Investigations on the Influence of the Axial Ligand in [Salophene]iron(III) Complexes on Biological Activity and Redox Behavior
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthetic Chemistry
2.1.1. Synthesis of the Compounds
2.1.2. Characterization of the Complexes
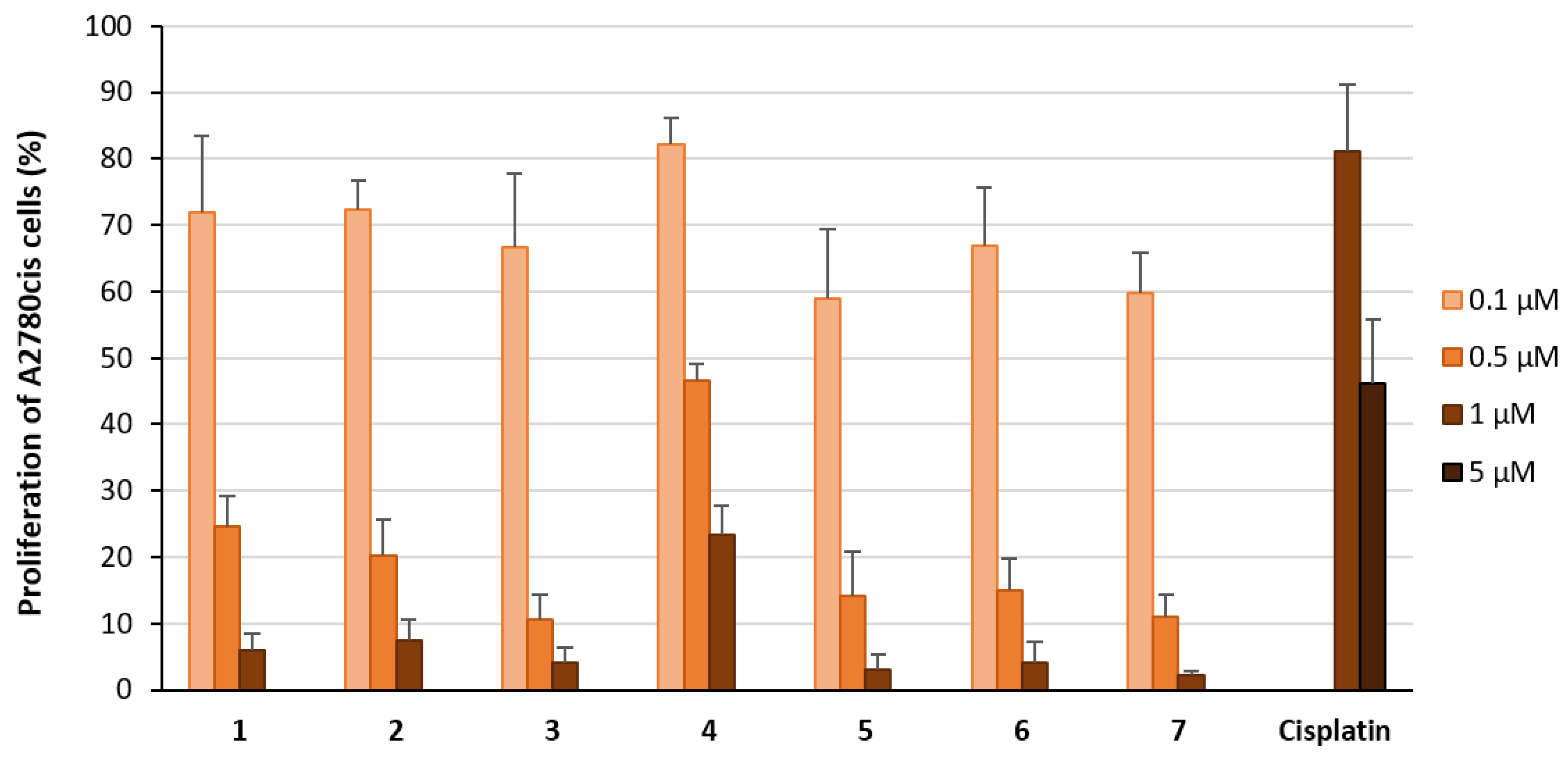
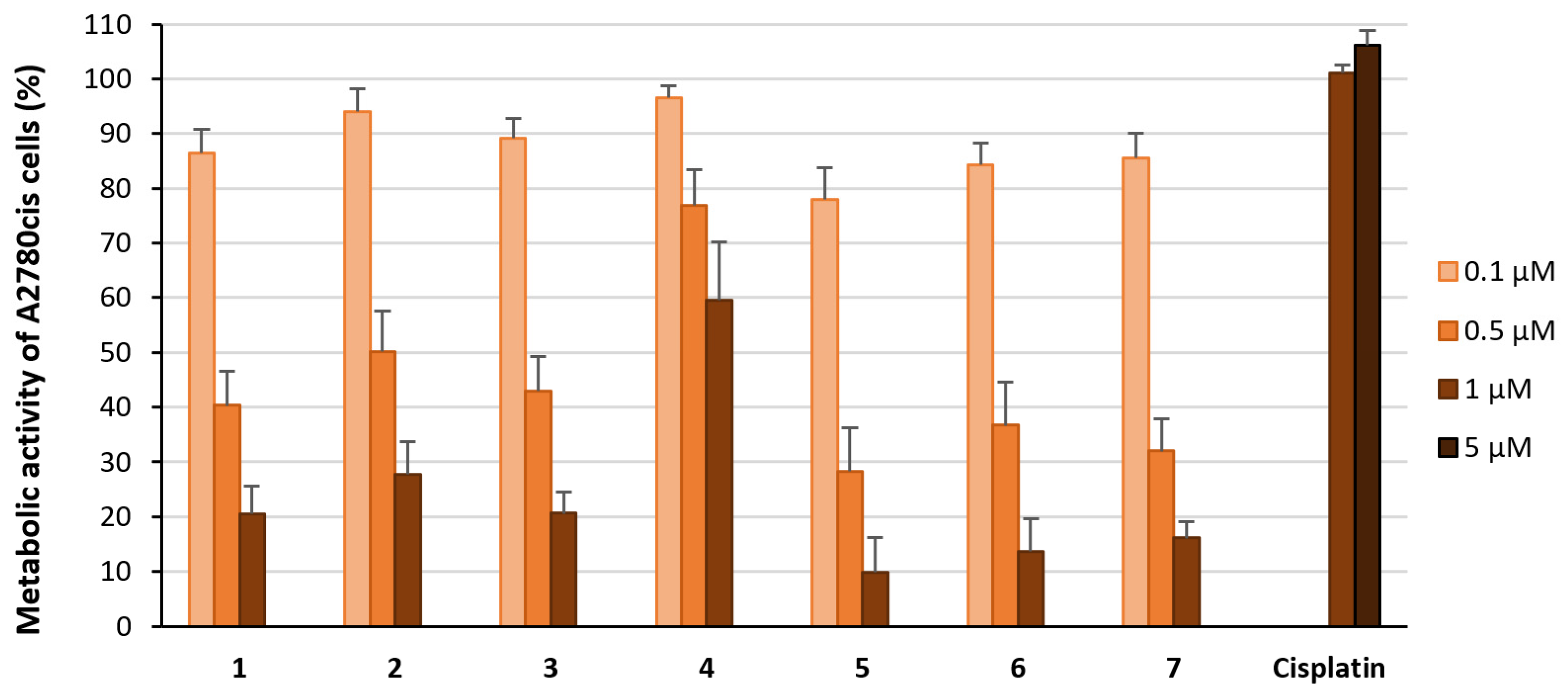
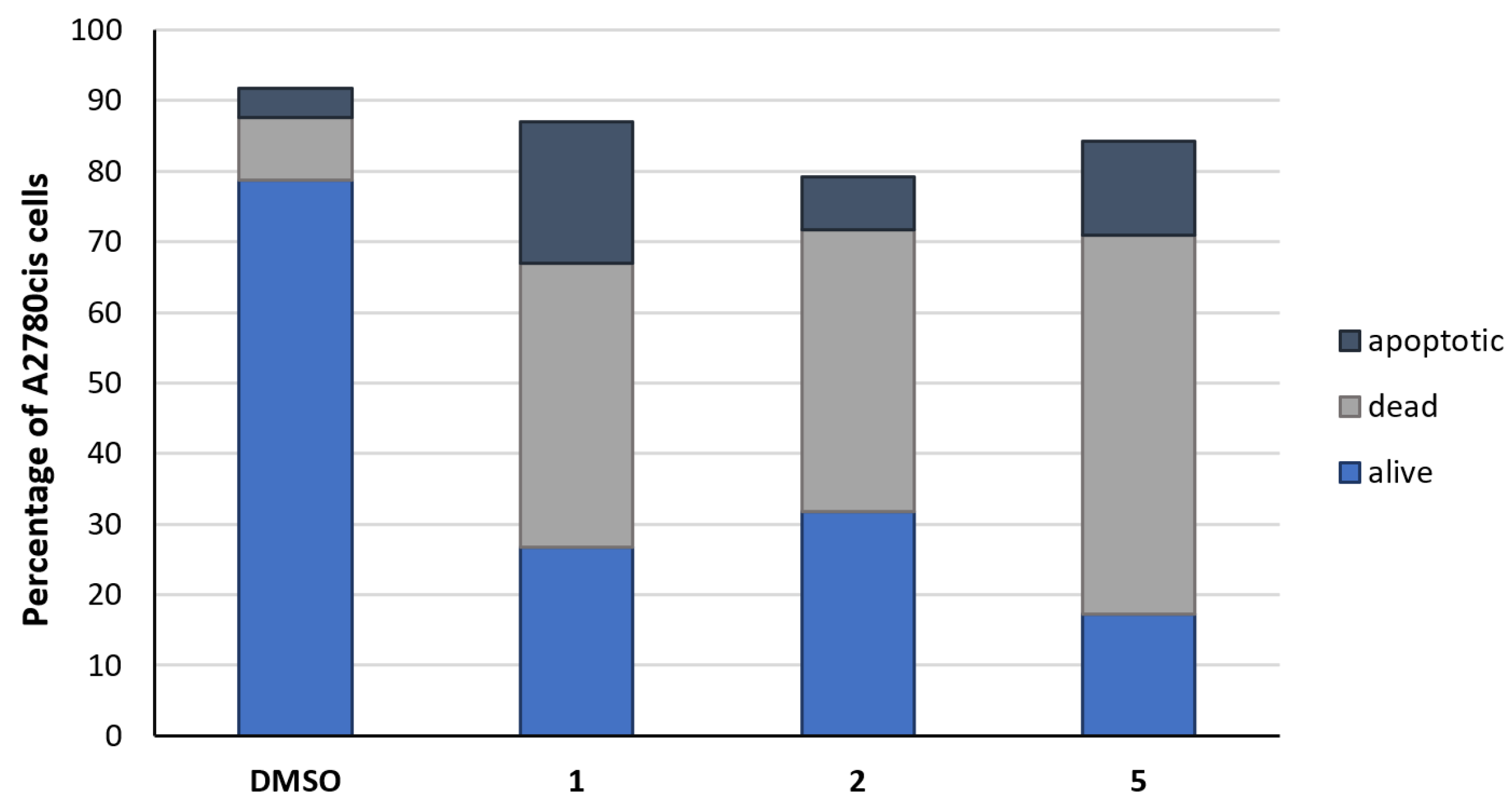
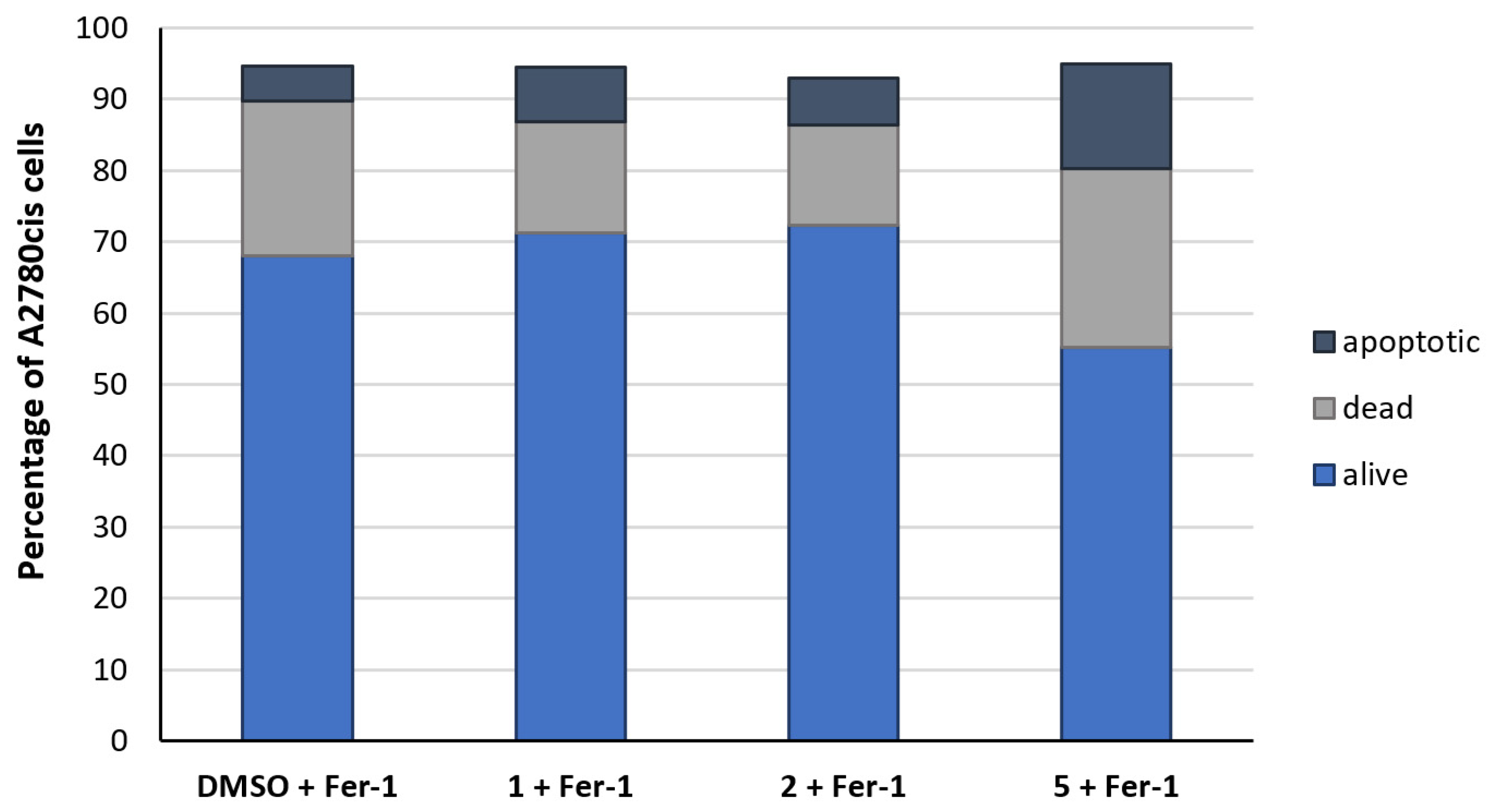
2.2. Biological Evaluation
2.2.1. Effect on Proliferation and Metabolic Activity
2.2.2. Cell-Death Induction
2.2.3. ROS Induction
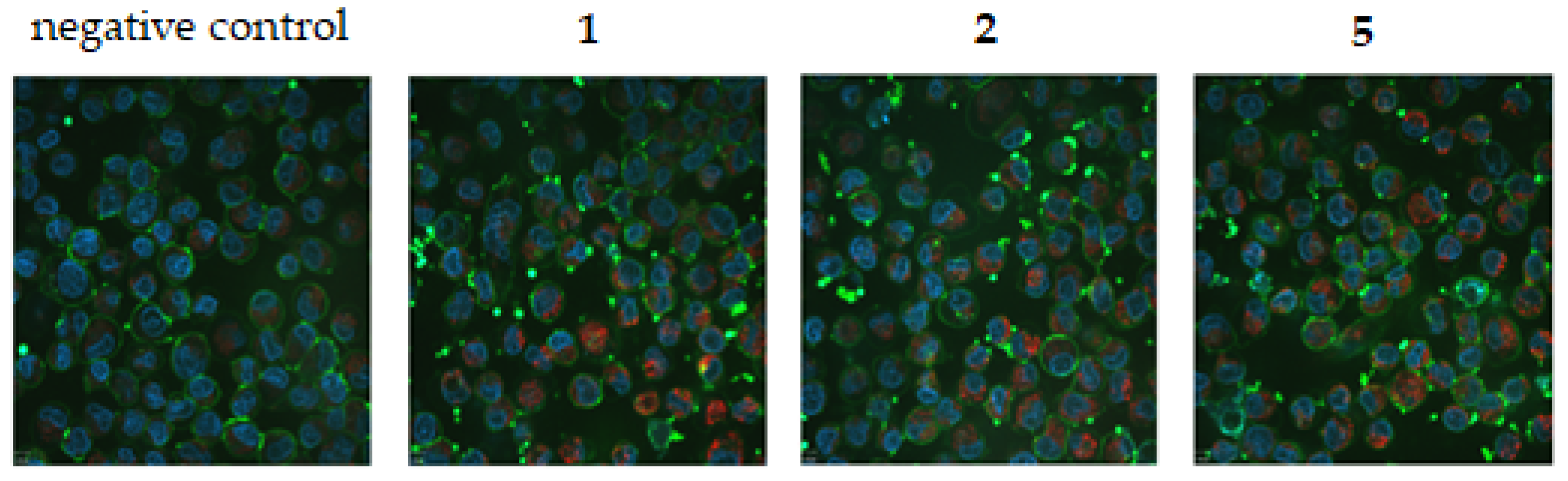
2.2.4. Lipid Peroxidation
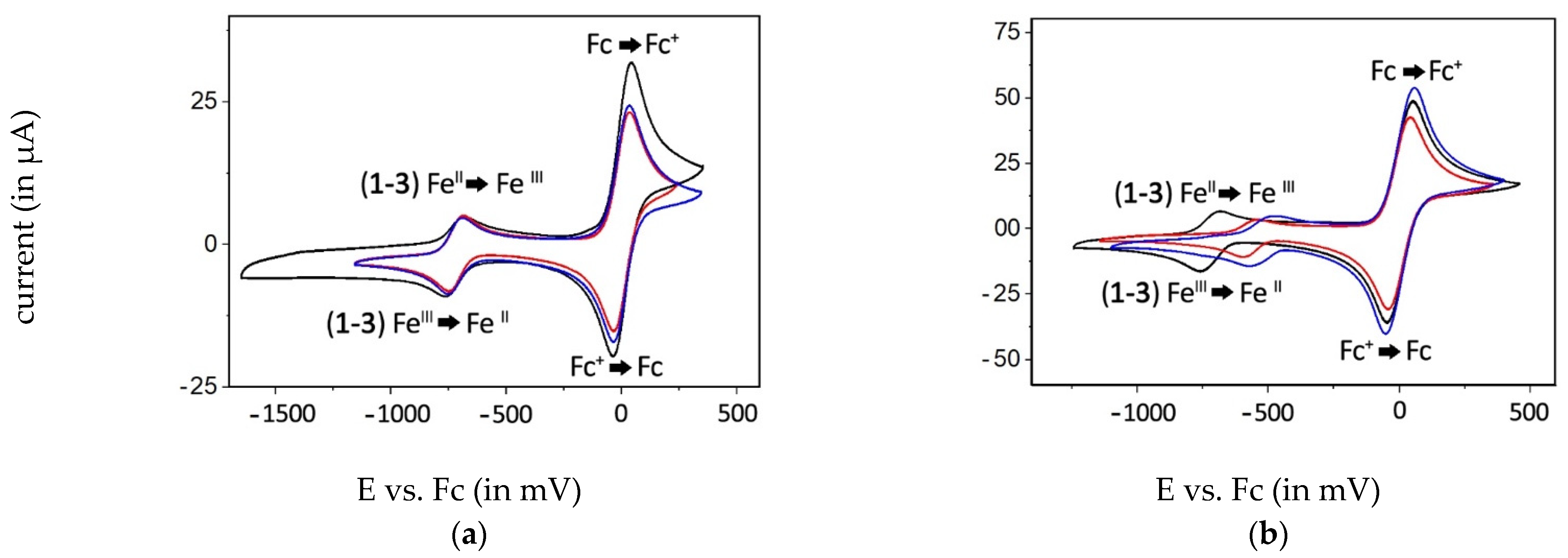
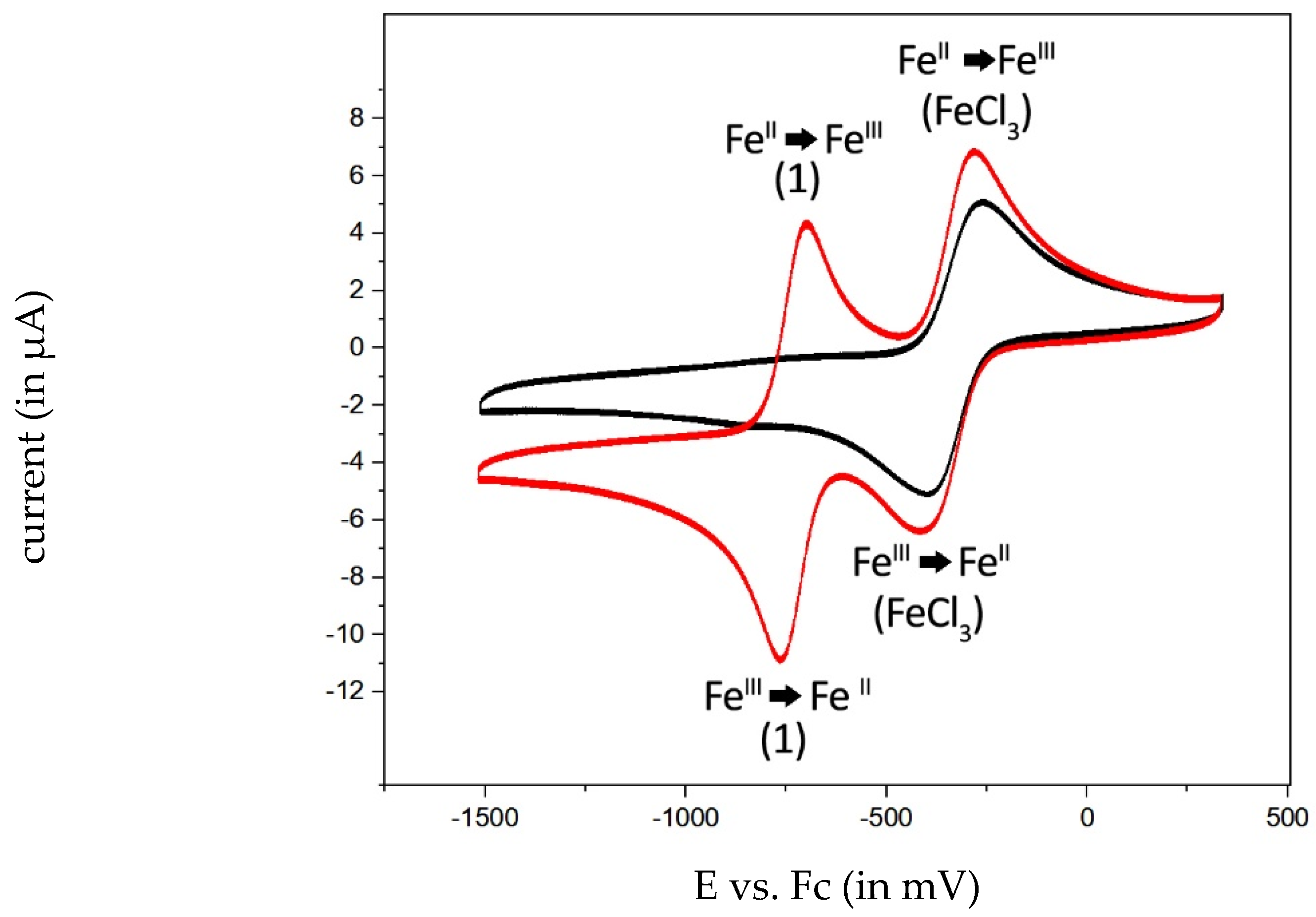
2.3. Electrochemical Behavior of the Complexes
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Compounds
3.2.1. Synthesis of the N,N′-disalicylidene-1,2-phenylenediamine Ligand [19]
3.2.2. Synthesis of the Complexes 1–3 (Method a)
3.2.3. Synthesis of Complex 4
3.2.4. Synthesis of the Complexes 5–6 (Method b)
3.2.5. Synthesis of Complex 7
3.3. Cyclic Voltammetry
3.3.1. Procedure of Sample Preparation for Cyclic Voltammetry
3.3.2. Analysis and Normalization of the Voltammograms
3.4. General Cell-Culture Methods
3.4.1. Analysis of Proliferation
3.4.2. Analysis of Metabolic Activity
3.4.3. Analysis of Caspases 3/7 Activation
3.4.4. Determination of Cell-Death and Indirect ROS Production by Flow Cytometry
3.4.5. ROS Staining
3.4.6. Determination of Mitochondrial Membrane Potential
3.4.7. Lipid Peroxidation Staining with BODIPY™ 581/591 C11
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Explanation |
| ΔΨ | Mitochondrial membrane potential |
| ACN | Acetonitrile |
| compd | Compound |
| cpm | Counts per minute |
| DAD | Diode array detector |
| DCM | Dichloromethane |
| DMF | Dimethylformamide |
| DMSO | Dimethyl sulfoxide |
| DMSO-d6 | Deuterated dimethyl sulfoxide |
| eq. | Equivalent |
| ESI | Electrospray ionization |
| EtOH | Ethanol |
| FBS | Fetal bovine serum |
| Fer-1 | Ferrostatin-1 |
| FITC | Fluorescein isothiocyanate |
| FT-IR | Fourier-transform infrared spectroscopy |
| HR-MS | High-resolution mass spectrometry |
| MeOH | Methanol |
| mROS | Mitochondrial reactive oxygen species |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NAC | N-acetyl-L-cysteine |
| Nec-1 | Necrostatin-1 |
| NMR | Nuclear magnetic resonance |
| HEPES | 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid |
| HPLC | High-performance liquid chromatography |
| Hz | Hertz |
| PBS | Phosphate-buffered saline |
| ppm | Parts per million |
| ROS | Reactive oxygen species |
| rcf | Relative centrifugal force |
| rt | Room temperature |
| RPMI | RPMI 1640 cell media |
| Salophene | N,N′-disalicylidene-1,2-phenylenediamine |
| SD | Standard deviation |
| SE | Standard error |
| TBAHFP | Tetrabutylammonium hexafluorophosphate |
References
- Böttcher, A.; Birnbaum, E.R.; Day, M.W.; Gray, H.B.; Grinstaff, M.W.; Labinger, J.A. How do electronegative substituents make metal complexes better catalysts for the oxidation of hydrocarbons by dioxygen? J. Mol. Catal. A Chem. 1997, 117, 229–242. [Google Scholar] [CrossRef]
- Ansari, K.I.; Kasiri, S.; Grant, J.D.; Mandal, S.S. Fe(III)-salen and salphen complexes induce caspase activation and apoptosis in human cells. J. Biomol. Screen. 2010, 16, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Würtenberger, I.; Follia, V.; Lerch, F.; Cwikla, C.; Fahrner, N.; Kalchschmidt, C.; Flögel, B.; Kircher, B.; Gust, R. Fluorinated Fe(III) salophene complexes: Optimization of tumor cell specific activity and utilization of fluorine labeling for in vitro analysis. J. Med. Chem. 2015, 58, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Vančo, J.; Šindelář, Z.; Dvořák, Z.; Trávníček, Z. Iron-salophen complexes involving azole-derived ligands: A new group of compounds with high-level and broad-spectrum in vitro antitumor activity. J. Inorg. Biochem. 2015, 142, 92–100. [Google Scholar] [CrossRef]
- Sagasser, J.; Ma, B.N.; Baecker, D.; Salcher, S.; Hermann, M.; Lamprecht, J.; Angerer, S.; Obexer, P.; Kircher, B.; Gust, R. A new approach in cancer treatment: Discovery of chlorido[N,N-disalicylidene-1,2-phenylenediamine]iron(III) complexes as ferroptosis inducers. J. Med. Chem. 2019, 62, 8053–8061. [Google Scholar] [CrossRef]
- Baecker, D.; Ma, B.N.; Sagasser, J.; Schultz, L.; Hörschläger, C.; Weinreich, M.; Steiner, L.; Kircher, B.; Gust, R. Amide and ester derivatives of chlorido[4-carboxy-1,2-disalicylideneaminobenzene]iron(III) as necroptosis and ferroptosis inducers. Dalton Trans. 2020, 49, 6842–6853. [Google Scholar] [CrossRef]
- Hille, A.; Ott, I.; Kitanovic, A.; Kitanovic, I.; Alborzinia, H.; Lederer, E.; Wölfl, S.; Metzler-Nolte, N.; Schäfer, S.; Sheldrick, W.S.; et al. [N,N-Bis(salicylidene)-1,2-phenylenediamine]metal complexes with cell death promoting properties. J. Biol. Inorg. Chem. 2009, 14, 711–725. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Hille, A.; Kitanovic, I.; Jesse, P.; Henze, G.; Wölfl, S.; Gust, R.; Prokop, A. [FeIII(salophene)Cl], a potent iron salophene complex overcomes multiple drug resistance in lymphoma and leukemia cells. Leuk. Res. 2011, 35, 387–393. [Google Scholar] [CrossRef]
- Bystrom, L.M.; Guzman, M.L.; Rivella, S. Iron and Reactive Oxygen Species: Friends or Foes of Cancer Cells? Antioxid. Redox Signal. 2012, 20, 1917–1924. [Google Scholar] [CrossRef]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef]
- El-Sheikh, M.Y.; Ashmawy, F.M.; Salem, I.A.; Zaki, A.B.; Nickel, U. Catalytic decomposition of hydrogen peroxide in the presence of N, N-bis(salicylidene)-o-phenylenediamineiron(III) sorbed on to Dowex-50W resin. Transit. Met. Chem. 1991, 16, 319–323. [Google Scholar] [CrossRef]
- Wenk, S.E.; Schultz, F.A. Electrochemistry of monomeric and oxo-bridged dimeric iron(III) complexes with the Schiff base ligand, N,N-ethylenebis(salicylidineimine). J. Electroanal. Chem. Interfacial Electrochem. 1979, 101, 89–99. [Google Scholar] [CrossRef]
- Carré, B.; Costes, J.P.; Tommasino, J.B.; De Montauzon, D.; Soulet, F.; Fabre, P.L. Electrochemical studies of Iron(III) Schiff base complexes—I. The monomeric FeIII(N2O2)Cl complexes. Polyhedron 1993, 12, 641–649. [Google Scholar] [CrossRef]
- Costes, J.P.; Tommasino, J.B.; Carré, B.; Soulet, F.; Fabre, P.L. Electrochemical studies of iron(III) Schiff base complexes—II. Dimeric μ-OXO[FeIII(N2O2)]2O complexes. Polyhedron 1995, 14, 771–780. [Google Scholar] [CrossRef]
- Ueda, T.; Inazuma, N.; Komatsu, D.; Yasuzawa, H.; Onda, A.; Guo, S.-X.; Bond, A.M. Comparison of chemical interactions with Li+ and catalytic reactivity of electrochemically generated [FeICl(L)]2− and [CoI(L)]− complexes (L = salen or salophen). Dalton Trans. 2013, 42, 11146–11154. [Google Scholar] [CrossRef] [PubMed]
- Hille, A.; Wolf, T.; Schumacher, P.; Ott, I.; Gust, R.; Kircher, B. Effects of Metal Salophene and Saldach Complexes on Lymphoma and Leukemia Cells. Arch. Pharm. 2011, 344, 217–223. [Google Scholar] [CrossRef]
- Hille, A.; Gust, R. Influence of methoxy groups on the antiproliferative effects of [FeIII(salophene-OMe)Cl] complexes. Eur. J. Med. Chem. 2010, 45, 5486–5492. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Boghaei, D.M.; Mohebi, S. Non-symmetrical tetradentate vanadyl Schiff base complexes derived from 1,2-phenylene diamine and 1,3-naphthalene diamine as catalysts for the oxidation of cyclohexene. Tetrahedron 2002, 58, 5357–5366. [Google Scholar] [CrossRef]
- El Deeb, S.; Ma, B.N.; Baecker, D.; Gust, R. Studies on the stability of the anticancer-active [N,N-bis(salicylidene)-1,2-phenylenediamine]chloridoiron(III) complex under pharmacological-like conditions. Inorg. Chim. Acta 2019, 487, 76–80. [Google Scholar] [CrossRef]
- El Deeb, S.; Ma, B.N.; Gust, R. Development and validation of a LC method for the separation and determination of the anticancer-active Fe(III) (4-methoxy-salophene) using the new second-generation monolith. J. Sep. Sci. 2012, 35, 3434–3438. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.; Arora, R.; Lahiri, S.; Amritphale, S.S.; Chandra, N. Synthesis and characterization of iron oxide nanoparticles by solvothermal method. Prot. Met. Phys. Chem. Surf. 2014, 50, 628–631. [Google Scholar] [CrossRef]
- Sanders-Loehr, J.; Wheeler, W.D.; Shiemke, A.K.; Averill, B.A.; Loehr, T.M. Electronic and Raman spectroscopic properties of oxo-bridged dinuclear iron centers in proteins and model compounds. J. Am. Chem. Soc. 1989, 111, 8084–8093. [Google Scholar] [CrossRef]
- Jana, S.; Chatterjee, S.; Chattopadhyay, S. Syntheses, characterization and X-ray crystal structures of hexa-coordinated monomeric and oxo-bridged dimeric Fe(III) compounds with salen-type Schiff bases. Polyhedron 2012, 48, 189–198. [Google Scholar] [CrossRef]
- Wang, H.; Braun, A.; Cramer, S.P.; Gee, L.B.; Yoda, Y. Nuclear Resonance Vibrational Spectroscopy: A Modern Tool to Pinpoint Site-Specific Cooperative Processes. Crystals 2021, 11, 909. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, M.; Branton, A.; Trivedi, D.; Nayak, G.; Bairwa, K.; Jana, S. Spectroscopic Characterization of Disodium Hydrogen Orthophosphate and Sodium Nitrate after Biofield Treatment. J. Chromatogr. Sep. Tech. 2015, 6, 1000282. [Google Scholar] [CrossRef]
- Azizitorghabeh, A.; Wang, J.; Ramsay, J.A.; Ghahreman, A. A review of thiocyanate gold leaching—Chemistry, thermodynamics, kinetics and processing. Miner. Eng. 2021, 160, 106689. [Google Scholar] [CrossRef]
- Edwards, H.G.M.; Lewis, I.R. Vibrational spectroscopic studies of iron(II) acetate. J. Mol. Struct. 1993, 296, 15–20. [Google Scholar] [CrossRef]
- Lange, T.S.; Kim, K.K.; Singh, R.K.; Strongin, R.M.; McCourt, C.K.; Brard, L. Iron(III)-salophene: An organometallic compound with selective cytotoxic and anti-proliferative properties in platinum-resistant ovarian cancer cells. PLoS ONE 2008, 3, e2303. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Zafarullah, M.; Li, W.Q.; Sylvester, J.; Ahmad, M. Molecular mechanisms of N-acetylcysteine actions. Cell. Mol. Life Sci. CMLS 2003, 60, 6–20. [Google Scholar] [CrossRef] [PubMed]






| Compound | FT-IR Vibrations | Reference |
|---|---|---|
| 2 | ν(NO2) = 1474 cm−1 ν(N=O) = 1279 cm−1 ν(N–O) = 979 cm−1 | [26] |
| 3 | ν(SCN) = 2036 cm−1 | [24,27] |
| 4 | ν(COO) = 1525 cm−1 | [28] |
| 5 | ν(C–H) = 3132, 3096, 3014, cm−1 ν(C–H) = 2926, 2852, 2786 cm−1 | [4] |
| 6 | ν(C–H) = 3111, 3046, 3012 cm−1 ν(C–H) = 2949, 2918, 2865, 2838 cm−1 | [4] |
| 7 | ν(Fe-O-Fe) = 749 cm−1 | [23,24] |
| Compound | Conc. [µM] | % of Cells with Lipid Peroxidation after | ||
|---|---|---|---|---|
| 8 h | 12 h | 24 h | ||
| neg. control | 0 | 0.1 | 0.1 | 0.1 |
| 1 | 0.1 | 0.6 | 0.3 | 0.3 |
| 1 | 0.5 | 7.2 | 7.4 | 0.7 |
| 2 | 0.1 | 2.6 | 3.7 | 0.2 |
| 2 | 0.5 | 11.2 | 10.5 | 0.4 |
| 5 | 0.1 | 0.6 | 0.3 | 0.3 |
| 5 | 0.5 | 7.7 | 6.2 | 0.7 |
| Compound | Standard Potentials (E1/2) vs. Fc in | |||
|---|---|---|---|---|
| DCM | ACN | DMSO | DMF | |
| 1 | −729 mV | −680 mV | −728 mV | −729 mV |
| 2 | −502 mV | −392 mV | −723 mV | n.d. |
| 3 | −572 mV | −515 mV | −724 mV | n.d. |
| 4 | −873 mV | −819 mV | −730/−1373 * mV | −1374 * mV |
| 5 | −660 mV | −665 mV | −722 mV | n.d. |
| 6 | −661 mV | −665 mV | −726 mV | n.d. |
| 7 | −1427 mV | −1376 mV | −724/−1403 mV | −1443 mV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Descher, H.; Strich, S.L.; Hermann, M.; Enoh, P.; Kircher, B.; Gust, R. Investigations on the Influence of the Axial Ligand in [Salophene]iron(III) Complexes on Biological Activity and Redox Behavior. Int. J. Mol. Sci. 2023, 24, 2173. https://doi.org/10.3390/ijms24032173
Descher H, Strich SL, Hermann M, Enoh P, Kircher B, Gust R. Investigations on the Influence of the Axial Ligand in [Salophene]iron(III) Complexes on Biological Activity and Redox Behavior. International Journal of Molecular Sciences. 2023; 24(3):2173. https://doi.org/10.3390/ijms24032173
Chicago/Turabian StyleDescher, Hubert, Sophie Luise Strich, Martin Hermann, Peter Enoh, Brigitte Kircher, and Ronald Gust. 2023. "Investigations on the Influence of the Axial Ligand in [Salophene]iron(III) Complexes on Biological Activity and Redox Behavior" International Journal of Molecular Sciences 24, no. 3: 2173. https://doi.org/10.3390/ijms24032173
APA StyleDescher, H., Strich, S. L., Hermann, M., Enoh, P., Kircher, B., & Gust, R. (2023). Investigations on the Influence of the Axial Ligand in [Salophene]iron(III) Complexes on Biological Activity and Redox Behavior. International Journal of Molecular Sciences, 24(3), 2173. https://doi.org/10.3390/ijms24032173








