active intermediates that are formed by the chemical, photochemical, or biochemical reduction in oxygen that, at least partially, triggers a chain of oxidative reactions. ROS are grouped into non-radical and radical species, the former including 1O2 and hydrogen peroxide (H2O2), and the latter including superoxide anions (O2•−), hydroxyl radicals (HO•), hydroperoxyl radicals (HO2•), alkoxyl radicals (RO•), and peroxyl radicals (ROO•) [7]. 1O2 is generated by energy transfer via chemical or photochemical pathways from an activated species to the oxygen molecule (triplet oxygen, 3O2) (Figure 1). Other species are formed by a series of reactions initiated by 1O2, or by certain one-electron reduction processes [7]. Therefore, ROS have been actively studied over the past three decades to determine whether they are beneficial or harmful.

Figure 1.
Relationship between energy levels of ground state triplet oxygen molecule 3O2 and singlet oxygen molecule 1O2. The ground state oxygen molecule, triplet oxygen 3O2 (1Σ−g), has two unpaired and spin-parallel electrons in π* antibonding orbitals. 3O2 (3Σ−g) in the ground state receives energy and is excited to the singlet state, forming singlet oxygen (1O2). 1O2 is the spin-flipped electron state of 3O2 (3Σ−g). There are two states of 1O2:1O2 (1Σ+g) and 1O2 (1Δg). 1O2 (1Σ+g) and 1O2 (1Δg) have energies that are 1.6 eV and 0.98 eV higher than the ground state 3O2 (3Σ−g), respectively. The lifetime of 1O2 (1Σ+g) is a few picoseconds, and it is rapidly converted to 1O2 (1Δg). Since the lifetime of 1O2 (1Δg) is several microseconds, which is significantly longer than that of 1O2 (1Σ+g), the 1O2 generated in vivo can be 1O2 (1Δg). The arrows indicate the direction of the electron spin of the highest occupied molecular orbital (HOMO).
High-energy radiations, such as X- and γ-rays, generate radical ROS directly by the ionization of H2O or oxygen, whereas various chemical and photochemical reactions produce 1O2 (Figure 2A), which in turn creates other ROS [8]. In the energy transfer mechanism, a photoactivated chromophore causes intersystem crossing to the triplet state, and triplet–triplet annihilation transfers part of the electronic energy to oxygen to generate 1O2 [9,10]. Since the energy of most photoactivated molecules is higher than the excitation energy of 1O2 (0.98 eV) (Figure 1), 1O2 is readily produced by endogenous chromophores activated by ultraviolet (UV) irradiation [11]. Hatz et al. showed that the lifetime of 1O2 generated by pulsed laser irradiation of a photosensitizer (5,10,15,20-tetrakis(N-methyl-4-pyridyl)-21H, 23H-phine (TMPyP)) incorporated into the nucleus of HeLa cells is about 3 μs. They also found that the generated 1O2 diffuses from the formation point to a sphere radius of about 100 nm [12,13]. On the other hand, Liang et al. created a system that generates organelle-specific 1O2 by irradiating 660 nm light using a photosensitizer that is locally expressed in the membrane, cytosol, endoplasmic reticulum, mitochondria, and nucleus [14]. Using this system, they observed differences in the irradiation energy required to induce cell death depending on the organelle in which the photosensitizer is expressed, as well as differences in the type of cell death (early apoptosis, necrosis, or late apoptosis) [14]. This result indicates that 1O2 generated in intracellular organelles may not spread to other organelles. These reports indicate that 1O2 generated by intracellular photosensitizers may induce cell death by oxidizing the components of the organelle containing the photosensitizer, rather than diffusing widely within the cell.
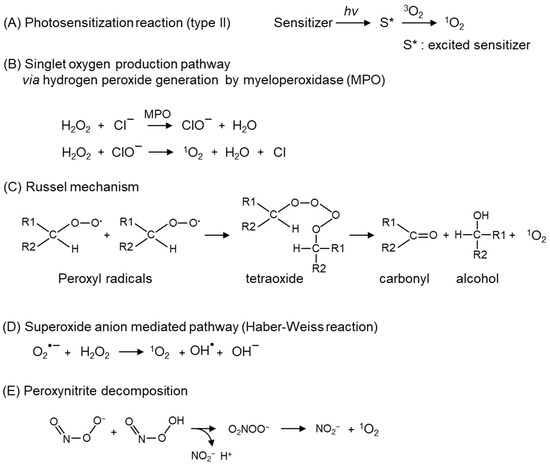
Figure 2.
Major 1O2 production mechanisms. 1O2 production pathways; (A) photochemical reaction, (B) reactions mediated by hydrogen peroxide produced by myeloperoxidase (MPO), (C) decomposition of peroxyl radicals (Russel mechanism), (D) reaction mediated by superoxide anion (O2•−), (E) pathway via peroxynitrite decomposition.
The secretion of myeloperoxidase (MPO) from phagocytes generates hypochlorite (ClO−), which reacts with H2O2 to form 1O2 (Figure 2B) [15]. In leukocytes, 1O2 generated via peroxidases and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase critically contributes to an antimicrobial role [16]. Another endogenous pathway of 1O2 is the degradation of lipid peroxides or ROO• via the Russel mechanism (Figure 2C) [17,18]. ROO• and lipid peroxides, which are the substrates of the Russel mechanism, are derived from the peroxidation of polyunsaturated fatty acids (PUFA) by auto-oxidation and enzymatic-oxidation, such as cytochrome c, lactoperoxidase [19], and lipoxygenases [20]. In addition, the light-independent generation pathways of 1O2 include reactions involving O2•− (Figure 2D) [21], peroxynitrite (Figure 2E) [22,23], and ozone [24,25,26]. Atmospheric particulates also catalyze the 1O2 generation [27,28].
3. Damage to Biomolecules by 1O2-Mediated Oxidation
Notably, because 1O2 has electrophilic properties, it attacks the π-bonds of the compound to form hydroperoxide by the ene reaction or endoperoxide by the 1,4-addition reaction (Figure 3A). 1O2 oxidatively modifies biomolecules, such as amino acids (Figure 3B) [29,30], nucleic acids (Figure 3C) [31,32], and lipids (Figure 3D) [33,34], either by a direct reaction or by the induction of ROS.
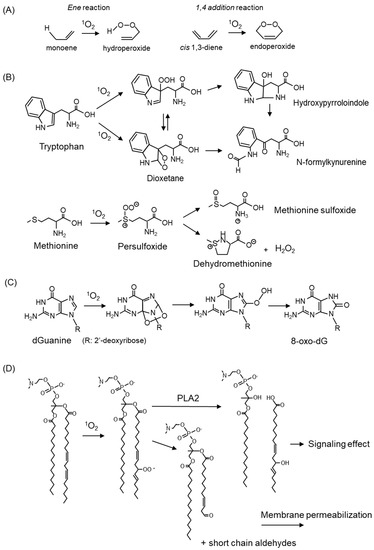
Figure 3.
1O2-mediated oxidation of amino acid, nucleic acid, and lipids. (A) Oxygenation reaction by 1O2. (B) Reaction products of tryptophan and methionine with 1O2. (C) Formation of 8-oxo-2′-deoxyguanosine (8-oxo-dG) by 1O2 oxidation to deoxyguanosine (dG). (D) Oxidative modification of fatty acids in membrane phospholipids by 1O2.
Amino acids, which are components of proteins, are the targets of 1O2. Among the 20 natural amino acids, tryptophan, histidine, tyrosine, and the sulfur-containing amino acids cysteine and methionine, are susceptible to reaction with 1O2 because of their structural properties. The reaction products of tryptophan and methionine with 1O2 are shown in Figure 3B. Tryptophan reacts with 1O2 to form a hydroperoxide at the C3 position as an intermediate, which undergoes ring closure to form hydroxypyrroloindole, and finally, N-formylkynurenine [35]. Tryptophan reacts with 1O2 to form C2-C3 cross-linked dioxetanes as intermediates, followed by the conversion to N-formylkynurenine [35]. Methionine reacts with 1O2 to form methionine sulfoxide and the cyclic product dehydromethionine via a persulfoxide intermediate [30]. Oxidative modifications to amino acids cause alterations in protein properties, resulting in cellular damage accompanied by reduced enzyme activity and disruption of the cell structure.
1O2 reacts specifically with the guanine portion of DNA bases converting to 7,8-dihydro-8-oxo-2′-deoxy-guanin (8-oxo-dG) (Figure 3C) [36]. 8-oxo-dG is a potential mutagenic substance because it pairs with adenine to cause a G → T transversion, and it is widely recognized as one of the indicators for evaluating oxidative DNA damage [37]. The details are discussed below in Section 5.1.4. on skin cancer.
Phospholipids in the membrane react with 1O2, resulting in the oxidative modification of fatty acids (Figure 3D). Oxidized fatty acids are cleaved and serve as signaling molecules involved in carcinogenesis and skin aging. The cleavage of short-chain aldehydes from fatty acids also results in membrane instability and increased permeability. The oxidation products derived from linoleic acid and cholesterol by 1O2 are described below.
1O2 also oxidatively modifies steroids [38], vitamins [39,40], carbohydrates, terpenes [41], and flavonoids [42]. In addition, 1O2 contributes to the stimulation of stress-activated kinases and the regulation of gene expression [43].
4. Detection Methods of 1O2 In Vivo and In Vitro
The oxidative modification of biomolecules by 1O2 has been related in several diseases, including skin and eye diseases [44]. The development of methods to precisely detect and quantify 1O2 contributes not only to an understanding of its roles in physiological and pathological conditions, but also in cancer therapy, by understanding the mechanism of PDT. Detection methods for 1O2 have been developed to verify 1O2 production in living systems [45]. The detection of 1O2 is mainly performed using the following methods: spectroscopic measurement of near-infrared (NIR) luminescence [46,47], electron spin resonance (ESR) with sterically hindered amine [48,49], fluorescence measurement by reaction with fluorescence probes [50,51], and the measurement of oxidation products produced by the reaction of 1O2 with biomolecules [52,53]. These methods were utilized for in vitro and in vivo experiments.
4.1. Direct Detection of 1O2
The measurement of 1O2 NIR luminescence is a reliable method for the direct spectroscopic detection of 1O2, as 1O2 emission (1270 nm) can be excited by an argon laser [54]. This luminescence emission was first used for the time-resolved detection of 1O2 in solution by Krasnovsky in 1976. This measurement method has been used as a standard technique for 1O2 formation yields, lifetimes, and deactivation constants in various solutions. The in vivo detection of 1O2 luminescence has been attempted previously, and it has been reported that 1O2 in a suspension of leukemia cells [55,56] and red cell ghost [57] can be detected using a sophisticated near-infrared photomultiplier device. However, this luminescence signal is weak because the inactivation of 1O2 is dominated by nonradiative pathways; typical phosphorescence yields are of the order of 10−5 to 10−7 [58]. In addition, these methods require the usage of deuterium oxide (D2O) to extend the lifetime of 1O2 and remove the 1270 nm emission absorption due to H2O [59]. This difficulty in detecting 1O2 in vivo can be attributed to the short lifetime of 1O2 in cells and tissues and the lack of sufficient sensitive detectors at NIR wavelengths.
ESR spectroscopy, which has been proposed for paramagnetic materials and organic compounds with unpaired electrons, has been used for the detection of free radicals [60]. To detect 1O2, a method was developed for measuring nitroxide radicals produced by the reaction of 1O2 with sterically hindered secondary amine probes such as 2,2,6,6-tetramethylpiperidine (TEMP) (Figure 4A) [61,62,63] and hydroxy-TEMP (Figure 4B) [64]. This method has been applied to the in vitro 1O2 scavenging activity of various substances in solution, and there is considerable evidence demonstrating the 1O2 scavenging effects of various substances using this method [48,65,66]. However, ESR spectroscopy is not considered suitable for the detection of intracellular 1O2, which may be because the time resolution in ESR measurements is not suitable for the short lifetime of 1O2 in cells existing in 1O2 quenching molecules.
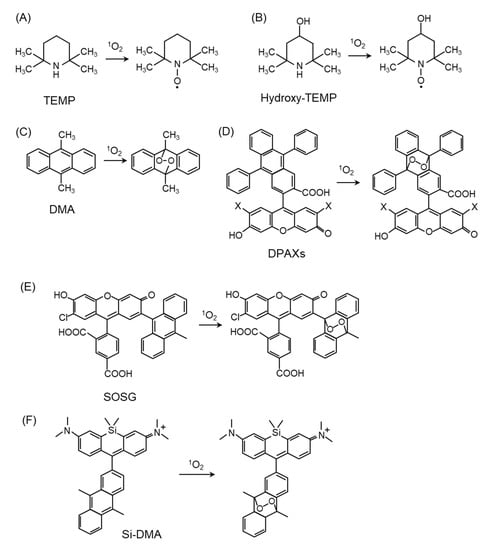
Figure 4.
Structures and oxidation modification of probes for 1O2 detection. (A) 2,2,6,6-tetramethylpiperidine (TEMP), (B) Hydroxy-TEMP, (C) 9,10-dimethylanthracene (DMA), (D) 9-[2-(3-Carboxy-9,10-diphenyl)anthryl]-6-hydroxy-3H-xanthen-3-ones (DPAXs), (E) Singlet Oxygen Sensor Green (SOSG), (F) silicone-containing rhodamine-9,10-dimethylanthracene (Si-DMA).
4.2. 1O2 Detection by Fluorescent Probes
Fluorescent probes are excellent sensors for the detection of ROS because of their high sensitivity, simplicity of data acquisition, and high spatial resolution in microscopic imaging [67,68,69]. Fluorescent probes, including 9,10-dimethylanthracene (DMA) (Figure 4C) [68,70,71] and 9-[2-(3-Carboxy-9,10-diphenyl)anthryl]-6-hydroxy-3H-xanthen-3-ones (DPAXs) (Figure 4D) [72], have been developed for the detection of 1O2 in neutral or basic aqueous solutions. These probes react specifically and rapidly with 1O2 to form stable endoperoxides with a high rate constant. Singlet Oxygen Sensor Green (SOSG) (Figure 4E) is a fluorescent probe for in vitro detection because of its high 1O2 selectivity [73,74,75]. To apply fluorescence probes in biological samples, the probes must penetrate the cell membrane and be localized in the cell. Recently, a far-red fluorescent probe consisting of DMA and silicon-containing rhodamine (Si-rhodamine) moieties, namely Si-DMA (Figure 4F), was developed for detecting 1O2 in the mitochondria. The advantages of Si-DMA are its cell-permeable ability and increased sensitivity to specifically detect mitochondrial 1O2 by the Si-rhodamine moiety [76]. It is important to precisely measure intracellular 1O2 generation to clarify the characteristics of fluorescence probes in terms of their signal-to-noise ratio and dynamic changes. We have demonstrated that intracellular 1O2 can be quantitatively measured using Si-DMA in living cells, as time-lapse imaging using Si-DMA provides an apparent signal-to-noise ratio in the treatments of a 1O2 generator and quencher [77]. Other fluorescent probes have been used to detect intracellular 1O2 using nanoparticles, including SOSG-based nanoprobes [50], super-pH-resolved nanosensors encoding SOSG [78], and biocompatible polymeric nanosensors encapsulating SOSG within their hydrophobic core [79]. The use of these fluorescence probes will improve our understanding of the 1O2 generation mechanism and the biological function of 1O2 in the physiological and pathological conditions of cultured cells.
4.3. Detection of 1O2-Mediated Peroxidation Products
1O2 generated in vivo quickly reacts with biomolecules, including lipids, proteins, and nuclei, and the subsequent comparatively stable oxidized products remain in the 1O2 generation site. This chemical property has been used for the development of in vivo ROS detection methods, and these oxidation products are widely used as oxidative stress biomarkers because they can be detected stably and correlate with oxidative stress status in vivo [80,81].
The oxidation product of linoleic acid, hydroxyoctadecadienoic acid (HODE), contains six isomers. 1O2 reacts with molecules containing double bonds to form hydroperoxides as primary products, which are subsequently reduced to hydroxides (Figure 5). 1O2 produces 9-, 10-, 12-, and 13-(Z,E)-hydroperoxyoctadecadienoic acid (HPODE) from linoleates; however, only 10- and 12-(Z,E)-HPODE are specific products by 1O2 because 9- and 13-(Z,E)-HPODE are also formed by both free radical- and enzyme-mediated oxidation. The chemical structures of 10- and 12-(Z,E)-HPODE differ significantly from those of 9- and 13-(Z,E)-HPODE: the latter contains a conjugated diene, while the former does not. These structural features cause differences in physiological functions; for example, 10- and 12-(Z,E)-HODE cause adaptive responses to UV-derived oxidative damage in cultured cells, whereas 9- and 13-(Z,E)-HODE do not [82,83].
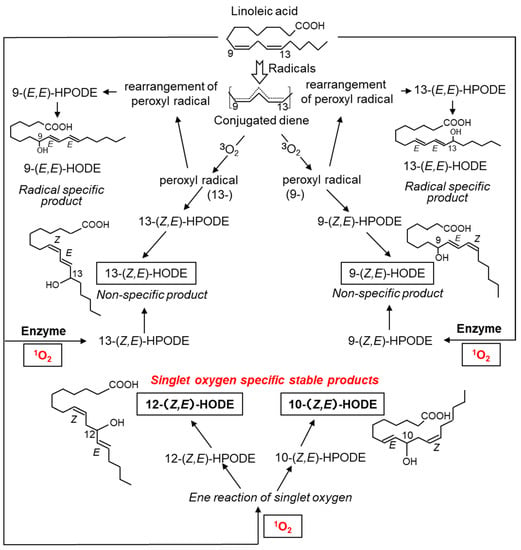
Figure 5.
Structures and formation mechanism of hydroxyoctadecadienoic acid (HODE), an oxidation product derived from linoleic acid. 1O2 oxidizes linoleic acid to produce 13-(Z,E)-HODE, 9-(Z,E)-HODE, 12-(Z,E)-HODE, and 10-(Z,E)-HODE. 13-(Z,E)-HODE and 9-(Z,E)-HODE are also produced by ROS other than 1O2 and by enzymatic oxidation reactions via lipid oxidases. 9-(E,E)-HODE and 13-(E,E)-HODE are produced in a radical-specific manner.
Cholesterol is an important lipid that constitutes biological membranes and undergoes oxidative modification to produce 7-, 24-, and 27-hydoroxycholesterol. Cholesterol, as well as unsaturated fatty acids, are substrates for the oxidative reaction of 1O2. When 1O2 reacts with cholesterol, it forms 5α-hydroperoxide (cholesterol 5α-OOH), 6α-hydroperoxide (cholesterol 6α-OOH), and 6β-hydroperoxide (cholesterol 6β-OOH) (Figure 6). Cholesterol 5α-OOH is produced in larger amounts than 6α/β-OOH. Therefore, cholesterol 5α-OOH could be used as a biomarker for oxidative reactions involving 1O2 in vivo. Furthermore, the Hock cleavage of cholesterol 5α-OOH converts to cholesterol 5,6-secosterol. Cholesterol 5,6-secosterol, also called ateronal, is involved in the development of cardiovascular diseases [84] and neurodegeneration [85].
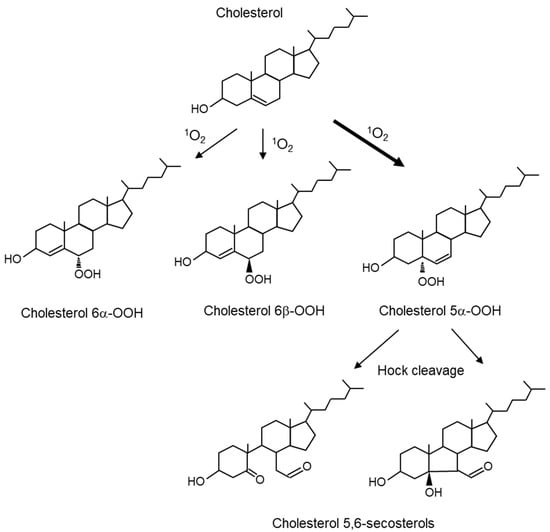
Figure 6.
Structures of cholesterol peroxides produced by 1O2-mediated oxidation reactions. 1O2 oxidizes cholesterol to produce 5α-hydroperoxide (cholesterol 5α-OOH), 6α-hydroperoxide (cholesterol 6α-OOH), and 6β-hydroperoxide (cholesterol 6β-OOH). Hock cleavage of cholesterol 5α-OOH converts it to cholesterol 5,6-secosterols.
We have developed a method to comprehensively measure lipid peroxidation and proposed the measurement of HODEs and hydroxycholesterol [86,87,88,89,90,91]. In this method, lipid components are extracted from biological samples after reduction and saponification. These pretreatments result in the measurement of both free and esterified forms of hydroperoxides and hydroxides as free hydroxides. HODEs and hydroxycholesterol are analyzed by liquid chromatography–tandem mass spectrometry (LC-MS/MS) and gas chromatography–mass spectrometry (GC-MS), respectively. Biological samples to be measured with this method include plasma, urine, erythrocytes, as well as cultured cells and tissues. This method allows for the measurement of not only radical-mediated oxidation products, but also 1O2-specific oxidation products using the HODEs isomer as an indicator [89,90,91], in other words, indirect in vivo 1O2 detection is possible. We have determined the relationship between 1O2-mediated oxidation and the early diagnosis of diabetes in humans and mice based on the production of 10- and 12-(Z,E)-HODEs [83,92,93], which will be discussed in more detail later. 1O2 measurements using 10- and 12-(Z,E)-HODEs as indicators can evaluate 1O2 generation not only in living cells, but also in humans and animals in vivo, and they can precisely analyze the involvement of 1O2 generation and diseases.
While ESR and fluorescent probes are useful for detecting 1O2 in vitro and in cells, it is difficult to use these tools to assess the degree of damage caused by 1O2 in tissues. Therefore, it is better to measure oxidation products that are relatively stable and retained in biological samples. Table 1 summarizes the methods for measuring 1O2 itself or its oxidation products and both their advantages and disadvantages. Table 2 summarizes reports of the measurements of linoleic acid- or cholesterol-derived oxidation products produced by 1O2-mediated oxidation reactions in human and animal samples. 1O2-derived lipid oxidation products have been measured in the blood and tissue samples obtained from patients with borderline diabetes, glaucoma, alcoholism, and atherosclerosis. In animal experiments, cholesterol 5α-hydroperoxide in the skin tissue was measured following UVA irradiation in mice. These results are important to understand the contribution of 1O2 to the pathogenesis of various disorders.

Table 1.
Characterization of 1O2 detection methods in vivo and in vitro.

Table 2.
Report on the results of the determination of linoleic acid- or cholesterol-derived peroxides produced by 1O2-mediated oxidation reactions using human and animal samples.
5. Diseases Involving 1O2 and Their Pathophysiology
5.1. Skin Diseases
The skin is the organ with the largest area exposed to sunlight. The skin is regularly exposed to sunlight and is at a high risk of oxidative stress. UV radiation from the sun generates ROS in keratinocytes, the major cells of the epidermis and the outermost layer of the skin [105]. UV exposure also increases the activities of cyclooxygenase and lipoxygenase, which produce eicosanoids from arachidonic acid. UV exposure of the skin causes erythema and acute inflammation, and long-term UV exposure causes photo-carcinogenesis [106] and skin aging [107]. Lipid oxidation on the skin surface is important in relation to sunburn, hyperpigmentation, wrinkle formation, freckles, atopic dermatitis, acne, and skin cancer.
UVA (320–400 nm) penetrates the dermis, whereas UVB (280–320 nm) only attacks the epidermis. Both UVA and UVB produce ROS and free radicals in the presence of photosensitizers (Figure 7). In type I reactions, an excited photosensitizer reacts with an organic compound to produce O2•− and lipid peroxyl radical (LOO•). In a type II reaction, the excited photosensitizer reacts with a triplet oxygen molecule, producing 1O2 primarily by energy transfer and, to a minor extent, O2•− by electron transfer.
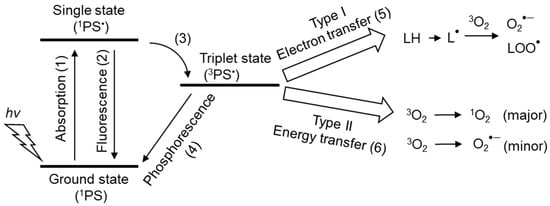
Figure 7.
Photosensitizers and the mechanisms of 1O2 production. (1) Photosensitizers (PS) absorb photons (hv) from light and transform them to the excited singlet state (1PS•). (2) 1PS• returns to the ground singlet state (1PS) by losing energy via fluorescence emission. (3) 1PS• was converted to the long-lived triplet state (3PS•). (4) 3PS• can return to the ground singlet state (1PS) via light emission (phosphorescence). (5) 3PS• forms organic radicals (L•) from organic compounds (LH) through the transfer of electrons in the type I photochemical reaction. L• affects oxygen (3O2) to produce ROS (superoxide anion O2•−, lipid peroxyl radical (LOO•). O2•− leads to the production of H2O2 and hydroxyl radicals (OH•), resulting in the induction of radical chain reactions. (6) In type II photochemical reactions, the energy of 3PS• is transferred to 3O2 to mainly produce 1O2, but O2•− is produced as a minor product via electron transfer from 3PS•.
5.1.1. Photosensitizers
Porphyrins such as protoporphyrin IX (Figure 8A) [108] have been examined as endogenous photosensitizers, and their derivatives [8] and 5-aminolevulinic acid [109], a component of porphyrins, have been investigated for application as PDT in therapeutic modalities against diseases such as cancer. Interestingly, coproporphyrin (Figure 8B) produced by Propionibacterium acnes, an acne-causing bacterium, also acts as a photosensitizer and generates 1O2, which is associated with skin inflammation [110,111]. Hemin (Figure 8C) [112] and chlorophyll (Figure 8D) [113], which are metal complexes of porphyrin and similar compounds, also exhibit photosensitizing effects. Hemin is an iron-containing protoporphyrin IX, and chlorophyll is a chemical responsible for absorbing light energy in the light reaction of photosynthesis. Pheophytin (Figure 8E), from which magnesium ions are removed during chlorophyll degradation, and pheophorbide (Figure 8F), from which the phytyl group is eliminated from pheophytin, also act as photosensitizers [114,115].
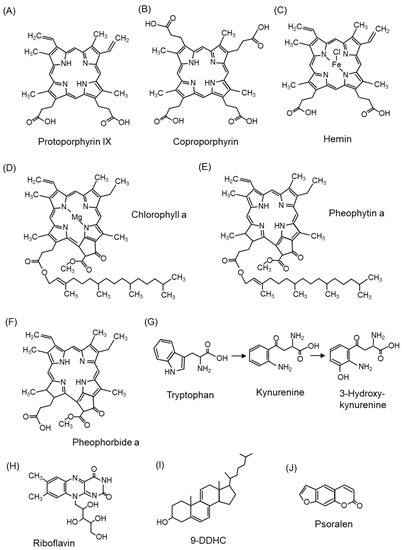
Figure 8.
The structures of photosensitizers. (A) Protoporphyrin IX, (B) coproporphyrin, (C) hemin, (D) chlorophyll a, (E) pheophytin a, (F) pheophorbide a, (G) tryptophan, (H) riboflavin, (I) cholesta-5,7,9(11)-trien-3beta-ol (9-DDHC), (J) psoralen.
In addition to porphyrins, tryptophan and tryptophan metabolites (kynurenine and 3-hydroxykynurenine) (Figure 8G) [116] and riboflavin (Figure 8H) [117,118] are endogenous photosensitizers. Riboflavin, a water-soluble vitamin B2, is distributed in the skin, eyes, brain, and blood vessels and increases the production of 1O2. Cholesta-5,7,9(11)-trien-3beta-ol (9-DDHC) (Figure 8I), a metabolite of 7-dehydrocholesterol (7-DHC), is a photosensitizer involved in rare cholesterol metabolism disorders [119]. Psoralen (Figure 8J), found in citrus fruits, is a dietary photosensitizer that causes photosensitivity [120,121].
5.1.2. Lipids in the Skin and Lipid Peroxidation Products Mediated by 1O2
The lipid composition of the epidermis is a mixture of free fatty acids, ceramide, and cholesterol at approximately 30–35% each [122]. The fatty acids in the epidermis include linoleic acid (21%), oleic acid (15%), palmitic acid (14%), stearic acid (11%), and arachidonic acid (6%) [123]. In contrast, the lipid composition of sebum is triacylglycerol (45–50%), wax ester (25%), squalene (Sq) (12%), and free fatty acids (10%) [122]. The fatty acid composition of sebum is palmitic acid (22%), palmitoleic acid (21%), oleic acid (15%), myristic acid (12%), and myristoleic acid (5%) [124]. The lipid composition of the sebum differs significantly from that of the epidermis.
Squalene (Sq) is a triterpene compound characteristically present in sebum, and six Sq monohydroperoxides (2-, 3-, 6-, 7-, 10-, and 11-OOH-Sq) are produced in similar amounts by the peroxidation of 1O2 (Figure 9) [125]. In contrast, 2- and 3-OOH-Sq were recently reported to be produced by free radical oxidation [125]. Considering these results, 6-, 7-, 10-, and 11-OOH-Sq may be the specific products of 1O2. SqOOHs are also associated with wrinkle formation [126] and inflammatory acne [127]. As SqOOHs are unstable, further photooxidation was revealed to produce 2-OOH-3-(1,2-dioxane)-Sq (Figure 9) as a secondary oxidation product [128]. SqOOH concentrations in sebum collected from skin exposed to tobacco heating products or electronic cigarette aerosols are lower than that in sebum from skin exposed to tobacco smoke, indicating consumer hygiene and cosmetic benefits [129].
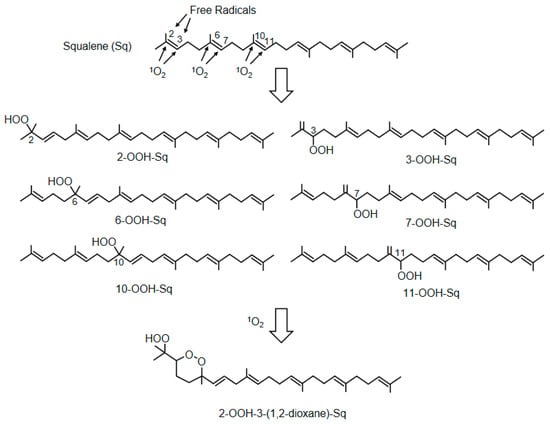
Figure 9.
The structures of squalene and its peroxidation products. Squalene (Sq) is converted by 1O2 to six monohydroperoxides (2-, 3-, 6-, 7-, 10-, and 11-OOH-Sq). The monohydroperoxides of Sq are further photo-oxidized to 2-OOH-3-(1,2-dioxane)-Sq.
Table 3 shows the results of SqOOH measurements in human and animal sebum samples. The fact that SqOOHs, which are specifically produced by 1O2, can be detected using sebum, which can be collected without severe invasion, makes it possible to assess the effects of pollutants, sunlight, and UV. In the future, techniques for the measurement of the SqOOH content in sebum could be further developed in the field of skin diseases, including cosmetology.
As mentioned above, 1O2 peroxidation products from cholesterol are caused by ene reactions to 5,6 double bonds to form cholesterol 5α-OOH, 6α-OOH, and 6β-OOH (Figure 6). However, cholesterol 5α-OOH is more abundant than cholesterol 6α- and 6β-OOH [52].
PUFAs in the skin are also target molecules for peroxidation by 1O2. UVA irradiation of hairless mice has also been reported to increase 10-HPODE and 12-HPODE levels (Figure 5), which are 1O2-specific lipid hydroperoxides of linoleic acid [130].

Table 3.
Report on the results of the determination of squalene-derived peroxides produced by oxidation reactions mediated by 1O2 using human and animal samples.
Table 3.
Report on the results of the determination of squalene-derived peroxides produced by oxidation reactions mediated by 1O2 using human and animal samples.
| 1O2-Mediated Oxidation Product | Sample Species | Disease/Model | Ref. |
|---|---|---|---|
| Squalene monohydroperoxides | Human | Increased by exposure of human skin to tobacco smoke | [131] |
| Human | Sunlight exposure increases SqOOH in sebum | [132] | |
| Human | UV irradiation to human sebum | [133,134,135] | |
| Human | SqOOH reduction in sebum by application of cosmetics | [136] | |
| Human | Human skin surface lipids | [137] | |
| Human | Increased SqOOH in the scalp due to high dandruff | [138] | |
| Human | Detection using human fingerprints | [139] | |
| Human | Increase in SqOOH in the sebum due to ambient dust and ozone | [140] | |
| Human | Less SqOOH in sebum exposed to tobacco heating products and electronic cigarettes compared to cigarette smoke exposure | [129] | |
| Pig | Cigarette smoke exposure to pig skin | [141] | |
| Rabbit | UVA irradiation of rabbit ears | [127] | |
| Guinea pig | Carotene reduces the increase in SqOOH caused by UV irradiation of the skin | [142] | |
| 2-OOH-3-(1,2-dioxane)-squalene | Human | Identification of cyclic peroxides of SqOOH | [128] |
5.1.3. Skin Aging
The main clinical sign of skin aging is the dysfunction of the dermis, with ultrastructural disruption of elastic fibers [143]. This is due to an imbalance in collagen remodeling relative to proteolysis that occurs in the extracellular matrix. UVA-irradiated cultured human fibroblasts and human epidermis have been shown to induce the expression of collagenase, a protease that degrades elastic fibers in the dermis [144]. Moreover, the addition of cholesterol oxidants derived from the 1O2 oxidation reaction to mouse fibroblasts causes them to accumulate in lipid rafts, as well as upregulates matrix metalloprotease-9 (MMP-9) activity [145]. The UVA irradiation of cells under protoporphyrin administration results in the upregulation of MMP-9 activity with the formation of cholesterol oxide [145]. This suggests that cholesterol oxidation products produced by 1O2 may alter the structure of lipid rafts, resulting in the activation of signaling pathways that induce MMP-9 expression, leading to skin aging.
5.1.4. Skin Cancer
DNA nucleobases are targets of oxidation reactions [44]. 1O2, as well as highly reactive HO•, are oxidants that can damage cellular DNA [146,147,148]. Since UVA is among the carcinogens to which organisms are exposed, it is important to understand the mechanisms that cause DNA damage [149]. UVA in sunlight is a potential source of oxidative DNA damage in the skin [150,151]. As discussed in the previous chapter, exposing skin to UVA radiation produces 1O2. The reactivity of 1O2 to nucleobases tends to occur in the order guanine > cytosine > adenine > uracil > thymine [152].
The introduction of an oxo group into the C8 of guanine and the addition of a hydrogen atom to the nitrogen of N7 yields 8-oxo-dG (Figure 3C). The presence of 8-oxo-dG in DNA causes problems in the S phase of the cell cycle. In the S phase, cells must replicate an exact copy of the genome. Unlike most other types of DNA damage, 8-oxo-dG does not stop the replication process but rather creates a point mutation. Normally, guanine pairs with cytosine (C), whereas 8-oxo-dG mimics thymine (T), thus forming a pair with adenine (A) [153]. Furthermore, the A:8-oxo-dG mispair does not cause helix distortion in the DNA backbone, thus avoiding correction by the error detection mechanism of replication polymerase [154]. This results in mutations that are essentially C:G→A:T mutations. The presence of C:G→A:T mutations in many cancers emphasizes the importance of 8-oxo-dG in cancer pathogenesis [155]. The amount of 8-oxo-dG produced is approximately 103/cell per day in normal cells, but increases to 105/cell in cancer cells [156]. 8-oxo-dG is also markedly increased in human skin cells after UVA irradiation [157]. There are reports that 8-oxo-dG is involved in photocarcinogenesis [158,159]. It has also been reported that 8-oxo-dG is increased in epidermal cells after chronic broadband UVB irradiation in Ogg1 knockout mice, which are unable to eliminate oxidized bases and are susceptible to skin cancer [160]. However, cyclobutane pyrimidine dimers observed in UV-irradiated DNA are also known to correlate with skin cancer development [161]. It is thought that DNA mutation due to 1O2 generation is not the only cause of UV-induced carcinogenesis, but that various mutagenic molecules are involved in the pathogenesis in a complex manner.
Furthermore, 8-oxo-dG has been used as a biomarker to evaluate the degree of oxidative stress, and its application has been attempted as a risk assessment for many diseases [162].
5.1.5. Porphyria
Porphyria is a rare genetic disorder caused by a deficiency in any of the eight enzymes involved in the heme metabolism, resulting in the accumulation of the photosensitizing substance porphyrin or its precursors [163]. Porphyrin accumulation in the skin also causes photosensitivity, resulting in burn-like skin lesions [164]. The nine porphyria are divided into “cutaneous porphyria” and “acute porphyria”. Among cutaneous porphyria, photosensitivity symptoms begin to appear shortly after birth for congenital erythropoietic porphyria (CEP) and hepato-erythropoietic porphyria (HEP), around age 5–6 for erythropoietic protoporphyria (EPP), and after age 50 for late-onset porphyria (PCT). Exposure to sunlight causes pain, itching, redness, and swelling, and, in severe cases, blister-like burns. Porphyrins accumulated in skin tissue become excited by the absorption of light and induce the production of 1O2, resulting in tissue and vascular damage due to complement activation. In addition, the release of histamine, kinins, and chemotactic factors is thought to cause skin damage [165]. Currently, there is no cure for porphyria, and photoprotection is the only way to treat this disease. Beta-carotene, a scavenger of 1O2, is widely used to treat photosensitivity caused by EPP [166,167], but it has also been reported to be less useful for photosensitivity associated with PCT [167,168].
The 1O2 produced via porphyrins causes protein oxidation and aggregation [169,170]. In a porphyria model mouse, in which porphyrin accumulation was induced by 3,5-diethoxycarbonyl-1,4-dihydrocollidine, protein aggregation in liver tissue was observed upon safelight exposure compared to non-exposure [171]. Moreover, overexposure to sunlight in EPP and PCT is associated with liver dysfunction, cirrhosis, gallstones, and acute liver failure, and the relationship between hepatic damage in porphyria and 1O2 production by irradiation is becoming clearer.
5.1.6. Smith–Lemli–Opitz Syndrome and Statin-Induced Skin Disorders
Smith–Lemli–Opitz syndrome (SLOS) is caused by mutations in the DHCR7 gene, which encodes 7-dehydrocholesterol (7-DHC) reductase, involved in the final step of cholesterol biosynthesis [172,173]. SLOS shows a cholesterol deficiency and increased 7-DHC in the plasma and tissues [173]. A variety of symptoms are recognized in SLOS, including growth retardation, microcephaly, intellectual disability, characteristic facial features, and external malformations; however, hypersensitivity to ultraviolet light is recognized as a symptom of SLOS [174]. Although 7-DHC itself does not absorb UVA and is not a direct source of photosensitivity in SLOS, 9-DDHC (Figure 8I), a 7-DHC metabolite found in the plasma of SLOS patients, is highly absorbable to UVA [119]. Furthermore, UVA exposure to 9-DDHC generates 1O2, providing a mechanism for the pathogenesis of photosensitivity in SLOS [119].
In addition, some statins inhibit 7-DHC reductase [175]; thus, the accumulation of 9-DDHC may also be involved in statin-induced skin photosensitivity [176] and cataracts [177].
5.1.7. Skin Disorders Caused by Exogenous Photosensitizers
Pheophorbide (Figure 8F), a degradation product of chlorophyll (Figure 8D), is a photosensitizing substance [178]. Pheophorbide is sometimes found in food, and the exposure of humans [179] and animals [180] to light after ingesting this compound can cause dermatitis due to photosensitivity. Cholesterol 5α-OOH (Figure 6) was detected in rat skin administered with pheophorbide and exposed to visible light [99].
The thiopurine prodrugs azathioprine and 6-mercaptopurine are widely prescribed for the treatment of leukemia and autoimmune diseases [181]. Although these drugs have shown therapeutic efficacy, long-term administration has been reported to increase the risk of basal cell carcinoma and squamous cell carcinoma by 10- and 65- to 250-fold, respectively [182]. These adverse effects are induced by sunlight exposure. Thiopurine derivatives absorb UVA, which is believed to be responsible for producing 1O2.
Ibuprofen and ketoprofen, both marketed as nonsteroidal anti-inflammatory drugs with antipyretic and analgesic properties, also produce 1O2 due to their photosensitizing effects and have been associated with adverse skin symptoms, including photosensitivity [183]. Some commonly used pharmaceutical compounds whose side effects include skin disorders associated with photosensitivity may also produce 1O2.
5.1.8. Diagnosis and Evaluation of Skin Diseases by Measuring 1O2-Mediated Products
In skin diseases, the measurement of 1O2-derived lipid peroxidation products in sebum, especially SqOOHs, may be useful in the diagnosis of skin aging and photosensitivity. On the other hand, while the diagnosis of porphyria and SLOS can be made by measuring porphyrin and 7-DHC, respectively, the measurement of 1O2-derived lipid peroxidation products in sebum may be useful in elucidating the pathogenesis and understanding a patient’s disease status. In skin cancer, 8-oxo-dG may be useful in assessing the risk of DNA mutations induced by UV, but it should be evaluated in combination with the analysis of compounds produced by UV irradiation, such as cyclobutane pyrimidine dimers.
5.2. Ophthalmological Diseases
Since sunlight directly impacts the skin and eyes, oxidative stress is involved not only in the skin, but also in the eyes. In this section, we focus on eye structures, light permeation, and dysfunctions, followed by diseases related to 1O2-mediated oxidative stress.
5.2.1. Structure and Optical Transparency of the Ocular Bulb
Light passes through the cornea, aqueous humor, and pupil through the lens and vitreous body, to reach the retina. The pupil regulates light, and the crystalline lens acts as the lens. The retina is a transparent membrane that lines the inside of the eyeball. It mainly consists of a layered structure of four types of cells: retinal ganglion cells, communication/glial cells, photoreceptors, and retinal pigment epithelium (RPE) cells of the vitreous. Light is received by photoreceptors; the pigment cell layer is located outside the photoreceptor layer. Under visible light, the light that passes through the eye differs depending on its wavelength, and UVA (320–400 nm), UVB (280–320 nm), and UVC (100–280 nm) are absorbed by the cornea and lens [184,185], although UV can reach the retina in an aphakic eye (i.e., an eye with no crystalline lens). In the human eye, light with a wavelength of 400–760 nm reaches the retina with little absorption from the cornea to the vitreous body. Since mid- and far-infrared rays are absorbed by water, they are absorbed by water in the cornea, lens, and aqueous humor and do not reach the fundus of the eye. Since near-infrared light has low water absorption, it penetrates the choroid [186].
5.2.2. Photooxidative Stress
Visual cells, which are photoreceptors, contain visual substances of chromoproteins that receive light. They are composed of two types of cone cells that sense colors and rod cells that sense the intensity (brightness and darkness) of light. Cone cells have photopsins, and rod cells have rhodopsins. 1O2 is produced by the oxygen present in photoreceptors [187]. In addition, photoreceptor cells and retinal pigment epithelial cells accumulate waste products, called lipofuscin, with aging [188], and these are also activated by light absorption to produce radicals [189,190].
Lipofuscin is considered a waste product in the lysosome, consisting primarily of 30–58% protein, 19–51% lipid-like substances (oxidation products of PUFA), carbohydrates, and trace metals (2%), including iron, copper, aluminum, zinc, zinc calcium, and manganese [191]. Lipofuscin cannot be degraded by lysosomal hydrolytic enzymes because of the polymerization and cross-linking of peptides with aldehydes, resulting in a plastic-like structure that is not biologically degradable and accumulates in neurons and cardiomyocytes with age. Retinal lipofuscin has been shown to exhibit strong photosensitizing properties when photoexcited in the presence of oxygen [189].
Recently, fluorescent bis-retinoid N-retinyl-N-retinylidene ethanolamine (A2E) (Figure 10A,B) has been implicated in photooxidation in the retina [192,193]. A2E is the main component of lipofuscin that accumulates in RPE cells during aging. Rhodopsin, a pigment protein present in photoreceptor cells, is composed of a protein moiety called opsin and 11-cis-retinal (Figure 10A,B). The retina contains vitamin A (retinol). Rhodopsin is broken down into opsin and all-trans-retinal when it absorbs light. The RPE regenerates all-trans-retinal to 11-cis-retinal (a process called the visual cycle) (Figure 10B). During this regeneration process, all-trans-retinal reacts with phospholipids and another all-trans-retinal generates A2E (Figure 10A,B). A2E, a major component of lipofuscin, generates 1O2 upon light stimulation, especially high-energy blue light [194].
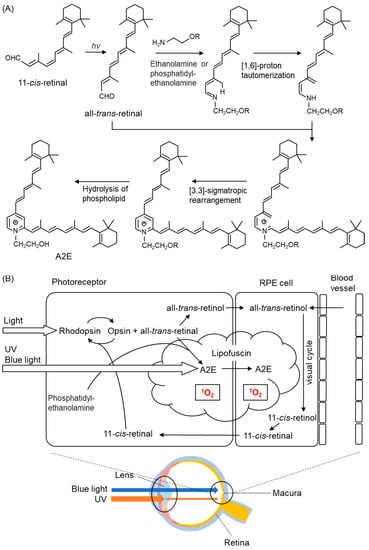
Figure 10.
The structures of biosynthesis of A2E. (A) After Schiff base formation between all-trans-retinal and ethanolamine or phosphatidylethanolamine, a [1,6]-proton tautomerization to enamine follows. After further Schiff base formation with a second molecule of all-trans-retinal, a [3,3]-sigmatropic rearrangement is followed by the hydrolysis of the linked phosphatidylethanolamine adduct, resulting in the formation of N-retinyl-N-retinylidene ethanolamine (A2E). (B) 1O2 production process mediated by A2E in the retina. Light irradiation of A2E accumulated in lipofuscin generates 1O2.
ROS and radicals generated by light act on the cell membrane lipids of photoreceptors to form lipid radicals [195,196]. Recently, photo-oxidative stress has been found to lead to retinal aging and is a factor in the onset of age-related macular degeneration [195,196].
5.2.3. Compounds Protecting against 1O2 in the Macular Pigment and Lens
The macula is susceptible to oxidative damage owing to the lack of a retinal inner layer, which is affected by intense light. Therefore, the retina has a macular pigment composed of lutein and zeaxanthin, which are carotenoids, and meso-zeaxanthin [197], which is converted from lutein by the RPE-65 enzyme [198,199].
The lens contains lutein and vitamin C, which have a protective mechanism against blue light, with an absorption peak at 400–500 nm. In addition, these macular pigments and carotenoids have an antioxidant ability to eliminate 1O2 and are thought to maintain retinal homeostasis [198].
5.2.4. Blue Light Hazard
Visible blue light (400–500 nm) from light-emitting diode (LED), mobile phones, and industrial equipment is increasingly being used, and we are susceptible to this in our daily lives. The accumulation of photo-oxidative stress caused by blue light, called blue light hazard, has been reported to cause chronic retinal changes [200,201].
Marie et al. exposed a 10 nm wide light band, range 390–520 nm, to primary retinal pigment epithelial cells treated with A2E and measured the precise action spectrum that produced the highest amount of ROS in the cells [194]. Of the spectrum of sunlight reaching the retina, blue light at 415–455 nm produced the highest amount of ROS and induced mitochondrial dysfunction. Lipofuscin and A2E accumulate in the RPE with aging, and blue light induce a photooxidative reaction that promotes cell death and angiogenesis. The need for the elderly to filter these wavelengths of light is emphasized.
Exposure to blue light (415–455 nm) for 15 h decreased activities of SOD and catalase and increased oxidized glutathione (GSSG) and ROS in A2E-treated RPE cells [194], suggesting that the balance between the oxidant and antioxidants is readily disrupted by massive blue light exposure [194].
5.2.5. Age-Related Macular Degeneration
Age-related macular degeneration (AMD) is a disease in which the photoreceptor cells in the macula of the central fundus of the eye are damaged. There are two types: the exudative type with neovascularization generated from the choroid that feeds the retina, and the atrophic type without it. AMD is a multifactorial disease characterized by high oxygen consumption, massive radiation exposure, and high PUFA content in the retina. Photooxidative stress is presumed to be one of the causes of retinal disorders such as AMD [200]. PUFAs in the membranes are targets for ROS, which drastically increases the susceptibility of the retina to photochemical damage. In addition, as described above, A2E-containing lipofuscin, which accumulates in RPE cells with aging, is activated by light, and 1O2 is generated from abundant oxygen in the retina. In the early stages of the disease, the body’s defense mechanism acts. However, the lesion begins to progress as the body’s defense mechanisms decline with age.
5.2.6. Glaucoma
Glaucoma is a progressive glaucomatous optic neuropathy that causes visual field loss and irreversible blindness [202,203,204]. The death and axon loss of retinal ganglion cells (RGCs) cause glaucomatous optic neuropathy [202]. Elevated intraocular pressure (IOP) is a primary risk factor for open-angle glaucoma (OAG) including primary OAG (POAG) [202]. In OAGs, the elevation of IOP is explained by a reduction in aqueous humor (AH) outflow at the trabecular meshwork (TM) due to qualitative and quantitative changes in the extracellular matrix in the TM [205]. Numerous reports have shown that various oxidative stressors induce RGC damage [206,207]. For example, antioxidant thioredoxins prevent glaucomatous tissue injury, specifically glutamate- and IOP-induced RGC death [208,209], indicating that oxidative stress is thought to be involved in IOP elevation and then RGC loss in POAG.
We previously demonstrated the involvement of oxidative stress in the pathogenesis of glaucoma by measuring AH and serum HODE levels derived from free radical-mediated oxidation [94,210,211], suggesting that systemic oxidation is at least partially involved in the disease. Furthermore, the levels of HODEs/LA (oxidized/parent molecules ratio, LA; linoleic acid) in AH correlated with those in serum, suggesting that ocular oxidative injury proceeds simultaneously with systemic oxidative stress [211]. The serum levels of 10- and 12-(Z,E)-HODEs/LA formed via 1O2 specific oxidation were correlated with IOP [94], which are indices of glaucoma severity derived from TM cell dysfunction. One possible process by which 1O2 is produced in the eye is type II photooxidation via a sensitizer present in the vicinity of the reaction milieu, similar to a cataract [212]. The specific region of oxidative injury has not been identified because sunlight does not reach TM cells. In addition, the pathways of the excretion and circulation of HODEs formed in the eyes remain undefined. However, our findings suggested that cerebrospinal HODE levels were well reflected in plasma levels [213,214]. Other studies have simultaneously analyzed the systemic and local redox status, suggesting that alterations in systemic oxidant and antioxidant levels reflect local redox status [215,216,217].
5.2.7. Cataract
Epidemiological approaches have indicated that sun exposure is a risk factor in age-related cataracts [218,219]. UVB is mostly filtered out by the cornea and aqueous humor [220]. For this reason, UVA, which occupies nearly 95% of the UV in sunlight, has been associated with cataracts [221]. It has been proposed that chromophores with UVA-visible light absorption properties, which accumulate in the lens with age, influence the development of cataracts through the ROS generation by photosensitizing reactions [222].
The major protein components of the lens are crystallins, of which there are three main types: α-, β-, and γ-crystallin. The most abundant of these is α-crystallin, which functions to maintain lens transparency and acts as a protective chaperone for the lens [223]. As α-crystallin is not turned over, damage to the amino acids/proteins constituting α-crystallin accumulates. This leads to changes in refraction and lens opacity (cataract formation) [224].
Tryptophan metabolites (kynurenine (Figure 8G), 3-hydroxykynurenine (Figure 8G), 3-hydroxykynurenine o-β-D-glucoside, and 4-(2-amino-3-hydroxyphenyl)-4-oxobutanoic acid o-β-D glucoside) are present in significant concentrations in the lens and act as endogenous chromophores and UV-absorbing filters [225]. These endogenous chromophores absorb UV and become excited but regenerate to their original ground state without producing radicals or 1O2. These chromophores undergo deamination under physiological conditions and are covalently bound to proteins (crystallin) via Michael addition reactions [226]. As covalent modification progresses gradually with age, the lens turns yellow. Paradoxically, tryptophan derivatives, which act as UV filters, have also been shown to generate 1O2 by acting as photosensitizers when bound to proteins [226]. However, the association between cataract development and 1O2 remains undefined and has not been confirmed in our studies [94,210].
5.2.8. 1O2-Derived Peroxidation Products in the Diagnosis and Evaluation of Ophthalmological Diseases
The measurement of 1O2-derived lipid peroxidation products in tissues removed during cataract and glaucoma surgery may provide assistance in elucidating the pathophysiology and in selecting the use of singlet oxygen scavengers. As our results indicate, measuring lipid oxides in circulation may also be useful for diagnosis and pathophysiological evaluation.
5.3. Diabetes Mellitus
5.3.1. Biomarkers for Diabetes and Diabetic Complications
Diabetes is characterized by a deficiency of the secretion or action of insulin, resulting in microvasculature damage to the retina, renal glomerulus, and heart, as well as peripheral neuropathy. Diabetes can be fatal if its complications include nephritis and atherosclerosis. Type 2 diabetes mellitus (T2D) indicates elevated blood glucose levels caused by the impairment of insulin secretion and insulin resistance. Prediabetic states, including impaired fasting glucose, impaired glucose tolerance (IGT), or slightly elevated blood glucose levels, may precede T2D for the year [227,228]. Progression from prediabetes to T2D can be prevented or delayed by improving diet and increasing physical activity.
Diabetic vascular complications include nephropathy, myocardial infarction, and glaucoma. The early detection of diabetes is important to prevent complications. Several studies have been conducted on the early detection of these complications. Biomarkers of diabetic nephropathy include urinary heme oxygenase-1 (HO-1) [229] and 8-hydroxydeoxyguanosine [230,231], and circulating microRNA 130b [232]. S-glutathionylation [233] and oxidized dityrosine-containing protein [234] are considered candidate markers of vasculopathy in diabetes. Angiotensin-II, protein kinase C (PKC), and advanced glycation end products (AGEs) activate NADPH oxidase and upregulate the production of ROS, leading to cardiac dysfunction [235,236]. The metallic elements selenium [237], copper, and zinc [238] are associated with the detection of diabetes risk in pregnant women. Recently, band 3 anion transport protein, also known as anion exchanger 1 or band 3, or solute carrier family 4 member 1, was proposed for the early detection of the glycation of hemoglobin leading to AGEs [239,240,241]. To completely detect diabetes and its complications, a combination of several biomarkers is necessary.
5.3.2. Diabetic Biomarkers of Lipid Peroxidation
Several studies have examined the association of oxidation products with diabetes pathology [242,243,244,245,246]. Griesser et al. revealed the interplay between lipid and protein modifications using animal models. A large cohort study showed that imbalances in the redox system contribute to the development of T2D [245]. Leinish et al. clarified that the structural and functional alterations in RNase A result from oxidation by 1O2 and ROO•, not solely from histidine and tyrosine cross-linking [246].
We recently found that 10- and 12-(Z,E)-HODE, 1O2-specific products derived from linoleic acid, significantly correlated with a risk index for impaired glucose tolerance and insulin resistance in oral glucose tolerance tests performed on healthy volunteers [92,247,248]. Free radical-specific lipid oxidation products, such as 9- and 13-(E,E)-HODE and 7β-hydroxycholesterol, and hydroxyeicosatetraenoic acids (HETEs), which are oxidation products derived from arachidonic acid, have not been detected. The process by which these 1O2-specific lipid oxidation products are produced in diabetic pathology is speculated to involve the reaction between H2O2 and ClO− derived from MPO (Figure 2B). This 1O2 generation mechanism is mediated by MPO from activated phagocytes [249,250] or eosinophils peroxidase [251,252]. Several reports have shown that neutrophil-derived MPO plays an important role in diabetic vascular injury (Figure 11) [253,254,255]. The source of H2O2 in diabetic pathology is thought to be NADPH oxidase in the vascular endothelial cells [253]. MPO released from activated neutrophils is known to bind to the vessel wall for several days [256,257]. The H2O2 derived from hyperglycemia-activated NADPH oxidase may be used by MPO bound to the vascular endothelium to produce HOCl, resulting in the production of 1O2 and vascular injury. Compounds that inhibit the activity of MPO, such as hydroxamic acids, hydrazides, and azides, have potentially harmful side effects. In contrast, quercetin was recently reported to inhibit MPO-dependent HOCl production and prevent vascular endothelial injury [258]. Onyango et al. reviewed the contribution of 1O2 to insulin resistance and reported that 1O2 generates bioactive aldehydes and induces mitochondrial DNA modification and endoplasmic reticulum stress, which lead to insulin resistance [259].
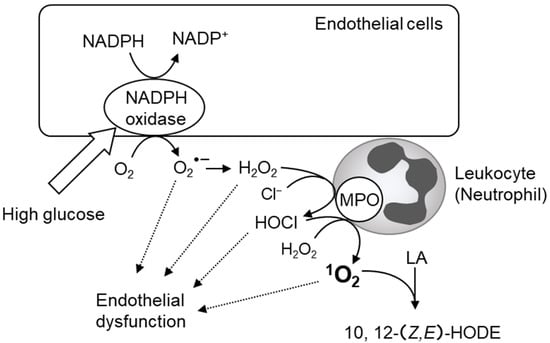
Figure 11.
Assumed scheme of 1O2 production via NADPH oxidase and myeloperoxidase (MPO) in vascular injury in diabetes mellitus. High glucose produces hydrogen peroxide (H2O2) by NADPH oxidase in vascular endothelial cells. 1O2 is generated via MPO in activated neutrophils bound to the vessel wall.
Clinicians may be able to manage and/or advise patients regarding their food and exercise habits before the onset of diabetes, by evaluating the levels of 1O2-induced lipid peroxidation products in the near future. In any event, more information and studies are needed to determine the pathological significance of 1O2 and 10,12-(Z,E)-HODE.
5.4. Bronchial Asthma
Bronchial asthma is characterized by chronic airway inflammation, reversible airflow obstruction, airway hyperresponsiveness, and structural changes in the airways, and its clinical manifestations include variable airway narrowing, cough, dyspnea, and wheezing [260]. Asthma is caused by multiple interactions between epigenetic regulation and environmental factors. Although this heterogeneity has made it difficult to define biologically distinct subgroups based on the differences in clinical phenotypes, recent advances in genetics and molecular biology have led to the pathogenetic classification of endotypes [261]. These characteristics of asthma may be helpful for understanding the pathophysiology and precision of medicine.
More than 300 million people worldwide suffer from asthma, of which approximately 10% have severe asthma. Symptom control in severe refractory asthma is difficult to achieve despite optimal treatment with high-dose inhaled corticosteroids (ICS) and long-acting adrenergic receptor β2 agonists [262]. Airway inflammation in asthma is typically involved in the submucosal infiltration of activated Th2 lymphocytes, neutrophils, eosinophils, mast cells, and macrophages [263,264]. Although Th2 cytokines and eosinophilic inflammation are typical factors in the development of mild to moderate asthma in response to ICS, a subset of asthmatics with Th2 high-eosinophil-predominant have a refractory course despite optimal treatment [265]. In more severe cases of steroid-resistance, the cellular environment is characterized by airway inflammation induced by Th1/Th2 cytokine expression and neutrophil, with poorly reversible airflow obstruction [265,266,267]. In adults, elevated neutrophil counts in sputum are related to disease severity [268] and the persistence of symptoms [269]. These reports suggest an important role for neutrophils in asthma and their association with a more steroid-refractory subtype.
Neutrophils are recruited to the site of pathogen invasion for immune defense [270,271]. Several proinflammatory mediators, including cytokines, chemokines, and complements, contribute to the regulation of lung neutrophilic recruitment. Accumulated neutrophils release chemotactic factors that attract monocytes and/or macrophages to the infection site and, subsequently, exacerbate airway inflammation [272]. In particular, Th17/IL-17 and IL-8 are involved in the pathogenesis of steroid-resistant asthma [273,274]. Neutrophil extracellular traps (NETs) comprising neutrophil DNA, whose formation is facilitated by proinflammatory cytokines, are also thought to be implicated in the pathological condition of neutrophilic asthma [274,275,276,277]. Activated neutrophils release not only inflammatory cytokines, but also ROS and MPO, which damage airway endothelial cells and exacerbate allergic inflammation [278,279,280,281]. Plasma MPO levels in asthmatic patients have been proposed as biomarkers for the evaluation of asthma severity in adults [277,282]. As described in Section 2 and Section 5.3.2, MPO contributes to the production of 1O2 mediated by the progression of the reaction with H2O2 and Cl− producing HClO. These suggest that 1O2 may be associated with pathogenesis phenotypes in refractory asthma. We have demonstrated a positive correlation between the levels of 1O2-mediated oxidation products 10- and 12-(Z,E)-HODEs, and the levels of MPO activity and IL-17-derived nerve growth factor (NGF) in the bronchoalveolar lavage fluid (BALF) of an asthmatic mouse model with mixed inflammation [97]. As increased NGF in the BALF of asthmatic patients induces smooth muscle hyperplasia in the airway [283,284] and anti-NGF antibodies improve airway hyperresponsiveness [285], NGF is also considered a therapeutic target for lung disease [286]. Interestingly, the generation of 1O2 with an endoperoxide [(3-(1, 4-epidioxy-4-methyl-1, 4-dihydro-1- naphthyl) propionic acid] increased NGF and IL-8 levels in human bronchial epithelial cells, suggesting that 1O2 generation may be an upstream event of increase in NGF.
Recent studies have described neutrophil-derived MPO as a potential biomarker for neutrophilic asthma [277,282]. Therefore, we would expect that 10, 12-(Z,E)-HODEs, which are elevated according to neutrophil recruitment, could also be a prominent biomarker with more stability than MPO for neutrophilic asthma. Glucocorticoids, which are used as the first choice for asthma therapy, fail to attenuate neutrophilic inflammation and may even promote neutrophil survival [268,287]. It seems reasonable to choose macrolide antibiotics for the treatment of asthma rather than steroids when the levels of 10- and 12-(Z,E)-HODEs are increased. However, the use of macrolides is not appropriate for the long-term treatment of neutrophilic asthma due to the risk of bacterial resistance. Although future studies are needed to determine how 1O2 is related to the pathological condition of asthma patients, 10- and 12-(Z,E)-HODEs may be useful in stratifying patients with refractory asthma and in selecting treatment options.
6. 1O2 Scavengers
The living body contains superoxide dismutase and catalase, which can scavenge ROS such as O2•− and H2O2, but it does not have enzymes that can scavenge 1O2. Substances that can effectively scavenge 1O2 depend on exogenous compounds such as dietary carotenoids.
6.1. Carotenoids
Carotenoids are plant pigments that are pro-vitamin A and potent scavengers of 1O2. The 1O2 scavenging of carotenoids is mainly based on physical scavenging. The excitation energy of 1O2 is transferred to carotenoids (singlet carotenoids; 1Car) to produce triplet oxygen (3O2) and triplet carotenoids (3Car). 3Car, which receives excitation energy, returns to ground state carotenoids by releasing heat as energy. Thus, carotenoids can repeatedly scavenge 1O2 [288].
1Car + 1O2 → 3Car + 3O2
Carotenoids are the most potent 1O2 scavengers found in nature, and carotenoids with 11 carbon atoms involved in the π-conjugation length, such as lycopene (Figure 12A), β-carotene (Figure 12B), and astaxanthin (Figure 11C), are the most efficient 1O2 scavengers [288]. Among carotenoids, studies have mainly been conducted on the products associated with the 1O2 scavenging of β-carotene, and β-carotene 5,8-endoperoxide (Figure 12B) is a specific product mediated by 1O2 oxidation [102,289,290]. In contrast, β-carotene 5,6-epoxide is formed by a free radical-mediated reaction. β-Carotene-5,8-endoperoxide has been detected in vitro [291,292] and in vivo [289]. Among carotenoids, lycopene has the strongest 1O2 scavenging capacity [293]. The second-order rate constants of physical quenching (kq) in ethanol/chloroform were 31,000 × 106 M−1s−1 for lycopene, 14,000 × 106 M−1s−1 for β-carotene, 8000 × 106 M−1s−1 for lutein, and 10,000 × 106 M−1s−1 for zeaxanthin, indicating that lycopene was the most efficient 1O2 quencher [294]. Lycopene and β-carotene also exhibited the fastest 1O2 scavenging rate constants under the conditions of the model membrane system using liposomes [295]. Unlike other carotenoids, lycopene does not possess a ring structure. Since 2-methyl-2-hepten-6-one (Figure 12A) and apo-6′-lycopenal (Figure 12A) were detected under 500 W light irradiation in the presence of methylene blue, a photosensitizer, lycopene is assumed to react with 1O2 via a dioxetane intermediate [296,297].
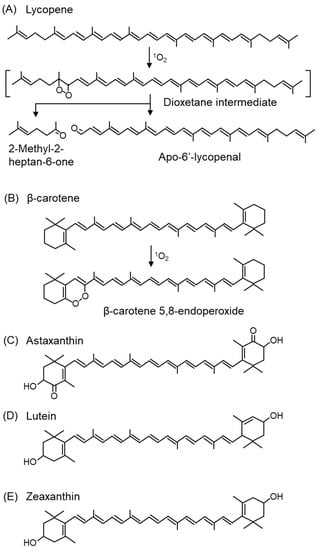
Figure 12.
The structures of carotenoid and peroxidation products from lycopene and β-carotene. (A) Lycopene, (B) β-carotene, (C) astaxanthin, (D) lutein, (E) zeaxanthin.
Carotenoids in plants exist in close proximity to chlorophyll in chloroplasts and scavenge 1O2 generated by the photosensitizing effect of chlorophyll during light absorption. Thus, carotenoids have a protective property against light stress in plants. In addition, carotenoids, such as xanthophylls (lutein (Figure 12D) and zeaxanthin (Figure 12E), specifically accumulate in the macula of the retina, which is an organ exposed to light. In sunlight, the energy of blue light (400 nm), in particular, is 100-fold greater than that of red light (590 nm) and causes severe damage to cells. Since lutein and zeaxanthin have absorption maxima for light at approximately 440 nm, these carotenoids can efficiently absorb blue light. Therefore, the accumulation of lutein and zeaxanthin in the retinal macula can prevent the oxidative degeneration of macular tissue and reduce the risk of AMD and other diseases. Lutein and zeaxanthin are also present in the lens, where they prevent the development of cataract [298,299]. The consumption of tomatoes rich in β-carotene and lycopene can reduce the formation of erythema in human skin due to UV irradiation [300,301].
6.2. Vitamin E (Tocopherol)
α-Tocopherol, a primary fat-soluble antioxidant in vivo, also has 1O2 scavenging capacity that is approximately 30–100 times weaker than that of carotenoids [302]. However, the 1O2 scavenging capacity of carotenoids is also reported to be reduced in liposomes, which are biomembrane models, compared to solution [303].Considering the level of tocopherols in vivo, the in vivo 1O2 scavenging activity of α-tocopherol is considered to be functional. Among tocopherols, α->β->γ->δ-tocopherol have 1O2 capacities in this order [304,305]. Tocotrienols, which are homologues of tocopherols, also have 1O2 scavenging ability in the order of α->β->γ->δ-tocotrienol [304].
The oxidation product of α-tocopherol produced by 1O2 is α-tocopherylquinone, which can be measured in biological samples [306,307,308]. α-Tocopherylquinone is not an 1O2-specific marker because it is also produced by ROS other than 1O2.
6.3. Other Compounds
As previously mentioned, PUFA is susceptible to oxidation by 1O2 and has 1O2 scavenging capacity, although it is very weak compared to carotenoids [309].
Bakuchiol (Figure 13A), a terpeno-phenolic compound, is a functional analog of retinol that has attracted attention in skincare for its ability to induce retinol gene expression and stimulate collagen production [310]. Bakuchiol protects retinol from degradation by 1O2 produced by H2O2 and lithium molybdenum. It was shown to inhibit the 1O2-induced peroxidation of squalene, prevent pore clogging, and reduce the onset of acne by 42% with topical bakuchiol treatment in 54 volunteers [311]. In addition, the 12-week application of a cream containing bakuchiol (0.5%) improved wrinkles and pigmentation [312].
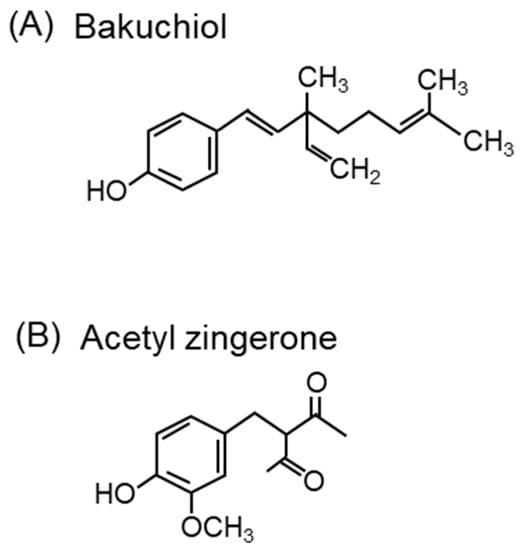
Figure 13.
Compounds with 1O2 scavenging activity other than carotenoids. The structures of (A) bakuchiol and (B) acetyl zingerone.
Zingerone (4-[4-hydroxy-3-methoxyphenyl]-2-butanone) from Zingiber officinale Roscoe (ginger) scavenges 1O2 [313]. The structure of zingerone is similar to that of turmeric-derived curcumin (1,7-bis[4-hydroxy-3-methoxyphenyl]-1,6-heptane-3,5-dione). Zingerone and curcumin can both prevent skin aging. Acetyl zingerone (3-(4-hydroxy-3-methoxybenzyl)pentane-2,4-dione) (Figure 13B), a multifunctional skincare ingredient, was synthesized using the molecular structures of zingerone and curcumin [314]. Curcumin has been shown to have 1O2 scavenging activity in an in vitro experimental system by ESR spectroscopy using TEMP as a spin-trap [315]. Acetyl zingerone, which has a higher 1O2 scavenging activity than α-tocopherol [316], decreases the expression of genes associated with extracellular matrix degradation (MMP-3 and cathepsin V) and inhibits the activity of MMP-1, -3, and -12 [314]. Clinical reports have demonstrated that treatment with a cream containing 1% acetyl zingerone applied twice daily for 8 weeks improves wrinkles, hyperpigmentation, and erythema [317].
7. Summary and Outlook
As previously described, diseases and dysfunctions mediated by or, at least, related to 1O2 can be categorized into two groups: those caused by the UV irradiation of photosensitizers accumulated in the living body, and those resulting from the activation of MPO in leukocytes. The former includes cutaneous photosensitivity and retinal diseases, which have been clinically investigated. For the latter, we have previously reported findings in diabetic patients, glaucoma patients, and in an asthma mouse model. MPO is expressed in leukocytes and is implicated in many inflammatory diseases [318]. MPO is associated with cardiovascular disease, atherosclerosis, glomerulonephritis, arthritis, and Alzheimer’s disease. Practical biomarkers are required for the early diagnosis, assessment, and progression of diseases, and for the evaluation of treatment efficacy. However, as summarized in Table 2 and Table 3, there are few reports on the analysis of lipid peroxidation products formed by 1O2 in clinical samples, except for SqOOHs. SqOOHs can be measured noninvasively as they are found in sebum; therefore, an increasing number of reports are available (Table 3), especially in the cosmetics and beauty industries. SqOOHs are lipid peroxidation products, but not specific products of 1O2. Since lipid peroxidation is a major oxidative injury in vivo, lipid peroxidation products, especially hydroxides, may be useful biomarkers. It should be noted, however, that lipid peroxidation products are generally not diagnostic indicators of specific diseases. Their usefulness should be enhanced by combination with other biomarkers. Further prospective studies and analysis of the correlation between lipid peroxidation products, specifically from 1O2, and the severity of disease progression should be conducted in the future. When disease risk can be assessed by a prominent biomarker, advice on diet, including carotenoids, and exercise habits can be provided.
Author Contributions
Writing—original draft preparation, K.M., A.U., M.S., M.T. and Y.Y.; writing—review and editing, K.M., A.U., M.S., M.T. and Y.Y.; visualization, K.M. and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partially supported by a Grant-in-Aid for JSPS KAKENHI Grant Number JP18K17951.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
Yasukazu Yoshida is an employee of LG Japan Lab, Inc. This paper has not received any form of financial support from LG Japan Lab, Inc. The findings of this paper do not result in any commercial or economic interest to LG Japan Lab, Inc. or any of the authors.
References
- Chen, Z.H.; Yoshida, Y.; Saito, Y.; Noguchi, N.; Niki, E. Adaptive response induced by lipid peroxidation products in cell cultures. FEBS Lett. 2006, 580, 479–483. [Google Scholar] [CrossRef]
- Haugaard, N. Cellular mechanisms of oxygen toxicity. Physiol. Rev. 1968, 48, 311–373. [Google Scholar] [CrossRef]
- Gottlieb, S.F. Effect of hyperbaric oxygen on microorganisms. Annu. Rev. Microbiol. 1971, 25, 111–152. [Google Scholar] [CrossRef]
- Gilbert, D.L. Symposium on oxygen. Introduction: Oxygen and life. Anesthesiology 1972, 37, 100–111. [Google Scholar] [CrossRef]
- Chance, B.; Sies, H.; Boveris, A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979, 59, 527–605. [Google Scholar] [CrossRef] [PubMed]
- Hackbarth, S.; Islam, W.; Fang, J.; Šubr, V.; Röder, B.; Etrych, T.; Maeda, H. Singlet oxygen phosphorescence detection in vivo identifies PDT-induced anoxia in solid tumors. Photochem. Photobiol. Sci. 2019, 18, 1304–1314. [Google Scholar] [CrossRef] [PubMed]
- Collin, F. Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 2407. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lv, H.; Sun, Y.; Zu, G.; Zhang, X.; Song, Y.; Zhao, F.; Wang, J. New porphyrin photosensitizers-Synthesis, singlet oxygen yield, photophysical properties and application in PDT. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2022, 279, 121447. [Google Scholar] [CrossRef]
- You, Z.Q.; Hsu, C.P.; Fleming, G.R. Triplet-triplet energy-transfer coupling: Theory and calculation. J. Chem. Phys. 2006, 124, 044506. [Google Scholar] [CrossRef]
- Dzebo, D.; Moth-Poulsen, K.; Albinsson, B. Robust triplet-triplet annihilation photon upconversion by efficient oxygen scavenging. Photochem. Photobiol. Sci. Off. J. Eur. Photochem. Assoc. Eur. Soc. Photobiol. 2017, 16, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Turro, N.J. Singlet oxygen and chemiluminescent organic reactions. In Modern Molecular Photochemistry; University Science Books: Melville, NY, USA, 1991; pp. 583–593. [Google Scholar]
- Hatz, S.; Poulsen, L.; Ogilby, P.R. Time-resolved singlet oxygen phosphorescence measurements from photosensitized experiments in single cells: Effects of oxygen diffusion and oxygen concentration. Photochem. Photobiol. 2008, 84, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.W.; Sinks, L.E.; Breitenbach, T.; Schack, N.B.; Vinogradov, S.A.; Ogilby, P.R. Single cell responses to spatially controlled photosensitized production of extracellular singlet oxygen. Photochem. Photobiol. 2011, 87, 1077–1091. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Kolodieznyi, D.; Creeger, Y.; Ballou, B.; Bruchez, M.P. Subcellular Singlet Oxygen and Cell Death: Location Matters. Front. Chem. 2020, 8, 592941. [Google Scholar] [CrossRef] [PubMed]
- Denys, G.A.; Devoe, N.C.; Gudis, P.; May, M.; Allen, R.C.; Stephens, J.T., Jr. Mechanism of Microbicidal Action of E-101 Solution, a Myeloperoxidase-Mediated Antimicrobial, and Its Oxidative Products. Infect. Immun. 2019, 87, e00261-19. [Google Scholar] [CrossRef]
- Neale, T.J.; Ullrich, R.; Ojha, P.; Poczewski, H.; Verhoeven, A.J.; Kerjaschki, D. Reactive oxygen species and neutrophil respiratory burst cytochrome b558 are produced by kidney glomerular cells in passive Heymann nephritis. Proc. Natl. Acad. Sci. USA 1993, 90, 3645–3649. [Google Scholar] [CrossRef] [PubMed]
- Russell, G.A. Deuterium-isotope Effects in the Autoxidation of Aralkyl Hydrocarbons. Mechanism of the Interaction of PEroxy Radicals1. J. Am. Chem. Soc. 1957, 79, 3871–3877. [Google Scholar] [CrossRef]
- Gonçalves, L.C.P.; Massari, J.; Licciardi, S.; Prado, F.M.; Linares, E.; Klassen, A.; Tavares, M.F.M.; Augusto, O.; Di Mascio, P.; Bechara, E.J.H. Singlet oxygen generation by the reaction of acrolein with peroxynitrite via a 2-hydroxyvinyl radical intermediate. Free Radic. Biol. Med. 2020, 152, 83–90. [Google Scholar] [CrossRef]
- Sun, S.; Bao, Z.; Ma, H.; Zhang, D.; Zheng, X. Singlet oxygen generation from the decomposition of alpha-linolenic acid hydroperoxide by cytochrome c and lactoperoxidase. Biochemistry 2007, 46, 6668–6673. [Google Scholar] [CrossRef]
- Chen, T.; Cohen, D.; Itkin, M.; Malitsky, S.; Fluhr, R. Lipoxygenase functions in 1O2 production during root responses to osmotic stress. Plant Physiol. 2021, 185, 1638–1651. [Google Scholar] [CrossRef]
- Khan, A.U.; Kasha, M. Singlet molecular oxygen in the Haber-Weiss reaction. Proc. Natl. Acad. Sci. USA 1994, 91, 12365–12367. [Google Scholar] [CrossRef]
- Miyamoto, S.; Ronsein, G.E.; Corrêa, T.C.; Martinez, G.R.; Medeiros, M.H.; Di Mascio, P. Direct evidence of singlet molecular oxygen generation from peroxynitrate, a decomposition product of peroxynitrite. Dalton Trans. 2009, 29, 5720–5729. [Google Scholar] [CrossRef] [PubMed]
- Bauer, G. The synergistic effect between hydrogen peroxide and nitrite, two long-lived molecular species from cold atmospheric plasma, triggers tumor cells to induce their own cell death. Redox Biol. 2019, 26, 101291. [Google Scholar] [CrossRef] [PubMed]
- Kanofsky, J.R.; Sima, P. Singlet oxygen production from the reactions of ozone with biological molecules. J. Biol. Chem. 1991, 266, 9039–9042. [Google Scholar] [CrossRef]
- Cerkovnik, J.; Erzen, E.; Koller, J.; Plesnicar, B. Evidence for HOOO radicals in the formation of alkyl hydrotrioxides (ROOOH) and hydrogen trioxide (HOOOH) in the ozonation of C-H bonds in hydrocarbons. J. Am. Chem. Soc. 2002, 124, 404–409. [Google Scholar] [CrossRef]
- Pershin, A.A.; Torbin, A.P.; Zagidullin, M.V.; Mebel, A.M.; Mikheyev, P.A.; Azyazov, V.N. Rate constants for collision-induced emission of O(2)(a(1)Δ(g)) with He, Ne, Ar, Kr, N(2), CO(2) and SF(6) as collisional partners. Phys. Chem. Chem. Phys. 2018, 20, 29677–29683. [Google Scholar] [CrossRef]
- Tao, F.; Gonzalez-Flecha, B.; Kobzik, L. Reactive oxygen species in pulmonary inflammation by ambient particulates. Free Radic. Biol. Med. 2003, 35, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Gurgueira, S.A.; Lawrence, J.; Coull, B.; Murthy, G.G.; González-Flecha, B. Rapid increases in the steady-state concentration of reactive oxygen species in the lungs and heart after particulate air pollution inhalation. Environ. Health Perspect. 2002, 110, 749–755. [Google Scholar] [CrossRef]
- Jensen, R.L.; Arnbjerg, J.; Ogilby, P.R. Reaction of singlet oxygen with tryptophan in proteins: A pronounced effect of the local environment on the reaction rate. J. Am. Chem. Soc. 2012, 134, 9820–9826. [Google Scholar] [CrossRef]
- Nascimento, R.O.; Prado, F.M.; Massafera, M.P.; Di Mascio, P.; Ronsein, G.E. Dehydromethionine is a common product of methionine oxidation by singlet molecular oxygen and hypohalous acids. Free Radic. Biol. Med. 2022, 187, 17–28. [Google Scholar] [CrossRef]
- Fouquerel, E.; Barnes, R.P.; Uttam, S.; Watkins, S.C.; Bruchez, M.P.; Opresko, P.L. Targeted and Persistent 8-Oxoguanine Base Damage at Telomeres Promotes Telomere Loss and Crisis. Mol. Cell 2019, 75, 117–130.e116. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Sun, Y.; Tsai, M.; Zhou, W.; Liu, J. Singlet O(2) Oxidation of a Deprotonated Guanine-Cytosine Base Pair and Its Entangling with Intra-Base-Pair Proton Transfer. Chemphyschem 2018, 19, 2645–2654. [Google Scholar] [CrossRef]
- Kato, S.; Shimizu, N.; Otoki, Y.; Ito, J.; Sakaino, M.; Sano, T.; Takeuchi, S.; Imagi, J.; Nakagawa, K. Determination of acrolein generation pathways from linoleic acid and linolenic acid: Increment by photo irradiation. NPJ Sci. Food 2022, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, A.; Ito, J.; Shimizu, N.; Kato, S.; Kobayashi, E.; Ohnari, H.; Sakata, O.; Naru, E.; Nakagawa, K. Linoleic acid and squalene are oxidized by discrete oxidation mechanisms in human sebum. Ann. N. Y. Acad. Sci. 2021, 1500, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Carroll, L.; Pattison, D.I.; Davies, J.B.; Anderson, R.F.; Lopez-Alarcon, C.; Davies, M.J. Superoxide radicals react with peptide-derived tryptophan radicals with very high rate constants to give hydroperoxides as major products. Free Radic. Biol. Med. 2018, 118, 126–136. [Google Scholar] [CrossRef]
- Moe, M.M.; Tsai, M.; Liu, J. Singlet Oxygen Oxidation of the Radical Cations of 8-Oxo-2′-deoxyguanosine and Its 9-Methyl Analogue: Dynamics, Potential Energy Surface, and Products Mediated by C5-O(2) -Addition. Chempluschem 2021, 86, 1243–1254. [Google Scholar] [CrossRef] [PubMed]
- Monzo-Beltran, L.; Vazquez-Tarragón, A.; Cerdà, C.; Garcia-Perez, P.; Iradi, A.; Sánchez, C.; Climent, B.; Tormos, C.; Vázquez-Prado, A.; Girbés, J.; et al. One-year follow-up of clinical, metabolic and oxidative stress profile of morbid obese patients after laparoscopic sleeve gastrectomy. 8-oxo-dG as a clinical marker. Redox Biol. 2017, 12, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Ponce, M.A.; Ramirez, J.A.; Galagovsky, L.R.; Gros, E.G.; Erra-Balsells, R. Singlet-oxygen ene reaction with 3β-substituted stigmastanes. An alternative pathway for the classical Schenck rearrangement. J. Chem. Soc. Perkin Trans. 2000, 2, 2351–2358. [Google Scholar] [CrossRef]
- Samuel, D.; Norrell, K.; Hilmey, D.G. Novel ring chemistry of vitamin B6 with singlet oxygen and an activated ene: Isolated products and identified intermediates suggesting an operable [3 + 2] cycloaddition. Org. Biomol. Chem. 2012, 10, 7278–7281. [Google Scholar] [CrossRef]
- Petrou, A.L.; Petrou, P.L.; Ntanos, T.; Liapis, A. A Possible Role for Singlet Oxygen in the Degradation of Various Antioxidants. A Meta-Analysis and Review of Literature Data. Antioxidants 2018, 7, 35. [Google Scholar] [CrossRef]
- Zeinali, N.; Oluwoye, I.; Altarawneh, M.K.; Almatarneh, M.H.; Dlugogorski, B.Z. Probing the Reactivity of Singlet Oxygen with Cyclic Monoterpenes. ACS Omega 2019, 4, 14040–14048. [Google Scholar] [CrossRef]
- Morales, J.; Günther, G.; Zanocco, A.L.; Lemp, E. Singlet oxygen reactions with flavonoids. A theoretical-experimental study. PLoS ONE 2012, 7, e40548. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.H.; Tsai, H.C.; Yu, P.L.; Chung, K.R. A Major Facilitator Superfamily Transporter-Mediated Resistance to Oxidative Stress and Fungicides Requires Yap1, Skn7, and MAP Kinases in the Citrus Fungal Pathogen Alternaria alternata. PLoS ONE 2017, 12, e0169103. [Google Scholar] [CrossRef] [PubMed]
- Di Mascio, P.; Martinez, G.R.; Miyamoto, S.; Ronsein, G.E.; Medeiros, M.H.G.; Cadet, J. Singlet Molecular Oxygen Reactions with Nucleic Acids, Lipids, and Proteins. Chem. Rev. 2019, 119, 2043–2086. [Google Scholar] [CrossRef] [PubMed]
- Kanofsky, J.R. Singlet oxygen production by biological systems. Chem. Biol. Interact. 1989, 70, 1–28. [Google Scholar] [CrossRef]
- Dong, S.; Xu, J.; Jia, T.; Xu, M.; Zhong, C.; Yang, G.; Li, J.; Yang, D.; He, F.; Gai, S.; et al. Upconversion-mediated ZnFe2O4 nanoplatform for NIR-enhanced chemodynamic and photodynamic therapy. Chem. Sci. 2019, 10, 4259–4271. [Google Scholar] [CrossRef]
- Choi, J.; Kim, S.Y. Synthesis of near-infrared-responsive hexagonal-phase upconversion nanoparticles with controllable shape and luminescence efficiency for theranostic applications. J. Biomater. Appl. 2022, 37, 646–658. [Google Scholar] [CrossRef]
- Takajo, T.; Kurihara, Y.; Iwase, K.; Miyake, D.; Tsuchida, K.; Anzai, K. Basic Investigations of Singlet Oxygen Detection Systems with ESR for the Measurement of Singlet Oxygen Quenching Activities. Chem. Pharm. Bull. 2020, 68, 150–154. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Quan, W.Z.; Cao, Y.; Niu, Q.; Lu, Y.; Xiao, X.; Cheng, L. Boosting the singlet oxygen production from H2O2 activation with highly dispersed Co-N-graphene for pollutant removal. RSC Adv 2022, 12, 17864–17872. [Google Scholar] [CrossRef]
- Ruiz-González, R.; Bresolí-Obach, R.; Gulías, Ò.; Agut, M.; Savoie, H.; Boyle, R.W.; Nonell, S.; Giuntini, F. NanoSOSG: A Nanostructured Fluorescent Probe for the Detection of Intracellular Singlet Oxygen. Angew Chem. Int. Ed. Engl. 2017, 56, 2885–2888. [Google Scholar] [CrossRef]
- Liu, H.; Carter, P.J.H.; Laan, A.C.; Eelkema, R.; Denkova, A.G. Singlet Oxygen Sensor Green is not a Suitable Probe for (1)O(2) in the Presence of Ionizing Radiation. Sci. Rep. 2019, 9, 8393. [Google Scholar] [CrossRef]
- Girotti, A.W.; Korytowski, W. Cholesterol as a singlet oxygen detector in biological systems. Methods Enzymol. 2000, 319, 85–100. [Google Scholar] [PubMed]
- Miyamoto, S.; Di Mascio, P. Lipid hydroperoxides as a source of singlet molecular oxygen. Subcell Biochem. 2014, 77, 3–20. [Google Scholar] [PubMed]
- Torinuki, W.; Miura, T. Singlet oxygen emission at 1270 nm in protoporphyrin solution excited by argon laser. Tohoku J. Exp. Med. 1983, 140, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.; Kanofsky, J.R. Direct observation of singlet oxygen phosphorescence at 1270 nm from L1210 leukemia cells exposed to polyporphyrin and light. Arch. Biochem. Biophys. 1991, 286, 70–75. [Google Scholar] [CrossRef]
- Baker, A.; Kanofsky, J.R. Time-resolved studies of singlet-oxygen emission from L1210 leukemia cells labeled with 5-(N-hexadecanoyl)amino eosin. A comparison with a one-dimensional model of singlet-oxygen diffusion and quenching. Photochem. Photobiol. 1993, 57, 720–727. [Google Scholar] [CrossRef]
- Oelckers, S.; Ziegler, T.; Michler, I.; Roder, B. Time-resolved detection of singlet oxygen luminescence in red-cell ghost suspensions: Concerning a signal component that can be attributed to 1O2 luminescence from the inside of a native membrane. J. Photochem. Photobiol. B 1999, 53, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, C.; Schmidt, R. Physical mechanisms of generation and deactivation of singlet oxygen. Chem. Rev. 2003, 103, 1685–1757. [Google Scholar] [CrossRef] [PubMed]
- Niedre, M.; Patterson, M.S.; Wilson, B.C. Direct near-infrared luminescence detection of singlet oxygen generated by photodynamic therapy in cells in vitro and tissues in vivo. Photochem. Photobiol. 2002, 75, 382–391. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef]
- Lion, Y.; Delmelle, M.; van de Vorst, A. New method of detecting singlet oxygen production. Nature 1976, 263, 442–443. [Google Scholar] [CrossRef]
- Moan, J.; Wold, E. Detection of singlet oxygen production by ESR. Nature 1979, 279, 450–451. [Google Scholar] [CrossRef]
- Dzwigaj, S.; Pezerat, H. Singlet oxygen-trapping reaction as a method of (1)O2 detection: Role of some reducing agents. Free Radic. Res. 1995, 23, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Victória, H.F.V.; Ferreira, D.C.; Filho, J.B.G.; Martins, D.C.S.; Pinheiro, M.V.B.; Sáfar, G.A.M.; Krambrock, K. Detection of singlet oxygen by EPR: The instability of the nitroxyl radicals. Free Radic. Biol. Med. 2022, 180, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.Y.; Choi, D.S. Electron spin resonance and luminescence spectroscopic observation and kinetic study of chemical and physical singlet oxygen quenching by resveratrol in methanol. J. Agric. Food Chem. 2010, 58, 11888–11895. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Maehata, Y.; Kawamura, Y.; Kusubata, M.; Hattori, S.; Tanaka, K.; Miyamoto, C.; Yoshino, F.; Yoshida, A.; Wada-Takahashi, S.; et al. Direct assessments of the antioxidant effects of the novel collagen peptide on reactive oxygen species using electron spin resonance spectroscopy. J. Pharmacol. Sci. 2011, 116, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Soh, N.; Katayama, Y.; Maeda, M. A fluorescent probe for monitoring nitric oxide production using a novel detection concept. Analyst 2001, 126, 564–566. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Miura, T.; Umezawa, N.; Urano, Y.; Kikuchi, K.; Higuchi, T.; Nagano, T. Rational design of fluorescein-based fluorescence probes. Mechanism-based design of a maximum fluorescence probe for singlet oxygen. J. Am. Chem. Soc. 2001, 123, 2530–2536. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Fernandes, E.; Lima, J.L. Fluorescence probes used for detection of reactive oxygen species. J. Biochem. Biophys. Methods 2005, 65, 45–80. [Google Scholar] [CrossRef]
- Corey, E.J.; Taylor, W.C. Study of the peroxidation of organic compounds by externally generated singlet oxygen molecules. J. Am. Chem. Soc. 1964, 86, 3881–3882. [Google Scholar] [CrossRef]
- Francis Wilkinson, W.P.H.; Ross, A.B. Rate Constants for the Decay and Reactions of the Lowest Electronically Excited Singlet State of Molecular Oxygen in Solution. J. Phys. Chem. Ref. 1995, 24, 663–1021. [Google Scholar]
- Umezawa, N.; Tanaka, K.; Urano, Y.; Kikuchi, K.; Higuchi, T.; Nagano, T. Novel Fluorescent Probes for Singlet Oxygen. Angew. Chem. Int. Ed. Engl. 1999, 38, 2899–2901. [Google Scholar] [CrossRef]
- Flors, C.; Fryer, M.J.; Waring, J.; Reeder, B.; Bechtold, U.; Mullineaux, P.M.; Nonell, S.; Wilson, M.T.; Baker, N.R. Imaging the production of singlet oxygen in vivo using a new fluorescent sensor, Singlet Oxygen Sensor Green. J. Exp. Bot. 2006, 57, 1725–1734. [Google Scholar] [CrossRef]
- Price, M.; Reiners, J.J.; Santiago, A.M.; Kessel, D. Monitoring singlet oxygen and hydroxyl radical formation with fluorescent probes during photodynamic therapy. Photochem. Photobiol. 2009, 85, 1177–1181. [Google Scholar] [CrossRef]
- Ragas, X.; Jimenez-Banzo, A.; Sanchez-Garcia, D.; Batllori, X.; Nonell, S. Singlet oxygen photosensitisation by the fluorescent probe Singlet Oxygen Sensor Green. Chem. Commun. 2009, 28, 2920–2922. [Google Scholar] [CrossRef]
- Kim, S.; Tachikawa, T.; Fujitsuka, M.; Majima, T. Far-red fluorescence probe for monitoring singlet oxygen during photodynamic therapy. J. Am. Chem. Soc. 2014, 136, 11707–11715. [Google Scholar] [CrossRef]
- Murotomi, K.; Umeno, A.; Sugino, S.; Yoshida, Y. Quantitative kinetics of intracellular singlet oxygen generation using a fluorescence probe. Sci. Rep. 2020, 10, 10616. [Google Scholar] [CrossRef]
- Chen, B.; Yang, Y.; Wang, Y.; Yan, Y.; Wang, Z.; Yin, Q.; Zhang, Q.; Wang, Y. Precise Monitoring of Singlet Oxygen in Specific Endocytic Organelles by Super-pH-Resolved Nanosensors. ACS Appl. Mater. Interfaces 2021, 13, 18533–18544. [Google Scholar] [CrossRef]
- Nath, P.; Hamadna, S.S.; Karamchand, L.; Foster, J.; Kopelman, R.; Amar, J.G.; Ray, A. Intracellular detection of singlet oxygen using fluorescent nanosensors. Analyst 2021, 146, 3933–3941. [Google Scholar] [CrossRef]
- Niki, E. Lipid peroxidation products as oxidative stress biomarkers. Biofactors 2008, 34, 171–180. [Google Scholar] [CrossRef]
- Niki, E. Assessment of antioxidant capacity in vitro and in vivo. Free Radic. Biol. Med. 2010, 49, 503–515. [Google Scholar] [CrossRef]
- Akazawa-Ogawa, Y.; Shichiri, M.; Nishio, K.; Yoshida, Y.; Niki, E.; Hagihara, Y. Singlet-oxygen-derived products from linoleate activate Nrf2 signaling in skin cells. Free Radic. Biol. Med. 2015, 79, 164–175. [Google Scholar] [CrossRef]
- Murotomi, K.; Umeno, A.; Yasunaga, M.; Shichiri, M.; Ishida, N.; Abe, H.; Yoshida, Y.; Nakajima, Y. Switching from singlet-oxygen-mediated oxidation to free-radical-mediated oxidation in the pathogenesis of type 2 diabetes in model mouse. Free Radic. Res. 2015, 49, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Luchetti, F.; Canonico, B.; Cesarini, E.; Betti, M.; Galluzzi, L.; Galli, L.; Tippins, J.; Zerbinati, C.; Papa, S.; Iuliano, L. 7-Ketocholesterol and 5,6-secosterol induce human endothelial cell dysfunction by differential mechanisms. Steroids 2015, 99, 204–211. [Google Scholar] [CrossRef]
- Dantas, L.S.; Chaves-Filho, A.B.; Coelho, F.R.; Genaro-Mattos, T.C.; Tallman, K.A.; Porter, N.A.; Augusto, O.; Miyamoto, S. Cholesterol secosterol aldehyde adduction and aggregation of Cu,Zn-superoxide dismutase: Potential implications in ALS. Redox Biol. 2018, 19, 105–115. [Google Scholar] [CrossRef]
- Niki, E.; Yoshida, Y. Biomarkers for oxidative stress: Measurement, validation, and application. J. Med. Invest. 2005, 52, 228–230. [Google Scholar] [CrossRef]
- Yoshida, Y.; Niki, E. Bio-markers of lipid peroxidation in vivo: Hydroxyoctadecadienoic acid and hydroxycholesterol. Biofactors 2006, 27, 195–202. [Google Scholar] [CrossRef]
- Yoshida, Y.; Hayakawa, M.; Habuchi, Y.; Itoh, N.; Niki, E. Evaluation of lipophilic antioxidant efficacy in vivo by the biomarkers hydroxyoctadecadienoic acid and isoprostane. Lipids 2007, 42, 463–472. [Google Scholar] [CrossRef]
- Yoshida, Y.; Kodai, S.; Takemura, S.; Minamiyama, Y.; Niki, E. Simultaneous measurement of F2-isoprostane, hydroxyoctadecadienoic acid, hydroxyeicosatetraenoic acid, and hydroxycholesterols from physiological samples. Anal. Biochem. 2008, 379, 105–115. [Google Scholar] [CrossRef]
- Yoshida, Y.; Umeno, A.; Shichiri, M. Lipid peroxidation biomarkers for evaluating oxidative stress and assessing antioxidant capacity in vivo. J. Clin. Biochem. Nutr. 2013, 52, 9–16. [Google Scholar] [CrossRef]
- Yoshida, Y.; Umeno, A.; Akazawa, Y.; Shichiri, M.; Murotomi, K.; Horie, M. Chemistry of lipid peroxidation products and their use as biomarkers in early detection of diseases. J. Oleo Sci. 2015, 64, 347–356. [Google Scholar] [CrossRef]
- Umeno, A.; Shichiri, M.; Ishida, N.; Hashimoto, Y.; Abe, K.; Kataoka, M.; Yoshino, K.; Hagihara, Y.; Aki, N.; Funaki, M.; et al. Singlet oxygen induced products of linoleates, 10- and 12-(Z,E)-hydroxyoctadecadienoic acids (HODE), can be potential biomarkers for early detection of type 2 diabetes. PLoS ONE 2013, 8, e63542. [Google Scholar] [CrossRef] [PubMed]
- Umeno, A.; Yoshino, K.; Hashimoto, Y.; Shichiri, M.; Kataoka, M.; Yoshida, Y. Multi-Biomarkers for Early Detection of Type 2 Diabetes, Including 10- and 12-(Z,E)-Hydroxyoctadecadienoic Acids, Insulin, Leptin, and Adiponectin. PLoS ONE 2015, 10, e0130971. [Google Scholar] [CrossRef] [PubMed]
- Umeno, A.; Tanito, M.; Kaidzu, S.; Takai, Y.; Horie, M.; Yoshida, Y. Comprehensive measurements of hydroxylinoleate and hydroxyarachidonate isomers in blood samples from primary open-angle glaucoma patients and controls. Sci. Rep. 2019, 9, 2171. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, U.; Spiteller, G. The course of enzymatically induced lipid peroxidation in homogenized porcine kidney tissue. Z. Naturforsch C J. Biosci. 1998, 53, 431–437. [Google Scholar] [CrossRef]
- Horie, M.; Miura, T.; Hirakata, S.; Hosoyama, A.; Sugino, S.; Umeno, A.; Murotomi, K.; Yoshida, Y.; Koike, T. Comparative analysis of the intestinal flora in type 2 diabetes and nondiabetic mice. Exp. Anim. 2017, 66, 405–416. [Google Scholar] [CrossRef]
- Ogawa, H.; Azuma, M.; Umeno, A.; Shimizu, M.; Murotomi, K.; Yoshida, Y.; Nishioka, Y.; Tsuneyama, K. Singlet oxygen -derived nerve growth factor exacerbates airway hyperresponsiveness in a mouse model of asthma with mixed inflammation. Allergol. Int. 2022, 71, 395–404. [Google Scholar] [CrossRef]
- Adachi, J.; Asano, M.; Naito, T.; Ueno, Y.; Imamichi, H.; Tatsuno, Y. Cholesterol hydroperoxides in erythrocyte membranes of alcoholic patients. Alcohol. Clin. Exp. Res. 1999, 23, 96s–100s. [Google Scholar] [CrossRef]
- Yamazaki, S.; Ozawa, N.; Hiratsuka, A.; Watanabe, T. Quantitative determination of cholesterol 5alpha-, 7alpha-, and 7beta-hydroperoxides in rat skin. Free Radic. Biol. Med. 1999, 27, 110–118. [Google Scholar] [CrossRef]
- Minami, Y.; Yokoi, S.; Setoyama, M.; Bando, N.; Takeda, S.; Kawai, Y.; Terao, J. Combination of TLC blotting and gas chromatography-mass spectrometry for analysis of peroxidized cholesterol. Lipids 2007, 42, 1055–1063. [Google Scholar] [CrossRef]
- Minami, Y.; Kawabata, K.; Kubo, Y.; Arase, S.; Hirasaka, K.; Nikawa, T.; Bando, N.; Kawai, Y.; Terao, J. Peroxidized cholesterol-induced matrix metalloproteinase-9 activation and its suppression by dietary beta-carotene in photoaging of hairless mouse skin. J. Nutr. Biochem. 2009, 20, 389–398. [Google Scholar] [CrossRef]
- Terao, J.; Minami, Y.; Bando, N. Singlet molecular oxygen-quenching activity of carotenoids: Relevance to protection of the skin from photoaging. J. Clin. Biochem. Nutr. 2011, 48, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Wentworth, P., Jr.; Nieva, J.; Takeuchi, C.; Galve, R.; Wentworth, A.D.; Dilley, R.B.; DeLaria, G.A.; Saven, A.; Babior, B.M.; Janda, K.D.; et al. Evidence for ozone formation in human atherosclerotic arteries. Science 2003, 302, 1053–1056. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Powers, E.T.; Nieva, J.; Huff, M.E.; Dendle, M.A.; Bieschke, J.; Glabe, C.G.; Eschenmoser, A.; Wentworth, P., Jr.; Lerner, R.A.; et al. Metabolite-initiated protein misfolding may trigger Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2004, 101, 4752–4757. [Google Scholar] [CrossRef] [PubMed]
- Elshafei, M.E.; Minamiyama, Y.; Ichikawa, H. Singlet oxygen from endoperoxide initiates an intracellular reactive oxygen species release in HaCaT keratinocytes. J. Clin. Biochem. Nutr. 2022, 71, 198–205. [Google Scholar] [CrossRef]
- Umar, S.A.; Shahid, N.H.; Nazir, L.A.; Tanveer, M.A.; Divya, G.; Archoo, S.; Raghu, S.R.; Tasduq, S.A. Pharmacological Activation of Autophagy Restores Cellular Homeostasis in Ultraviolet-(B)-Induced Skin Photodamage. Front. Oncol. 2021, 11, 726066. [Google Scholar] [CrossRef]
- Mokrzyński, K.; Krzysztyńska-Kuleta, O.; Zawrotniak, M.; Sarna, M.; Sarna, T. Fine Particulate Matter-Induced Oxidative Stress Mediated by UVA-Visible Light Leads to Keratinocyte Damage. Int. J. Mol. Sci. 2021, 22, 10645. [Google Scholar] [CrossRef] [PubMed]
- Requena, M.B.; Russignoli, P.E.; Vollet-Filho, J.D.; Salvio, A.G.; Fortunato, T.C.; Pratavieira, S.; Bagnato, V.S. Use of dermograph for improvement of PpIX precursor’s delivery in photodynamic therapy: Experimental and clinical pilot studies. Photodiagnosis Photodyn. Ther. 2020, 29, 101599. [Google Scholar] [CrossRef]
- Pinto, M.A.F.; Ferreira, C.B.R.; de Lima, B.E.S.; Molon, Â.C.; Ibarra, A.M.C.; Cecatto, R.B.; Dos Santos Franco, A.L.; Rodrigues, M. Effects of 5-ALA mediated photodynamic therapy in oral cancer stem cells. J. Photochem. Photobiol. B 2022, 235, 112552. [Google Scholar] [CrossRef]
- Arakane, K.; Ryu, A.; Hayashi, C.; Masunaga, T.; Shinmoto, K.; Mashiko, S.; Nagano, T.; Hirobe, M. Singlet oxygen (1 delta g) generation from coproporphyrin in Propionibacterium acnes on irradiation. Biochem. Biophys. Res. Commun. 1996, 223, 578–582. [Google Scholar] [CrossRef]
- Patwardhan, S.V.; Richter, C.; Vogt, A.; Blume-Peytavi, U.; Canfield, D.; Kottner, J. Measuring acne using Coproporphyrin III, Protoporphyrin IX, and lesion-specific inflammation: An exploratory study. Arch. Dermatol. Res. 2017, 309, 159–167. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, X.; Wang, Z.; Sun, J.; He, Z.; Sun, B.; Luo, C. Molecularly Self-Engineered Nanoamplifier for Boosting Photodynamic Therapy via Cascade Oxygen Elevation and Lipid ROS Accumulation. ACS Appl. Mater Interfaces 2022, 14, 38497–38505. [Google Scholar] [CrossRef] [PubMed]
- Uliana, M.P.; da Cruz Rodrigues, A.; Ono, B.A.; Pratavieira, S.; de Oliveira, K.T.; Kurachi, C. Photodynamic Inactivation of Microorganisms Using Semisynthetic Chlorophyll a Derivatives as Photosensitizers. Molecules 2022, 27, 5769. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, M.; Azami, C.; Koyama, K.; Rutherford, A.W.; Rappaport, F.; Boussac, A. Modification of the pheophytin redox potential in Thermosynechococcus elongatus Photosystem II with PsbA3 as D1. Biochim. Biophys. Acta 2014, 1837, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Braun, M.S.; Wink, M. Chlorophyll and Chlorophyll Derivatives Interfere with Multi-Drug Resistant Cancer Cells and Bacteria. Molecules 2019, 24, 2968. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Poojary, M.M.; Skibsted, L.H.; Lund, M.N. Cleavage of Disulfide Bonds in Cystine by UV-B Illumination Mediated by Tryptophan or Tyrosine as Photosensitizers. J. Agric. Food Chem. 2020, 68, 6900–6909. [Google Scholar] [CrossRef]
- Ionita, G.; Matei, I. Application of Riboflavin Photochemical Properties in Hydrogel Synthesis. In Biophysical Chemistry Advance Applications; InTech Open: London, UK, 2020. [Google Scholar]
- Wangsuwan, S.; Meephansan, J. Comparative Study Of Photodynamic Therapy With Riboflavin-Tryptophan Gel And 13% 5-Aminolevulinic Acid In The Treatment Of Mild To Moderate Acne Vulgaris. Clin. Cosmet. Investig. Dermatol. 2019, 12, 805–814. [Google Scholar] [CrossRef]
- Chignell, C.F.; Kukielczak, B.M.; Sik, R.H.; Bilski, P.J.; He, Y.Y. Ultraviolet A sensitivity in Smith-Lemli-Opitz syndrome: Possible involvement of cholesta-5,7,9(11)-trien-3 beta-ol. Free Radic. Biol. Med. 2006, 41, 339–346. [Google Scholar] [CrossRef]
- Bode, C.W.; Hänsel, W. 5-(3-Phenylpropoxy)psoralen and 5-(4-phenylbutoxy)psoralen: Mechanistic studies on phototoxicity. Pharmazie 2005, 60, 225–228. [Google Scholar]
- Lee, K.P.; Girijala, R.L.; Chon, S.Y. Phytophotodermatitis due to a Citrus-Based Hand Sanitizer: A Case Report. Korean J. Fam. Med. 2022, 43, 271–273. [Google Scholar] [CrossRef]
- Pappas, A. Epidermal surface lipids. Dermatoendocrinology 2009, 1, 72–76. [Google Scholar] [CrossRef]
- Chapkin, R.S.; Ziboh, V.A.; Marcelo, C.L.; Voorhees, J.J. Metabolism of essential fatty acids by human epidermal enzyme preparations: Evidence of chain elongation. J. Lipid Res. 1986, 27, 945–954. [Google Scholar] [CrossRef]
- Ni Raghallaigh, S.; Bender, K.; Lacey, N.; Brennan, L.; Powell, F.C. The fatty acid profile of the skin surface lipid layer in papulopustular rosacea. Br. J. Dermatol. 2012, 166, 279–287. [Google Scholar]
- Shimizu, N.; Ito, J.; Kato, S.; Otoki, Y.; Goto, M.; Eitsuka, T.; Miyazawa, T.; Nakagawa, K. Oxidation of squalene by singlet oxygen and free radicals results in different compositions of squalene monohydroperoxide isomers. Sci. Rep. 2018, 8, 9116. [Google Scholar] [CrossRef] [PubMed]
- Chiba, K.; Kawakami, K.; Sone, T.; Onoue, M. Characteristics of skin wrinkling and dermal changes induced by repeated application of squalene monohydroperoxide to hairless mouse skin. Skin Pharmacol. Appl. Skin Physiol. 2003, 16, 242–251. [Google Scholar] [CrossRef]
- Chiba, K.; Yoshizawa, K.; Makino, I.; Kawakami, K.; Onoue, M. Comedogenicity of squalene monohydroperoxide in the skin after topical application. J. Toxicol. Sci. 2000, 25, 77–83. [Google Scholar] [CrossRef]
- Khalifa, S.; Enomoto, M.; Kato, S.; Nakagawa, K. Novel Photoinduced Squalene Cyclic Peroxide Identified, Detected, and Quantified in Human Skin Surface Lipids. Antioxidants 2021, 10, 1760. [Google Scholar] [CrossRef]
- Dalrymple, A.; McEwan, M.; Brandt, M.; Bielfeldt, S.; Bean, E.J.; Moga, A.; Coburn, S.; Hardie, G. A novel clinical method to measure skin staining reveals activation of skin damage pathways by cigarette smoke. Skin Res. Technol. 2022, 28, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Minami, Y.; Yokoyama, K.; Bando, N.; Kawai, Y.; Terao, J. Occurrence of singlet oxygen oxygenation of oleic acid and linoleic acid in the skin of live mice. Free Radic. Res. 2008, 42, 197–204. [Google Scholar] [CrossRef]
- Egawa, M.; Kohno, Y.; Kumano, Y. Oxidative effects of cigarette smoke on the human skin. Int. J. Cosmet. Sci. 1999, 21, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Ibusuki, D.; Suzuki, Y.; Yamashita, S.; Higuchi, O.; Oikawa, S.; Miyazawa, T. Ion-trap tandem mass spectrometric analysis of squalene monohydroperoxide isomers in sunlight-exposed human skin. J. Lipid Res. 2007, 48, 2779–2787. [Google Scholar] [CrossRef] [PubMed]
- Ekanayake-Mudiyanselage, S.; Tavakkol, A.; Polefka, T.G.; Nabi, Z.; Elsner, P.; Thiele, J.J. Vitamin E delivery to human skin by a rinse-off product: Penetration of alpha-tocopherol versus wash-out effects of skin surface lipids. Skin Pharmacol. Physiol. 2005, 18, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Ekanayake Mudiyanselage, S.; Hamburger, M.; Elsner, P.; Thiele, J.J. Ultraviolet a induces generation of squalene monohydroperoxide isomers in human sebum and skin surface lipids in vitro and in vivo. J. Invest. Dermatol. 2003, 120, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Auffray, B. Protection against singlet oxygen, the main actor of sebum squalene peroxidation during sun exposure, using Commiphora myrrha essential oil. Int. J. Cosmet. Sci. 2007, 29, 23–29. [Google Scholar] [CrossRef]
- Lens, M.; Podesta Marty, M.H. Assessment of the kinetics of the antioxidative capacity of topical antioxidants. J. Drugs Dermatol. 2011, 10, 262–267. [Google Scholar] [PubMed]
- Shimizu, N.; Ito, J.; Kato, S.; Eitsuka, T.; Saito, T.; Nishida, H.; Miyazawa, T.; Nakagawa, K. Evaluation of squalene oxidation mechanisms in human skin surface lipids and shark liver oil supplements. Ann. N. Y. Acad. Sci. 2019, 1457, 158–165. [Google Scholar] [CrossRef]
- Jourdain, R.; Moga, A.; Vingler, P.; El Rawadi, C.; Pouradier, F.; Souverain, L.; Bastien, P.; Amalric, N.; Breton, L. Exploration of scalp surface lipids reveals squalene peroxide as a potential actor in dandruff condition. Arch. Dermatol. Res. 2016, 308, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Mountfort, K.A.; Bronstein, H.; Archer, N.; Jickells, S.M. Identification of oxidation products of squalene in solution and in latent fingerprints by ESI-MS and LC/APCI-MS. Anal. Chem. 2007, 79, 2650–2657. [Google Scholar] [CrossRef]
- Curpen, S.; Francois-Newton, V.; Moga, A.; Hosenally, M.; Petkar, G.; Soobramaney, V.; Ruchaia, B.; Lutchmanen Kolanthan, V.; Roheemun, N.; Sokeechand, B.N.; et al. A novel method for evaluating the effect of pollution on the human skin under controlled conditions. Skin Res. Technol. 2020, 26, 50–60. [Google Scholar] [CrossRef]
- Bielfeldt, S.; Jung, K.; Laing, S.; Moga, A.; Wilhelm, K.P. Anti-pollution effects of two antioxidants and a chelator-Ex vivo electron spin resonance and in vivo cigarette smoke model assessments in human skin. Skin Res. Technol. 2021, 27, 1092–1099. [Google Scholar] [CrossRef]
- Someya, K.; Totsuka, Y.; Murakoshi, M.; Kitano, H.; Miyazawa, T. The antioxidant effect of palm fruit carotene on skin lipid peroxidation in guinea pigs as estimated by chemiluminescence-HPLC method. J. Nutr. Sci. Vitaminol. 1994, 40, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Pena, A.M.; Baldeweck, T.; Decencière, E.; Koudoro, S.; Victorin, S.; Raynaud, E.; Ngo, B.; Bastien, P.; Brizion, S.; Tancrède-Bohin, E. In vivo multiphoton multiparametric 3D quantification of human skin aging on forearm and face. Sci. Rep. 2022, 12, 14863. [Google Scholar] [CrossRef] [PubMed]
- Scharffetter, K.; Wlaschek, M.; Hogg, A.; Bolsen, K.; Schothorst, A.; Goerz, G.; Krieg, T.; Plewig, G. UVA irradiation induces collagenase in human dermal fibroblasts in vitro and in vivo. Arch. Dermatol. Res. 1991, 283, 506–511. [Google Scholar] [CrossRef]
- Nakamura, T.; Noma, A.; Shimada, S.; Ishii, N.; Bando, N.; Kawai, Y.; Terao, J. Non-selective distribution of isomeric cholesterol hydroperoxides to microdomains in cell membranes and activation of matrix metalloproteinase activity in a model of dermal cells. Chem. Phys. Lipids 2013, 174, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Douki, T.; Ravanat, J.L. Oxidatively generated damage to the guanine moiety of DNA: Mechanistic aspects and formation in cells. Acc. Chem. Res. 2008, 41, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Douki, T.; Ravanat, J.L. Oxidatively generated damage to cellular DNA by UVB and UVA radiation. Photochem. Photobiol. 2015, 91, 140–155. [Google Scholar] [CrossRef] [PubMed]
- Ravanat, J.L.; Di Mascio, P.; Martinez, G.R.; Medeiros, M.H.; Cadet, J. Singlet oxygen induces oxidation of cellular DNA. J. Biol. Chem. 2000, 275, 40601–40604. [Google Scholar] [CrossRef] [PubMed]
- Yagura, T.; Schuch, A.P.; Garcia, C.C.M.; Rocha, C.R.R.; Moreno, N.C.; Angeli, J.P.F.; Mendes, D.; Severino, D.; Bianchini Sanchez, A.; Di Mascio, P.; et al. Direct participation of DNA in the formation of singlet oxygen and base damage under UVA irradiation. Free Radic. Biol. Med. 2017, 108, 86–93. [Google Scholar] [CrossRef]
- Cadet, J.; Sage, E.; Douki, T. Ultraviolet radiation-mediated damage to cellular DNA. Mutat. Res. 2005, 571, 3–17. [Google Scholar] [CrossRef]
- Mouret, S.; Baudouin, C.; Charveron, M.; Favier, A.; Cadet, J.; Douki, T. Cyclobutane pyrimidine dimers are predominant DNA lesions in whole human skin exposed to UVA radiation. Proc. Natl. Acad. Sci. USA 2006, 103, 13765–13770. [Google Scholar] [CrossRef]
- Prat, F.; Hou, C.-C.; Foote, C.S. Determination of the Quenching Rate Constants of Singlet Oxygen by Derivatized Nucleosides in Nonaqueous Solution. J. Am. Chem. Soc. 1997, 119, 5051–5052. [Google Scholar] [CrossRef]
- David, S.S.; O’Shea, V.L.; Kundu, S. Base-excision repair of oxidative DNA damage. Nature 2007, 447, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Hsu, G.W.; Ober, M.; Carell, T.; Beese, L.S. Error-prone replication of oxidatively damaged DNA by a high-fidelity DNA polymerase. Nature 2004, 431, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Greenman, C.; Stephens, P.; Smith, R.; Dalgliesh, G.L.; Hunter, C.; Bignell, G.; Davies, H.; Teague, J.; Butler, A.; Stevens, C.; et al. Patterns of somatic mutation in human cancer genomes. Nature 2007, 446, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, T.; Barnes, D.E. Repair of endogenous DNA damage. Cold Spring Harb. Symp. Quant. Biol. 2000, 65, 127–133. [Google Scholar] [CrossRef]
- Greinert, R.; Volkmer, B.; Henning, S.; Breitbart, E.W.; Greulich, K.O.; Cardoso, M.C.; Rapp, A. UVA-induced DNA double-strand breaks result from the repair of clustered oxidative DNA damages. Nucleic Acids Res. 2012, 40, 10263–10273. [Google Scholar] [CrossRef] [PubMed]
- Agar, N.S.; Halliday, G.M.; Barnetson, R.S.; Ananthaswamy, H.N.; Wheeler, M.; Jones, A.M. The basal layer in human squamous tumors harbors more UVA than UVB fingerprint mutations: A role for UVA in human skin carcinogenesis. Proc. Natl. Acad. Sci. USA 2004, 101, 4954–4959. [Google Scholar] [CrossRef]
- Kunisada, M.; Sakumi, K.; Tominaga, Y.; Budiyanto, A.; Ueda, M.; Ichihashi, M.; Nakabeppu, Y.; Nishigori, C. 8-Oxoguanine formation induced by chronic UVB exposure makes Ogg1 knockout mice susceptible to skin carcinogenesis. Cancer Res. 2005, 65, 6006–6010. [Google Scholar] [CrossRef]
- Kunisada, M.; Kumimoto, H.; Ishizaki, K.; Sakumi, K.; Nakabeppu, Y.; Nishigori, C. Narrow-band UVB induces more carcinogenic skin tumors than broad-band UVB through the formation of cyclobutane pyrimidine dimer. J. Invest. Dermatol. 2007, 127, 2865–2871. [Google Scholar] [CrossRef]
- Zhang, R.B.; Eriksson, L.A. A triplet mechanism for the formation of cyclobutane pyrimidine dimers in UV-irradiated DNA. J. Phys. Chem. B 2006, 110, 7556–7562. [Google Scholar] [CrossRef]
- You, Y.; Zhu, F.; Li, Z.; Zhang, L.; Xie, Y.; Chinnathambi, A.; Alahmadi, T.A.; Lu, B. Phyllanthin prevents diethylnitrosamine (DEN) induced liver carcinogenesis in rats and induces apoptotic cell death in HepG2 cells. Biomed. Pharmacother. 2021, 137, 111335. [Google Scholar] [CrossRef]
- Yuan, H.; Guo, L.; Su, Q.; Su, X.; Wen, Y.; Wang, T.; Yang, P.; Xu, M.; Li, F. Afterglow Amplification for Fast and Sensitive Detection of Porphyria in Whole Blood. ACS Appl. Mater. Interfaces 2021, 13, 27991–27998. [Google Scholar] [CrossRef]
- Ventura, P.; Brancaleoni, V.; Di Pierro, E.; Graziadei, G.; Macrì, A.; Carmine Guida, C.; Nicolli, A.; Rossi, M.T.; Granata, F.; Fiorentino, V.; et al. Clinical and molecular epidemiology of erythropoietic protoporphyria in Italy. Eur. J. Dermatol. 2020, 30, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Balwani, M. Erythropoietic Protoporphyria and X-Linked Protoporphyria: Pathophysiology, genetics, clinical manifestations, and management. Mol. Genet. Metab. 2019, 128, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Mathews-Roth, M.M.; Pathak, M.A.; Fitzpatrick, T.B.; Harber, L.C.; Kass, E.H. Beta-carotene as a photoprotective agent in erythropoietic protoporphyria. N. Engl. J. Med. 1970, 282, 1231–1234. [Google Scholar] [CrossRef] [PubMed]
- Black, H.S.; Boehm, F.; Edge, R.; Truscott, T.G. The Benefits and Risks of Certain Dietary Carotenoids that Exhibit both Anti- and Pro-Oxidative Mechanisms-A Comprehensive Review. Antioxidants 2020, 9, 264. [Google Scholar] [CrossRef]
- Mathews-Roth, M.M.; Pathak, M.A.; Fitzpatrick, T.B.; Harber, L.H.; Kass, E.H. Beta carotene therapy for erythropoietic protoporphyria and other photosensitivity diseases. Arch. Dermatol. 1977, 113, 1229–1232. [Google Scholar] [CrossRef]
- Maitra, D.; Bragazzi Cunha, J.; Elenbaas, J.S.; Bonkovsky, H.L.; Shavit, J.A.; Omary, M.B. Porphyrin-Induced Protein Oxidation and Aggregation as a Mechanism of Porphyria-Associated Cell Injury. Cell Mol Gastroenterol. Hepatol. 2019, 8, 535–548. [Google Scholar] [CrossRef]
- Maitra, D.; Carter, E.L.; Richardson, R.; Rittié, L.; Basrur, V.; Zhang, H.; Nesvizhskii, A.I.; Osawa, Y.; Wolf, M.W.; Ragsdale, S.W.; et al. Oxygen and Conformation Dependent Protein Oxidation and Aggregation by Porphyrins in Hepatocytes and Light-Exposed Cells. Cell Mol. Gastroenterol. Hepatol. 2019, 8, 659–682.e651. [Google Scholar] [CrossRef]
- Maitra, D.; Elenbaas, J.S.; Whitesall, S.E.; Basrur, V.; D’Alecy, L.G.; Omary, M.B. Ambient Light Promotes Selective Subcellular Proteotoxicity after Endogenous and Exogenous Porphyrinogenic Stress. J. Biol. Chem. 2015, 290, 23711–23724. [Google Scholar] [CrossRef]
- Tomita, H.; Hines, K.M.; Herron, J.M.; Li, A.; Baggett, D.W.; Xu, L. 7-Dehydrocholesterol-derived oxysterols cause neurogenic defects in Smith-Lemli-Opitz syndrome. Elife 2022, 11, e67141. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, C.; Ma, L.; Zhang, Y.; Liu, Z.; Chen, L.; Wang, R.; Luan, Y.; Rao, Y. Measurement of 7-dehydrocholesterol and cholesterol in hair can be used in the diagnosis of Smith-Lemli-Opitz syndrome. J. Lipid Res. 2022, 63, 100228. [Google Scholar] [CrossRef]
- Koczok, K.; Horváth, L.; Korade, Z.; Mezei, Z.A.; Szabó, G.P.; Porter, N.A.; Kovács, E.; Mirnics, K.; Balogh, I. Biochemical and Clinical Effects of Vitamin E Supplementation in Hungarian Smith-Lemli-Opitz Syndrome Patients. Biomolecules 2021, 11, 1228. [Google Scholar] [CrossRef] [PubMed]
- Quiec, D.; Mazière, C.; Auclair, M.; Santus, R.; Gardette, J.; Redziniak, G.; Franchi, J.; Dubertret, L.; Mazière, J.C. Lovastatin enhances the photocytotoxicity of UVA radiation towards cultured N.C.T.C. 2544 human keratinocytes: Prevention by cholesterol supplementation and by a cathepsin inhibitor. Biochem. J. 1995, 310 Pt 1, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Alrashidi, A.; Rhodes, L.E.; Sharif, J.C.H.; Kreeshan, F.C.; Farrar, M.D.; Ahad, T. Systemic drug photosensitivity-Culprits, impact and investigation in 122 patients. Photodermatol. Photoimmunol. Photomed. 2020, 36, 441–451. [Google Scholar] [CrossRef]
- Chen, H.L.; Chang, H.M.; Wu, H.J.; Lin, Y.C.; Chang, Y.H.; Chang, Y.C.; Lee, W.H.; Chang, C.T. Effect of hydrophilic and lipophilic statins on early onset cataract: A nationwide case-control study. Regul. Toxicol. Pharmacol. 2021, 124, 104970. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, B.W.; Lin, L.N.; Zan, X.L.; Zhang, G.C.; Chen, G.S.; Ji, J.Y.; Ma, W.H. Key factors affecting photoactivated fungicidal activity of sodium pheophorbide a against Pestalotiopsis neglecta. Pestic. Biochem. Physiol. 2020, 167, 104584. [Google Scholar] [CrossRef]
- Kobayashi, T.; Sugaya, K.; Onose, J.I.; Abe, N. Peppermint (Mentha piperita L.) extract effectively inhibits cytochrome P450 3A4 (CYP3A4) mRNA induction in rifampicin-treated HepG2 cells. Biosci. Biotechnol. Biochem. 2019, 83, 1181–1192. [Google Scholar] [CrossRef]
- Puschner, B.; Chen, X.; Read, D.; Affolter, V.K. Alfalfa hay induced primary photosensitization in horses. Vet. J. 2016, 211, 32–38. [Google Scholar] [CrossRef]
- Bayoumy, A.B.; Crouwel, F.; Chanda, N.; Florin, T.H.J.; Buiter, H.J.C.; Mulder, C.J.J.; de Boer, N.K.H. Advances in Thiopurine Drug Delivery: The Current State-of-the-Art. Eur. J. Drug Metab. Pharmacokinet. 2021, 46, 743–758. [Google Scholar] [CrossRef]
- Euvrard, S.; Kanitakis, J.; Claudy, A. Skin cancers after organ transplantation. N. Engl. J. Med. 2003, 348, 1681–1691. [Google Scholar] [CrossRef]
- Bignon, E.; Marazzi, M.; Besancenot, V.; Gattuso, H.; Drouot, G.; Morell, C.; Eriksson, L.A.; Grandemange, S.; Dumont, E.; Monari, A. Ibuprofen and ketoprofen potentiate UVA-induced cell death by a photosensitization process. Sci. Rep. 2017, 7, 8885. [Google Scholar] [CrossRef] [PubMed]
- Zawadzka, M.; Ràcz, B.; Ambrosini, D.; Görbitz, C.H.; Morth, J.P.; Wilkins, A.L.; Østeby, A.; Elgstøen, K.B.P.; Lundanes, E.; Rise, F.; et al. Searching for a UV-filter in the eyes of high-flying birds. Sci. Rep. 2021, 11, 273. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.L.; Lim, H.W.; Mohammad, T.F. Sunscreens and Photoaging: A Review of Current Literature. Am. J. Clin. Dermatol. 2021, 22, 819–828. [Google Scholar] [CrossRef]
- Behar-Cohen, F.; Martinsons, C.; Viénot, F.; Zissis, G.; Barlier-Salsi, A.; Cesarini, J.P.; Enouf, O.; Garcia, M.; Picaud, S.; Attia, D. Light-emitting diodes (LED) for domestic lighting: Any risks for the eye? Prog. Retin. Eye Res. 2011, 30, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.X.; Zhou, W.C.; Zhu, X.G. Mitochondria as Potential Targets and Initiators of the Blue Light Hazard to the Retina. Oxid. Med. Cell. Longev. 2019, 2019, 6435364. [Google Scholar] [CrossRef] [PubMed]
- Kaarniranta, K.; Blasiak, J.; Liton, P.; Boulton, M.; Klionsky, D.J.; Sinha, D. Autophagy in age-related macular degeneration. Autophagy 2023, 19, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Różanowska, M.B.; Pawlak, A.; Różanowski, B. Products of Docosahexaenoate Oxidation as Contributors to Photosensitising Properties of Retinal Lipofuscin. Int. J. Mol. Sci. 2021, 22, 3525. [Google Scholar] [CrossRef]
- Höhn, A.; Grune, T. Lipofuscin: Formation, effects and role of macroautophagy. Redox Biol. 2013, 1, 140–144. [Google Scholar] [CrossRef]
- Singh Kushwaha, S.; Patro, N.; Kumar Patro, I. A Sequential Study of Age-Related Lipofuscin Accumulation in Hippocampus and Striate Cortex of Rats. Ann. Neurosci. 2018, 25, 223–233. [Google Scholar] [CrossRef]
- Gao, Z.; Liao, Y.; Chen, C.; Liao, C.; He, D.; Chen, J.; Ma, J.; Liu, Z.; Wu, Y. Conversion of all-trans-retinal into all-trans-retinal dimer reflects an alternative metabolic/antidotal pathway of all-trans-retinal in the retina. J. Biol. Chem. 2018, 293, 14507–14519. [Google Scholar] [CrossRef]
- Parish, C.A.; Hashimoto, M.; Nakanishi, K.; Dillon, J.; Sparrow, J. Isolation and one-step preparation of A2E and iso-A2E, fluorophores from human retinal pigment epithelium. Proc. Natl. Acad. Sci. USA 1998, 95, 14609–14613. [Google Scholar] [CrossRef] [PubMed]
- Marie, M.; Bigot, K.; Angebault, C.; Barrau, C.; Gondouin, P.; Pagan, D.; Fouquet, S.; Villette, T.; Sahel, J.A.; Lenaers, G.; et al. Light action spectrum on oxidative stress and mitochondrial damage in A2E-loaded retinal pigment epithelium cells. Cell Death Dis. 2018, 9, 287. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, P.S.; Li, B.; Vachali, P.P.; Gorusupudi, A.; Shyam, R.; Henriksen, B.S.; Nolan, J.M. Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog. Retin. Eye Res. 2016, 50, 34–66. [Google Scholar] [CrossRef] [PubMed]
- Lem, D.W.; Davey, P.G.; Gierhart, D.L.; Rosen, R.B. A Systematic Review of Carotenoids in the Management of Age-Related Macular Degeneration. Antioxidants 2021, 10, 1255. [Google Scholar] [CrossRef] [PubMed]
- Shyam, R.; Gorusupudi, A.; Nelson, K.; Horvath, M.P.; Bernstein, P.S. RPE65 has an additional function as the lutein to meso-zeaxanthin isomerase in the vertebrate eye. Proc. Natl. Acad. Sci. USA 2017, 114, 10882–10887. [Google Scholar] [CrossRef] [PubMed]
- Johra, F.T.; Bepari, A.K.; Bristy, A.T.; Reza, H.M. A Mechanistic Review of β-Carotene, Lutein, and Zeaxanthin in Eye Health and Disease. Antioxidants 2020, 9, 1046. [Google Scholar] [CrossRef] [PubMed]
- Mizdrak, J.; Hains, P.G.; Truscott, R.J.; Jamie, J.F.; Davies, M.J. Tryptophan-derived ultraviolet filter compounds covalently bound to lens proteins are photosensitizers of oxidative damage. Free Radic. Biol. Med. 2008, 44, 1108–1119. [Google Scholar] [CrossRef]
- Ouyang, X.; Yang, J.; Hong, Z.; Wu, Y.; Xie, Y.; Wang, G. Mechanisms of blue light-induced eye hazard and protective measures: A review. Biomed. Pharmacother. 2020, 130, 110577. [Google Scholar] [CrossRef]
- Boulton, M.; Rozanowska, M.; Rozanowski, B.; Wess, T. The photoreactivity of ocular lipofuscin. Photochem. Photobiol. Sci. Off. J. Eur. Photochem. Assoc. Eur. Soc. Photobiol. 2004, 3, 759–764. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Khaw, P.T. Primary open-angle glaucoma. Lancet 2004, 363, 1711–1720. [Google Scholar] [CrossRef]
- Foster, A.; Resnikoff, S. The impact of Vision 2020 on global blindness. Eye 2005, 19, 1133–1135. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M.; Kaidzu, S.; Takai, Y.; Ohira, A. Status of systemic oxidative stresses in patients with primary open-angle glaucoma and pseudoexfoliation syndrome. PLoS ONE 2012, 7, e49680. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, J.A.; Murphy, C.G. Outflow obstruction in pigmentary and primary open angle glaucoma. Arch. Ophthalmol. 1992, 110, 1769–1778. [Google Scholar] [CrossRef] [PubMed]
- Himori, N.; Yamamoto, K.; Maruyama, K.; Ryu, M.; Taguchi, K.; Yamamoto, M.; Nakazawa, T. Critical role of Nrf2 in oxidative stress-induced retinal ganglion cell death. J. Neurochem. 2013, 127, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, Y.; Maruyama, K.; Yamamoto, K.; Omodaka, K.; Yasuda, M.; Himori, N.; Ryu, M.; Nishiguchi, K.M.; Nakazawa, T. The role of calpain in an in vivo model of oxidative stress-induced retinal ganglion cell damage. Biochem. Biophys. Res. Commun. 2014, 451, 510–515. [Google Scholar] [CrossRef]
- Inomata, Y.; Nakamura, H.; Tanito, M.; Teratani, A.; Kawaji, T.; Kondo, N.; Yodoi, J.; Tanihara, H. Thioredoxin inhibits NMDA-induced neurotoxicity in the rat retina. J. Neurochem. 2006, 98, 372–385. [Google Scholar] [CrossRef]
- Munemasa, Y.; Ahn, J.H.; Kwong, J.M.; Caprioli, J.; Piri, N. Redox proteins thioredoxin 1 and thioredoxin 2 support retinal ganglion cell survival in experimental glaucoma. Gene Ther. 2009, 16, 17–25. [Google Scholar] [CrossRef]
- Umeno, A.; Tanito, M.; Kaidzu, S.; Takai, Y.; Yoshida, Y. Involvement of free radical-mediated oxidation in the pathogenesis of pseudoexfoliation syndrome detected based on specific hydroxylinoleate isomers. Free Radic. Biol. Med. 2020, 147, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Umeno, A.; Yoshida, Y.; Kaidzu, S.; Tanito, M. Positive Association between Aqueous Humor Hydroxylinoleate Levels and Intraocular Pressure. Molecules 2022, 27, 2215. [Google Scholar] [CrossRef]
- Schafheimer, N.; King, J. Tryptophan cluster protects human γD-crystallin from ultraviolet radiation-induced photoaggregation in vitro. Photochem. Photobiol. 2013, 89, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Hayakawa, M.; Habuchi, Y.; Niki, E. Evaluation of the dietary effects of coenzyme Q in vivo by the oxidative stress marker, hydroxyoctadecadienoic acid and its stereoisomer ratio. Biochim. Biophys. Acta 2006, 1760, 1558–1568. [Google Scholar] [CrossRef] [PubMed]
- Hirashima, Y.; Doshi, M.; Hayashi, N.; Endo, S.; Akazawa, Y.; Shichiri, M.; Yoshida, Y. Plasma platelet-activating factor-acetyl hydrolase activity and the levels of free forms of biomarker of lipid peroxidation in cerebrospinal fluid of patients with aneurysmal subarachnoid hemorrhage. Neurosurgery 2012, 70, 602–609. [Google Scholar] [CrossRef]
- Fan Gaskin, J.C.; Shah, M.H.; Chan, E.C. Oxidative Stress and the Role of NADPH Oxidase in Glaucoma. Antioxidants 2021, 10, 238. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Medina, J.J.; Rubio-Velazquez, E.; Lopez-Bernal, M.D.; Cobo-Martinez, A.; Zanon-Moreno, V.; Pinazo-Duran, M.D.; Del-Rio-Vellosillo, M. Glaucoma and Antioxidants: Review and Update. Antioxidants 2020, 9, 1031. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Li, S.; Cao, W.; Sun, X. The Association of Oxidative Stress Status with Open-Angle Glaucoma and Exfoliation Glaucoma: A Systematic Review and Meta-Analysis. J. Ophthalmol. 2019, 2019, 1803619. [Google Scholar] [CrossRef]
- Neale, R.E.; Purdie, J.L.; Hirst, L.W.; Green, A.C. Sun exposure as a risk factor for nuclear cataract. Epidemiology 2003, 14, 707–712. [Google Scholar] [CrossRef]
- Garźon-Chavez, D.R.; Quentin, E.; Harrison, S.L.; Parisi, A.V.; Butler, H.J.; Downs, N.J. The geospatial relationship of pterygium and senile cataract with ambient solar ultraviolet in tropical Ecuador. Photochem. Photobiol. Sci. Off. J. Eur. Photochem. Assoc. Eur. Soc. Photobiol. 2018, 17, 1075–1083. [Google Scholar] [CrossRef]
- Dillon, J.; Atherton, S.J. Time resolved spectroscopic studies on the intact human lens. Photochem. Photobiol. 1990, 51, 465–468. [Google Scholar] [CrossRef]
- Kessel, L.; Eskildsen, L.; Lundeman, J.H.; Jensen, O.B.; Larsen, M. Optical effects of exposing intact human lenses to ultraviolet radiation and visible light. BMC Ophthalmol. 2011, 11, 41. [Google Scholar] [CrossRef]
- Davies, M.J.; Truscott, R.J. Photo-oxidation of proteins and its role in cataractogenesis. J. Photochem. Photobiol. B 2001, 63, 114–125. [Google Scholar] [CrossRef]
- Ehrenshaft, M.; Zhao, B.; Andley, U.P.; Mason, R.P.; Roberts, J.E. Immunological detection of N-formylkynurenine in porphyrin-mediated photooxided lens α-crystallin. Photochem. Photobiol. 2011, 87, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Benedek, G.B. Theory of transparency of the eye. Appl. Opt. 1971, 10, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Ávila, F.; Ravello, N.; Zanocco, A.L.; Gamon, L.F.; Davies, M.J.; Silva, E. 3-Hydroxykynurenine bound to eye lens proteins induces oxidative modifications in crystalline proteins through a type I photosensitizing mechanism. Free Radic. Biol. Med. 2019, 141, 103–114. [Google Scholar] [CrossRef]
- Parker, N.R.; Jamie, J.F.; Davies, M.J.; Truscott, R.J. Protein-bound kynurenine is a photosensitizer of oxidative damage. Free Radic. Biol. Med. 2004, 37, 1479–1489. [Google Scholar] [CrossRef]
- Ratter-Rieck, J.M.; Maalmi, H.; Trenkamp, S.; Zaharia, O.P.; Rathmann, W.; Schloot, N.C.; Straßburger, K.; Szendroedi, J.; Herder, C.; Roden, M. Leukocyte Counts and T-Cell Frequencies Differ Between Novel Subgroups of Diabetes and Are Associated With Metabolic Parameters and Biomarkers of Inflammation. Diabetes 2021, 70, 2652–2662. [Google Scholar] [CrossRef] [PubMed]
- Penno, G.; Solini, A.; Orsi, E.; Bonora, E.; Fondelli, C.; Trevisan, R.; Vedovato, M.; Cavalot, F.; Zerbini, G.; Lamacchia, O.; et al. Insulin resistance, diabetic kidney disease, and all-cause mortality in individuals with type 2 diabetes: A prospective cohort study. BMC Med. 2021, 19, 66. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, Y.; Liu, X.; Nie, Y.; Zhao, Z. Urinary heme oxygenase-1 as a potential biomarker for early diabetic nephropathy. Nephrology 2017, 22, 58–64. [Google Scholar] [CrossRef]
- Xu, G.W.; Yao, Q.H.; Weng, Q.F.; Su, B.L.; Zhang, X.; Xiong, J.H. Study of urinary 8-hydroxydeoxyguanosine as a biomarker of oxidative DNA damage in diabetic nephropathy patients. J. Pharm. Biomed. Anal. 2004, 36, 101–104. [Google Scholar] [CrossRef]
- Maschirow, L.; Khalaf, K.; Al-Aubaidy, H.A.; Jelinek, H.F. Inflammation, coagulation, endothelial dysfunction and oxidative stress in prediabetes--Biomarkers as a possible tool for early disease detection for rural screening. Clin. Biochem. 2015, 48, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Zhou, Y.H.; Wu, C.; Shao, Y.; Lu, C.L.; Wang, Q.Y. The changes in miR-130b levels in human serum and the correlation with the severity of diabetic nephropathy. Diabetes Metab. Res. Rev. 2015, 31, 717–724. [Google Scholar] [CrossRef]
- Sánchez-Gómez, F.J.; Espinosa-Díez, C.; Dubey, M.; Dikshit, M.; Lamas, S. S-glutathionylation: Relevance in diabetes and potential role as a biomarker. Biol. Chem. 2013, 394, 1263–1280. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Wang, J.; Xie, C.; Yang, Y.; Tian, J.W.; Xue, Y.M.; Hou, F.F. Increased plasma advanced oxidation protein products is an early marker of endothelial dysfunction in type 2 diabetes patients without albuminuria 2. J. Diabetes 2014, 6, 417–426. [Google Scholar] [CrossRef]
- Ceriello, A.; Testa, R.; Genovese, S. Clinical implications of oxidative stress and potential role of natural antioxidants in diabetic vascular complications. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Paneni, F.; Costantino, S.; Cosentino, F. Molecular mechanisms of vascular dysfunction and cardiovascular biomarkers in type 2 diabetes. Cardiovasc. Diagn. Ther. 2014, 4, 324–332. [Google Scholar] [PubMed]
- Molnar, J.; Garamvolgyi, Z.; Herold, M.; Adanyi, N.; Somogyi, A.; Rigo, J., Jr. Serum selenium concentrations correlate significantly with inflammatory biomarker high-sensitive CRP levels in Hungarian gestational diabetic and healthy pregnant women at mid-pregnancy. Biol. Trace Elem. Res. 2008, 121, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Yu, J.Y.; Jenkins, A.J.; Nankervis, A.J.; Hanssen, K.F.; Henriksen, T.; Lorentzen, B.; Garg, S.K.; Menard, M.K.; Hammad, S.M.; et al. Trace elements as predictors of preeclampsia in type 1 diabetic pregnancy. Nutr. Res. 2015, 35, 421–430. [Google Scholar] [CrossRef]
- Morabito, R.; Remigante, A.; Spinelli, S.; Vitale, G.; Trichilo, V.; Loddo, S.; Marino, A. High Glucose Concentrations Affect Band 3 Protein in Human Erythrocytes. Antioxidants 2020, 9, 365. [Google Scholar] [CrossRef]
- Chai, J.F.; Kao, S.L.; Wang, C.; Lim, V.J.; Khor, I.W.; Dou, J.; Podgornaia, A.I.; Chothani, S.; Cheng, C.Y.; Sabanayagam, C.; et al. Genome-Wide Association for HbA1c in Malay Identified Deletion on SLC4A1 that Influences HbA1c Independent of Glycemia. J. Clin. Endocrinol. Metab. 2020, 105, dgaa658. [Google Scholar] [CrossRef]
- Zhekova, H.R.; Jiang, J.; Wang, W.; Tsirulnikov, K.; Kayık, G.; Khan, H.M.; Azimov, R.; Abuladze, N.; Kao, L.; Newman, D.; et al. CryoEM structures of anion exchanger 1 capture multiple states of inward- and outward-facing conformations. Commun. Biol. 2022, 5, 1372. [Google Scholar] [CrossRef]
- Kostopoulou, E.; Kalaitzopoulou, E.; Papadea, P.; Skipitari, M.; Rojas Gil, A.P.; Spiliotis, B.E.; Georgiou, C.D. Oxidized lipid-associated protein damage in children and adolescents with type 1 diabetes mellitus: New diagnostic/prognostic clinical markers. Pediatr. Diabetes 2021, 22, 1135–1142. [Google Scholar] [CrossRef]
- Veitch, S.; Njock, M.S.; Chandy, M.; Siraj, M.A.; Chi, L.; Mak, H.; Yu, K.; Rathnakumar, K.; Perez-Romero, C.A.; Chen, Z.; et al. MiR-30 promotes fatty acid beta-oxidation and endothelial cell dysfunction and is a circulating biomarker of coronary microvascular dysfunction in pre-clinical models of diabetes. Cardiovasc. Diabetol. 2022, 21, 31. [Google Scholar] [CrossRef] [PubMed]
- Griesser, E.; Vemula, V.; Raulien, N.; Wagner, U.; Reeg, S.; Grune, T.; Fedorova, M. Cross-talk between lipid and protein carbonylation in a dynamic cardiomyocyte model of mild nitroxidative stress. Redox Biol 2017, 11, 438–455. [Google Scholar] [CrossRef]
- Schöttker, B.; Xuan, Y.; Gào, X.; Anusruti, A.; Brenner, H. Oxidatively Damaged DNA/RNA and 8-Isoprostane Levels Are Associated with the Development of Type 2 Diabetes at Older Age: Results From a Large Cohort Study. Diabetes Care 2020, 43, 130–136. [Google Scholar] [CrossRef]
- Leinisch, F.; Mariotti, M.; Hägglund, P.; Davies, M.J. Structural and functional changes in RNAse A originating from tyrosine and histidine cross-linking and oxidation induced by singlet oxygen and peroxyl radicals. Free Radic. Biol. Med. 2018, 126, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S.; Martinez, G.R.; Medeiros, M.H.; Di Mascio, P. Singlet molecular oxygen generated by biological hydroperoxides. J. Photochem. Photobiol. B 2014, 139, 24–33. [Google Scholar] [CrossRef]
- Umeno, A.; Fukui, T.; Hashimoto, Y.; Kataoka, M.; Hagihara, Y.; Nagai, H.; Horie, M.; Shichiri, M.; Yoshino, K.; Yoshida, Y. Early diagnosis of type 2 diabetes based on multiple biomarkers and non-invasive indices. J. Clin. Biochem. Nutr. 2018, 62, 187–194. [Google Scholar] [CrossRef]
- Hampton, M.B.; Kettle, A.J.; Winterbourn, C.C. Inside the neutrophil phagosome: Oxidants, myeloperoxidase, and bacterial killing. Blood 1998, 92, 3007–3017. [Google Scholar] [CrossRef]
- Badwey, J.A.; Karnovsky, M.L. Active oxygen species and the functions of phagocytic leukocytes. Annu. Rev. Biochem. 1980, 49, 695–726. [Google Scholar] [CrossRef] [PubMed]
- Kanofsky, J.R. Singlet oxygen production from the peroxidase-catalyzed oxidation of indole-3-acetic acid. J. Biol. Chem. 1988, 263, 14171–14175. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S.; Martinez, G.R.; Rettori, D.; Augusto, O.; Medeiros, M.H.; Di Mascio, P. Linoleic acid hydroperoxide reacts with hypochlorous acid, generating peroxyl radical intermediates and singlet molecular oxygen. Proc. Natl. Acad. Sci. USA 2006, 103, 293–298. [Google Scholar] [CrossRef]
- Tian, R.; Ding, Y.; Peng, Y.Y.; Lu, N. Myeloperoxidase amplified high glucose-induced endothelial dysfunction in vasculature: Role of NADPH oxidase and hypochlorous acid. Biochem. Biophys. Res. Commun. 2017, 484, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, J.; Jennings, L.K. Leukocyte-derived myeloperoxidase amplifies high-glucose--induced endothelial dysfunction through interaction with high-glucose—Stimulated, vascular non—Leukocyte-derived reactive oxygen species. Diabetes 2004, 53, 2950–2959. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, J.; Jacobs, J.D.; Jennings, L.K. Interaction of myeloperoxidase with vascular NAD(P)H oxidase-derived reactive oxygen species in vasculature: Implications for vascular diseases. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H2563–H2572. [Google Scholar] [CrossRef] [PubMed]
- Eiserich, J.P.; Baldus, S.; Brennan, M.L.; Ma, W.; Zhang, C.; Tousson, A.; Castro, L.; Lusis, A.J.; Nauseef, W.M.; White, C.R.; et al. Myeloperoxidase, a leukocyte-derived vascular NO oxidase. Science 2002, 296, 2391–2394. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, C.; Klinke, A.; Adam, M.; Baldus, S.; Sperandio, M. Myeloperoxidase: A leukocyte-derived protagonist of inflammation and cardiovascular disease. Antioxid. Redox Signal 2013, 18, 692–713. [Google Scholar] [CrossRef]
- Tian, R.; Jin, Z.; Zhou, L.; Zeng, X.P.; Lu, N. Quercetin Attenuated Myeloperoxidase-Dependent HOCl Generation and Endothelial Dysfunction in Diabetic Vasculature. J. Agric. Food Chem. 2021, 69, 404–413. [Google Scholar] [CrossRef]
- Onyango, A.N. The Contribution of Singlet Oxygen to Insulin Resistance. Oxid. Med. Cell. Longev. 2017, 2017, 8765972. [Google Scholar] [CrossRef]
- Nakamura, Y.; Tamaoki, J.; Nagase, H.; Yamaguchi, M.; Horiguchi, T.; Hozawa, S.; Ichinose, M.; Iwanaga, T.; Kondo, R.; Nagata, M.; et al. Japanese guidelines for adult asthma 2020. Allergol. Int. 2020, 69, 519–548. [Google Scholar] [CrossRef]
- Kaur, R.; Chupp, G. Phenotypes and endotypes of adult asthma: Moving toward precision medicine. J. Allergy Clin. Immunol. 2019, 144, 1–12. [Google Scholar] [CrossRef]
- Hekking, P.W.; Wener, R.R.; Amelink, M.; Zwinderman, A.H.; Bouvy, M.L.; Bel, E.H. The prevalence of severe refractory asthma. J. Allergy Clin. Immunol. 2015, 135, 896–902. [Google Scholar] [CrossRef]
- Hammad, H.; Lambrecht, B.N. The basic immunology of asthma. Cell 2021, 184, 1469–1485. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Hammad, H. The immunology of asthma. Nat. Immunol. 2015, 16, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Trevor, J.L.; Deshane, J.S. Refractory asthma: Mechanisms, targets, and therapy. Allergy 2014, 69, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, P.G.; Khashayar, R.; Lazarus, S.C.; Janson, S.; Avila, P.; Boushey, H.A.; Segal, M.; Fahy, J.V. Relationship between airway inflammation, hyperresponsiveness, and obstruction in asthma. J. Allergy Clin. Immunol. 2001, 108, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Shaw, D.E.; Berry, M.A.; Hargadon, B.; McKenna, S.; Shelley, M.J.; Green, R.H.; Brightling, C.E.; Wardlaw, A.J.; Pavord, I.D. Association between neutrophilic airway inflammation and airflow limitation in adults with asthma. Chest 2007, 132, 1871–1875. [Google Scholar] [CrossRef]
- Moore, W.C.; Hastie, A.T.; Li, X.; Li, H.; Busse, W.W.; Jarjour, N.N.; Wenzel, S.E.; Peters, S.P.; Meyers, D.A.; Bleecker, E.R.; et al. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J. Allergy Clin. Immunol. 2014, 133, 1557–1563. [Google Scholar] [CrossRef]
- Jatakanon, A.; Uasuf, C.; Maziak, W.; Lim, S.; Chung, K.F.; Barnes, P.J. Neutrophilic inflammation in severe persistent asthma. Am. J. Respir. Crit. Care Med. 1999, 160, 1532–1539. [Google Scholar] [CrossRef]
- Rosales, C. Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types? Front. Physiol. 2018, 9, 113. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Yang, F.; Xu, Y.; Feng, C.; Zhao, Y. The regulatory roles of neutrophils in adaptive immunity. Cell Commun. Signal 2019, 17, 147. [Google Scholar] [CrossRef]
- Giacalone, V.D.; Margaroli, C.; Mall, M.A.; Tirouvanziam, R. Neutrophil Adaptations upon Recruitment to the Lung: New Concepts and Implications for Homeostasis and Disease. Int. J. Mol. Sci. 2020, 21, 851. [Google Scholar] [CrossRef]
- Wills-Karp, M. Neutrophil ghosts worsen asthma. Sci. Immunol. 2018, 3, eaau0112. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Kolls, J.K. Neutrophilic Inflammation in Asthma and Association with Disease Severity. Trends Immunol. 2017, 38, 942–954. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Yu, M.; Zhong, Y.; Wang, L.; Huang, H. Characteristics and Role of Neutrophil Extracellular Traps in Asthma. Inflammation. 2022, 45, 6–13. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Qin, L.; He, R.; Hu, C. Neutrophil Extracellular Trapping Network Promotes the Pathogenesis of Neutrophil-associated Asthma through Macrophages. Immunol. Invest. 2021, 50, 544–561. [Google Scholar] [CrossRef]
- Varricchi, G.; Modestino, L.; Poto, R.; Cristinziano, L.; Gentile, L.; Postiglione, L.; Spadaro, G.; Galdiero, M.R. Neutrophil extracellular traps and neutrophil-derived mediators as possible biomarkers in bronchial asthma. Clin. Exp. Med. 2022, 22, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Huang, Y.; Li, J.; Huang, J.; Zhang, L.; Feng, J.; Li, J.; Xia, Q.; Zhao, Q.; Huang, L.; et al. Eosinophil extracellular traps drive asthma progression through neuro-immune signals. Nat. Cell Biol. 2021, 23, 1060–1072. [Google Scholar] [CrossRef]
- Michaeloudes, C.; Abubakar-Waziri, H.; Lakhdar, R.; Raby, K.; Dixey, P.; Adcock, I.M.; Mumby, S.; Bhavsar, P.K.; Chung, K.F. Molecular mechanisms of oxidative stress in asthma. Mol. Aspects Med. 2022, 85, 101026. [Google Scholar] [CrossRef]
- Crisford, H.; Sapey, E.; Rogers, G.B.; Taylor, S.; Nagakumar, P.; Lokwani, R.; Simpson, J.L. Neutrophils in asthma: The good, the bad and the bacteria. Thorax 2021, 76, 835–844. [Google Scholar] [CrossRef]
- Andreadis, A.A.; Hazen, S.L.; Comhair, S.A.; Erzurum, S.C. Oxidative and nitrosative events in asthma. Free Radic Biol. Med. 2003, 35, 213–225. [Google Scholar] [CrossRef]
- Obaid Abdullah, S.; Ramadan, G.M.; Makki Al-Hindy, H.A.; Mousa, M.J.; Al-Mumin, A.; Jihad, S.; Hafidh, S.; Kadhum, Y. Serum Myeloperoxidase as a Biomarker of Asthma Severity Among Adults: A Case Control Study. Rep. Biochem. Mol. Biol. 2022, 11, 182–189. [Google Scholar] [CrossRef]
- Olgart Hoglund, C.; de Blay, F.; Oster, J.P.; Duvernelle, C.; Kassel, O.; Pauli, G.; Frossard, N. Nerve growth factor levels and localisation in human asthmatic bronchi. Eur. Respir. J. 2002, 20, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, H.; Azuma, M.; Tsunematsu, T.; Morimoto, Y.; Kondo, M.; Tezuka, T.; Nishioka, Y.; Tsuneyama, K. Neutrophils induce smooth muscle hyperplasia via neutrophil elastase-induced FGF-2 in a mouse model of asthma with mixed inflammation. Clin. Exp. Allergy 2018, 48, 1715–1725. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Kou, L.; Kong, L. Anti-nerve growth factor antibody improves airway hyperresponsiveness by down-regulating RhoA. J. Asthma 2018, 55, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Li, S.; Tang, L. Nerve Growth Factor: A Potential Therapeutic Target for Lung Diseases. Int. J. Mol. Sci. 2021, 22, 9112. [Google Scholar] [CrossRef] [PubMed]
- Saffar, A.S.; Ashdown, H.; Gounni, A.S. The molecular mechanisms of glucocorticoids-mediated neutrophil survival. Curr. Drug Targets 2011, 12, 556–562. [Google Scholar] [CrossRef]
- Tamura, H.; Ishikita, H. Quenching of Singlet Oxygen by Carotenoids via Ultrafast Superexchange Dynamics. J. Phys. Chem. A 2020, 124, 5081–5088. [Google Scholar] [CrossRef]
- Stratton, S.P.; Schaefer, W.H.; Liebler, D.C. Isolation and identification of singlet oxygen oxidation products of beta-carotene. Chem. Res. Toxicol. 1993, 6, 542–547. [Google Scholar] [CrossRef]
- Zbyradowski, M.; Duda, M.; Wisniewska-Becker, A.; Heriyanto; Rajwa, W.; Fiedor, J.; Cvetkovic, D.; Pilch, M.; Fiedor, L. Triplet-driven chemical reactivity of β-carotene and its biological implications. Nat. Commun. 2022, 13, 2474. [Google Scholar] [CrossRef]
- Montenegro, M.A.; Nazareno, M.A.; Durantini, E.N.; Borsarelli, C.D. Singlet molecular oxygen quenching ability of carotenoids in a reverse-micelle membrane mimetic system. Photochem. Photobiol. 2002, 75, 353–361. [Google Scholar] [CrossRef]
- Ramel, F.; Birtic, S.; Cuiné, S.; Triantaphylidès, C.; Ravanat, J.L.; Havaux, M. Chemical quenching of singlet oxygen by carotenoids in plants. Plant Physiol. 2012, 158, 1267–1278. [Google Scholar] [CrossRef]
- Kruk, J.; Szymańska, R. Singlet oxygen oxidation products of carotenoids, fatty acids and phenolic prenyllipids. J. Photochem. Photobiol. B 2021, 216, 112148. [Google Scholar] [CrossRef]
- Di Mascio, P.; Devasagayam, T.P.; Kaiser, S.; Sies, H. Carotenoids, tocopherols and thiols as biological singlet molecular oxygen quenchers. Biochem. Soc. Trans. 1990, 18, 1054–1056. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, A.; McGarvey, D.J.; Truscott, T.G.; Rancan, F.; Böhm, F. Singlet oxygen quenching by dietary carotenoids in a model membrane environment. Arch. Biochem. Biophys. 2003, 412, 47–54. [Google Scholar] [CrossRef]
- Ukai, N.; Lu, Y.; Etoh, H.; Yagi, A.; Ina, K.; Oshima, S.; Ojima, F.; Sakamoto, H.; Ishiguro, Y. Photosensitized Oxygenation of Lycopene. Biosci. Biotechnol. Biochem. 1994, 58, 1718–1719. [Google Scholar] [CrossRef]
- Naik, P.; Kumar, M. Receptor interactions of constituents of Zingiber officinalis and Solanum lycopersicum on COX. J. Pharmacogn. Phytochem. 2019, 8, 4637–4641. [Google Scholar]
- Hayashi, R.; Hayashi, S.; Machida, S. Changes in Aqueous Humor Lutein Levels of Patients with Cataracts after a 6-Week Course of Lutein-Containing Antioxidant Supplementation. Curr. Eye Res. 2022, 47, 1016–1023. [Google Scholar] [CrossRef]
- Sideri, O.; Tsaousis, K.T.; Li, H.J.; Viskadouraki, M.; Tsinopoulos, I.T. The potential role of nutrition on lens pathology: A systematic review and meta-analysis. Surv. Ophthalmol. 2019, 64, 668–678. [Google Scholar] [CrossRef]
- Groten, K.; Marini, A.; Grether-Beck, S.; Jaenicke, T.; Ibbotson, S.H.; Moseley, H.; Ferguson, J.; Krutmann, J. Tomato Phytonutrients Balance UV Response: Results from a Double-Blind, Randomized, Placebo-Controlled Study. Skin Pharmacol. Physiol. 2019, 32, 101–108. [Google Scholar] [CrossRef]
- Fam, V.W.; Holt, R.R.; Keen, C.L.; Sivamani, R.K.; Hackman, R.M. Prospective Evaluation of Mango Fruit Intake on Facial Wrinkles and Erythema in Postmenopausal Women: A Randomized Clinical Pilot Study. Nutrients 2020, 12, 3381. [Google Scholar] [CrossRef]
- Ouchi, A.; Aizawa, K.; Iwasaki, Y.; Inakuma, T.; Terao, J.; Nagaoka, S.; Mukai, K. Kinetic study of the quenching reaction of singlet oxygen by carotenoids and food extracts in solution. Development of a singlet oxygen absorption capacity (SOAC) assay method. J. Agric. Food Chem. 2010, 58, 9967–9978. [Google Scholar] [CrossRef]
- Fukuzawa, K.; Inokami, Y.; Tokumura, A.; Terao, J.; Suzuki, A. Rate constants for quenching singlet oxygen and activities for inhibiting lipid peroxidation of carotenoids and alpha-tocopherol in liposomes. Lipids 1998, 33, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Foote, C.S.; Ching, T.Y.; Geller, G.G. Chemistry of singlet oxygen. XVIII. Rates of reaction and quenching of alpha-tocopherol and singlet oxygen. Photochem. Photobiol. 1974, 20, 511–513. [Google Scholar] [PubMed]
- Gruszka, J.; Pawlak, A.; Kruk, J. Tocochromanols, plastoquinol, and other biological prenyllipids as singlet oxygen quenchers-determination of singlet oxygen quenching rate constants and oxidation products. Free Radic. Biol. Med. 2008, 45, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Shichiri, M.; Yoshida, Y.; Ishida, N.; Hagihara, Y.; Iwahashi, H.; Tamai, H.; Niki, E. α-Tocopherol suppresses lipid peroxidation and behavioral and cognitive impairments in the Ts65Dn mouse model of Down syndrome. Free Radic. Biol. Med. 2011, 50, 1801–1811. [Google Scholar] [CrossRef] [PubMed]
- Shichiri, M.; Harada, N.; Ishida, N.; Komaba, L.K.; Iwaki, S.; Hagihara, Y.; Niki, E.; Yoshida, Y. Oxidative stress is involved in fatigue induced by overnight deskwork as assessed by increase in plasma tocopherylhydroqinone and hydroxycholesterol. Biol. Psychol. 2013, 94, 527–533. [Google Scholar] [CrossRef]
- Shichiri, M.; Ishida, N.; Hagihara, Y.; Yoshida, Y.; Kume, A.; Suzuki, H. Probucol induces the generation of lipid peroxidation products in erythrocytes and plasma of male cynomolgus macaques. J. Clin. Biochem. Nutr. 2019, 64, 129–142. [Google Scholar] [CrossRef]
- Krasnovsky, A.A.; Kagan, V.E.; Minin, A.A. Quenching of singlet oxygen luminescence by fatty acids and lipids: Contribution of physical and chemical mechanisms. FEBS Lett. 1983, 155, 233–236. [Google Scholar] [CrossRef]
- Chaudhuri, R.K.; Bojanowski, K. Bakuchiol: A retinol-like functional compound revealed by gene expression profiling and clinically proven to have anti-aging effects. Int. J. Cosmet. Sci. 2014, 36, 221–230. [Google Scholar] [CrossRef]
- Chaudhuri, R.K.; Marchio, F. Bakuchiol in the Management of Acne-affected Skin. Cosmet. Toilet. 2011, 126, 502–510. [Google Scholar]
- Dhaliwal, S.; Rybak, I.; Ellis, S.R.; Notay, M.; Trivedi, M.; Burney, W.; Vaughn, A.R.; Nguyen, M.; Reiter, P.; Bosanac, S.; et al. Prospective, randomized, double-blind assessment of topical bakuchiol and retinol for facial photoageing. Br. J. Dermatol. 2019, 180, 289–296. [Google Scholar] [CrossRef]
- Krishnakantha, T.P.; Lokesh, B.R. Scavenging of superoxide anions by spice principles. Indian J. Biochem. Biophys. 1993, 30, 133–134. [Google Scholar]
- Swindell, W.R.; Bojanowski, K.; Chaudhuri, R.K. A Zingerone Analog, Acetyl Zingerone, Bolsters Matrisome Synthesis, Inhibits Matrix Metallopeptidases, and Represses IL-17A Target Gene Expression. J. Invest. Dermatol. 2020, 140, 602–614.e615. [Google Scholar] [CrossRef] [PubMed]
- Das, K.C.; Das, C.K. Curcumin (diferuloylmethane), a singlet oxygen ((1)O(2)) quencher. Biochem. Biophys. Res. Commun. 2002, 295, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, R.K.; Meyer, T.; Premi, S.; Brash, D. Acetyl zingerone: An efficacious multifunctional ingredient for continued protection against ongoing DNA damage in melanocytes after sun exposure ends. Int. J. Cosmet. Sci. 2020, 42, 36–45. [Google Scholar] [CrossRef]
- Dhaliwal, S.; Rybak, I.; Pourang, A.; Burney, W.; Haas, K.; Sandhu, S.; Crawford, R.; Sivamani, R.K. Randomized double-blind vehicle controlled study of the effects of topical acetyl zingerone on photoaging. J. Cosmet. Dermatol. 2021, 20, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Aratani, Y. Myeloperoxidase: Its role for host defense, inflammation, and neutrophil function. Arch. Biochem. Biophys. 2018, 640, 47–52. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).