Pre-Molten, Wet, and Dry Molten Globules en Route to the Functional State of Proteins
Abstract
1. Introduction
2. Molten Globule as an (Un)Folding Intermediate
3. Potential Functionality of Folding Intermediates
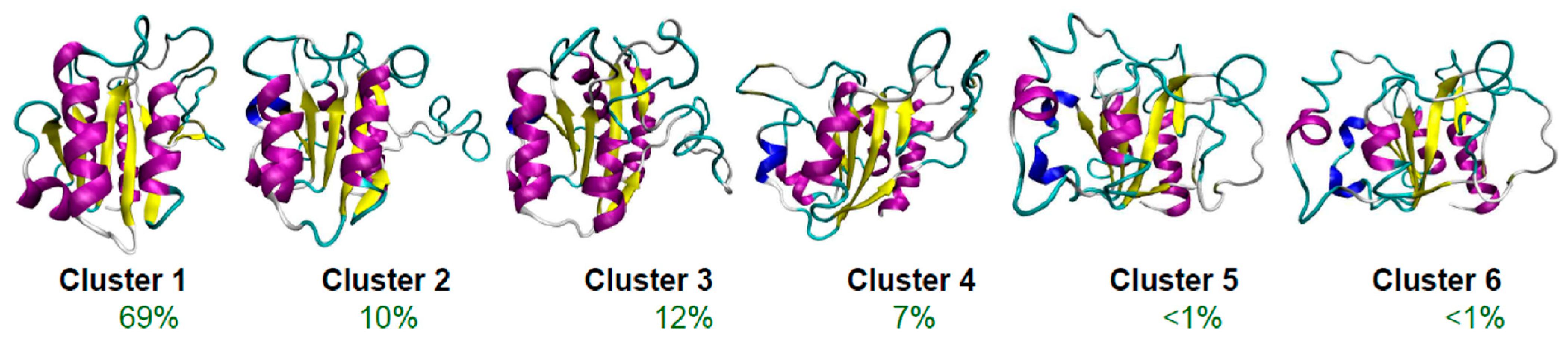
4. How Can One Find Molten Globules, and Where Can They Be Found?
5. Baroenzymology, Cryoenzymology and Molten Globules
6. Molten Globules and Intrinsic Disorder in Proteins
7. Engineered Molten Globules
8. Macromolecular Crowding and Molten Globules
9. Interactions of Nanomaterials with Molten Globules
10. Dry and Wet Molten Globules
11. Pre-Molten Globule States
12. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lesk, A. Introduction to Protein Science: Architecture, Function, and Genomics; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Anfinsen, C.B. Principles that govern the folding of protein chains. Science 1973, 181, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Anfinsen, C.B.; Haber, E.; Sela, M.; White, F.H., Jr. The kinetics of formation of native ribonuclease during oxidation of the reduced polypeptide chain. Proc. Natl. Acad. Sci. USA 1961, 47, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- White, F.H., Jr. Regeneration of native secondary and tertiary structures by air oxidation of reduced ribonuclease. J. Biol. Chem. 1961, 236, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Anfinsen, C.B.; Haber, E. Studies on the reduction and re-formation of protein disulfide bonds. J. Biol. Chem. 1961, 236, 1361–1363. [Google Scholar] [CrossRef]
- Seckler, R.; Jaenicke, R. Protein folding and protein refolding. FASEB J. 1992, 6, 2545–2552. [Google Scholar] [CrossRef]
- Tsytlonok, M.; Itzhaki, L.S. The how’s and why’s of protein folding intermediates. Arch. Biochem. Biophys. 2013, 531, 14–23. [Google Scholar] [CrossRef]
- Privalov, P.L. Stability of proteins: Small globular proteins. Adv. Protein Chem. 1979, 33, 167–241. [Google Scholar] [CrossRef] [PubMed]
- Tanford, C. Protein denaturation. Adv. Protein Chem. 1968, 23, 121–282. [Google Scholar] [CrossRef]
- Gil’manshin, R.I.; Dolgikh, D.A.; Ptitsyn, O.B.; Finkel’shtein, A.V.; Shakhnovich, E.I. Protein globule without the unique three-dimensional structure: Experimental data for alpha-lactalbumins and general model. Biofizika 1982, 27, 1005–1016. [Google Scholar]
- Dolgikh, D.A.; Gilmanshin, R.I.; Brazhnikov, E.V.; Bychkova, V.E.; Semisotnov, G.V.; Venyaminov, S.; Ptitsyn, O.B. Alpha-Lactalbumin: Compact state with fluctuating tertiary structure? FEBS Lett. 1981, 136, 311–315. [Google Scholar] [CrossRef]
- Ohgushi, M.; Wada, A. ‘Molten-globule state’: A compact form of globular proteins with mobile side-chains. FEBS Lett. 1983, 164, 21–24. [Google Scholar] [CrossRef]
- Ptitsyn, O.B.; Dolgikh, D.A.; Gil’manshin, R.I.; Shakhnovich, E.I.; Finkel’shtein, A.V. Fluctuating state of the protein globule. Mol. Biol. 1983, 17, 569–576. [Google Scholar]
- Kuwajima, K. The molten globule state as a clue for understanding the folding and cooperativity of globular-protein structure. Proteins 1989, 6, 87–103. [Google Scholar] [CrossRef]
- Ptitsyn, O.B. Structures of folding intermediates. Curr. Opin. Struct. Biol. 1995, 5, 74–78. [Google Scholar] [CrossRef]
- Ptitsyn, O.B. Molten globule and protein folding. Adv. Protein Chem. 1995, 47, 83–229. [Google Scholar] [CrossRef]
- Arai, M.; Kuwajima, K. Role of the molten globule state in protein folding. Adv. Protein Chem. 2000, 53, 209–282. [Google Scholar] [CrossRef]
- Bychkova, V.E.; Semisotnov, G.V.; Balobanov, V.A.; Finkelstein, A.V. The Molten Globule Concept: 45 Years Later. Biochemistry 2018, 83, S33–S47. [Google Scholar] [CrossRef]
- Baldwin, R.L.; Rose, G.D. Molten globules, entropy-driven conformational change and protein folding. Curr. Opin. Struct. Biol. 2013, 23, 4–10. [Google Scholar] [CrossRef]
- Balobanov, V.A.; Katina, N.S.; Finkelstein, A.V.; Bychkova, V.E. Intermediate States of Apomyoglobin: Are They Parts of the Same Area of Conformations Diagram? Biochemistry 2017, 82, 625–631. [Google Scholar] [CrossRef]
- Kharakoz, D.P.; Bychkova, V.E. Molten globule of human alpha-lactalbumin: Hydration, density, and compressibility of the interior. Biochemistry 1997, 36, 1882–1890. [Google Scholar] [CrossRef]
- Georlette, D.; Blaise, V.; Bouillenne, F.; Damien, B.; Thorbjarnardottir, S.H.; Depiereux, E.; Gerday, C.; Uversky, V.N.; Feller, G. Adenylation-dependent conformation and unfolding pathways of the NAD+-dependent DNA ligase from the thermophile Thermus scotoductus. Biophys. J. 2004, 86, 1089–1104. [Google Scholar] [CrossRef]
- Georlette, D.; Blaise, V.; Dohmen, C.; Bouillenne, F.; Damien, B.; Depiereux, E.; Gerday, C.; Uversky, V.N.; Feller, G. Cofactor binding modulates the conformational stabilities and unfolding patterns of NAD(+)-dependent DNA ligases from Escherichia coli and Thermus scotoductus. J. Biol. Chem. 2003, 278, 49945–49953. [Google Scholar] [CrossRef]
- Uversky, V.N.; Ptitsyn, O.B. Further evidence on the equilibrium “pre-molten globule state”: Four-state guanidinium chloride-induced unfolding of carbonic anhydrase B at low temperature. J. Mol. Biol. 1996, 255, 215–228. [Google Scholar] [CrossRef]
- Ptitsyn, O.B.; Bychkova, V.E.; Uversky, V.N. Kinetic and equilibrium folding intermediates. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1995, 348, 35–41. [Google Scholar] [CrossRef]
- Uversky, V.N.; Ptitsyn, O.B. “Partly folded” state, a new equilibrium state of protein molecules: Four-state guanidinium chloride-induced unfolding of beta-lactamase at low temperature. Biochemistry 1994, 33, 2782–2791. [Google Scholar] [CrossRef]
- Ptitsyn, O.B. Stages in the mechanism of self-organization of protein molecules. Dokl. Akad. Nauk. SSSR 1973, 210, 1213–1215. [Google Scholar]
- Sarkar, S.S.; Udgaonkar, J.B.; Krishnamoorthy, G. Unfolding of a small protein proceeds via dry and wet globules and a solvated transition state. Biophys. J. 2013, 105, 2392–2402. [Google Scholar] [CrossRef]
- Acharya, N.; Jha, S.K. Dry Molten Globule-Like Intermediates in Protein Folding, Function, and Disease. J. Phys. Chem. B 2022, 126, 8614–8622. [Google Scholar] [CrossRef]
- Acharya, N.; Mishra, P.; Jha, S.K. A dry molten globule-like intermediate during the base-induced unfolding of a multidomain protein. Phys. Chem. Chem. Phys. 2017, 19, 30207–30216. [Google Scholar] [CrossRef]
- de Oliveira, G.A.P.; Silva, J.L. The push-and-pull hypothesis in protein unfolding, misfolding and aggregation. Biophys. Chem. 2017, 231, 20–26. [Google Scholar] [CrossRef]
- Neumaier, S.; Kiefhaber, T. Redefining the dry molten globule state of proteins. J. Mol. Biol. 2014, 426, 2520–2528. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.K.; Marqusee, S. Kinetic evidence for a two-stage mechanism of protein denaturation by guanidinium chloride. Proc. Natl. Acad. Sci. USA 2014, 111, 4856–4861. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, R.L.; Frieden, C.; Rose, G.D. Dry molten globule intermediates and the mechanism of protein unfolding. Proteins 2010, 78, 2725–2737. [Google Scholar] [CrossRef] [PubMed]
- Reiner, A.; Henklein, P.; Kiefhaber, T. An unlocking/relocking barrier in conformational fluctuations of villin headpiece subdomain. Proc. Natl. Acad. Sci. USA 2010, 107, 4955–4960. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.K.; Udgaonkar, J.B. Direct evidence for a dry molten globule intermediate during the unfolding of a small protein. Proc. Natl. Acad. Sci. USA 2009, 106, 12289–12294. [Google Scholar] [CrossRef] [PubMed]
- Rami, B.R.; Udgaonkar, J.B. Mechanism of formation of a productive molten globule form of barstar. Biochemistry 2002, 41, 1710–1716. [Google Scholar] [CrossRef] [PubMed]
- Kiefhaber, T.; Labhardt, A.M.; Baldwin, R.L. Direct NMR evidence for an intermediate preceding the rate-limiting step in the unfolding of ribonuclease A. Nature 1995, 375, 513–515. [Google Scholar] [CrossRef]
- Finkelstein, A.V.; Shakhnovich, E.I. Theory of cooperative transitions in protein molecules. II. Phase diagram for a protein molecule in solution. Biopolymers 1989, 28, 1681–1694. [Google Scholar] [CrossRef]
- Gupta, M.N.; Uversky, V.N. Macromolecular crowding: How it affects protein structure, disorder, and catalysis. In Structure and Intrinsic Disorder in Enzymology; Elsevier: Amsterdam, The Netherlands, 2023; pp. 353–376. [Google Scholar]
- Gupta, M.N.; Uversky, V.N. Structure and disorder: Protein functions depend on this new binary transforming lock-and-key into structure-function continuum. In Structure and Intrinsic Disorder in Enzymology; Elsevier: Amsterdam, The Netherlands, 2023; pp. 127–148. [Google Scholar]
- Edsall, J.T. Hsien Wu and the First Theory of Protein Denaturation (1931). Adv. Protein Chem. 1995, 46, 1–5. [Google Scholar] [CrossRef]
- Wu, H. Studies of Denaturation of Proteins XIII. A Theory of Denaturation. Chin. J. Physiol. 1931, 5, 321–344. [Google Scholar]
- Anson, M.L.; Mirsky, A.E. On Some General Properties of Proteins. J. Gen. Physiol. 1925, 9, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Mirsky, A.E.; Pauling, L. On the Structure of Native, Denatured, and Coagulated Proteins. Proc. Natl. Acad. Sci. USA 1936, 22, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Neurath, H.; Greenstein, J.P.; Putnam, F.W.; Erickson, J.A. The chemistry of protein denaturation. Chem. Rev. 1944, 34, 157–265. [Google Scholar] [CrossRef]
- Greenstein, J.P. Sulfhydryl groups in proteins: I. Egg albumin in solutions of urea, guanidine, and their derivatives. J. Biol. Chem. 1938, 125, 501–513. [Google Scholar] [CrossRef]
- Greenstein, J.P. Sulfhydryl groups in proteins: II. Edestin, Excelsin, and Globin in solutions of guanidine hydrochloride, urea, and their derivatives. J. Biol. Chem. 1939, 128, 233–240. [Google Scholar] [CrossRef]
- Greenstein, J.P. Sulfhydryl groups in proteins: III. The effect on egg albumin of various salts of guanidine. J. Biol. Chem. 1939, 130, 519–526. [Google Scholar] [CrossRef]
- Neurath, H.; Cooper, G.R.; Erickson, J.O. The denaturation of proteins and its apparent reversal: I. horse serum albumin. J. Biol. Chem. 1942, 142, 249–263. [Google Scholar] [CrossRef]
- Neurath, H.; Cooper, G.R.; Erickson, J.O. The denaturation of proteins and its apparent reversal: II. horse serum pseudoglobulin. J. Biol. Chem. 1942, 142, 265–276. [Google Scholar] [CrossRef]
- Aune, K.C.; Salahuddin, A.; Zarlengo, M.H.; Tanford, C. Evidence for residual structure in acid- and heat-denatured proteins. J. Biol. Chem. 1967, 242, 4486–4489. [Google Scholar] [CrossRef]
- Wong, K.P.; Tanford, C. Denaturation of bovine carbonic anhydrase B by guanidine hydrochloride. A process involving separable sequential conformational transitions. J. Biol. Chem. 1973, 248, 8518–8523. [Google Scholar] [CrossRef]
- Wong, K.P.; Hamlin, L.M. Acid denaturation of bovine carbonic anhydrase B. Biochemistry 1974, 13, 2678–2683. [Google Scholar] [CrossRef] [PubMed]
- Dobson, C.M. Protein folding. Solid evidence for molten globules. Curr. Biol. 1994, 4, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N. Protein folding revisited. A polypeptide chain at the folding-misfolding-nonfolding cross-roads: Which way to go? Cell. Mol. Life Sci. 2003, 60, 1852–1871. [Google Scholar] [CrossRef] [PubMed]
- Tcherkasskaya, O.; Uversky, V.N. Polymeric aspects of protein folding: A brief overview. Protein Pept. Lett. 2003, 10, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Uverskii, V.N. How many molten globules states exist? Biofizika 1998, 43, 416–421. [Google Scholar]
- Ikeguchi, M.; Fujino, M.; Kato, M.; Kuwajima, K.; Sugai, S. Transition state in the folding of alpha-lactalbumin probed by the 6–120 disulfide bond. Protein Sci. 1998, 7, 1564–1574. [Google Scholar] [CrossRef]
- Ptitsyn, O. How molten is the molten globule? Nat. Struct. Biol. 1996, 3, 488–490. [Google Scholar] [CrossRef]
- Fink, A.L. Compact intermediates states in protein folding. Subcell. Biochem. 1995, 24, 27–53. [Google Scholar] [CrossRef]
- Ewbank, J.J.; Creighton, T.E.; Hayer-Hartl, M.K.; Ulrich Hartl, F. What is the molten globule? Nat. Struct. Biol. 1995, 2, 10–11. [Google Scholar] [CrossRef]
- Fink, A.L. Compact intermediate states in protein folding. Annu. Rev. Biophys. Biomol. Struct. 1995, 24, 495–522. [Google Scholar] [CrossRef]
- Fink, A.L. Molten globules. Methods Mol. Biol. 1995, 40, 343–360. [Google Scholar] [CrossRef] [PubMed]
- Vassilenko, K.S.; Uversky, V.N. Native-like secondary structure of molten globules. Biochim. Biophys. Acta 2002, 1594, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Redfield, C. Using nuclear magnetic resonance spectroscopy to study molten globule states of proteins. Methods 2004, 34, 121–132. [Google Scholar] [CrossRef]
- Krishna, M.M.; Hoang, L.; Lin, Y.; Englander, S.W. Hydrogen exchange methods to study protein folding. Methods 2004, 34, 51–64. [Google Scholar] [CrossRef]
- Bracken, C. NMR spin relaxation methods for characterization of disorder and folding in proteins. J. Mol. Graph. Model. 2001, 19, 3–12. [Google Scholar] [CrossRef]
- Bose, H.S.; Whittal, R.M.; Baldwin, M.A.; Miller, W.L. The active form of the steroidogenic acute regulatory protein, StAR, appears to be a molten globule. Proc. Natl. Acad. Sci. USA 1999, 96, 7250–7255. [Google Scholar] [CrossRef] [PubMed]
- Eliezer, D.; Yao, J.; Dyson, H.J.; Wright, P.E. Structural and dynamic characterization of partially folded states of apomyoglobin and implications for protein folding. Nat. Struct. Biol. 1998, 5, 148–155. [Google Scholar] [CrossRef]
- Wu, L.C.; Laub, P.B.; Elove, G.A.; Carey, J.; Roder, H. A noncovalent peptide complex as a model for an early folding intermediate of cytochrome c. Biochemistry 1993, 32, 10271–10276. [Google Scholar] [CrossRef]
- Chyan, C.L.; Wormald, C.; Dobson, C.M.; Evans, P.A.; Baum, J. Structure and stability of the molten globule state of guinea-pig alpha-lactalbumin: A hydrogen exchange study. Biochemistry 1993, 32, 5681–5691. [Google Scholar] [CrossRef]
- Jeng, M.F.; Englander, S.W.; Elove, G.A.; Wand, A.J.; Roder, H. Structural description of acid-denatured cytochrome c by hydrogen exchange and 2D NMR. Biochemistry 1990, 29, 10433–10437. [Google Scholar] [CrossRef]
- Bushnell, G.W.; Louie, G.V.; Brayer, G.D. High-resolution three-dimensional structure of horse heart cytochrome c. J. Mol. Biol. 1990, 214, 585–595. [Google Scholar] [CrossRef]
- Baum, J.; Dobson, C.M.; Evans, P.A.; Hanley, C. Characterization of a partly folded protein by NMR methods: Studies on the molten globule state of guinea pig alpha-lactalbumin. Biochemistry 1989, 28, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Hsu, D.J.; Leshchev, D.; Kosheleva, I.; Kohlstedt, K.L.; Chen, L.X. Unfolding bovine alpha-lactalbumin with T-jump: Characterizing disordered intermediates via time-resolved x-ray solution scattering and molecular dynamics simulations. J. Chem. Phys. 2021, 154, 105101. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N.; Karnoup, A.S.; Segel, D.J.; Seshadri, S.; Doniach, S.; Fink, A.L. Anion-induced folding of Staphylococcal nuclease: Characterization of multiple equilibrium partially folded intermediates. J. Mol. Biol. 1998, 278, 879–894. [Google Scholar] [CrossRef]
- Semisotnov, G.V.; Kihara, H.; Kotova, N.V.; Kimura, K.; Amemiya, Y.; Wakabayashi, K.; Serdyuk, I.N.; Timchenko, A.A.; Chiba, K.; Nikaido, K.; et al. Protein globularization during folding. A study by synchrotron small-angle X-ray scattering. J. Mol. Biol. 1996, 262, 559–574. [Google Scholar] [CrossRef]
- Eliezer, D.; Chiba, K.; Tsuruta, H.; Doniach, S.; Hodgson, K.O.; Kihara, H. Evidence of an associative intermediate on the myoglobin refolding pathway. Biophys. J. 1993, 65, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, M.; Hagihara, Y.; Mihara, K.; Goto, Y. Molten globule of cytochrome c studied by small angle X-ray scattering. J. Mol. Biol. 1993, 229, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, M.; Kuwajima, K.; Tokunaga, F.; Goto, Y. Structural characterization of the molten globule of alpha-lactalbumin by solution X-ray scattering. Protein Sci. 1997, 6, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N. Use of fast protein size-exclusion liquid chromatography to study the unfolding of proteins which denature through the molten globule. Biochemistry 1993, 32, 13288–13298. [Google Scholar] [CrossRef]
- Uversky, V.N. Molten globular enzymes. In Structure and Intrinsic Disorder in Enzymology; Elsevier: Amsterdam, The Netherlands, 2023; pp. 303–325. [Google Scholar]
- Fontana, A.; de Laureto, P.P.; Spolaore, B.; Frare, E.; Picotti, P.; Zambonin, M. Probing protein structure by limited proteolysis. Acta Biochim. Pol. 2004, 51, 299–321. [Google Scholar] [CrossRef]
- Fontana, A.; Polverino de Laureto, P.; De Filippis, V.; Scaramella, E.; Zambonin, M. Probing the partly folded states of proteins by limited proteolysis. Fold. Des. 1997, 2, R17–R26. [Google Scholar] [CrossRef] [PubMed]
- Merrill, A.R.; Cohen, F.S.; Cramer, W.A. On the nature of the structural change of the colicin E1 channel peptide necessary for its translocation-competent state. Biochemistry 1990, 29, 5829–5836. [Google Scholar] [CrossRef] [PubMed]
- Polverino de Laureto, P.; De Filippis, V.; Di Bello, M.; Zambonin, M.; Fontana, A. Probing the molten globule state of alpha-lactalbumin by limited proteolysis. Biochemistry 1995, 34, 12596–12604. [Google Scholar] [CrossRef]
- Polverino de Laureto, P.; Frare, E.; Gottardo, R.; Fontana, A. Molten globule of bovine alpha-lactalbumin at neutral pH induced by heat, trifluoroethanol, and oleic acid: A comparative analysis by circular dichroism spectroscopy and limited proteolysis. Proteins 2002, 49, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Polverino de Laureto, P.; Frare, E.; Gottardo, R.; Van Dael, H.; Fontana, A. Partly folded states of members of the lysozyme/lactalbumin superfamily: A comparative study by circular dichroism spectroscopy and limited proteolysis. Protein Sci. 2002, 11, 2932–2946. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N.; Winter, S.; Lober, G. Use of fluorescence decay times of 8-ANS-protein complexes to study the conformational transitions in proteins which unfold through the molten globule state. Biophys. Chem. 1996, 60, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Palleros, D.R.; Fink, A.L. Protein conformational changes induced by 1,1’-bis(4-anilino-5-naphthalenesulfonic acid): Preferential binding to the molten globule of DnaK. Biochemistry 1994, 33, 7536–7546. [Google Scholar] [CrossRef] [PubMed]
- Semisotnov, G.V.; Rodionova, N.A.; Razgulyaev, O.I.; Uversky, V.N.; Gripas, A.F.; Gilmanshin, R.I. Study of the “molten globule” intermediate state in protein folding by a hydrophobic fluorescent probe. Biopolymers 1991, 31, 119–128. [Google Scholar] [CrossRef]
- Regan, L. Molten globules move into action. Proc. Natl. Acad. Sci. USA 2003, 100, 3553–3554. [Google Scholar] [CrossRef]
- Uversky, V.N. Protein intrinsic disorder and structure-function continuum. Prog. Mol. Biol. Transl. Sci. 2019, 166, 1–17. [Google Scholar] [CrossRef]
- Uversky, V.N. Dancing Protein Clouds: The Strange Biology and Chaotic Physics of Intrinsically Disordered Proteins. J. Biol. Chem. 2016, 291, 6681–6688. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N. Unusual biophysics of intrinsically disordered proteins. Biochim. Biophys. Acta 2013, 1834, 932–951. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N.; Semisotnov, G.V.; Pain, R.H.; Ptitsyn, O.B. ‘All-or-none’ mechanism of the molten globule unfolding. FEBS Lett. 1992, 314, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N.; Ptitsyn, O.B. All-or-none solvent-induced transitions between native, molten globule and unfolded states in globular proteins. Fold. Des. 1996, 1, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Ptitsyn, O.B.; Uversky, V.N. The molten globule is a third thermodynamical state of protein molecules. FEBS Lett. 1994, 341, 15–18. [Google Scholar] [CrossRef]
- Pande, V.S.; Rokhsar, D.S. Is the molten globule a third phase of proteins? Proc. Natl. Acad. Sci. USA 1998, 95, 1490–1494. [Google Scholar] [CrossRef]
- Bychkova, V.E.; Pain, R.H.; Ptitsyn, O.B. The ‘molten globule’ state is involved in the translocation of proteins across membranes? FEBS Lett. 1988, 238, 231–234. [Google Scholar] [CrossRef]
- Song, M.; Shao, H.; Mujeeb, A.; James, T.L.; Miller, W.L. Molten-globule structure and membrane binding of the N-terminal protease-resistant domain (63-193) of the steroidogenic acute regulatory protein (StAR). Biochem. J. 2001, 356, 151–158. [Google Scholar] [CrossRef]
- Bose, H.S.; Baldwin, M.A.; Miller, W.L. Evidence that StAR and MLN64 act on the outer mitochondrial membrane as molten globules. Endocr. Res. 2000, 26, 629–637. [Google Scholar] [CrossRef]
- Ren, J.; Kachel, K.; Kim, H.; Malenbaum, S.E.; Collier, R.J.; London, E. Interaction of diphtheria toxin T domain with molten globule-like proteins and its implications for translocation. Science 1999, 284, 955–957. [Google Scholar] [CrossRef]
- van der Goot, F.G.; Lakey, J.H.; Pattus, F. The molten globule intermediate for protein insertion or translocation through membranes. Trends Cell. Biol. 1992, 2, 343–348. [Google Scholar] [CrossRef] [PubMed]
- van der Goot, F.G.; Gonzalez-Manas, J.M.; Lakey, J.H.; Pattus, F. A ‘molten-globule’ membrane-insertion intermediate of the pore-forming domain of colicin A. Nature 1991, 354, 408–410. [Google Scholar] [CrossRef] [PubMed]
- Banuelos, S.; Muga, A. Binding of molten globule-like conformations to lipid bilayers. Structure of native and partially folded alpha-lactalbumin bound to model membranes. J. Biol. Chem. 1995, 270, 29910–29915. [Google Scholar] [CrossRef]
- Watts, A. Biophysics of the membrane interface. Biochem. Soc. Trans. 1995, 23, 959–965. [Google Scholar] [CrossRef]
- Bychkova, V.E.; Basova, L.V.; Balobanov, V.A. How membrane surface affects protein structure. Biochemistry 2014, 79, 1483–1514. [Google Scholar] [CrossRef] [PubMed]
- Narizhneva, N.V.; Uversky, V.N. Decrease of dielectric constant transforms the protein molecule into the molten globule state. Biochemistry 1998, 63, 448–455. [Google Scholar]
- Uversky, V.N.; Narizhneva, N.V.; Kirschstein, S.O.; Winter, S.; Lober, G. Conformational transitions provoked by organic solvents in beta-lactoglobulin: Can a molten globule like intermediate be induced by the decrease in dielectric constant? Fold. Des. 1997, 2, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Bychkova, V.E.; Dujsekina, A.E.; Klenin, S.I.; Tiktopulo, E.I.; Uversky, V.N.; Ptitsyn, O.B. Molten globule-like state of cytochrome c under conditions simulating those near the membrane surface. Biochemistry 1996, 35, 6058–6063. [Google Scholar] [CrossRef] [PubMed]
- Bychkova, V.E.; Dujsekina, A.E.; Fantuzzi, A.; Ptitsyn, O.B.; Rossi, G.L. Release of retinol and denaturation of its plasma carrier, retinol-binding protein. Fold. Des. 1998, 3, 285–291. [Google Scholar] [CrossRef]
- Uversky, V.N.; Narizhneva, N.V. Effect of natural ligands on the structural properties and conformational stability of proteins. Biochemistry 1998, 63, 420–433. [Google Scholar]
- Uversky, V.N.; Narizhneva, N.V.; Ivanova, T.V.; Tomashevski, A.Y. Rigidity of human alpha-fetoprotein tertiary structure is under ligand control. Biochemistry 1997, 36, 13638–13645. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N.; Narizhneva, N.V.; Ivanova, T.V.; Kirkitadze, M.D.; Tomashevski, A. Ligand-free form of human alpha-fetoprotein: Evidence for the molten globule state. FEBS Lett. 1997, 410, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, R.S.; Indu, S.; Varadarajan, R. Identification and thermodynamic characterization of molten globule states of periplasmic binding proteins. Biochemistry 2007, 46, 10339–10352. [Google Scholar] [CrossRef]
- Rajaraman, K.; Raman, B.; Rao, C.M. Molten-globule state of carbonic anhydrase binds to the chaperone-like alpha-crystallin. J. Biol. Chem. 1996, 271, 27595–27600. [Google Scholar] [CrossRef]
- Martin, J.; Langer, T.; Boteva, R.; Schramel, A.; Horwich, A.L.; Hartl, F.U. Chaperonin-mediated protein folding at the surface of groEL through a ‘molten globule’-like intermediate. Nature 1991, 352, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Bai, J.H.; Park, Y.D.; Zhou, H.M. Aggregation of creatine kinase during refolding and chaperonin-mediated folding of creatine kinase. Int. J. Biochem. Cell Biol. 2001, 33, 279–286. [Google Scholar] [CrossRef]
- Hayer-Hartl, M.K.; Ewbank, J.J.; Creighton, T.E.; Hartl, F.U. Conformational specificity of the chaperonin GroEL for the compact folding intermediates of alpha-lactalbumin. EMBO J. 1994, 13, 3192–3202. [Google Scholar] [CrossRef]
- Braig, K.; Simon, M.; Furuya, F.; Hainfeld, J.F.; Horwich, A.L. A polypeptide bound by the chaperonin groEL is localized within a central cavity. Proc. Natl. Acad. Sci. USA 1993, 90, 3978–3982. [Google Scholar] [CrossRef]
- Melki, R.; Cowan, N.J. Facilitated folding of actins and tubulins occurs via a nucleotide-dependent interaction between cytoplasmic chaperonin and distinctive folding intermediates. Mol. Cell Biol. 1994, 14, 2895–2904. [Google Scholar] [CrossRef]
- Zhou, B.; Tian, K.; Jing, G. An in vitro peptide folding model suggests the presence of the molten globule state during nascent peptide folding. Protein Eng. 2000, 13, 35–39. [Google Scholar] [CrossRef]
- Bychkova, V.E.; Ptitsyn, O.B. The molten globule in vitro and in vivo. Chemtracts Biochem. Molec. Biol. 1993, 4, 133–163. [Google Scholar]
- Bychkova, V.E.; Ptitsyn, O.B. Folding intermediates are involved in genetic diseases? FEBS Lett. 1995, 359, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Bychkova, V.E.; Dolgikh, D.A.; Balobanov, V.A.; Finkelstein, A.V. The molten globule state of a globular protein in a cell is more or less frequent case rather than an exception. Molecules 2022, 27, 4361. [Google Scholar] [CrossRef] [PubMed]
- Griko, Y.V.; Freire, E.; Privalov, P.L. Energetics of the alpha-lactalbumin states: A calorimetric and statistical thermodynamic study. Biochemistry 1994, 33, 1889–1899. [Google Scholar] [CrossRef]
- Goto, Y.; Fink, A.L. Acid-induced folding of heme proteins. Methods Enzym. 1994, 232, 3–15. [Google Scholar] [CrossRef]
- Kuroda, Y.; Kidokoro, S.; Wada, A. Thermodynamic characterization of cytochrome c at low pH. Observation of the molten globule state and of the cold denaturation process. J. Mol. Biol. 1992, 223, 1139–1153. [Google Scholar] [CrossRef]
- Hughson, F.M.; Wright, P.E.; Baldwin, R.L. Structural characterization of a partly folded apomyoglobin intermediate. Science 1990, 249, 1544–1548. [Google Scholar] [CrossRef]
- Goto, Y.; Takahashi, N.; Fink, A.L. Mechanism of acid-induced folding of proteins. Biochemistry 1990, 29, 3480–3488. [Google Scholar] [CrossRef]
- Goto, Y.; Calciano, L.J.; Fink, A.L. Acid-induced folding of proteins. Proc. Natl. Acad. Sci. USA 1990, 87, 573–577. [Google Scholar] [CrossRef]
- Maheshwari, D.; Yadav, R.; Rastogi, R.; Jain, A.; Tripathi, S.; Mukhopadhyay, A.; Arora, A. Structural and Biophysical Characterization of Rab5a from Leishmania Donovani. Biophys. J. 2018, 115, 1217–1230. [Google Scholar] [CrossRef]
- Wahiduzzaman; Dar, M.A.; Haque, M.A.; Idrees, D.; Hassan, M.I.; Islam, A.; Ahmad, F. Characterization of folding intermediates during urea-induced denaturation of human carbonic anhydrase II. Int. J. Biol. Macromol. 2017, 95, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Khan, P.; Prakash, A.; Haque, M.A.; Islam, A.; Hassan, M.I.; Ahmad, F. Structural basis of urea-induced unfolding: Unraveling the folding pathway of hemochromatosis factor E. Int. J. Biol. Macromol. 2016, 91, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, G.; Mandal, D.K. Differing structural characteristics of molten globule intermediate of peanut lectin in urea and guanidine-HCl. Int. J. Biol. Macromol. 2012, 51, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.K.; Samaddar, S.; Banerjee, R.; Lahiri, S.; Bhattacharyya, A.; Roy, S. Glutamate counteracts the denaturing effect of urea through its effect on the denatured state. J. Biol. Chem. 2003, 278, 36077–36084. [Google Scholar] [CrossRef] [PubMed]
- Garcia, P.; Serrano, L.; Rico, M.; Bruix, M. An NMR view of the folding process of a CheY mutant at the residue level. Structure 2002, 10, 1173–1185. [Google Scholar] [CrossRef]
- Cymes, G.D.; Grosman, C.; Delfino, J.M.; Wolfenstein-Todel, C. Detection and characterization of an ovine placental lactogen stable intermediate in the urea-induced unfolding process. Protein Sci. 1996, 5, 2074–2079. [Google Scholar] [CrossRef]
- Das, B.K.; Bhattacharyya, T.; Roy, S. Characterization of a urea induced molten globule intermediate state of glutaminyl-tRNA synthetase from Escherichia coli. Biochemistry 1995, 34, 5242–5247. [Google Scholar] [CrossRef]
- Rodionova, N.A.; Semisotnov, G.V.; Kutyshenko, V.P.; Uverskii, V.N.; Bolotina, I.A. Staged equilibrium of carbonic anhydrase unfolding in strong denaturants. Mol. Biol. 1989, 23, 683–692. [Google Scholar]
- Kuznetsova, I.M.; Stepanenko, O.V.; Turoverov, K.K.; Zhu, L.; Zhou, J.M.; Fink, A.L.; Uversky, V.N. Unraveling multistate unfolding of rabbit muscle creatine kinase. Biochim. Biophys. Acta 2002, 1596, 138–155. [Google Scholar] [CrossRef]
- Zerovnik, E.; Jerala, R.; Kroon-Zitko, L.; Turk, V.; Pain, R.H. Denaturation of stefin B by GuHCl, pH and heat; evidence for molten globule intermediates. Biol. Chem. Hoppe Seyler 1992, 373, 453–458. [Google Scholar] [CrossRef]
- Powell, L.M.; Pain, R.H. Effects of glycosylation on the folding and stability of human, recombinant and cleaved alpha 1-antitrypsin. J. Mol. Biol. 1992, 224, 241–252. [Google Scholar] [CrossRef]
- Christensen, H.; Pain, R.H. Molten globule intermediates and protein folding. Eur. Biophys. J. 1991, 19, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Ptitsyn, O.B.; Pain, R.H.; Semisotnov, G.V.; Zerovnik, E.; Razgulyaev, O.I. Evidence for a molten globule state as a general intermediate in protein folding. FEBS Lett. 1990, 262, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Magsumov, T.; Ziying, L.; Sedov, I. Comparative study of the protein denaturing ability of different organic cosolvents. Int. J. Biol. Macromol. 2020, 160, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Prasanna Kumari, N.K.; Jagannadham, M.V. Deciphering the molecular structure of cryptolepain in organic solvents. Biochimie 2012, 94, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Santucci, R.; Polizio, F.; Desideri, A. Formation of a molten-globule-like state of cytochrome c induced by high concentrations of glycerol. Biochimie 1999, 81, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Wicar, S.; Mulkerrin, M.G.; Bathory, G.; Khundkar, L.H.; Karger, B.L. Conformational changes in the reversed phase liquid chromatography of recombinant human growth hormone as a function of organic solvent: The molten globule state. Anal. Chem. 1994, 66, 3908–3915. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, G.; Kumar, T.K.; Arunkumar, A.I.; Yu, C. 2,2,2-Trifluoroethanol induces helical conformation in an all beta-sheet protein. Biochem. Biophys. Res. Commun. 1996, 222, 33–37. [Google Scholar] [CrossRef]
- Dubey, V.K.; Shah, A.; Jagannadham, M.V.; Kayastha, A.M. Effect of organic solvents on the molten globule state of procerain: Beta-sheet to alpha-helix switchover in presence of trifluoroethanol. Protein Pept. Lett. 2006, 13, 545–547. [Google Scholar] [CrossRef]
- Hirota-Nakaoka, N.; Goto, Y. Alcohol-induced denaturation of beta-lactoglobulin: A close correlation to the alcohol-induced alpha-helix formation of melittin. Bioorg. Med. Chem. 1999, 7, 67–73. [Google Scholar] [CrossRef]
- Kuwata, K.; Hoshino, M.; Era, S.; Batt, C.A.; Goto, Y. alpha-->beta transition of beta-lactoglobulin as evidenced by heteronuclear NMR. J. Mol. Biol. 1998, 283, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Shiraki, K.; Nishikawa, K.; Goto, Y. Trifluoroethanol-induced stabilization of the alpha-helical structure of beta-lactoglobulin: Implication for non-hierarchical protein folding. J. Mol. Biol. 1995, 245, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Hirota, N.; Mizuno, K.; Goto, Y. Cooperative alpha-helix formation of beta-lactoglobulin and melittin induced by hexafluoroisopropanol. Protein Sci. 1997, 6, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Konuma, T.; Sakurai, K.; Yagi, M.; Goto, Y.; Fujisawa, T.; Takahashi, S. Highly Collapsed Conformation of the Initial Folding Intermediates of beta-Lactoglobulin with Non-Native alpha-Helix. J. Mol. Biol. 2015, 427, 3158–3165. [Google Scholar] [CrossRef]
- Uversky, V.N. A multiparametric approach to studies of self-organization of globular proteins. Biochemistry 1999, 64, 250–266. [Google Scholar]
- Jacobs, M.D.; Fox, R.O. Staphylococcal nuclease folding intermediate characterized by hydrogen exchange and NMR spectroscopy. Proc. Natl. Acad. Sci. USA 1994, 91, 449–453. [Google Scholar] [CrossRef]
- Alam Khan, M.K.; Das, U.; Rahaman, M.H.; Hassan, M.I.; Srinivasan, A.; Singh, T.P.; Ahmad, F. A single mutation induces molten globule formation and a drastic destabilization of wild-type cytochrome c at pH 6.0. J. Biol. Inorg. Chem. 2009, 14, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Jennings, P.A.; Wright, P.E. Formation of a molten globule intermediate early in the kinetic folding pathway of apomyoglobin. Science 1993, 262, 892–896. [Google Scholar] [CrossRef]
- Radford, S.E.; Dobson, C.M.; Evans, P.A. The folding of hen lysozyme involves partially structured intermediates and multiple pathways. Nature 1992, 358, 302–307. [Google Scholar] [CrossRef]
- Shokri, M.M.; Khajeh, K.; Alikhajeh, J.; Asoodeh, A.; Ranjbar, B.; Hosseinkhani, S.; Sadeghi, M. Comparison of the molten globule states of thermophilic and mesophilic alpha-amylases. Biophys. Chem. 2006, 122, 58–65. [Google Scholar] [CrossRef]
- Gloss, L.M.; Topping, T.B.; Binder, A.K.; Lohman, J.R. Kinetic folding of Haloferax volcanii and Escherichia coli dihydrofolate reductases: Haloadaptation by unfolded state destabilization at high ionic strength. J. Mol. Biol. 2008, 376, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Qvist, J.; Ortega, G.; Tadeo, X.; Millet, O.; Halle, B. Hydration dynamics of a halophilic protein in folded and unfolded states. J. Phys. Chem. B 2012, 116, 3436–3444. [Google Scholar] [CrossRef] [PubMed]
- Franzetti, B.; Schoehn, G.; Garcia, D.; Ruigrok, R.W.; Zaccai, G. Characterization of the proteasome from the extremely halophilic archaeon Haloarcula marismortui. Archaea 2002, 1, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N. Hypothesis: The unfolding power of protein dielectricity. Intrinsically Disord. Proteins 2013, 1, e25725. [Google Scholar] [CrossRef]
- Gupta, M.N.; Batra, R.; Tyagi, R.; Sharma, A. Polarity index: The guiding solvent parameter for enzyme stability in aqueous-organic cosolvent mixtures. Biotechnol. Prog. 1997, 13, 284–288. [Google Scholar] [CrossRef]
- Bocedi, A.; Gambardella, G.; Cattani, G.; Bartolucci, S.; Limauro, D.; Pedone, E.; Iavarone, F.; Castagnola, M.; Ricci, G. Ultra-rapid glutathionylation of chymotrypsinogen in its molten globule-like conformation: A comparison to archaeal proteins. Sci. Rep. 2020, 10, 8943. [Google Scholar] [CrossRef]
- van der Lee, R.; Buljan, M.; Lang, B.; Weatheritt, R.J.; Daughdrill, G.W.; Dunker, A.K.; Fuxreiter, M.; Gough, J.; Gsponer, J.; Jones, D.T.; et al. Classification of intrinsically disordered regions and proteins. Chem. Rev. 2014, 114, 6589–6631. [Google Scholar] [CrossRef]
- Uversky, V.N. A decade and a half of protein intrinsic disorder: Biology still waits for physics. Protein Sci. 2013, 22, 693–724. [Google Scholar] [CrossRef]
- Uversky, V.N.; Dunker, A.K. Understanding protein non-folding. Biochim. Biophys. Acta 2010, 1804, 1231–1264. [Google Scholar] [CrossRef]
- Dunker, A.K.; Silman, I.; Uversky, V.N.; Sussman, J.L. Function and structure of inherently disordered proteins. Curr. Opin. Struct. Biol. 2008, 18, 756–764. [Google Scholar] [CrossRef]
- Rantalainen, K.I.; Uversky, V.N.; Permi, P.; Kalkkinen, N.; Dunker, A.K.; Makinen, K. Potato virus A genome-linked protein VPg is an intrinsically disordered molten globule-like protein with a hydrophobic core. Virology 2008, 377, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, C.J.; Meng, J.; Yang, J.Y.; Yang, M.Q.; Uversky, V.N.; Dunker, A.K. Flexible nets: Disorder and induced fit in the associations of p53 and 14-3-3 with their partners. BMC Genom. 2008, 9 (Suppl. S1), S1. [Google Scholar] [CrossRef] [PubMed]
- Kokai, E.; Tantos, A.; Vissi, E.; Szoor, B.; Tompa, P.; Gausz, J.; Alphey, L.; Friedrich, P.; Dombradi, V. CG15031/PPYR1 is an intrinsically unstructured protein that interacts with protein phosphatase Y. Arch. Biochem. Biophys. 2006, 451, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Dunker, A.K.; Cortese, M.S.; Romero, P.; Iakoucheva, L.M.; Uversky, V.N. Flexible nets. The roles of intrinsic disorder in protein interaction networks. FEBS J. 2005, 272, 5129–5148. [Google Scholar] [CrossRef]
- Fink, A.L. Natively unfolded proteins. Curr. Opin. Struct. Biol. 2005, 15, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Tompa, P. Intrinsically unstructured proteins. Trends Biochem. Sci. 2002, 27, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N. Natively unfolded proteins: A point where biology waits for physics. Protein Sci. 2002, 11, 739–756. [Google Scholar] [CrossRef]
- Dyson, H.J.; Wright, P.E. Coupling of folding and binding for unstructured proteins. Curr. Opin. Struct. Biol. 2002, 12, 54–60. [Google Scholar] [CrossRef]
- Uversky, V.N. What does it mean to be natively unfolded? Eur. J. Biochem. 2002, 269, 2–12. [Google Scholar] [CrossRef]
- Dunker, A.K.; Obradovic, Z. The protein trinity--linking function and disorder. Nat. Biotechnol. 2001, 19, 805–806. [Google Scholar] [CrossRef]
- Dunker, A.K.; Lawson, J.D.; Brown, C.J.; Williams, R.M.; Romero, P.; Oh, J.S.; Oldfield, C.J.; Campen, A.M.; Ratliff, C.M.; Hipps, K.W.; et al. Intrinsically disordered protein. J. Mol. Graph. Model. 2001, 19, 26–59. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.I.; Hatakeyama, S.; Nakayama, K. Regulation of the cell cycle at the G1-S transition by proteolysis of cyclin E and p27Kip1. Biochem. Biophys. Res. Commun. 2001, 282, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Alny, C.B.; Heiber-Langer, I.; Inserm, R.L. New trends in baro-enzymology. Int. J. High Press. Res. 1994, 12, 187–191. [Google Scholar] [CrossRef]
- Roy, I.; Gupta, M.N. Applications of Three Phase Partitioning and Macro-(Affinity Ligand) Facilitated Three Phase Partitioning in Protein Refolding In Three Phase Partitionin; Roy, I., Gupta, M.N., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 197–222. [Google Scholar]
- Watanabe, M.; Aizawa, T.; Demura, M.; Nitta, K. Effect of hydrostatic pressure on conformational changes of canine milk lysozyme between the native, molten globule, and unfolded states. Biochim. Biophys. Acta 2004, 1702, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Dunker, A.K.; Powers, J.R.; Clark, S.; Swanson, B.G. Beta-lactoglobulin molten globule induced by high pressure. J. Agric. Food Chem. 2001, 49, 3236–3243. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.L.; Silveira, C.F.; Correia, A., Jr.; Pontes, L. Dissociation of a native dimer to a molten globule monomer. Effects of pressure and dilution on the association equilibrium of arc repressor. J. Mol. Biol. 1992, 223, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, T.; Kato, M.; Inoko, Y. Structural characterization of lactate dehydrogenase dissociation under high pressure studied by synchrotron high-pressure small-angle X-ray scattering. Biochemistry 1999, 38, 6411–6418. [Google Scholar] [CrossRef]
- Da Poian, A.T.; Johnson, J.E.; Silva, J.L. Differences in pressure stability of the three components of cowpea mosaic virus: Implications for virus assembly and disassembly. Biochemistry 1994, 33, 8339–8346. [Google Scholar] [CrossRef]
- Gaspar, L.P.; Johnson, J.E.; Silva, J.L.; Da Poian, A.T. Partially folded states of the capsid protein of cowpea severe mosaic virus in the disassembly pathway. J. Mol. Biol. 1997, 273, 456–466. [Google Scholar] [CrossRef]
- Ruan, K.; Lange, R.; Bec, N.; Balny, C. A stable partly denatured state of trypsin induced by high hydrostatic pressure. Biochem. Biophys. Res. Commun. 1997, 239, 150–154. [Google Scholar] [CrossRef]
- Dumoulin, M.; Ueno, H.; Hayashi, R.; Balny, C. Contribution of the carbohydrate moiety to conformational stability of the carboxypeptidase Y high pressure study. Eur. J. Biochem. 1999, 262, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Masson, P.; Fortier, P.L.; Albaret, C.; Clery, C.; Guerra, P.; Lockridge, O. Structural and hydration changes in the active site gorge of phosporhylated butyrylcholinesterase accompanying the aging process. Chem. Biol. Interact. 1999, 119–120, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Seemann, H.; Winter, R.; Royer, C.A. Volume, expansivity and isothermal compressibility changes associated with temperature and pressure unfolding of Staphylococcal nuclease. J. Mol. Biol. 2001, 307, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Trovaslet, M.; Dallet-Choisy, S.; Meersman, F.; Heremans, K.; Balny, C.; Legoy, M.D. Fluorescence and FTIR study of pressure-induced structural modifications of horse liver alcohol dehydrogenase (HLADH). Eur. J. Biochem. 2003, 270, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Marchal, S.; Shehi, E.; Harricane, M.C.; Fusi, P.; Heitz, F.; Tortora, P.; Lange, R. Structural instability and fibrillar aggregation of non-expanded human ataxin-3 revealed under high pressure and temperature. J. Biol. Chem. 2003, 278, 31554–31563. [Google Scholar] [CrossRef]
- Smeller, L.; Meersman, F.; Heremans, K. Stable misfolded states of human serum albumin revealed by high-pressure infrared spectroscopic studies. Eur. Biophys. J. 2008, 37, 1127–1132. [Google Scholar] [CrossRef]
- Marion, J.; Trovaslet, M.; Martinez, N.; Masson, P.; Schweins, R.; Nachon, F.; Trapp, M.; Peters, J. Pressure-induced molten globule state of human acetylcholinesterase: Structural and dynamical changes monitored by neutron scattering. Phys. Chem. Chem. Phys. 2015, 17, 3157–3163. [Google Scholar] [CrossRef]
- Giannoglou, M.; Alexandrakis, Z.; Stavros, P.; Katsaros, G.; Katapodis, P.; Nounesis, G.; Taoukis, P. Effect of high pressure on structural modifications and enzymatic activity of a purified X-prolyl dipeptidyl aminopeptidase from Streptococcus thermophilus. Food Chem. 2018, 248, 304–311. [Google Scholar] [CrossRef]
- Silva, J.L.; Oliveira, A.C.; Gomes, A.M.; Lima, L.M.; Mohana-Borges, R.; Pacheco, A.B.; Foguel, D. Pressure induces folding intermediates that are crucial for protein-DNA recognition and virus assembly. Biochim. Biophys. Acta 2002, 1595, 250–265. [Google Scholar] [CrossRef]
- Tsai, C.J.; Maizel, J.V., Jr.; Nussinov, R. The hydrophobic effect: A new insight from cold denaturation and a two-state water structure. Crit. Rev. Biochem. Mol. Biol. 2002, 37, 55–69. [Google Scholar] [CrossRef]
- Privalov, P.L.; Griko Yu, V.; Venyaminov, S.; Kutyshenko, V.P. Cold denaturation of myoglobin. J. Mol. Biol. 1986, 190, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Privalov, P.L. Cold denaturation of proteins. Crit. Rev. Biochem. Mol. Biol. 1990, 25, 281–305. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.P.; Freire, E. Thermodynamics of structural stability and cooperative folding behavior in proteins. Adv. Protein Chem. 1992, 43, 313–361. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.L.; Schellman, J.A. Low-temperature unfolding of a mutant of phage T4 lysozyme. 1. Equilibrium studies. Biochemistry 1989, 28, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Mizuguchi, M.; Hashimoto, D.; Sakurai, M.; Nitta, K. Cold denaturation of alpha-lactalbumin. Proteins 2000, 38, 407–413. [Google Scholar] [CrossRef]
- Dzwolak, W.; Kato, M.; Shimizu, A.; Taniguchi, Y. FTIR study on heat-induced and pressure-assisted cold-induced changes in structure of bovine alpha-lactalbumin: Stabilizing role of calcium ion. Biopolymers 2001, 62, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Yajima, T.; Fujiwara, K.; Arai, M.; Ito, K.; Shimizu, A.; Kihara, H.; Kuwajima, K.; Amemiya, Y.; Ikeguchi, M. Helical and expanded conformation of equine beta-lactoglobulin in the cold-denatured state. J. Mol. Biol. 2005, 350, 338–348. [Google Scholar] [CrossRef]
- Babu, C.R.; Hilser, V.J.; Wand, A.J. Direct access to the cooperative substructure of proteins and the protein ensemble via cold denaturation. Nat. Struct. Mol. Biol. 2004, 11, 352–357. [Google Scholar] [CrossRef]
- Nelson, C.J.; LaConte, M.J.; Bowler, B.E. Direct detection of heat and cold denaturation for partial unfolding of a protein. J. Am. Chem. Soc. 2001, 123, 7453–7454. [Google Scholar] [CrossRef]
- Kumar, R.; Prabhu, N.P.; Rao, D.K.; Bhuyan, A.K. The alkali molten globule state of horse ferricytochrome c: Observation of cold denaturation. J. Mol. Biol. 2006, 364, 483–495. [Google Scholar] [CrossRef]
- Jakob, U.; Kriwacki, R.; Uversky, V.N. Conditionally and transiently disordered proteins: Awakening cryptic disorder to regulate protein function. Chem. Rev. 2014, 114, 6779–6805. [Google Scholar] [CrossRef] [PubMed]
- Bardwell, J.C.; Jakob, U. Conditional disorder in chaperone action. Trends Biochem. Sci. 2012, 37, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Follis, A.V.; Chipuk, J.E.; Fisher, J.C.; Yun, M.K.; Grace, C.R.; Nourse, A.; Baran, K.; Ou, L.; Min, L.; White, S.W.; et al. PUMA binding induces partial unfolding within BCL-xL to disrupt p53 binding and promote apoptosis. Nat. Chem. Biol. 2013, 9, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N.; Oldfield, C.J.; Dunker, A.K. Showing your ID: Intrinsic disorder as an ID for recognition, regulation and cell signaling. J. Mol. Recognit. 2005, 18, 343–384. [Google Scholar] [CrossRef]
- Prakash, S.; Matouschek, A. Protein unfolding in the cell. Trends Biochem. Sci. 2004, 29, 593–600. [Google Scholar] [CrossRef]
- Kukreja, R.; Singh, B. Biologically active novel conformational state of botulinum, the most poisonous poison. J. Biol. Chem. 2005, 280, 39346–39352. [Google Scholar] [CrossRef]
- Cai, S.; Singh, B.R. Role of the disulfide cleavage induced molten globule state of type a botulinum neurotoxin in its endopeptidase activity. Biochemistry 2001, 40, 15327–15333. [Google Scholar] [CrossRef]
- Kumar, R.; Chang, T.-W.; Singh, B.R. Evolutionary Traits of Toxins. In Biological Toxins and Bioterrorism. Toxinology; Gopalakrishnakone, P., Balali-Mood, M., Llewellyn, L., Singh, B.R., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 527–557. [Google Scholar] [CrossRef]
- Uversky, V.N. Recent Developments in the Field of Intrinsically Disordered Proteins: Intrinsic Disorder-Based Emergence in Cellular Biology in Light of the Physiological and Pathological Liquid-Liquid Phase Transitions. Annu. Rev. Biophys. 2021, 50, 135–156. [Google Scholar] [CrossRef]
- Kulkarni, P.; Bhattacharya, S.; Achuthan, S.; Behal, A.; Jolly, M.K.; Kotnala, S.; Mohanty, A.; Rangarajan, G.; Salgia, R.; Uversky, V. Intrinsically Disordered Proteins: Critical Components of the Wetware. Chem. Rev. 2022, 122, 6614–6633. [Google Scholar] [CrossRef]
- Salgueiro, M.; Camporeale, G.; Conci, J.; Sousa, B.; Visentin, A.; Corbat, A.; Grecco, H.; de Oliveira, G.A.; de Prat-Gay, G. Molten Globule Driven Liquid-Liquid Phase Separation at the Center of Viral Factory Assembly. Biophys. J. 2020, 118, 215a. [Google Scholar] [CrossRef]
- Ambadipudi, S.; Biernat, J.; Riedel, D.; Mandelkow, E.; Zweckstetter, M. Liquid-liquid phase separation of the microtubule-binding repeats of the Alzheimer-related protein Tau. Nat. Commun. 2017, 8, 275. [Google Scholar] [CrossRef] [PubMed]
- Darling, A.L.; Breydo, L.; Rivas, E.G.; Gebru, N.T.; Zheng, D.; Baker, J.D.; Blair, L.J.; Dickey, C.A.; Koren, J., 3rd; Uversky, V.N. Repeated repeat problems: Combinatorial effect of C9orf72-derived dipeptide repeat proteins. Int. J. Biol. Macromol. 2019, 127, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Ford, L.K.; Fioriti, L. Coiled-Coil Motifs of RNA-Binding Proteins: Dynamicity in RNA Regulation. Front. Cell Dev. Biol. 2020, 8, 607947. [Google Scholar] [CrossRef]
- Franklin, J.M.; Guan, K.L. YAP/TAZ phase separation for transcription. Nat. Cell Biol. 2020, 22, 357–358. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Singh, N.; Kumar, R.; Patel, K.; Pandey, S.; Datta, D.; Mahato, J.; Panigrahi, R.; Navalkar, A.; Mehra, S.; et al. alpha-Synuclein aggregation nucleates through liquid-liquid phase separation. Nat. Chem. 2020, 12, 705–716. [Google Scholar] [CrossRef]
- Hernandez-Sanchez, I.E.; Maruri-Lopez, I.; Martinez-Martinez, C.; Janis, B.; Jimenez-Bremont, J.F.; Covarrubias, A.A.; Menze, M.A.; Graether, S.P.; Thalhammer, A. LEAfing through literature: Late embryogenesis abundant proteins coming of age-achievements and perspectives. J. Exp. Bot. 2022, 73, 6525–6546. [Google Scholar] [CrossRef]
- Shih, P.Y.; Fang, Y.L.; Shankar, S.; Lee, S.P.; Hu, H.T.; Chen, H.; Wang, T.F.; Hsia, K.C.; Hsueh, Y.P. Phase separation and zinc-induced transition modulate synaptic distribution and association of autism-linked CTTNBP2 and SHANK3. Nat. Commun. 2022, 13, 2664. [Google Scholar] [CrossRef]
- Soltys, K.; Ozyhar, A. Ordered structure-forming properties of the intrinsically disordered AB region of hRXRgamma and its ability to promote liquid-liquid phase separation. J. Steroid Biochem. Mol. Biol. 2020, 198, 105571. [Google Scholar] [CrossRef]
- Leontiev, V.V.; Uversky, V.N.; Gudkov, A.T. Comparative stability of dihydrofolate reductase mutants in vitro and in vivo. Protein Eng. 1993, 6, 81–84. [Google Scholar] [CrossRef]
- Protasova, N.; Kireeva, M.L.; Murzina, N.V.; Murzin, A.G.; Uversky, V.N.; Gryaznova, O.I.; Gudkov, A.T. Circularly permuted dihydrofolate reductase of E. coli has functional activity and a destabilized tertiary structure. Protein Eng. 1994, 7, 1373–1377. [Google Scholar] [CrossRef]
- Uversky, V.N.; Kutyshenko, V.P.; Protasova, N.; Rogov, V.V.; Vassilenko, K.S.; Gudkov, A.T. Circularly permuted dihydrofolate reductase possesses all the properties of the molten globule state, but can resume functional tertiary structure by interaction with its ligands. Protein Sci. 1996, 5, 1844–1851. [Google Scholar] [CrossRef] [PubMed]
- MacBeath, G.; Kast, P.; Hilvert, D. Redesigning enzyme topology by directed evolution. Science 1998, 279, 1958–1961. [Google Scholar] [CrossRef] [PubMed]
- Vamvaca, K.; Vogeli, B.; Kast, P.; Pervushin, K.; Hilvert, D. An enzymatic molten globule: Efficient coupling of folding and catalysis. Proc. Natl. Acad. Sci. USA 2004, 101, 12860–12864. [Google Scholar] [CrossRef] [PubMed]
- Pervushin, K.; Vamvaca, K.; Vogeli, B.; Hilvert, D. Structure and dynamics of a molten globular enzyme. Nat. Struct. Mol. Biol. 2007, 14, 1202–1206. [Google Scholar] [CrossRef] [PubMed]
- Vamvaca, K.; Jelesarov, I.; Hilvert, D. Kinetics and thermodynamics of ligand binding to a molten globular enzyme and its native counterpart. J. Mol. Biol. 2008, 382, 971–977. [Google Scholar] [CrossRef]
- Woycechowsky, K.J.; Choutko, A.; Vamvaca, K.; Hilvert, D. Relative tolerance of an enzymatic molten globule and its thermostable counterpart to point mutation. Biochemistry 2008, 47, 13489–13496. [Google Scholar] [CrossRef] [PubMed]
- Walter, K.U.; Vamvaca, K.; Hilvert, D. An active enzyme constructed from a 9-amino acid alphabet. J. Biol. Chem. 2005, 280, 37742–37746. [Google Scholar] [CrossRef] [PubMed]
- Mannervik, B.; Danielson, U.H. Glutathione transferases--structure and catalytic activity. CRC Crit. Rev. Biochem. 1988, 23, 283–337. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.N. Structure, catalytic mechanism, and evolution of the glutathione transferases. Chem. Res. Toxicol. 1997, 10, 2–18. [Google Scholar] [CrossRef]
- Honaker, M.T.; Acchione, M.; Zhang, W.; Mannervik, B.; Atkins, W.M. Enzymatic detoxication, conformational selection, and the role of molten globule active sites. J. Biol. Chem. 2013, 288, 18599–18611. [Google Scholar] [CrossRef]
- Hou, L.; Honaker, M.T.; Shireman, L.M.; Balogh, L.M.; Roberts, A.G.; Ng, K.C.; Nath, A.; Atkins, W.M. Functional promiscuity correlates with conformational heterogeneity in A-class glutathione S-transferases. J. Biol. Chem. 2007, 282, 23264–23274. [Google Scholar] [CrossRef] [PubMed]
- Blikstad, C.; Shokeer, A.; Kurtovic, S.; Mannervik, B. Emergence of a novel highly specific and catalytically efficient enzyme from a naturally promiscuous glutathione transferase. Biochim. Biophys. Acta 2008, 1780, 1458–1463. [Google Scholar] [CrossRef] [PubMed]
- Nath, A.; Atkins, W.M. A quantitative index of substrate promiscuity. Biochemistry 2008, 47, 157–166. [Google Scholar] [CrossRef]
- Stojanovski, B.M.; Breydo, L.; Hunter, G.A.; Uversky, V.N.; Ferreira, G.C. Catalytically active alkaline molten globular enzyme: Effect of pH and temperature on the structural integrity of 5-aminolevulinate synthase. Biochim. Biophys. Acta 2014, 1844, 2145–2154. [Google Scholar] [CrossRef] [PubMed]
- Bemporad, F.; Gsponer, J.; Hopearuoho, H.I.; Plakoutsi, G.; Stati, G.; Stefani, M.; Taddei, N.; Vendruscolo, M.; Chiti, F. Biological function in a non-native partially folded state of a protein. EMBO J. 2008, 27, 1525–1535. [Google Scholar] [CrossRef]
- Bemporad, F.; Capanni, C.; Calamai, M.; Tutino, M.L.; Stefani, M.; Chiti, F. Studying the folding process of the acylphosphatase from Sulfolobus solfataricus. A comparative analysis with other proteins from the same superfamily. Biochemistry 2004, 43, 9116–9126. [Google Scholar] [CrossRef]
- Alexandrescu, A.T.; Shortle, D. Backbone dynamics of a highly disordered 131 residue fragment of staphylococcal nuclease. J. Mol. Biol. 1994, 242, 527–546. [Google Scholar] [CrossRef]
- Alexandrescu, A.T.; Abeygunawardana, C.; Shortle, D. Structure and dynamics of a denatured 131-residue fragment of staphylococcal nuclease: A heteronuclear NMR study. Biochemistry 1994, 33, 1063–1072. [Google Scholar] [CrossRef]
- Gillespie, J.R.; Shortle, D. Characterization of long-range structure in the denatured state of staphylococcal nuclease. II. Distance restraints from paramagnetic relaxation and calculation of an ensemble of structures. J. Mol. Biol. 1997, 268, 170–184. [Google Scholar] [CrossRef]
- Gillespie, J.R.; Shortle, D. Characterization of long-range structure in the denatured state of staphylococcal nuclease. I. Paramagnetic relaxation enhancement by nitroxide spin labels. J. Mol. Biol. 1997, 268, 158–169. [Google Scholar] [CrossRef]
- Li, Y.; Jing, G. Double point mutant F34W/W140F of staphylococcal nuclease is in a molten globule state but highly competent to fold into a functional conformation. J. Biochem. 2000, 128, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Makarov, M.; Meng, J.; Tretyachenko, V.; Srb, P.; Brezinova, A.; Giacobelli, V.G.; Bednarova, L.; Vondrasek, J.; Dunker, A.K.; Hlouchova, K. Enzyme catalysis prior to aromatic residues: Reverse engineering of a dephospho-CoA kinase. Protein Sci. 2021, 30, 1022–1034. [Google Scholar] [CrossRef]
- Johnsson, K.; Allemann, R.K.; Widmer, H.; Benner, S.A. Synthesis, structure and activity of artificial, rationally designed catalytic polypeptides. Nature 1993, 365, 530–532. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, I.M.; Zaslavsky, B.Y.; Breydo, L.; Turoverov, K.K.; Uversky, V.N. Beyond the excluded volume effects: Mechanistic complexity of the crowded milieu. Molecules 2015, 20, 1377–1409. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, I.M.; Turoverov, K.K.; Uversky, V.N. What macromolecular crowding can do to a protein. Int. J. Mol. Sci. 2014, 15, 23090–23140. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, S.B.; Trach, S.O. Estimation of macromolecule concentrations and excluded volume effects for the cytoplasm of Escherichia coli. J. Mol. Biol. 1991, 222, 599–620. [Google Scholar] [CrossRef]
- van den Berg, B.; Ellis, R.J.; Dobson, C.M. Effects of macromolecular crowding on protein folding and aggregation. EMBO J. 1999, 18, 6927–6933. [Google Scholar] [CrossRef]
- Rivas, G.; Ferrone, F.; Herzfeld, J. Life in a crowded world. EMBO Rep. 2004, 5, 23–27. [Google Scholar] [CrossRef]
- Ellis, R.J.; Minton, A.P. Cell biology: Join the crowd. Nature 2003, 425, 27–28. [Google Scholar] [CrossRef]
- Balcells, C.; Pastor, I.; Vilaseca, E.; Madurga, S.; Cascante, M.; Mas, F. Macromolecular crowding effect upon in vitro enzyme kinetics: Mixed activation-diffusion control of the oxidation of NADH by pyruvate catalyzed by lactate dehydrogenase. J. Phys. Chem. B 2014, 118, 4062–4068. [Google Scholar] [CrossRef]
- Zhou, H.X.; Rivas, G.; Minton, A.P. Macromolecular crowding and confinement: Biochemical, biophysical, and potential physiological consequences. Annu. Rev. Biophys. 2008, 37, 375–397. [Google Scholar] [CrossRef] [PubMed]
- McPhie, P.; Ni, Y.S.; Minton, A.P. Macromolecular crowding stabilizes the molten globule form of apomyoglobin with respect to both cold and heat unfolding. J. Mol. Biol. 2006, 361, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Sasahara, K.; McPhie, P.; Minton, A.P. Effect of dextran on protein stability and conformation attributed to macromolecular crowding. J. Mol. Biol. 2003, 326, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Parray, Z.A.; Ahmad, F.; Alajmi, M.F.; Hussain, A.; Hassan, M.I.; Islam, A. Formation of molten globule state in horse heart cytochrome c under physiological conditions: Importance of soft interactions and spectroscopic approach in crowded milieu. Int. J. Biol. Macromol. 2020, 148, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Nasreen, K.; Ahamad, S.; Ahmad, F.; Hassan, M.I.; Islam, A. Macromolecular crowding induces molten globule state in the native myoglobin at physiological pH. Int. J. Biol. Macromol. 2018, 106, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Roque, A.; Ponte, I.; Suau, P. Macromolecular crowding induces a molten globule state in the C-terminal domain of histone H1. Biophys. J. 2007, 93, 2170–2177. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, R.; Sharma, D.; Garg, M.; Kumar, V.; Agarwal, M.C. Macromolecular crowding-induced molten globule states of the alkali pH-denatured proteins. Biochim. Biophys. Acta Proteins Proteom. 2018, 1866, 1102–1114. [Google Scholar] [CrossRef]
- Permyakov, E.A.; Berliner, L.J. alpha-Lactalbumin: Structure and function. FEBS Lett. 2000, 473, 269–274. [Google Scholar] [CrossRef]
- Svensson, M.; Hakansson, A.; Mossberg, A.K.; Linse, S.; Svanborg, C. Conversion of alpha-lactalbumin to a protein inducing apoptosis. Proc. Natl. Acad. Sci. USA 2000, 97, 4221–4226. [Google Scholar] [CrossRef]
- Zhang, D.L.; Wu, L.J.; Chen, J.; Liang, Y. Effects of macromolecular crowding on the structural stability of human alpha-lactalbumin. Acta Biochim. Biophys. Sin. 2012, 44, 703–711. [Google Scholar] [CrossRef]
- Candotti, M.; Orozco, M. The Differential Response of Proteins to Macromolecular Crowding. PLoS Comput. Biol. 2016, 12, e1005040. [Google Scholar] [CrossRef] [PubMed]
- Norde, W. Adsorption of proteins from solution at the solid-liquid interface. Adv. Colloid Interface Sci. 1986, 25, 267–340. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.N.; Khare, S.K.; Sinha, R. An Overview of Interactions between Microorganisms and Nanomaterials. In Interfaces between Nanomaterials and Microbes; CRC Press: Boca Raton, FL, USA, 2021; pp. 17–37. [Google Scholar]
- Saptarshi, S.R.; Duschl, A.; Lopata, A.L. Interaction of nanoparticles with proteins: Relation to bio-reactivity of the nanoparticle. J. Nanobiotechnology 2013, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.N.; Roy, I. How Corona Formation Impacts Nanomaterials as Drug Carriers. Mol. Pharm. 2020, 17, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Billsten, P.; Freskgard, P.O.; Carlsson, U.; Jonsson, B.H.; Elwing, H. Adsorption to silica nanoparticles of human carbonic anhydrase II and truncated forms induce a molten-globule-like structure. FEBS Lett. 1997, 402, 67–72. [Google Scholar] [CrossRef]
- Colvin, V.L.; Kulinowski, K.M. Nanoparticles as catalysts for protein fibrillation. Proc. Natl. Acad. Sci. USA 2007, 104, 8679–8680. [Google Scholar] [CrossRef]
- Bhattacharya, J.; Choudhuri, U.; Siwach, O.; Sen, P.; Dasgupta, A.K. Interaction of hemoglobin and copper nanoparticles: Implications in hemoglobinopathy. Nanomedicine 2006, 2, 191–199. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Basu, S.; Singha, S.; Patra, H.K. Inner-View of Nanomaterial Incited Protein Conformational Changes: Insights into Designable Interaction. Research 2018, 2018, 9712832. [Google Scholar] [CrossRef]
- Denisov, V.P.; Jonsson, B.H.; Halle, B. Hydration of denatured and molten globule proteins. Nat. Struct. Biol. 1999, 6, 253–260. [Google Scholar] [CrossRef]
- Hoeltzli, S.D.; Frieden, C. Stopped-flow NMR spectroscopy: Real-time unfolding studies of 6-19F-tryptophan-labeled Escherichia coli dihydrofolate reductase. Proc. Natl. Acad. Sci. USA 1995, 92, 9318–9322. [Google Scholar] [CrossRef]
- Hua, L.; Zhou, R.; Thirumalai, D.; Berne, B.J. Urea denaturation by stronger dispersion interactions with proteins than water implies a 2-stage unfolding. Proc. Natl. Acad. Sci. USA 2008, 105, 16928–16933. [Google Scholar] [CrossRef] [PubMed]
- Acharya, N.; Mishra, P.; Jha, S.K. Evidence for Dry Molten Globule-Like Domains in the pH-Induced Equilibrium Folding Intermediate of a Multidomain Protein. J. Phys. Chem. Lett. 2016, 7, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Jeng, M.F.; Englander, S.W. Stable submolecular folding units in a non-compact form of cytochrome c. J. Mol. Biol. 1991, 221, 1045–1061. [Google Scholar] [CrossRef] [PubMed]
- Chaffotte, A.; Guillou, Y.; Delepierre, M.; Hinz, H.J.; Goldberg, M.E. The isolated C-terminal (F2) fragment of the Escherichia coli tryptophan synthase beta 2-subunit folds into a stable, organized nonnative conformation. Biochemistry 1991, 30, 8067–8074. [Google Scholar] [CrossRef]
- Chaffotte, A.F.; Guijarro, J.I.; Guillou, Y.; Delepierre, M.; Goldberg, M.E. The “pre-molten globule,” a new intermediate in protein folding. J. Protein Chem. 1997, 16, 433–439. [Google Scholar] [CrossRef]
- Samaddar, S.; Mandal, A.K.; Mondal, S.K.; Sahu, K.; Bhattacharyya, K.; Roy, S. Solvation dynamics of a protein in the pre molten globule state. J. Phys. Chem. B 2006, 110, 21210–21215. [Google Scholar] [CrossRef]
- Alam Khan, M.K.; Rahaman, M.H.; Hassan, M.I.; Singh, T.P.; Moosavi-Movahedi, A.A.; Ahmad, F. Conformational and thermodynamic characterization of the premolten globule state occurring during unfolding of the molten globule state of cytochrome c. J. Biol. Inorg. Chem. 2010, 15, 1319–1329. [Google Scholar] [CrossRef]
- Khan, M.K.; Rahaman, H.; Ahmad, F. Conformation and thermodynamic stability of pre-molten and molten globule states of mammalian cytochromes-c. Metallomics 2011, 3, 327–338. [Google Scholar] [CrossRef]
- Parray, Z.A.; Ahamad, S.; Ahmad, F.; Hassan, M.I.; Islam, A. First evidence of formation of pre-molten globule state in myoglobin: A macromolecular crowding approach towards protein folding in vivo. Int. J. Biol. Macromol. 2019, 126, 1288–1294. [Google Scholar] [CrossRef]
- Yameen, D.; Siraj, S.; Parray, Z.A.; Masood, M.; Islam, A.; Haque, M.M. Soft interactions versus hard core repulsions: A journey of cytochrome c from acid-induced denaturation to native protein via pre-molten globule and molten globule conformations exploiting dextran and its monomer glucose. J. Mol. Liq. 2022, 366, 120257. [Google Scholar] [CrossRef]
- Garcia-Fandino, R.; Bernado, P.; Ayuso-Tejedor, S.; Sancho, J.; Orozco, M. Defining the nature of thermal intermediate in 3 state folding proteins: Apoflavodoxin, a study case. PLoS Comput. Biol. 2012, 8, e1002647. [Google Scholar] [CrossRef]
- Gupta, M.N. (Ed.) Thermostability of Enzymes; Springer: Berlin/Heidelberg, Germany, 1993. [Google Scholar]
- Purich, D.L. Enzyme Kinetics: Catalysis and Control: A Reference of Theory and Best-Practice Methods; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Avadhani, V.S.; Mondal, S.; Banerjee, S. Mapping Protein Structural Evolution upon Unfolding. Biochemistry 2022, 61, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, J.; Chandra, S.; Varadarajan, R. Deep mutational scanning to probe specificity determinants in proteins. In Structure and Intrinsic Disorder in Enzymology; Elsevier: Amsterdam, The Netherlands, 2023; pp. 31–71. [Google Scholar]
- Gupta, M.N.; Alam, A.; Hasnain, S.E. Protein promiscuity in drug discovery, drug-repurposing and antibiotic resistance. Biochimie 2020, 175, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Pandey, S.; Ehtesham, N.Z.; Hasnain, S.E. Medical implications of protein moonlighting. Indian J. Med. Res. 2019, 149, 322. [Google Scholar] [PubMed]
- Xue, B.; Uversky, V.N. Intrinsic disorder in proteins involved in the innate antiviral immunity: Another flexible side of a molecular arms race. J. Mol. Biol. 2014, 426, 1322–1350. [Google Scholar] [CrossRef]
- Gupta, M.N.; Roy, I. Drugs, host proteins and viral proteins: How their promiscuities shape antiviral design. Biol. Rev. Camb. Philos. Soc. 2021, 96, 205–222. [Google Scholar] [CrossRef]
- Ahmad, J.; Farhana, A.; Pancsa, R.; Arora, S.K.; Srinivasan, A.; Tyagi, A.K.; Babu, M.M.; Ehtesham, N.Z.; Hasnain, S.E. Contrasting Function of Structured N-Terminal and Unstructured C-Terminal Segments of Mycobacterium tuberculosis PPE37 Protein. mBio 2018, 9, e01712-17. [Google Scholar] [CrossRef]
- Mukherjee, J.; Gupta, M.N. Increasing importance of protein flexibility in designing biocatalytic processes. Biotechnol. Rep. 2015, 6, 119–123. [Google Scholar] [CrossRef]
- Hu, G.; Wu, Z.; Wang, K.; Uversky, V.N.; Kurgan, L. Untapped Potential of Disordered Proteins in Current Druggable Human Proteome. Curr. Drug Targets 2016, 17, 1198–1205. [Google Scholar] [CrossRef]
- Uversky, V.N. Proteins without unique 3D structures: Biotechnological applications of intrinsically unstable/disordered proteins. Biotechnol. J. 2015, 10, 356–366. [Google Scholar] [CrossRef]
- Uversky, V.N. Intrinsically disordered proteins and novel strategies for drug discovery. Expert Opin. Drug Discov. 2012, 7, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Dunker, A.K.; Uversky, V.N. Drugs for ‘protein clouds’: Targeting intrinsically disordered transcription factors. Curr. Opin. Pharm. 2010, 10, 782–788. [Google Scholar] [CrossRef]
- Coskuner, O.; Uversky, V.N. Intrinsically disordered proteins in various hypotheses on the pathogenesis of Alzheimer’s and Parkinson’s diseases. Prog. Mol. Biol. Transl. Sci. 2019, 166, 145–223. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N. Intrinsic Disorder, Protein-Protein Interactions, and Disease. Adv. Protein Chem. Struct. Biol. 2018, 110, 85–121. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N.; Dave, V.; Iakoucheva, L.M.; Malaney, P.; Metallo, S.J.; Pathak, R.R.; Joerger, A.C. Pathological unfoldomics of uncontrolled chaos: Intrinsically disordered proteins and human diseases. Chem. Rev. 2014, 114, 6844–6879. [Google Scholar] [CrossRef]
- Uversky, V.N. Wrecked regulation of intrinsically disordered proteins in diseases: Pathogenicity of deregulated regulators. Front. Mol. Biosci. 2014, 1, 6. [Google Scholar] [CrossRef]
- Uversky, V.N. Flexible nets of malleable guardians: Intrinsically disordered chaperones in neurodegenerative diseases. Chem. Rev. 2011, 111, 1134–1166. [Google Scholar] [CrossRef]
- Uversky, V.N. Targeting intrinsically disordered proteins in neurodegenerative and protein dysfunction diseases: Another illustration of the D(2) concept. Expert Rev. Proteom. 2010, 7, 543–564. [Google Scholar] [CrossRef]
- Uversky, V.N. Intrinsic disorder in proteins associated with neurodegenerative diseases. Front. Biosci. 2009, 14, 5188–5238. [Google Scholar] [CrossRef]
- Uversky, V.N.; Oldfield, C.J.; Dunker, A.K. Intrinsically disordered proteins in human diseases: Introducing the D2 concept. Annu. Rev. Biophys. 2008, 37, 215–246. [Google Scholar] [CrossRef]
- Uversky, V.N. A protein-chameleon: Conformational plasticity of alpha-synuclein, a disordered protein involved in neurodegenerative disorders. J. Biomol. Struct. Dyn. 2003, 21, 211–234. [Google Scholar] [CrossRef] [PubMed]
- Blundell, T.L.; Gupta, M.N.; Hasnain, S.E. Intrinsic disorder in proteins: Relevance to protein assemblies, drug design and host-pathogen interactions. Prog. Biophys. Mol. Biol. 2020, 156, 34–42. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, M.N.; Uversky, V.N. Pre-Molten, Wet, and Dry Molten Globules en Route to the Functional State of Proteins. Int. J. Mol. Sci. 2023, 24, 2424. https://doi.org/10.3390/ijms24032424
Gupta MN, Uversky VN. Pre-Molten, Wet, and Dry Molten Globules en Route to the Functional State of Proteins. International Journal of Molecular Sciences. 2023; 24(3):2424. https://doi.org/10.3390/ijms24032424
Chicago/Turabian StyleGupta, Munishwar Nath, and Vladimir N. Uversky. 2023. "Pre-Molten, Wet, and Dry Molten Globules en Route to the Functional State of Proteins" International Journal of Molecular Sciences 24, no. 3: 2424. https://doi.org/10.3390/ijms24032424
APA StyleGupta, M. N., & Uversky, V. N. (2023). Pre-Molten, Wet, and Dry Molten Globules en Route to the Functional State of Proteins. International Journal of Molecular Sciences, 24(3), 2424. https://doi.org/10.3390/ijms24032424






