Genes Encoding Structurally Conserved Serpins in the Wheat Genome: Identification and Expression Profiles during Plant Development and Abiotic and Biotic Stress
Abstract
1. Introduction
2. Results
2.1. Serpin Identification
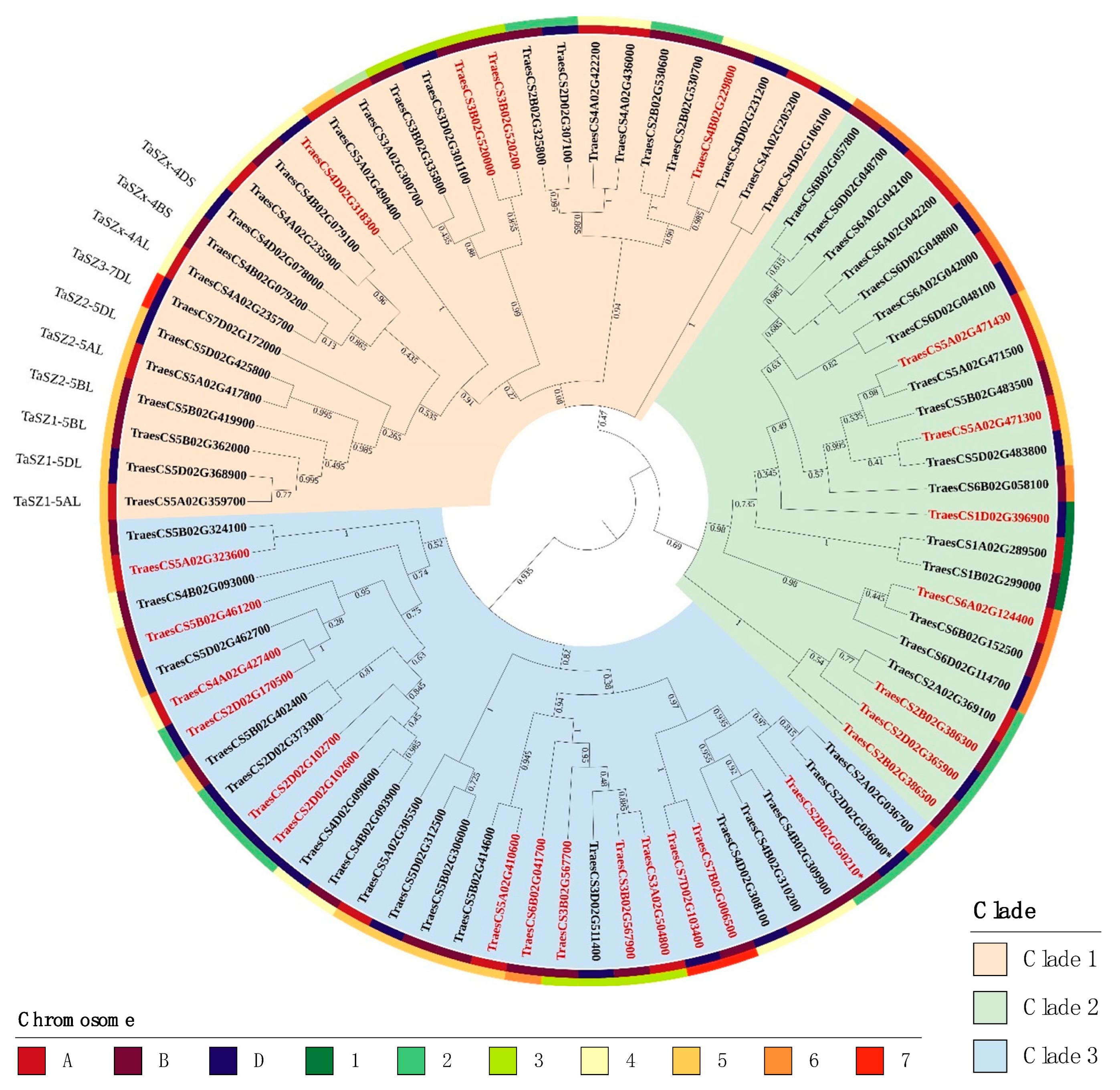
2.2. Phylogenetic Analysis
2.3. Genome Distribution and Gene Duplication of the Wheat Serpin Gene Complement
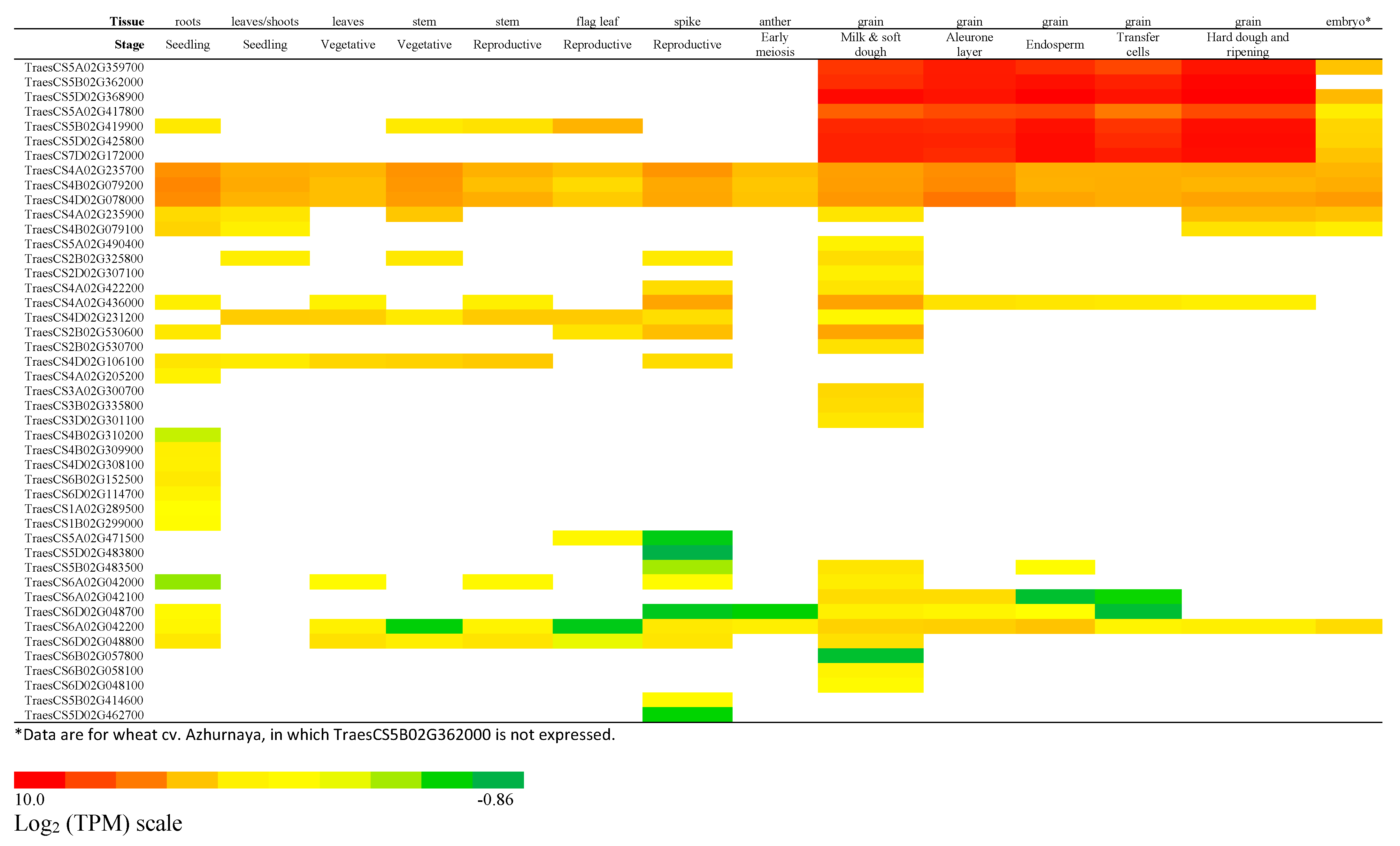
2.4. Expression Profile of Wheat Serpin Genes
2.4.1. Highly Expressed Grain-Specific Serpins and the Correction of the TraesCS5B02G362000 Sequence
2.4.2. Serpin Genes Expressed Ubiquitously and Tissue-Specifically
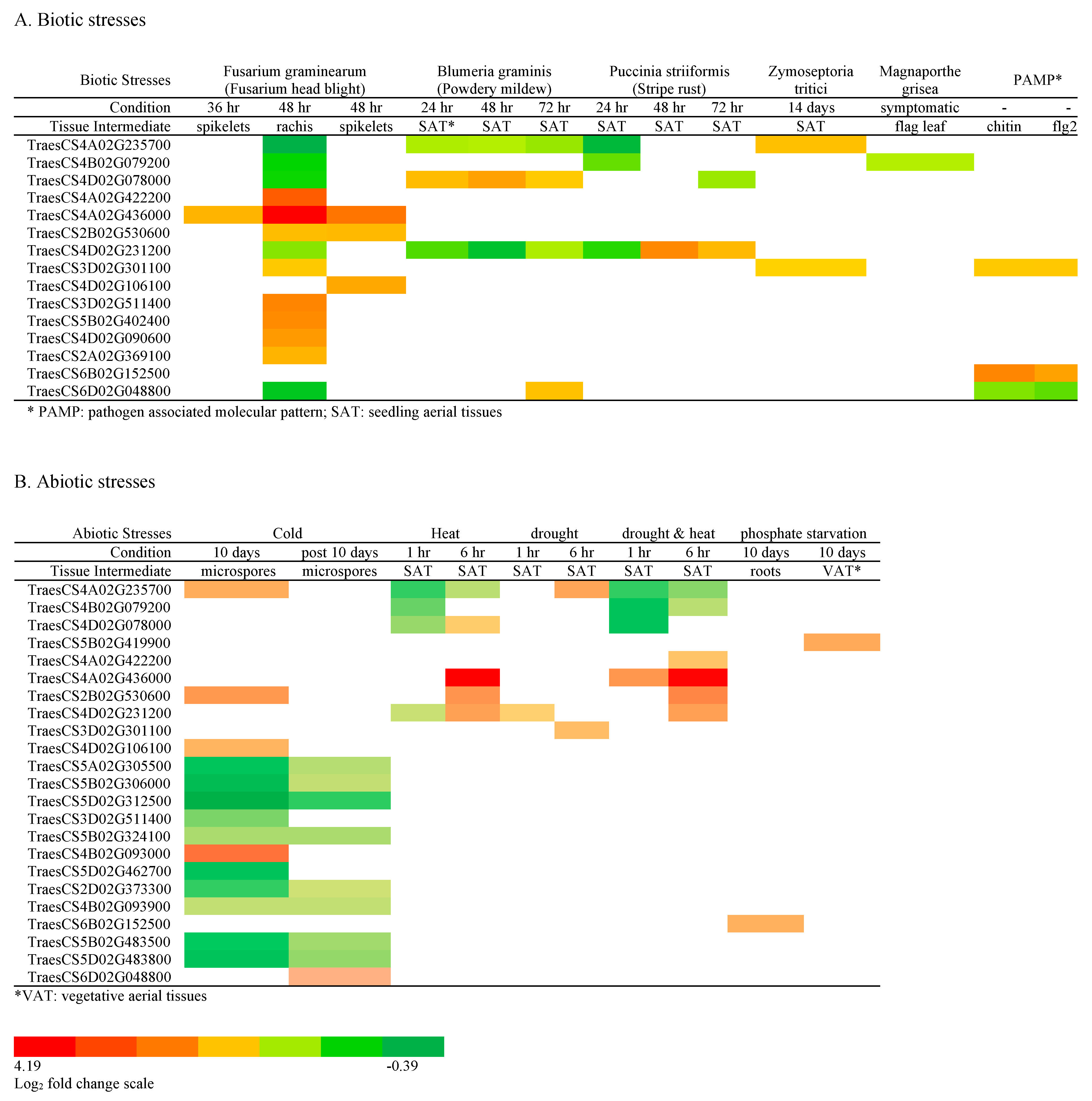
2.4.3. Serpin Genes Responsive to Abiotic or Biotic Stress
3. Discussion
4. Materials and Methods
4.1. Reference Serpin Lists
4.2. Serpin Gene Identification
4.3. Phylogenetic Analysis
4.4. Chromosomal Locations, Homeologous Genes, and Gene Duplication
4.5. Expression Analysis—Wheat Tissues
4.6. Expression Analysis—Abiotic and Biotic Stresses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benbow, H.R.; Jermiin, L.S.; Doohan, F.M. Serpins: Genome-wide characterisation and expression analysis of the serine protease inhibitor family in Triticum aestivum. G3-Genes Genomes Genet. 2019, 9, 2709–2722. [Google Scholar] [CrossRef] [PubMed]
- Atchley, W.R.; Lokot, T.; Wollenberg, K.; Dress, A.; Ragg, H. Phylogenetic analyses of amino acid variation in the serpin proteins. Mol. Biol. Evol. 2001, 18, 1502–1511. [Google Scholar] [CrossRef] [PubMed]
- Irving, J.A.; Steenbakkers, P.J.M.; Lesk, A.M.; Op den Camp, H.J.M.; Pike, R.N.; Whisstock, J.C. Serpins in prokaryotes. Mol. Biol. Evol. 2002, 19, 1881–1890. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.H.; Hejgaard, J.; Saunders, N.F.W.; Cavicchioli, R.; Curmi, P.M.G. Serpins in unicellular eukarya, archaea, and bacteria: Sequence analysis and evolution. J. Mol. Evol. 2004, 59, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Law, R.H.P.; Zhang, Q.; McGowan, S.; Buckle, A.M.; Silverman, G.A.; Wong, W.; Rosado, C.J.; Langendorf, C.G.; Pike, R.N.; Bird, P.I.; et al. An overview of the serpin superfamily. Genome Biol. 2006, 7, 216. [Google Scholar] [CrossRef]
- Huntington, J.A. Serpin structure, function and dysfunction. J. Thromb. Haemost. 2011, 9, 26–34. [Google Scholar] [CrossRef]
- Jiang, R.; Zhang, B.; Kurokawa, K.; So, Y.I.; Kim, E.H.; Hwang, H.O.; Lee, J.H.; Shiratsuchi, A.; Zhang, J.; Nakanishi, Y.; et al. 93-kDa twin-domain serine protease inhibitor (serpin) has a regulatory function on the beetle Toll proteolytic signaling cascade. J. Biol. Chem. 2011, 286, 35087–35095. [Google Scholar] [CrossRef]
- Gettins, P.G.W. Serpin structure, mechanism, and function. Chem. Rev. 2002, 102, 4751–4803. [Google Scholar] [CrossRef]
- Zhou, A.; Wei, Z.; Read, R.J.; Carrell, R.W. Structural mechanism for the carriage and release of thyroxine in the blood. Proc. Natl. Acad. Sci. USA 2006, 103, 13321–13326. [Google Scholar] [CrossRef]
- Zhou, A.; Wei, Z.; Stanley, P.L.D.; Read, R.J.; Stein, P.E.; Carrell, R.W. The S-to-R transition of corticosteroid-binding globulin and the mechanism of hormone release. J. Mol. Biol. 2008, 380, 244–251. [Google Scholar] [CrossRef]
- Huntington, J.A.; Read, R.J.; Carrell, R.W. Structure of a serpin-protease complex shows inhibition by deformation. Nature 2000, 407, 923–926. [Google Scholar] [CrossRef]
- Carrell, R.W.; Owen, M.C. Plakalbumin, alpha1-antitrypsin, antithrombin and the mechanism of inflammatory thrombosis. Nature 1985, 317, 730–732. [Google Scholar] [CrossRef]
- Hejgaard, J. Free and protein-bound β-amylases of barley grain. Characterization by two-dimensional immunoelectrophoresis. Physiol. Plant. 1976, 38, 293–299. [Google Scholar] [CrossRef]
- Roberts, T.H.; Hejgaard, J. Serpins in plants and green algae. Funct. Integr. Genom. 2008, 8, 1–27. [Google Scholar] [CrossRef]
- Rehman, S.; Jørgensen, B.; Aziz, E.; Batool, R.; Naseer, S.; Rasmussen, S.K. Genome wide identification and comparative analysis of the serpin gene family in brachypodium and barley. Plants 2020, 9, 1439. [Google Scholar] [CrossRef]
- Rosenkrands, I.; Hejgaard, J.; Rasmussen, S.K.; Bjørn, S.E. Serpins from wheat grain. FEBS Lett. 1994, 343, 75–80. [Google Scholar] [CrossRef]
- Rasmussen, S.K.; Dahl, S.W.; Norgard, A.; Hejgaard, J. A recombinant wheat serpin with inhibitory activity. Plant Mol. Biol. 1996, 30, 673–677. [Google Scholar] [CrossRef]
- Dahl, S.W.; Rasmussen, S.E.; Hejgaard, J. Heterologous expression of three plant serpins with distinct inhibitory specificities. J. Biol. Chem. 1996, 271, 25083–25088. [Google Scholar] [CrossRef]
- Dahl, S.W.; Rasmussen, S.K.; Petersen, L.C.; Hejgaard, J. Inhibition of coagulation factors by recombinant barley serpin BSZx. FEBS Lett. 1996, 394, 165–168. [Google Scholar] [CrossRef]
- Hejgaard, J. Inhibitory serpins from rye grain with glutamine as P1 and P2 residues in the reactive center. FEBS Lett. 2001, 488, 149–153. [Google Scholar] [CrossRef]
- Hejgaard, J. Inhibitory plant serpins with a sequence of three glutamine residues in the reactive center. Biol. Chem. 2005, 386, 1319–1323. [Google Scholar] [CrossRef] [PubMed]
- Ostergaard, H.; Rasmussen, S.K.; Roberts, T.H.; Hejgaard, J. Inhibitory serpins from wheat grain with reactive centers resembling glutamine-rich repeats of prolamin storage proteins-cloning and characterization of five major molecular forms. J. Biol. Chem. 2000, 275, 33272–33279. [Google Scholar] [CrossRef] [PubMed]
- Hejgaard, J.; Hauge, S. Serpins of oat (Avena sativa) grain with distinct reactive centres and inhibitory specificity. Physiol. Plant. 2002, 116, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Yoo, B.C.; Aoki, K.; Xiang, Y.; Campbell, L.R.; Hull, R.J.; Xoconostle-Cázares, B.; Monzer, J.; Lee, J.Y.; Ullman, D.E.; Lucas, W.J. Characterization of Cucurbita maxima phloem serpin-1 (CmPS-1). A developmentally regulated elastase inhibitor. J. Biol. Chem. 2000, 275, 35122–35128. [Google Scholar] [CrossRef] [PubMed]
- Lampl, N.; Budai-Hadrian, O.; Davydov, O.; Joss, T.V.; Harrop, S.J.; Curmi, P.M.G.; Roberts, T.H.; Fluhr, R. Arabidopsis AtSerpin1, crystal structure and in vivo interaction with its target protease RESPONSIVE TO DESICCATION-21 (RD21). J. Biol. Chem. 2010, 285, 13550–13560. [Google Scholar] [CrossRef] [PubMed]
- Lampl, N.; Alkan, N.; Davydov, O.; Fluhr, R. Set-point control of RD21 protease activity by AtSerpin1 controls cell death in Arabidopsis. Plant J. 2013, 74, 498–510. [Google Scholar] [CrossRef]
- Johnson, E.T.; Skory, C.D.; Naumann, T.A.; Jairajpuri, M.A.; Dowd, P.F. Three sorghum serpin recombinant proteins inhibit midgut trypsin activity and growth of corn earworm. Agri Gene 2016, 2, 11–16. [Google Scholar] [CrossRef]
- Hejgaard, J.; Boisen, S. High-lysine proteins in Hiproly barley breeding: Identification, nutritional significance and new screening methods. Hereditas 1980, 93, 311–320. [Google Scholar] [CrossRef]
- Hejgaard, J.; Roberts, T.H. Plant Serpins. In Molecular and Cellular Aspects of the Serpinopathies and Disorders in Serpin Activity; Silverman, G.A., Lomas, D.A., Eds.; World Scientific: Hackensack, NJ, USA, 2007; pp. 279–300. [Google Scholar]
- Cohen, M.; Fluhr, R. Noncanonical interactions between serpin and β-amylase in barley grain improve β-amylase activity in vitro. Plant Direct. 2018, 2, e00054. [Google Scholar] [CrossRef]
- Petersen, G.; Seberg, O.; Yde, M.; Berthelsen, K. Phylogenetic relationships of Triticum and Aegilops and evidence for the origin of the A, B, and D genomes of common wheat (Triticum aestivum). Mol. Phylogenet. Evol. 2006, 39, 70–82. [Google Scholar] [CrossRef]
- Lukaszewski, A.J.; Alberti, A.; Sharpe, A.; Kilian, A.; Stanca, A.M.; Keller, B.; Clavijo, B.J.; Friebe, B.; Gill, B.; Wulff, B.; et al. A chromosome-based draft sequence of the hexaploid bread wheat (Triticum aestivum) genome. Science 2014, 345, 1251788. [Google Scholar]
- Tutar, Y. Pseudogenes. Comp. Funct. Genom. 2012, 2012, 424526. [Google Scholar] [CrossRef]
- Ramírez-González, R.H.; Borrill, P.; Lang, D.; Harrington, S.A.; Brinton, J.; Venturini, L.; Davey, M.; Jacobs, J.; Van Ex, F.; Pasha, A.; et al. The transcriptional landscape of polyploid wheat. Science 2018, 361, eaar6089. [Google Scholar] [CrossRef]
- Appels, R.; Eversole, K.; Feuillet, C.; Keller, B.; Rogers, J.; Stein, N.; Pozniak, C.J.; Choulet, F.; Distelfeld, A.; Poland, J.; et al. Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science 2018, 361, eaar7191. [Google Scholar]
- Wilkinson, P.A.; Winfield, M.O.; Barker, G.L.A.; Allen, A.M.; Burridge, A.; Coghill, J.A.; Edwards, K.J. Cerealsdb 2.0: An integrated resource for plant breeders and scientists. BMC Bioinform. 2012, 13, 219. [Google Scholar] [CrossRef]
- EnsemblePlants. Available online: https://plants.ensembl.org/Triticum_aestivum/Info/Index (accessed on 1 February 2019).
- Vercammen, D.; Belenghi, B.; van de Cotte, B.; Beunens, T.; Gavigan, J.A.; De Rycke, R.; Brackenier, A.; Inzé, D.; Harris, J.L.; Van Breusegem, F. Serpin1 of Arabidopsis thaliana is a suicide inhibitor for metacaspase 9. J. Mol. Biol. 2006, 364, 625–636. [Google Scholar] [CrossRef]
- Fluhr, R.; Lampl, N.; Roberts, T.H. Serpin protease inhibitors in plant biology. Physiol. Plant. 2012, 145, 95–102. [Google Scholar] [CrossRef]
- expVIP. Available online: http://www.wheat-expression.com/ (accessed on 1 February 2019).
- Wu, M.J.; McKay, S.; Howes, N.; Chin, J.; Hegedus, E. Identification of novel serpin isoforms and serpin polymorphisms among Australian wheat cultivars. J. Cereal Sci. 2012, 55, 202–209. [Google Scholar] [CrossRef]
- Ma, J.; Stiller, J.; Berkman, P.J.; Wei, Y.; Rogers, J.; Feuillet, C.; Dolezel, J.; Mayer, K.F.; Eversole, K.; Zheng, Y.L.; et al. Sequence-based analysis of translocations and inversions in bread wheat (Triticum aestivum L.). PLoS ONE 2013, 8, e79329. [Google Scholar] [CrossRef]
- Expression Altas. Available online: https://www.ebi.ac.uk/gxa/home (accessed on 1 February 2021).
- Genevestigator. Available online: https://genevestigator.com/ (accessed on 1 February 2022).
- Cane, K.; Sharp, P.J.; Eagles, H.A.; Eastwood, R.F.; Hollamby, G.J.; Kuchel, H.; Lu, M.; Martin, P.J. The effects on grain quality traits of a grain serpin protein and the VPM1 segment in southern Australian wheat breeding. Aust. J. Agric. Res. 2008, 59, 883–890. [Google Scholar] [CrossRef]
- Roberts, T.H.; Marttila, S.; Rasmussen, S.K.; Hejgaard, J. Differential gene expression for suicide-substrate serine proteinase inhibitors (serpins) in vegetative and grain tissues of barley. J. Exp. Bot. 2003, 54, 2251–2263. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.-W.; Atwell, B.J.; Roberts, T.H. Serpin genes ATSRP2 and ATSRP3 are required for normal growth sensitivity to a DNA alkylating agent in Arabidopsis. BMC Plant Biol. 2009, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, L.; Singh, P.K.; Singh, S.; Nandi, A.K. Down-regulation of rice serpin gene OsSRP-LRS exaggerates stress-induced cell death. J. Plant Biol. 2015, 58, 327–332. [Google Scholar] [CrossRef]
- Francis, S.E.; Ersoy, R.A.; Ahn, J.W.; Atwell, B.J.; Roberts, T.H. Serpins in rice: Protein sequence analysis, phylogeny and gene expression during development. BMC Genom. 2012, 13, 449. [Google Scholar] [CrossRef]
- Van De Peer, Y.; Maere, S.; Meyer, A. The evolutionary significance of ancient genome duplications. Nat. Rev. Gen. 2009, 10, 725–732. [Google Scholar] [CrossRef]
- Wu, X.; Liu, H.; Li, X.; Tian, Y.; Mahecha, M.D. Responses of winter wheat yields to warming-mediated vernalization variations across temperate Europe. Front. Ecol. Evol. 2017, 5, 126. [Google Scholar] [CrossRef]
- Dusenge, M.E.; Duarte, A.G.; Way, D.A. Plant carbon metabolism and climate change: Elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytol. 2019, 221, 32–49. [Google Scholar] [CrossRef]
- UniprotKB. Available online: https://www.uniprot.org (accessed on 1 March 2021).
- Clustal Omega. Available online: https://www.ebi.ac.uk/Tools/msa/clustalo/ (accessed on 1 March 2021).
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. Mega X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. The rapid generation of mutation data matrices from protein sequences. Bioinformatics 1992, 8, 275–282. [Google Scholar] [CrossRef]
- iTOL. Available online: https://itol.embl.de (accessed on 1 September 2022).
- IWGSC RefSeq Annotation. Available online: https://urgi.versailles.inra.fr/jbrowseiwgsc/gmod_jbrowse/ (accessed on 1 February 2019).
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. Tbtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Borrill, P.; Ramirez-Gonzalez, R.; Uauy, C. Expvip: A customizable RNA-seq data analysis and visualization platform. Plant Physiol. 2016, 170, 2172–2186. [Google Scholar] [CrossRef]
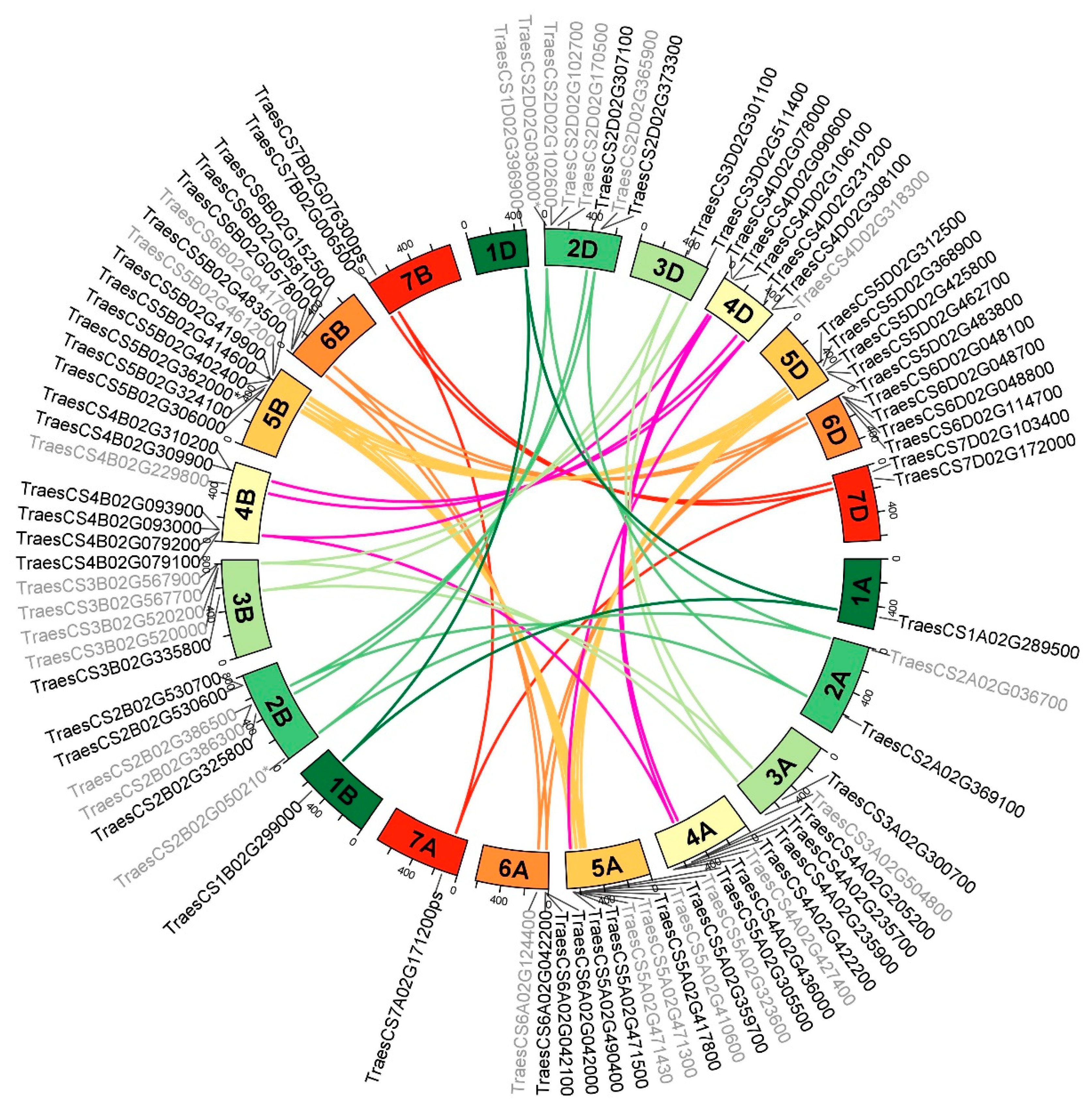

| Chromosome | Subgenome | |||
|---|---|---|---|---|
| A | B | D | Total | |
| 1 | 1 | 1 | 1 | 3 |
| 2 | 2 | 6 | 7 | 15 |
| 3 | 2 | 5 | 2 | 9 |
| 4 | 6 | 7 | 6 | 19 |
| 5 | 9 | 8 | 5 | 22 |
| 6 | 4 | 4 | 4 | 12 |
| 7 | 0 | 1 | 2 | 3 |
| Total | 24 | 32 | 27 | 83 |
| Clade | Wheat Serpin Gene | Biotic Stress * | Abiotic Stress |
|---|---|---|---|
| Clade I | TraesCS4A02G235700 | Zt | cold, drought |
| TraesCS4B02G079200 | Xt | ||
| TraesCS4D02G078000 | Bg | heat | |
| TraesCS5B02G419900 | P-starvation | ||
| TraesCS4A02G422200 | Fg, Xt | drought and heat | |
| TraesCS4A02G436000 | Fg, Xt | heat, drought, and heat | |
| TraesCS2B02G530600 | Fg, Xt | cold, heat, drought, and heat | |
| TraesCS4D02G231200 | Ps | heat, drought, drought, and heat | |
| TraesCS3D02G301100 | Fg, Zt, chitin, flg22 | drought | |
| TraesCS4D02G106100 | Fg, Xt | cold | |
| Clade II | TraesCS6D02G048800 | Bg | |
| TraesCS6B02G152500 | chitin, flg22 | P-starvation | |
| TraesCS2A02G369100 | Fg | ||
| Clade III | TraesCS3D02G511400 | Fg | |
| TraesCS5B02G402400 | Fg | ||
| TraesCS4D02G090600 | Fg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, C.; Huang, T.-C.; Roberts, T.H. Genes Encoding Structurally Conserved Serpins in the Wheat Genome: Identification and Expression Profiles during Plant Development and Abiotic and Biotic Stress. Int. J. Mol. Sci. 2023, 24, 2707. https://doi.org/10.3390/ijms24032707
Dong C, Huang T-C, Roberts TH. Genes Encoding Structurally Conserved Serpins in the Wheat Genome: Identification and Expression Profiles during Plant Development and Abiotic and Biotic Stress. International Journal of Molecular Sciences. 2023; 24(3):2707. https://doi.org/10.3390/ijms24032707
Chicago/Turabian StyleDong, Chongmei, Ting-Chun Huang, and Thomas H. Roberts. 2023. "Genes Encoding Structurally Conserved Serpins in the Wheat Genome: Identification and Expression Profiles during Plant Development and Abiotic and Biotic Stress" International Journal of Molecular Sciences 24, no. 3: 2707. https://doi.org/10.3390/ijms24032707
APA StyleDong, C., Huang, T.-C., & Roberts, T. H. (2023). Genes Encoding Structurally Conserved Serpins in the Wheat Genome: Identification and Expression Profiles during Plant Development and Abiotic and Biotic Stress. International Journal of Molecular Sciences, 24(3), 2707. https://doi.org/10.3390/ijms24032707







