Abstract
Meniere’s disease (MD) is one of the most complicated diseases in the otologic clinic. The complexity of MD is partially due to the multifactorial etiological mechanisms and the heterogenous symptoms, including episodic vertigo, hearing loss, aural fullness and tinnitus. As a result, the diagnosis of MD and differentiating MD from other diseases with similar symptoms, such as vestibular migraine (VM), is challenging. In addition, it is difficult to predict the progression of hearing loss and the frequency of vertigo attacks. Detailed studies have revealed that functional markers, such as pure tone audiometry (PTA), electrocochleography (ECochG), vestibular evoked myogenic potential (VEMP), caloric test, video head impulse test (vHIT) and magnetic resonance imaging (MRI) could help to evaluate MD with different hearing levels and frequency of vertigo attacks. Investigations of molecular markers such as autoimmunity, inflammation, protein signatures, vasopressin and circadian clock genes in MD are still underway. This review will summarize these functional and molecular markers, address how these markers are associated with hearing loss and vertigo attacks in MD, and analyze the results of the markers between MD and VM.
1. Introduction
Meniere’s disease (MD) is a heterogeneous inner ear disorder with complex symptoms, including episodic vertigo, sensorineural hearing loss and aural symptoms such as aural fullness or tinnitus. The incidence and prevalence of MD are varied and range from 3.5 per 100,000 to 513 per 100,000 [1]. The most classic pathogenesis of MD is endolymphatic hydrops (EH). According to the published temporal bone finding from the histopathological studies, EH of pars inferior structures was found in 98.8–100% of all confirmed cases [2,3,4]. Different pathogeneses of MD were claimed in recent decades, including anatomic or structural changes, vasopressin, autoimmune, allergy, migraine-related, genetic theories, and so on [5,6,7,8,9,10,11,12,13,14,15]. In fact, it is a multifactorial disease with more than one etiology converging into characteristic symptomatology [16].
The clinical heterogeneity makes the diagnosis of MD a challenge in the clinic since we lack good objective markers and exact examination standards but only depend on subjective symptoms and signs. In 2015, the American Academy of Otolaryngology–Head and Neck Surgery Foundation (AAO-HNSF) revised the diagnostic criteria for two MD categories: Definite MD and probable MD (Table 1) [17,18]. These diagnostic criteria are important in diagnosing MD and distinguishing MD from other causes of vertigo with similar symptoms. However, because of the variable clinical presentation of MD, it needs to take a period of time to observe the clinical manifestations to make an accurate diagnosis of definite MD.

Table 1.
Diagnostic criteria of MD according to 2015 AAO-HNS Equilibrium Committee [17].
Fluctuating low-tone hearing loss is the most characteristic finding in early MD. It was hypothesized that the distention of basilar membranes in EH starts from the apex and causes low-frequency hearing loss [19]. Therefore, the severity of cochlear EH can be evaluated by the degree of hearing loss. Therefore, the most commonly used staging of MD is based on the average of pure tone thresholds at 0.5, 1, 2 and 3 kHz of the audiogram: stage I, less than 26 dB; stage II, 26–40 dB; stage III, 41 to 70 dB; stage IV, more than 70 dB [20]. In general, the history of hearing loss in MD is usually progressive but sometimes inconsistent between different subtypes of MD patients [21,22]. In addition, the frequency of vertigo attacks and vestibular hypofunction seem not able to predict the hearing outcome of MD [22,23]. However, no specific markers could be used to correlate with the hearing results of MD patients in the clinic.
The frequency of vertigo attacks is another concern for patients with MD since vertigo episodes could affect the quality of life. Although environmental factors might precipitate the attacks of MD, these factors are generally unspecific, and the factors that could induce vertigo in one patient may not affect another one. Therefore, the investigation for markers to predict vertigo attacks is essential for MD patients. On the other hand, clinicians need available markers to differentiate MD from vestibular migraine (VM), a disease comprising similar symptoms to MD. It is crucial since the prognosis and treatment strategies of MD and VM are different.
The diagnosis of MD is based on the diagnostic criteria of MD. Although several audiovestibular markers have been used to evaluate MD in the clinic, clinicians and patients are frequently confused with the results. In addition, there is no consensus regarding the role of molecular markers based on the etiological mechanisms of MD. In this review, we will overview the recent studies about the markers of functional testing used in the clinic and the recent development of magnetic resonance imaging (MRI) to evaluate MD. In addition, the molecular markers investigated in recent years will be reviewed. We will also focus on the functional and molecular markers in different hearing levels/staging and vertigo attacks/remission. In addition, we will look at the articles to see whether these markers could help to differentiate MD from VM.
2. Functional Markers for MD
2.1. Pure Tone Audiometry (PTA)
PTA is the most widely used hearing examination to identify hearing threshold levels and to determine the hearing loss degree, type and configuration. According to current AAO-HNS diagnostic criteria, one of the essential conditions of definite MD is the documented fluctuating low- to mid-frequency sensorineural hearing loss, which must be examined by the PTA [17]. Therefore, PTA is a necessary examination for suspected MD patients. In early MD presentation, hearing loss usually fluctuates or is subtle, making it difficult to catch a positive finding using PTA, and the suspected patient always undergoes several PTAs. In addition, the reliability and accuracy of PTA results depend on the cooperation of patients. However, PTA is still the basic test to accurately diagnose and determine the stage of MD.
During the natural course of MD, the hearing loss pattern could shift from the early stages of lower-frequency hearing loss to the late stage of high-frequency hearing loss, showing a “flat type audiogram” in PTA [23]. Therefore, PTA could be used in predicting the course of the disease. It was also observed that MD patients with middle- and high-frequency hearing loss at the initial visit had a poor prognosis in relation to hearing loss [21]. On the other hand, patients with primary MD exhibit moderate-to-severe hearing loss within 5–10 years, whereas patients with migraine-related MD or VM tend to recover and fluctuate for a long time [21,24].
Although a fluctuating sensorineural hearing loss, which affects low frequencies, is the major initial finding of PTA in MD, other similar diseases, such as acute low-tone hearing loss (ALHL), also characterizes similar PTAs. In particular, recurrent hearing loss is not uncommon in ALHL [25] and was previously thought to be “cochlear MD” [26]. Because of the clinical similarity of recurrent low-tone hearing loss with MD, one may wonder whether the recurrence of low-tone hearing loss is a sign of EH and whether it will progress to definite MD. In a study conducted by Yamasoba et al., the author followed patients with initial ALHL without vertigo for a minimum of 3 years, and only 11% developed clinical MD [27]. In a later study by Junicho et al., they also found that not all ALHL suffered from EH, even if they had a vertigo attack at the onset [28]. The reason is probably that recurrent ALHL might be a common sign of several diseases, such as MD, sudden deafness, and VM [29]. Recently, it was also observed that recurrent low-tone hearing loss possibly occurred in patients with migraine without vertigo, which was proposed as the symptoms of “cochlear migraine” [30]. Therefore, one cannot solely use PTA to diagnose MD, and definite MD has to be confirmed according to the diagnosis criteria.
In terms of vertigo attacks, fluctuating hearing loss is not always related to vestibular symptoms. Sometimes there is a time delay between hearing loss and vertigo [31]. During the natural course of the disease, some studies assume that the frequency of vertigo increases during the early stage of MD and may then keep stable or without vertigo attack for several years [32,33]. However, it was also observed that some patients with long-term MD still suffered from frequent vertigo attacks [34]. Compared to the progression of hearing loss in typical MD, the frequency of vertigo attacks is difficult to predict. The relationship between PTA and vertigo attacks during the course of MD needs further elucidation.
2.2. Electrocochleography (ECochG)
ECochG is an objective examination conducted to record the electrical potentials generated in the inner ear and auditory nerve in response to sound stimulation in order to detect the distortion of the basilar membrane due to EH [14]. Three main basic potentials in ECochG are the action potential (AP), the cochlear microphonics (CM) and the summating potential (SP). Since Gibson et al. proposed the abnormal findings of the ECochG in MD decades ago [35], several other studies showed that the amplitude of the AP and SP ratio could identify EH or MD. Although several researchers tried to use ECochG to diagnose MD, a standardized cut-off value to confirm EH is lacking. A recent systemic review revealed that the sensitivity of ECochG is about 66.7–85.7%, while the specificity is about 80–100% [36]. The problem of low sensitivity is possibly due to the fact that patients with probable MD may not have developed cochlear changes that result in an abnormal ECochG [37]. In addition, an elevated SP/AP ratio can be observed in other inner ear diseases, such as superior semicircular canal dehiscence [36]. In addition, the SP/AP ratio does not recover even if vertigo attacks disappear in MD [38]. Because the extratympanic electrode to measure the SP and AP ratio provided low specificity and sensitivity [39,40], transtympanic ECochG was developed to enhance the sensitivity [41,42]. Other methods to increase sensitivity include the usage of tone burst stimuli [43,44] and the measurement of the SP/AP area ratio [45], SP bias ratio [46], and graphic angle [47].
Since a positive ECochG represents EH, the correlation between ECochG results and the audiological symptoms of MD has been investigated. In the study conducted by Hornibrrok et al., the “positive” ECochG group had a significantly higher proportion of participants who showed asymmetrical hearing thresholds than the “negative” ECochG group [42]. In fact, a significant association between an enlarged SP/AP ratio and the degree of hearing loss was noted, showing that patients with advanced staging had a higher possibility of an enlarged SP/AP ratio [48]. Another study by Takeda et al. found that the incidence of an enhanced SP was increased in cases with MD with a pure-tone average exceeding 31 dB [49]. Therefore, they concluded that ECochG is more likely to be positive in patients with longer periods of cochlear and vestibular symptoms. However, in their study, the elevated SP/AP ratio may persist even in glycerol-induced hearing gain. This implied that even though ECochG might be used as the diagnostic tool in MD, the usefulness of ECochG as the marker for hearing loss in MD needs further investigation.
Another important topic is whether ECochG could differentiate MD from VM. Since EH is a distinct feature of MD, Mertines et al. observed a higher proportion of abnormal ECochG in MD than in VM [50]. However, EH still possibly occurred in patients of VM, which revealed a higher SP/AP [51]. Therefore, ECochG could not be used as the sole tool to differentiate MD and VM.
2.3. Vestibular Evoked Myogenic Potential (VEMP)
VEMP is a vestibular function technique used to evaluate the function of the utricle and saccule. Generally, VEMPs can be recorded from the contracted sternocleidomastoid muscle (cervical VEMPs or cVEMPs) to assess saccule function, while the inferior oblique muscle (ocular VEMPs or oVEMPs) to assess utricle function [52,53]. Air-conduction sound (ACS) and bone-conduction vibration (BCV) are the most frequently applied in clinical VEMP settings [54]. Compared to a healthy control, MD patients presented both cVEMP and oVEMP larger amplitudes when using BCV than ACS and both lower response rates when using ACS than BCV [55,56]. Another parameter of VEMP is the interaural amplitude difference (IAD) ratio. The higher ratio may indicate lower vestibular function [57]. In a meta-analysis, the sensitivity and specificity of cVEMP for identifying EH were 49% and 95% [58]. In fact, the evidence is insufficient to determine whether VEMP is useful for diagnosing MD. Therefore, VEMP might not be used as a reliable marker to diagnose MD but could serve as an adjuvant measurement of vestibular dysfunction [59] or as a component of the inner ear battery test for mapping the topographic involvement of EH in MD [56].
In addition to evaluating the inner ear function in MD, there are some implications of VEMP to help clinicians and MD patients. For example, since saccule is the second most frequent site of EH, VEMP was also used to assess the stage of MD. In a study conducted by Young et al., the IAD ratio of VEMP increased in the advanced stage of MD [57]. They then concluded that VEMP might provide another aid for evaluating the staging of MD in addition to the hearing test. Interestingly, because the saccule is spare in acute low-tone hearing loss, VEMP test may also be used to differentiate acute low-tone hearing loss from MD [60,61]. Additionally, the VEMP could also help predict vertigo attack frequency [62] and identify asymptomatic EH in the unaffected ear for evolving bilateral MD [63]. Of note, the result of VEMP could differ between quiescence and acute attack status [64]. Therefore, heterogeneous stages and disease status should be put into consideration during the interpretation of VEMP in MD patients.
In recent years, several studies attempted to use VEMP to differentiate MD from VM. Reduced click-evoked cVEMP and oVEMP amplitudes were observed in MD and VM compared to the control group [65,66]. However, it was found that the MD group showed reduced tone-evoked amplitudes for oVEMP [65] and a higher prevalence of increased IAD ratios compared to the VM group [66]. Additionally, the affected ears of MD had higher percentages of absent cVEMP and oVEMP responses [67]. Moreover, the IAD ratio in MD patients appeared to increase or remain stable over time, whereas VM patients showed fluctuating or stable IAD ratios. These studies implied that VEMP might be a potential functional marker for differentiating MD and VM, but more studies are needed to clarify this.
2.4. Caloric Test/Video Head Impulse Test (vHIT)
The caloric test and vHIT are frequently used to evaluate the vestibular function of the semicircular canal. In the caloric test, the horizontal semicircular canals can be stimulated via warm and cool water or air and then the change in endolymph gravity or thermal-induced pressure is expected [68,69] to reflect the low-frequency stimulation of the horizontal semicircular canal. It was noted that about 47–67% of patients with MD have unilateral canal weakness [70,71]. Caloric responses are usually diminished during the attacks of MD [72], and the incidence of canal paresis in the caloric test is higher in the advanced stage of MD [73]. In contrast, the abnormality rate of vHIT, a test that uses high-frequency stimulation to evaluate the function of six semicircular canals, varied between studies. The fluctuation of vestibulo–ocular reflex (VOR) function during and between acute vertigo attacks might explain the inconsistent results of vHIT among studies [72,74,75]. In addition, no differences in abnormal vHIT results between different stages and duration of MD were observed [73,76]. In general, the caloric test can detect vestibular abnormalities better than vHIT, and the discordance between the caloric test and vHIT was thought to be a marker for MD [70,77].
Since MD and VM share similar clinical features of vestibular symptoms, both diseases could exhibit abnormalities in the caloric test compared to healthy controls. However, the incidences of an abnormal caloric test are higher in MD than in VM [66,78]. Although horizontal VOR (hVOR) deficit could be found in VM patients, its incidence is lower than in MD [78,79]. It was suggested that vestibular testing with the caloric test still seems more sensitive for detecting hVOR pathology than vHIT when differentiating MD from VM [79].
2.5. MRI
Since EH is one of the most predominant histological demonstrations in MD, Nakashima et al. first proffered the visualization of EH after the intratympanic injection of gadolinium under MRI imaging in humans. MRI with three-dimensional fluid-attenuated inversion recovery (3D-FLAIR) is considered one of the most straightforward tools to recognize the EH directly in certain MD cases [80]. Later on, the same team demonstrated the EH in MD after the intravenous administration of gadolinium [81,82,83]. Recently, new“HYDROPS” (a hybrid of the reversed image of positive endolymph signal and native image of positive perilymph signal) and the advanced techniques after the intravenous administration of single-dose gadodiamide were claimed to improve the contrast in the production of a positive endolymph image and positive perilymph images [84]. Meanwhile, several MRI grading systems were used to quantitatively calibrate the enlargement of the endolymphatic spaces. For example, the three-stage system to record the perilymphatic and vestibular space and the four-stage system to include cochlear EH to ameliorate MD diagnosis [85,86] were proposed. Recently, significant correlations between the hearing level and the EH degree when using MRI were shown [87,88,89,90]. On the other side, there was no significant relationship between the extent or duration of vertigo and EH presentation when using MRI [87,90]. Although EH in MRI was associated with vertigo attacks in MD patients [87,88,89,91,92], the stability of EH was still observed during and after vertigo attacks [93]. As a result, the specific MRI stage correlated to hearing level and vertigo attacks in MD patients has not yet been well established but complemented MRI images can provide additional information to evaluate EH in MD patients [88,90,94,95,96,97,98].
Although the EH can be visualized in MRI and is considered a specific characteristic in MD, some studies found that EH can also be present in some VM patients [99,100,101,102,103]. Particularly, EH shown in MRI often correlated with auditory symptoms both in MD and VM [102]. However, a higher incidence of EH was observed in MD compared to VM [102,103]. On the other hand, Leng et al. reported the significance of the anatomical variations of these two diseases via non-contrast MRI, indicating that compared with the VM patients, patients with unilateral MD exhibited a shorter distance between the vertical part of the posterior semicircular canal and the posterior fossa with poorer vestibular aqueducts visibility in MRI [104]. However, a low diagnostic value was noted using these radiological variations. As such, the usage of MRI as a single functional image marker to differentiate MD and VM remains insufficient and needs further evidence.
2.6. The Problems and Future Directions of Functional Markers for MD
Although traditional markers such as ECochG/caloric tests have been used in MD diagnosis and evaluation for years, newer examinations such as VEMP/vHIT have recently been largely investigated. However, the relatively low sensitivity of these tools (particularly for the early stage of MD) is still a major problem for clinicians when using these tests as markers for diagnosing MD. In particular, the fluctuating features of MD symptoms might affect the results of functional markers. Therefore, the diagnosis of MD still needs to depend on the clinical diagnosis criteria. Further studies are necessary to evaluate the results according to different stages, duration of disease and vertigo attack phase to elucidate the role of functional markers in MD. So far, these tests could still provide complementary information to assess the vestibular function in MD patients in the clinic [105]. In recent years, MRI has become a cutting-edge method for evaluating EH in MD. However, it should be noted that EH could also occur in the healthy ear and various otological disorders. In addition, not all types of EH can be visualized in MRI [95]. Moreover, the spatial resolution of MRI could affect the interpretation. Advanced techniques and protocols to improve image acquisition and interpretation in the future would be helpful for clinicians to use MRI to predict EH in MD.
3. Molecular Markers for MD
3.1. Immunological/Autoimmunity Markers
Many studies have revealed that some autoimmune diseases were associated with MD. For instance, higher prevalences of systemic lupus erythematosus, ankylosing spondylitis or rheumatoid arthritis were observed in MD groups than in the general population [10,106]. Therefore, autoimmunity was thought as one of the possible causes of MD and the endolymphatic sac may play an important role in the immuno-mediated reaction. It has been speculated that one-third of MD causes seem to be of an autoimmune origin [107].
Several immunological markers were investigated to see whether they could differentiate MD patients from healthy controls. For example, heat shock proteins (HSPs) play an essential role in chaperoning functions, protein folding and protecting cells from physiological and ototoxic stresses [108]. The immunoglobulin G (IgG) antibodies to HSP70 (68-kD protein) were elevated in 30% of the MD group compared to 5% in the control group [109]. However, the results were varied in other reports, showing that 7.7% to 27% of MD cases were positive for HSP70 antibodies [110,111]. In fact, the detection of HSP70 antibodies in diagnosing MD is controversial because of the high prevalence of antiHSP70 antibodies in healthy subjects and the lack of association with disease activity [112].
Circulating immune complexes (CICs), the molecules comprising multiple antigens and antibodies, can damage targeted organs or tissues via complement activation with or without deposition to modulate the inflammation and immune reaction. In MD patients, some studies found elevated serum CICs and postulated that endolymphatic sac function was interfered with by CICs [113,114,115,116,117]. Additionally, increased total IgG, C3 and anti-type II collagen antibodies were found in MD [114,118,119,120].
Immunoglobulin E (IgE), induced by type I allergic reactions, is another immunological marker investigated in MD. It was reported that elevated total serum IgE was observed in patients in MD [116,121]. Interestingly, a recent study revealed that a high level of IgE was noted in patients with acute low-tone sudden sensorineural hearing loss, and a higher IgE level was correlated with an enhanced SP/AP ratio, which might be used as a predictor for MD [122]. In addition, Zhang et al. observed that IgE was correlated to the grading of EH, hearing stage and the functional level of MD patients [123], implying the association of allergy with the clinical severity of MD.
3.2. Inflammatory Markers
Since the immune system could be involved in the pathogenesis of MD, inflammatory responses may possibly occur in the inner ear [124]. As a result, several studies have investigated different cytokines and chemokines to see if they can be used as possible markers for MD. For example, Frejo et al. revealed that MD patients had higher basal levels of IL-1β, IL-1RA, IL-6 and TNF-α compared to healthy controls [125]. In addition, they observed the bimodal distribution of IL-1β levels in two different subgroups of MD, suggesting that a subset of MD patients have higher basal levels of proinflammatory cytokines. Later, they observed that patients with MD or VM have different proinflammatory signatures and concluded that the cytokine panel with IL- 1β, CCL3, CCL22,and CXCL1 levels might help to differentiate MD from VM [126].
The association of immunological and inflammatory markers with hearing loss and the stage of MD was recently investigated by Zhang et al. [127]. They checked CIC, HSP70 and TNF-α in the serum in MD patients with different hearing levels and staging. In addition to the increased concentration of these markers in MD, they observed that the phase of the pure tone average was positively associated with the concentration of CIC, HSP70 and TNF-α. In addition, the concentration of these markers was also increased in the group with severe EH compared to mild and moderate EH, implying that these immunological and inflammatory markers have the potential to reflect the EH severity and staging of MD.
3.3. Protein Signatures
The development of proteomics and protein array analysis in recent years has made it possible to survey possible protein markers in diseases. Kim et al. used Protoarray to investigate the proteins in sera from MD patients and controls. They observed higher signals of immunoglobulin heavy constant gamma 1 (IGHG1), the regulator of G-protein signaling 10 (RGS10), transcript variant 2, chromosome 2 open reading frame 34 (C2orf34), and SH3-domain GRB2-like endophilin B1 (SH3GLB1) with 80% sensitivity and specificity [128]. The authors imply that multiple antibodies or antigens might cause autoimmune reactions in the inner ear of MD.
In another study using proteomics to analyze the plasma from 15 MD patients and 12 healthy controls, upregulated complement factor-B and H, fibrinogen α-chain, β-actin, pigment epithelium-derived factor and fibrinogen γ-chain were found in MD, while vitamin D-binding protein, apolipoprotein A-1 and β-2-glycoprotein were downregulated compared to the control group [129]. They further used Western blotting to investigate plasma protein expression in different stages of MD patients [130]. They observed increased fibrinogen α- and γ-chain expression in stage III and decreased β-2-glycoprotein expression in stage IV patients. Stage I individuals have a higher expression of complement factor H and B proteins. They concluded that a set of plasma proteins might be used as a tool for a biomarker-oriented diagnosis and MD staging.
3.4. Vasopressin
Vasopressin, also known as antidiuretic hormone (ADH) or arginine vasopressin (AVP), is a nonapeptide that acts on water metabolism via vasopressin receptors. It was hypothesized that plasma vasopressin elevation and subsequent vasopressin type-2 receptor (V2R)-cyclic AMP (cAMP)-protein kinase A (PKA) activation in the endolymphatic sac might lead to the intracellular translocation of aquaporin-2 (AQP2) from the luminal side to the basolateral side with endosomal trapping, resulting in EH in the inner ear [131].
In previous decades, vasopressin has probably been the most investigated marker in MD. Takeda et al. first compared plasma vasopressin levels in patients with MD with other types of vertigo. They observed that vasopressin levels were higher in MD patients. In addition, vasopressin was significantly higher in the acute phase than remission phase [132]. Later on, Aoki et al. also showed increased vasopressin levels, osmolality and stress scores in the acute phase of MD [133,134]. However, there is no significant correlation between vasopressin levels, osmolality and stress score. Particularly, the patients with abnormally high vasopressin in the acute phase were resistant to conservative treatments for vertigo attacks [135]. They thus thought that the elevation of vasopressin in MD might be related to the pathogenesis of MD attacks.
In contrast, there are some studies showing conflicting results. Lim et al. compared the vasopressin levels of unilateral MD patients within one week of acute vertigo with 31 healthy volunteers, and they did not find statistical differences [136]. Similarly, Hornibrook et al. evaluated vasopressin levels in 80 patients with MD who were diagnosed using conventional symptoms and ECochG [137]. They still could not find the differences between the MD subjects and the normal controls. In addition, vasopressin levels did not correlate to the stage of MD. A recent meta-analysis revealed that the discrepancy of vasopressin levels between previous studies might be due to a couple of selection biases and confounding factors: unilateral vs. bilateral MD, acute vs. remission phase, the sensitivity of measurement and psychological stress might affect the levels of vasopressin [138].
Although there is no consensus regarding the usage of vasopressin as a diagnostic marker for MD, most publications agree that vasopressin might reflect the status of MD attack in MD patients [8], probably because the overexpression and hyperactivity of V2R in the endolymphatic sac of MD patients develop EH and vertigo attacks after vasopressin elevation [139]. A pilot study from Kitahara et al. observed that interventions to decrease vasopressin levels by adequate water intake, tympanic ventilation tube insertion and sleeping in darkness are useful to control MD [140]. Interestingly, the authors also found that in patients with intractable MD, vasopressin levels were reduced after endolymphatic sac surgery, and long-lasting plasma low vasopressin levels were associated with good surgical outcomes. Their findings implied that plasma vasopressin levels might be a feasible marker to reflect the disease status of MD [131].
3.5. Circadian Clock Genes
The circadian clock is present in eukaryotes with a 24 h cycle, and daily rhythmic changes can be observed in many physiological processes. In mammals, the circadian clock genes regulate circadian rhythms through transcriptional-translational feedback loops. Once the circadian clock is dysregulated or disrupted due to light changes, the expression of circadian clock genes is altered [141]. Evidence shows that circadian disruption is associated with an increased risk of several diseases, and altered circadian clock gene expression was frequently observed in patients with these disorders [142,143].
Previous studies have proven that circadian clock genes have time-dependent variation patterns in the peripheral blood leukocytes of healthy subjects [142,144]. Because the precipitating factors for vertigo attacks in MD, such as a high-salt diet, caffeine and stress, might affect the circadian clock [145], we recently investigated the expression of circadian clock genes from the peripheral blood leukocytes of unilateral MD patients with recent vertigo attacks within one week to reflect the gene expression in the active status of MD [146]. We observed significantly decreased PER1 and increased CLOCK gene expression in the MD group compared to a healthy control group using real-time quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) analysis. Particularly, the area under the receiver operating characteristic (ROC) curve (AUC) is higher in the PER1 gene to predict the diagnosis of MD (Figure 1). Further immunocytochemical analysis for PER1 in PB leukocytes also revealed the lower percentage of PER1-positive cells in the peripheral blood of MD patients [146]. These results implied that the expression of PER1 might be a potential marker of MD.
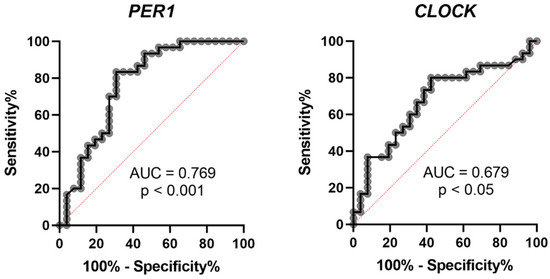
Figure 1.
Receiver operating characteristic (ROC) curve of PER1 and CLOCK to predict the diagnosis of MD. AUC: area under the curve.
Another implication of PER1 as a marker of MD is to see if PER1 expression is associated with disease severity. In the subgroup analyses of the circadian clock genes in groups with different dizziness handicaps and hearing levels, the expression of PER1 was not different between the patients with mild-to-moderate and severe dizziness handicaps but is significantly lower in patients with stage 3 and 4 than with stage 1 and 2 [146]. The down expression of PER1 was also significantly correlated to the pure tone average and speech reception threshold of the affected ear, implying that PER1 might also be a potential marker for evaluating the hearing levels of MD patients.
The mechanism of altered PER1 in the pathogenesis of MD and its effects on hearing levels still need further investigation. We hypothesized that since PER1 in PB leukocytes may reflect the human circadian system [144], its dysregulation might affect cellular glutathione peroxidase-related reactive oxygen species fluctuation and augment oxidative stress in the hair cells [147]. In addition, our data were evaluated using MD patients in the acute phase, which was deemed to be a consequence of precipitating factors; it is reasonable to speculate that PER1 expression may be different between the patients in acute and remission phases. Future exploration is necessary to evaluate the role of circadian clock genes as markers of MD.
3.6. The Problems and Future Directions of Molecular Markers for MD
Although the aforementioned molecular markers have been investigated based on the possible mechanisms of MD, such as autoimmunity, inflammation, hormone and circadian clock alteration, the design of most studies is cross-sectional. The reason is probably that the diagnosis of definite MD needs long-term follow-up, and longitudinal studies to evaluate the potential molecular markers are difficult. In addition, the expression of the molecular markers might be confounded by environmental factors. For example, plasma vasopressin levels might change due to stress and the circadian clock. Furthermore, different disease statuses, such as active vs. remission phase, unilateral MD vs. bilateral MD and the severity of EH and hearing loss might affect the results of these molecular markers. Therefore, a longitudinal design to evaluate targeted molecular markers and appropriately analyze the results by subgrouping MD patients would be mandatory to develop suitable molecular markers for MD. Last, since the specificity of the molecular markers from the peripheral blood may be limited by environmental factors, it would be more valuable to check the expression of targeted markers in the inner ear fluid (during the endolymphatic sac surgery) to accurately understand the role of these markers in the etiopathogenesis of MD [128].
In recent years, there has been a growing theory about the connection between migraine and MD. It was hypothesized that because of similar clinical manifestations, epidemiological factors and pathophysiological considerations [148], MD and migraine may be different regional manifestations of the same pathology. Another researcher also proposed that MD might be a “cochleovestibular migraine”, which resembles a combination of symptoms from cochlear and vestibular migraine [12]. If the hypothesis is true, the molecular markers for migraine and MD might be similar if the samples were gathered from the peripheral blood. Indeed, migraineurs had higher levels of serum inflammatory markers such as proinflammatory cytokines [149,150]. Increased plasma vasopressin levels are observed in migraine patients during an attack [151,152]. However, it is not clear whether the migraine biomarkers such as glutamate, calcitonin gene-related peptide (CGRP) and pituitary adenylate cyclase-activating peptide-38 (PACAP-38) are also markers of MD or can be used to differentiate migraine from MD [153]. The comparison of MD and migraine markers between the patients with MD and VM will help to elucidate the interplay between migraine and MD. In addition, since the diagnosis of MD and VM is based on the clinical diagnostic criteria, the development of a useful molecular marker to tell MD from VM would be a new direction toward the precise diagnosis of MD.
4. Conclusions and Future Perspectives
MD is a disease that is difficult to diagnose, particularly in the early stage wherein not all of the typical symptoms are present. The diagnosis of MD is based on the clinical diagnostic criteria. However, the development of functional and molecular markers for MD is important for helping clinicians diagnose MD correctly and evaluate inner ear status. Table 2 summarizes the markers for MD based on different aspects. For functional markers, ECochG and MRI have a relatively high sensitivity. Therefore, these tests could be used to confirm the EH in MD in ambiguous cases and those with atypical symptoms. The incidence of abnormalities in PTA, ECochG, VEMP, caloric test and MRI are higher in patients with high levels of hearing loss. Therefore, the positive results in these tests could reflect the advanced stages of MD. However, we shall keep in mind that the results of VEMP, caloric test and vHIT may be different in active and quiescence status. As a result, the abnormalities of these tests may reflect recent vertigo attacks. All functional markers might help clinicians to differentiate between MD and VM. For molecular markers, the majority of them might differentiate MD and healthy control as well as correlate to the hearing stage. However, further investigation is necessary to elucidate their expression between and during vertigo attacks and to know whether they are helpful in differentiating MD and VM. Looking ahead, although we have numerous papers to investigate the role of functional and molecular markers in MD, the duplication and verification of these markers are mandatory for proof of future usefulness in the clinic. There is still a long way to go to obtain ideal markers for MD right now, but we could still combine several markers to help diagnose and evaluate MD status. For instance, the combination of function markers, molecular markers, and 3D-FLAIR MRI may be a better strategy for helping in the diagnosis and evaluation of MD. However, before a perfect protocol of markers is developed, clinicians shall keep in mind that it is still essential to diagnose MD using the latest diagnostic criteria.

Table 2.
Summary of functional and molecular markers for MD.
Author Contributions
Conceptualization, C.-H.Y.; writing—original draft preparation, C.-H.Y., K.-H.L.; writing—review, editing C.-H.Y., K.-H.L., M.-Y.Y., C.-F.H.; supervision, C.-H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
C.-H.Y.’s work on circadian clock genes in Meniere’s disease is funded by grants from the Ministry of Science and Technology of Taiwan (MOST 105-2314-B-182A-074-) and Chang Gung Memorial Hospital (CMRPG8H1431).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available to the corresponding authors upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Harris, J.P.; Alexander, T.H. Current-day prevalence of Ménière’s syndrome. Audiol. Neurootol. 2010, 15, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Paparella, M.M. Pathology of Meniere’s disease. Ann. Otol. Rhinol. Laryngol. Suppl. 1984, 112, 31–35. [Google Scholar] [CrossRef]
- Gluth, M.B. On the Relationship Between Menière’s Disease and Endolymphatic Hydrops. Otol. Neurotol. 2020, 41, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Merchant, S.N.; Adams, J.C.; Nadol, J.B., Jr. Pathophysiology of Meniere’s syndrome: Are symptoms caused by endolymphatic hydrops? Otol. Neurotol. 2005, 26, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Foster, C.A.; Breeze, R.E. Endolymphatic hydrops in Ménière’s disease: Cause, consequence, or epiphenomenon? Otol. Neurotol. 2013, 34, 1210–1214. [Google Scholar] [CrossRef] [PubMed]
- Bächinger, D.; Luu, N.N.; Kempfle, J.S.; Barber, S.; Zürrer, D.; Lee, D.J.; Curtin, H.D.; Rauch, S.D.; Nadol, J.B., Jr.; Adams, J.C.; et al. Vestibular Aqueduct Morphology Correlates With Endolymphatic Sac Pathologies in Menière’s Disease-A Correlative Histology and Computed Tomography Study. Otol. Neurotol. 2019, 40, e548–e555. [Google Scholar] [CrossRef]
- Sando, I.; Ikeda, M. Pneumatization and thickness of the petrous bone in patients with Meniere’s disease. A histopathological study. Ann. Otol. Rhinol. Laryngol. Suppl. 1985, 118, 2–5. [Google Scholar] [CrossRef]
- Takeda, T.; Takeda, S.; Kakigi, A.; Okada, T.; Nishioka, R.; Taguchi, D.; Nishimura, M.; Nakatani, H. Hormonal aspects of Ménière’s disease on the basis of clinical and experimental studies. ORL J. Otorhinolaryngol Relat. Spec. 2010, 71 (Suppl. 1), 1–9. [Google Scholar] [CrossRef]
- Kakigi, A.; Takeda, T. Antidiuretic hormone and osmolality in patients with Ménière’s disease. ORL J. Otorhinolaryngol. Relat. Spec. 2009, 71, 11–13. [Google Scholar] [CrossRef]
- Gazquez, I.; Soto-Varela, A.; Aran, I.; Santos, S.; Batuecas, A.; Trinidad, G.; Perez-Garrigues, H.; Gonzalez-Oller, C.; Acosta, L.; Lopez-Escamez, J.A. High prevalence of systemic autoimmune diseases in patients with Menière’s disease. PLoS ONE 2011, 6, e26759. [Google Scholar] [CrossRef]
- Koo, J.W.; Oh, S.H.; Chang, S.O.; Park, M.H.; Lim, M.J.; Yoo, T.J.; Kim, C.S. Association of HLA-DR and type II collagen autoimmunity with Meniere’s disease. Tissue Antigens 2003, 61, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Sarna, B.; Abouzari, M.; Lin, H.W.; Djalilian, H.R. A hypothetical proposal for association between migraine and Meniere’s disease. Med. Hypotheses 2020, 134, 109430. [Google Scholar] [CrossRef] [PubMed]
- Ghavami, Y.; Mahboubi, H.; Yau, A.Y.; Maducdoc, M.; Djalilian, H.R. Migraine features in patients with Meniere’s disease. Laryngoscope 2016, 126, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Martinez, A.; Lopez-Escamez, J.A. Genetic architecture of Meniere’s disease. Hearth Res. 2020, 397, 107872. [Google Scholar] [CrossRef] [PubMed]
- Takeda, T.; Takeda, S.; Egami, N.; Kakigi, A.; Nishioka, R.; Yamasoba, T. Type 1 allergy-induced endolymphatic hydrops and the suppressive effect of leukotriene receptor antagonist. Otol. Neurotol. 2012, 33, 886–890. [Google Scholar] [CrossRef]
- Rizk, H.G.; Mehta, N.K.; Qureshi, U.; Yuen, E.; Zhang, K.; Nkrumah, Y.; Lambert, P.R.; Liu, Y.F.; McRackan, T.R.; Nguyen, S.A.; et al. Pathogenesis and Etiology of Meniere Disease: A Scoping Review of a Century of Evidence. JAMA Otolaryngol. Head Neck Surg. 2022, 148, 360–368. [Google Scholar] [CrossRef]
- Goebel, J.A. 2015 Equilibrium Committee amendment to the 1995 AAO-HNS guidelines for the definition of Meniere’s disease. Otolaryngol. Head Neck Surg. 2016, 154, 403–404. [Google Scholar] [CrossRef]
- Lopez-Escamez, J.A.; Carey, J.; Chung, W.-H.; Goebel, J.A.; Magnusson, M.; Mandalà, M.; Newman-Toker, D.E.; Strupp, M.; Suzuki, M.; Trabalzini, F. Diagnostic criteria for Menière’s disease. J. Vestib. Res. 2015, 25, 1–7. [Google Scholar] [CrossRef]
- Nakashima, T.; Pyykko, I.; Arroll, M.A.; Casselbrant, M.L.; Foster, C.A.; Manzoor, N.F.; Megerian, C.A.; Naganawa, S.; Young, Y.H. Meniere’s disease. Nat. Rev. Dis Prim. 2016, 2, 16028. [Google Scholar] [CrossRef] [PubMed]
- Committee on Hearing and Equilibrium. Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Menière’s disease. Otolaryngol. Head Neck Surg. 1995, 113, 181–185. [Google Scholar]
- Chen, H.L.; Tan, C.T.; Lai, J.T.; Liu, T.C. Long-term hearing progression of Ménière’s disease. Ear Nose Throat J. 2022, 1455613221074149. [Google Scholar] [CrossRef] [PubMed]
- Sato, G.; Sekine, K.; Matsuda, K.; Ueeda, H.; Horii, A.; Nishiike, S.; Kitahara, T.; Uno, A.; Imai, T.; Inohara, H.; et al. Long-term prognosis of hearing loss in patients with unilateral Ménière’s disease. Acta Otolaryngol. 2014, 134, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Huppert, D.; Strupp, M.; Brandt, T. Long-term course of Menière’s disease revisited. Acta Otolaryngol. 2010, 130, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Wang, D.; Ren, T.; Wang, W. Auditory Manifestations of Vestibular Migraine. Front. Neurol. 2022, 13, 944001. [Google Scholar] [CrossRef] [PubMed]
- Fushiki, H.; Junicho, M.; Kanazawa, Y.; Aso, S.; Watanabe, Y. Prognosis of sudden low-tone loss other than acute low-tone sensorineural hearing loss. Acta Otolaryngol. 2010, 130, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Committee on Hearing and Equilibrium. Report of Subcommittee on Equilibrium and its Measurement. Meniere’s disease: Criteria for diagnosis and evaluation of therapy for reporting. Trans. Am. Acad. Ophthalmol. Otolaryngol. 1972, 76, 1462–1464. [Google Scholar]
- Yamasoba, T.; Kikuchi, S.; Sugasawa, M.; Yagi, M.; Harada, T. Acute low-tone sensorineural hearing loss without vertigo. Arch. Otolaryngol. Head Neck Surg. 1994, 120, 532–535. [Google Scholar] [CrossRef]
- Junicho, M.; Aso, S.; Fujisaka, M.; Watanabe, Y. Prognosis of low-tone sudden deafness—Does it inevitably progress to Meniere’s disease? Acta Otolaryngol. 2008, 128, 304–308. [Google Scholar] [CrossRef]
- Xue, J.; Ma, X.; Lin, Y.; Shan, H.; Yu, L. Audiological Findings in Patients with Vestibular Migraine and Migraine: History of Migraine May Be a Cause of Low-Tone Sudden Sensorineural Hearing Loss. Audiol. Neuro-Otol. 2020, 25, 209–214. [Google Scholar] [CrossRef]
- Lai, J.T.; Liu, T.C. Proposal for a New Diagnosis for Cochlear Migraine. JAMA Otolaryngol. Head Neck Surg. 2018, 144, 185–186. [Google Scholar] [CrossRef]
- Pyykkö, I.; Nakashima, T.; Yoshida, T.; Zou, J.; Naganawa, S. Meniere’s disease: A reappraisal supported by a variable latency of symptoms and the MRI visualisation of endolymphatic hydrops. BMJ Open 2013, 3, e001555. [Google Scholar] [CrossRef] [PubMed]
- Perez-Garrigues, H.; Lopez-Escamez, J.A.; Perez, P.; Sanz, R.; Orts, M.; Marco, J.; Barona, R.; Tapia, M.C.; Aran, I.; Cenjor, C.; et al. Time course of episodes of definitive vertigo in Meniere’s disease. Arch. Otolaryngol. Head Neck Surg. 2008, 134, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- Green, J.D., Jr.; Blum, D.J.; Harner, S.G. Longitudinal followup of patients with Menière’s disease. Otolaryngol. Head Neck Surg. 1991, 104, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Havia, M.; Kentala, E. Progression of symptoms of dizziness in Ménière’s disease. Arch. Otolaryngol. Head Neck Surg. 2004, 130, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Gibson, W.P.; Moffat, D.A.; Ramsden, R.T. Clinical electrocochleography in the diagnosis and management of Meneère’s disorder. Audiology 1977, 16, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Ziylan, F.; Smeeing, D.P.; Stegeman, I.; Thomeer, H.G. Click Stimulus Electrocochleography Versus MRI With Intratympanic Contrast in Ménière’s Disease: A Systematic Review. Otol. Neurotol. 2016, 37, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.H.; Kim, K.W.; Chang, J.; Jun, H.S.; Kwon, E.H.; Choi, J.Y.; Im, G.J.; Chae, S.W.; Jung, H.H.; Choi, J. Can we use electrocochleography as a clinical tool in the diagnosis of Meniere’s disease during the early symptomatic period? Acta Otolaryngol. 2014, 134, 771–775. [Google Scholar] [CrossRef]
- Orchik, D.J.; Shea, J.J., Jr.; Ge, N.N. Summating potential and action potential ratio in Meniere’s disease before and after treatment. Am. J. Otol. 1998, 19, 478–482. [Google Scholar]
- Kim, H.H.; Kumar, A.; Battista, R.A.; Wiet, R.J. Electrocochleography in patients with Meniere’s disease. Am. J. Otolaryngol. 2005, 26, 128–131. [Google Scholar] [CrossRef]
- Pappas, D.G., Jr.; Pappas, D.G., Sr.; Carmichael, L.; Hyatt, D.P.; Toohey, L.M. Extratympanic electrocochleography: Diagnostic and predictive value. Am. J. Otol. 2000, 21, 81–87. [Google Scholar] [CrossRef]
- Ghosh, S.; Gupta, A.K.; Mann, S.S. Can electrocochleography in Meniere’s disease be noninvasive? J. Otolaryngol. 2002, 31, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Hornibrook, J.; Kalin, C.; Lin, E.; O’Beirne, G.A.; Gourley, J. Transtympanic Electrocochleography for the Diagnosis of Ménière’s Disease. Int. J. Otolaryngol. 2012, 2012, 852714. [Google Scholar] [CrossRef] [PubMed]
- Gibson, W.P. A comparison of two methods of using transtympanic electrocochleography for the diagnosis of Meniere’s disease: Click summating potential/action potential ratio measurements and tone burst summating potential measurements. Acta Otolaryngol. Suppl. 2009, 129, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Hornibrook, J.; Flook, E.; Greig, S.; Babbage, M.; Goh, T.; Coates, M.; Care, R.; Bird, P. MRI Inner Ear Imaging and Tone Burst Electrocochleography in the Diagnosis of Ménière’s Disease. Otol. Neurotol. 2015, 36, 1109–1114. [Google Scholar] [CrossRef]
- Al-momani, M.O.; Ferraro, J.A.; Gajewski, B.J.; Ator, G. Improved sensitivity of electrocochleography in the diagnosis of Meniere’s disease. Int. J. Audiol. 2009, 48, 811–819. [Google Scholar] [CrossRef]
- Iseli, C.; Gibson, W. A comparison of three methods of using transtympanic electrocochleography for the diagnosis of Meniere’s disease: Click summating potential measurements, tone burst summating potential amplitude measurements, and biasing of the summating potential using a low frequency tone. Acta Otolaryngol. 2010, 130, 95–101. [Google Scholar]
- Lopes Kde, C.; Munhoz, M.S.; Santos, M.A.; Moraes, M.F.; Chaves, A.G. Graphic angle measure as an electrocochleography evaluation parameter. Braz. J. Otorhinolaryngol. 2011, 77, 214–220. [Google Scholar] [CrossRef]
- Ge, X.; Shea, J.J., Jr. Transtympanic electrocochleography: A 10-year experience. Otol. Neurotol. 2002, 23, 799–805. [Google Scholar] [CrossRef]
- Takeda, T.; Kakigi, A. The clinical value of extratympanic electrocochleography in the diagnosis of Ménière’s disease. ORL J. Otorhinolaryngol. Relat. Spec. 2010, 72, 196–204. [Google Scholar] [CrossRef]
- Martines, F.; Dispenza, F.; Montalbano, C.; Priola, R.; Torrente, A.; La Gumina, R.; Brighina, F.; Galletti, F.; Salvago, P. Comparison of Electrocochleography and Video Head Impulse Test findings in Vestibular Migraine and Ménière Disease: A Preliminary Study. J. Int Adv. Otol. 2020, 16, 183–189. [Google Scholar] [CrossRef]
- Yollu, U.; Uluduz, D.U.; Yilmaz, M.; Yener, H.M.; Akil, F.; Kuzu, B.; Kara, E.; Hayir, D.; Ceylan, D.; Korkut, N. Vestibular migraine screening in a migraine-diagnosed patient population, and assessment of vestibulocochlear function. Clin. Otolaryngol. 2017, 42, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Curthoys, I.S. A critical review of the neurophysiological evidence underlying clinical vestibular testing using sound, vibration and galvanic stimuli. Clin. Neurophysiol. 2010, 121, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Maheu, M.; Alvarado-Umanzor, J.M.; Delcenserie, A.; Champoux, F. The Clinical Utility of Vestibular-Evoked Myogenic Potentials in the Diagnosis of Ménière’s Disease. Front. Neurol. 2017, 8, 415. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Weng, W.J.; Jaw, F.S.; Young, Y.H. Ocular and cervical vestibular-evoked myogenic potentials: A study to determine whether air- or bone-conducted stimuli are optimal. Ear Hearth 2010, 31, 283–288. [Google Scholar] [CrossRef]
- Taylor, R.L.; Wijewardene, A.A.; Gibson, W.P.; Black, D.A.; Halmagyi, G.M.; Welgampola, M.S. The vestibular evoked-potential profile of Ménière’s disease. Clin. Neurophysiol. 2011, 122, 1256–1263. [Google Scholar] [CrossRef]
- Huang, C.H.; Wang, S.J.; Young, Y.H. Localization and prevalence of hydrops formation in Ménière’s disease using a test battery. Audiol. Neuro-Otol. 2011, 16, 41–48. [Google Scholar] [CrossRef]
- Young, Y.H.; Huang, T.W.; Cheng, P.W. Assessing the stage of Meniere’s disease using vestibular evoked myogenic potentials. Arch. Otolaryngol. Head Neck Surg. 2003, 129, 815–818. [Google Scholar] [CrossRef]
- Zhang, S.; Leng, Y.; Liu, B.; Shi, H.; Lu, M.; Kong, W. Diagnostic Value of Vestibular Evoked Myogenic Potentials in Endolymphatic Hydrops: A Meta-Analysis. Sci Rep. 2015, 5, 14951. [Google Scholar] [CrossRef]
- Fife, T.D.; Colebatch, J.G.; Kerber, K.A.; Brantberg, K.; Strupp, M.; Lee, H.; Walker, M.F.; Ashman, E.; Fletcher, J.; Callaghan, B.; et al. Practice guideline: Cervical and ocular vestibular evoked myogenic potential testing: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2017, 89, 2288–2296. [Google Scholar] [CrossRef]
- Wu, C.L.; Young, Y.H. Vestibular evoked myogenic potentials in acute low-tone sensorineural hearing loss. Laryngoscope 2004, 114, 2172–2175. [Google Scholar] [CrossRef]
- Young, Y.H.; Wu, C.C.; Wu, C.H. Augmentation of vestibular evoked myogenic potentials: An indication for distended saccular hydrops. Laryngoscope 2002, 112, 509–512. [Google Scholar] [CrossRef]
- Wu, P.H.; Chang, C.M.; Lo, W.C.; Wang, C.T.; Wen, M.H.; Huang, T.W.; Young, Y.H.; Cheng, P.W. Prediction of Unilateral Meniere’s Disease Attack Using Inner Ear Test Battery. Ear Hearth 2020, 41, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.Y.; Timmer, F.C.; Oriel, B.S.; Zhou, G.; Guinan, J.J.; Kujawa, S.G.; Herrmann, B.S.; Merchant, S.N.; Rauch, S.D. Vestibular evoked myogenic potentials (VEMP) can detect asymptomatic saccular hydrops. Laryngoscope 2006, 116, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Manzari, L.; Tedesco, A.R.; Burgess, A.M.; Curthoys, I.S. Ocular and cervical vestibular-evoked myogenic potentials to bone conducted vibration in Ménière’s disease during quiescence vs during acute attacks. Clin. Neurophysiol. 2010, 121, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Zuniga, M.G.; Janky, K.L.; Schubert, M.C.; Carey, J.P. Can vestibular-evoked myogenic potentials help differentiate Ménière disease from vestibular migraine? Otolaryngol. Head Neck Surg. 2012, 146, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Egami, N.; Fujimoto, C.; Kinoshita, M.; Yamasoba, T.; Iwasaki, S. Vestibular Evoked Myogenic Potentials in Vestibular Migraine: Do They Help Differentiating From Menière’s Disease? Ann. Otol. Rhinol. Laryngol. 2016, 125, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Rizk, H.G.; Liu, Y.F.; Strange, C.C.; Van Ausdal, C.H.; English, R.C.; McRackan, T.R.; Meyer, T.A. Predictive Value of Vestibular Evoked Myogenic Potentials in the Diagnosis of Menière’s Disease and Vestibular Migraine. Otol. Neurotol. 2020, 41, 828–835. [Google Scholar] [CrossRef]
- O’Neill, G. The caloric stimulus: Mechanisms of heat transfer. Br. J. Audiol. 1995, 29, 87–94. [Google Scholar] [CrossRef]
- Valli, P.; Buizza, A.; Botta, L.; Zucca, G.; Ghezzi, L.; Valli, S. Convection, buoyancy or endolymph expansion: What is the actual mechanism responsible for the caloric response of semicircular canals? J. Vestib Res. 2002, 12, 155–165. [Google Scholar] [CrossRef]
- Cordero-Yanza, J.A.; Arrieta Vázquez, E.V.; Hernaiz Leonardo, J.C.; Mancera Sánchez, J.; Hernández Palestina, M.S.; Pérez-Fernández, N. Comparative study between the caloric vestibular and the video-head impulse tests in unilateral Menière’s disease. Acta Otolaryngol. 2017, 137, 1178–1182. [Google Scholar] [CrossRef]
- Shin, J.E.; Kim, C.H.; Park, H.J. Vestibular abnormality in patients with Meniere’s disease and migrainous vertigo. Acta Otolaryngol. 2013, 133, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.U.; Kim, H.J.; Koo, J.W.; Kim, J.S. Comparison of caloric and head-impulse tests during the attacks of Meniere’s disease. Laryngoscope 2017, 127, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Limviriyakul, S.; Luangsawang, C.; Suvansit, K.; Prakairungthong, S.; Thongyai, K.; Atipas, S. Video head impulse test and caloric test in definite Ménière’s disease. Eur. Arch. Otorhinolaryngol. 2020, 277, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Kaci, B.; Nooristani, M.; Mijovic, T.; Maheu, M. Usefulness of Video Head Impulse Test Results in the Identification of Meniere’s Disease. Front. Neurol. 2020, 11, 581527. [Google Scholar] [CrossRef]
- Mahringer, A.; Rambold, H.A. Caloric test and video-head-impulse: A study of vertigo/dizziness patients in a community hospital. Eur. Arch. Otorhinolaryngol. 2014, 271, 463–472. [Google Scholar] [CrossRef]
- van Esch, B.F.; Abolhosseini, K.; Masius-Olthof, S.; van der Zaag-Loonen, H.J.; van Benthem, P.P.G.; Bruintjes, T.D. Video-head impulse test results in patients with Menière’s disease related to duration and stage of disease. J. Vestib Res. 2018, 28, 401–407. [Google Scholar] [CrossRef]
- Hannigan, I.P.; Welgampola, M.S.; Watson, S.R.D. Dissociation of caloric and head impulse tests: A marker of Meniere’s disease. J. Neurol. 2021, 268, 431–439. [Google Scholar] [CrossRef]
- Blödow, A.; Heinze, M.; Bloching, M.B.; von Brevern, M.; Radtke, A.; Lempert, T. Caloric stimulation and video-head impulse testing in Ménière’s disease and vestibular migraine. Acta Otolaryngol. 2014, 134, 1239–1244. [Google Scholar] [CrossRef]
- Yilmaz, M.S.; Egilmez, O.K.; Kara, A.; Guven, M.; Demir, D.; Genc Elden, S. Comparison of the results of caloric and video head impulse tests in patients with Meniere’s disease and vestibular migraine. Eur. Arch. Otorhinolaryngol. 2021, 278, 1829–1834. [Google Scholar] [CrossRef]
- Nakashima, T.; Naganawa, S.; Sugiura, M.; Teranishi, M.; Sone, M.; Hayashi, H.; Nakata, S.; Katayama, N.; Ishida, I.M. Visualization of endolymphatic hydrops in patients with Meniere’s disease. Laryngoscope 2007, 117, 415–420. [Google Scholar] [CrossRef]
- Naganawa, S.; Yamazaki, M.; Kawai, H.; Bokura, K.; Sone, M.; Nakashima, T. Imaging of Ménière’s disease after intravenous administration of single-dose gadodiamide: Utility of subtraction images with different inversion time. Magn. Reson. Med. Sci. 2012, 11, 213–219. [Google Scholar] [CrossRef]
- Naganawa, S.; Suzuki, K.; Nakamichi, R.; Bokura, K.; Yoshida, T.; Sone, M.; Homann, G.; Nakashima, T.; Ikeda, M. Semi-quantification of endolymphatic size on MR imaging after intravenous injection of single-dose gadodiamide: Comparison between two types of processing strategies. Magn. Reson. Med. Sci. 2013, 12, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Naganawa, S.; Teranishi, M.; Tagaya, M.; Nakata, S.; Sone, M.; Otake, H.; Kato, K.; Iwata, T.; Nishio, N. Endolymphatic hydrops revealed by intravenous gadolinium injection in patients with Ménière’s disease. Acta Otolaryngol. 2010, 130, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Naganawa, S.; Kawai, H.; Taoka, T.; Sone, M. Improved HYDROPS: Imaging of Endolymphatic Hydrops after Intravenous Administration of Gadolinium. Magn. Reson. Med. Sci. 2017, 16, 357–361. [Google Scholar] [CrossRef]
- Bernaerts, A.; Vanspauwen, R.; Blaivie, C.; van Dinther, J.; Zarowski, A.; Wuyts, F.L.; Vanden Bossche, S.; Offeciers, E.; Casselman, J.W.; De Foer, B. The value of four stage vestibular hydrops grading and asymmetric perilymphatic enhancement in the diagnosis of Menière’s disease on MRI. Neuroradiology 2019, 61, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Baráth, K.; Schuknecht, B.; Naldi, A.M.; Schrepfer, T.; Bockisch, C.J.; Hegemann, S.C. Detection and grading of endolymphatic hydrops in Menière disease using MR imaging. AJNR Am. J. Neuroradiol. 2014, 35, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Jasińska, A.; Lachowska, M.; Wnuk, E.; Pierchała, K.; Rowiński, O.; Niemczyk, K. Correlation between magnetic resonance imaging classification of endolymphatic hydrops and clinical manifestations and audiovestibular test results in patients with definite Ménière’s disease. Auris Nasus Larynx 2022, 49, 34–45. [Google Scholar] [CrossRef]
- Han, S.C.; Kim, Y.S.; Kim, Y.; Lee, S.-Y.; Song, J.-J.; Choi, B.Y.; Kim, J.-S.; Bae, Y.J.; Koo, J.-W. Correlation of clinical parameters with endolymphatic hydrops on MRI in Meniere’s disease. Front. Neurol. 2022, 13, 937703. [Google Scholar] [CrossRef]
- Shi, S.; Guo, P.; Li, W.; Wang, W. Clinical Features and Endolymphatic Hydrops in Patients With MRI Evidence of Hydrops. Ann. Otol. Rhinol. Laryngol. 2019, 128, 286–292. [Google Scholar] [CrossRef]
- Zhang, W.; Hui, L.; Zhang, B.; Ren, L.; Zhu, J.; Wang, F.; Li, S. The Correlation Between Endolymphatic Hydrops and Clinical Features of Meniere Disease. Laryngoscope 2021, 131, E144–E150. [Google Scholar] [CrossRef]
- Nakada, T.; Teranishi, M.; Sugiura, S.; Uchida, Y.; Naganawa, S.; Sone, M. Imaging of endolymphatic hydrops on a vertigo attack of Meniere’s disease. Nagoya J. Med. Sci. 2021, 83, 209–216. [Google Scholar]
- Chen, W.; Geng, Y.; Niu, Y.; Lin, M.; Lin, N.; Sha, Y. Endolymphatic Hydrops Magnetic Resonance Imaging in Menire’s Disease Patients on a Vertigo Attack. Otol. Neurotol. 2022, 43, 489–493. [Google Scholar] [CrossRef]
- Fukushima, M.; Akahani, S.; Inohara, H.; Takeda, N. Stability of Endolymphatic Hydrops in Ménière Disease Shown by 3-Tesla Magnetic Resonance Imaging During and After Vertigo Attacks. JAMA Otolaryngol. Head Neck Surg. 2019, 145, 583–585. [Google Scholar] [CrossRef]
- Zhang, D.G.; Shi, H.L.; Fan, Z.M.; Wang, G.B.; Han, Y.C.; Li, Y.W.; Wang, H.B. Visualization of endolymphatic hydrops in 3D-FLAIR MRI after intratympanic Gd-DTPA administration in Meniere’s disease patients. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2013, 48, 628–633. [Google Scholar] [PubMed]
- Lin, K.T.; Lu, C.J.; Young, Y.H. Predicting positive cochlear endolymphatic hydrops on magnetic resonance images. Laryngoscope Investig. Otolaryngol. 2022, 7, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Zhang, F.; Zheng, H.; Sun, X.; Chen, J.; Chen, J.; Liu, Y.; Wang, L.; Wang, W.; Li, S.; et al. The Correlation of a 2D Volume-Referencing Endolymphatic-Hydrops Grading System With Extra-Tympanic Electrocochleography in Patients With Definite Ménière’s Disease. Front. Neurol. 2020, 11, 595038. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.T.; Lu, C.J.; Young, Y.H. Magnetic resonance imaging: Role on diagnosing all types of endolymphatic hydrops. J. Med. Assoc. 2022, 121, 1325–1333. [Google Scholar] [CrossRef]
- Kazemi, M.A.; Ghasemi, A.; Casselman, J.W.; Shafiei, M.; Zarandy, M.M.; Sharifian, H.; Hashemi, H.; Firouznia, K.; Moradi, B.; Kasani, K.; et al. Correlation of semi-quantitative findings of endolymphatic hydrops in MRI with the audiometric findings in patients with Meniere’s disease. J. Otol. 2022, 17, 123–129. [Google Scholar] [CrossRef]
- Kirsch, V.; Becker-Bense, S.; Berman, A.; Kierig, E.; Ertl-Wagner, B.; Dieterich, M. Transient endolymphatic hydrops after an attack of vestibular migraine: A longitudinal single case study. J. Neurol. 2018, 265, 51–53. [Google Scholar] [CrossRef]
- Gürkov, R.; Kantner, C.; Strupp, M.; Flatz, W.; Krause, E.; Ertl-Wagner, B. Endolymphatic hydrops in patients with vestibular migraine and auditory symptoms. Eur. Arch. Oto-Rhino-Laryngol. 2014, 271, 2661–2667. [Google Scholar] [CrossRef]
- Lee, B.N.; Hwang, S.-B.; Kang, J.-J.; Oh, S.-Y. Endolymphatic Hydrops in Vestibular Migraine Associated with Menière’s Disease: A Report of Two Cases. Res. Vestib. Sci. 2021, 20, 156–160. [Google Scholar] [CrossRef]
- Oh, S.Y.; Dieterich, M.; Lee, B.N.; Boegle, R.; Kang, J.J.; Lee, N.R.; Gerb, J.; Hwang, S.B.; Kirsch, V. Endolymphatic Hydrops in Patients With Vestibular Migraine and Concurrent Meniere’s Disease. Front. Neurol. 2021, 12, 594481. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Guo, P.; Ren, T.; Wang, W. Magnetic resonance imaging of intratympanic gadolinium helps differentiate vestibular migraine from Ménière disease. Laryngoscope 2017, 127, 2382–2388. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Lei, P.; Chen, C.; Liu, Y.; Xia, K.; Liu, B. Non-contrast MRI of Inner Ear Detected Differences of Endolymphatic Drainage System Between Vestibular Migraine and Unilateral Ménière’s Disease. Front. Neurol. 2022, 13, 814518. [Google Scholar] [CrossRef] [PubMed]
- Basura, G.J.; Adams, M.E.; Monfared, A.; Schwartz, S.R.; Antonelli, P.J.; Burkard, R.; Bush, M.L.; Bykowski, J.; Colandrea, M.; Derebery, J. Clinical practice guideline: Ménière’s disease. Otolaryngol. Head Neck Surg. 2020, 162, S1–S55. [Google Scholar] [CrossRef]
- Caulley, L.; Quimby, A.; Karsh, J.; Ahrari, A.; Tse, D.; Kontorinis, G. Autoimmune arthritis in Ménière’s disease: A systematic review of the literature. Semin. Arthritis Rheum. 2018, 48, 141–147. [Google Scholar] [CrossRef]
- Greco, A.; Gallo, A.; Fusconi, M.; Marinelli, C.; Macri, G.F.; de Vincentiis, M. Meniere’s disease might be an autoimmune condition? Autoimmun. Rev. 2012, 11, 731–738. [Google Scholar] [CrossRef]
- De Maio, A. Extracellular Hsp70: Export and function. Curr. Protein Pept. Sci. 2014, 15, 225–231. [Google Scholar] [CrossRef]
- Gottschlich, S.; Billings, P.B.; Keithley, E.M.; Weisman, M.H.; Harris, J.P. Assessment of serum antibodies in patients with rapidly progressive sensorineural hearing loss and Menière’s disease. Laryngoscope 1995, 105, 1347–1352. [Google Scholar] [CrossRef] [PubMed]
- DiBerardino, F.; Cesarani, A.; Hahn, A.; Alpini, D. Viral infection and serum antibodies to heat shock protein 70 in the acute phase of Ménière’s disease. Int. Tinnitus J. 2007, 13, 90–93. [Google Scholar]
- Ruckenstein, M.J.; Prasthoffer, A.; Bigelow, D.C.; Von Feldt, J.M.; Kolasinski, S.L. Immunologic and serologic testing in patients with Ménière’s disease. Otol. Neurotol. 2002, 23, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Rauch, S.D.; Zurakowski, D.; Bloch, D.B.; Bloch, K.J. Anti-heat shock protein 70 antibodies in Meniere’s disease. Laryngoscope 2000, 110, 1516–1521. [Google Scholar] [CrossRef] [PubMed]
- Brookes, G.B. Circulating immune complexes in Meniere’s disease. Arch. Otolaryngol. Head Neck Surg. 1986, 112, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, K.; Ohashi, T.; Urushibata, T.; Kenmochi, M.; Akagi, M. Antibodies of type II collagen and immune complexes in Menière’s disease. Acta Otolaryngol. Suppl. 1996, 522, 79–85. [Google Scholar] [PubMed]
- Derebery, M.J.; Rao, V.S.; Siglock, T.J.; Linthicum, F.H.; Nelson, R.A. Menière’s disease: An immune complex-mediated illness? Laryngoscope 1991, 101, 225–229. [Google Scholar]
- Hsu, L.; Zhu, X.N.; Zhao, Y.S. Immunoglobulin E and circulating immune complexes in endolymphatic hydrops. Ann. Otol. Rhinol. Laryngol. 1990, 99, 535–538. [Google Scholar] [CrossRef]
- Savastano, M.; Giacomelli, L.; Marioni, G. Non-specific immunological determinations in Meniere’s disease: Any role in clinical practice? Eur. Arch. Otorhinolaryngol. 2007, 264, 15–19. [Google Scholar] [CrossRef]
- Perez Garrigues, H.; Carmona, E.; Morera, C.; Sanchez-Cuenca, J.M. Circulating auto-antibodies in Ménière’s disease. Ann. Otolaryngol. Chir. Cervicofac. 1995, 112, 225–228. [Google Scholar]
- Muiño, J.C.; Carreras, R.; Ocampo, M.A.; Ferrero, M.; Romero Piffiguer, M.D.; Landa, C.; Beltramo, D. The importance of IgG auto-antibodies, anti-collagen type II specific in Menière’s disease and progressive hearing loss. Rev. Fac. Cien Med. Univ. Nac. Cordoba 1999, 56, 71–80. [Google Scholar]
- Derebery, M.J. Allergic and immunologic features of Ménière’s disease. Otolaryngol. Clin. North. Am. 2011, 44, 655–666. [Google Scholar] [CrossRef]
- Keles, E.; Gödekmerdan, A.; Kalidağ, T.; Kaygusuz, I.; Yalçin, S.; Cengiz Alpay, H.; Aral, M. Meniere’s disease and allergy: Allergens and cytokines. J. Laryngol. Otol. 2004, 118, 688–693. [Google Scholar] [CrossRef]
- Ma, Y.; Sun, Q.; Zhang, K.; Bai, L.; Du, L. High level of IgE in acute low-tone sensorineural hearing loss: A predictor for recurrence and Meniere Disease transformation. Am. J. Otolaryngol. 2021, 42, 102856. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Lyu, Y.; Guo, J.; Liu, J.; Song, Y.; Fan, Z.; Li, X.; Li, N.; Zhang, D.; Wang, H. Bidirectional Transport of IgE by CD23 in the Inner Ear of Patients with Meniere’s Disease. J. Immunol. 2022, 208, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Frejo, L.; Lopez-Escamez, J.A. Cytokines and Inflammation in Meniere Disease. Clin. Exp. Otorhinolaryngol. 2022, 15, 49–59. [Google Scholar] [CrossRef]
- Frejo, L.; Gallego-Martinez, A.; Requena, T.; Martin-Sanz, E.; Amor-Dorado, J.C.; Soto-Varela, A.; Santos-Perez, S.; Espinosa-Sanchez, J.M.; Batuecas-Caletrio, A.; Aran, I.; et al. Proinflammatory cytokines and response to molds in mononuclear cells of patients with Meniere disease. Sci. Rep. 2018, 8, 5974. [Google Scholar] [CrossRef] [PubMed]
- Flook, M.; Frejo, L.; Gallego-Martinez, A.; Martin-Sanz, E.; Rossi-Izquierdo, M.; Amor-Dorado, J.C.; Soto-Varela, A.; Santos-Perez, S.; Batuecas-Caletrio, A.; Espinosa-Sanchez, J.M.; et al. Differential Proinflammatory Signature in Vestibular Migraine and Meniere Disease. Front. Immunol 2019, 10, 1229. [Google Scholar] [CrossRef]
- Zhang, S.; Gong, Y.; Liang, Y.; Wang, B.; Gao, W.; Xu, Q. Cyclophosphamide inhibits the progression of Meniere’s disease by reducing the generation of circulating immune complex. Exp. Med. 2021, 22, 1177. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, J.Y.; Lee, H.J.; Gi, M.; Kim, B.G.; Choi, J.Y. Autoimmunity as a candidate for the etiopathogenesis of Meniere’s disease: Detection of autoimmune reactions and diagnostic biomarker candidate. PLoS ONE 2014, 9, e111039. [Google Scholar] [CrossRef] [PubMed]
- Chiarella, G.; Saccomanno, M.; Scumaci, D.; Gaspari, M.; Faniello, M.C.; Quaresima, B.; Di Domenico, M.; Ricciardi, C.; Petrolo, C.; Cassandro, C.; et al. Proteomics in Ménière disease. J. Cell Physiol. 2012, 227, 308–312. [Google Scholar] [CrossRef]
- Chiarella, G.; Di Domenico, M.; Petrolo, C.; Saccomanno, M.; Rothenberger, R.; Giordano, A.; Costanzo, F.; Cassandro, E.; Cuda, G. A proteomics-driven assay defines specific plasma protein signatures in different stages of Ménière’s disease. J. Cell Biochem. 2014, 115, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, T.; Okayasu, T.; Ito, T.; Fujita, H.; Ueda, K. Endolymphatic Sac Drainage Surgery and Plasma Stress Hormone Vasopressin Levels in Meniere’s Disease. Front. Neurol. 2021, 12, 722217. [Google Scholar] [CrossRef] [PubMed]
- Takeda, T.; Kakigi, A.; Saito, H. Antidiuretic hormone (ADH) and endolymphatic hydrops. Acta Otolaryngol. Suppl. 1995, 519, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.; Ando, K.; Kuze, B.; Mizuta, K.; Hayashi, T.; Ito, Y. The association of antidiuretic hormone levels with an attack of Meniere’s disease. Clin. Otolaryngol. 2005, 30, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.; Asai, M.; Nishihori, T.; Mizuta, K.; Ito, Y.; Ando, K. The relevance of an elevation in the plasma vasopressin levels to the pathogenesis of Meniere’s attack. J. Neuroendocr. 2007, 19, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.; Hayashi, H.; Kuze, B.; Mizuta, K.; Ito, Y. The association of the plasma vasopressin level during attacks with a prognosis of Meniere’s disease. Int. J. Audiol. 2010, 49, 1–6. [Google Scholar] [CrossRef]
- Lim, J.S.; Lange, M.E.; Megerian, C.A. Serum antidiuretic hormone levels in patients with unilateral Meniere’s disease. Laryngoscope 2003, 113, 1321–1326. [Google Scholar] [CrossRef]
- Hornibrook, J.; George, P.; Gourley, J. Vasopressin in definite Meniere’s disease with positive electrocochleographic findings. Acta Otolaryngol. 2011, 131, 613–617. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, J.; Dong, L.; Fan, W.; Zhang, J.; Wu, C. A Mysterious Role of Arginine Vasopressin Levels in Ménière’s Disease-Meta-analysis of Clinical Studies. Otol. Neurotol. 2017, 38, 161–167. [Google Scholar] [CrossRef]
- Kitahara, T.; Doi, K.; Maekawa, C.; Kizawa, K.; Horii, A.; Kubo, T.; Kiyama, H. Meniere’s attacks occur in the inner ear with excessive vasopressin type-2 receptors. J. Neuroendocr. 2008, 20, 1295–1300. [Google Scholar] [CrossRef]
- Kitahara, T.; Okamoto, H.; Fukushima, M.; Sakagami, M.; Ito, T.; Yamashita, A.; Ota, I.; Yamanaka, T. A Two-Year Randomized Trial of Interventions to Decrease Stress Hormone Vasopressin Production in Patients with Meniere’s Disease-A Pilot Study. PLoS ONE 2016, 11, e0158309. [Google Scholar] [CrossRef]
- Yang, C.-H.; Hwang, C.-F.; Chuang, J.-H.; Lian, W.-S.; Wang, F.-S.; Huang, E.I.; Yang, M.-Y. Constant Light Dysregulates Cochlear Circadian Clock and Exacerbates Noise-Induced Hearing Loss. Int. J. Mol. Sci. 2020, 21, 7535. [Google Scholar] [PubMed]
- Yang, M.-Y.; Yang, W.-C.; Lin, P.-M.; Hsu, J.-F.; Hsiao, H.-H.; Liu, Y.-C.; Tsai, H.-J.; Chang, C.-S.; Lin, S.-F. Altered expression of circadian clock genes in human chronic myeloid leukemia. J. Biol. Rhythm. 2011, 26, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Hwang, C.F.; Lin, P.M.; Chuang, J.H.; Hsu, C.M.; Lin, S.F.; Yang, M.Y. Sleep Disturbance and Altered Expression of Circadian Clock Genes in Patients With Sudden Sensorineural Hearing Loss. Medicine 2015, 94, e978. [Google Scholar] [CrossRef]
- Fukuya, H.; Emoto, N.; Nonaka, H.; Yagita, K.; Okamura, H.; Yokoyama, M. Circadian expression of clock genes in human peripheral leukocytes. Biochem. Biophys. Res. Commun. 2007, 354, 924–928. [Google Scholar] [CrossRef] [PubMed]
- Rauch, S.D. Clinical hints and precipitating factors in patients suffering from Meniere’s disease. Otolaryngol. Clin. North. Am. 2010, 43, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Hwang, C.F.; Tsai, N.W.; Yang, M.Y. Expression of circadian clock genes in leukocytes of patients with Meniere’s disease. Laryngoscope Investig. Otolaryngol. 2022, 7, 584–591. [Google Scholar] [CrossRef]
- Sun, Q.; Yang, Y.; Wang, Z.; Yang, X.; Gao, Y.; Zhao, Y.; Ge, W.; Liu, J.; Xu, X.; Guan, W. PER1 interaction with GPX1 regulates metabolic homeostasis under oxidative stress. Redox Biol. 2020, 37, 101694. [Google Scholar] [PubMed]
- Alicandri-Ciufelli, M.; Aggazzotti-Cavazza, E.; Cunsolo, E.; Marchioni, D.; Monzani, D.; Genovese, E.; Presutti, L. Is Ménière’s disease the ‘inner ear migraine’? A neurovascular region-based hypothesis supported by epidemiological appraisal and pathophysiological considerations. Hearth Balance Commun. 2016, 14, 63–69. [Google Scholar] [CrossRef]
- Martami, F.; Razeghi Jahromi, S.; Togha, M.; Ghorbani, Z.; Seifishahpar, M.; Saidpour, A. The serum level of inflammatory markers in chronic and episodic migraine: A case-control study. Neurol. Sci 2018, 39, 1741–1749. [Google Scholar] [CrossRef]
- Togha, M.; Razeghi Jahromi, S.; Ghorbani, Z.; Ghaemi, A.; Rafiee, P. Evaluation of Inflammatory State in Migraineurs: A Case-control Study. Iran. J. Allergy Asthma Immunol. 2020, 19, 83–90. [Google Scholar] [CrossRef]
- Hampton, K.K.; Esack, A.; Peatfield, R.C.; Grant, P.J. Elevation of plasma vasopressin in spontaneous migraine. Cephalalgia 1991, 11, 249–250. [Google Scholar] [CrossRef] [PubMed]
- Hasselblatt, M.; Köhler, J.; Volles, E.; Ehrenreich, H. Simultaneous monitoring of endothelin-1 and vasopressin plasma levels in migraine. Neuroreport 1999, 10, 423–425. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, K.S.; Dhillon, H.; Velly, A.M. The role of a potential biomarker in patients with migraine: Review and new insights. Expert Rev. Neurother. 2021, 21, 817–831. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).