Rodent Animal Models of Endometriosis-Associated Pain: Unmet Needs and Resources Available for Improving Translational Research in Endometriosis
Abstract
1. Introduction
| Treatment | Mechanism of Action | Side Effects | Ref. | |
|---|---|---|---|---|
| Hormonal treatment | Combined oral contraceptives and progestins | Ovulation and decidualization suppression | Erratic bleeding, nausea, headaches, breast tenderness | [16,18,23,34] |
| Progesterone | Produce endometrial atrophy inducing decidualization of endometrial cells | Abnormal uterine bleeding, vomiting, breast discomfort, and depression | [18,35,36] | |
| Gonadotrophin releasing Hormone (GnRH) agonists | Ovarian suppression | Vaginal dryness, hot flushes, reduced bone mineral density, mood instability | [16,18,23,34] | |
| Gonadotrophin releasing Hormone (GnRH) antagonists | Ovarian suppression | Vaginal dryness, hot flushes, reduced bone mineral density | [16,18,34] | |
| Aromatase inhibitors | Blocking estrogen produced locally in endometriotic lesions | Vasomotor symptoms, decreased libido, loss of calcium in the bones, nausea, and headaches | [16,18,23,34] | |
| Non-hormonal treatment | NSAIDs | COX2 inhibitors | Potential ulcers, bleeding, perforation of the stomach and intestine, increased risk of heart attack, stroke, and related cardiovascular conditions | [16,18,23,34,37] |
| Neuromodulatory drugs | Central nervous system desensitization | Dizziness, tiredness, drowsiness, change in mood, visual disturbance, shortness of breath, among others | [16,18,34,38] | |
| Surgery | Remove the lesion by surgery | Possibility of disease recurrence and development of postsurgical pain | [18,23,32,33] | |
2. Results
2.1. Endometriosis Models in Rodents: Limits and Solutions
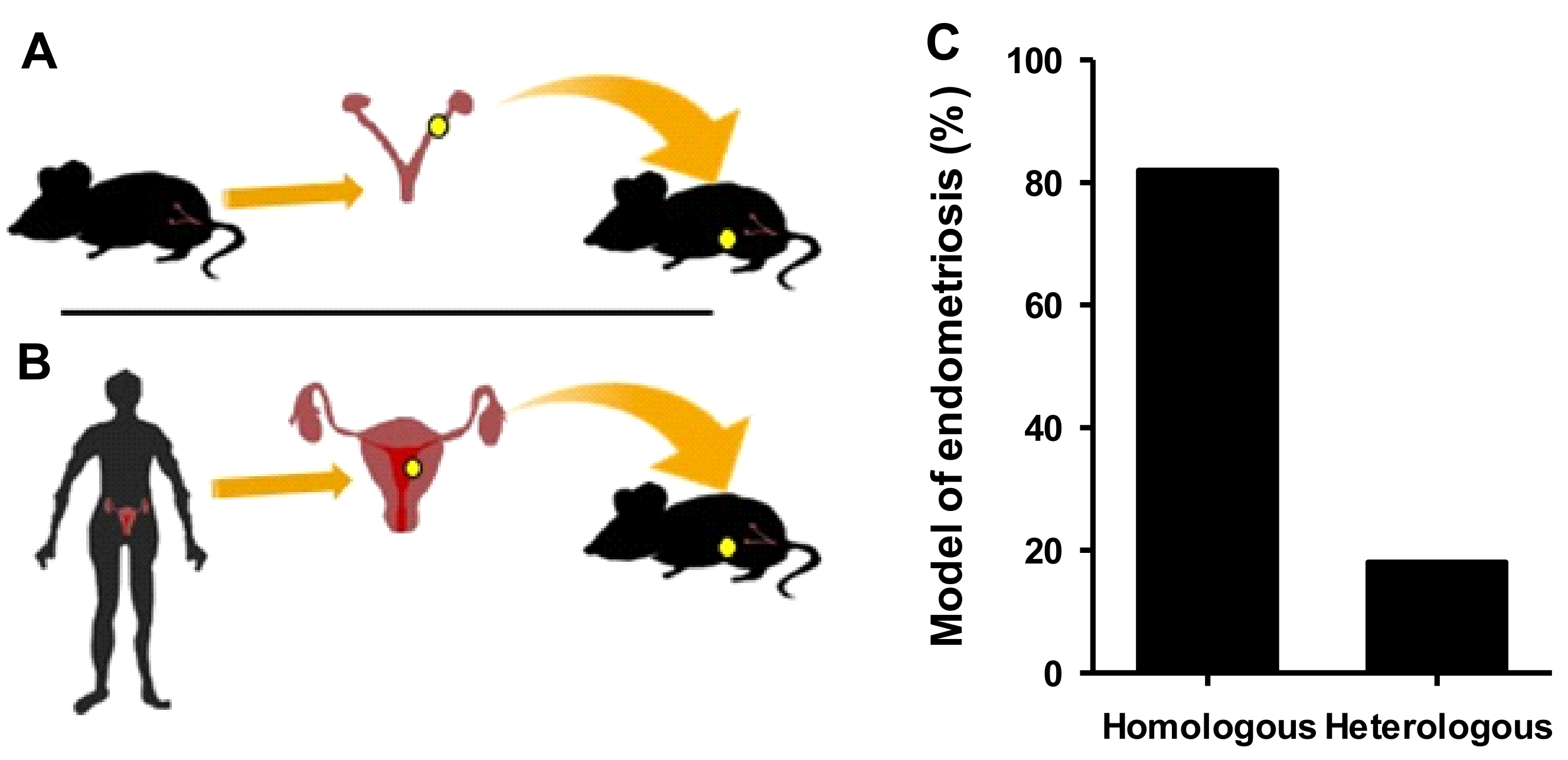
2.1.1. Homologous Models
2.1.2. Heterologous Models
2.2. Pain Assessment
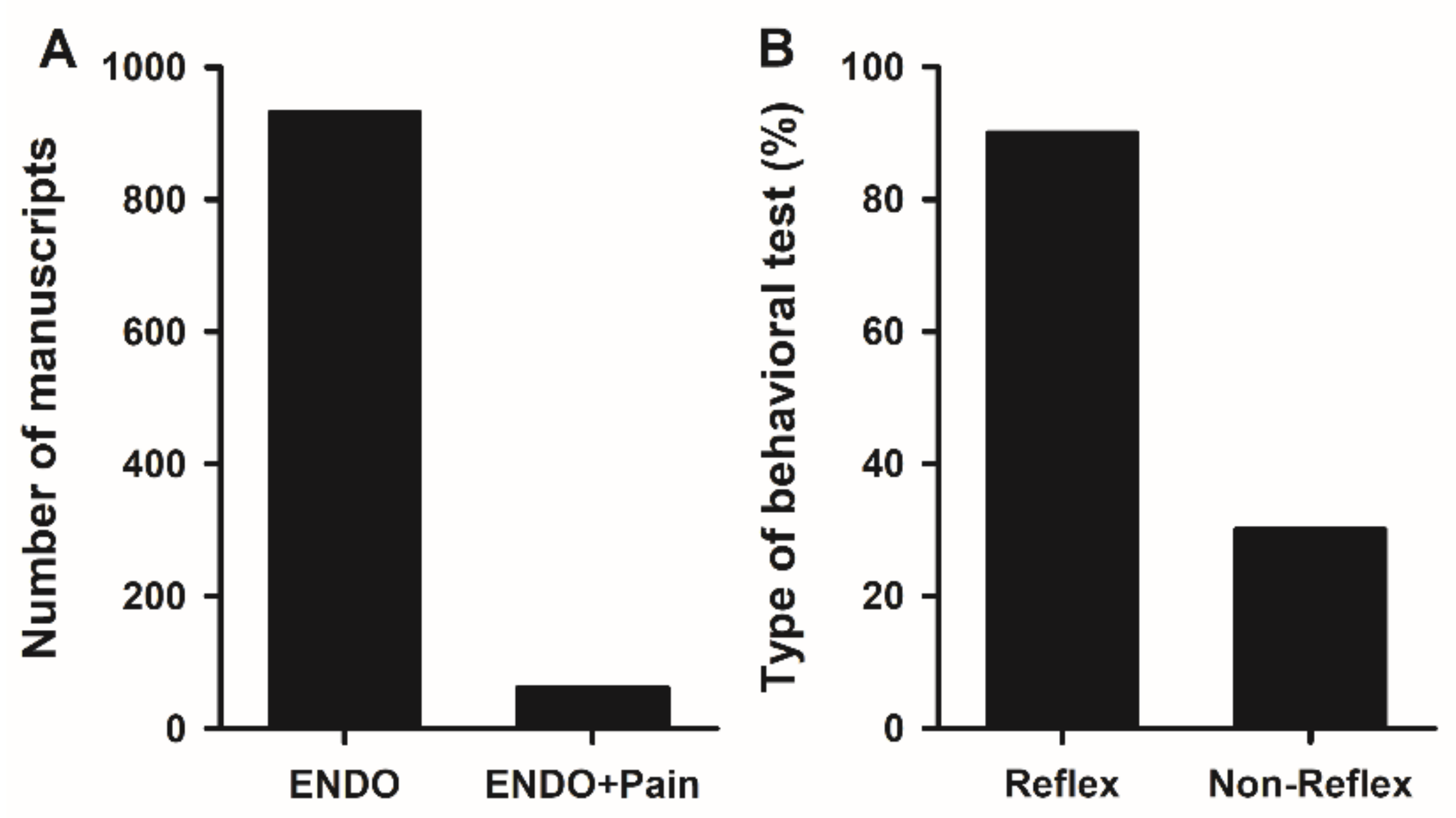
2.2.1. Study of Endometriosis-Induced Pain in Rodents
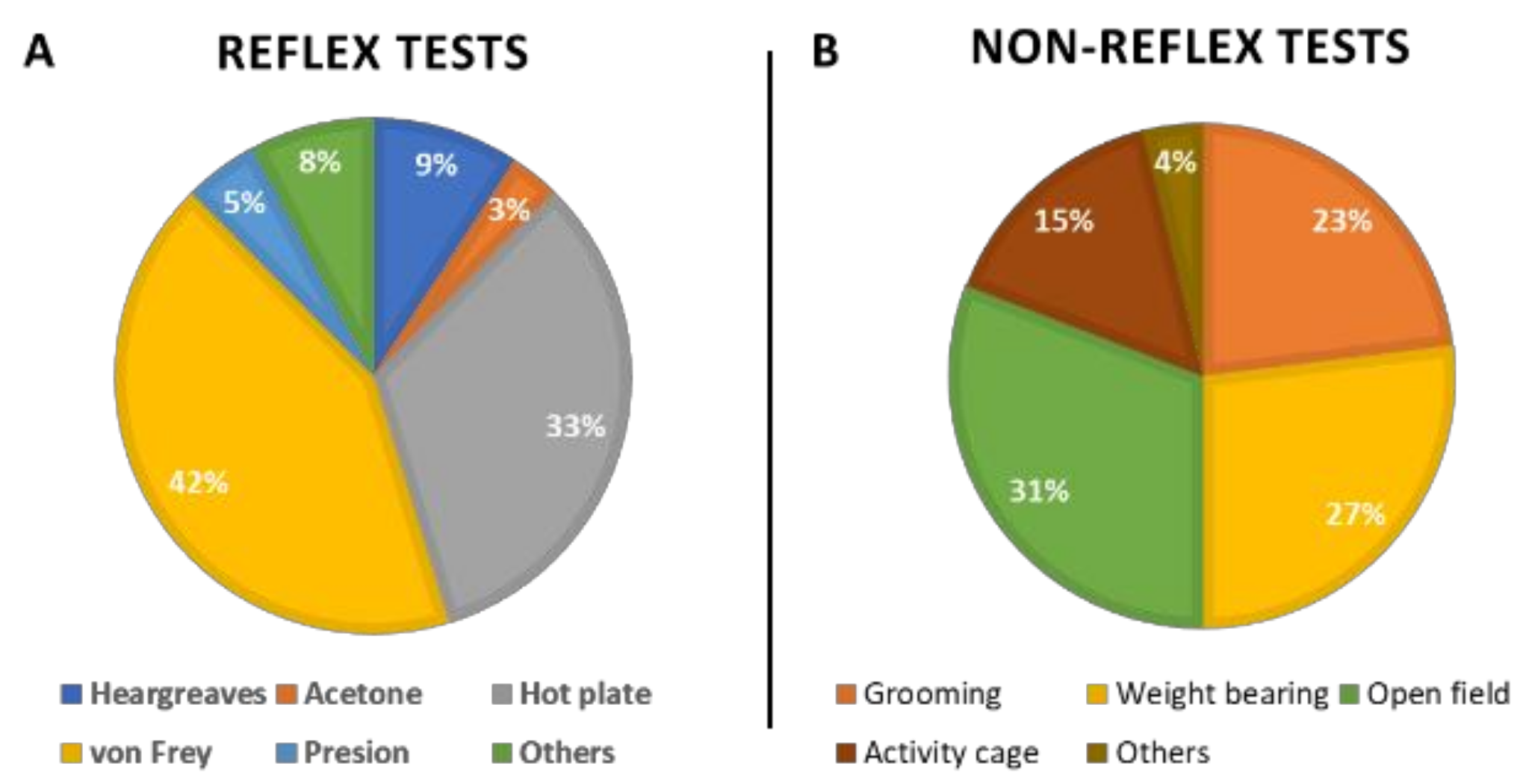
2.2.2. Reflex Tests
Mechanical Hyperalgesia
Thermal Hyperalgesia
2.2.3. Non-Reflex Tests
Pain-like Behavioral Responses
Grooming
Open Field
Locomotor Activity
Burrowing and Nesting
Grimace Scale
Weight Bearing
Skinner Box
Novel-Object Recognition
3. Experimental Section
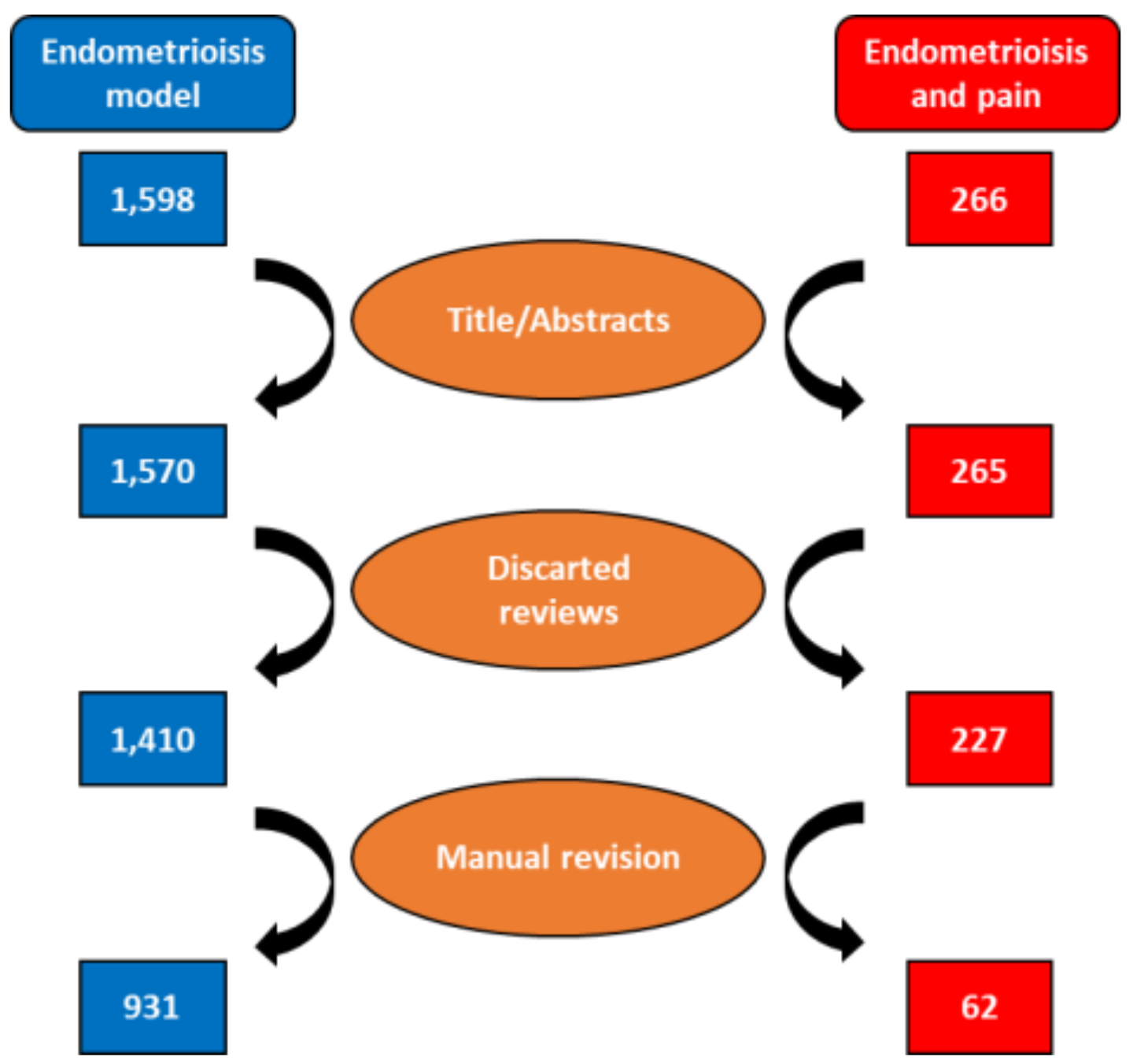
3.1. Identification of Manuscripts That Studied Endometriosis in Rodents and Model Type Used
3.2. Identification of Manuscripts That Studied Pain
3.3. Study of Behavioral Tests Used in Endometriosis Rodent Models
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zondervan, K.T.; Becker, C.M.; Koga, K.; Missmer, S.A.; Taylor, R.N.; Vigano, P. Endometriosis. Nat. Rev. Dis. Primers 2018, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Giudice, L.C. Clinical practice. Endometriosis. N. Engl. J. Med. 2010, 362, 2389–2398. [Google Scholar] [CrossRef] [PubMed]
- Shafrir, A.; Farland, L.; Shah, D.; Harris, H.; Kvaskoff, M.; Zondervan, K.; Missmer, S. Risk for and consequences of endometriosis: A critical epidemiologic review. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 51, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Adamson, G.D.; Kennedy, S.; Hummelshoj, L. Creating Solutions in Endometriosis: Global Collaboration through the World Endometriosis Research Foundation. J. Endometr. Pelvic Pain Disord. 2010, 2, 3–6. [Google Scholar] [CrossRef]
- Bank, W. Population Projection Tables by Country and Group. Available online: https://genderdata.worldbank.org/topics/population/ (accessed on 4 November 2022).
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef]
- Hudelist, G.; Fritzer, N.; Thomas, A.; Niehues, C.; Oppelt, P.; Haas, D.; Tammaa, A.; Salzer, H. Diagnostic delay for endometriosis in Austria and Germany: Causes and possible consequences. Hum. Reprod. 2012, 27, 3412–3416. [Google Scholar] [CrossRef]
- Holoch, K.J.; Lessey, B.A. Endometriosis and infertility. Clin. Obstet. Gynecol. 2010, 53, 429–438. [Google Scholar] [CrossRef]
- Simoens, S.; Dunselman, G.; Dirksen, C.; Hummelshoj, L.; Bokor, A.; Brandes, I.; Brodszky, V.; Canis, M.; Colombo, G.L.; DeLeire, T.; et al. The burden of endometriosis: Costs and quality of life of women with endometriosis and treated in referral centres. Hum. Reprod. 2012, 27, 1292–1299. [Google Scholar] [CrossRef]
- Falcone, T.; Flyckt, R. Clinical Management of Endometriosis. Obstet. Gynecol. 2018, 131, 557–571. [Google Scholar] [CrossRef]
- Simoens, S.; Hummelshoj, L.; Dunselman, G.; Brandes, I.; Dirksen, C.; D’Hooghe, T. EndoCost Consortium Endometriosis Cost Assessment (the EndoCost Study): A Cost-of-Illness Study Protocol. Gynecol. Obstet. Investig. 2011, 71, 170–176. [Google Scholar] [CrossRef]
- Falcone, T.; Lebovic, D.I. Clinical Management of Endometriosis. Obstet. Gynecol. 2011, 118, 691–705. [Google Scholar] [CrossRef]
- Soliman, A.M.; Surrey, E.S.; Bonafede, M.; Nelson, J.K.; Vora, J.B.; Agarwal, S.K. Health Care Utilization and Costs Associated with Endometriosis Among Women with Medicaid Insurance. J. Manag. Care Spéc. Pharm. 2019, 25, 566–572. [Google Scholar] [CrossRef]
- Husby, G.K.; Haugen, R.S.; Moen, M.H. Diagnostic delay in women with pain and endometriosis. Acta Obstet. Gynecol. Scand. 2003, 82, 649–653. [Google Scholar] [CrossRef]
- Surrey, E.; Soliman, A.M.; Trenz, H.; Blauer-Peterson, C.; Sluis, A. Impact of Endometriosis Diagnostic Delays on Healthcare Resource Utilization and Costs. Adv. Ther. 2020, 37, 1087–1099. [Google Scholar] [CrossRef]
- Saunders, P.T.K.; Horne, A.W. Endometriosis: Etiology, pathobiology, and therapeutic prospects. Cell 2021, 184, 2807–2824. [Google Scholar] [CrossRef]
- Nisenblat, V.; Bossuyt, P.M.M.; Farquhar, C.; Johnson, N.; Hull, M.L. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst. Rev. 2016, 2, CD009591. [Google Scholar] [CrossRef]
- Horne, A.W.; Missmer, S.A. Pathophysiology, diagnosis, and management of endometriosis. BMJ 2022, 379, e070750. [Google Scholar] [CrossRef]
- Liang, Y.; Wu, J.; Wang, W.; Xie, H.; Yao, S. Pro-endometriotic niche in endometriosis. Reprod. Biomed. Online 2019, 38, 549–559. [Google Scholar] [CrossRef]
- Ścieżyńska, A.; Komorowski, M.; Soszyńska, M.; Malejczyk, J. NK Cells as Potential Targets for Immunotherapy in Endometriosis. J. Clin. Med. 2019, 8, 1468. [Google Scholar] [CrossRef]
- Symons, L.K.; Miller, J.E.; Tyryshkin, K.; Monsanto, S.P.; Marks, R.M.; Lingegowda, H.; Vanderbeck, K.; Childs, T.; Young, S.L.; Lessey, B.A.; et al. Neutrophil recruitment and function in endometriosis patients and a syngeneic murine model. FASEB J. 2020, 34, 1558–1575. [Google Scholar] [CrossRef]
- Xu, H.; Zhao, J.; Lu, J.; Sun, X. Ovarian endometrioma infiltrating neutrophils orchestrate immunosuppressive microenvironment. J. Ovarian Res. 2020, 13, 44–48. [Google Scholar] [CrossRef] [PubMed]
- França, P.R.d.C.; Lontra, A.C.P.; Fernandes, P.D. Endometriosis: A Disease with Few Direct Treatment Options. Molecules 2022, 27, 4034. [Google Scholar] [CrossRef] [PubMed]
- Vallvé-Juanico, J.; Houshdaran, S.; Giudice, L.C. The endometrial immune environment of women with endometriosis. Hum. Reprod. Updat. 2019, 25, 565–592. [Google Scholar] [CrossRef] [PubMed]
- Mechsner, S. Endometriosis, an Ongoing Pain-Step-by-Step Treatment. J. Clin. Med. 2022, 11, 467. [Google Scholar] [CrossRef]
- Coxon, L.; Horne, A.W.; Vincent, K. Pathophysiology of endometriosis-associated pain: A review of pelvic and central nervous system mechanisms. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 51, 53–67. [Google Scholar] [CrossRef]
- Chauhan, S.; More, A.; Chauhan, V.; Kathane, A. Endometriosis: A Review of Clinical Diagnosis, Treatment, and Pathogenesis. Cureus 2022, 14, 28864. [Google Scholar] [CrossRef]
- Abesadze, E.; Chiantera, V.; Sehouli, J.; Mechsner, S. Post-operative management and follow-up of surgical treatment in the case of rectovaginal and retrocervical endometriosis. Arch. Gynecol. Obstet. 2020, 302, 957–967. [Google Scholar] [CrossRef]
- He, W.; Liu, X.; Zhang, Y.; Guo, S.-W. Generalized Hyperalgesia in Women With Endometriosis and Its Resolution Following a Successful Surgery. Reprod. Sci. 2010, 17, 1099–1111. [Google Scholar] [CrossRef]
- Simanski, C.J.; Althaus, A.; Hoederath, S.; Kreutz, K.W.; Hoederath, P.; Lefering, R.; Pape-Köhler, C.; Neugebauer, E.A. Incidence of Chronic Postsurgical Pain (CPSP) after General Surgery. Pain Med. 2014, 15, 1222–1229. [Google Scholar] [CrossRef]
- Althaus, A.; Hinrichs-Rocker, A.; Chapman, R.; Becker, O.A.; Lefering, R.; Simanski, C.; Weber, F.; Moser, K.-H.; Joppich, R.; Trojan, S.; et al. Development of a risk index for the prediction of chronic post-surgical pain. Eur. J. Pain 2012, 16, 901–910. [Google Scholar] [CrossRef]
- Shakiba, K.; Bena, J.F.; McGill, K.M.; Minger, J.; Falcone, T. Surgical treatment of endometriosis: A 7-year follow-up on the requirement for further surgery. Obstet. Gynecol. 2008, 111, 1285–1292. [Google Scholar] [CrossRef]
- Guo, S.-W. Recurrence of endometriosis and its control. Hum. Reprod. Updat. 2009, 15, 441–461. [Google Scholar] [CrossRef]
- Carneiro, M.M. Deciding on the appropriate pharmacotherapy for the treatment of endometriosis. Expert Opin. Pharmacother. 2023, 24, 1–5. [Google Scholar] [CrossRef]
- Razzi, S.; Luisi, S.; Calonaci, F.; Altomare, A.; Bocchi, C.; Petraglia, F. Efficacy of vaginal danazol treatment in women with recurrent deeply infiltrating endometriosis. Fertil. Steril. 2007, 88, 789–794. [Google Scholar] [CrossRef]
- Berlanda, N.; Somigliana, E.; Vigano’, P.; Vercellini, P.P. Safety of medical treatments for endometriosis. Expert Opin. Drug Saf. 2016, 15, 21–30. [Google Scholar] [CrossRef]
- Park, Y.; Cho, Y.J.; Sung, N.; Park, M.J.; Guan, X.; Gibbons, W.E.; O’Malley, B.W.; Han, S.J. Oleuropein suppresses endometriosis progression and improves the fertility of mice with endometriosis. J. Biomed. Sci. 2022, 29, 1–20. [Google Scholar] [CrossRef]
- Horne, A.W.; Vincent, K.; Hewitt, C.A.; Middleton, L.J.; Koscielniak, M.; Szubert, W.; Doust, A.M.; Daniels, J.P.; Abdi, S.; Acharya, S.; et al. Gabapentin for chronic pelvic pain in women (GaPP2): A multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2020, 396, 909–917. [Google Scholar] [CrossRef]
- Bruner-Tran, K.L.; Carvalho-Macedo, A.C.; Duleba, A.J.; Crispens, M.A.; Osteen, K.G. Experimental endometriosis in immunocompromised mice after adoptive transfer of human leukocytes. Fertil. Steril. 2010, 93, 2519–2524. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, X.; Lu, X.; Jiang, J. Effects of inhibiting the PI3K/Akt/mTOR signaling pathway on the pain of sciatic endometriosis in a rat model. Can. J. Physiol. Pharmacol. 2019, 97, 963–970. [Google Scholar] [CrossRef]
- Nagira, K.; Taniguchi, F.; Nakamura, K.; Tokita, Y.; Tsuchiya, N.; Khine, Y.M.; Harada, T. Tokishakuyakusan, a Kampo medicine, attenuates endometriosis-like lesions and hyperalgesia in murine with endometriosis-like symptoms. Am. J. Reprod. Immunol. 2019, 82, e13182. [Google Scholar] [CrossRef]
- Greaves, E.; Rosser, M.; Saunders, P.T.K. Endometriosis-Associated Pain—Do Preclinical Rodent Models Provide a Good Platform for Translation? Adv. Anat. Embryol. Cell Biol. 2020, 232, 25–55. [Google Scholar]
- Gonzalez-Cano, R.; Montilla-Garcia, A.; Ruiz-Cantero, M.C.; Bravo-Caparros, I.; Tejada, M.A.; Nieto, F.R.; Cobos, E.J. The search for translational pain outcomes to refine analgesic development: Where did we come from and where are we going? Neurosci. Biobehav. Rev. 2020, 113, 238–261. [Google Scholar] [CrossRef] [PubMed]
- Yezierski, R.P.; Hansson, P. Inflammatory and Neuropathic Pain From Bench to Bedside: What Went Wrong? J. Pain 2018, 19, 571–588. [Google Scholar] [CrossRef] [PubMed]
- Nunez-Badinez, P.; De Leo, B.; Laux-Biehlmann, A.; Hoffmann, A.; Zollner, T.M.; Saunders, P.T.K.; Simitsidellis, I.; Charrua, A.; Cruz, F.; Gomez, R.; et al. Preclinical models of endometriosis and interstitial cystitis/bladder pain syndrome: An Innovative Medicines Initiative-PainCare initiative to improve their value for translational research in pelvic pain. Pain 2021, 162, 2349–2365. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, M.; Saito, Y.; Tanaka-Hattori, N.; Takeda, K.; Ihara, T.; Sugamata, M. Expression Profile of Extracellular Matrix and Adhesion Molecules in the Development of Endometriosis in a Mouse Model. Reprod. Sci. 2012, 19, 1365–1372. [Google Scholar] [CrossRef]
- Cheng, C.-W.; Licence, D.; Cook, E.; Luo, F.; Arends, M.J.; Smith, S.K.; Print, C.G.; Charnock-Jones, D.S. Activation of mutated K-ras in donor endometrial epithelium and stroma promotes lesion growth in an intact immunocompetent murine model of endometriosis. J. Pathol. 2011, 224, 261–269. [Google Scholar] [CrossRef]
- Greaves, E.; Cousins, F.L.; Murray, A.; Esnal-Zufiaurre, A.; Fassbender, A.; Horne, A.W.; Saunders, P.T. A Novel Mouse Model of Endometriosis Mimics Human Phenotype and Reveals Insights into the Inflammatory Contribution of Shed Endometrium. Am. J. Pathol. 2014, 184, 1930–1939. [Google Scholar] [CrossRef]
- Ferrero, H.; Buigues, A.; Martínez, J.; Simón, C.; Pellicer, A.; Gómez, R. A novel homologous model for noninvasive monitoring of endometriosis progression. Biol. Reprod. 2017, 96, 302–312. [Google Scholar] [CrossRef]
- Buigues, A.; Ferrero, H.; Martínez, J.; Pellicer, N.; Pellicer, A.; Gómez, R. Evaluation of PAI-1 in endometriosis using a homologous immunocompetent mouse model. Biol. Reprod. 2018, 99, 326–335. [Google Scholar] [CrossRef]
- Grümmer, R. Animal models in endometriosis research. Hum. Reprod. Updat. 2006, 12, 641–649. [Google Scholar] [CrossRef]
- Grümmer, R.; Schwarzer, F.; Bainczyk, K.; Hess-Stumpp, H.; Regidor, P.-A.; Schindler, A.E.; Winterhager, E. Peritoneal endometriosis: Validation of an in-vivo model. Hum. Reprod. 2001, 16, 1736–1743. [Google Scholar] [CrossRef]
- Tejada, M.A.; Santos-Llamas, A.I.; Escriva, L.; Tarin, J.J.; Cano, A.; Fernández-Ramírez, M.J.; Nunez-Badinez, P.; De Leo, B.; Saunders, P.T.K.; Vidal, V.; et al. Identification of Altered Evoked and Non-Evoked Responses in a Heterologous Mouse Model of Endometriosis-Associated Pain. Biomedicines 2022, 10, 501. [Google Scholar] [CrossRef]
- Mogil, J.S. Animal models of pain: Progress and challenges. Nat. Rev. Neurosci. 2009, 10, 283–294. [Google Scholar] [CrossRef]
- Fattori, V.; Franklin, N.S.; Cano, R.G.; Peterse, D.; Ghalali, A.; Madrian, E.; Verri, W.A.; Andrews, N.; Woolf, C.J.; Rogers, M.S. Nonsurgical mouse model of endometriosis-associated pain that responds to clinically active drugs. Pain 2020, 161, 1321–1331. [Google Scholar] [CrossRef]
- Forster, R.; Sarginson, A.; Velichkova, A.; Hogg, C.; Dorning, A.; Horne, A.W.; Saunders, P.T.K.; Greaves, E. Macrophage-derived insulin-like growth factor-1 is a key neurotrophic and nerve-sensitizing factor in pain associated with endometriosis. FASEB J. 2019, 33, 11210–11222. [Google Scholar] [CrossRef]
- Zheng, P.; Jia, S.; Guo, D.; Chen, S.; Zhang, W.; Cheng, A.; Xie, W.; Sun, G.; Leng, J.; Lang, J. Central Sensitization-Related Changes in Brain Function Activity in a Rat Endometriosis-Associated Pain Model. J. Pain Res. 2020, 13, 95–107. [Google Scholar] [CrossRef]
- Berkley, K.J.; Cason, A.; Jacobs, H.; Bradshaw, H.; Wood, E. Vaginal hyperalgesia in a rat model of endometriosis. Neurosci. Lett. 2001, 306, 185–188. [Google Scholar] [CrossRef]
- Maddern, J.; Grundy, L.; Harrington, A.; Schober, G.; Castro, J.; Brierley, S.M. A syngeneic inoculation mouse model of endometriosis that develops multiple comorbid visceral and cutaneous pain like behaviours. Pain 2022, 163, 1622–1635. [Google Scholar] [CrossRef]
- Liu, M.; Liu, X.; Zhang, Y.; Guo, S.-W. Valproic Acid and Progestin Inhibit Lesion Growth and Reduce Hyperalgesia in Experimentally Induced Endometriosis in Rats. Reprod. Sci. 2012, 19, 360–373. [Google Scholar] [CrossRef]
- Zhou, Q.; Price, D.; Caudle, R.M.; Verne, N.G. Visceral and somatic hypersensitivity in a subset of rats following TNBS-induced colitis. Pain 2008, 134, 9–15. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, S.; Qiu, C.; Sun, Y.; Li, W.; Jiang, J.; Zhang, J.-M. Fractalkine/CX3CR1 Contributes to Endometriosis-Induced Neuropathic Pain and Mechanical Hypersensitivity in Rats. Front. Cell. Neurosci. 2018, 12, 495. [Google Scholar] [CrossRef] [PubMed]
- Pereira, F.; Medeiros, F.D.C.; Rocha, H.A.L.; Silva, K.S.D. Effects of omega-6/3 and omega-9/6 nutraceuticals on pain and fertility in peritoneal endometriosis in rats. Acta Cir. Bras. 2019, 34, e201900405. [Google Scholar] [CrossRef] [PubMed]
- González-Cano, R.; Artacho-Cordón, A.; Romero, L.; Tejada, M.A.; Nieto, F.R.; Merlos, M.; Cañizares, F.J.; Cendán, C.M.; Fernández-Segura, E.; Baeyens, J.M. Urinary bladder sigma-1 receptors: A new target for cystitis treatment. Pharmacol. Res. 2020, 155, 104724. [Google Scholar] [CrossRef] [PubMed]
- Giamberardino, M.A.; Berkley, K.J.; Affaitati, G.; Lerza, R.; Centurione, L.; Lapenna, D.; Vecchiet, L. Influence of endometriosis on pain behaviors and muscle hyperalgesia induced by a ureteral calculosis in female rats. Pain 2002, 95, 247–257. [Google Scholar] [CrossRef]
- Hassan, A.M.; Jain, P.; Mayerhofer, R.; Fröhlich, E.E.; Farzi, A.; Reichmann, F.; Herzog, H.; Holzer, P. Visceral hyperalgesia caused by peptide YY deletion and Y2 receptor antagonism. Sci. Rep. 2017, 7, 40968. [Google Scholar] [CrossRef]
- Cordaro, M.; Trovato Salinaro, A.; Siracusa, R.; D’Amico, R.; Impellizzeri, D.; Scuto, M.; Ontario, M.L.; Interdonato, L.; Crea, R.; Fusco, R.; et al. Hidrox((R)) and Endometriosis: Biochemical Evaluation of Oxidative Stress and Pain. Antioxidants 2021, 10, 720. [Google Scholar] [CrossRef]
- Jain, P.; Hassan, A.M.; Koyani, C.N.; Mayerhofer, R.; Reichmann, F.; Farzi, A.; Schuligoi, R.; Malle, E.; Holzer, P. Behavioral and molecular processing of visceral pain in the brain of mice: Impact of colitis and psychological stress. Front. Behav. Neurosci. 2015, 9, 177. [Google Scholar] [CrossRef]
- Leung, V.S.; Benoit-Biancamano, M.-O.; Pang, D.S. Performance of behavioral assays: The Rat Grimace Scale, burrowing activity and a composite behavior score to identify visceral pain in an acute and chronic colitis model. PAIN Rep. 2019, 4, e718. [Google Scholar] [CrossRef]
- George, R.P.; Howarth, G.S.; Whittaker, A.L. Use of the Rat Grimace Scale to Evaluate Visceral Pain in a Model of Chemotherapy-Induced Mucositis. Animals 2019, 9, 678. [Google Scholar] [CrossRef]
- Gruen, M.; Laux-Biehlmann, A.; Zollner, T.M.; Nagel, J. Use of dynamic weight bearing as a novel end-point for the assessment of abdominal pain in the LPS-induced peritonitis model in the rat. J. Neurosci. Methods 2014, 232, 118–124. [Google Scholar] [CrossRef]
- Laux-Biehlmann, A.; Boyken, J.; Dahllöf, H.; Schmidt, N.; Zollner, T.; Nagel, J. Dynamic weight bearing as a non-reflexive method for the measurement of abdominal pain in mice. Eur. J. Pain 2016, 20, 742–752. [Google Scholar] [CrossRef]
- Persoons, E.; De Clercq, K.; Van den Eynde, C.; Pinto, S.J.P.C.; Luyten, K.; Van Bree, R.; Tomassetti, C.; Voets, T.; Vriens, J. Mimicking Sampson’s Retrograde Menstrual Theory in Rats: A New Rat Model for Ongoing Endometriosis-Associated Pain. Int. J. Mol. Sci. 2020, 21, 2326. [Google Scholar] [CrossRef]
- Van Aken, M.A.; Groothuis, P.G.; Panagiotou, M.; van Duin, M.; Nap, A.W.; van Rijn, T.C.; Kozicz, T.; Braat, D.D.; Peeters, A.B. An objective and automated method for evaluating abdominal hyperalgesia in a rat model for endometriosis. Lab. Anim. 2020, 54, 365–372. [Google Scholar] [CrossRef]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef]
- Tejada, M.A.; Montilla-García, A.; Cronin, S.J.; Cikes, D.; Sánchez-Fernández, C.; González-Cano, R.; Ruiz-Cantero, M.C.; Penninger, J.M.; Vela, J.M.; Baeyens, J.M.; et al. Sigma-1 receptors control immune-driven peripheral opioid analgesia during inflammation in mice. Proc. Natl. Acad. Sci. USA 2017, 114, 8396–8401. [Google Scholar] [CrossRef]
- Leuchtweis, J.; Imhof, A.-K.; Montechiaro, F.; Schaible, H.-G.; Boettger, M. Validation of the digital pressure application measurement (PAM) device for detection of primary mechanical hyperalgesia in rat and mouse antigen-induced knee joint arthritis. Methods Find. Exp. Clin. Pharmacol. 2010, 32, 575–583. [Google Scholar] [CrossRef]
- Hargreaves, K.; Dubner, R.; Brown, F.; Flores, C.; Joris, J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988, 32, 77–88. [Google Scholar] [CrossRef]
- Iuvone, T.; Affaitati, G.; De Filippis, D.; Lopopolo, M.; Grassia, G.; Lapenna, D.; Negro, L.; Costantini, R.; Vaia, M.; Cipollone, F.; et al. Ultramicronized palmitoylethanolamide reduces viscerovisceral hyperalgesia in a rat model of endometriosis plus ureteral calculosis: Role of mast cells. Pain 2016, 157, 80–91. [Google Scholar] [CrossRef]
- Oz, H.S. Multiorgan chronic inflammatory hepatobiliary pancreatic murine model deficient in tumor necrosis factor receptors 1 and 2. World J. Gastroenterol. 2016, 22, 4988–4998. [Google Scholar] [CrossRef]
- Pitzer, C.; La Porta, C.; Treede, R.-D.; Tappe-Theodor, A. Inflammatory and neuropathic pain conditions do not primarily evoke anxiety-like behaviours in C57BL/6 mice. Eur. J. Pain 2019, 23, 285–306. [Google Scholar] [CrossRef]
- Harada, T.; Takahashi, H.; Kaya, H.; Inoki, R. A test for analgesics as an indicator of locomotor activity in writhing mice. Arch. Int. Pharmacodyn. Ther. 1979, 242, 273–284. [Google Scholar]
- Sałat, K.; Cios, A.; Wyska, E.; Salat, R.; Mogilski, S.; Filipek, B.; Więckowski, K.; Malawska, B. Antiallodynic and antihyperalgesic activity of 3-[4-(3-trifluoromethyl-phenyl)-piperazin-1-yl]-dihydrofuran-2-one compared to pregabalin in chemotherapy-induced neuropathic pain in mice. Pharmacol. Biochem. Behav. 2014, 122, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Luna-Sanchez, M.; Diaz-Casado, E.; Barca, E.; Tejada, M.A.; Montilla-Garcia, A.; Cobos, E.J.; Escames, G.; Acuna-Castroviejo, D.; Quinzii, C.M.; Lopez, L.C. The clinical heterogeneity of coenzyme Q10 deficiency results from genotypic differences in the Coq9 gene. EMBO Mol. Med. 2015, 7, 670–687. [Google Scholar] [CrossRef] [PubMed]
- Deacon, R. Burrowing: A sensitive behavioural assay, tested in five species of laboratory rodents. Behav. Brain Res. 2009, 200, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Alexander, K.S.; Rodriguez, T.R.; Sarfo, A.N.; Patton, T.B.; Miller, L. Effects of monoamine uptake inhibitors on pain-related depression of nesting in mice. Behav. Pharmacol. 2019, 30, 463–470. [Google Scholar] [CrossRef]
- Tappe-Theodor, A.; King, T.; Morgan, M.M. Pros and Cons of Clinically Relevant Methods to Assess Pain in Rodents. Neurosci. Biobehav. Rev. 2019, 100, 335–343. [Google Scholar] [CrossRef]
- Tuttle, A.H.; Molinaro, M.J.; Jethwa, J.F.; Sotocinal, S.G.; Prieto, J.C.; Styner, M.A.; Mogil, J.S.; Zylka, M.J. A deep neural network to assess spontaneous pain from mouse facial expressions. Mol. Pain 2018, 14. [Google Scholar] [CrossRef]
- Schött, E.; Berge, O.-G.; Ängeby-Möller, K.; Hammarström, G.; Dalsgaard, C.-J.; Brodin, E. Weight bearing as an objective measure of arthritic pain in the rat. J. Pharmacol. Toxicol. Methods 1994, 31, 79–83. [Google Scholar] [CrossRef]
- Hamers, F.P.; Lankhorst, A.J.; van Laar, T.J.; Veldhuis, W.B.; Gispen, W.H. Automated Quantitative Gait Analysis During Overground Locomotion in the Rat: Its Application to Spinal Cord Contusion and Transection Injuries. J. Neurotrauma 2001, 18, 187–201. [Google Scholar] [CrossRef]
- Liu, L.; Tian, D.; Liu, C.; Yu, K.; Bai, J. Metformin Enhances Functional Recovery of Peripheral Nerve in Rats with Sciatic Nerve Crush Injury. Experiment 2019, 25, 10067–10076. [Google Scholar] [CrossRef]
- Olechowski, C.J.; Tenorio, G.; Sauve, Y.; Kerr, B.J. Changes in nociceptive sensitivity and object recognition in experimental autoimmune encephalomyelitis (EAE). Exp. Neurol. 2013, 241, 113–121. [Google Scholar] [CrossRef]
- Carcolé, M.; Zamanillo, D.; Merlos, M.; Fernández-Pastor, B.; Cabañero, D.; Maldonado, R. Blockade of the Sigma-1 Receptor Relieves Cognitive and Emotional Impairments Associated to Chronic Osteoarthritis Pain. Front. Pharmacol. 2019, 10, 468. [Google Scholar] [CrossRef]
- Negus, S.S.; Vanderah, T.W.; Brandt, M.R.; Bilsky, E.J.; Becerra, L.; Borsook, D. Preclinical Assessment of Candidate Analgesic Drugs: Recent Advances and Future Challenges. Experiment 2006, 319, 507–514. [Google Scholar] [CrossRef]
| Behavioural Test | Differences vs. Control | Ref. | ||
|---|---|---|---|---|
| Reflex tests | Mechanical hyperalgesia | Von Frey | ↑↑↑ | [40,53,55,56,57] |
| Randall-Selitto | No data for endometriosis/visceral pain | |||
| pressure application measurement | No data for endometriosis/visceral pain | |||
| vaginal distension | ↑↑ | [58,59] | ||
| Thermal hyperalgesia | Holt plate | ↑↑ | [41,57,60] | |
| Hargreaves | ↑↑↑ | [40,61] | ||
| Acetone | ↑↑↑ | [62] | ||
| Non-reflex tests | Pain-like behavioural responses | ↑↑↑ | [63,64,65] | |
| Grooming | ↑↑ | [56,66] | ||
| Open field | ↑↑ | [57,67] | ||
| Locomotor activity | ↑ | [53,68] | ||
| Borrowing | ↑ | [69] | ||
| Nesting | ↑↑ | [53] | ||
| Grimace scale | ↑↑ | [66,70] | ||
| Weight bearing | ↑↑ | [55,71,72,73] | ||
| Skinner box | ↑ | [74] | ||
| Novel-object recognition | No data for endometriosis/visceral pain | |||
| Test | Brief Description | Ref. |
|---|---|---|
| Activity cage | Time and distance spent by animals near walls, in center or in total | [68,83] |
| Activity wheels | Total distance voluntarily travelled by animals on the wheel | [84] |
| Automated home cage | Distance travelled and interactions between animals in the same home cage, also during in dark phases when the rodents are more active | [53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tejada, M.A.; Antunez, C.; Nunez-Badinez, P.; De Leo, B.; Saunders, P.T.; Vincent, K.; Cano, A.; Nagel, J.; Gomez, R. Rodent Animal Models of Endometriosis-Associated Pain: Unmet Needs and Resources Available for Improving Translational Research in Endometriosis. Int. J. Mol. Sci. 2023, 24, 2422. https://doi.org/10.3390/ijms24032422
Tejada MA, Antunez C, Nunez-Badinez P, De Leo B, Saunders PT, Vincent K, Cano A, Nagel J, Gomez R. Rodent Animal Models of Endometriosis-Associated Pain: Unmet Needs and Resources Available for Improving Translational Research in Endometriosis. International Journal of Molecular Sciences. 2023; 24(3):2422. https://doi.org/10.3390/ijms24032422
Chicago/Turabian StyleTejada, Miguel A., Carles Antunez, Paulina Nunez-Badinez, Bianca De Leo, Philippa T. Saunders, Katy Vincent, Antonio Cano, Jens Nagel, and Raul Gomez. 2023. "Rodent Animal Models of Endometriosis-Associated Pain: Unmet Needs and Resources Available for Improving Translational Research in Endometriosis" International Journal of Molecular Sciences 24, no. 3: 2422. https://doi.org/10.3390/ijms24032422
APA StyleTejada, M. A., Antunez, C., Nunez-Badinez, P., De Leo, B., Saunders, P. T., Vincent, K., Cano, A., Nagel, J., & Gomez, R. (2023). Rodent Animal Models of Endometriosis-Associated Pain: Unmet Needs and Resources Available for Improving Translational Research in Endometriosis. International Journal of Molecular Sciences, 24(3), 2422. https://doi.org/10.3390/ijms24032422








