CRISPR/Cas9-Targeted Disruption of Two Highly Homologous Arabidopsis thaliana DSS1 Genes with Roles in Development and the Oxidative Stress Response
Abstract
1. Introduction
2. Results
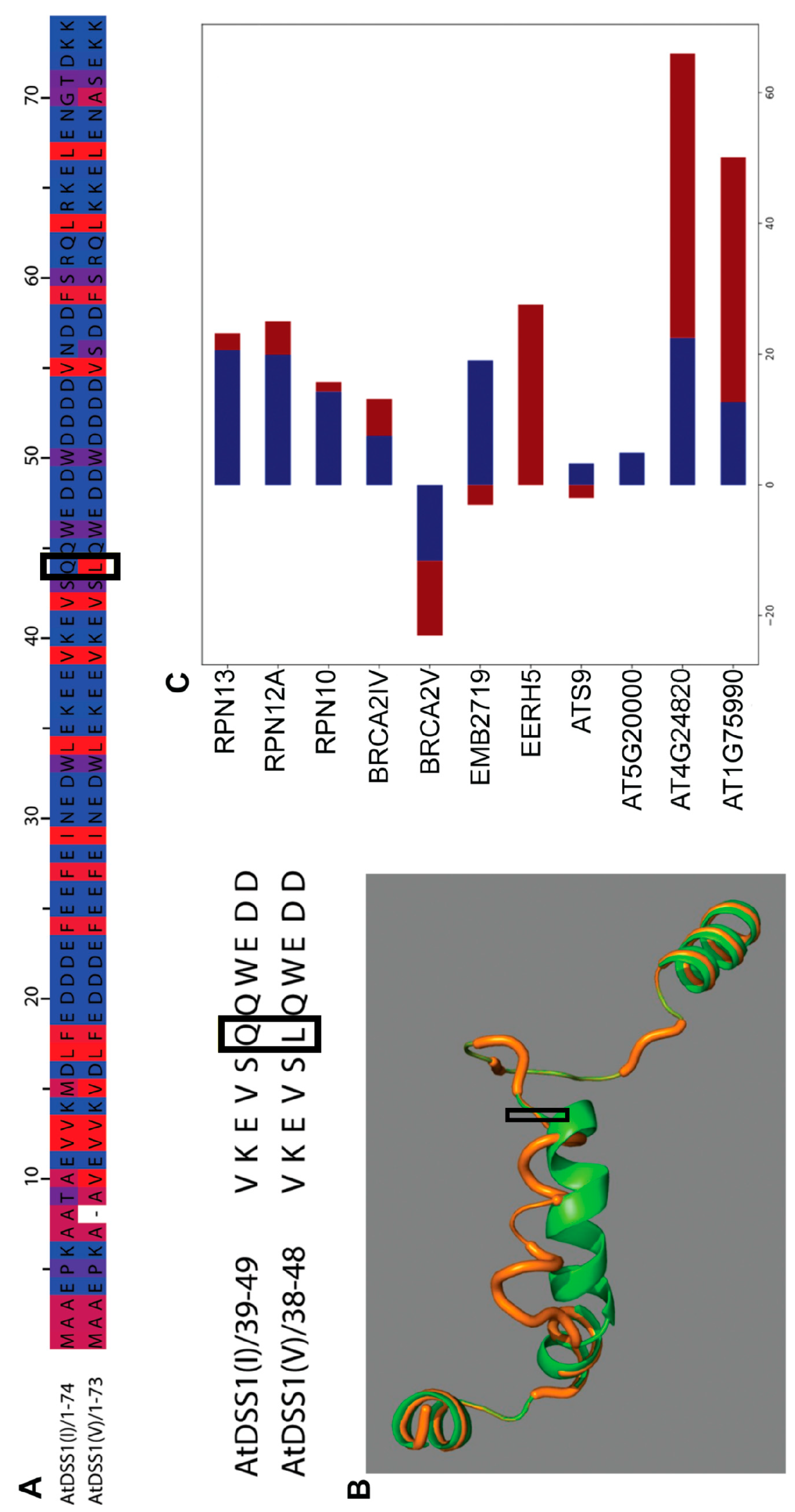
2.1. In Silico Comparative Analysis of DSS1(I) and DSS1(V) Proteins and Prediction of Their Specific Interactomes
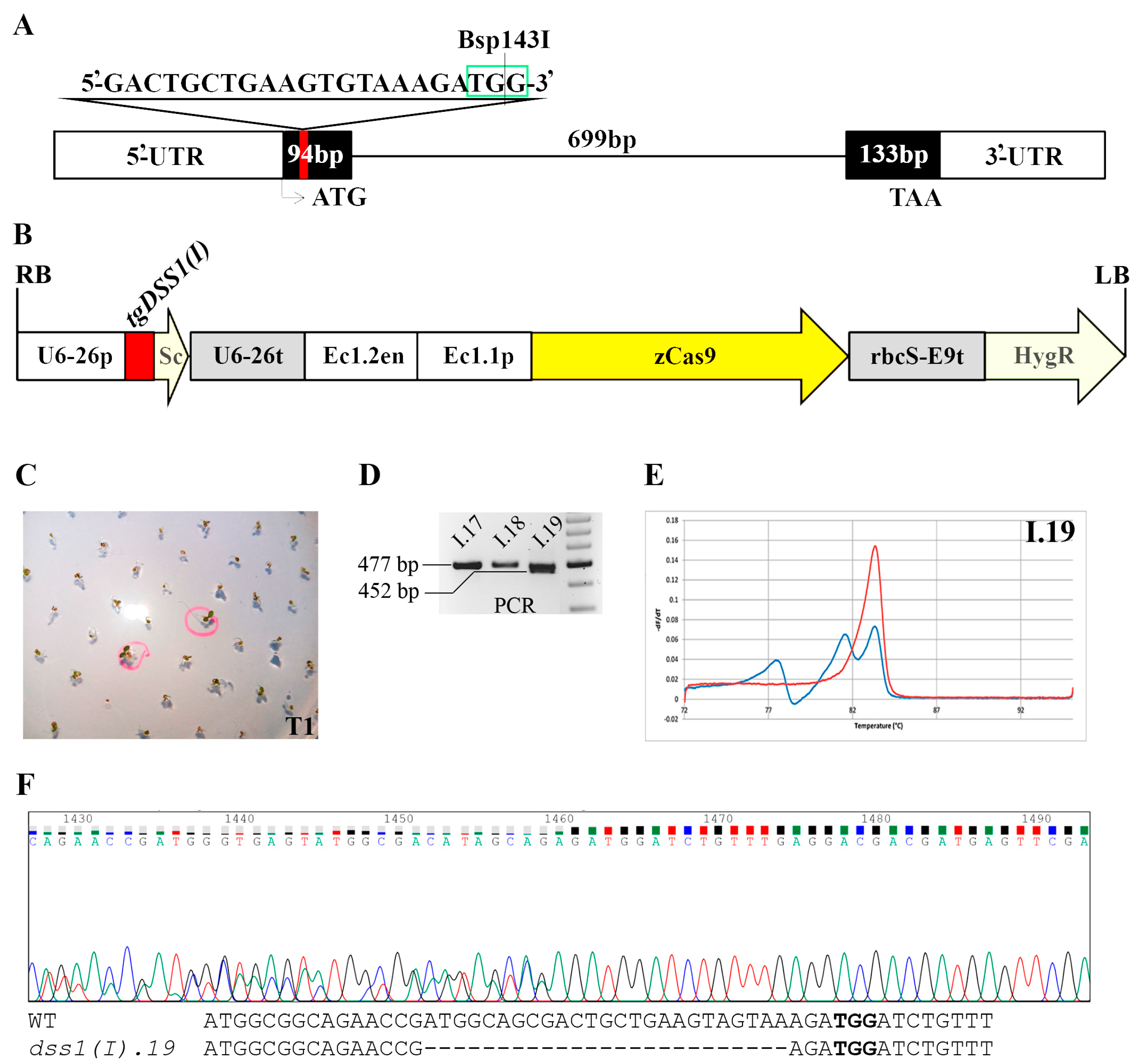
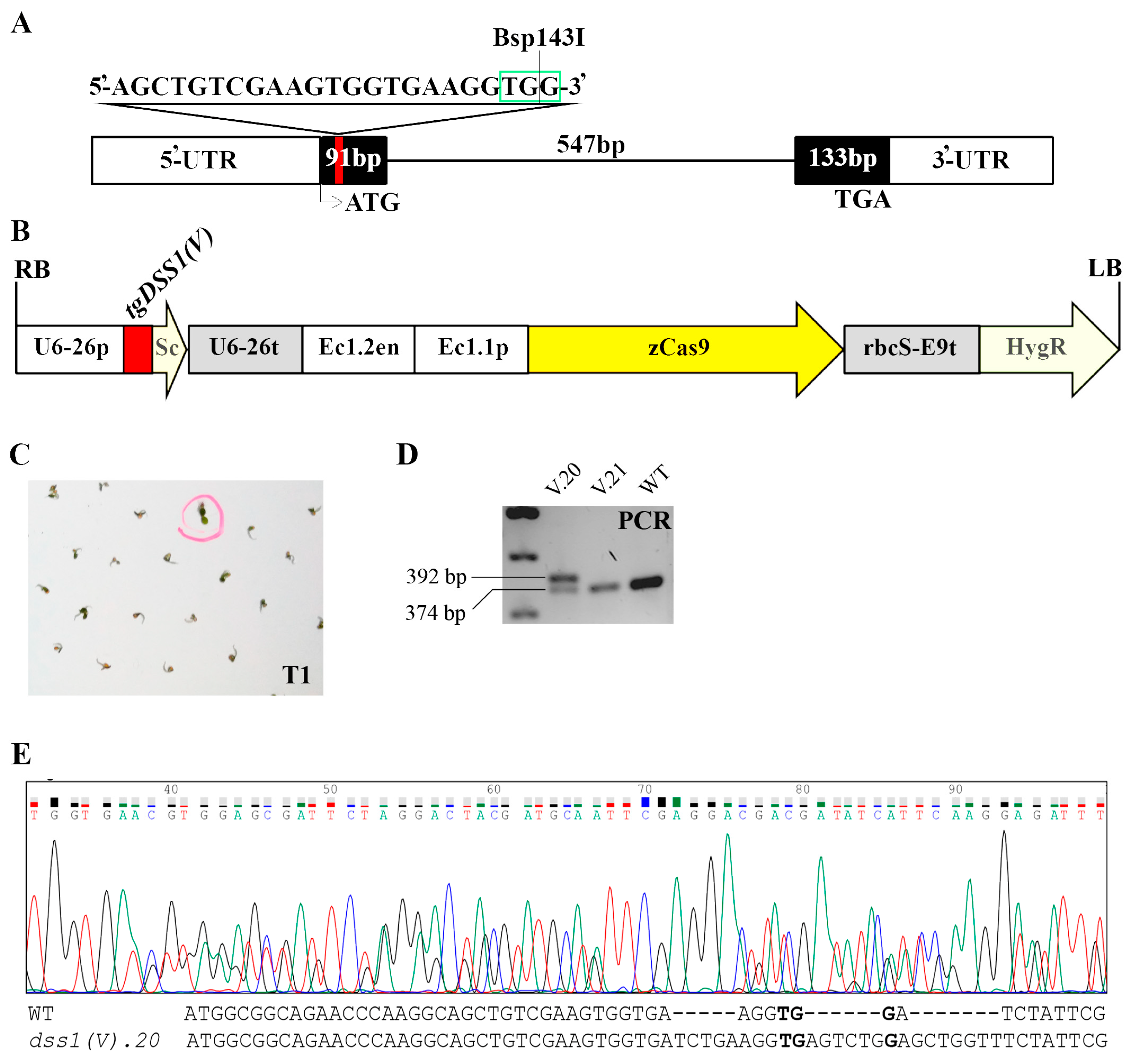
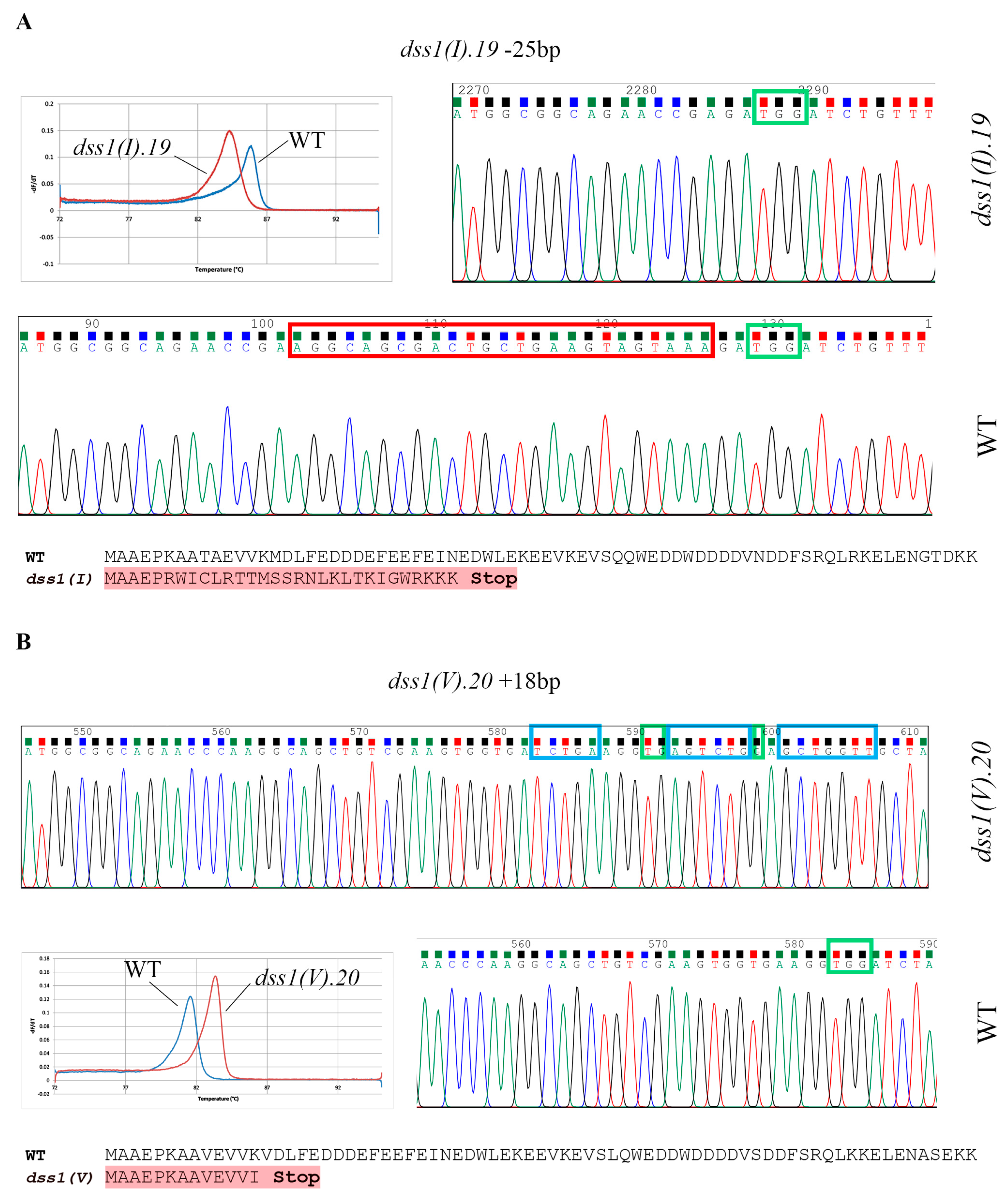
2.2. CRISPR/Cas9 Based Mutagenesis of Two Highly Homologous DSS1 Genes
2.3. Phenotypic Characterization of Arabidopsis dss1(I) and dss1(V) Mutant Lines
2.4. Sensitivity of Atdss1(I) and Atdss1(V) Lines to Oxidative Stress
3. Discussion
4. Materials and Methods
4.1. Plant Material and Treatment
4.2. In Silico Analysis of Protein Structure
4.3. sgRNA Design
4.4. Construction of the CRISPR/Cas9 Binary Vector and Plant Transformation
4.5. Genomic DNA Isolation
4.6. Workflow for Screening Gene-CRISPR/Cas9-Modified Plants
4.7. Off-Target Site Analysis
4.8. RNA Isolation and cDNA Synthesis
4.9. PCR and Gene Expression
4.10. Lipid Peroxidation Measurement
4.11. Protein Isolation and Immunoblot Analysis
4.12. OxyBlot Analysis
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crackower, M. Characterization of the Split Hand/Split Foot Malformation Locus SHFM1 at 7q21.3-Q22.1 and Analysis of a Candidate Gene for Its Expression during Limb Development. Hum. Mol. Genet. 1996, 5, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Dunker, A.K.; Garner, E.; Guilliot, S.; Romero, P.; Albrecht, K.; Hart, J.; Obradovic, Z.; Kissinger, C.; Villafranca, J.E. Protein Disorder and the Evolution of Molecular Recognition: Theory, Predictions and Observations. In Proceedings of the Pac Symp Biocomput, Maui, Hawaii, 4–9 January 1998; Volume 3, pp. 473–484. [Google Scholar]
- Kragelund, B.B.; Schenstrøm, S.M.; Rebula, C.A.; Panse, V.G.; Hartmann-Petersen, R. DSS1/Sem1, a Multifunctional and Intrinsically Disordered Protein. Trends Biochem. Sci. 2016, 41, 446–459. [Google Scholar] [CrossRef] [PubMed]
- Tompa, P. Intrinsically Unstructured Proteins. Trends Biochem. Sci. 2002, 27, 527–533. [Google Scholar] [CrossRef]
- Uversky, V.N. Natively Unfolded Proteins: A Point Where Biology Waits for Physics. Protein Sci. 2002, 11, 739–756. [Google Scholar] [CrossRef]
- Dyson, H.J.; Wright, P.E. Intrinsically Unstructured Proteins and Their Functions. Nat. Rev. Mol. Cell Biol. 2005, 6, 197–208. [Google Scholar] [CrossRef]
- Ruidiaz, S.F.; Dreier, J.E.; Hartmann-Petersen, R.; Kragelund, B.B. The Disordered PCI -Binding Human Proteins CSNAP and DSS1 Have Diverged in Structure and Function. Protein Sci. 2021, 30, 2069–2082. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi, M.; Li, X.; Velichutina, I.; Hochstrasser, M.; Kobayashi, H. Sem1, the Yeast Ortholog of a Human BRCA2-Binding Protein, is a Component of the Proteasome Regulatory Particle That Enhances Proteasome Stability. J. Cell Sci. 2004, 117, 6447–6454. [Google Scholar] [CrossRef]
- Krogan, N.J.; Lam, M.H.Y.; Fillingham, J.; Keogh, M.-C.; Gebbia, M.; Li, J.; Datta, N.; Cagney, G.; Buratowski, S.; Emili, A.; et al. Proteasome Involvement in the Repair of DNA Double-Strand Breaks. Mol. Cell 2004, 16, 1027–1034. [Google Scholar] [CrossRef]
- Sone, T.; Saeki, Y.; Toh-e, A.; Yokosawa, H. Sem1p Is a Novel Subunit of the 26 S Proteasome from Saccharomyces Cerevisiae. J. Biol. Chem. 2004, 279, 28807–28816. [Google Scholar] [CrossRef]
- Yang, H. BRCA2 Function in DNA Binding and Recombination from a BRCA2-DSS1-SsDNA Structure. Science 2002, 297, 1837–1848. [Google Scholar] [CrossRef]
- Kojic, M.; Yang, H.; Kostrub, C.F.; Pavletich, N.P.; Holloman, W.K. The BRCA2-Interacting Protein DSS1 Is Vital for DNA Repair, Recombination, and Genome Stability in Ustilago Maydis. Mol. Cell 2003, 12, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Le, H.P.; Ma, X.; Vaquero, J.; Brinkmeyer, M.; Guo, F.; Heyer, W.-D.; Liu, J. DSS1 and SsDNA Regulate Oligomerization of BRCA2. Nucleic Acids Res. 2020, 48, 7818–7833. [Google Scholar] [CrossRef] [PubMed]
- Wilmes, G.M.; Bergkessel, M.; Bandyopadhyay, S.; Shales, M.; Braberg, H.; Cagney, G.; Collins, S.R.; Whitworth, G.B.; Kress, T.L.; Weissman, J.S.; et al. A Genetic Interaction Map of RNA-Processing Factors Reveals Links between Sem1/Dss1-Containing Complexes and MRNA Export and Splicing. Mol. Cell 2008, 32, 735–746. [Google Scholar] [CrossRef]
- Faza, M.B.; Kemmler, S.; Jimeno, S.; González-Aguilera, C.; Aguilera, A.; Hurt, E.; Panse, V.G. Sem1 Is a Functional Component of the Nuclear Pore Complex–Associated Messenger RNA Export Machinery. J. Cell Biol. 2009, 184, 833–846. [Google Scholar] [CrossRef]
- Pick, E.; Hofmann, K.; Glickman, M.H. PCI Complexes: Beyond the Proteasome, CSN, and EIF3 Troika. Mol. Cell 2009, 35, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Kolog Gulko, M.; Heinrich, G.; Gross, C.; Popova, B.; Valerius, O.; Neumann, P.; Ficner, R.; Braus, G.H. Sem1 Links Proteasome Stability and Specificity to Multicellular Development. PLoS Genet. 2018, 14, e1007141. [Google Scholar] [CrossRef]
- Tomko, R.J.; Hochstrasser, M. The Intrinsically Disordered Sem1 Protein Functions as a Molecular Tether during Proteasome Lid Biogenesis. Mol. Cell 2014, 53, 433–443. [Google Scholar] [CrossRef]
- Reed, R.G.; Jobin, G.W.; Tomko, R.J. Sem1/DSS1 Accelerates ATP-Dependent Substrate Unfolding by the Proteasome through a Conformation-Dependent Intercomplex Contact. bioRxiv 2022. [Google Scholar] [CrossRef]
- Liu, J.; Doty, T.; Gibson, B.; Heyer, W.-D. Human BRCA2 Protein Promotes RAD51 Filament Formation on RPA-Covered Single-Stranded DNA. Nat. Struct. Mol. Biol. 2010, 17, 1260–1262. [Google Scholar] [CrossRef]
- Jeyasekharan, A.D.; Liu, Y.; Hattori, H.; Pisupati, V.; Jonsdottir, A.B.; Rajendra, E.; Lee, M.; Sundaramoorthy, E.; Schlachter, S.; Kaminski, C.F.; et al. A Cancer-Associated BRCA2 Mutation Reveals Masked Nuclear Export Signals Controlling Localization. Nat. Struct. Mol. Biol. 2013, 20, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, C.N.; Bystol, K.M.; Li, B.; Serrano, L.; Brenneman, M.A. Depletion of DSS1 Protein Disables Homologous Recombinational Repair in Human Cells. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2010, 694, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Vaithiyalingam, S.; San Filippo, J.; Maranon, D.G.; Jimenez-Sainz, J.; Fontenay, G.V.; Kwon, Y.; Leung, S.G.; Lu, L.; Jensen, R.B.; et al. Promotion of BRCA2-Dependent Homologous Recombination by DSS1 via RPA Targeting and DNA Mimicry. Mol. Cell 2015, 59, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Z.; Ke, J.; Hong, J.; Zhu, Z.; Niu, L. Structural Assembly of the Nucleic-Acid-Binding Thp3–Csn12–Sem1 Complex Functioning in MRNA Splicing. Nucleic Acids Res. 2022, 50, 8882–8897. [Google Scholar] [CrossRef] [PubMed]
- Ellisdon, A.M.; Dimitrova, L.; Hurt, E.; Stewart, M. Structural Basis for the Assembly and Nucleic Acid Binding of the TREX-2 Transcription-Export Complex. Nat. Struct. Mol. Biol. 2012, 19, 328–336. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, F.-M.; Huang, J.; Junco, J.J.; Maffi, S.K.; Pridgen, H.I.; Catano, G.; Dang, H.; Ding, X.; Yang, F.; et al. DSSylation, a Novel Protein Modification Targets Proteins Induced by Oxidative Stress, and Facilitates Their Degradation in Cells. Protein Cell 2014, 5, 124–140. [Google Scholar] [CrossRef]
- Dray, E.; Siaud, N.; Dubois, E.; Doutriaux, M.-P. Interaction between Arabidopsis Brca2 and Its Partners Rad51, Dmc1, and Dss1. Plant Physiol. 2006, 140, 1059–1069. [Google Scholar] [CrossRef]
- Lu, Q.; Tang, X.; Tian, G.; Wang, F.; Liu, K.; Nguyen, V.; Kohalmi, S.E.; Keller, W.A.; Tsang, E.W.T.; Harada, J.J.; et al. Arabidopsis Homolog of the Yeast TREX-2 MRNA Export Complex: Components and Anchoring Nucleoporin. Plant J. 2009, 61, 259–270. [Google Scholar] [CrossRef]
- Jäntti, J.; Lahdenranta, J.; Olkkonen, V.M.; Söderlund, H.; Keränen, S. SEM1, a Homologue of the Split Hand/Split Foot Malformation Candidate Gene Dss1, Regulates Exocytosis and Pseudohyphal Differentiation in Yeast. Proc. Natl. Acad. Sci. USA 1999, 96, 909–914. [Google Scholar] [CrossRef]
- Pispa, J.; Palmén, S.; Holmberg, C.I.; Jäntti, J.C. Elegans Dss-1 Is Functionally Conserved and Required for Oogenesis and Larval Growth. BMC Dev. Biol. 2008, 8, 51. [Google Scholar] [CrossRef]
- Nikolić, I.P.; Nešić, S.B.; Samardžić, J.T.; Timotijević, G.S. Intrinsically Disordered Protein AtDSS1(v) Participates in Plant Defense Response to Oxidative Stress. Protoplasma 2021, 258, 779–792. [Google Scholar] [CrossRef]
- Jang, G.; Joung, Y.H. CRISPR/Cas-Mediated Genome Editing for Crop Improvement: Current Applications and Future Prospects. Plant Biotechnol. Rep. 2018, 13, 1–10. [Google Scholar] [CrossRef]
- Liu, W.; Zhu, X.; Lei, M.; Xia, Q.; Botella, J.R.; Zhu, J.-K.; Mao, Y. A Detailed Procedure for CRISPR/Cas9-Mediated Gene Editing in Arabidopsis Thaliana. Sci. Bull. 2015, 60, 1332–1347. [Google Scholar] [CrossRef]
- Liu, H.; Ding, Y.; Zhou, Y.; Jin, W.; Xie, K.; Chen, L.-L. CRISPR-P 2.0: An Improved CRISPR-Cas9 Tool for Genome Editing in Plants. Mol. Plant 2017, 10, 530–532. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, D.; Ramasamy, K.; Sellamuthu, G.; Jayabalan, S.; Venkataraman, G. CRISPR for Crop Improvement: An Update Review. Front. Plant Sci. 2018, 9, 985. [Google Scholar] [CrossRef] [PubMed]
- Baysal, C.; Bortesi, L.; Zhu, C.; Farré, G.; Schillberg, S.; Christou, P. CRISPR/Cas9 Activity in the Rice OsBEIIb Gene Does Not Induce Off-Target Effects in the Closely Related Paralog OsBEIIa. Mol. Breeding 2016, 36, 108. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.-K. Abiotic Stress Responses in Plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Parvin, K.; Bhuiyan, T.F.; Anee, T.I.; Nahar, K.; Hossen, M.S.; Zulfiqar, F.; Alam, M.M.; Fujita, M. Regulation of ROS Metabolism in Plants under Environmental Stress: A Review of Recent Experimental Evidence. Int. J. Mol. Sci. 2020, 21, 8695. [Google Scholar] [CrossRef] [PubMed]
- Chmielowska-BÄk, J.; IzbiaÅska, K.; Deckert, J. Products of Lipid, Protein and RNA Oxidation as Signals and Regulators of Gene Expression in Plants. Front. Plant Sci. 2015, 6, 405. [Google Scholar] [CrossRef] [PubMed]
- Kmiecik, P.; Leonardelli, M.; Teige, M. Novel Connections in Plant Organellar Signalling Link Different Stress Responses and Signalling Pathways. J. Exp. Bot. 2016, 67, 3793–3807. [Google Scholar] [CrossRef]
- Roy, B.; Noren, S.K.; Mandal, A.B.; Basu, A.K. Genetic Engineering for Abiotic Stress Tolerance in Agricultural Crops. Biotechnology 2011, 10, 1–22. [Google Scholar] [CrossRef]
- Esmaeili, N.; Shen, G.; Zhang, H. Genetic Manipulation for Abiotic Stress Resistance Traits in Crops. Front. Plant Sci. 2022, 13, 1011985. [Google Scholar] [CrossRef] [PubMed]
- Bozorgmehr, J.E.H. The Effect of Functional Compensation among Duplicate Genes Can Constrain Their Evolutionary Divergence. J. Genet. 2012, 91, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Panchy, N.; Lehti-Shiu, M.; Shiu, S.-H. Evolution of Gene Duplication in Plants. Plant Physiol. 2016, 171, 2294–2316. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Yan, L.; Zheng, Z.; Zhang, Y.; Zhan, H.; Tian, Y.; Zhang, T.; Li, R.; Gong, X.; Xu, M.; et al. Editing Gene Families by CRISPR/Cas9: Accelerating the Isolation of Multiple Transgene-Free Null Mutant Combinations with Much Reduced Labor-Intensive Analysis. Plant Biotechnol. J. 2021, 20, 241–243. [Google Scholar] [CrossRef]
- Hahn, F.; Nekrasov, V. CRISPR/Cas Precision: Do We Need to Worry about Off-Targeting in Plants? Plant Cell Rep. 2018, 38, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Durrant, W.E.; Song, J.; Spivey, N.W.; Dong, X. Arabidopsis BRCA2 and RAD51 Proteins Are Specifically Involved in Defense Gene Transcription during Plant Immune Responses. Proc. Natl. Acad. Sci. USA 2010, 107, 22716–22721. [Google Scholar] [CrossRef]
- Zdarska, M.; Dobisová, T.; Gelová, Z.; Pernisová, M.; Dabravolski, S.; Hejátko, J. Illuminating Light, Cytokinin, and Ethylene Signalling Crosstalk in Plant Development. J. Exp. Bot. 2015, 66, 4913–4931. [Google Scholar] [CrossRef]
- Vaseva, I.I.; Vandenbussche, F.; Simon, D.; Vissenberg, K.; Van Der Straeten, D. Cell Type Specificity of Plant Hormonal Signals: Case Studies and Reflections on Ethylene. Russ. J. Plant Physiol. 2016, 63, 577–586. [Google Scholar] [CrossRef]
- Shi, H.; Shen, X.; Liu, R.; Xue, C.; Wei, N.; Deng, X.; Zhong, S. The Red Light Receptor Phytochrome B Directly Enhances Substrate-E3 Ligase Interactions to Attenuate Ethylene Responses. Dev. Cell 2016, 39, 597–610. [Google Scholar] [CrossRef]
- Seeliger, K.; Dukowic-Schulze, S.; Wurz-Wildersinn, R.; Pacher, M.; Puchta, H. BRCA2 Is a Mediator of RAD51- and DMC1-Facilitated Homologous Recombination in Arabidopsis Thaliana. New Phytol. 2011, 193, 364–375. [Google Scholar] [CrossRef]
- Yang, H.; Li, Q.; Fan, J.; Holloman, W.K.; Pavletich, N.P. The BRCA2 Homologue Brh2 Nucleates RAD51 Filament Formation at a DsDNA–SsDNA Junction. Nature 2005, 433, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Kojic, M.; Zhou, Q.; Lisby, M.; Holloman, W.K. Brh2-Dss1 Interplay Enables Properly Controlled Recombination in Ustilago Maydis. Mol. Cell Biol. 2005, 25, 2547–2557. [Google Scholar] [CrossRef]
- Furger, A. Promoter Proximal Splice Sites Enhance Transcription. Genes. Dev. 2002, 16, 2792–2799. [Google Scholar] [CrossRef] [PubMed]
- Heyn, P.; Kalinka, A.T.; Tomancak, P.; Neugebauer, K.M. Introns and Gene Expression: Cellular Constraints, Transcriptional Regulation, and Evolutionary Consequences. BioEssays 2014, 37, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Gallegos, J.E.; Rose, A.B. Intron DNA Sequences Can Be More Important than the Proximal Promoter in Determining the Site of Transcript Initiation. Plant Cell 2017, 29, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Ambros, V.; Lee, R.C.; Lavanway, A.; Williams, P.T.; Jewell, D. MicroRNAs and Other Tiny Endogenous RNAs in C. Elegans. Curr. Biol. 2003, 13, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-L.; Chang, D.; Wu, D.-Y.; Ying, S.-Y. A Novel RNA Splicing-Mediated Gene Silencing Mechanism Potential for Genome Evolution. Biochem. Biophys. Res. Commun. 2003, 310, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A. Identification of Mammalian MicroRNA Host Genes and Transcription Units. Genome Res. 2004, 14, 1902–1910. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Shao, C. Large-Scale Identification of Mirtrons in Arabidopsis and Rice. PLoS ONE 2012, 7, e31163. [Google Scholar] [CrossRef]
- Zhu, F.; Zhang, Q.; Che, Y.; Zhu, P.; Zhang, Q.; Ji, Z. Glutathione Contributes to Resistance Responses to TMV through a Differential Modulation of Salicylic Acid and Reactive Oxygen Species. Mol. Plant Pathol. 2021, 22, 1668–1687. [Google Scholar] [CrossRef]
- Uzilday, B.; Ozgur, R.; Sekmen, A.H.; Turkan, I. Endoplasmic Reticulum Stress Regulates Glutathione Metabolism and Activities of Glutathione Related Enzymes in Arabidopsis. Funct. Plant Biol. 2018, 45, 284. [Google Scholar] [CrossRef] [PubMed]
- Marston, N.J.; Richards, W.J.; Hughes, D.; Bertwistle, D.; Marshall, C.J.; Ashworth, A. Interaction between the product of the breast cancer susceptibility gene BRCA2 and DSS1, a protein functionally conserved from yeast to mammals. Mol. Cell Biol. 1999, 19, 4633–4642. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.J.; Kuhn, M.; Stark, M.; Chaffron, S.; Creevey, C.; Muller, J.; Doerks, T.; Julien, P.; Roth, A.; Simonovic, M.; et al. STRING 8--a Global View on Proteins and Their Functional Interactions in 630 Organisms. Nucleic Acids Res. 2009, 37 (Suppl. S1), D412–D416. [Google Scholar] [CrossRef]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J.E. The Phyre2 Web Portal for Protein Modeling, Prediction and Analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Luan, B.; Wu, J.; Shi, Y. An Atomic Structure of the Human 26S Proteasome. Nat. Struct. Mol. Biol. 2016, 23, 778–785. [Google Scholar] [CrossRef]
- Duhovny, D.; Nussinov, R.; Wolfson, H.J. Efficient Unbound Docking of Rigid Molecules. In Algorithms in Bioinformatics; Guigó, R., Gusfield, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2002; Volume 2452, pp. 185–200. [Google Scholar] [CrossRef]
- Andrusier, N.; Nussinov, R.; Wolfson, H.J. FireDock: Fast Interaction Refinement in Molecular Docking. Proteins. Struct. Funct. Genet. 2007, 69, 139–159. [Google Scholar] [CrossRef]
- Zhang, C.; Vasmatzis, G.; Cornette, J.L.; DeLisi, C. Determination of Atomic Desolvation Energies from the Structures of Crystallized Proteins. J. Mol. Biol. 1997, 267, 707–726. [Google Scholar] [CrossRef]
- Labun, K.; Montague, T.G.; Gagnon, J.A.; Thyme, S.B.; Valen, E. CHOPCHOP V2: A Web Tool for the next Generation of CRISPR Genome Engineering. Nucleic Acids Res. 2016, 44, W272–W276. [Google Scholar] [CrossRef]
- Inoue, H.; Nojima, H.; Okayama, H. High Efficiency Transformation of Escherichia Coli with Plasmids. Gene 1990, 96, 23–28. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral Dip: A Simplified Method For Agrobacterium-Mediated Transformation of Arabidopsis Thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, E.K.; Chen, T.; Amendola, M.; van Steensel, B. Easy Quantitative Assessment of Genome Editing by Sequence Trace Decomposition. Nucleic Acids Res. Spec. Publ. 2014, 42, e168. [Google Scholar] [CrossRef] [PubMed]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the Thiobarbituric Acid-Reactive-Substances Assay for Estimating Lipid Peroxidation in Plant Tissues Containing Anthocyanin and Other Interfering Compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolić, I.; Samardžić, J.; Stevanović, S.; Miljuš-Đukić, J.; Milisavljević, M.; Timotijević, G. CRISPR/Cas9-Targeted Disruption of Two Highly Homologous Arabidopsis thaliana DSS1 Genes with Roles in Development and the Oxidative Stress Response. Int. J. Mol. Sci. 2023, 24, 2442. https://doi.org/10.3390/ijms24032442
Nikolić I, Samardžić J, Stevanović S, Miljuš-Đukić J, Milisavljević M, Timotijević G. CRISPR/Cas9-Targeted Disruption of Two Highly Homologous Arabidopsis thaliana DSS1 Genes with Roles in Development and the Oxidative Stress Response. International Journal of Molecular Sciences. 2023; 24(3):2442. https://doi.org/10.3390/ijms24032442
Chicago/Turabian StyleNikolić, Ivana, Jelena Samardžić, Strahinja Stevanović, Jovanka Miljuš-Đukić, Mira Milisavljević, and Gordana Timotijević. 2023. "CRISPR/Cas9-Targeted Disruption of Two Highly Homologous Arabidopsis thaliana DSS1 Genes with Roles in Development and the Oxidative Stress Response" International Journal of Molecular Sciences 24, no. 3: 2442. https://doi.org/10.3390/ijms24032442
APA StyleNikolić, I., Samardžić, J., Stevanović, S., Miljuš-Đukić, J., Milisavljević, M., & Timotijević, G. (2023). CRISPR/Cas9-Targeted Disruption of Two Highly Homologous Arabidopsis thaliana DSS1 Genes with Roles in Development and the Oxidative Stress Response. International Journal of Molecular Sciences, 24(3), 2442. https://doi.org/10.3390/ijms24032442






