Aging Effects on Optic Nerve Neurodegeneration
Abstract
1. Introduction
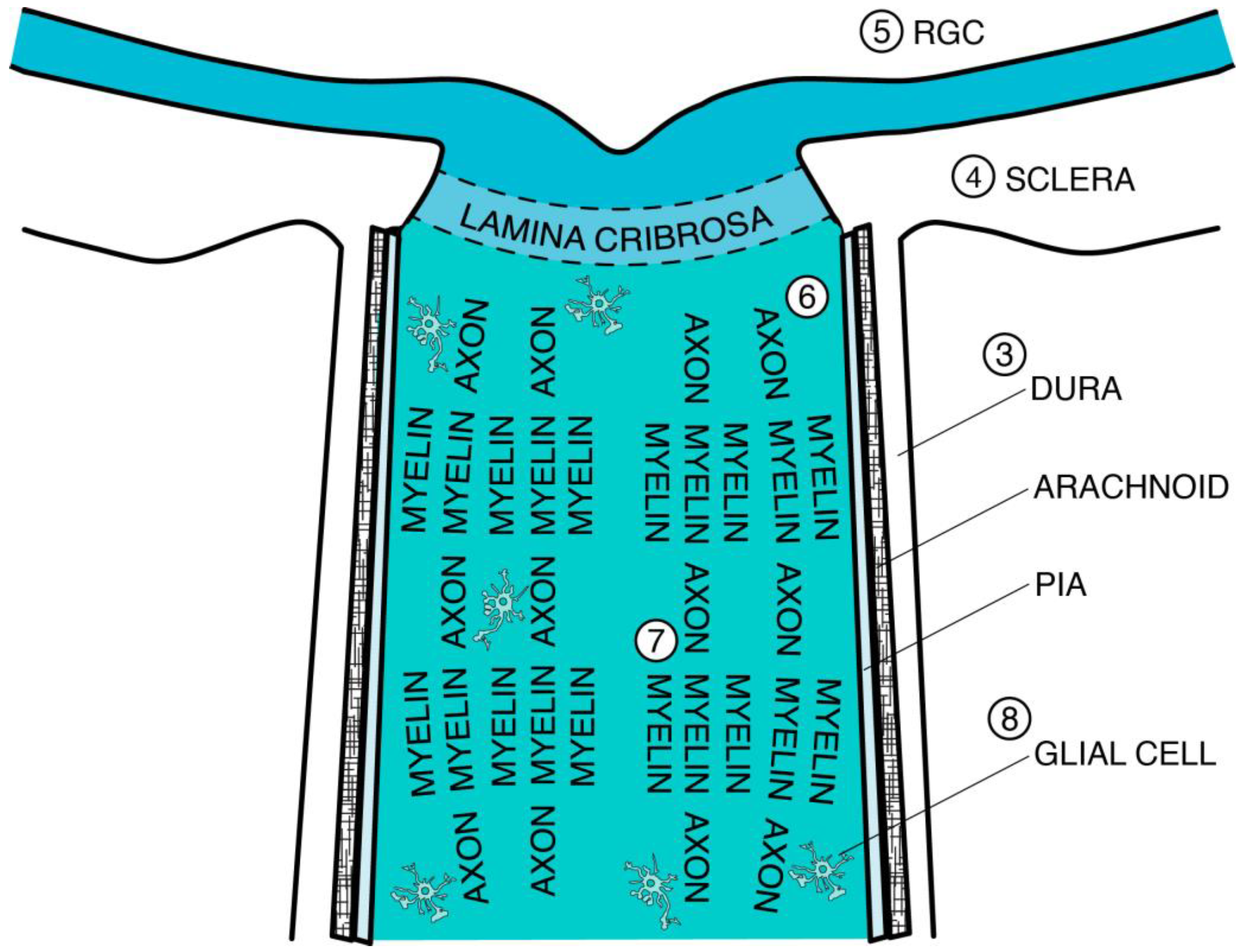
2. Optic Nerve Development
2.1. Embryology
2.2. Newborn to Elderly
3. Connective Tissue: Thickening, Stiffening, and Weakening
3.1. Elastic Fibers
3.2. Collagen Fibers
3.2.1. Elevated Lysyl Oxidase (LOX) Expression
3.2.2. Advanced Glycation End Products (AGEs)
3.2.3. Receptor for AGE (RAGE)
3.2.4. Other Molecular Mechanisms
3.2.5. Mechanical Considerations
3.3. Laminin
Matrix Metalloproteases (MMPs)
3.4. Dura Mater
3.5. Arachnoid and Subarachnoid Space
3.6. Pia Mater and Septa
3.7. Lamina Cribrosa (LC)
3.7.1. Collagen Deposition
3.7.2. Increased Cribriform Plate Rigidity
3.7.3. Unique Vascularity
3.7.4. Thicker Astrocyte Basement Membranes
4. Decreased Optic Canal Expansion
4.1. Scleral Stiffening
Central Serous Chorioretinopathy (CSCR)
4.2. Optic Nerve Head (ONH) Impacts
5. Retinal Ganglion Cell (RGC): Loss and Injury
5.1. Embryonic
5.2. Age-Dependent Loss of RGC and Axons
5.3. Aging and Injury
5.4. Mitochondrial Dysfunction in RGC
Reactive Oxygen Species (ROS)
5.5. Autophagy
5.6. Melanopsin-Expressing RGC (ipRGC)
5.7. Diabetic Retinopathy (DR)
5.8. Glaucoma
5.9. Lack of De Novo RGC Regeneration
6. Axon: Age-Dependent Genetic Regulation of Regeneration, Diminished Density, Swelling, and Metabolic Dysfunction
6.1. Embryonic
6.2. Limitations of Axonal Regeneration
6.3. Loss of Axonal Density
6.3.1. Childhood and Adulthood
6.3.2. After Age 60
6.4. Axonal Swelling
6.4.1. Below Age 65
6.4.2. After Age 70
6.5. Metabolic Dysfunction
NAD+ and Senescence
7. Myelin: Loss of Thickness and Age-Dependent Failure of Remyelination
7.1. Newborn
7.2. Childhood and Adult
7.3. After Age 70
8. Glial Cell: Reactivity, Remodeling, Hypertrophy, Inclusions, Migration, and Polarization
8.1. Reactive Astrocytosis
8.2. Remodeling
8.3. Hypertrophy
8.4. Inclusions
8.4.1. Corpora Amylacea (CA)
8.4.2. Myelin Debris and Lipids
8.5. Migration
8.6. Microglial Polarization
9. Lipofuscin (LF)
10. Other Lesions
10.1. Vascular Degeneration
10.2. Cavernous Degeneration
11. Conclusions and Next Steps
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akinrodoye, M.A.; Lui, F. Neuroanatomy, Somatic Nervous System. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Vrabec, J.P.; Levin, L.A. The Neurobiology of Cell Death in Glaucoma. Eye 2007, 21, S11–S14. [Google Scholar] [CrossRef]
- Inman, D.M.; Harun-Or-Rashid, M. Metabolic Vulnerability in the Neurodegenerative Disease Glaucoma. Front. Neurosci. 2017, 11, 146. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, S.; Pan, Y.; Jin, M.; Li, J.; Luo, Y.; Sun, X.; Li, G. Diabetic Retinopathy: Involved Cells, Biomarkers, and Treatments. Front. Pharmacol. 2022, 13, 953691. [Google Scholar] [CrossRef]
- Takahashi, H.; Chihara, E. Impact of Diabetic Retinopathy on Quantitative Retinal Nerve Fiber Layer Measurement and Glaucoma Screening. Investig. Opthalmology Vis. Sci. 2008, 49, 687–692. [Google Scholar] [CrossRef]
- Catalani, E.; Cervia, D. Diabetic Retinopathy: A Matter of Retinal Ganglion Cell Homeostasis. Neural Regen. Res. 2020, 15, 1253. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, A.; Zou, M.; Zhang, Y.; Jin, L.; Li, Y.; Zheng, D.; Jin, G.; Congdon, N. Time Trends, Associations and Prevalence of Blindness and Vision Loss Due to Glaucoma: An Analysis of Observational Data from the Global Burden of Disease Study 2017. BMJ Open 2022, 12, e053805. [Google Scholar] [CrossRef]
- Purola, P.K.M.; Ojamo, M.U.I.; Gissler, M.; Uusitalo, H.M.T. Changes in Visual Impairment Due to Diabetic Retinopathy During 1980–2019 Based on Nationwide Register Data. Diabetes Care 2022, 45, 2020–2027. [Google Scholar] [CrossRef]
- Allison, K.; Patel, D.; Alabi, O. Epidemiology of Glaucoma: The Past, Present, and Predictions for the Future. Cureus 2020, 12, 11686. [Google Scholar] [CrossRef]
- Teo, Z.L.; Tham, Y.-C.; Yu, M.; Chee, M.L.; Rim, T.H.; Cheung, N.; Bikbov, M.M.; Wang, Y.X.; Tang, Y.; Lu, Y.; et al. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045. Ophthalmology 2021, 128, 1580–1591. [Google Scholar] [CrossRef]
- World Population Prospects 2022; Methodology of the United Nations Population Estimates and Projections; Population Division, Department of Economic and Social Affairs, United Nations: New York, NY, USA, 2022.
- Dolman, C.L.; McCormick, A.Q.; Drance, S.M. Aging of the Optic Nerve. Arch. Ophthalmol. 1980, 98, 2053–2058. [Google Scholar] [CrossRef]
- Erler, J.T.; Weaver, V.M. Three-Dimensional Context Regulation of Metastasis. Clin. Exp. Metastasis 2009, 26, 35–49. [Google Scholar] [CrossRef]
- Hernandez, M.R. Ultrastructural Immunocytochemical Analysis of Elastin in the Human Lamina Cribrosa. Changes in Elastic Fibers in Primary Open-Angle Glaucoma. Investig. Ophthalmol. Vis. Sci. 1992, 33, 2891–2903. [Google Scholar]
- Kagan, H.M. Lysyl Oxidase: Mechanism, Regulation and Relationship to Liver Fibrosis. Pathol. Res. Pract. 1994, 190, 910–919. [Google Scholar] [CrossRef]
- Sherratt, M.J. Tissue Elasticity and the Ageing Elastic Fibre. AGE 2009, 31, 305–325. [Google Scholar] [CrossRef]
- Shin, A.; Park, J.; Le, A.; Poukens, V.; Demer, J.L. Bilaminar Mechanics of the Human Optic Nerve Sheath. Curr. Eye Res. 2020, 45, 854–863. [Google Scholar] [CrossRef]
- Morrison, J.C. Optic Nerve Head Extracellular Matrix in Primary Optic Atrophy and Experimental Glaucoma. Arch. Ophthalmol. 1990, 108, 1020. [Google Scholar] [CrossRef]
- Morrison, J.C. Structural Proteins of the Neonatal and Adult Lamina Cribrosa. Arch. Ophthalmol. 1989, 107, 1220–1224. [Google Scholar] [CrossRef]
- Roberts, C.J.; Dupps, W.J.; Downs, J.C. Biomechanics of the Eye; Kugler Publications: Amsterdam, The Netherlands, 2018; ISBN 978-90-6299-250-8. [Google Scholar]
- Humphrey, J.D. Mechanisms of Vascular Remodeling in Hypertension. Am. J. Hypertens. 2021, 34, 432–441. [Google Scholar] [CrossRef]
- Sherratt, M.J. Age-Related Tissue Stiffening: Cause and Effect. Adv. Wound Care 2013, 2, 11–17. [Google Scholar] [CrossRef]
- Liu, B.; McNally, S.; Kilpatrick, J.I.; Jarvis, S.P.; O’Brien, C.J. Aging and Ocular Tissue Stiffness in Glaucoma. Surv. Ophthalmol. 2018, 63, 56–74. [Google Scholar] [CrossRef]
- Pehrsson, M.; Mortensen, J.H.; Manon-Jensen, T.; Bay-Jensen, A.-C.; Karsdal, M.A.; Davies, M.J. Enzymatic Cross-Linking of Collagens in Organ Fibrosis—Resolution and Assessment. Expert Rev. Mol. Diagn. 2021, 21, 1049–1064. [Google Scholar] [CrossRef] [PubMed]
- Tezel, G.; Luo, C.; Yang, X. Accelerated Aging in Glaucoma: Immunohistochemical Assessment of Advanced Glycation End Products in the Human Retina and Optic Nerve Head. Investig. Opthalmology Vis. Sci. 2007, 48, 1201–1211. [Google Scholar] [CrossRef] [PubMed]
- Bailey, A.J.; Paul, R.G.; Knott, L. Mechanisms of Maturation and Ageing of Collagen. Mech. Ageing Dev. 1998, 106, 1–56. [Google Scholar] [CrossRef] [PubMed]
- Csiszar, K. Lysyl Oxidases: A Novel Multifunctional Amine Oxidase Family. Prog. Nucleic Acid Res. Mol. Biol. 2001, 70, 1–32. [Google Scholar] [CrossRef]
- Wang, X.; Rumpel, H.; Lim, W.E.H.; Baskaran, M.; Perera, S.A.; Nongpiur, M.E.; Aung, T.; Milea, D.; Girard, M.J.A. Finite Element Analysis Predicts Large Optic Nerve Head Strains During Horizontal Eye Movements. Investig. Opthalmol. Vis. Sci. 2016, 57, 2452–2462. [Google Scholar] [CrossRef]
- Zwirner, J.; Scholze, M.; Waddell, J.N.; Ondruschka, B.; Hammer, N. Mechanical Properties of Human Dura Mater in Tension—An Analysis at an Age Range of 2 to 94 Years. Sci. Rep. 2019, 9, 16655. [Google Scholar] [CrossRef]
- Fleischman, D.; Berdahl, J.P.; Zaydlarova, J.; Stinnett, S.; Fautsch, M.P.; Allingham, R.R. Cerebrospinal Fluid Pressure Decreases with Older Age. PLoS ONE 2012, 7, e52664. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, L. Biomechanics of Injury and Prevention; Springer: Singapore, 2022; ISBN 9789811642685. [Google Scholar]
- Fazio, M.A.; Clark, M.E.; Bruno, L.; Girkin, C.A. In Vivo Optic Nerve Head Mechanical Response to Intraocular and Cerebrospinal Fluid Pressure: Imaging Protocol and Quantification Method. Sci. Rep. 2018, 8, 12639. [Google Scholar] [CrossRef]
- Killer, H.; Pircher, A. Normal Tension Glaucoma: Review of Current Understanding and Mechanisms of the Pathogenesis. Eye 2018, 32, 924–930. [Google Scholar] [CrossRef]
- Hopkins, A.A.; Murphy, R.; Irnaten, M.; Wallace, D.M.; Quill, B.; O’Brien, C. The Role of Lamina Cribrosa Tissue Stiffness and Fibrosis as Fundamental Biomechanical Drivers of Pathological Glaucoma Cupping. Am. J. Physiol. Cell Physiol. 2020, 319, C611–C623. [Google Scholar] [CrossRef]
- Albon, J. Age Related Compliance of the Lamina Cribrosa in Human Eyes. Br. J. Ophthalmol. 2000, 84, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Downs, J.C.; Girkin, C.A. Lamina Cribrosa in Glaucoma. Curr. Opin. Ophthalmol. 2017, 28, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Stowell, C.; Burgoyne, C.F.; Tamm, E.R.; Ethier, C.R.; Dowling, J.E.; Downs, C.; Ellisman, M.H.; Fisher, S.; Fortune, B.; Fruttiger, M.; et al. Biomechanical Aspects of Axonal Damage in Glaucoma: A Brief Review. Exp. Eye Res. 2017, 157, 13–19. [Google Scholar] [CrossRef]
- Gogola, A.; Jan, N.-J.; Brazile, B.; Lam, P.; Lathrop, K.L.; Chan, K.C.; Sigal, I.A. Spatial Patterns and Age-Related Changes of the Collagen Crimp in the Human Cornea and Sclera. Investig. Ophthalmol. Vis. Sci. 2018, 59, 2987. [Google Scholar] [CrossRef]
- Boote, C.; Sigal, I.A.; Grytz, R.; Hua, Y.; Nguyen, T.D.; Girard, M.J.A. Scleral Structure and Biomechanics. Prog. Retin. Eye Res. 2020, 74, 100773. [Google Scholar] [CrossRef] [PubMed]
- Girard, M.J.A.; Suh, J.-K.F.; Bottlang, M.; Burgoyne, C.F.; Downs, J.C. Scleral Biomechanics in the Aging Monkey Eye. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5226–5237. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.; Cone, F.E.; Nguyen, T.D.; Coudrillier, B.; Pease, M.E.; Steinhart, M.R.; Oglesby, E.N.; Jefferys, J.L.; Quigley, H.A. Studies of Scleral Biomechanical Behavior Related to Susceptibility for Retinal Ganglion Cell Loss in Experimental Mouse Glaucoma. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1767–1780. [Google Scholar] [CrossRef]
- Coudrillier, B.; Tian, J.; Alexander, S.; Myers, K.M.; Quigley, H.A.; Nguyen, T.D. Biomechanics of the Human Posterior Sclera: Age- and Glaucoma-Related Changes Measured Using Inflation Testing. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1714–1728. [Google Scholar] [CrossRef]
- Vurgese, S.; Panda-Jonas, S.; Jonas, J.B. Scleral Thickness in Human Eyes. PLoS ONE 2012, 7, e29692. [Google Scholar] [CrossRef]
- Fernández-Vigo, J.I.; Shi, H.; Burgos-Blasco, B.; Fernández-Aragón, S.; de-Pablo-Gómez-de-Liaño, L.; Kudsieh, B.; Macarro-Merino, A.; Ángel Fernández-Vigo, J. Anterior Scleral Thickness Dimensions by Swept-Source Optical Coherence Tomography. Clin. Exp. Optom. 2022, 105, 13–19. [Google Scholar] [CrossRef]
- Spaide, R.F.; Gemmy Cheung, C.M.; Matsumoto, H.; Kishi, S.; Boon, C.J.F.; van Dijk, E.H.C.; Mauget-Faysse, M.; Behar-Cohen, F.; Hartnett, M.E.; Sivaprasad, S.; et al. Venous Overload Choroidopathy: A Hypothetical Framework for Central Serous Chorioretinopathy and Allied Disorders. Prog. Retin. Eye Res. 2022, 86, 100973. [Google Scholar] [CrossRef] [PubMed]
- Buckhurst, H.D.; Gilmartin, B.; Cubbidge, R.P.; Logan, N.S. Measurement of Scleral Thickness in Humans Using Anterior Segment Optical Coherent Tomography. PLoS ONE 2015, 10, e0132902. [Google Scholar] [CrossRef] [PubMed]
- Read, S.A.; Alonso-Caneiro, D.; Vincent, S.J.; Bremner, A.; Fothergill, A.; Ismail, B.; McGraw, R.; Quirk, C.J.; Wrigley, E. Anterior Eye Tissue Morphology: Scleral and Conjunctival Thickness in Children and Young Adults. Sci. Rep. 2016, 6, 33796. [Google Scholar] [CrossRef] [PubMed]
- Schlatter, B.; Beck, M.; Frueh, B.E.; Tappeiner, C.; Zinkernagel, M. Evaluation of Scleral and Corneal Thickness in Keratoconus Patients. J. Cataract Refract. Surg. 2015, 41, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; McPheeters, S.; Johnson, G.; Utzinger, U.; Vande Geest, J.P. Microstructural Differences in the Human Posterior Sclera as a Function of Age and Race. Investig. Opthalmol. Vis. Sci. 2011, 52, 821. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.G.; Young, R.D. Scleral Structure, Organisation and Disease. A Review. Exp. Eye Res. 2004, 78, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Rada, J.A.; Achen, V.R.; Penugonda, S.; Schmidt, R.W.; Mount, B.A. Proteoglycan Composition in the Human Sclera during Growth and Aging. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1639–1648. [Google Scholar]
- Malik, N.S.; Moss, S.J.; Ahmed, N.; Furth, A.J.; Wall, R.S.; Meek, K.M. Ageing of the Human Corneal Stroma: Structural and Biochemical Changes. Biochim. Biophys. Acta 1992, 1138, 222–228. [Google Scholar] [CrossRef]
- Schultz, D.S.; Lotz, J.C.; Lee, S.M.; Trinidad, M.L.; Stewart, J.M. Structural Factors That Mediate Scleral Stiffness. Investig. Opthalmology Vis. Sci. 2008, 49, 4232. [Google Scholar] [CrossRef]
- Daxer, A.; Misof, K.; Grabner, B.; Ettl, A.; Fratzl, P. Collagen Fibrils in the Human Corneal Stroma: Structure and Aging. Investig. Ophthalmol. Vis. Sci. 1998, 39, 644–648. [Google Scholar]
- Coudrillier, B.; Pijanka, J.; Jefferys, J.; Sorensen, T.; Quigley, H.A.; Boote, C.; Nguyen, T.D. Collagen Structure and Mechanical Properties of the Human Sclera: Analysis for the Effects of Age. J. Biomech. Eng. 2015, 137, 041006. [Google Scholar] [CrossRef] [PubMed]
- Danford, F.L.; Yan, D.; Dreier, R.A.; Cahir, T.M.; Girkin, C.A.; Vande Geest, J.P. Differences in the Region- and Depth-Dependent Microstructural Organization in Normal Versus Glaucomatous Human Posterior Sclerae. Investig. Opthalmol. Vis. Sci. 2013, 54, 7922–7932. [Google Scholar] [CrossRef] [PubMed]
- Jan, N.-J.; Sigal, I.A. Collagen Fiber Recruitment: A Microstructural Basis for the Nonlinear Response of the Posterior Pole of the Eye to Increases in Intraocular Pressure. Acta Biomater. 2018, 72, 295–305. [Google Scholar] [CrossRef]
- Murienne, B.J.; Jefferys, J.L.; Quigley, H.A.; Nguyen, T.D. The Effects of Glycosaminoglycan Degradation on the Mechanical Behavior of the Posterior Porcine Sclera. Acta Biomater. 2015, 12, 195–206. [Google Scholar] [CrossRef]
- Murienne, B.J.; Chen, M.L.; Quigley, H.A.; Nguyen, T.D. The Contribution of Glycosaminoglycans to the Mechanical Behaviour of the Posterior Human Sclera. J. R. Soc. Interface 2016, 13, 20160367. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.N.; Berry, M.; Logan, A.; Blanch, R.J.; Ahmed, Z. Caspases in Retinal Ganglion Cell Death and Axon Regeneration. Cell Death Discov. 2017, 3, 17032. [Google Scholar] [CrossRef]
- Fazio, M.A.; Grytz, R.; Morris, J.S.; Bruno, L.; Gardiner, S.K.; Girkin, C.A.; Downs, J.C. Age-Related Changes in Human Peripapillary Scleral Strain. Biomech. Model. Mechanobiol. 2014, 13, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Downs, J.C. Optic Nerve Head Biomechanics in Aging and Disease. Exp. Eye Res. 2015, 133, 19–29. [Google Scholar] [CrossRef]
- Geraghty, B.; Jones, S.W.; Rama, P.; Akhtar, R.; Elsheikh, A. Age-Related Variations in the Biomechanical Properties of Human Sclera. J. Mech. Behav. Biomed. Mater. 2012, 16, 181–191. [Google Scholar] [CrossRef]
- Pease, M.E.; Oglesby, E.N.; Cone-Kimball, E.; Jefferys, J.L.; Steinhart, M.R.; Kim, A.J.; Hanes, J.; Quigley, H.A. Scleral Permeability Varies by Mouse Strain and Is Decreased by Chronic Experimental Glaucoma. Investig. Opthalmol. Vis. Sci. 2014, 55, 2564. [Google Scholar] [CrossRef]
- Steinhart, M.R.; Cone-Kimball, E.; Nguyen, C.; Nguyen, T.D.; Pease, M.E.; Chakravarti, S.; Oglesby, E.N.; Quigley, H.A. Susceptibility to Glaucoma Damage Related to Age and Connective Tissue Mutations in Mice. Exp. Eye Res. 2014, 119, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Sigal, I.A.; Yang, H.; Roberts, M.D.; Grimm, J.L.; Burgoyne, C.F.; Demirel, S.; Downs, J.C. IOP-Induced Lamina Cribrosa Deformation and Scleral Canal Expansion: Independent or Related? Investig. Opthalmol. Vis. Sci. 2011, 52, 9023. [Google Scholar] [CrossRef] [PubMed]
- Mikelberg, F.S.; Yidegiligne, H.M.; White, V.A.; Schulzer, M. Relation between Optic Nerve Axon Number and Axon Diameter to Scleral Canal Area. Ophthalmology 1991, 98, 60–63. [Google Scholar] [CrossRef]
- Rao, R.C.; Tchedre, K.T.; Malik, M.T.A.; Coleman, N.; Fang, Y.; Marquez, V.E.; Chen, D.F. Dynamic Patterns of Histone Lysine Methylation in the Developing Retina. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6784–6792. [Google Scholar] [CrossRef]
- Mayordomo, R.; Valenciano, A.I.; de la Rosa, E.J.; Hallbook, F. Generation of Retinal Ganglion Cells Is Modulated by Caspase-Dependent Programmed Cell Death. Eur. J. Neurosci. 2003, 18, 1744–1750. [Google Scholar] [CrossRef]
- Chavarría, T.; Baleriola, J.; Mayordomo, R.; de Pablo, F.; de la Rosa, E.J. Early Neural Cell Death Is an Extensive, Dynamic Process in the Embryonic Chick and Mouse Retina. Sci. World J. 2013, 2013, 627240. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Chen, H.; Xie, X.; Libby, R.T.; Tian, N.; Gan, L. BARHL2 Differentially Regulates the Development of Retinal Amacrine and Ganglion Neurons. J. Neurosci. 2009, 29, 3992–4003. [Google Scholar] [CrossRef]
- Anderson, S.R.; Zhang, J.; Steele, M.R.; Romero, C.O.; Kautzman, A.G.; Schafer, D.P.; Vetter, M.L. Complement Targets Newborn Retinal Ganglion Cells for Phagocytic Elimination by Microglia. J. Neurosci. 2019, 39, 2025–2040. [Google Scholar] [CrossRef]
- Pelzel, H.R.; Schlamp, C.L.; Nickells, R.W. Histone H4 Deacetylation Plays a Critical Role in Early Gene Silencing during Neuronal Apoptosis. BMC Neurosci. 2010, 11, 62. [Google Scholar] [CrossRef] [PubMed]
- Harwerth, R.S.; Wheat, J.L.; Rangaswamy, N.V. Age-Related Losses of Retinal Ganglion Cells and Axons. Investig. Opthalmol. Vis. Sci. 2008, 49, 4437. [Google Scholar] [CrossRef]
- Ahmed, Z.; Kalinski, H.; Berry, M.; Almasieh, M.; Ashush, H.; Slager, N.; Brafman, A.; Spivak, I.; Prasad, N.; Mett, I.; et al. Ocular Neuroprotection by SiRNA Targeting Caspase-2. Cell Death Dis. 2011, 2, e173. [Google Scholar] [CrossRef] [PubMed]
- Vigneswara, V.; Berry, M.; Logan, A.; Ahmed, Z. Pharmacological Inhibition of Caspase-2 Protects Axotomised Retinal Ganglion Cells from Apoptosis in Adult Rats. PLoS ONE 2012, 7, e53473. [Google Scholar] [CrossRef] [PubMed]
- Vigneswara, V.; Akpan, N.; Berry, M.; Logan, A.; Troy, C.M.; Ahmed, Z. Combined Suppression of CASP2 and CASP6 Protects Retinal Ganglion Cells from Apoptosis and Promotes Axon Regeneration through CNTF-Mediated JAK/STAT Signalling. Brain J. Neurol. 2014, 137, 1656–1675. [Google Scholar] [CrossRef] [PubMed]
- Vigneswara, V.; Ahmed, Z. Long-Term Neuroprotection of Retinal Ganglion Cells by Inhibiting Caspase-2. Cell Death Discov. 2016, 2, 16044. [Google Scholar] [CrossRef] [PubMed]
- Vigneswara, V.; Berry, M.; Logan, A.; Ahmed, Z. Caspase-2 Is Upregulated after Sciatic Nerve Transection and Its Inhibition Protects Dorsal Root Ganglion Neurons from Apoptosis after Serum Withdrawal. PLoS ONE 2013, 8, e57861. [Google Scholar] [CrossRef] [PubMed]
- Gaub, P.; Tedeschi, A.; Puttagunta, R.; Nguyen, T.; Schmandke, A.; Di Giovanni, S. HDAC Inhibition Promotes Neuronal Outgrowth and Counteracts Growth Cone Collapse through CBP/P300 and P/CAF-Dependent P53 Acetylation. Cell Death Differ. 2010, 17, 1392–1408. [Google Scholar] [CrossRef]
- Lebrun-Julien, F.; Suter, U. Combined HDAC1 and HDAC2 Depletion Promotes Retinal Ganglion Cell Survival After Injury Through Reduction of P53 Target Gene Expression. ASN Neuro 2015, 7, 1759091415593066. [Google Scholar] [CrossRef]
- Ma, E.L.; Kwong, J.M.; Lee, J.C.; Caprioli, J. Aging Is Associated with Increased Reactive Gliosis and Accelerated Retinal Ganglion Cell Loss after Optic Nerve Injury. Investig. Ophthalmol. Vis. Sci. 2022, 63, 927-A0396. [Google Scholar]
- Watkins, T.A.; Wang, B.; Huntwork-Rodriguez, S.; Yang, J.; Jiang, Z.; Eastham-Anderson, J.; Modrusan, Z.; Kaminker, J.S.; Tessier-Lavigne, M.; Lewcock, J.W.; et al. DLK Initiates a Transcriptional Program That Couples Apoptotic and Regenerative Responses to Axonal Injury. Proc. Natl. Acad. Sci. USA 2013, 110, 4039–4044. [Google Scholar] [CrossRef]
- Fernandes, K.A.; Harder, J.M.; John, S.W.; Shrager, P.; Libby, R.T. DLK-Dependent Signaling Is Important for Somal but Not Axonal Degeneration of Retinal Ganglion Cells Following Axonal Injury. Neurobiol. Dis. 2014, 69, 108–116. [Google Scholar] [CrossRef]
- Lewis, S. Retinal Protection. Nat. Rev. Neurosci. 2021, 22, 590–591. [Google Scholar] [CrossRef]
- Guo, X.; Zhou, J.; Starr, C.; Mohns, E.J.; Li, Y.; Chen, E.P.; Yoon, Y.; Kellner, C.P.; Tanaka, K.; Wang, H.; et al. Preservation of Vision after CaMKII-Mediated Protection of Retinal Ganglion Cells. Cell 2021, 184, 4299–4314.e12. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, H.M.; Schlamp, C.L.; Nickells, R.W. Targeting HDAC3 Activity with RGFP966 Protects Against Retinal Ganglion Cell Nuclear Atrophy and Apoptosis After Optic Nerve Injury. J. Ocul. Pharmacol. Ther. 2018, 34, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Ashok, A.; Pooranawattanakul, S.; Tai, W.L.; Cho, K.-S.; Utheim, T.P.; Cestari, D.M.; Chen, D.F. Epigenetic Regulation of Optic Nerve Development, Protection, and Repair. Int. J. Mol. Sci. 2022, 23, 8927. [Google Scholar] [CrossRef] [PubMed]
- Madsen, A.S.; Kristensen, H.M.E.; Lanz, G.; Olsen, C.A. The Effect of Various Zinc Binding Groups on Inhibition of Histone Deacetylases 1-11. ChemMedChem 2014, 9, 614–626. [Google Scholar] [CrossRef] [PubMed]
- Younes, A.; Oki, Y.; Bociek, R.G.; Kuruvilla, J.; Fanale, M.; Neelapu, S.; Copeland, A.; Buglio, D.; Galal, A.; Besterman, J.; et al. Mocetinostat for Relapsed Classical Hodgkin’s Lymphoma: An Open-Label, Single-Arm, Phase 2 Trial. Lancet Oncol. 2011, 12, 1222–1228. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.L.; Lukas, T.J.; Yuan, M.; Neufeld, A.H. Age-Related Increase in Mitochondrial DNA Damage and Loss of DNA Repair Capacity in the Neural Retina. Neurobiol. Aging 2010, 31, 2002–2010. [Google Scholar] [CrossRef]
- Eells, J.T. Mitochondrial Dysfunction in the Aging Retina. Biology 2019, 8, 31. [Google Scholar] [CrossRef]
- Wiley, C.D.; Velarde, M.C.; Lecot, P.; Liu, S.; Sarnoski, E.A.; Freund, A.; Shirakawa, K.; Lim, H.W.; Davis, S.S.; Ramanathan, A.; et al. Mitochondrial Dysfunction Induces Senescence with a Distinct Secretory Phenotype. Cell Metab. 2016, 23, 303–314. [Google Scholar] [CrossRef]
- Hurley, J.B.; Lindsay, K.J.; Du, J. Glucose, Lactate, and Shuttling of Metabolites in Vertebrate Retinas: Lactate Shuttle in the Retina. J. Neurosci. Res. 2015, 93, 1079–1092. [Google Scholar] [CrossRef]
- Bell, K.; Rosignol, I.; Sierra-Filardi, E.; Rodriguez-Muela, N.; Schmelter, C.; Cecconi, F.; Grus, F.; Boya, P. Age Related Retinal Ganglion Cell Susceptibility in Context of Autophagy Deficiency. Cell Death Discov. 2020, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-C.; Liu, P.-F.; Chang, C.-H.; Lin, Y.-C.; Chen, Y.-J.; Shu, C.-W. The Interplay of Autophagy and Oxidative Stress in the Pathogenesis and Therapy of Retinal Degenerative Diseases. Cell Biosci. 2022, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, M.; Tsujimura, S.; Okajima, K. A Quantitative Analysis of the Contribution of Melanopsin to Brightness Perception. Sci. Rep. 2019, 9, 7568. [Google Scholar] [CrossRef]
- Ecker, J.L.; Dumitrescu, O.N.; Wong, K.Y.; Alam, N.M.; Chen, S.-K.; LeGates, T.; Renna, J.M.; Prusky, G.T.; Berson, D.M.; Hattar, S.; et al. Melanopsin-Expressing Retinal Ganglion-Cell Photoreceptors: Cellular Diversity and Role in Pattern Vision. Neuron 2010, 67, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M. Clinical Factors Affecting Pupillary Light Reflex Parameters: A Single-centre, Cross-sectional Study. Ophthalmic Physiol. Opt. 2021, 41, 952–960. [Google Scholar] [CrossRef]
- Esquiva, G.; Hannibal, J. Melanopsin-Expressing Retinal Ganglion Cells in Aging and Disease. Histol. Histopathol. 2019, 34, 1299–1311. [Google Scholar] [CrossRef]
- Hudson, B.I.; Stickland, M.H.; Futers, T.S.; Grant, P.J. Effects of Novel Polymorphisms in the RAGE Gene on Transcriptional Regulation and Their Association with Diabetic Retinopathy. Diabetes 2001, 50, 1505–1511. [Google Scholar] [CrossRef]
- Huttunen, H.J.; Kuja-Panula, J.; Rauvala, H. Receptor for Advanced Glycation End Products (RAGE) Signaling Induces CREB-Dependent Chromogranin Expression during Neuronal Differentiation. J. Biol. Chem. 2002, 277, 38635–38646. [Google Scholar] [CrossRef]
- Vlassara, H.; Bucala, R.; Striker, L. Pathogenic Effects of Advanced Glycosylation: Biochemical, Biologic, and Clinical Implications for Diabetes and Aging. Lab. Investig. J. Technol. Methods Pathol. 1994, 70, 138–151. [Google Scholar]
- Friedlander, M.; Theesfeld, C.L.; Sugita, M.; Fruttiger, M.; Thomas, M.A.; Chang, S.; Cheresh, D.A. Involvement of Integrins Alpha v Beta 3 and Alpha v Beta 5 in Ocular Neovascular Diseases. Proc. Natl. Acad. Sci. USA 1996, 93, 9764–9769. [Google Scholar] [CrossRef]
- Kolb, H. Simple Anatomy of the Retina. In Webvision: The Organization of the Retina and Visual System; Kolb, H., Fernandez, E., Nelson, R., Eds.; University of Utah Health Sciences Center: Salt Lake City, UT, USA, 1995. [Google Scholar]
- Simó, R.; Hernández, C. Neurodegeneration in the Diabetic Eye: New Insights and Therapeutic Perspectives. Trends Endocrinol. Metab. 2014, 25, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. Biochemistry and Molecular Cell Biology of Diabetic Complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.C. Integrins in the Optic Nerve Head: Potential Roles in Glaucomatous Optic Neuropathy (an American Ophthalmological Society Thesis). Trans. Am. Ophthalmol. Soc. 2006, 104, 453–477. [Google Scholar]
- Almasieh, M.; Wilson, A.M.; Morquette, B.; Cueva Vargas, J.L.; di Polo, A. The Molecular Basis of Retinal Ganglion Cell Death in Glaucoma. Prog. Retin. Eye Res. 2012, 31, 152–181. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic Reactive Astrocytes Are Induced by Activated Microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Guttenplan, K.A.; Stafford, B.K.; El-Danaf, R.N.; Adler, D.I.; Münch, A.E.; Weigel, M.K.; Huberman, A.D.; Liddelow, S.A. Neurotoxic Reactive Astrocytes Drive Neuronal Death after Retinal Injury. Cell Rep. 2020, 31, 107776. [Google Scholar] [CrossRef] [PubMed]
- Knöferle, J.; Koch, J.C.; Ostendorf, T.; Michel, U.; Planchamp, V.; Vutova, P.; Tönges, L.; Stadelmann, C.; Brück, W.; Bähr, M.; et al. Mechanisms of Acute Axonal Degeneration in the Optic Nerve in Vivo. Proc. Natl. Acad. Sci. USA 2010, 107, 6064–6069. [Google Scholar] [CrossRef]
- Kaufman, P.L.; Lütjen Drecoll, E.; Croft, M.A. Presbyopia and Glaucoma: Two Diseases, One Pathophysiology? The 2017 Friedenwald Lecture. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1801–1812. [Google Scholar] [CrossRef]
- Ju, W.-K.; Perkins, G.A.; Kim, K.-Y.; Bastola, T.; Choi, W.-Y.; Choi, S.-H. Glaucomatous Optic Neuropathy: Mitochondrial Dynamics, Dysfunction and Protection in Retinal Ganglion Cells. Prog. Retin. Eye Res. 2022; 101136, in press. [Google Scholar] [CrossRef]
- Zhou, H.; Su, J.; Hu, X.; Zhou, C.; Li, H.; Chen, Z.; Xiao, Q.; Wang, B.; Wu, W.; Sun, Y.; et al. Glia-to-Neuron Conversion by CRISPR-CasRx Alleviates Symptoms of Neurological Disease in Mice. Cell 2020, 181, 590–603.e16. [Google Scholar] [CrossRef]
- Xie, Y.; Zhou, J.; Chen, B. Critical Examination of Ptbp1-Mediated Glia-to-Neuron Conversion in the Mouse Retina. Cell Rep. 2022, 39, 110960. [Google Scholar] [CrossRef]
- Hoang, T.; Kim, D.W.; Appel, H.; Pannullo, N.A.; Leavey, P.; Ozawa, M.; Zheng, S.; Yu, M.; Peachey, N.S.; Blackshaw, S.; et al. Genetic Loss of Function of Ptbp1 Does Not Induce Glia-to-Neuron Conversion in Retina. Cell Rep. 2022, 39, 110849. [Google Scholar] [CrossRef] [PubMed]
- Repka, M.X.; Quigley, H.A. The Effect of Age on Normal Human Optic Nerve Fiver Number and Diameter. Ophthalmology 1989, 96, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Provis, J.M.; van Driel, D.; Billson, F.A.; Russell, P. Human Fetal Optic Nerve: Overproduction and Elimination of Retinal Axons during Development. J. Comp. Neurol. 1985, 238, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, J.W. The Struggle to Make CNS Axons Regenerate: Why Has It Been so Difficult? Neurochem. Res. 2020, 45, 144–158. [Google Scholar] [CrossRef]
- Yun, M. Changes in Regenerative Capacity through Lifespan. Int. J. Mol. Sci. 2015, 16, 25392–25432. [Google Scholar] [CrossRef]
- Gauthier, A.C.; Liu, J. Epigenetics and Signaling Pathways in Glaucoma. BioMed Res. Int. 2017, 2017, 5712341. [Google Scholar] [CrossRef]
- Schmitt, H.M.; Schlamp, C.L.; Nickells, R.W. Role of HDACs in Optic Nerve Damage-Induced Nuclear Atrophy of Retinal Ganglion Cells. Neurosci. Lett. 2016, 625, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Patil, K.; Sharma, S.C. Broad Spectrum Caspase Inhibitor Rescues Retinal Ganglion Cells after Ischemia. NeuroReport 2004, 15, 981–984. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Pappas, A.C.; Wang, R.; Seifert, P.; Sun, D.; Jakobs, T.C. Ultrastructural Morphology of the Optic Nerve Head in Aged and Glaucomatous Mice. Investig. Opthalmology Vis. Sci. 2018, 59, 3984. [Google Scholar] [CrossRef] [PubMed]
- Pardo, I.D.; Otis, D.; Ritenour, H.N.; Bailey, S.; Masek-Hammerman, K.; Dowty, H.V.; Bolon, B.; Palazzi, X. Spontaneous Axonal Dystrophy in the Brain and Spinal Cord in Naïve Beagle Dogs. Toxicol. Pathol. 2020, 48, 694–701. [Google Scholar] [CrossRef]
- Bastian, C.; Zaleski, J.; Stahon, K.; Parr, B.; McCray, A.; Day, J.; Brunet, S.; Baltan, S. NOS3 Inhibition Confers Post-Ischemic Protection to Young and Aging White Matter Integrity by Conserving Mitochondrial Dynamics and Miro-2 Levels. J. Neurosci. 2018, 38, 6247–6266. [Google Scholar] [CrossRef] [PubMed]
- Schmidlin, C.J.; Dodson, M.B.; Madhavan, L.; Zhang, D.D. Redox Regulation by NRF2 in Aging and Disease. Free. Radic. Biol. Med. 2019, 134, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S. Emerging Insights into the Metabolic Alterations in Aging Using Metabolomics. Metabolites 2019, 9, 301. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.L.; Pasini, S.; Lambert, W.S.; D’Alessandro, K.B.; Yao, V.; Risner, M.L.; Calkins, D.J. Redistribution of Metabolic Resources through Astrocyte Networks Mitigates Neurodegenerative Stress. Proc. Natl. Acad. Sci. USA 2020, 117, 18810–18821. [Google Scholar] [CrossRef] [PubMed]
- Harun-Or-Rashid, M.; Pappenhagen, N.; Palmer, P.G.; Smith, M.A.; Gevorgyan, V.; Wilson, G.N.; Crish, S.D.; Inman, D.M. Structural and Functional Rescue of Chronic Metabolically Stressed Optic Nerves through Respiration. J. Neurosci. 2018, 38, 5122–5139. [Google Scholar] [CrossRef]
- Jassim, A.H.; Coughlin, L.; Harun-Or-Rashid, M.; Kang, P.T.; Chen, Y.-R.; Inman, D.M. Higher Reliance on Glycolysis Limits Glycolytic Responsiveness in Degenerating Glaucomatous Optic Nerve. Mol. Neurobiol. 2019, 56, 7097–7112. [Google Scholar] [CrossRef]
- Magoon, E.H. Development of Myelin in Human Optic Nerve and Tract: A Light and Electron Microscopic Study. Arch. Ophthalmol. 1981, 99, 655–659. [Google Scholar] [CrossRef]
- Abdellatif, A.; Attia, H. Aging, Myelination, and the Optic Nerve. In Factors Affecting Neurological Aging; Elsevier: Amsterdam, The Netherlands, 2021; pp. 301–311. ISBN 978-0-12-817990-1. [Google Scholar]
- Pierre, W.C.; Smith, P.L.P.; Londono, I.; Chemtob, S.; Mallard, C.; Lodygensky, G.A. Neonatal Microglia: The Cornerstone of Brain Fate. Brain Behav. Immun. 2017, 59, 333–345. [Google Scholar] [CrossRef]
- Guo, S.; Wang, H.; Yin, Y. Microglia Polarization from M1 to M2 in Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 815347. [Google Scholar] [CrossRef]
- Moyon, S.; Ma, D.; Huynh, J.L.; Coutts, D.J.C.; Zhao, C.; Casaccia, P.; Franklin, R.J.M. Efficient Remyelination Requires DNA Methylation. Eneuro 2017, 4, ENEURO.0336-16.2017. [Google Scholar] [CrossRef]
- Li, E.; Bestor, T.H.; Jaenisch, R. Targeted Mutation of the DNA Methyltransferase Gene Results in Embryonic Lethality. Cell 1992, 69, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Jackson-Grusby, L.; Beard, C.; Possemato, R.; Tudor, M.; Fambrough, D.; Csankovszki, G.; Dausman, J.; Lee, P.; Wilson, C.; Lander, E.; et al. Loss of Genomic Methylation Causes P53-Dependent Apoptosis and Epigenetic Deregulation. Nat. Genet. 2001, 27, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Unterberger, A.; Andrews, S.D.; Weaver, I.C.G.; Szyf, M. DNA Methyltransferase 1 Knockdown Activates a Replication Stress Checkpoint. Mol. Cell. Biol. 2006, 26, 7575–7586. [Google Scholar] [CrossRef] [PubMed]
- Seritrakul, P.; Gross, J.M. Tet-Mediated DNA Hydroxymethylation Regulates Retinal Neurogenesis by Modulating Cell-Extrinsic Signaling Pathways. PLoS Genet. 2017, 13, e1006987. [Google Scholar] [CrossRef]
- Attia, H.; Taha, M.; Abdellatif, A. Effects of Aging on the Myelination of the Optic Nerve in Rats. Int. J. Neurosci. 2019, 129, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Brommer, B.; Tian, X.; Krishnan, A.; Meer, M.; Wang, C.; Vera, D.L.; Zeng, Q.; Yu, D.; Bonkowski, M.S.; et al. Reprogramming to Recover Youthful Epigenetic Information and Restore Vision. Nature 2020, 588, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T.P.; McDowell, C.M.; Liu, Y.; Wagner, A.H.; Thole, D.; Faga, B.P.; Wordinger, R.J.; Braun, T.A.; Clark, A.F. Optic Nerve Crush Induces Spatial and Temporal Gene Expression Patterns in Retina and Optic Nerve of BALB/CJ Mice. Mol. Neurodegener. 2014, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Suter, T.A.C.S.; Wang, J.; Meng, H.; He, Z. Utilizing Mouse Optic Nerve Crush to Examine CNS Remyelination. Star Protoc. 2021, 2, 100796. [Google Scholar] [CrossRef] [PubMed]
- Sarrazin, N.; Chavret-Reculon, E.; Bachelin, C.; Felfli, M.; Arab, R.; Gilardeau, S.; Brazhnikova, E.; Dubus, E.; Yahia Cherif, L.; Lorenceau, J.; et al. Failed Remyelination of the Nonhuman Primate Optic Nerve Leads to Axon Degeneration, Retinal Damages, and Visual Dysfunction. Proc. Natl. Acad. Sci. USA 2022, 119, e2115973119. [Google Scholar] [CrossRef]
- Wagner, S.; Tagaya, M.; Koziol, J.A.; Quaranta, V.; del Zoppo, G.J. Rapid Disruption of an Astrocyte Interaction with the Extracellular Matrix Mediated by Integrin Alpha 6 Beta 4 during Focal Cerebral Ischemia/Reperfusion. Stroke 1997, 28, 858–865. [Google Scholar] [CrossRef]
- Cambier, S.; Gline, S.; Mu, D.; Collins, R.; Araya, J.; Dolganov, G.; Einheber, S.; Boudreau, N.; Nishimura, S.L. Integrin Alpha(v)Beta8-Mediated Activation of Transforming Growth Factor-Beta by Perivascular Astrocytes: An Angiogenic Control Switch. Am. J. Pathol. 2005, 166, 1883–1894. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, J.; Zheng, J.; Qin, S. Reactive Astrocytes in Neurodegenerative Diseases. Aging Dis. 2019, 10, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Mayorga-Weber, G.; Rivera, F.J.; Castro, M.A. Neuron-glia (Mis)Interactions in Brain Energy Metabolism during Aging. J. Neurosci. Res. 2022, 100, 835–854. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Barres, B.A. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef]
- García-Bermúdez, M.Y.; Freude, K.K.; Mouhammad, Z.A.; van Wijngaarden, P.; Martin, K.K.; Kolko, M. Glial Cells in Glaucoma: Friends, Foes, and Potential Therapeutic Targets. Front. Neurol. 2021, 12, 624983. [Google Scholar] [CrossRef]
- Fernandez de Castro, J.P.; Mullins, R.F.; Manea, A.M.; Hernandez, J.; Wallen, T.; Kuehn, M.H. Lipofuscin in Human Glaucomatous Optic Nerves. Exp. Eye Res. 2013, 111, 61–66. [Google Scholar] [CrossRef]
- Castaño, A.; Herrera, A.J.; Cano, J.; Machado, A. Lipopolysaccharide Intranigral Injection Induces Inflammatory Reaction and Damage in Nigrostriatal Dopaminergic System. J. Neurochem. 1998, 70, 1584–1592. [Google Scholar] [CrossRef]
- Zamanian, J.L.; Xu, L.; Foo, L.C.; Nouri, N.; Zhou, L.; Giffard, R.G.; Barres, B.A. Genomic Analysis of Reactive Astrogliosis. J. Neurosci. 2012, 32, 6391–6410. [Google Scholar] [CrossRef]
- Cooper, M.L.; Collyer, J.W.; Calkins, D.J. Astrocyte Remodeling without Gliosis Precedes Optic Nerve Axonopathy. Acta Neuropathol. Commun. 2018, 6, 38. [Google Scholar] [CrossRef]
- Sandell, J.H.; Peters, A. Effects of Age on Nerve Fibers in the Rhesus Monkey Optic Nerve. J. Comp. Neurol. 2001, 429, 541–553. [Google Scholar] [CrossRef]
- Means, J.C.; Lopez, A.A.; Koulen, P. Estrogen Protects Optic Nerve Head Astrocytes Against Oxidative Stress by Preventing Caspase-3 Activation, Tau Dephosphorylation at Ser422 and the Formation of Tau Protein Aggregates. Cell. Mol. Neurobiol. 2021, 41, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Avendano, J.; Rodrigues, M.M.; Hackett, J.J.; Gaskins, R. Corpora Amylacea of the Optic Nerve and Retina: A Form of Neuronal Degeneration. Investig. Ophthalmol. Vis. Sci. 1980, 19, 550–555. [Google Scholar]
- Kubota, T.; Holbach, L.M.; Naumann, G.O.H. Corpora Amylacea in Glaucomatous and Non-Glaucomatous Optic Nerve and Retina. Graefe’s Arch. Clin. Exp. Ophthalmol. 1993, 231, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Woodford, B.; Tso, M.O.M. An Ultrastructural Study of the Corpora Amylacea of the Optic Nerve Head and Retina. Am. J. Ophthalmol. 1980, 90, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Riba, M.; del Valle, J.; Augé, E.; Vilaplana, J.; Pelegrí, C. From Corpora Amylacea to Wasteosomes: History and Perspectives. Ageing Res. Rev. 2021, 72, 101484. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Yokoo, H.; Kakita, A.; Takahashi, H.; Harigaya, Y.; Ikota, H.; Nakazato, Y. Phagocytized Corpora Amylacea as a Histological Hallmark of Astrocytic Injury in Neuromyelitis Optica. Neuropathology 2012, 32, 587–594. [Google Scholar] [CrossRef]
- Bathini, P.; Mottas, A.; Jaquet, M.; Brai, E.; Alberi, L. Progressive Signaling Changes in the Olfactory Nerve of Patients with Alzheimer’s Disease. Neurobiol. Aging 2019, 76, 80–95. [Google Scholar] [CrossRef]
- Loeffler, K.U.; Edward, D.P.; Tso, M.O. Tau-2 Immunoreactivity of Corpora Amylacea in the Human Retina and Optic Nerve. Investig. Ophthalmol. Vis. Sci. 1993, 34, 2600–2603. [Google Scholar]
- Nag, T.; Wadhwa, S. Accumulation of Lipid Inclusions in Astrocytes of Aging Human Optic Nerve. Acta Biol. Hung. 2012, 63, 54–64. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Tang, Y.; Le, W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wei, Y.-Z.; Wang, G.-Q.; Li, D.-D.; Shi, J.-S.; Zhang, F. Targeting MAPK Pathways by Naringenin Modulates Microglia M1/M2 Polarization in Lipopolysaccharide-Stimulated Cultures. Front. Cell. Neurosci. 2019, 12, 531. [Google Scholar] [CrossRef]
- Li, H.-Y.; Huang, M.; Luo, Q.-Y.; Hong, X.; Ramakrishna, S.; So, K.-F. Lycium Barbarum (Wolfberry) Increases Retinal Ganglion Cell Survival and Affects Both Microglia/Macrophage Polarization and Autophagy after Rat Partial Optic Nerve Transection. Cell Transplant. 2019, 28, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Damani, M.R.; Zhao, L.; Fontainhas, A.M.; Amaral, J.; Fariss, R.N.; Wong, W.T. Age-Related Alterations in the Dynamic Behavior of Microglia: Age-Related Changes in Microglial Behavior. Aging Cell 2011, 10, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Moreno-García, A.; Kun, A.; Calero, O.; Medina, M.; Calero, M. An Overview of the Role of Lipofuscin in Age-Related Neurodegeneration. Front. Neurosci. 2018, 12, 464. [Google Scholar] [CrossRef]
- Huang, J.-D.; Presley, J.B.; Chimento, M.F.; Curcio, C.A.; Johnson, M. Age-Related Changes in Human Macular Bruch’s Membrane as Seen by Quick-Freeze/Deep-Etch. Exp. Eye Res. 2007, 85, 202–218. [Google Scholar] [CrossRef] [PubMed]
- Hammer, M.; Schultz, R.; Hasan, S.; Sauer, L.; Klemm, M.; Kreilkamp, L.; Zweifel, L.; Augsten, R.; Meller, D. Fundus Autofluorescence Lifetimes and Spectral Features of Soft Drusen and Hyperpigmentation in Age-Related Macular Degeneration. Transl. Vis. Sci. Technol. 2020, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Ben Ami, T.; Hong, S.; Heintzmann, R.; Gerig, G.; Ablonczy, Z.; Curcio, C.A.; Ach, T.; Smith, R.T. Hyperspectral Autofluorescence Imaging of Drusen and Retinal Pigment Epithelium in Donor Eyes with Age-Related Macular Degeneration. Retina 2016, 36, S127–S136. [Google Scholar] [CrossRef] [PubMed]
- De Jong, P.T.V.M. Age-Related Macular Degeneration. N. Engl. J. Med. 2006, 355, 1474–1485. [Google Scholar] [CrossRef]
- Wilkins, J.M.; Pomeranz, H.D. Visual Manifestations of Visible and Buried Optic Disc Drusen. J. Neuro-Ophthalmol. 2004, 24, 125–129. [Google Scholar] [CrossRef]
- Giarelli, L.; Falconieri, G.; Cameron, J.D.; Pheley, A.M. Schnabel Cavernous Degeneration: A Vascular Change of the Aging Eye. Arch. Pathol. Lab. Med. 2003, 127, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- Bales, T.R.; Lopez, M.J.; Clark, J. Embryology, Eye. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Sylvester, P.E.; Ari, K. The Size and Growth of the Human Optic Nerve. J. Neurol. Neurosurg. Psychiatry 1961, 24, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Kohler, C.; van den Dorpel, H.; Scholl, H.P.N.; Meyer, P.; Killer, H.E.; Neutzner, A. The Extracellular Matrix Composition of the Optic Nerve Subarachnoid Space. Exp. Eye Res. 2020, 200, 108250. [Google Scholar] [CrossRef] [PubMed]
- Reinhard, J.; Wiemann, S.; Hildebrandt, S.; Faissner, A. Extracellular Matrix Remodeling in the Retina and Optic Nerve of a Novel Glaucoma Mouse Model. Biology 2021, 10, 169. [Google Scholar] [CrossRef]
- Thornalley, P.J. Cell Activation by Glycated Proteins. AGE Receptors, Receptor Recognition Factors and Functional Classification of AGEs. Cell. Mol. Biol. 1998, 44, 1013–1023. [Google Scholar]
- Schmidt, A.M.; Yan, S.D.; Yan, S.F.; Stern, D.M. The Biology of the Receptor for Advanced Glycation End Products and Its Ligands. Biochim. Biophys. Acta Mol. Cell Res. 2000, 1498, 99–111. [Google Scholar] [CrossRef]
- Gao, X.; Lin, C.; Lu, L.; Liang, G.; Chen, Z.; Zhang, X. RAGE, NF-kappaB, p21 expressions in mouse spiral ganglion cells. J. Clin. Otorhinolaryngol. Head Neck Surg. 2014, 28, 265–268. [Google Scholar]
- Hammes, H.P.; Hoerauf, H.; Alt, A.; Schleicher, E.; Clausen, J.T.; Bretzel, R.G.; Laqua, H. Nε(Carboxymethyl)Lysin and the AGE Receptor RAGE Colocalize in Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1855–1859. [Google Scholar]
- Wagenseil, J.E.; Mecham, R.P. Elastin in Large Artery Stiffness and Hypertension. J. Cardiovasc. Transl. Res. 2012, 5, 264–273. [Google Scholar] [CrossRef]
- Cullum, N.A.; Mahon, J.; Stringer, K.; McLean, W.G. Glycation of Rat Sciatic Nerve Tubulin in Experimental Diabetes Mellitus. Diabetologia 1991, 34, 387–389. [Google Scholar] [CrossRef]
- Mackic, J.B.; Stins, M.; McComb, J.G.; Calero, M.; Ghiso, J.; Kim, K.S.; Yan, S.D.; Stern, D.; Schmidt, A.M.; Frangione, B.; et al. Human Blood-Brain Barrier Receptors for Alzheimer’s Amyloid-Beta 1- 40. Asymmetrical Binding, Endocytosis, and Transcytosis at the Apical Side of Brain Microvascular Endothelial Cell Monolayer. J. Clin. Investig. 1998, 102, 734–743. [Google Scholar] [CrossRef]
- Lander, H.M.; Tauras, J.M.; Ogiste, J.S.; Hori, O.; Moss, R.A.; Schmidt, A.M. Activation of the Receptor for Advanced Glycation End Products Triggers a P21ras-Dependent Mitogen-Activated Protein Kinase Pathway Regulated by Oxidant Stress. J. Biol. Chem. 1997, 272, 17810–17814. [Google Scholar] [CrossRef] [PubMed]
- Deora, A.A.; Win, T.; Vanhaesebroeck, B.; Lander, H.M. A Redox-Triggered Ras-Effector Interaction. J. Biol. Chem. 1998, 273, 29923–29928. [Google Scholar] [CrossRef] [PubMed]
- Applebaum-Bowden, D.; Haffner, S.M.; Hartsook, E.; Luk, K.H.; Albers, J.J.; Hazzard, W.R. Down-Regulation of the Low-Density Lipoprotein Receptor by Dietary Cholesterol. Am. J. Clin. Nutr. 1984, 39, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Juranek, J.; Mukherjee, K.; Kordas, B.; Załęcki, M.; Korytko, A.; Zglejc-Waszak, K.; Szuszkiewicz, J.; Banach, M. Role of RAGE in the Pathogenesis of Neurological Disorders. Neurosci. Bull. 2022, 38, 1248–1262. [Google Scholar] [CrossRef]
- Andreassen, T.T.; Seyer-Hansen, K.; Bailey, A.J. Thermal Stability, Mechanical Properties and Reducible Cross-Links of Rat Tail Tendon in Experimental Diabetes. Biochim. Biophys. Acta 1981, 677, 313–317. [Google Scholar] [CrossRef]
- Winlove, C.P.; Parker, K.H.; Avery, N.C.; Bailey, A.J. Interactions of Elastin and Aorta with Sugars in Vitro and Their Effects on Biochemical and Physical Properties. Diabetologia 1996, 39, 1131–1139. [Google Scholar] [CrossRef]
- Brüel, A.; Oxlund, H. Changes in Biomechanical Properties, Composition of Collagen and Elastin, and Advanced Glycation Endproducts of the Rat Aorta in Relation to Age. Atherosclerosis 1996, 127, 155–165. [Google Scholar] [CrossRef]
- Reiser, K.M. Nonenzymatic Glycation of Collagen in Aging and Diabetes. Proc. Soc. Exp. Biol. Med. 1998, 218, 23–37. [Google Scholar] [CrossRef]
- Robins, S.P. Biochemistry and Functional Significance of Collagen Cross-Linking. Biochem. Soc. Trans. 2007, 35, 849–852. [Google Scholar] [CrossRef]
- Aumailley, M. The Laminin Family. Cell Adhes. Migr. 2013, 7, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Kohno, T.; Sorgente, N.; Ishibashi, T.; Goodnight, R.; Ryan, S.J. Immunofluorescent Studies of Fibronectin and Laminin in the Human Eye. Investig. Ophthalmol. Vis. Sci. 1987, 28, 506–514. [Google Scholar]
- Chintala, S.K.; Zhang, X.; Austin, J.S.; Fini, M.E. Deficiency in Matrix Metalloproteinase Gelatinase B (MMP-9) Protects against Retinal Ganglion Cell Death after Optic Nerve Ligation. J. Biol. Chem. 2002, 277, 47461–47468. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cheng, M.; Chintala, S.K. Kainic Acid–Mediated Upregulation of Matrix Metalloproteinase-9 Promotes Retinal Degeneration. Investig. Opthalmol. Vis. Sci. 2004, 45, 2374–2383. [Google Scholar] [CrossRef]
- Morrison, J.C. Ultrastructural Location of Extracellular Matrix Components in the Optic Nerve Head. Arch. Ophthalmol. 1989, 107, 123–129. [Google Scholar] [CrossRef]
- Wipff, P.-J.; Rifkin, D.B.; Meister, J.-J.; Hinz, B. Myofibroblast Contraction Activates Latent TGF-Β1 from the Extracellular Matrix. J. Cell Biol. 2007, 179, 1311–1323. [Google Scholar] [CrossRef]
- Burgoyne, C.F. A Biomechanical Paradigm for Axonal Insult within the Optic Nerve Head in Aging and Glaucoma. Exp. Eye Res. 2011, 93, 120–132. [Google Scholar] [CrossRef]
- Lambert, W.; Agarwal, R.; Howe, W.; Clark, A.F.; Wordinger, R.J. Neurotrophin and Neurotrophin Receptor Expression by Cells of the Human Lamina Cribrosa. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2315–2323. [Google Scholar]
- Desmoulière, A.; Geinoz, A.; Gabbiani, F.; Gabbiani, G. Transforming Growth Factor-Beta 1 Induces Alpha-Smooth Muscle Actin Expression in Granulation Tissue Myofibroblasts and in Quiescent and Growing Cultured Fibroblasts. J. Cell Biol. 1993, 122, 103–111. [Google Scholar] [CrossRef]
- Chambers, R.C.; Leoni, P.; Kaminski, N.; Laurent, G.J.; Heller, R.A. Global Expression Profiling of Fibroblast Responses to Transforming Growth Factor-Beta1 Reveals the Induction of Inhibitor of Differentiation-1 and Provides Evidence of Smooth Muscle Cell Phenotypic Switching. Am. J. Pathol. 2003, 162, 533–546. [Google Scholar] [CrossRef]
- Kirwan, R.P.; Crean, J.K.; Fenerty, C.H.; Clark, A.F.; O’Brien, C.J. Effect of Cyclical Mechanical Stretch and Exogenous Transforming Growth Factor-Beta1 on Matrix Metalloproteinase-2 Activity in Lamina Cribrosa Cells from the Human Optic Nerve Head. J. Glaucoma 2004, 13, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, R.P.; Fenerty, C.H.; Crean, J.; Wordinger, R.J.; Clark, A.F.; O’Brien, C.J. Influence of Cyclical Mechanical Strain on Extracellular Matrix Gene Expression in Human Lamina Cribrosa Cells in Vitro. Mol. Vis. 2005, 11, 798–810. [Google Scholar] [PubMed]
- Grytz, R.; Fazio, M.A.; Libertiaux, V.; Bruno, L.; Gardiner, S.; Girkin, C.A.; Downs, J.C. Age- and Race-Related Differences in Human Scleral Material Properties. Investig. Ophthalmol. Vis. Sci. 2014, 55, 8163–8172. [Google Scholar] [CrossRef] [PubMed]
- Waxman, S.; Brazile, B.L.; Yang, B.; Lee, P.-Y.; Hua, Y.; Gogola, A.L.; Lam, P.; Voorhees, A.P.; Rizzo, J.F.; Jakobs, T.C.; et al. Lamina Cribrosa Vessel and Collagen Beam Networks Are Distinct. Exp. Eye Res. 2022, 215, 108916. [Google Scholar] [CrossRef] [PubMed]
- Brazile, B.L.; Hua, Y.; Jan, N.-J.; Wallace, J.; Gogola, A.; Sigal, I.A. Thin Lamina Cribrosa Beams Have Different Collagen Microstructure Than Thick Beams. Investig. Opthalmology Vis. Sci. 2018, 59, 4653. [Google Scholar] [CrossRef]
- An, L.; Johnstone, M.; Wang, R.K. Optical Microangiography Provides Correlation between Microstructure and Microvasculature of Optic Nerve Head in Human Subjects. J. Biomed. Opt. 2012, 17, 116018. [Google Scholar] [CrossRef]
- Hynes, R.O.; Lively, J.C.; McCarty, J.H.; Taverna, D.; Francis, S.E.; Hodivala-Dilke, K.; Xiao, Q. The Diverse Roles of Integrins and Their Ligands in Angiogenesis. Cold Spring Harb. Lab. Press 2002, 67, 143–153. [Google Scholar] [CrossRef]
- McCarty, J.H.; Monahan-Earley, R.A.; Brown, L.F.; Keller, M.; Gerhardt, H.; Rubin, K.; Shani, M.; Dvorak, H.F.; Wolburg, H.; Bader, B.L.; et al. Defective Associations between Blood Vessels and Brain Parenchyma Lead to Cerebral Hemorrhage in Mice Lacking Alphav Integrins. Mol. Cell. Biol. 2002, 22, 7667–7677. [Google Scholar] [CrossRef] [PubMed]
- Tun, T.A.; Wang, X.; Baskaran, M.; Nongpiur, M.E.; Tham, Y.-C.; Perera, S.A.; Strouthidis, N.G.; Aung, T.; Cheng, C.-Y.; Girard, M.J.A.; et al. Variation of Peripapillary Scleral Shape with Age. Investig. Opthalmol. Vis. Sci. 2019, 60, 3275. [Google Scholar] [CrossRef]
- Spaide, R.F.; Meyerle, C.B. CSCR (Central Serous Chorioretinopathy). In Albert and Jakobiec’s Principles and Practice of Ophthalmology; Albert, D., Miller, J., Azar, D., Young, L.H., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–26. ISBN 978-3-319-90495-5. [Google Scholar]
- Spaide, R.F.; Fisher, Y.L.; Ngo, W.K.; Barbazetto, I. Regional Scleral Thickness as A Risk Factor for Central Serous Chorioretinopathy. Retina 2022, 42, 1231–1237. [Google Scholar] [CrossRef]
- Auten, R.L.; Davis, J.M. Oxygen Toxicity and Reactive Oxygen Species: The Devil Is in the Details. Pediatr. Res. 2009, 66, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Ramanathan, A. The Aging Metabolome—Biomarkers to Hub Metabolites. Proteomics 2020, 20, 1800407. [Google Scholar] [CrossRef]
- Jonas, J.B.; Schmidt, A.M.; Müller-Bergh, J.A.; Schlötzer-Schrehardt, U.M.; Naumann, G.O. Human Optic Nerve Fiber Count and Optic Disc Size. Investig. Ophthalmol. Vis. Sci. 1992, 33, 2012–2018. [Google Scholar]
- Błaszczyk, J.W. Energy Metabolism Decline in the Aging Brain—Pathogenesis of Neurodegenerative Disorders. Metabolites 2020, 10, 450. [Google Scholar] [CrossRef] [PubMed]
- Yazdankhah, M.; Shang, P.; Ghosh, S.; Hose, S.; Liu, H.; Weiss, J.; Fitting, C.S.; Bhutto, I.A.; Zigler, J.S.; Qian, J.; et al. Role of Glia in Optic Nerve. Prog. Retin. Eye Res. 2021, 81, 100886. [Google Scholar] [CrossRef] [PubMed]
| Tissue (Broad) | Tissue (Subtype, Process, Age, or Disease) | Summary of Molecular Mechanisms | Citation |
|---|---|---|---|
| Connective tissue ECM | Elastin | LOX oxidation Decreased quality Further research needed on elastin quantity and relative importance of ECM composition and organization | [12,13,14,15,16,17,18,19,20,21,22,23] |
| Collagen | Elevated LOX expression AGE accumulation RAGE “two-hit” hypothesis ECM remodeling Calcium and lipid accumulation MMPs Mechanical considerations Thickening, stiffening | [23,24,25,26,27] | |
| Dura mater | 2nd most ON elastic fibers Calcifications Mechanical considerations Less stiff | [12,16,17,28,29] | |
| Arachnoid Subarachnoid space | CSFP decreases (linked to normal tension glaucoma) Thickening, more coarse trabeculae, more prominent granulations | [12,30,31,32,33] | |
| Pia mater Septa | Thickening, expands into fibrous sleeve | [12] | |
| Lamina Cribrosa (LC) | Most ON elastic fibers Collagen deposition AGE and RAGE ECM remodeling Myofibroblast contraction, TGF-β, mechanotransduction TGF-β1 upregulation MMP-2 Increased cribriform plate rigidity (plastic flow) Unique vascularity (thin and thick beams) Thicker astrocyte basement membranes Integrins α2β1, α3β1, α6β1, and α6β4 | [12,18,19,34,35,36,37] | |
| Optic (scleral) canal | Sclera | Decreased elastin Increased collagen AGE Mechanical considerations V-shaped PPS CSCR Further research is needed on scleral stiffening and/or thickening | [31,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59] |
| Optic nerve head (ONH) | Age-dependent structural changes may alter the biochemical environment RGC loss Smaller optic canal area associated with more axons | [12,20,35,41,60,61,62,63,64,65,66,67] | |
| Retinal ganglion cell (RGC) | Embryonic | Caspase-3 neuronal pruning Histone lysine methylation (H3K9me2, H3K27me3) Histone methyl transferases (G9a (EHMT2) and Ezh2 (EZH2)) Barhl2 (BARHL2) Transcriptional gene control Microglial phagocytosis (complement proteins) | [60,68,69,70,71,72] |
| Age-dependent loss | Mechanical and biochemical RGC loss RNFL thinning Caspases (inflammatory, apoptotic) HAT HDAC | [12,60,73,74,75,76,77,78,79,80,81] | |
| Aging and injury | Upregulated DLK CaMKII-CREB signaling interference Caspases HDAC upregulation | [73,75,76,77,78,79,82,83,84,85,86,87,88,89,90] | |
| Mitochondria | Functional decline ROS Worsened mtDNA repair pathways (lost PARP1, MYH, NTH1) Low NRF2 | [91,92,93,94] | |
| Autophagy | Age-related decline Decreased Atg7 (ATG7) and Beclin-1 (BECN1) Normal tension glaucoma (Optn, Tbk1, and Opa-1) Autophagy activators (AMPK inducer, mTORC1 inhibitors) may decrease ocular disease severity | [74,95,96,97,98,99,100] | |
| ipRGC | Decreased density and plexus Lipofuscin accumulation | [74,97,98,99,100] | |
| Diabetic retinopathy (DR) | Decreased oxygen Abnormal metabolism Polyol and hexosamine pathways DAG-PKC synthesis Free radicals AGE and RAGE Caspases Bax Fas Integrins | [4,5,6,60,61,101,102,103,104,105,106,107] | |
| Glaucoma | Failed axonal transport Derived neurotrophic factor Pro-neurotrophins Apoptotic signals (intrinsic, extrinsic) Caspases AGE and RAGE Integrins Mitochondrial dysfunction (DRP1, OPA1, CoQ10, SOD2, and Mic60) ECM remodeling Trabecular meshwork stiffening | [12,25,60,108,109,110,111,112,113,114] | |
| Regeneration | Adult RGCs do not regenerate | [115,116,117] | |
| Axon | Embryonic | Embryonic RGCs regenerate Deletion of PTEN, IL22, or SOCS3 (activate mTOR and STAT3 pathways) and Notch, Hh, and mTOR signal pathways (regulate JAK/STAT pathway) propel cell growth and regeneration Caspases | [60,88,118,119,120,121,122,123,124] |
| Loss of density | ~50–70% of axons eliminated in gestation General (nonlinear) density loss after age 60 May not be associated with visual loss or pathology | [12] | |
| Swelling | Most notable after age 70 NOS AGE and RAGE | [2,12,25,102,112,125,126,127] | |
| Metabolic dysfunction | Hypometabolism Decreased respiration, ATP production, spare capacity Altered redox homeostasis Low NRF2 ROS Decreased NAD+ levels may induce senescence | [3,128,129,130,131,132] | |
| Myelin | Newborn | Newborn ON unmyelinated Oligodendrocytes DNA methylation imperative for myelination (DNMT1, DNMT3A) | [12,88,133,134,135,136,137,138,139,140] |
| Childhood and adult | Dense myelin sheaths age 2 Remyelination by DNA demethylation, hydroxymethylation (TET1, TET2) | [12,88,137,141] | |
| After age 70 | Decreased myelin packing density Morphological alterations Ballooning sheaths Separated lamellae Oligodendrocyte degeneration ECM remodeling Decreased growth factors Mitigation of hydroxymethylation Oct4 (Pou5f1), Sox2, and Klf4 (OSK) may contribute | [88,134,142,143,144,145,146] | |
| Glial cell | Reactive astrocytosis | ROS Decreased ATP production Neurotoxins Classical complement Neurotrophic factors TGF-β1, TNF, CASP3, and p53 “Two-hit” model of injury and neuroinflammatory mediators | [88,110,111,130,147,148,149,150,151,152,153,154,155] |
| Remodeling | Gap-junction coupling of Cx43 Organizational deterioration Glial coverage increases | [130,156] | |
| Hypertrophy | Glial filaments in processes Oxidative stress (ROS) Caspases Tau protein Tau dephosphorylation at Ser422 | [72,157,158] | |
| Inclusions | Corpora amylacea (CA) Myelin debris Lipids | [12,159,160,161,162,163,164,165,166] | |
| Migration | Integrins | [104,108] | |
| Microglial polarization | M1/M2 polarization imbalance MAPK signaling JNK inactivation Altered cytokine production Increased activation marker expression Abnormal morphologies Dynamic behavior changes | [86,136,167,168,169,170,171] | |
| Lipofuscin (LF) | May actively induce pro-inflammatory glial cell phenotypes | [12,153,172,173,174,175,176,177] | |
| Vascular degeneration * | Hyalinization of arterioles Integrins α3β1, α6β1, and α6β4, along with α5β1 and αvβ1 | [12,82,104,125] | |
| Cavernous degeneration * | Common in neurodegeneration Likely due to vascular disease (not glaucoma) | [12,178] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coleman-Belin, J.; Harris, A.; Chen, B.; Zhou, J.; Ciulla, T.; Verticchio, A.; Antman, G.; Chang, M.; Siesky, B. Aging Effects on Optic Nerve Neurodegeneration. Int. J. Mol. Sci. 2023, 24, 2573. https://doi.org/10.3390/ijms24032573
Coleman-Belin J, Harris A, Chen B, Zhou J, Ciulla T, Verticchio A, Antman G, Chang M, Siesky B. Aging Effects on Optic Nerve Neurodegeneration. International Journal of Molecular Sciences. 2023; 24(3):2573. https://doi.org/10.3390/ijms24032573
Chicago/Turabian StyleColeman-Belin, Janet, Alon Harris, Bo Chen, Jing Zhou, Thomas Ciulla, Alice Verticchio, Gal Antman, Michael Chang, and Brent Siesky. 2023. "Aging Effects on Optic Nerve Neurodegeneration" International Journal of Molecular Sciences 24, no. 3: 2573. https://doi.org/10.3390/ijms24032573
APA StyleColeman-Belin, J., Harris, A., Chen, B., Zhou, J., Ciulla, T., Verticchio, A., Antman, G., Chang, M., & Siesky, B. (2023). Aging Effects on Optic Nerve Neurodegeneration. International Journal of Molecular Sciences, 24(3), 2573. https://doi.org/10.3390/ijms24032573










