Evaluation of Oxidative Stress and Metabolic Profile in a Preclinical Kidney Transplantation Model According to Different Preservation Modalities
Abstract
1. Introduction
2. Results
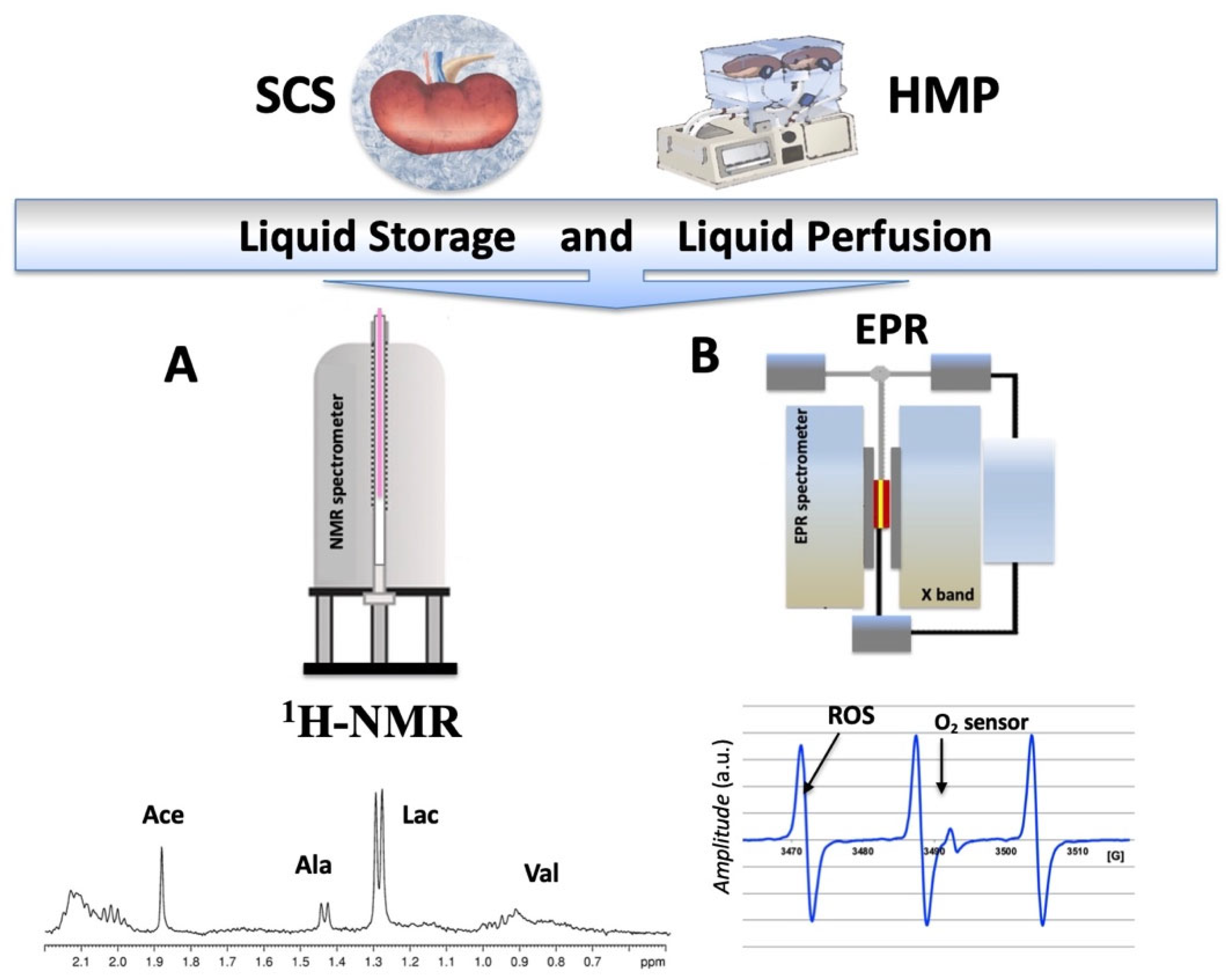
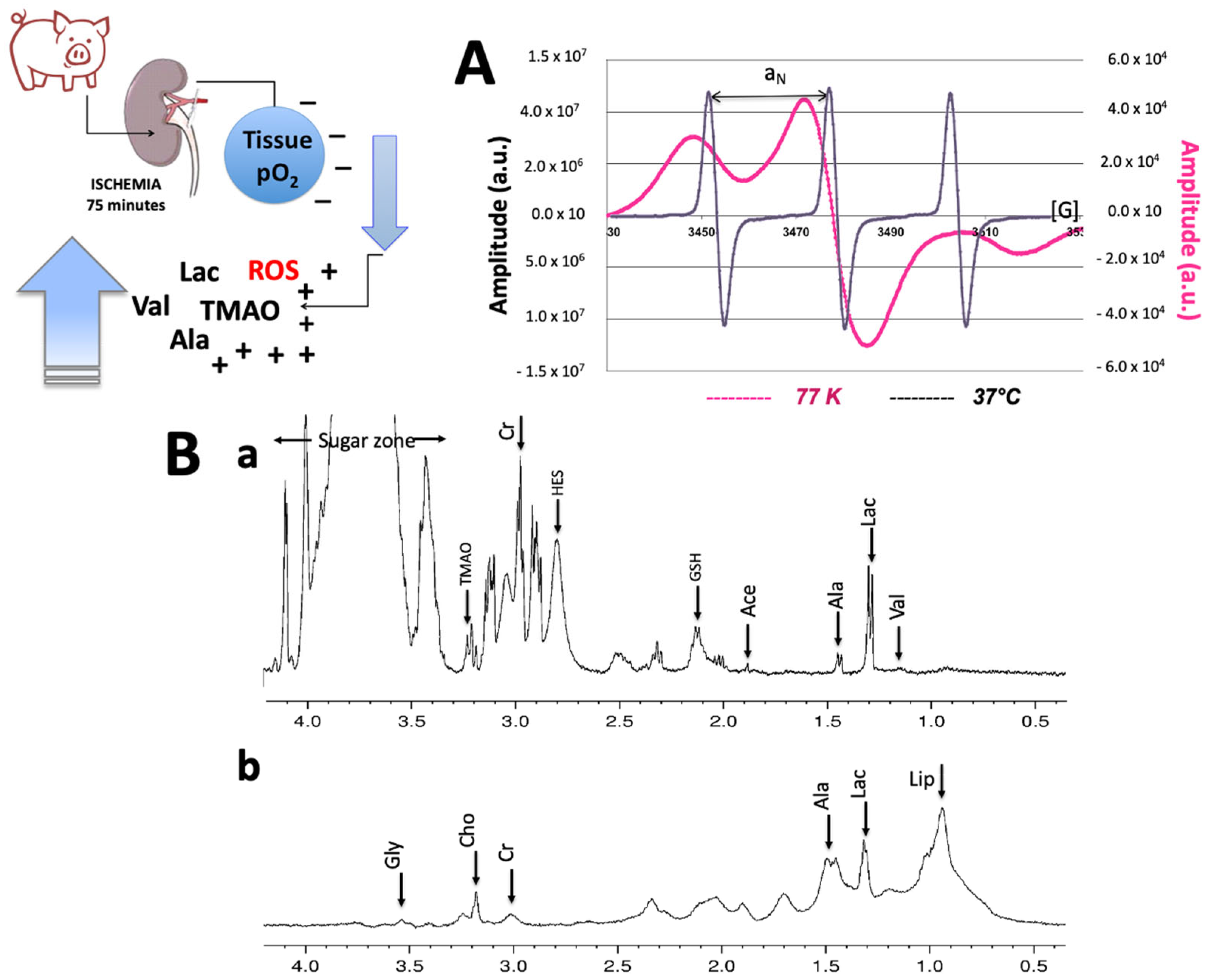
2.1. NMR and EPR Procedure
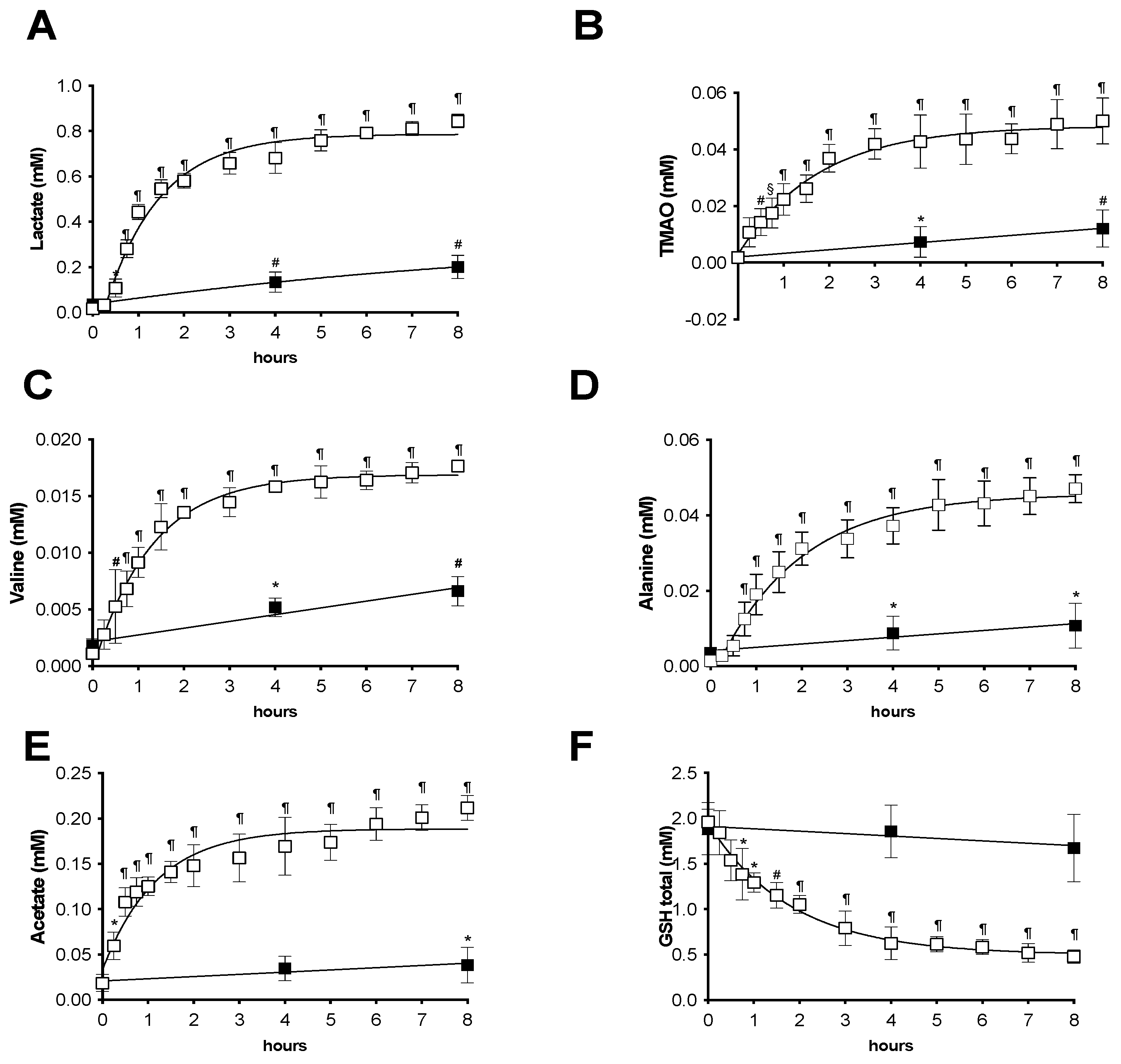
2.2. Analysis during the Preservation Procedure
1H-NMR Analysis from Preservation Solutions
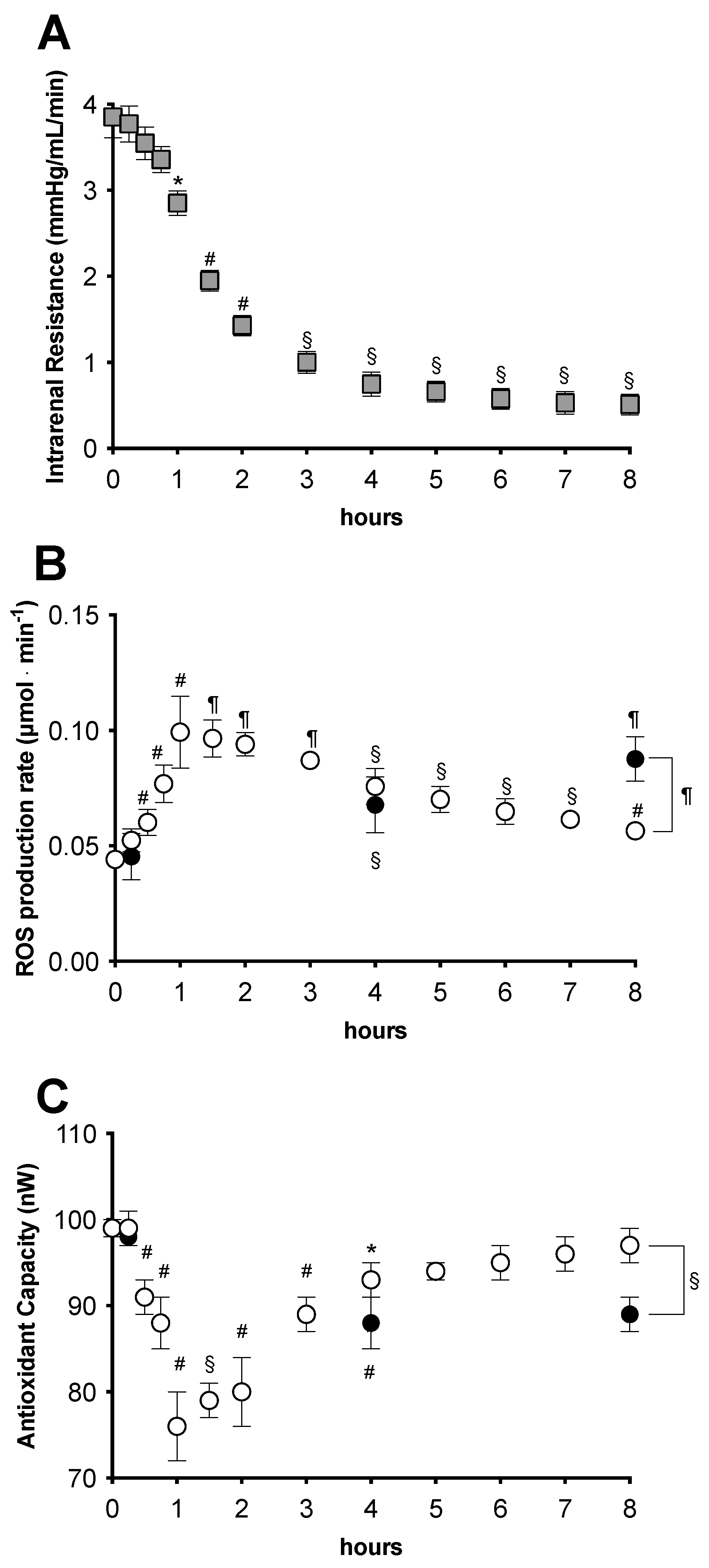
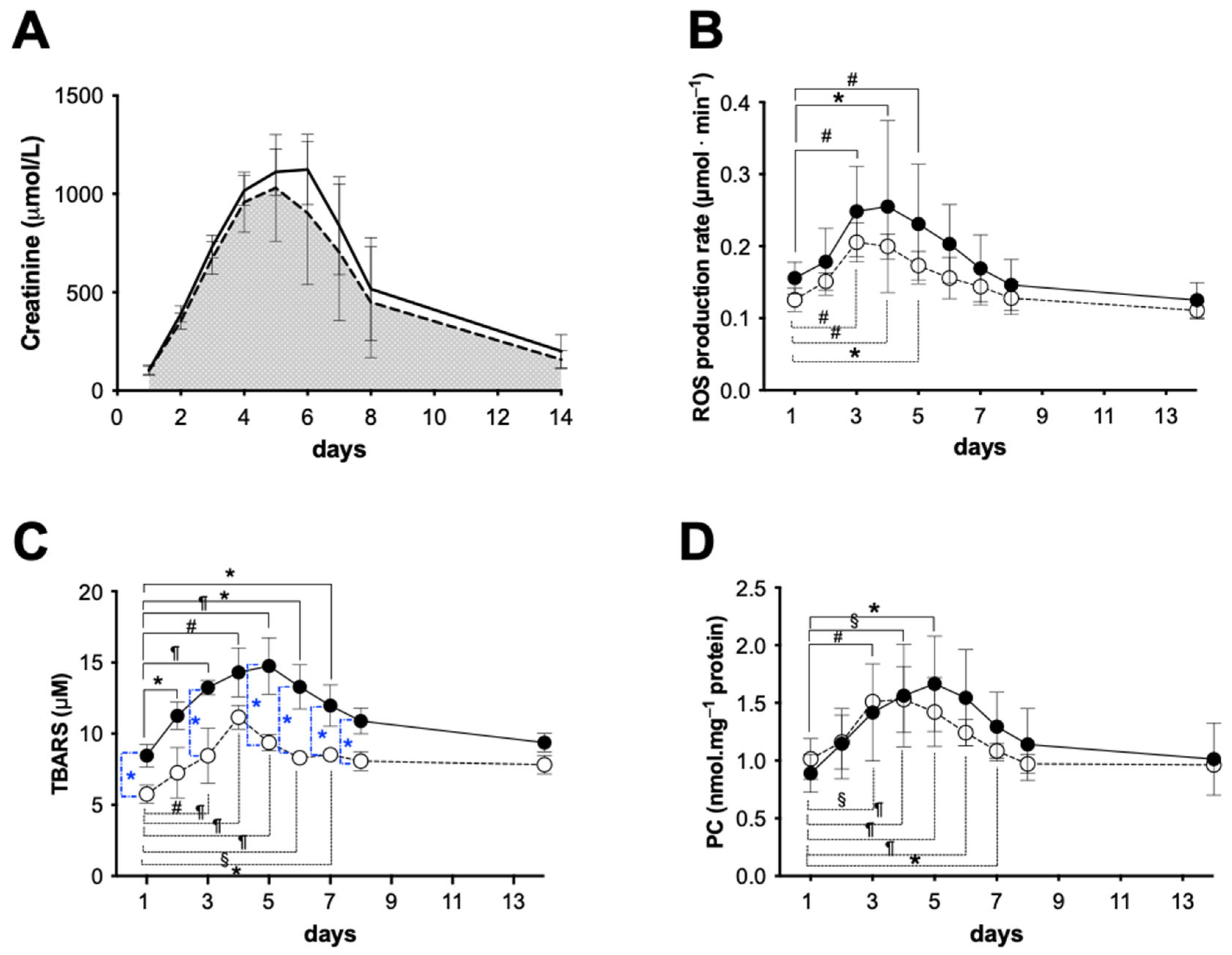
2.3. Functional Analysis during the Survival Period
2.3.1. Early Functional Recovery
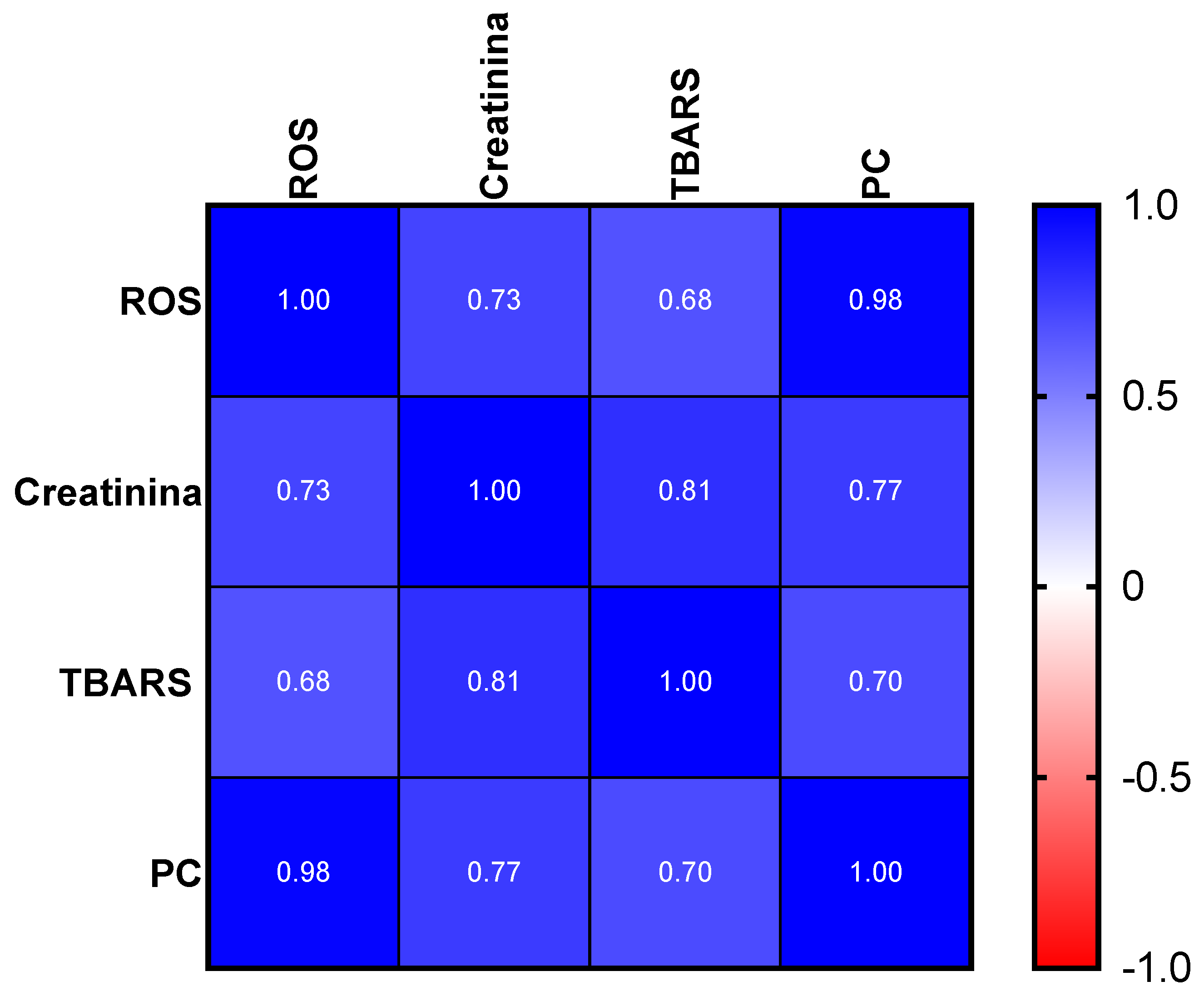
2.3.2. Oxidative Stress Evaluation
2.4. 1HNMR and EPR Tissue Analysis
2.5. Histological Evaluation
3. Discussion
4. Materials and Methods
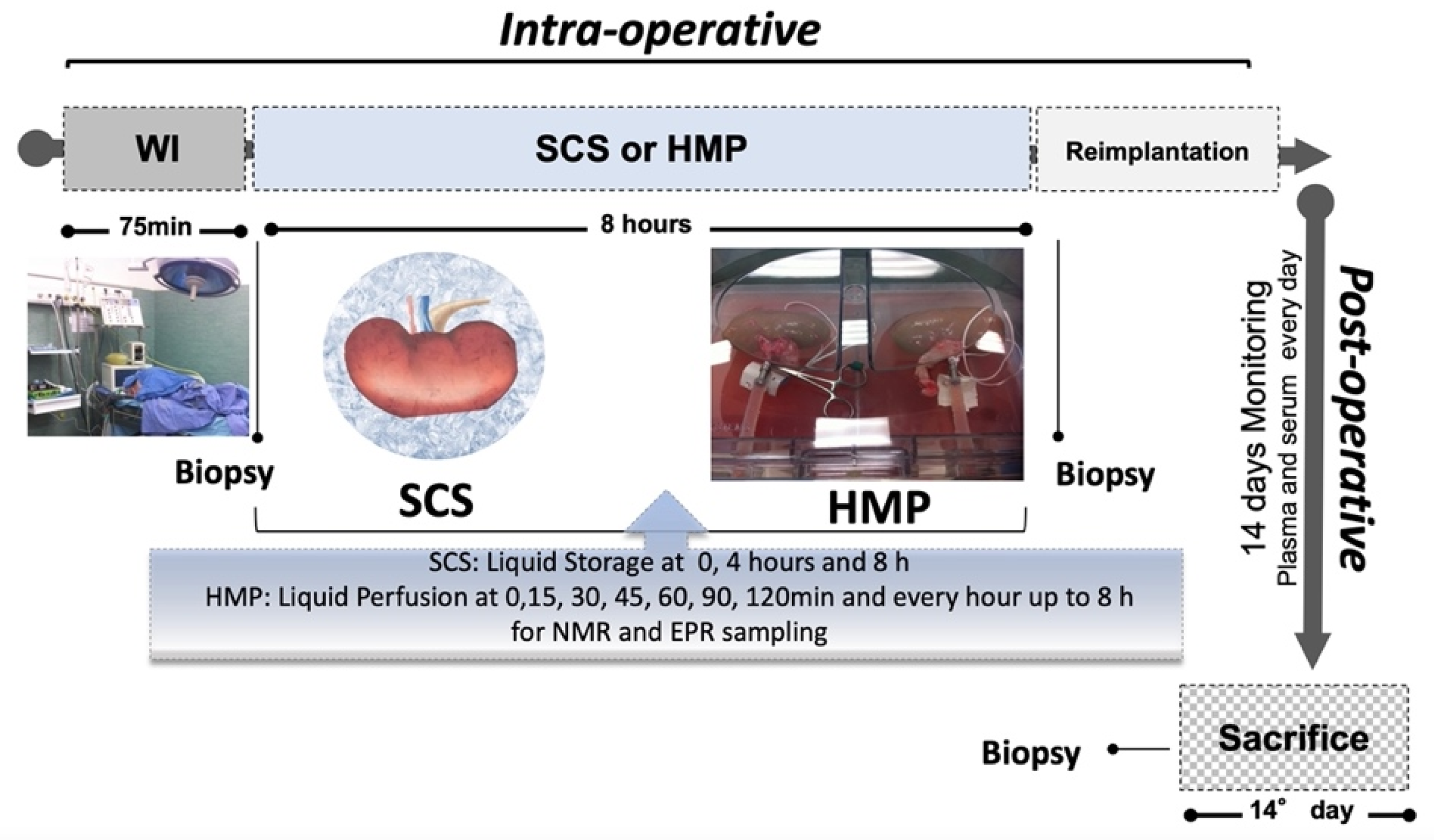
4.1. Animal Model and Surgical Procedure
4.2. Post-Surgical Evaluation Procedure
4.3. Sampling Methods
4.3.1. Perfusate Sampling
4.3.2. Blood Sampling
4.3.3. Tissue Sampling
4.4. Measurements
4.4.1. EPR Measurements
4.4.2. Antioxidant Capacity
4.4.3. Enzymatic Assays
Thiobarbituric-Acid-Reactive Substances (TBARS)
Protein Carbonyls (PC)
4.4.4. 1H-NMR Analysis
4.5. Histological Evaluation
4.6. Renal Function Recovery Evaluation
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | Adenosine Triphosphate |
| 1H NMR | Proton Nuclear Magnetic Resonance |
| CMH | 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine |
| CP | 3-Carboxy-2,2,5,5-tetramethyl-1-pyrrolidinyloxy |
| DCD | Donation after Cardiac Death |
| DETC | Sodium Diethyldithio-Carbamate Trihydrate |
| DF | Deferoxamine Methane-Sulfonate Salt |
| DGF | Delayed Graft Function |
| EPR | Electron Paramagnetic Resonance |
| ECD | Expanded Criteria Donor |
| HE | Hematoxylin and Eosin |
| MDA | Malondialdehyde |
| PC | Protein Carbonyls |
| HMP | Hypothermic Machine Perfusion |
| ROS | Reactive Oxygen Species |
| SCD | Standard Criteria Donors |
| SCS | Static Cold Storage |
| TAC | Total Antioxidant Capacity |
| TBARS | Thiobarbituric-Acid-Reactive Substances |
| WIT | Warm Ischemia Time |
References
- Wolfe, R.A.; Ashby, V.B.; Milford, E.L.; Ojo, A.O.; Ettenger, R.E.; Agodoa, L.Y.; Held, P.J.; Port, F.K. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N. Engl. J. Med. 1999, 41, 1725–1730. [Google Scholar] [CrossRef]
- Tonelli, M.; Wiebe, N.; Knoll, G.; Bello, A.; Browne, S.; Jadhav, D.; Klarenbach, S.; Gill, J. Systematic review: Kidney trans‚Äê plantation compared with dialysis in clinically relevant outcomes. Am. J. Transplant. 2011, 11, 2093. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Gruessner, A.; Agopian, V.G.; Khalpey, Z.; Riaz, I.B.; Kaplan, B.; Halazun, K.J.; Busuttil, R.W.; SGruessner, R.W.G. Survival benefit of solid-organ transplant in the United States. JAMA Surg. 2015, 150, 252–259. [Google Scholar] [CrossRef]
- Morrissey, P.E.; Monaco, A.P. Donation after circulatory death: Cur- rent practices, ongoing challenges, and potential improvements. Transplantation 2014, 97, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.S.; Ojo, A. The alphabet soup of kidney transplantation: SCD, DCD, ECD–fundamentals for the practicing nephrologist. Clin. J. Am. Soc. Nephrol. 2009, 4, 1827–1831. [Google Scholar] [CrossRef] [PubMed]
- Darius, T.; Gianello, P.; Vergauwen, M.; Mourad, N.; Buemi, A.; De Meyer, M.; Mourad, M. The effect on early renal function of various dynamic preservation strategies in a preclinical pig ischemia-reperfusion autotransplant model. Am. J. Transplant. 2019, 19, 752–762. [Google Scholar] [CrossRef]
- Alshahrani, M.; Alotaibi, M.; Bhutto, B. Assessing the Outcome of Adult Kidney Transplantation from a Deceased Expanded Criteria Donor: A Descriptive Study. Cureus 2020, 12, e11199. [Google Scholar] [CrossRef]
- Barreda, M.; Redondo-Pachón, D.; Miñambres García, E.; Rodrigo Calabia, E. Kidney transplant outcome of expanded criteria donors after circulatory death. Nefrologia 2022, 42, 135–144. [Google Scholar]
- Akoh, J.A. Kidney donation after cardiac death. World J. Nephrol. 2012, 1, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Salahudeen, A.K. Consequences of cold ischemic injury of kidneys in clinical transplantation. J. Investig. Med. 2004, 52, 296–298. [Google Scholar] [CrossRef]
- Salahudeen, A.K. Cold ischemic injury of transplanted organs: Some new strategies against an old problem. Am. J. Transplant. 2004, 4, 1. [Google Scholar] [CrossRef]
- Norma, A.; Palomeque, J.; Carbonell, T. Oxidative stress and antioxidant activity in hypothermia and rewarming: Can RONS modulate the beneficial effects of therapeutic hypothermia? Oxid. Med. Cell. Longev. 2013, 2013, 957054. [Google Scholar]
- Koetting, M.; Frotscher, C.; Minor, T. Hypothermic reconditioning after cold storage improves postischemic graft function in isolated porcine kidneys. Transplant. Int. 2010, 23, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, D.P.; Gallinat, A.; Swoboda, S.; Wohlschlaeger, J.; Rauen, U.; Paul, A.; Minor, T. Influence of oxygen concentration during hypothermic machine perfusion on porcine kidneys from donation after circulatory death. Transplantation 2014, 98, 944–950. [Google Scholar] [CrossRef]
- Thuillier, R.; Allain, G.; Celhay, O.; Hebrard, W.; Barrou, B.; Badet, L.; Leuvenink, H.; Hauet, T. Benefits of active oxygenation during hypothermic machine perfusion of kidneys in a preclinical model of deceased after cardiac death donors. J. Surg. Res. 2013, 184, 1174–1181. [Google Scholar] [CrossRef]
- Darius, T.; Vergauwen, M.; Smith, T.; Gerin, I.; Joris, V.; Mueller, M.; Aydin, S.; Muller, X.; Schlegel, A.; Nath, J.; et al. Brief O uploading during continuous hypothermic machine perfusion is simple yet effective oxygenation method to improve initial kidney function in a porcine autotransplant model. Am. J. Transplant. 2020, 20, 030–2043. [Google Scholar] [CrossRef] [PubMed]
- Perico, N.; Cattaneo, D.; Sayegh, M.H.; Remuzzi, G. Delayed graft function in kidney transplantation. Lancet 2004, 364, 1814–1827. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, J.; Otarashvili, G.; Meszaros, A.; Ebner, S.; Weissenbachwer, A.; Cardini, B.; Oberhuber, R.; Resch, T.; Ofner, D.; Schneeberger, S.; et al. Restoring Mitochondrial Function While Avoiding Redox Stress: The Key to Preventing Ischemia/Reperfusion Injury in Machine Perfused Liver Grafts? Int. J. Mol. Sci. 2020, 21, 3132. [Google Scholar] [CrossRef]
- Theodore, K.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Ischemia/Reperfusion. Compr. Physiol. 2016, 7, 113–170. [Google Scholar]
- Micò-Carnero, M.; Zaouali, M.A.; Rojano-Alfinso, C.; Maroto-Serrat, C.; Abdennebi, H.B.; Peralta, C. A Potential Route to Reduce Ischemia/Reperfusion Injury in Organ Preservation. Cells 2022, 11, 2763. [Google Scholar] [CrossRef]
- Fonseca, I.; Reguengo, H.; Almeida, M.; Dias, L.; Martins, L.S.; Pedroso, S.; Santos, J.; Lobato, L.; Henriques, A.C.; Mendonça, D. Oxidative stress in kidney transplantation: Malondialdehyde is an early predictive marker of graft dysfunction. Transplantation 2014, 97, 1058–1065. [Google Scholar] [CrossRef]
- O’Callaghan, J.M.; Knight, S.R.; Morgan, R.D.; Morris, P.J. Preservation solutions for static cold storage of kidney allografts: A systematic review and meta-analysis. Am. J. Transplant. 2012, 12, 896–906. [Google Scholar] [CrossRef]
- Tingle, S.J.; Figueiredo, R.S.; Moir, J.A.; Goodfellow, M.; Talbot, D.; Wilson, C.H. Machine perfusion preservation versus static cold storage for deceased donor kidney transplantation. Cochrane Database Syst. Rev. 2019, 3, CD011671. [Google Scholar] [CrossRef]
- Belzer, F.O.; Southard, J.H. Principles of solid-organ preservation by cold storage. Transplantation 1988, 45, 673–676. [Google Scholar] [CrossRef]
- Peng, P.; Ding, Z.; He, Y.; Zhang, J.; Wang, X.; Yang, Z. Hypothermic machine perfusion versus static cold storage in deceased donor kidney transplantation: A systematic review and meta-analysis of randomized controlled trials. Artif. Organs 2019, 43, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Feng, L.; Pan, M.; Gao, Y. Optimizing livers for transplantation using machine perfusion versus cold storage in large animal studies and human studies: A systematic review and meta-analysis. Biomed. Res. Int. 2018, 2018, 9180757. [Google Scholar] [CrossRef] [PubMed]
- Weberskirch, S.; Katou, S.; Reuter, S.; Kneifel, F.; Morgul, M.H.; Becker, F.; Houben, P.; Pascher, A.; Vogel, T.; Radunz, S. Dynamic Parameters of Hypothermic Machine Perfusion—An Image of Initial Graft Function in Adult Kidney Transplantation? J. Clin. Med. 2022, 11, 5698. [Google Scholar] [CrossRef] [PubMed]
- Nath, J.; Smith, T.B.; Patel, K.; Ebbs, S.R.; Hollis, A.; Tennant, D.A.; Ludwig, C.; Ready, A.R. Metabolic differences between cold stored and machine perfused porcine kidneys: A 1H NMR based study. Cryobiology 2017, 74, 115–120. [Google Scholar] [CrossRef]
- Dikalov, S.; Griendling, K.K.; Harrison, D.G. Measurement of reactive oxygen species in cardiovascular studies. Hypertension 2007, 49, 717–727. [Google Scholar] [CrossRef]
- Suzen, S.; Gurer-Orhan, H.; Saso, L. Detection of Reactive Oxygen and Nitrogen Species by Electron Paramagnetic Resonance (EPR) Technique. Molecules 2017, 22, 181. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Gussoni, M.; Montorsi, M.; Porcelli, S.; Vezzoli, A. A quantitative method to monitor reactive oxygen species production by electron paramagnetic resonance in physiological and pathological conditions. Oxid. Med. Cell. Longev. 2014, 2014, 306179. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Vezzoli, A.; Maderna, L.; Gregorini, F.; Montorsi, M.; Moretti, S.; Greco, F.; Cova, M.; Gussoni, M. R(+)-Thioctic Acid Effects on Oxidative Stress and Peripheral Neuropathy in Type II Diabetic Patients: Preliminary Results by Electron Paramagnetic Resonance and Electroneurography. Oxid. Med. Cell. Longev. 2018, 10, 2018, 1767265. [Google Scholar] [CrossRef]
- Cova, E.; Inghilleri, S.; Pandolfi, L.; Morosini, M.; Magni, S.; Colombo, M.; Piloni, D.; Finetti, C.; Ceccarelli, G.; Benedetti, L.; et al. Bioengineered gold nanoparticles targeted to mesenchymal cells from patients with bronchiolitis obliterans syndrome does not rise the inflammatory response and can be safely inhaled by rodents. Nanotoxicology 2017, 11, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Rivellini, C.; Porrello, E.; Dina, G.; Mrakic-Sposta, S.; Vezzoli, A.; Bacigaluppi, M.; Gullotta, G.S.; Chaabane, l.; Leocani, L.; Marenna, S.; et al. JAB1 deletion in oligodendrocytes causes senescence-induced inflammation and neurodegeneration in mice. J. Clin. Investig. 2022, 132, e145071. [Google Scholar] [CrossRef]
- Malacrida, S.; De Lazzari, F.; Mrakic-Sposta, S.; Vezzoli, A.; Zordan, M.A.; Bisaglia, M.; Menti, G.M.; Meda, N.; Frighetto, G.; Bosco, G.; et al. Lifespan and ROS levels in different Drosophila melanogaster strains after 24 h hypoxia exposure. Biol. Open 2022, 11, bio059386. [Google Scholar] [CrossRef]
- Vezzoli, A.; Gussoni, M.; Greco, F.; Zetta, L. Effects of temperature and extracellular pH on metabolites: Kinetics of anaerobic metabolism in resting muscle by 31P- and 1H-NMR spectroscopy. J. Exp. Biol. 2003, 206 Pt 17, 3043–3052. [Google Scholar] [CrossRef][Green Version]
- Vezzoli, A.; Gussoni, M.; Greco, F.; Zetta, L.; Cerretelli, P. Temperature and pH dependence of energy balance by (31)P- and (1)H-MRS in anaerobic frog muscle. Biochim. Biophys. Acta 2004, 1608, 163–170. [Google Scholar] [CrossRef][Green Version]
- Gussoni, M.; Cremonini, M.A.; Vezzoli, A.; Greco, F.; Zetta, L. A quantitative method to assess muscle tissue oxygenation in vivo by monitoring 1H nuclear magnetic resonance myoglobin resonances. Anal. Biochem. 2010, 400, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Scorciapino, M.A.; Spiga, E.; Vezzoli, A.; Mrakic-Sposta, S.; Russo, R.; Fink, B.; Casu, M.; Gussoni, M.; Ceccarelli, M. Structure-function paradigm in human myoglobin: How a single-residue substitution affects NO reactivity at low pO2. Am. Chem. Soc. 2013, 135, 7534–7544. [Google Scholar] [CrossRef]
- Pasini, G.; Greco, F.; Cremonini, M.A.; Brandolini, A.; Consonni, R.; Gussoni, M. Structural and Nutritional Properties of Pasta from Triticum monococcum and Triticum durum Species. A Combined 1H NMR, MRI, and Digestibility Study. J. Agric. Food Chem. 2015, 63, 5072–5082. [Google Scholar] [CrossRef] [PubMed]
- Rossard, L.; Favreau, F.; Demars, J.; Robert, R.; Nadeau, C.; Cau, J.; Thuillier, R.; Hauet, T. Evaluation of early regenerative processes in a preclinical pig model of acute kidney injury. Curr. Mol. Med. 2012, 12, 502–505. [Google Scholar] [PubMed]
- Codas, R.; Thuillier, R.; Hauet, T.; Badet, L. Renoprotective effect of pulsatile perfusion machine RM3: Pathophysiological and kidney injury biomarker characterization in a preclinical model of autotransplanted pig. BJU Int. 2012, 109, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Nasr, M.; Sigdel, T.; Sarwal, M. Advances in diagnostics for transplant rejection. Expert Rev. Mol. Diagn. 2016, 16, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://www.accessdata.fda.gov/cdrh_docs/pdf5/K053169.pdf (accessed on 1 January 2022).
- Situmorang, G.R.; Sheerin, N.S. Ischaemia reperfusion injury: Mechanisms of progression to chronic graft dysfunction. Pediatr. Nephrol. 2019, 34, 951–963. [Google Scholar] [CrossRef]
- Zhao, H.; Alam, A.; Pac Soo, A.; George, A.J.T.; Ma, D. Ischemia-Reperfusion Injury Reduces Long Term Renal Graft Survival: Mechanism and Beyond. EBioMedicine 2018, 28, 31–42. [Google Scholar] [CrossRef]
- Patrono, D.; Roggio, D.; Mazzeo, A.T.; Catalano, G.; Mazza, E.; Rizza, G.; Gambella, A.; Rigo, F.; Leone, N.; Elia, V.; et al. Clinical assessment of liver metabolism during hypothermic oxygenated machine perfusion using microdialysis. Artif. Organs 2022, 46, 281–295. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Granger, N.I.; Kvietys, P.R. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox. Biol. 2015, 6, 524–551. [Google Scholar] [CrossRef]
- Tejchman, K.; Sierocka, A.; Kotfis, K.; Kotowski, M.; Dolegowska, B.; Ostrowski, M.; Sienko, J. Assessment of Oxidative Stress Markers in Hypothermic Preservation of Transplanted Kidneys. Antioxidants 2021, 10, 1263. [Google Scholar] [CrossRef] [PubMed]
- Carcy, R.; Cougnon, M.; Poet, M.; Durandy, M.; Sicard, A.; Counillon, L.; Blondeau, N.; Hauet, T.; Tauc, M.; Pisani, D.F. Targeting oxidative stress, a crucial challenge in renal transplantation outcome. Radic. Biol. Med. 2021, 69, 258–270. [Google Scholar] [CrossRef]
- Darius, T.; Vergauwen, M.; Smith, T.B.; Patel, K.; Craps, J.; Joris, V.; Aydin, S.; Ury, B.; Buemi, A.; De Meyer, M.; et al. Influence of Different Partial Pressures of Oxygen During Continuous Hypothermic Machine Perfusion in a Pig Kidney Ischemia-reperfusion Autotransplant Model. Transplantation 2020, 104, 731–774. [Google Scholar] [CrossRef] [PubMed]
- Hosgood, S.A.; Yang, B.; Bagul, A.; Mohamed, I.H.; Nicholson, M.L. A comparison of hypothermic machine perfusion versus static cold storage in an experimental model of renal ischemia reperfusion injury. Transplantation 2010, 89, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Endemann, D.H.; Schiffrin, E.L. Endothelial Dysfunction. JASN 2004, 15, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Kohen, R.; Nyska, A. Oxidation of biological systems: Oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol. Pathol. 2002, 30, 620–650. [Google Scholar] [CrossRef]
- Wishart, D.S. Metabolomics in monitoring kidney transplants. Curr. Opin. Nephrol. Hyperten. 2006, 15, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Aquilano, K.; Baldelli, S.; Ciriolo. M.R. Glutathione: New roles in redox signaling for an old antioxidant. Front. Pharmacol. 2014, 5, 196. [Google Scholar] [CrossRef]
- Shokes, D.A.; Halloran, P.F. Delayed Graft Function in Renal Transplantation: Etiology, Management and Long-term Significance. J. Urol. 1996, 155, 1831–1840. [Google Scholar] [CrossRef] [PubMed]
- Gutteridge, J.M. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin. Chem. 1995, 41 Pt 2, 1819–1828. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Mylonas, C.; Kouretas, D. Lipid peroxidation and tissue damage. In Vivo 1999, 13, 295–309. [Google Scholar]
- Gianazza, E.; Brioschi, M.; Fernandez, A.M.; Banfi, C. Lipoxidation in cardiovascular diseases. Redox. Biol. 2019, 23, 101119. [Google Scholar] [CrossRef] [PubMed]
- Silver, E.H.; Szabo, S. Role of lipid peroxidation in tissue injury after hepatic ischemia. Exp. Mol. Pathol. 1983, 38, 69–76. [Google Scholar] [CrossRef]
- Semmelmann, A.; Neeff, H.; Sommer, O.; Thomusch, O.; Hopt, U.T.; Von Dobschuetz, E. Evaluation of preservation solutions by ESR-spectroscopy: Superior effects of University of Wisconsin over Histidine-Tryptophan-Ketoglutarate in reducing renal reactive oxygen species. Kidney Int. 2007, 71, 875–881. [Google Scholar] [CrossRef][Green Version]
- Chen, Y.; Shi, J.; Xia, T.C.; Xu, R.; He, X.; Xia, Y. Preservation Solution for kidney transplantation: History, advances and mechanisms. Cell Transplantation. 2019, 28, 1472–1489. [Google Scholar] [CrossRef]
- Ostroóżka-Cieslik, A. The Effect of Antioxidant Added to Preservation Solution on the Protection of Kidneys before Transplantation. Int. J. Mol. Sci. 2022, 23, 3141. [Google Scholar] [CrossRef] [PubMed]
- Rampino, T.; Abelli, M.; Ticozzelli, E.; Gregorini, M.; Bosio, F.; Piotti, G.; Bedino, G.; Esposito, P.; Balenzano, C.T.; Geraci, P.; et al. Non-heart-beating-donor transplant: The first experience in Italy. G Ital. Nefrol. 2010, 27, 56–68. [Google Scholar]
- Treska, V.; Kobr, J.; Hasman, D.; Racek, J.; Trefil, L.; Reischig, T.; Hes, O.; Kuntscher, V.; Molacek, J.; Treska, I. Ischemia-reperfusion injury in kidney transplantation from non-heart-beating donor--do antioxidants or antiinflammatory drugs play any role? Bratisl. Lek. Listy 2009, 110, 133–136. [Google Scholar] [PubMed]
- Catena, F.; Coccolini, F.; Montori, G.; Vallicelli, C.; Amaduzzi, A.; Ercolani, G.; Ravaioli, M.; Del Gaudio, M.; Schiavina, R.; Brunocilla, E.; et al. Kidney Preservation: Review of Present and Future Perspective. Transplant. Proc. 2013, 45, 3170–3177. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Gussoni, M.; Montorsi, M.; Porcelli, S.; Vezzoli, A. Assessment of a standardized ROS production profile in humans by electron paramagnetic resonance. Oxid. Med. Cell. Longev. 2012, 2012, 973927. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Gussoni, M.; Porcelli, S.; Pugliese, L.; Pavei, G.; Bellistri, G.; Montorsi, M.; Tacchini, P.; Vezzoli, A. Training effects on ROS production determined by electron paramagnetic resonance in master swimmers. Oxid. Med. Cell. Longev. 2015, 2015, 804794. [Google Scholar] [CrossRef]
- Mrakic Sposta, S.; Montorsi, M.; Porcelli, S.; Marzorati, M.; Healey, B.; Dellanoce, C.; Vezzoli, A. Effects of Prolonged Exposure to Hypobaric Hypoxia on Oxidative Stress: Overwintering in Antarctic Concordia Station. Oxid. Med. Cell. Longev. 2022, 2022, 4430032. [Google Scholar] [CrossRef] [PubMed]
- Dikalov, S.I.; Polienko, Y.F.; Kirilyuk, I. Electron paramagnetic resonance measurements of reactive oxygen species by cyclic hydroxylamine spin probes. Antioxid. Redox. Signal. 2018, 28, 1433–1443. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Roussel, C.; Lagger, G.; Tacchini, P.; Girault, H.H. Antioxidant sensors based on DNA-modified electrodes. Anal. Chem. 2005, 77, 7687–7694. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Su, B.; Lagger, G.; Tacchini, P.; Girault, H.H. Antioxidant redox sensors based on DNA modified carbon screen-printed electrodes. Anal. Chem. 2006, 78, 6879–6884. [Google Scholar] [CrossRef]
- Beckonert, O.; Keun, H.C.; Ebbels, T.M.D.; Bundy, J.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat. Protoc. 2007, 2, 2692–2703. [Google Scholar] [CrossRef]
- Bathini, V.; McGregor, T.; McAlister, V.C.; Luke, P.P.; Sener, A. Renal perfusion pump vs cold storage for donation after cardiac death kidneys: A systematic review. J. Urol. 2013, 189, 2214–2220. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mrakic-Sposta, S.; Vezzoli, A.; Cova, E.; Ticcozzelli, E.; Montorsi, M.; Greco, F.; Sepe, V.; Benzoni, I.; Meloni, F.; Arbustini, E.; et al. Evaluation of Oxidative Stress and Metabolic Profile in a Preclinical Kidney Transplantation Model According to Different Preservation Modalities. Int. J. Mol. Sci. 2023, 24, 1029. https://doi.org/10.3390/ijms24021029
Mrakic-Sposta S, Vezzoli A, Cova E, Ticcozzelli E, Montorsi M, Greco F, Sepe V, Benzoni I, Meloni F, Arbustini E, et al. Evaluation of Oxidative Stress and Metabolic Profile in a Preclinical Kidney Transplantation Model According to Different Preservation Modalities. International Journal of Molecular Sciences. 2023; 24(2):1029. https://doi.org/10.3390/ijms24021029
Chicago/Turabian StyleMrakic-Sposta, Simona, Alessandra Vezzoli, Emanuela Cova, Elena Ticcozzelli, Michela Montorsi, Fulvia Greco, Vincenzo Sepe, Ilaria Benzoni, Federica Meloni, Eloisa Arbustini, and et al. 2023. "Evaluation of Oxidative Stress and Metabolic Profile in a Preclinical Kidney Transplantation Model According to Different Preservation Modalities" International Journal of Molecular Sciences 24, no. 2: 1029. https://doi.org/10.3390/ijms24021029
APA StyleMrakic-Sposta, S., Vezzoli, A., Cova, E., Ticcozzelli, E., Montorsi, M., Greco, F., Sepe, V., Benzoni, I., Meloni, F., Arbustini, E., Abelli, M., & Gussoni, M. (2023). Evaluation of Oxidative Stress and Metabolic Profile in a Preclinical Kidney Transplantation Model According to Different Preservation Modalities. International Journal of Molecular Sciences, 24(2), 1029. https://doi.org/10.3390/ijms24021029








