Active Glycogen Synthase in the Liver Prevents High-Fat Diet-Induced Glucose Intolerance, Decreases Food Intake, and Lowers Body Weight
Abstract
1. Introduction
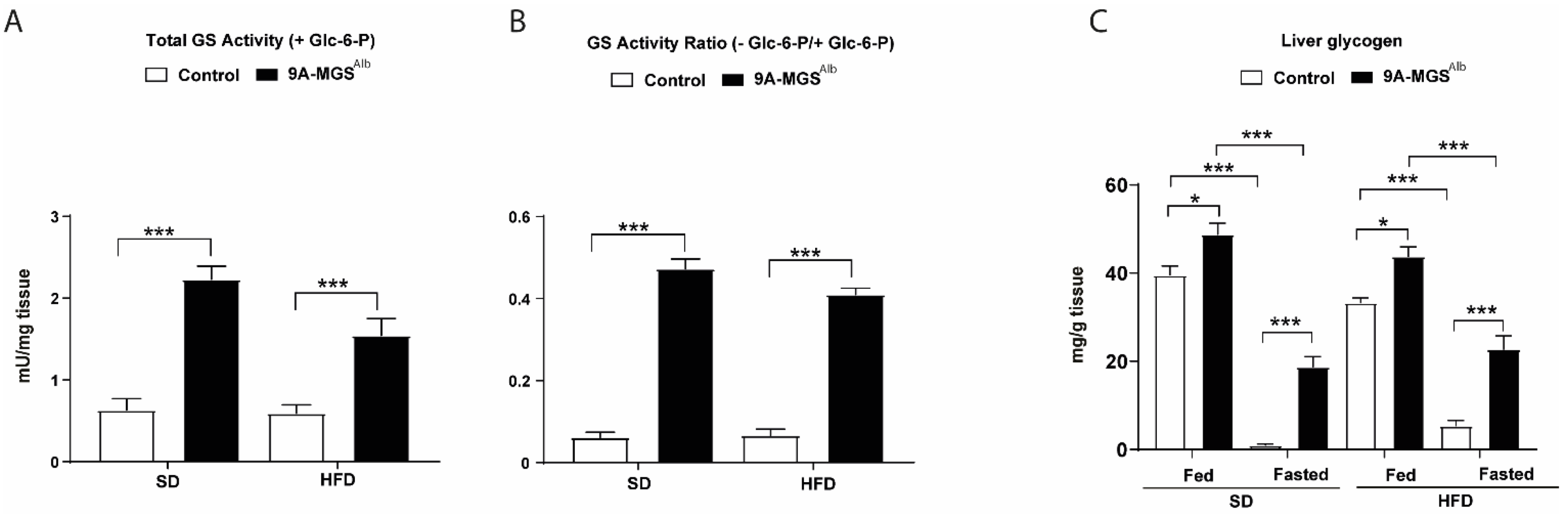
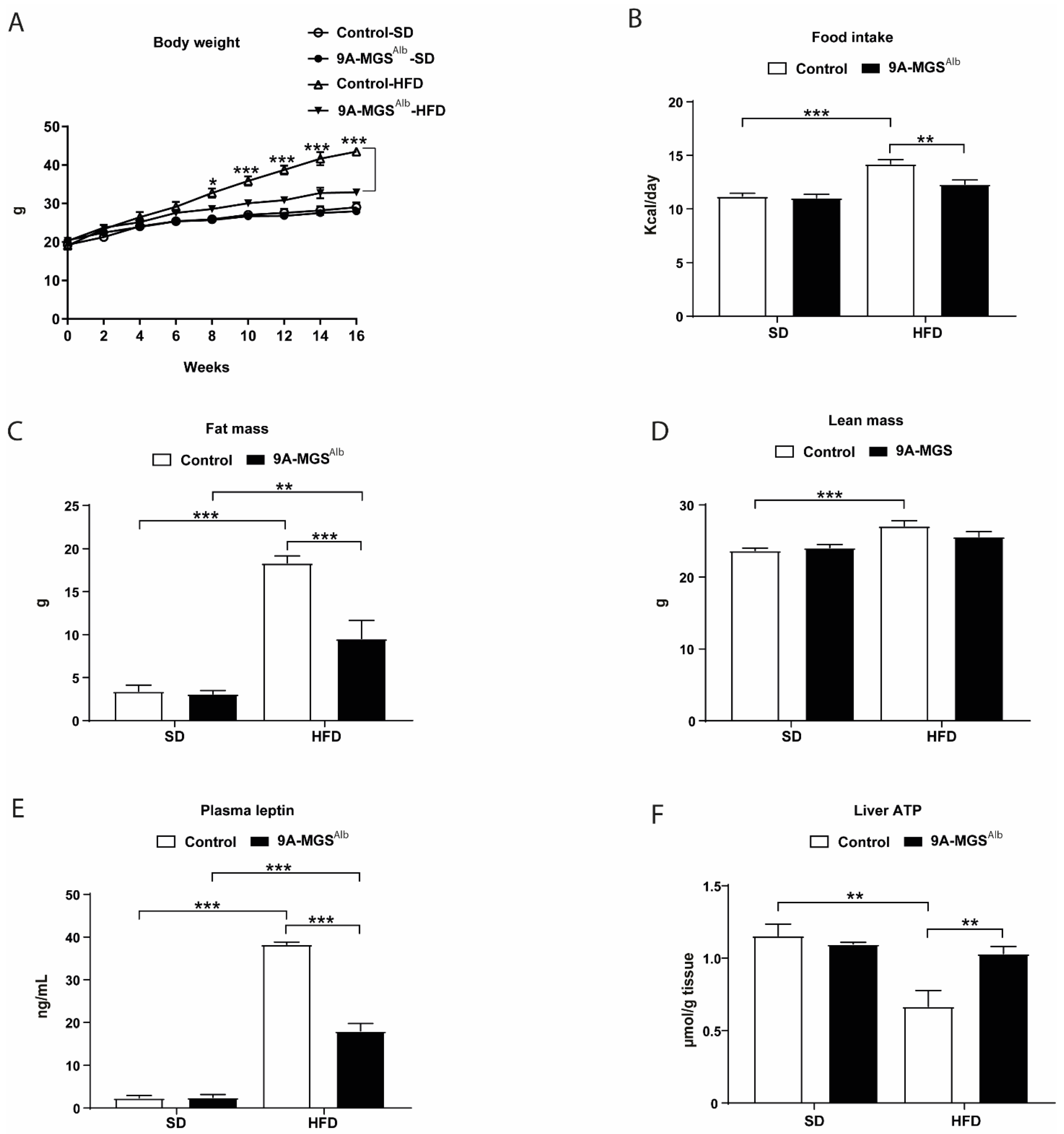
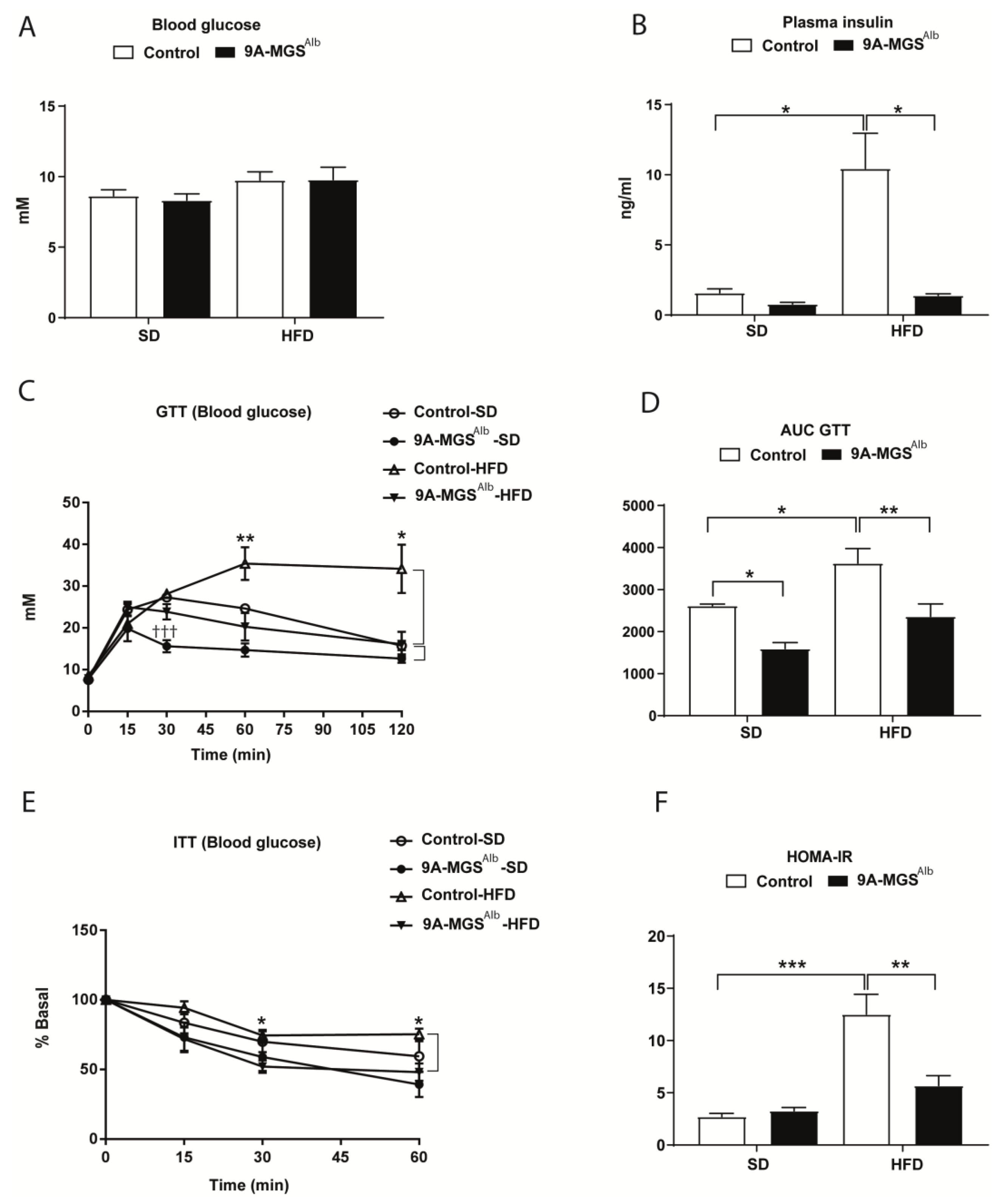
2. Results
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Body Mass Composition
4.3. Metabolic Activity
4.4. Measurement of Cumulative Food Intake
4.5. Blood Parameters
4.6. Tissue Preparation and Biochemical Analysis
4.7. Glucose Tolerance, Insulin Tolerance Test and HOMA-IR
4.8. RNA Preparation and Quantitative RT-PCR
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Roach, P.J.; Depaoli-Roach, A.A.; Hurley, T.D.; Tagliabracci, V.S. Glycogen and its metabolism: Some new developments and old themes. Biochem. J. 2012, 441, 763–787. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.H.; Brown, N.E.; Larner, J. Molecular characteristics of the totally dependent and independent forms of glycogen synthase of rabbit skeletal muscle. II. Some chemical characteristics of the enzyme protein and of its change on interconversion. Biochim. Biophys. Acta 1971, 242, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Agius, L. Role of glycogen phosphorylase in liver glycogen metabolism. Mol. Asp. Med. 2015, 46, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B.; Brady, M.J.; O’Doherty, R.M.; Saltiel, A.R. Organizing glucose disposal: Emerging roles of the glycogen targeting subunits of protein phosphatase-1. Diabetes 2000, 49, 1967–1977. [Google Scholar] [CrossRef] [PubMed]
- Printen, J.A.; Brady, M.J.; Saltiel, A.R. PTG, a protein phosphatase 1-binding protein with a role in glycogen metabolism. Science 1997, 275, 1475–1478. [Google Scholar] [CrossRef]
- Brady, M.J.; Printen, J.A.; Mastick, C.C.; Saltiel, A.R. Role of protein targeting to glycogen (PTG) in the regulation of protein phosphatase-1 activity. J. Biol. Chem. 1997, 272, 20198–20204. [Google Scholar] [CrossRef]
- Berman, H.K.; O’Doherty, R.M.; Anderson, P.; Newgard, C.B. Overexpression of protein targeting to glycogen (PTG) in rat hepatocytes causes profound activation of glycogen synthesis independent of normal hormone- and substrate-mediated regulatory mechanisms. J. Biol. Chem. 1998, 273, 26421–26425. [Google Scholar] [CrossRef]
- Roach, P.J.; Cheng, C.; Huang, D.; Lin, A.; Mu, J.; Skurat, A.V.; Wilson, W.; Zhai, L. Novel aspects of the regulation of glycogen storage. J. Basic Clin. Physiol. Pharmacol. 1998, 9, 139–151. [Google Scholar] [CrossRef]
- Lopez-Soldado, I.; Zafra, D.; Duran, J.; Adrover, A.; Calbo, J.; Guinovart, J.J. Liver glycogen reduces food intake and attenuates obesity in a high-fat diet-fed mouse model. Diabetes 2015, 64, 796–807. [Google Scholar] [CrossRef]
- Lopez-Soldado, I.; Bertini, A.; Adrover, A.; Duran, J.; Guinovart, J.J. Maintenance of liver glycogen during long-term fasting preserves energy state in mice. FEBS Lett. 2020, 594, 1698–1710. [Google Scholar] [CrossRef]
- Lopez-Soldado, I.; Guinovart, J.J.; Duran, J. Hepatic overexpression of protein targeting to glycogen attenuates obesity and improves hyperglycemia in db/db mice. Front. Endocrinol. 2022, 13, 969924. [Google Scholar] [CrossRef]
- Duran, J.; Tevy, M.F.; Garcia-Rocha, M.; Calbo, J.; Milan, M.; Guinovart, J.J. Deleterious effects of neuronal accumulation of glycogen in flies and mice. EMBO Mol. Med. 2012, 4, 719–729. [Google Scholar] [CrossRef]
- Testoni, G.; Duran, J.; Garcia-Rocha, M.; Vilaplana, F.; Serrano, A.L.; Sebastian, D.; Lopez-Soldado, I.; Sullivan, M.A.; Slebe, F.; Vilaseca, M.; et al. Lack of Glycogenin Causes Glycogen Accumulation and Muscle Function Impairment. Cell Metab. 2017, 26, 256–266.e254. [Google Scholar] [CrossRef]
- Duran, J.; Hervera, A.; Markussen, K.H.; Varea, O.; Lopez-Soldado, I.; Sun, R.C.; Del Rio, J.A.; Gentry, M.S.; Guinovart, J.J. Astrocytic glycogen accumulation drives the pathophysiology of neurodegeneration in Lafora disease. Brain A J. Neurol. 2021, 144, 2349–2360. [Google Scholar] [CrossRef]
- Duran, J.; Gruart, A.; Garcia-Rocha, M.; Delgado-Garcia, J.M.; Guinovart, J.J. Glycogen accumulation underlies neurodegeneration and autophagy impairment in Lafora disease. Hum. Mol. Genet. 2014, 23, 3147–3156. [Google Scholar] [CrossRef]
- Postic, C.; Shiota, M.; Niswender, K.D.; Jetton, T.L.; Chen, Y.; Moates, J.M.; Shelton, K.D.; Lindner, J.; Cherrington, A.D.; Magnuson, M.A. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J. Biol. Chem. 1999, 274, 305–315. [Google Scholar] [CrossRef]
- Yen, C.L.; Stone, S.J.; Cases, S.; Zhou, P.; Farese, R.V., Jr. Identification of a gene encoding MGAT1, a monoacylglycerol acyltransferase. Proc. Natl. Acad. Sci. USA 2002, 99, 8512–8517. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, J.W. Monoacylglycerol O-acyltransferase 1 (MGAT1) localizes to the ER and lipid droplets promoting triacylglycerol synthesis. BMB Rep. 2017, 50, 367–372. [Google Scholar] [CrossRef]
- Schmid, A.I.; Szendroedi, J.; Chmelik, M.; Krssak, M.; Moser, E.; Roden, M. Liver ATP synthesis is lower and relates to insulin sensitivity in patients with type 2 diabetes. Diabetes Care 2011, 34, 448–453. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Z.; Li, S.; Zhang, Y.; Jia, S.; Li, J.; Chi, Y.; Miao, Y.; Guan, Y.; Yang, J. Hepatic overexpression of ATP synthase beta subunit activates PI3K/Akt pathway to ameliorate hyperglycemia of diabetic mice. Diabetes 2014, 63, 947–959. [Google Scholar] [CrossRef]
- Lopez-Soldado, I.; Fuentes-Romero, R.; Duran, J.; Guinovart, J.J. Effects of hepatic glycogen on food intake and glucose homeostasis are mediated by the vagus nerve in mice. Diabetologia 2017, 60, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Mehran, A.E.; Templeman, N.M.; Brigidi, G.S.; Lim, G.E.; Chu, K.Y.; Hu, X.; Botezelli, J.D.; Asadi, A.; Hoffman, B.G.; Kieffer, T.J.; et al. Hyperinsulinemia drives diet-induced obesity independently of brain insulin production. Cell Metab. 2012, 16, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Soldado, I.; Guinovart, J.J.; Duran, J. Increasing hepatic glycogen moderates the diabetic phenotype in insulin-deficient Akita mice. J. Biol. Chem. 2021, 296, 100498. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.M.; Kou, K.; Chen, Z.; Pietka, T.A.; Kumar, M.; Korenblat, K.M.; Lee, K.; Ahn, K.; Fabbrini, E.; Klein, S.; et al. Evidence for regulated monoacylglycerol acyltransferase expression and activity in human liver. J. Lipid Res. 2012, 53, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Ko, E.H.; Kim, J.E.; Kim, E.; Lee, H.; Choi, H.; Yu, J.H.; Kim, H.J.; Seong, J.K.; Kim, K.S.; et al. Nuclear receptor PPARgamma-regulated monoacylglycerol O-acyltransferase 1 (MGAT1) expression is responsible for the lipid accumulation in diet-induced hepatic steatosis. Proc. Natl. Acad. Sci. USA 2012, 109, 13656–13661. [Google Scholar] [CrossRef]
- Nassir, F.; Rector, R.S.; Hammoud, G.M.; Ibdah, J.A. Pathogenesis and Prevention of Hepatic Steatosis. Gastroenterol. Hepatol. 2015, 11, 167–175. [Google Scholar]
- Garcia-Rocha, M.; Roca, A.; De La Iglesia, N.; Baba, O.; Fernandez-Novell, J.M.; Ferrer, J.C.; Guinovart, J.J. Intracellular distribution of glycogen synthase and glycogen in primary cultured rat hepatocytes. Biochem. J. 2001, 357, 17–24. [Google Scholar] [CrossRef]
- Chan, T.M.; Exton, J.H. A rapid method for the determination of glycogen content and radioactivity in small quantities of tissue or isolated hepatocytes. Anal. Biochem. 1976, 71, 96–105. [Google Scholar] [CrossRef]
- Ros, S.; Garcia-Rocha, M.; Calbo, J.; Guinovart, J.J. Restoration of hepatic glycogen deposition reduces hyperglycaemia, hyperphagia and gluconeogenic enzymes in a streptozotocin-induced model of diabetes in rats. Diabetologia 2011, 54, 2639–2648. [Google Scholar] [CrossRef]
- Salmon, D.M.; Flatt, J.P. Effect of dietary fat content on the incidence of obesity among ad libitum fed mice. Int. J. Obes. 1985, 9, 443–449. [Google Scholar]
- Ros, S.; Zafra, D.; Valles-Ortega, J.; Garcia-Rocha, M.; Forrow, S.; Dominguez, J.; Calbo, J.; Guinovart, J.J. Hepatic overexpression of a constitutively active form of liver glycogen synthase improves glucose homeostasis. J. Biol. Chem. 2010, 285, 37170–37177. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Soldado, I.; Guinovart, J.J.; Duran, J. Active Glycogen Synthase in the Liver Prevents High-Fat Diet-Induced Glucose Intolerance, Decreases Food Intake, and Lowers Body Weight. Int. J. Mol. Sci. 2023, 24, 2574. https://doi.org/10.3390/ijms24032574
López-Soldado I, Guinovart JJ, Duran J. Active Glycogen Synthase in the Liver Prevents High-Fat Diet-Induced Glucose Intolerance, Decreases Food Intake, and Lowers Body Weight. International Journal of Molecular Sciences. 2023; 24(3):2574. https://doi.org/10.3390/ijms24032574
Chicago/Turabian StyleLópez-Soldado, Iliana, Joan J. Guinovart, and Jordi Duran. 2023. "Active Glycogen Synthase in the Liver Prevents High-Fat Diet-Induced Glucose Intolerance, Decreases Food Intake, and Lowers Body Weight" International Journal of Molecular Sciences 24, no. 3: 2574. https://doi.org/10.3390/ijms24032574
APA StyleLópez-Soldado, I., Guinovart, J. J., & Duran, J. (2023). Active Glycogen Synthase in the Liver Prevents High-Fat Diet-Induced Glucose Intolerance, Decreases Food Intake, and Lowers Body Weight. International Journal of Molecular Sciences, 24(3), 2574. https://doi.org/10.3390/ijms24032574





