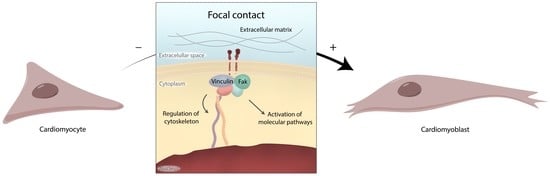
Cardiac Differentiation Promotes Focal Adhesions Assembly through Vinculin Recruitment
Abstract
:1. Introduction
2. Results
2.1. Increase in Cardiac Markers in Differentiated Cells
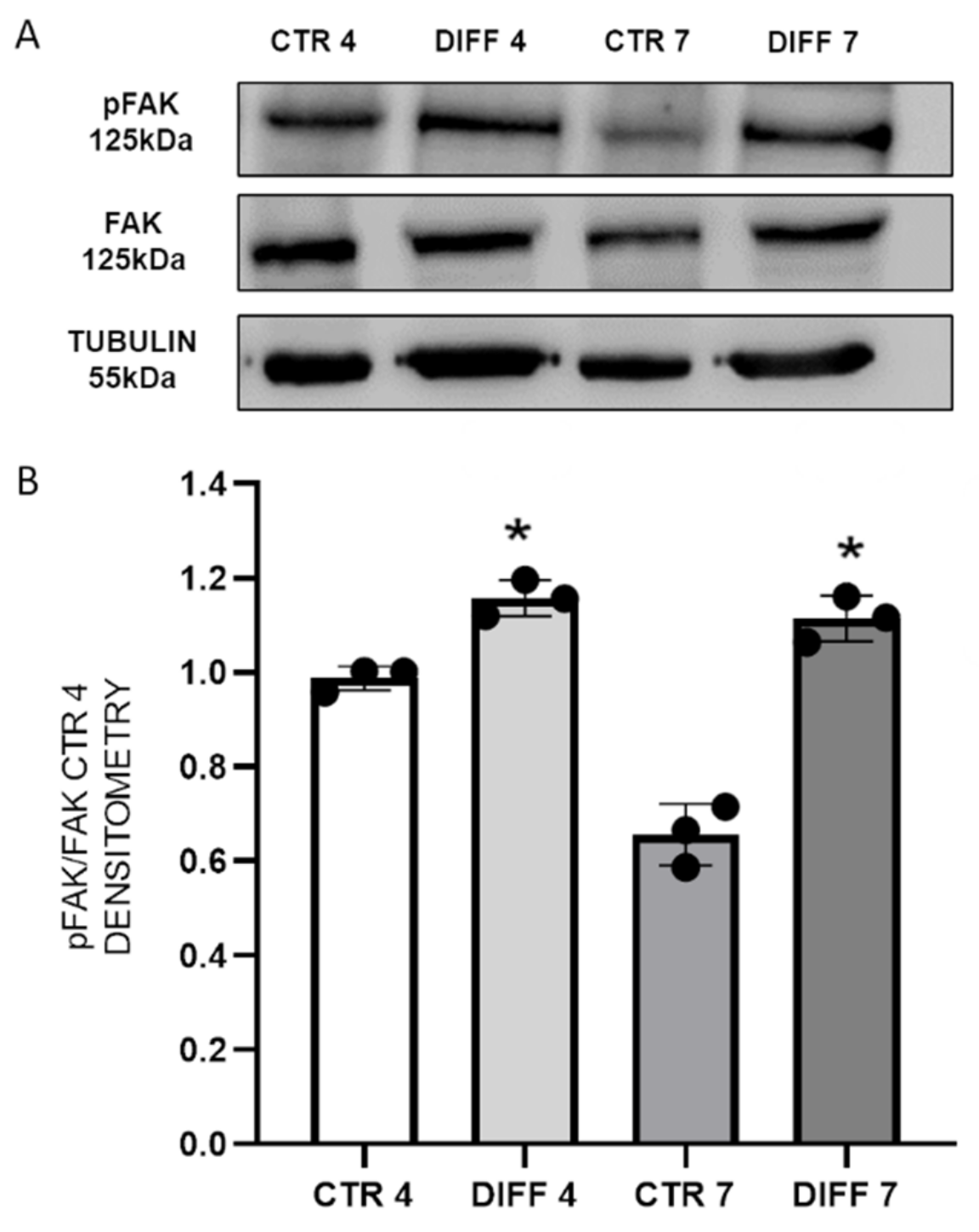
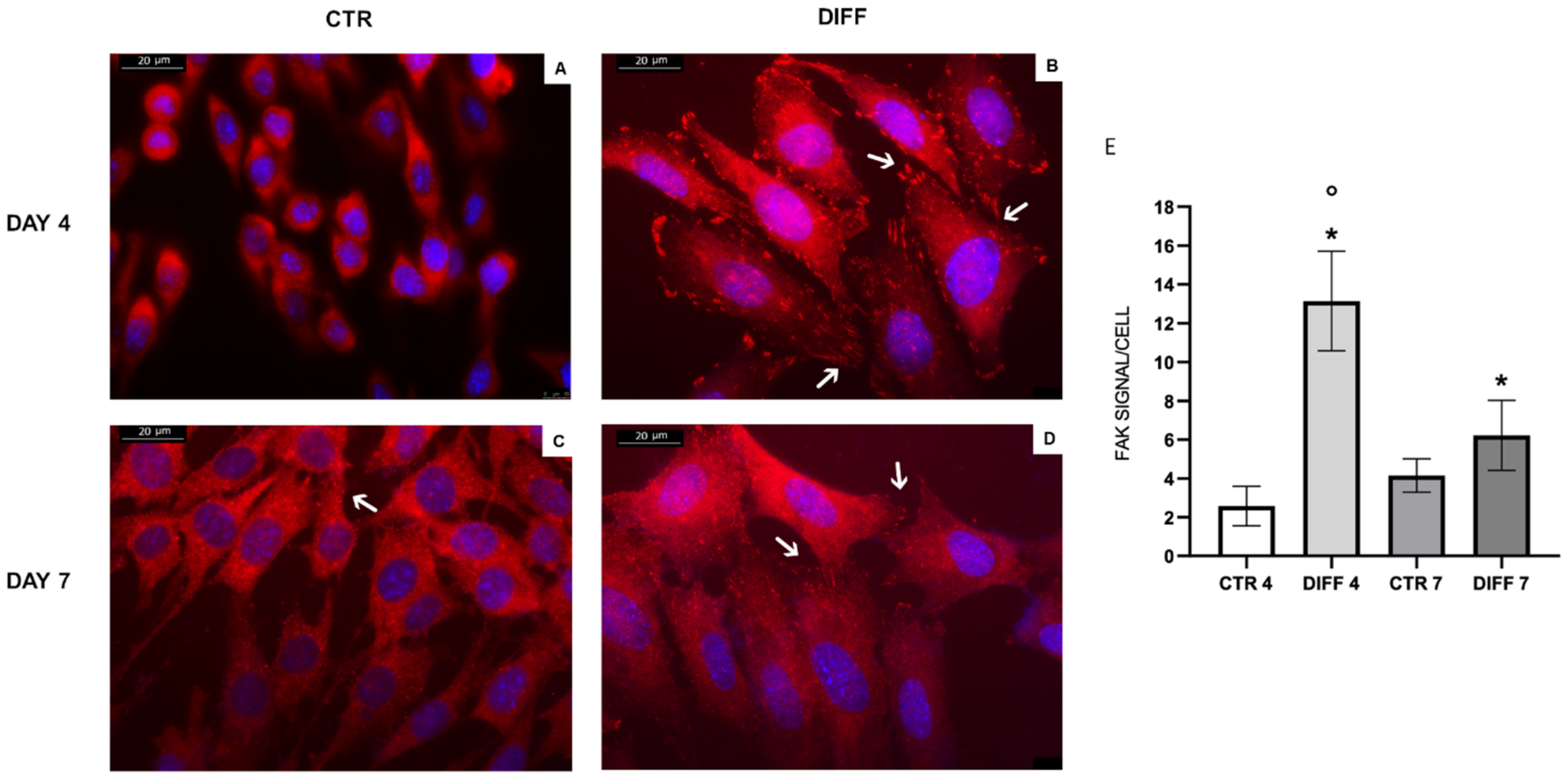
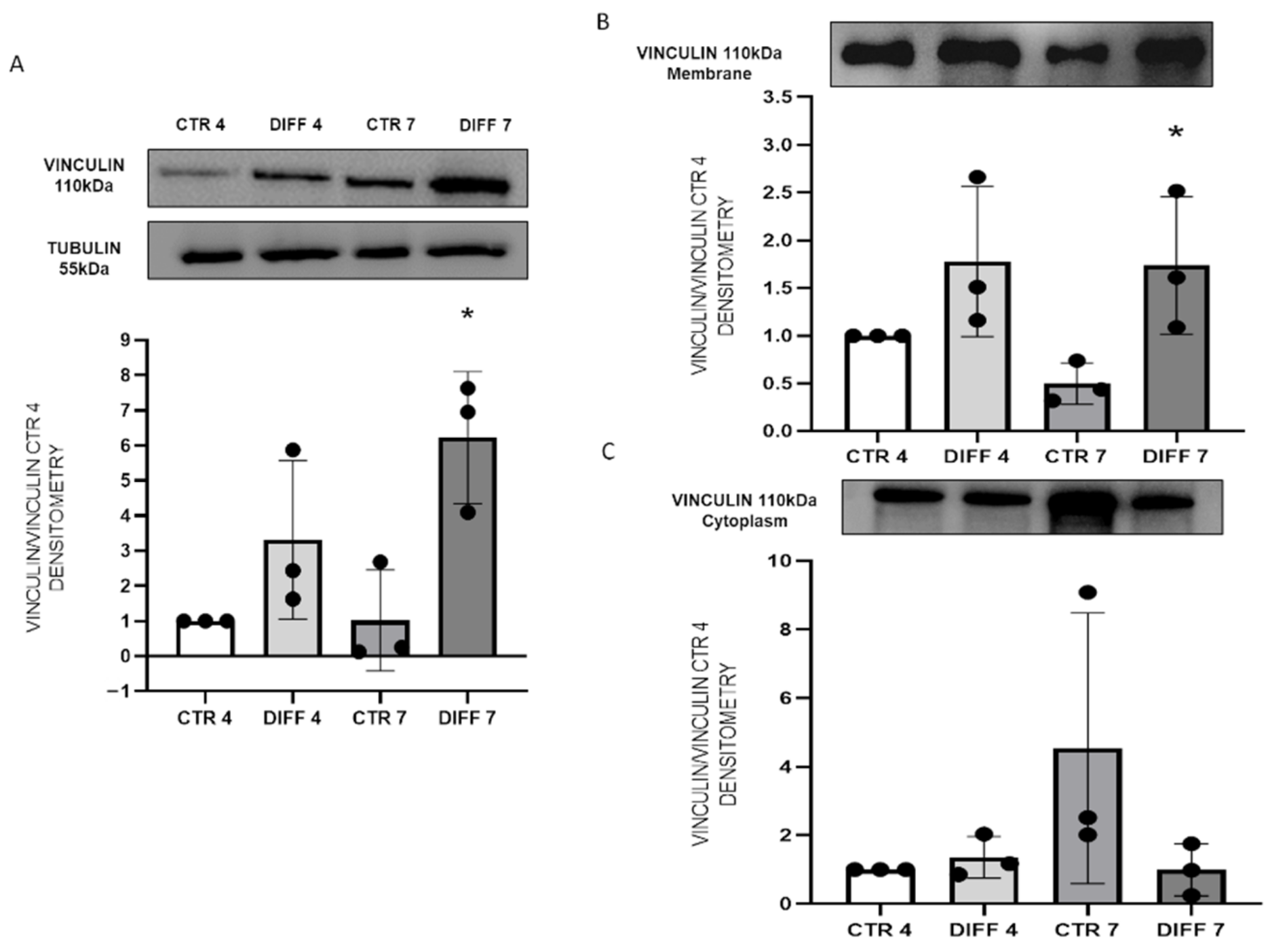
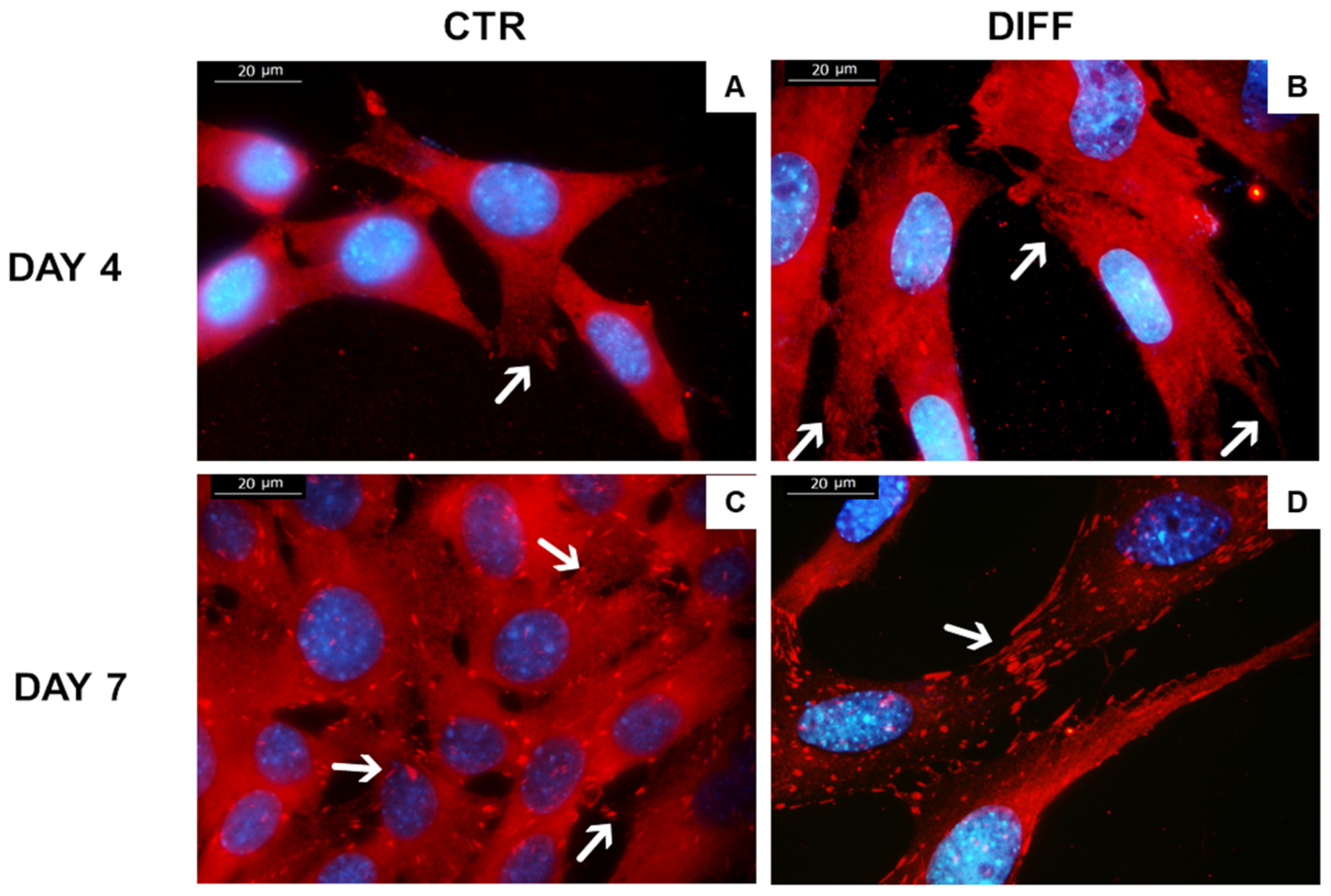
2.2. Activation of FAK and Vinculin Translocation during Cardiomyocyte Differentiation
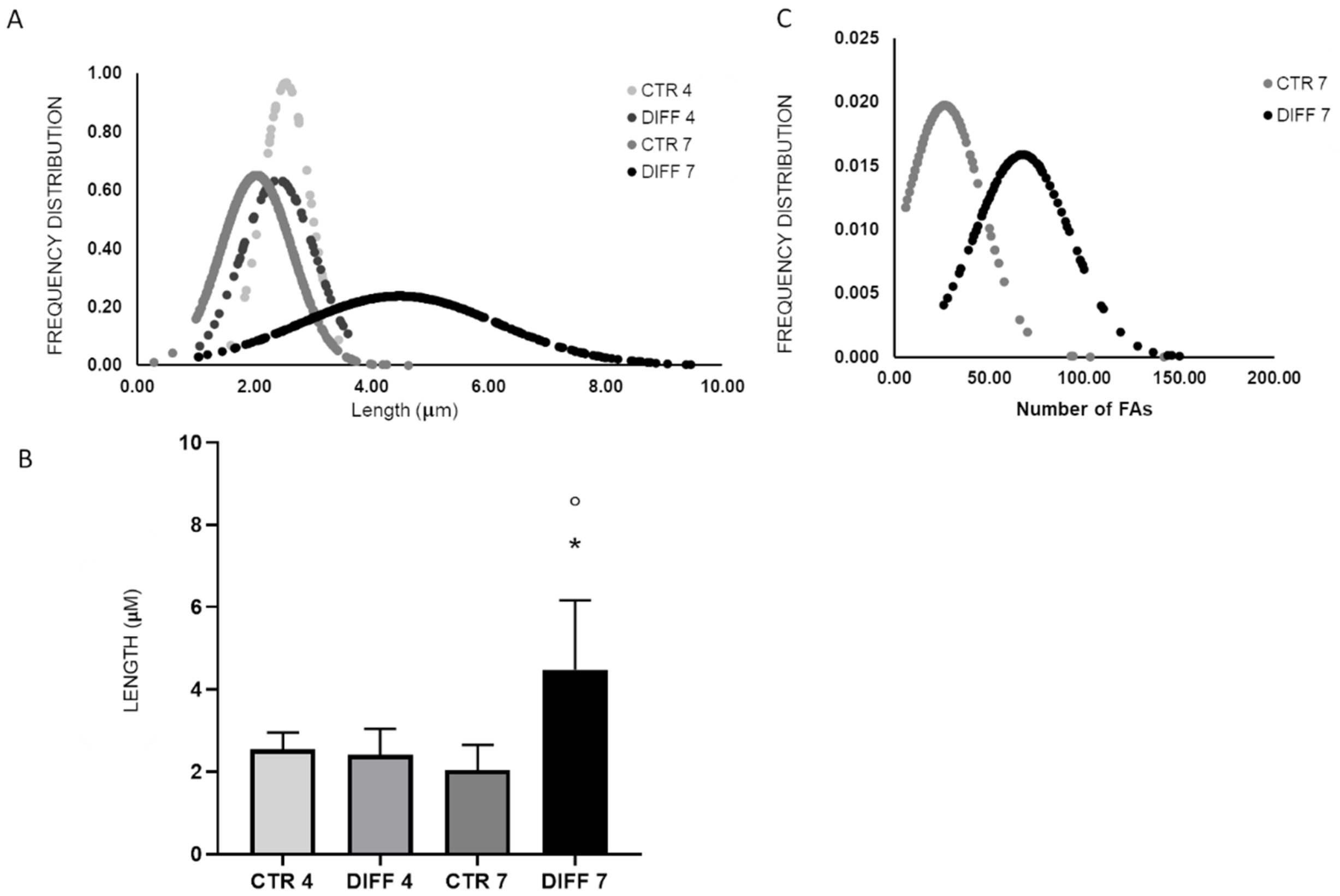
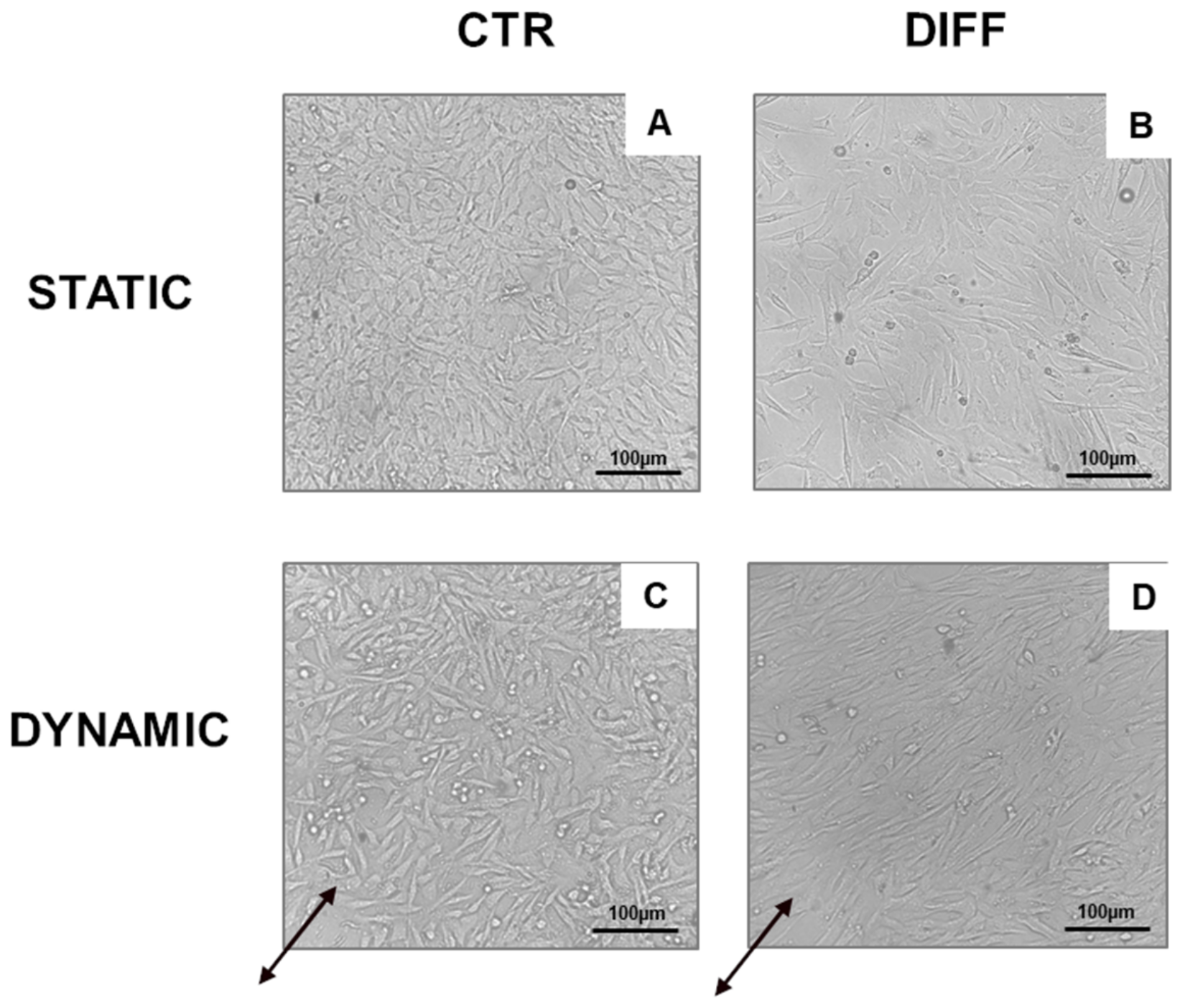
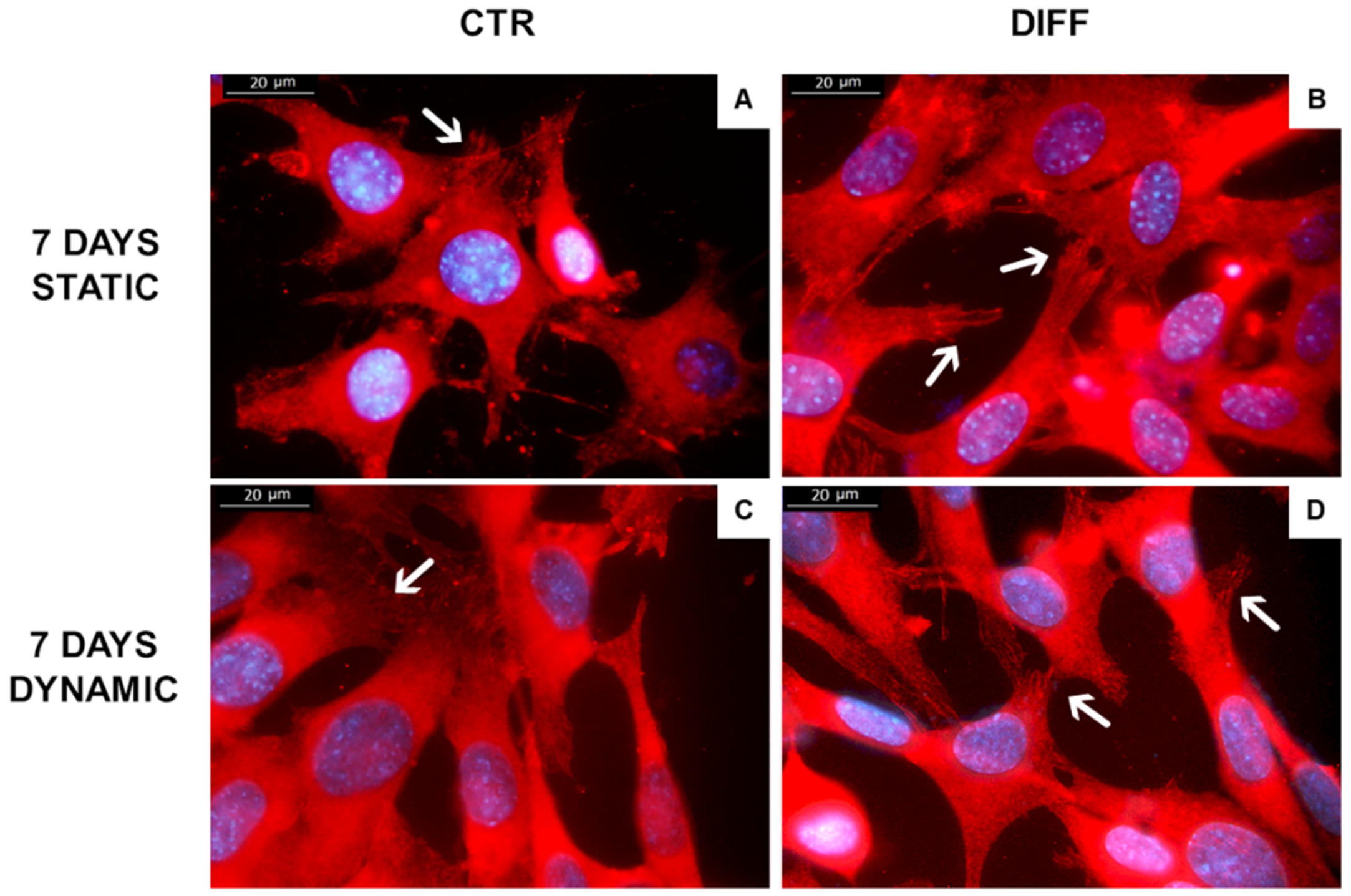
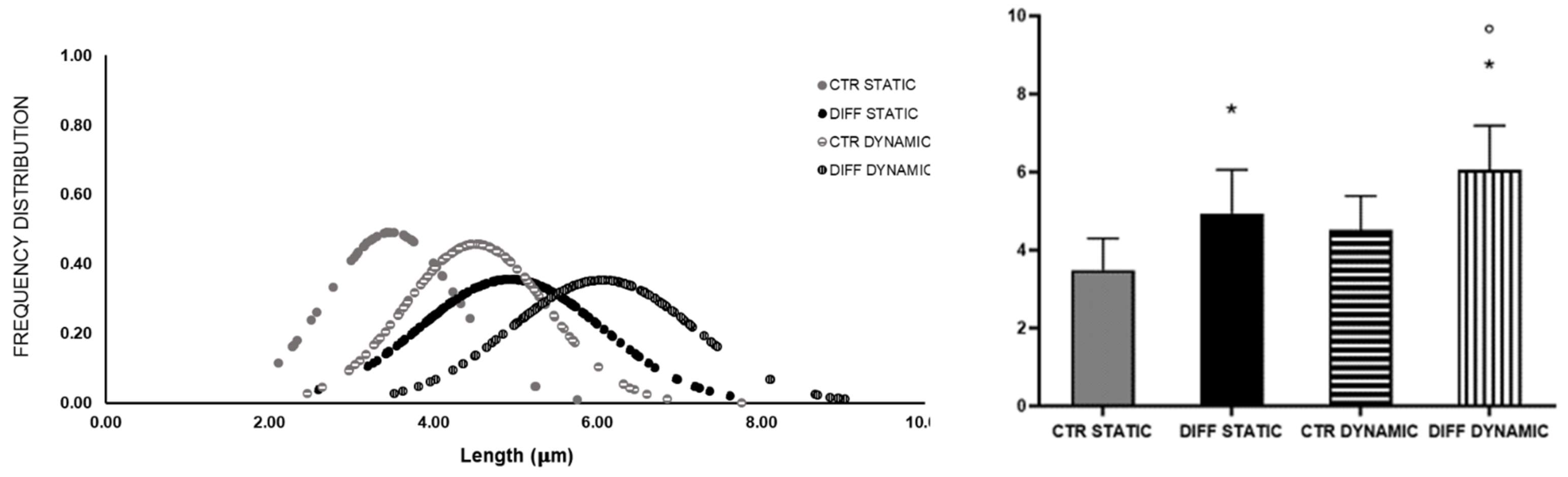
2.3. Activation of Focal Adhesions upon Mechanical Stress
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Total RNA Isolation and Real-Time Quantitative PCR (qRT-PCR)
4.3. Western Blot Analysis
4.4. Densitometry
4.5. Mechanical Stimulation
4.6. Cell Morphology
4.7. Immunofluorescence Analysis
4.8. Morphometric Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geiger, B.; Bershadsky, A. Assembly and Mechanosensory Function of Focal Contacts. Curr. Opin Cell. Biol. 2001, 13, 584–592. [Google Scholar] [CrossRef]
- Legerstee, K.; Houtsmuller, A.B. A Layered View on Focal Adhesions. Biology 2021, 10, 1189. [Google Scholar] [CrossRef]
- Kresh, J.Y.; Chopra, A. Intercellular and Extracellular Mechanotransduction in Cardiac Myocytes. Pflug. Arch. 2011, 462, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Samarel, A.M. Focal Adhesion Signaling in Heart Failure. Pflug. Arch. 2014, 466, 1101–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laukaitis, C.M.; Webb, D.J.; Donais, K.; Horwitz, A.F. Differential Dynamics of Alpha 5 Integrin, Paxillin, and Alpha-Actinin during Formation and Disassembly of Adhesions in Migrating Cells. J. Cell. Biol. 2001, 153, 1427–1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webb, D.J.; Donais, K.; Whitmore, L.A.; Thomas, S.M.; Turner, C.E.; Parsons, J.T.; Horwitz, A.F. FAK–Src Signalling through Paxillin, ERK and MLCK Regulates Adhesion Disassembly. Nat. Cell. Biol. 2004, 6, 154–161. [Google Scholar] [CrossRef]
- Wiseman, P.W.; Brown, C.M.; Webb, D.J.; Hebert, B.; Johnson, N.L.; Squier, J.A.; Ellisman, M.H.; Horwitz, A.F. Spatial Mapping of Integrin Interactions and Dynamics during Cell Migration by Image Correlation Microscopy. J. Cell Sci. 2004, 117, 5521–5534. [Google Scholar] [CrossRef] [Green Version]
- Kanchanawong, P.; Shtengel, G.; Pasapera, A.M.; Ramko, E.B.; Davidson, M.W.; Hess, H.F.; Waterman, C.M. Nanoscale Architecture of Integrin-Based Cell Adhesions. Nature 2010, 468, 580–584. [Google Scholar] [CrossRef] [Green Version]
- Humphries, J.D.; Wang, P.; Streuli, C.; Geiger, B.; Humphries, M.J.; Ballestrem, C. Vinculin Controls Focal Adhesion Formation by Direct Interactions with Talin and Actin. J. Cell. Biol. 2007, 179, 1043–1057. [Google Scholar] [CrossRef] [Green Version]
- Goldmann, W.H. Role of Vinculin in Cellular Mechanotransduction. Cell. Biol. Int. 2016, 40, 241–256. [Google Scholar] [CrossRef]
- Huveneers, S.; Oldenburg, J.; Spanjaard, E.; van der Krogt, G.; Grigoriev, I.; Akhmanova, A.; Rehmann, H.; de Rooij, J. Vinculin Associates with Endothelial VE-Cadherin Junctions to Control Force-Dependent Remodeling. J. Cell. Biol. 2012, 196, 641–652. [Google Scholar] [CrossRef] [Green Version]
- Bakolitsa, C.; Cohen, D.M.; Bankston, L.A.; Bobkov, A.A.; Cadwell, G.W.; Jennings, L.; Critchley, D.R.; Craig, S.W.; Liddington, R.C. Structural Basis for Vinculin Activation at Sites of Cell Adhesion. Nature 2004, 430, 583–586. [Google Scholar] [CrossRef]
- Bays, J.L.; DeMali, K.A. Vinculin in Cell-Cell and Cell-Matrix Adhesions. Cell. Mol. Life Sci. 2017, 74, 2999–3009. [Google Scholar] [CrossRef] [Green Version]
- Demali, K.A. Vinculin--a Dynamic Regulator of Cell Adhesion. Trends Biochem. Sci. 2004, 29, 565–567. [Google Scholar] [CrossRef]
- Stupack, D.G. Integrins as a Distinct Subtype of Dependence Receptors. Cell Death. Differ. 2005, 12, 1021–1030. [Google Scholar] [CrossRef] [Green Version]
- Ross, R.S.; Borg, T.K. Integrins and the Myocardium. Circ. Res. 2001, 88, 1112–1119. [Google Scholar] [CrossRef] [Green Version]
- Tirziu, D.; Giordano, F.J.; Simons, M. Cell Communications in the Heart. Circulation 2010, 122, 928–937. [Google Scholar] [CrossRef] [Green Version]
- Kovacic-Milivojević, B.; Roediger, F.; Almeida, E.A.; Damsky, C.H.; Gardner, D.G.; Ilić, D. Focal Adhesion Kinase and P130Cas Mediate Both Sarcomeric Organization and Activation of Genes Associated with Cardiac Myocyte Hypertrophy. Mol. Biol. Cell. 2001, 12, 2290–2307. [Google Scholar] [CrossRef]
- Kaushik, G.; Spenlehauer, A.; Sessions, A.O.; Trujillo, A.S.; Fuhrmann, A.; Fu, Z.; Venkatraman, V.; Pohl, D.; Tuler, J.; Wang, M.; et al. Vinculin Network-Mediated Cytoskeletal Remodeling Regulates Contractile Function in the Aging Heart. Sci. Transl. Med. 2015, 7, 292ra99. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Baribault, H.; Adamson, E.D. Vinculin Knockout Results in Heart and Brain Defects during Embryonic Development. Development 1998, 125, 327–337. [Google Scholar] [CrossRef]
- Zemljic-Harpf, A.E.; Miller, J.C.; Henderson, S.A.; Wright, A.T.; Manso, A.M.; Elsherif, L.; Dalton, N.D.; Thor, A.K.; Perkins, G.A.; McCulloch, A.D.; et al. Cardiac-Myocyte-Specific Excision of the Vinculin Gene Disrupts Cellular Junctions, Causing Sudden Death or Dilated Cardiomyopathy. Mol. Cell. Biol. 2007, 27, 7522–7537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, P.; Andersen, P.; Hassel, D.; Kaynak, B.L.; Limphong, P.; Juergensen, L.; Kwon, C.; Srivastava, D. Fibronectin Mediates Mesendodermal Cell Fate Decisions. Development 2013, 140, 2587–2596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lian, X.; Hsiao, C.; Wilson, G.; Zhu, K.; Hazeltine, L.B.; Azarin, S.M.; Raval, K.K.; Zhang, J.; Kamp, T.J.; Palecek, S.P. Robust Cardiomyocyte Differentiation from Human Pluripotent Stem Cells via Temporal Modulation of Canonical Wnt Signaling. Proc. Natl. Acad Sci. USA 2012, 109, E1848–E1857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, C.; Chen, G.; Kuan, S.-F.; Zhang, D.H.; Schlaepfer, D.D.; Hu, J. FAK/PYK2 Promotes the Wnt/β-Catenin Pathway and Intestinal Tumorigenesis by Phosphorylating GSK3β. eLife 2015, 4, e10072. [Google Scholar] [CrossRef] [PubMed]
- Dobner, S.; Amadi, O.; Lee, R. Cardiovascular Mechanotransduction. Muscle 2012, 1, 173–186, ISBN 978-0-12-381510-1. [Google Scholar]
- Stoppel, W.L.; Kaplan, D.L.; Black, L.D. Electrical and Mechanical Stimulation of Cardiac Cells and Tissue Constructs. Adv. Drug. Deliv. Rev. 2016, 96, 135–155. [Google Scholar] [CrossRef] [Green Version]
- Happe, C.L.; Engler, A.J. Mechanical Forces Reshape Differentiation Cues That Guide Cardiomyogenesis. Circ. Res. 2016, 118, 296–310. [Google Scholar] [CrossRef] [Green Version]
- Jacot, J.G.; Martin, J.C.; Hunt, D.L. Mechanobiology of Cardiomyocyte Development. J. Biomech. 2010, 43, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Sheehy, S.P.; Grosberg, A.; Parker, K.K. The Contribution of Cellular Mechanotransduction to Cardiomyocyte Form and Function. Biomech. Model Mechanobiol. 2012, 11, 1227–1239. [Google Scholar] [CrossRef] [Green Version]
- Sit, B.; Gutmann, D.; Iskratsch, T. Costameres, Dense Plaques and Podosomes: The Cell Matrix Adhesions in Cardiovascular Mechanosensing. J. Muscle Res. Cell. Motil. 2019, 40, 197–209. [Google Scholar] [CrossRef] [Green Version]
- Martino, F.; Perestrelo, A.R.; Vinarský, V.; Pagliari, S.; Forte, G. Cellular Mechanotransduction: From Tension to Function. Front. Physiol. 2018, 9, 824. [Google Scholar] [CrossRef]
- Lammerding, J.; Kamm, R.D.; Lee, R.T. Mechanotransduction in Cardiac Myocytes. Ann. N. Y. Acad. Sci. 2004, 1015, 53–70. [Google Scholar] [CrossRef]
- Harston, R.K.; Kuppuswamy, D. Integrins Are the Necessary Links to Hypertrophic Growth in Cardiomyocytes. J. Signal. Transduct. 2011, 2011, 521742. [Google Scholar] [CrossRef] [Green Version]
- Münch, J.; Abdelilah-Seyfried, S. Sensing and Responding of Cardiomyocytes to Changes of Tissue Stiffness in the Diseased Heart. Front. Cell Dev. Biol. 2021, 9, 642840. [Google Scholar] [CrossRef]
- Sharp, W.W.; Simpson, D.G.; Borg, T.K.; Samarel, A.M.; Terracio, L. Mechanical Forces Regulate Focal Adhesion and Costamere Assembly in Cardiac Myocytes. Am. J. Physiol. 1997, 273, H546–H556. [Google Scholar] [CrossRef]
- del Rio, A.; Perez-Jimenez, R.; Liu, R.; Roca-Cusachs, P.; Fernandez, J.M.; Sheetz, M.P. Stretching Single Talin Rod Molecules Activates Vinculin Binding. Science 2009, 323, 638–641. [Google Scholar] [CrossRef]
- Ohashi, K.; Fujiwara, S.; Mizuno, K. Roles of the Cytoskeleton, Cell Adhesion and Rho Signalling in Mechanosensing and Mechanotransduction. J. Biochem. 2017, 161, 245–254. [Google Scholar] [CrossRef] [Green Version]
- Carisey, A.; Tsang, R.; Greiner, A.M.; Nijenhuis, N.; Heath, N.; Nazgiewicz, A.; Kemkemer, R.; Derby, B.; Spatz, J.; Ballestrem, C. Vinculin Regulates the Recruitment and Release of Core Focal Adhesion Proteins in a Force-Dependent Manner. Curr. Biol. 2013, 23, 271–281. [Google Scholar] [CrossRef] [Green Version]
- Samarel, A.M. Costameres, Focal Adhesions, and Cardiomyocyte Mechanotransduction. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H2291–H2301. [Google Scholar] [CrossRef]
- Pietronave, S.; Zamperone, A.; Oltolina, F.; Colangelo, D.; Follenzi, A.; Novelli, E.; Diena, M.; Pavesi, A.; Consolo, F.; Fiore, G.B.; et al. Monophasic and Biphasic Electrical Stimulation Induces a Precardiac Differentiation in Progenitor Cells Isolated from Human Heart. Stem Cells Dev. 2014, 23, 888–898. [Google Scholar] [CrossRef] [Green Version]
- Kawaguchi, N. Adult Cardiac-Derived Stem Cells: Differentiation and Survival Regulators. Vitam. Horm. 2011, 87, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Potthoff, M.; Olson, E. MEF2: A central regulator of diverse developmental programs. Development 2007, 134, 4131–4140. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, M.; Courilleau, D.; Jullian, J.-C.; Fortin, D.; Ventura-Clapier, R.; Blondeau, J.-P.; Garnier, A. A Cardiac-Specific Robotized Cellular Assay Identified Families of Human Ligands as Inducers of PGC-1α Expression and Mitochondrial Biogenesis. PLoS ONE 2012, 7, e46753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsumi, A.; Naoe, T.; Matsushita, T.; Kaibuchi, K.; Schwartz, M.A. Integrin Activation and Matrix Binding Mediate Cellular Responses to Mechanical Stretch. J. Biol. Chem. 2005, 280, 16546–16549. [Google Scholar] [CrossRef] [Green Version]
- Schaller, M.D. Biochemical Signals and Biological Responses Elicited by the Focal Adhesion Kinase. Biochim. Biophys. Acta 2001, 1540, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, M.A.; Shattil, S.J. Signaling Networks Linking Integrins and Rho Family GTPases. Trends Biochem. Sci. 2000, 25, 388–391. [Google Scholar] [CrossRef]
- Zhang, S.J.; Truskey, G.A.; Kraus, W.E. Effect of Cyclic Stretch on Beta1D-Integrin Expression and Activation of FAK and RhoA. Am. J. Physiol. Cell Physiol. 2007, 292, C2057–C2069. [Google Scholar] [CrossRef] [Green Version]
- Schlaepfer, D.D.; Hauck, C.R.; Sieg, D.J. Signaling through Focal Adhesion Kinase. Prog. Biophys Mol. Biol. 1999, 71, 435–478. [Google Scholar] [CrossRef] [Green Version]
- Ivankovic-Dikic, I.; Grönroos, E.; Blaukat, A.; Barth, B.U.; Dikic, I. Pyk2 and FAK Regulate Neurite Outgrowth Induced by Growth Factors and Integrins. Nat. Cell Biol. 2000, 2, 574–581. [Google Scholar] [CrossRef]
- Torsoni, A.S.; Constancio, S.S.; Nadruz, W.; Hanks, S.K.; Franchini, K.G. Focal Adhesion Kinase Is Activated and Mediates the Early Hypertrophic Response to Stretch in Cardiac Myocytes. Circ. Res. 2003, 93, 140–147. [Google Scholar] [CrossRef] [Green Version]
- Merkel, C.D.; Li, Y.; Raza, Q.; Stolz, D.B.; Kwiatkowski, A.V. Vinculin anchors contractile actin to the cardiomyocyte adherens junction. Mol. Biol. Cell. 2019, 30, 2639–2650. [Google Scholar] [CrossRef]
- Chen, H.; Cohen, D.M.; Choudhury, D.M.; Kioka, N.; Craig, S.W. Spatial Distribution and Functional Significance of Activated Vinculin in Living Cells. J. Cell. Biol. 2005, 169, 459–470. [Google Scholar] [CrossRef] [Green Version]
- Garoffolo, G.; Pesce, M. Mechanotransduction in the Cardiovascular System: From Developmental Origins to Homeostasis and Pathology. Cells 2019, 8, 1607. [Google Scholar] [CrossRef] [Green Version]
- Carton, F.; Malatesta, M. In Vitro Models of Biological Barriers for Nanomedical Research. Int. J. Mol. Sci. 2022, 23, 8910. [Google Scholar] [CrossRef]
- Jorba, I.; Mostert, D.; Hermans, L.H.L.; van der Pol, A.; Kurniawan, N.A.; Bouten, C.V.C. In Vitro Methods to Model Cardiac Mechanobiology in Health and Disease. Tissue Eng. Part C Methods 2021, 27, 139–151. [Google Scholar] [CrossRef]
- Thompson, M.S.; Epari, D.R.; Bieler, F.; Duda, G.N. In vitro models for bone mechanobiology: Applications in bone regeneration and tissue engineering. Proc. Inst. Mech. Eng. H 2010, 224, 1533–1541. [Google Scholar] [CrossRef]
- Uyeda, T.Q.P.; Iwadate, Y.; Umeki, N.; Nagasaki, A.; Yumura, S. Stretching Actin Filaments within Cells Enhances Their Affinity for the Myosin II Motor Domain. PLoS ONE 2011, 6, e26200. [Google Scholar] [CrossRef] [Green Version]
- Dhein, S.; Schreiber, A.; Steinbach, S.; Apel, D.; Salameh, A.; Schlegel, F.; Kostelka, M.; Dohmen, P.M.; Mohr, F.W. Mechanical Control of Cell Biology. Effects of Cyclic Mechanical Stretch on Cardiomyocyte Cellular Organization. Prog. Biophys. Mol. Biol. 2014, 115, 93–102. [Google Scholar] [CrossRef]
- Boccafoschi, F.; Bosetti, M.; Sandra, P.M.; Leigheb, M.; Cannas, M. Effects of Mechanical Stress on Cell Adhesion: A Possible Mechanism for Morphological Changes. Cell Adh. Migr. 2010, 4, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, R.; Gunawan, F.; Ramadass, R.; Beisaw, A.; Konzer, A.; Mullapudi, S.T.; Gentile, A.; Maischein, H.-M.; Graumann, J.; Stainier, D.Y.R. Mechanical Forces Regulate Cardiomyocyte Myofilament Maturation via the VCL-SSH1-CFL Axis. Dev. Cell. 2019, 51, 62–77.e5. [Google Scholar] [CrossRef]
- Golji, J.; Mofrad, M.R.K. A Molecular Dynamics Investigation of Vinculin Activation. Biophys. J. 2010, 99, 1073–1081. [Google Scholar] [CrossRef] [PubMed]

| Gene | Forward Primers | Reverse Primers | T.m. a |
|---|---|---|---|
| β-actin | gatgacccagatcatgtttgaga | gtctccggagtccatcacaat | 53 °C |
| gata-4 | caactgccagactaccaccac | ccatggagcttcatgtagagg | 52 °C |
| mef2c | tgctgtgcgactgtgagattg | cgtgcggctcgttgtactcg | 59 °C |
| nkx2.5 | tccgagcctggtagggaaa | aggtcaacaaaaggggatgga | 55 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carton, F.; Casarella, S.; Di Francesco, D.; Zanella, E.; D'urso, A.; Di Nunno, L.; Fusaro, L.; Cotella, D.; Prat, M.; Follenzi, A.; et al. Cardiac Differentiation Promotes Focal Adhesions Assembly through Vinculin Recruitment. Int. J. Mol. Sci. 2023, 24, 2444. https://doi.org/10.3390/ijms24032444
Carton F, Casarella S, Di Francesco D, Zanella E, D'urso A, Di Nunno L, Fusaro L, Cotella D, Prat M, Follenzi A, et al. Cardiac Differentiation Promotes Focal Adhesions Assembly through Vinculin Recruitment. International Journal of Molecular Sciences. 2023; 24(3):2444. https://doi.org/10.3390/ijms24032444
Chicago/Turabian StyleCarton, Flavia, Simona Casarella, Dalila Di Francesco, Emma Zanella, Annarita D'urso, Luca Di Nunno, Luca Fusaro, Diego Cotella, Maria Prat, Antonia Follenzi, and et al. 2023. "Cardiac Differentiation Promotes Focal Adhesions Assembly through Vinculin Recruitment" International Journal of Molecular Sciences 24, no. 3: 2444. https://doi.org/10.3390/ijms24032444
APA StyleCarton, F., Casarella, S., Di Francesco, D., Zanella, E., D'urso, A., Di Nunno, L., Fusaro, L., Cotella, D., Prat, M., Follenzi, A., & Boccafoschi, F. (2023). Cardiac Differentiation Promotes Focal Adhesions Assembly through Vinculin Recruitment. International Journal of Molecular Sciences, 24(3), 2444. https://doi.org/10.3390/ijms24032444