Enhanced Antitumor Efficacy of Radium-223 and Enzalutamide in the Intratibial LNCaP Prostate Cancer Model
Abstract
1. Introduction
2. Results
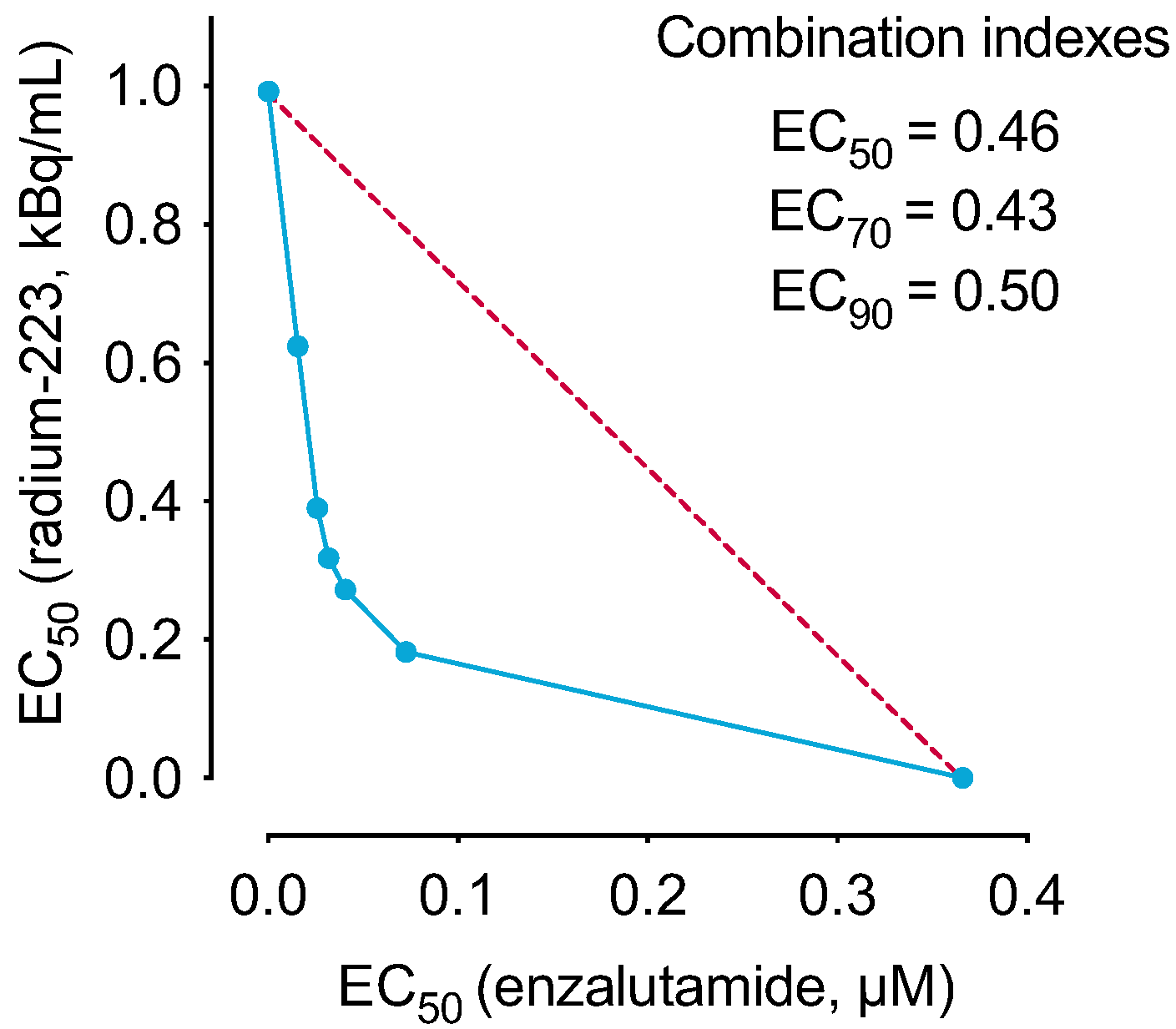
2.1. The Combination of Radium-223 and Enzalutamide Has Synergistic Antiproliferative Efficacy In Vitro
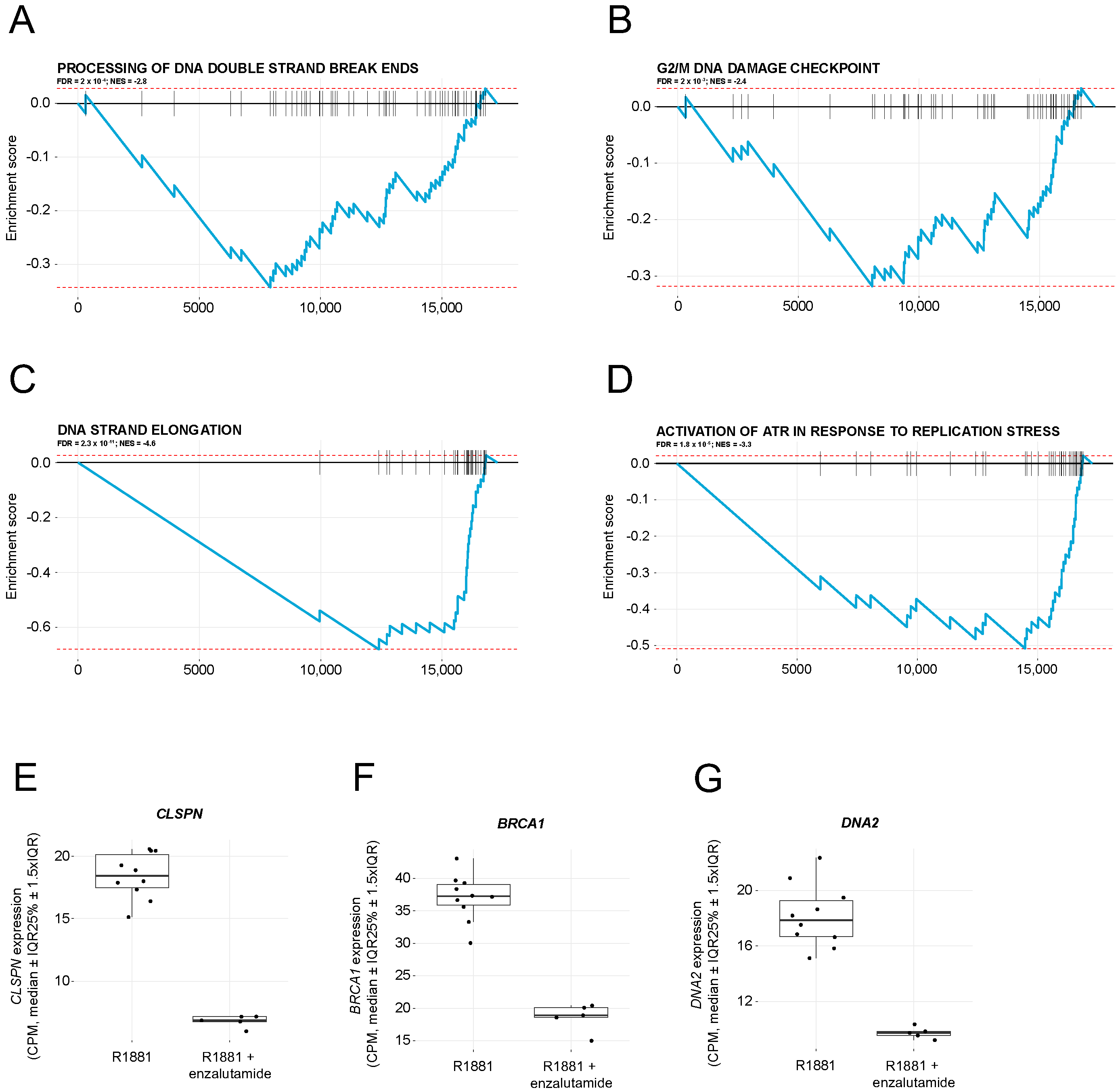
2.2. Enzalutamide Treatment Downregulates Pathways Involved in DNA Damage Repair
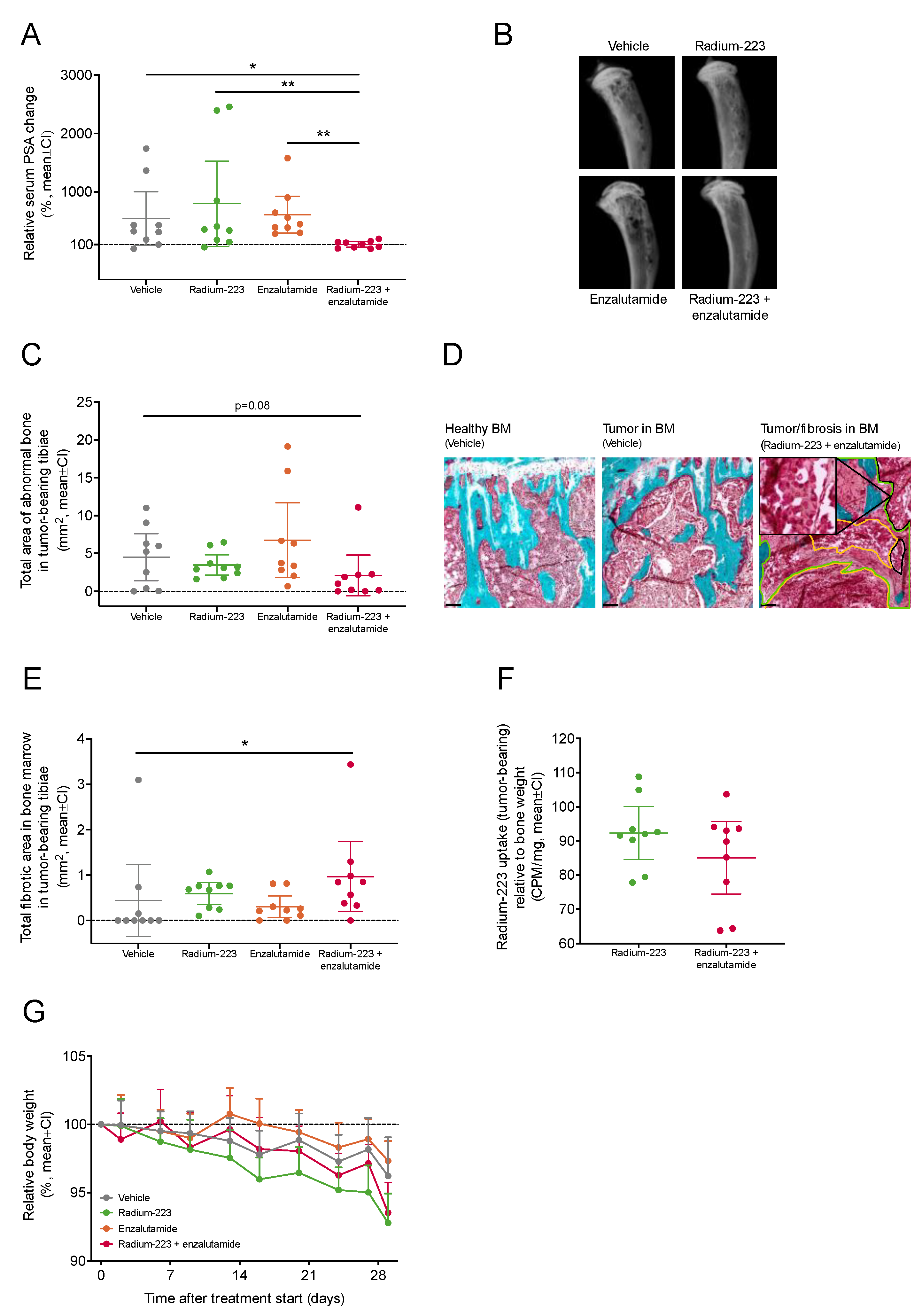
2.3. Combination Treatment with Radium-223 and Enzalutamide Exhibits Enhanced Antitumor Efficacy In Vivo
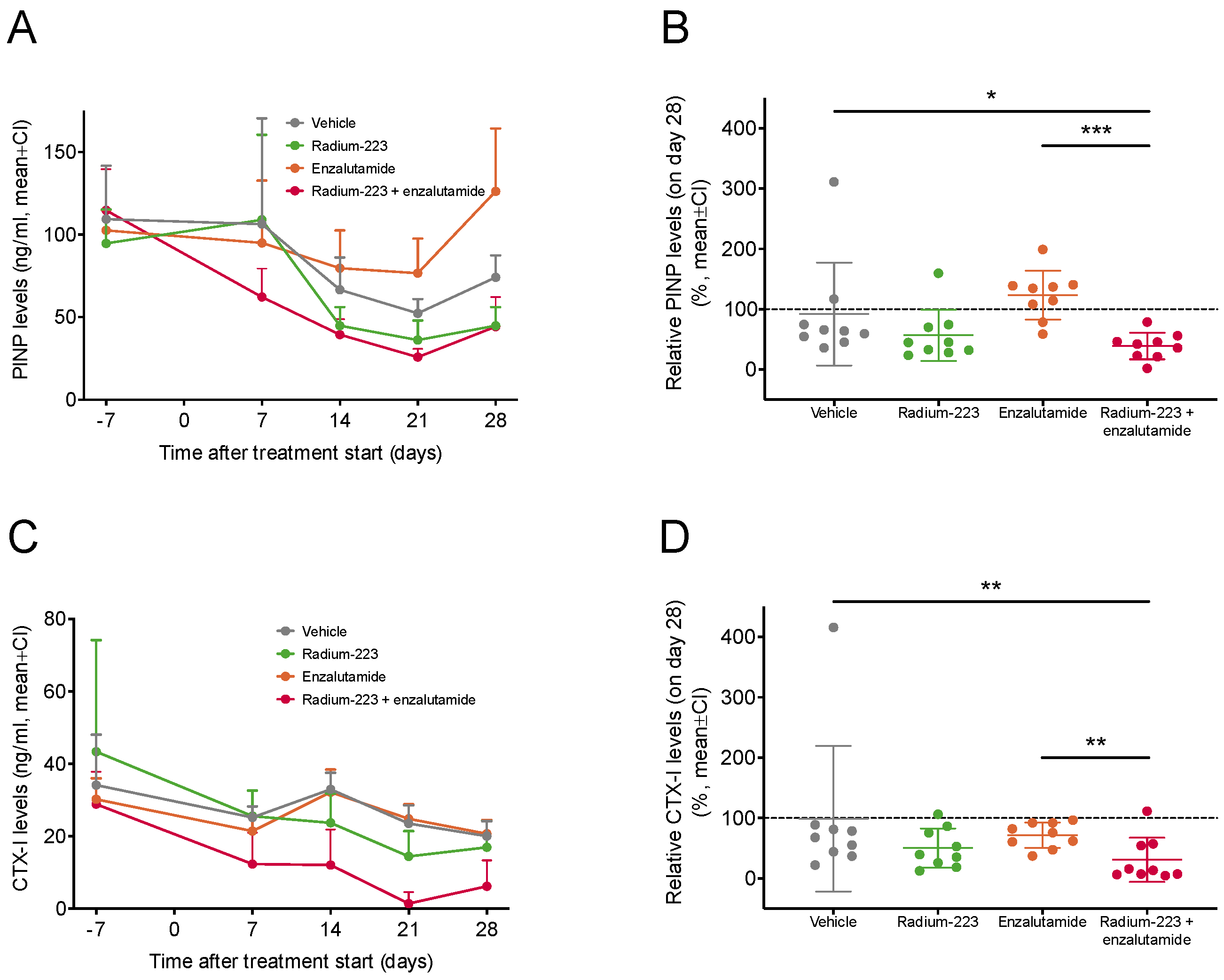
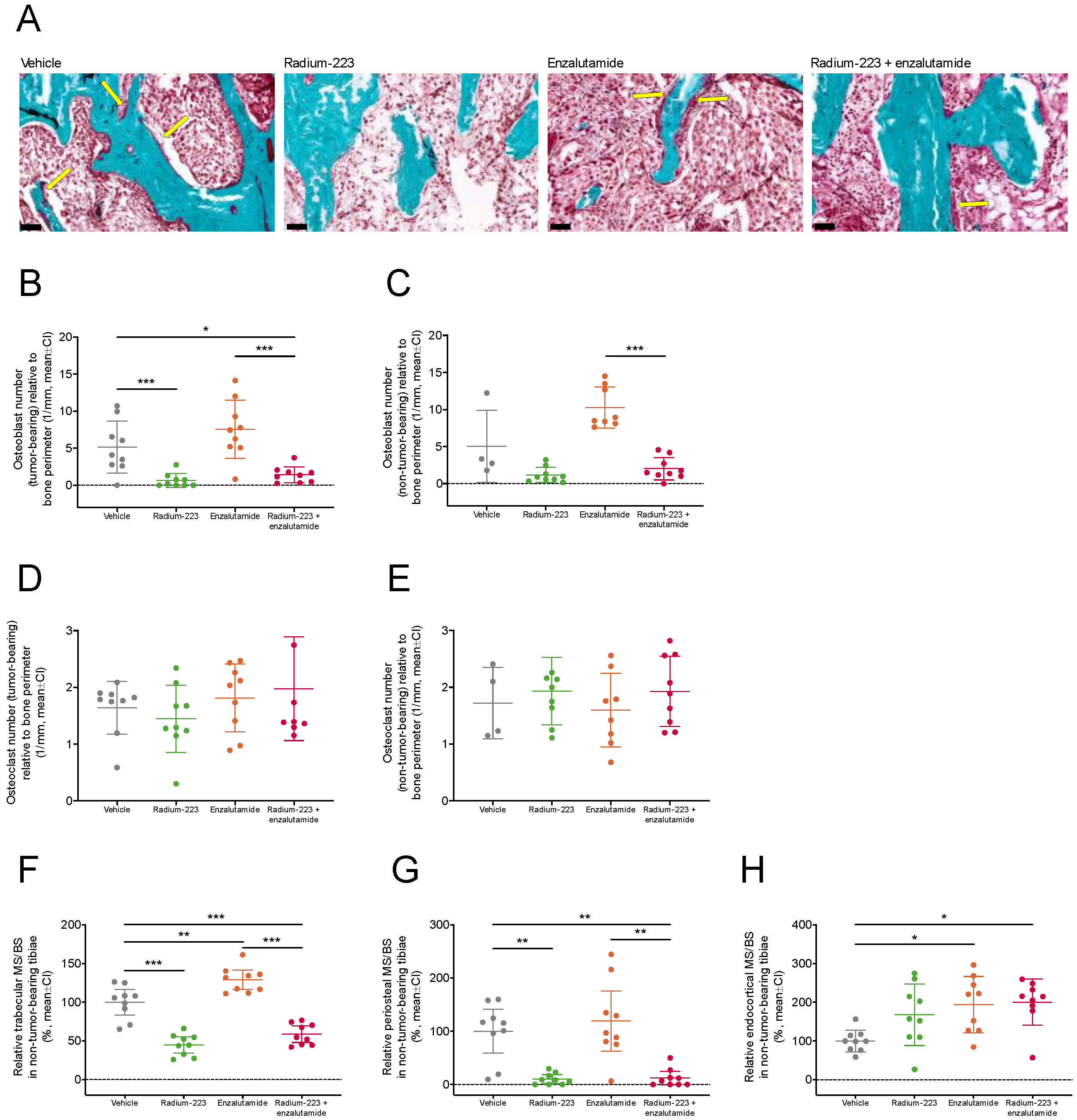
2.4. Radium-223 and Enzalutamide Combination Treatment Inhibits Abnormal Bone Turnover
2.5. Concurrent Enzalutamide Administration Does Not Impair Radium-223-Specific Antitumor Effects in Bone or Its Ability to Inhibit Abnormal Bone Formation
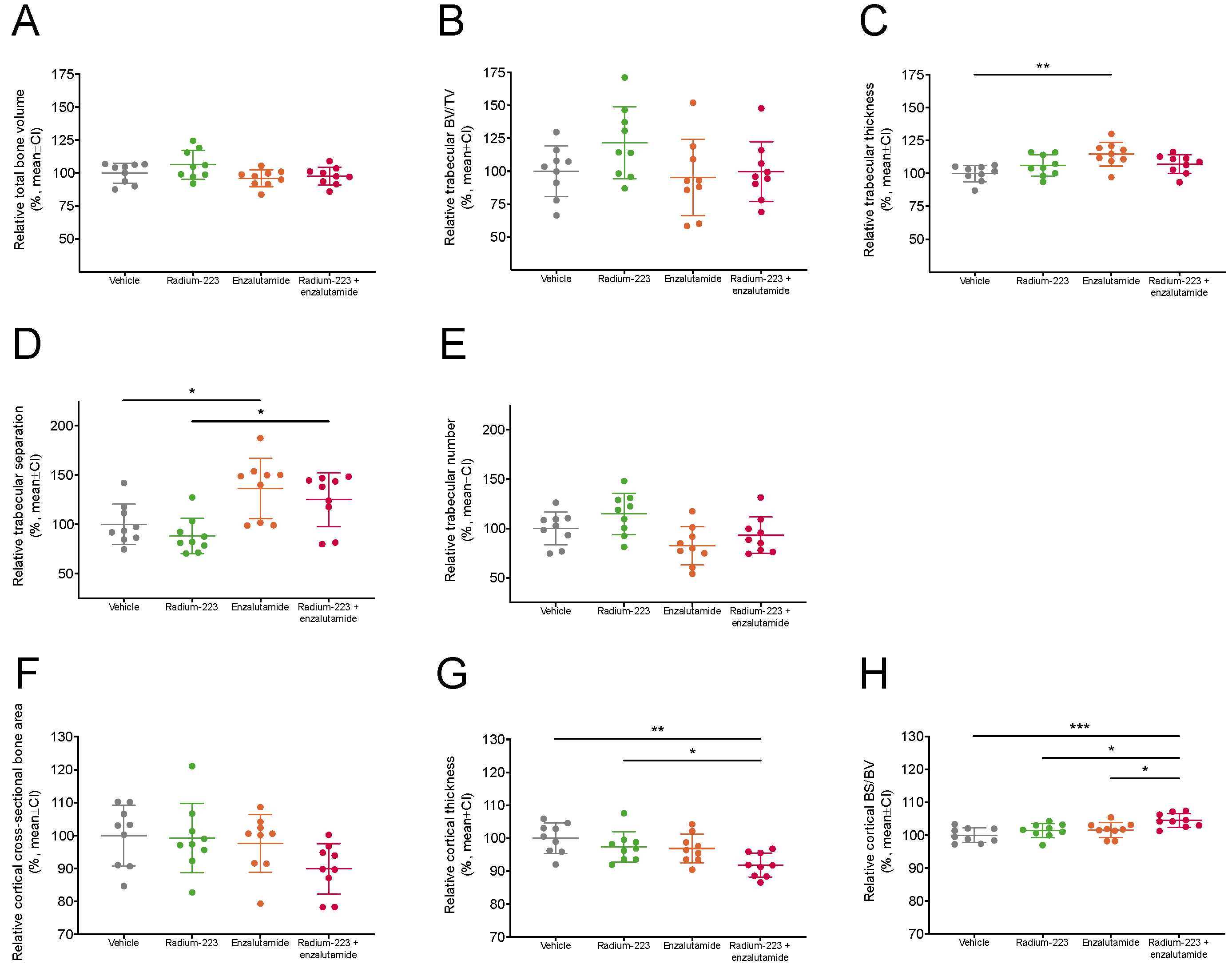
2.6. Radium-223 and Enzalutamide Combination Treatment Does Not Affect Bone Microarchitecture
3. Discussion
4. Materials and Methods
4.1. In Vitro Cell Viability Assay
4.2. In Vitro Androgen Stimulation Assay and RNA Sequencing
4.3. Intratibial LNCaP Xenograft Model
4.4. PSA and Biochemical Markers of Bone Turnover
4.5. Radiography of Tumor-Bearing Tibiae
4.6. Ex Vivo Analyses
4.7. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, J.M.; Parker, C. The safety and efficacy of radium-223 dichloride for the treatment of advanced prostate cancer. Expert. Rev. Anticancer Ther. 2016, 16, 911–918. [Google Scholar] [CrossRef]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fossa, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Suominen, M.I.; Wilson, T.; Kakonen, S.M.; Scholz, A. The Mode-of-Action of Targeted Alpha Therapy Radium-223 as an Enabler for Novel Combinations to Treat Patients with Bone Metastasis. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Fizazi, K.; Saad, F.; Taplin, M.E.; Sternberg, C.N.; Miller, K.; de Wit, R.; Mulders, P.; Chi, K.N.; Shore, N.D.; et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 2012, 367, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.J.; Szmulewitz, R.Z.; Petrylak, D.P.; Holzbeierlein, J.; Villers, A.; Azad, A.; Alcaraz, A.; Alekseev, B.; Iguchi, T.; Shore, N.D.; et al. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy With Enzalutamide or Placebo in Men With Metastatic Hormone-Sensitive Prostate Cancer. J. Clin. Oncol. 2019, 37, 2974–2986. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Fizazi, K.; Saad, F.; Rathenborg, P.; Shore, N.; Ferreira, U.; Ivashchenko, P.; Demirhan, E.; Modelska, K.; Phung, D.; et al. Enzalutamide in Men with Nonmetastatic, Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2018, 378, 2465–2474. [Google Scholar] [CrossRef]
- Cattrini, C.; Caffo, O.; De Giorgi, U.; Mennitto, A.; Gennari, A.; Olmos, D.; Castro, E. Apalutamide, Darolutamide and Enzalutamide for Nonmetastatic Castration-Resistant Prostate Cancer (nmCRPC): A Critical Review. Cancers 2022, 14, 1792. [Google Scholar] [CrossRef]
- Evans, C.P.; Higano, C.S.; Keane, T.; Andriole, G.; Saad, F.; Iversen, P.; Miller, K.; Kim, C.S.; Kimura, G.; Armstrong, A.J.; et al. The PREVAIL Study: Primary Outcomes by Site and Extent of Baseline Disease for Enzalutamide-treated Men with Chemotherapy-naïve Metastatic Castration-resistant Prostate Cancer. Eur. Urol. 2016, 70, 675–683. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Lin, P.; Tombal, B.; Saad, F.; Higano, C.S.; Joshua, A.M.; Parli, T.; Rosbrook, B.; van Os, S.; Beer, T.M. Five-year Survival Prediction and Safety Outcomes with Enzalutamide in Men with Chemotherapy-naïve Metastatic Castration-resistant Prostate Cancer from the PREVAIL Trial. Eur. Urol. 2020, 78, 347–357. [Google Scholar] [CrossRef]
- Ghashghaei, M.; Niazi, T.M.; Heravi, M.; Bekerat, H.; Trifiro, M.; Paliouras, M.; Muanza, T. Enhanced radiosensitization of enzalutamide via schedule dependent administration to androgen-sensitive prostate cancer cells. Prostate 2018, 78, 64–75. [Google Scholar] [CrossRef]
- Ghashghaei, M.; Niazi, T.M.; Aguilar-Mahecha, A.; Klein, K.O.; Greenwood, C.M.T.; Basik, M.; Muanza, T.M. Identification of a Radiosensitivity Molecular Signature Induced by Enzalutamide in Hormone-sensitive and Hormone-resistant Prostate Cancer Cells. Sci. Rep. 2019, 9, 8838. [Google Scholar] [CrossRef] [PubMed]
- Sekhar, K.R.; Wang, J.; Freeman, M.L.; Kirschner, A.N. Radiosensitization by enzalutamide for human prostate cancer is mediated through the DNA damage repair pathway. PLoS ONE 2019, 14, e0214670. [Google Scholar] [CrossRef] [PubMed]
- Shore, N.D.; Schellhammer, P.F.; Tutrone, R.F.; Mariados, N.F.; Harrelson, S.S. Open Label Phase II Study of Enzalutamide With Concurrent Administration of Radium 223 Dichloride in Patients With Castration-Resistant Prostate Cancer. Clin. Genitourin. Cancer 2020, 18, 416–422. [Google Scholar] [CrossRef]
- Maughan, B.L.; Kessel, A.; McFarland, T.R.; Sayegh, N.; Nussenzveig, R.; Hahn, A.W.; Hoffman, J.M.; Morton, K.; Sirohi, D.; Kohli, M.; et al. Radium-223 plus Enzalutamide Versus Enzalutamide in Metastatic Castration-Refractory Prostate Cancer: Final Safety and Efficacy Results. Oncologist 2021, 26, 1006-e2129. [Google Scholar] [CrossRef] [PubMed]
- Tombal, B.F.; Loriot, Y.; Saad, F.; McDermott, R.S.; Elliott, T.; Rodriguez-Vida, A.; Nole, F.; Fournier, B.; Collette, L.; Gillessen, S. Decreased fracture rate by mandating bone-protecting agents in the EORTC 1333/PEACE III trial comparing enzalutamide and Ra223 versus enzalutamide alone: An interim safety analysis. J. Clin. Oncol. 2019, 37 (Suppl. 15), 5007. [Google Scholar] [CrossRef]
- Gillessen, S.; Choudhury, A.; Rodriguez-Vida, A.; Nole, F.; Diaz, E.G.; Roumeguere, T.A.; Daugaard, G.; Loriot, Y.; Saad, F.; McDermott, R.S.; et al. Decreased fracture rate by mandating bone protecting agents in the EORTC 1333/PEACEIII trial combining Ra223 with enzalutamide versus enzalutamide alone: An updated safety analysis. J. Clin. Oncol. 2021, 39 (Suppl. 15), 5002. [Google Scholar] [CrossRef]
- Linder, S.; van der Poel, H.G.; Bergman, A.M.; Zwart, W.; Prekovic, S. Enzalutamide therapy for advanced prostate cancer: Efficacy, resistance and beyond. Endocr. Relat. Cancer 2018, 26, R31–R52. [Google Scholar] [CrossRef]
- Shore, N.; Higano, C.S.; George, D.J.; Sternberg, C.N.; Saad, F.; Tombal, B.; Miller, K.; Kalinovsky, J.; Jiao, X.; Tangirala, K.; et al. Concurrent or layered treatment with radium-223 and enzalutamide or abiraterone/prednisone: Real-world clinical outcomes in patients with metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2020, 23, 680–688. [Google Scholar] [CrossRef]
- Triggiani, L.; Colosini, A.; Buglione, M.; Pasinetti, N.; Orizio, F.; Bardoscia, L.; Borghetti, P.; Maddalo, M.; Spiazzi, L.; Magrini, S.M.; et al. Exploring the Role of Enzalutamide in Combination with Radiation Therapy: An In Vitro Study. Anticancer Res. 2018, 38, 3487–3492. [Google Scholar] [CrossRef]
- Spratt, D.E.; Evans, M.J.; Davis, B.J.; Doran, M.G.; Lee, M.X.; Shah, N.; Wongvipat, J.; Carnazza, K.E.; Klee, G.G.; Polkinghorn, W.; et al. Androgen Receptor Upregulation Mediates Radioresistance after Ionizing Radiation. Cancer Res. 2015, 75, 4688–4696. [Google Scholar] [CrossRef]
- Suominen, M.I.; Fagerlund, K.M.; Rissanen, J.P.; Konkol, Y.M.; Morko, J.P.; Peng, Z.; Alhoniemi, E.J.; Laine, S.K.; Corey, E.; Mumberg, D.; et al. Radium-223 Inhibits Osseous Prostate Cancer Growth by Dual Targeting of Cancer Cells and Bone Microenvironment in Mouse Models. Clin. Cancer Res. 2017, 23, 4335–4346. [Google Scholar] [CrossRef] [PubMed]
- Wengner, A.M.; Scholz, A.; Haendler, B. Targeting DNA Damage Response in Prostate and Breast Cancer. Int. J. Mol. Sci. 2020, 21, 8273. [Google Scholar] [CrossRef] [PubMed]
- Polkinghorn, W.R.; Parker, J.S.; Lee, M.X.; Kass, E.M.; Spratt, D.E.; Iaquinta, P.J.; Arora, V.K.; Yen, W.F.; Cai, L.; Zheng, D.; et al. Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov. 2013, 3, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Chini, C.C.; Chen, J. Human claspin is required for replication checkpoint control. J. Biol. Chem. 2003, 278, 30057–30062. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Li, K.; Stewart, G.S.; Elledge, S.J. Human Claspin works with BRCA1 to both positively and negatively regulate cell proliferation. Proc. Natl. Acad. Sci. USA 2004, 101, 6484–6489. [Google Scholar] [CrossRef] [PubMed]
- Nimonkar, A.V.; Genschel, J.; Kinoshita, E.; Polaczek, P.; Campbell, J.L.; Wyman, C.; Modrich, P.; Kowalczykowski, S.C. BLM-DNA2-RPA-MRN and EXO1-BLM-RPA-MRN constitute two DNA end resection machineries for human DNA break repair. Genes Dev. 2011, 25, 350–362. [Google Scholar] [CrossRef]
- Efstathiou, E.; Titus, M.; Wen, S.; Hoang, A.; Karlou, M.; Ashe, R.; Tu, S.M.; Aparicio, A.; Troncoso, P.; Mohler, J.; et al. Molecular characterization of enzalutamide-treated bone metastatic castration-resistant prostate cancer. Eur. Urol. 2015, 67, 53–60. [Google Scholar] [CrossRef]
- Suominen, M.I.; Knuuttila, M.; Sjöholm, B.; Alhoniemi, E.; Mumberg, D.; Halleen, J.M.; Käkönen, S.-M.; Scholz, A. Abstract 6286: The effects of prednisone, abiraterone acetate and radium-223 dichloride on bone in the intratibial LNCaP prostate cancer model. Cancer Res. 2020, 80 (Suppl. 16), 6286. [Google Scholar] [CrossRef]
- Agarwal, N.; Nussenzveig, R.; Hahn, A.W.; Hoffman, J.M.; Morton, K.; Gupta, S.; Batten, J.; Thorley, J.; Hawks, J.; Santos, V.S.; et al. Prospective Evaluation of Bone Metabolic Markers as Surrogate Markers of Response to Radium-223 Therapy in Metastatic Castration-resistant Prostate Cancer. Clin. Cancer Res. 2020, 26, 2104–2110. [Google Scholar] [CrossRef]
- Wu, J.; Movérare-Skrtic, S.; Börjesson, A.E.; Lagerquist, M.K.; Sjögren, K.; Windahl, S.H.; Koskela, A.; Grahnemo, L.; Islander, U.; Wilhelmson, A.S.; et al. Enzalutamide Reduces the Bone Mass in the Axial But Not the Appendicular Skeleton in Male Mice. Endocrinology 2016, 157, 969–977. [Google Scholar] [CrossRef]
- McDermott, R.S.; Greene, J.; McCaffrey, J.; Parker, I.; Helanova, S.; Baird, A.M.; Teiserskiene, A.; Lim, M.; Matthews, H.; Deignan, O.; et al. Radium-223 in combination with enzalutamide in metastatic castration-resistant prostate cancer: A multi-centre, phase II open-label study. Ther. Adv. Med. Oncol. 2021, 13, 17588359211042691. [Google Scholar] [CrossRef] [PubMed]
- Trieu, J.; Chang, M.; Rojas, V.; Varada, N.; Cao, Y.; Anderson, M.; Vogelzang, N.J. Lower Fracture Rates in Patients Treated with Radium-223, Abiraterone or Enzalutamide, When Given Concurrently with Bone Health Agents: A Real-World Analysis. Clin. Genitourin. Cancer 2022, 20, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Korotkevich, G.; Sukhov, V.; Budin, N.; Shpak, B.; Artyomov, M.N.; Sergushichev, A. Fast gene set enrichment analysis. bioRxiv 2021. [Google Scholar] [CrossRef]
- Gillespie, M.; Jassal, B.; Stephan, R.; Milacic, M.; Rothfels, K.; Senff-Ribeiro, A.; Griss, J.; Sevilla, C.; Matthews, L.; Gong, C.; et al. The reactome pathway knowledgebase 2022. Nucleic Acids Res. 2021, 50, D687–D692. [Google Scholar] [CrossRef] [PubMed]
- Liberzon, A.; Subramanian, A.; Pinchback, R.; Thorvaldsdóttir, H.; Tamayo, P.; Mesirov, J.P. Molecular signatures database (MSigDB) 3.0. Bioinformatics 2011, 27, 1739–1740. [Google Scholar] [CrossRef]
- Horoszewicz, J.S.; Leong, S.S.; Kawinski, E.; Karr, J.P.; Rosenthal, H.; Chu, T.M.; Mirand, E.A.; Murphy, G.P. LNCaP model of human prostatic carcinoma. Cancer Res. 1983, 43, 1809–1818. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef]
- van’t Hof, R.J. Analysis of bone architecture in rodents using microcomputed tomography. Methods Mol. Biol. 2012, 816, 461–476. [Google Scholar] [PubMed]
- Engelke, K.; Prevrhal, S.; Genant, H.K. Macro- and Microimaging of Bone Architecture. In Principles of Bone Biology; Academic Press: San Diego, CA, USA, 2008; pp. 1905–1942. [Google Scholar] [CrossRef]
- Dempster, D.W. Histomorphometric analysis of bone remodeling. In Principles of Bone Biology; Academic Press: San Diego, CA, USA, 2008; pp. 447–463. [Google Scholar]
- Erben, R.G.; Glosmann, M. Histomorphometry in rodents. Methods Mol. Biol. 2012, 816, 279–303. [Google Scholar] [PubMed]
- Dempster, D.W.; Compston, J.E.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R.; Parfitt, A.M. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 2013, 28, 2–17. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suominen, M.I.; Knuuttila, M.; Schatz, C.A.; Schlicker, A.; Vääräniemi, J.; Sjöholm, B.; Alhoniemi, E.; Haendler, B.; Mumberg, D.; Käkönen, S.-M.; et al. Enhanced Antitumor Efficacy of Radium-223 and Enzalutamide in the Intratibial LNCaP Prostate Cancer Model. Int. J. Mol. Sci. 2023, 24, 2189. https://doi.org/10.3390/ijms24032189
Suominen MI, Knuuttila M, Schatz CA, Schlicker A, Vääräniemi J, Sjöholm B, Alhoniemi E, Haendler B, Mumberg D, Käkönen S-M, et al. Enhanced Antitumor Efficacy of Radium-223 and Enzalutamide in the Intratibial LNCaP Prostate Cancer Model. International Journal of Molecular Sciences. 2023; 24(3):2189. https://doi.org/10.3390/ijms24032189
Chicago/Turabian StyleSuominen, Mari I., Matias Knuuttila, Christoph A. Schatz, Andreas Schlicker, Jukka Vääräniemi, Birgitta Sjöholm, Esa Alhoniemi, Bernard Haendler, Dominik Mumberg, Sanna-Maria Käkönen, and et al. 2023. "Enhanced Antitumor Efficacy of Radium-223 and Enzalutamide in the Intratibial LNCaP Prostate Cancer Model" International Journal of Molecular Sciences 24, no. 3: 2189. https://doi.org/10.3390/ijms24032189
APA StyleSuominen, M. I., Knuuttila, M., Schatz, C. A., Schlicker, A., Vääräniemi, J., Sjöholm, B., Alhoniemi, E., Haendler, B., Mumberg, D., Käkönen, S.-M., & Scholz, A. (2023). Enhanced Antitumor Efficacy of Radium-223 and Enzalutamide in the Intratibial LNCaP Prostate Cancer Model. International Journal of Molecular Sciences, 24(3), 2189. https://doi.org/10.3390/ijms24032189






