Immunopharmacological Activities of Luteolin in Chronic Diseases
Abstract
1. Introduction
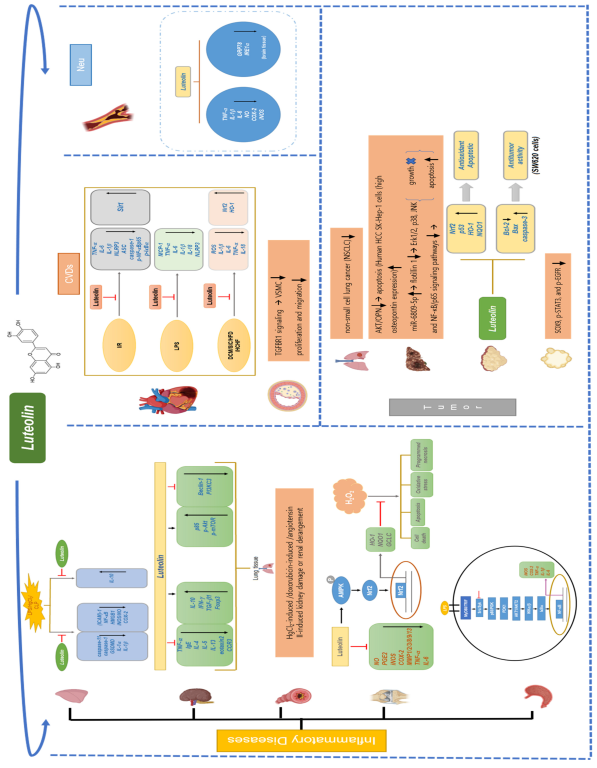
2. Anti-Inflammatory Activity of Luteolin
2.1. Gastritis
2.2. Arthritis
2.3. Asthma
2.4. Renal Damage
2.5. Lung Injury
3. Anti-Cardiovascular Disease Activity of Luteolin
3.1. Myocardial Infarction
3.2. Atherosclerosis
4. Anti-Tumor Activity of Luteolin
4.1. Lung Cancer
4.2. Hepatocellular Carcinoma
4.3. Colorectal Cancer
4.4. Pancreatic Cancer
4.5. Other Cancers
5. Anti-Neurodegenerative Disease Activity of Luteolin
5.1. Alzheimer’s Disease
5.2. Parkinson’s Disease
6. Anti-Inflammatory Properties of Luteolin in Other Diseases
7. DEGs Related to Anti-Inflammatory Cytokines/Chemokines in the GEO Dataset
8. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Luteolin | Luteolin (3′, 4′, 5,7-tetrahydroxyflavone) |
| TNF-α | Tumor Necrosis Factor-α |
| IL-1 | Interleukin-1 |
| COX-2 | Cyclooxygenase-2 |
| MMP | Matrix Metalloproteinases |
| DMM | Destabilization of the medial meniscus |
| OVA | Ovalbumin Antigen |
| AHR | Airway Hyper Responsiveness |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| M1 | Activated Macrophages |
| M2 | Alternatively Activated Macrophages |
| Th2 | T Helper2 |
| WHO | World Health Organization |
| CVDs | Cardiovascular Diseases |
| MI | Myocardial Infarction |
| AS | Atherosclerosis |
| CK-MB | Creatine Kinase Isoenzymes |
| LDH | Lactate Dehydrogenase |
| LPS | Lipopolysaccharide |
| DCM | Diabetic Cardiomyopathy |
| VSMC | Vascular Smooth Muscle Cell |
| TGFBR1 | Transforming Growth Factor-β Receptor 1 |
| ICAM-1 | Intercellular Adhesion Molecule-1 |
| HgCl2 | Mercury Chloride |
| HCC | Hepatocellular Carcinoma |
| OPN | Osteopontin |
| ER | Endoplasmic Reticulum |
| FLOT1 | Flotillin 1 |
| CRC | Colorectal Cancer |
| PDAC | Pancreatic Ductal Adenocarcinoma |
| DPYD | Dihydropyrimidine Dehydrogenase |
| CK19 | Cytokeratin-19 |
| NQO1 | Quinone-acceptor 1 |
| HO-1 | Heme Oxygenase-1 |
| AngII | Angiotensin II |
| BC | Breast Cancer |
| TNBC | Triple-negative Breast Cancer |
| hTERT | Human Telomerase Reverse Transcriptase |
| RANKL | Receptor activator of nuclear factor κB ligand |
| iNOS | Nitric Oxide Synthase |
| BMP-2 | one morphogenetic protein-2 |
| AD | Alzheimer’s disease |
| PD | Parkinson’s disease |
| OA | Osteoarthritis |
| AR | Allergic Rhinitis |
| DEGs | Differentially Expressed Genes |
References
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant Flavonoids: Classification, Distribution, Biosynthesis, and Antioxidant Activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Wen, K.; Fang, X.; Yang, J.; Yao, Y.; Nandakumar, K.S.; Salem, M.L.; Cheng, K. Recent Research on Flavonoids and Their Biomedical Applications. Curr. Med. Chem. 2020, 28, 1042–1066. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.C.; Samman, S. Flavonoids—Chemistry, Metabolism, Cardioprotective Effects, and Dietary Sources. J. Nutr. Biochem. 1996, 7, 66–76. [Google Scholar] [CrossRef]
- Weng, C.J.; Yen, G.C. Flavonoids, a Ubiquitous Dietary Phenolic Subclass, Exert Extensive in Vitro Anti-Invasive and in Vivo Anti-Metastatic Activities. Cancer Metastasis Rev. 2012, 31, 323–351. [Google Scholar] [CrossRef]
- Somerset, S.M.; Johannot, L. Dietary Flavonoid Sources in Australian Adults. Nutr. Cancer 2008, 60, 442–449. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef]
- Calis, Z.; Mogulkoc, R.; Baltaci, A.K. The Roles of Flavonols/Flavonoids in Neurodegeneration and Neuroinflammation. Mini-Reviews Med. Chem. 2019, 20, 1475–1488. [Google Scholar] [CrossRef]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food Sources, Bioavailability, Metabolism, and Bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef]
- Aziz, N.; Kim, M.Y.; Cho, J.Y. Anti-Inflammatory Effects of Luteolin: A Review of in Vitro, in Vivo, and in Silico Studies. J. Ethnopharmacol. 2018, 225, 342–358. [Google Scholar] [CrossRef]
- Lopez-Lazaro, M. Distribution and Biological Activities of the Flavonoid Luteolin. Mini-Rev. Med. Chem. 2008, 9, 31–59. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and Physiological Roles of Inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Stone, W.L.; Basit, H.; Burns, B. Pathology, Inflammation. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Hannoodee, S.; Nasuruddin, D.N. Acute Inflammatory Response. In Nature; StatPearls Publishing: Treasure Island, FL, USA, 1965; Volume 206, p. 20. [Google Scholar]
- Porcelli, E.G. Chronic Inflammation. J. Am. Dent. Assoc. 2018, 149, 750–751. [Google Scholar] [CrossRef]
- Shacter, E.; Weitzman, S.A. Chronic Inflammation and Cancer. Oncology 2002, 16, 217–226. [Google Scholar]
- Varela, M.L.; Mogildea, M.; Moreno, I.; Lopes, A. Acute Inflammation and Metabolism. Inflammation 2018, 41, 1115–1127. [Google Scholar] [CrossRef]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Detection of Inflammatory Biomarkers in Saliva and Urine: Potential in Diagnosis, Prevention, and Treatment for Chronic Diseases. Exp. Biol. Med. 2016, 241, 783–799. [Google Scholar] [CrossRef]
- Liu, D.; Huang, P.; Li, X.; Ge, M.; Luo, G.; Hei, Z. Using Inflammatory and Oxidative Biomarkers in Urine to Predict Early Acute Kidney Injury in Patients Undergoing Liver Transplantation. Biomarkers 2014, 19, 424–429. [Google Scholar] [CrossRef]
- Rhodus, N.L.; Cheng, B.; Myers, S.; Miller, L.; Ho, V.; Ondrey, F. The Feasibility of Monitoring NF-ΚB Associated Cytokines: TNF-α, IL-1α, IL-6, and IL-8 in Whole Saliva for the Malignant Transformation of Oral Lichen Planus. Mol. Carcinog. 2005, 44, 77–82. [Google Scholar] [CrossRef]
- Fei, J.; Liang, B.; Jiang, C.; Ni, H.; Wang, L. Luteolin Inhibits IL-1β-Induced Inflammation in Rat Chondrocytes and Attenuates Osteoarthritis Progression in a Rat Model. Biomed. Pharmacother. 2019, 109, 1586–1592. [Google Scholar] [CrossRef]
- Boeing, T.; da Silva, L.M.; Mariott, M.; de Andrade, S.F.; de Souza, P. Diuretic and Natriuretic Effect of Luteolin in Normotensive and Hypertensive Rats: Role of Muscarinic Acetylcholine Receptors. Pharmacol. Rep. 2017, 69, 1121–1124. [Google Scholar] [CrossRef]
- Nagata, M.; Toyonaga, K.; Ishikawa, E.; Haji, S.; Okahashi, N.; Takahashi, M.; Izumi, Y.; Imamura, A.; Takato, K.; Hideharu, I.; et al. Helicobacter Pylori Metabolites Exacerbate Gastritis through C-Type Lectin Receptors. J. Exp. Med. 2021, 218, e20200815. [Google Scholar] [CrossRef] [PubMed]
- Radziejewska, I.; Borzym-Κluczyk, M.; Leszczyńska, K. Luteolin Alters MUC1 Extracellular Domain, ST Antigen, ADAM-17, IL-8, IL-10 and NF-κB Expression in Helicobacter Pylori-infected Gastric Cancer CRL-1739 Cells: A Preliminary Study. Biomed. Reports 2021, 14, 1. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, J.G.; Hong, Y.H.; Shin, K.K.; Kim, J.K.; Kim, Y.D.; Yoon, K.D.; Kim, K.H.; Yoo, B.C.; Sung, G.H.; et al. Sauropus Brevipes Ethanol Extract Negatively Regulates Inflammatory Responses in Vivo and in Vitro by Targeting Src, Syk and IRAK1. Pharm. Biol. 2021, 59, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.H.; Kim, J.H.; Cho, J.Y. Ranunculus Bulumei Methanol Extract Exerts Anti-Inflammatory Activity by Targeting Src/Syk in Nf-Κb Signaling. Biomolecules 2020, 10, 546. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-H. Research of Pathogenesis and Novel Therapeutics in Arthritis. Int. J. Mol. Sci. 2019, 20, 1646. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Ye, J.; Xia, Z.; Cheng, B. Effect of Luteolin on Apoptosis, MAPK and JNK Signaling Pathways in Guinea Pig Chondrocyte with Osteoarthritis. Cell. Mol. Biol. 2019, 65, 91–95. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, L.; Liu, Y.; Huang, C.; Xia, W.; Zhou, H.; Zhou, Z.; Zhou, X. Luteolin Protects Chondrocytes from H2O2-Induced Oxidative Injury and Attenuates Osteoarthritis Progression by Activating AMPK-Nrf2 Signaling. Oxid. Med. Cell. Longev. 2022, 2022, 5635797. [Google Scholar] [CrossRef]
- Gans, M.D.; Gavrilova, T. Understanding the Immunology of Asthma: Pathophysiology, Biomarkers, and Treatments for Asthma Endotypes. Paediatr. Respir. Rev. 2020, 36, 118–127. [Google Scholar] [CrossRef]
- Sockrider, M.; Fussner, L. What Is Asthma? Am. J. Respir. Crit. Care Med. 2020, 202, P25–P26. [Google Scholar] [CrossRef]
- Seumois, G.; Ramírez-Suástegui, C.; Schmiedel, B.J.; Liang, S.; Peters, B.; Sette, A.; Vijayanand, P. Single-Cell Transcriptomic Analysis of Allergen-Specific T Cells in Allergy and Asthma. Sci. Immunol. 2020, 5, eaba6087. [Google Scholar] [CrossRef]
- Lan, F.; Zhang, N.; Bachert, C.; Zhang, L. Stability of Regulatory T Cells in T Helper 2–Biased Allergic Airway Diseases. Allergy Eur. J. Allergy Clin. Immunol. 2020, 75, 1914–1922. [Google Scholar] [CrossRef]
- Kim, S.H.; Saba, E.; Kim, B.K.; Yang, W.K.; Park, Y.C.; Shin, H.J.; Han, C.K.; Lee, Y.C.; Rhee, M.H. Luteolin Attenuates Airway Inflammation by Inducing the Transition of CD4+CD25– to CD4+CD25+ Regulatory T Cells. Eur. J. Pharmacol. 2018, 820, 53–64. [Google Scholar] [CrossRef]
- Li, J.; Liu, G.; Li, L.; Yao, Z.; Huang, J. Research Progress on the Effect of Autophagy-Lysosomal Pathway on Tumor Drug Resistance. Exp. Cell Res. 2020, 389, 111925. [Google Scholar] [CrossRef]
- Gross, A.S.; Graef, M. Mechanisms of Autophagy in Metabolic Stress Response. J. Mol. Biol. 2020, 432, 28–52. [Google Scholar] [CrossRef]
- Wang, S.; Wuniqiemu, T.; Tang, W.; Teng, F.; Bian, Q.; Yi, L.; Qin, J.; Zhu, X.; Wei, Y.; Dong, J. Luteolin Inhibits Autophagy in Allergic Asthma by Activating PI3K/Akt/MTOR Signaling and Inhibiting Beclin-1-PI3KC3 Complex. Int. Immunopharmacol. 2021, 94, 107460. [Google Scholar] [CrossRef]
- Yang, H.; Rao, Z. Structural Biology of SARS-CoV-2 and Implications for Therapeutic Development. Nat. Rev. Microbiol. 2021, 19, 685–700. [Google Scholar] [CrossRef]
- Choi, H.G.; Wee, J.H.; Kim, S.Y.; Kim, J.H.; Il Kim, H.; Park, J.Y.; Park, S.; Il Hwang, Y.; Jang, S.H.; Jung, K.S. Association between Asthma and Clinical Mortality/Morbidity in COVID-19 Patients Using Clinical Epidemiologic Data from Korean Disease Control and Prevention. Allergy Eur. J. Allergy Clin. Immunol. 2021, 76, 921–924. [Google Scholar] [CrossRef]
- Xie, Y.Z.; Peng, C.W.; Su, Z.Q.; Huang, H.T.; Liu, X.H.; Zhan, S.F.; Huang, X.F. A Practical Strategy for Exploring the Pharmacological Mechanism of Luteolin Against COVID-19/Asthma Comorbidity: Findings of System Pharmacology and Bioinformatics Analysis. Front. Immunol. 2022, 12, 769011. [Google Scholar] [CrossRef]
- Shen, J.; Sun, X.; Pan, B.; Cao, S.; Cao, J.; Che, D.; Liu, F.; Zhang, S.; Yu, Y. IL-17 Induces Macrophages to M2-like Phenotype via NF-ΚB. Cancer Manag. Res. 2018, 10, 4217–4228. [Google Scholar] [CrossRef]
- Brenot, A.; Knolhoff, B.L.; Denardo, D.G.; Longmore, G.D. SNAIL1 Action in Tumor Cells Influences Macrophage Polarization and Metastasis in Breast Cancer through Altered GM-CSF Secretion. Oncogenesis 2018, 7, 32. [Google Scholar] [CrossRef]
- Lee, H.S.; Park, D.E.; Bae, B.; Oh, K.; Jung, J.W.; Lee, D.S.; Kim, I.G.; Cho, S.H.; Kang, H.R. Tranglutaminase 2 Contributes to the Asthmatic Inflammation by Modulating Activation of Alveolar Macrophages. Immunity, Inflamm. Dis. 2021, 9, 871–882. [Google Scholar] [CrossRef]
- Gong, B.; Zheng, Y.; Li, J.; Lei, H.; Liu, K.; Tang, J.; Peng, Y. Luteolin Activates M2 Macrophages and Suppresses M1 Macrophages by Upregulation of Hsa_circ_0001326 in THP-1 Derived Macrophages. Bioengineered 2022, 13, 5079–5090. [Google Scholar] [CrossRef] [PubMed]
- Xuan, X.; Sun, Z.; Yu, C.; Chen, J.; Chen, M.; Wang, Q.; Li, L. Network Pharmacology-Based Study of the Protective Mechanism of Conciliatory Anti-Allergic Decoction on Asthma. Allergol. Immunopathol. 2020, 48, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Lu, D.; You, J.; Liu, T.; Sun, J.; Lu, Y.; Pan, J.; Li, Y.; Liu, C. Integrated Plasma Pharmacochemistry and Network Pharmacology to Explore the Mechanism of Gerberae Piloselloidis Herba in Treatment of Allergic Asthma. J. Ethnopharmacol. 2022, 298, 115624. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.J.; Wu, C.J.; Wang, S.D.; Kao, S.T. Qi-Wei-Du-Qi-Wan and Its Major Constituents Exert an Anti-Asthmatic Effect by Inhibiting Mast Cell Degranulation. J. Ethnopharmacol. 2020, 254, 112406. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, M.; Yuan, F.; Zeng, L.; Zhao, K.; Sun, T.; Li, Z.; Liu, R. Exploration of the Molecular Mechanisms of Hyssopus Cuspidatus Boriss Treatment of Asthma in an MRNA-MiRNA Network via Bioinformatics Analysis. Biomed Res. Int. 2022, 2022, 7111901. [Google Scholar] [CrossRef]
- Xu, X.; Yu, Z.; Han, B.; Li, S.; Sun, Y.; Du, Y.; Wang, Z.; Gao, D.; Zhang, Z. Luteolin Alleviates Inorganic Mercury-Induced Kidney Injury via Activation of the AMPK/MTOR Autophagy Pathway. J. Inorg. Biochem. 2021, 224, 111583. [Google Scholar] [CrossRef]
- Tan, X.; Liu, B.; Lu, J.; Li, S.; Baiyun, R.; Lv, Y.; Lu, Q.; Zhang, Z. Dietary Luteolin Protects against HgCl2-Induced Renal Injury via Activation of Nrf2-Mediated Signaling in Rat. J. Inorg. Biochem. 2018, 179, 24–31. [Google Scholar] [CrossRef]
- Owumi, S.E.; Lewu, D.O.; Arunsi, U.O.; Oyelere, A.K. Luteolin Attenuates Doxorubicin-Induced Derangements of Liver and Kidney by Reducing Oxidative and Inflammatory Stress to Suppress Apoptosis. Hum. Exp. Toxicol. 2021, 40, 1656–1672. [Google Scholar] [CrossRef]
- Liu, Y.-S.; Yang, Q.; Li, S.; Luo, L.; Liu, H.-Y.; Li, X.-Y.; Gao, Z.-N. Luteolin Attenuates Angiotensin II-induced Renal Damage in Apolipoprotein E-deficient Mice. Mol. Med. Rep. 2021, 23, 1. [Google Scholar] [CrossRef]
- Zhang, Z.T.; Zhang, D.Y.; Xie, K.; Wang, C.J.; Xu, F. Luteolin Activates Tregs to Promote IL-10 Expression and Alleviating Caspase-11-Dependent Pyroptosis in Sepsis-Induced Lung Injury. Int. Immunopharmacol. 2021, 99, 107914. [Google Scholar] [CrossRef]
- Xie, K.; Chai, Y.S.; Lin, S.H.; Xu, F.; Wang, C.J. Luteolin Regulates the Differentiation of Regulatory T Cells and Activates IL-10-Dependent Macrophage Polarization against Acute Lung Injury. J. Immunol. Res. 2021, 2021, 8883962. [Google Scholar] [CrossRef]
- Erdoğan, A.; Erdoğan, M.A.; Kara, A.Y.; Bora, S.; Yiğittürk, G.; Erbaş, O. Effect of Fl Uid Resuscitation on Acute Lung Injury in a Rat Model of Sepsis. Bratisl. Lek. Listy 2021, 122, 280–286. [Google Scholar]
- Rungsung, S.; Singh, T.U.; Rabha, D.J.; Kumar, T.; Cholenahalli Lingaraju, M.; Parida, S.; Paul, A.; Sahoo, M.; Kumar, D. Luteolin Attenuates Acute Lung Injury in Experimental Mouse Model of Sepsis. Cytokine 2018, 110, 333–343. [Google Scholar] [CrossRef]
- Liu, B.; Yu, H.; Baiyun, R.; Lu, J.; Li, S.; Bing, Q.; Zhang, X.; Zhang, Z. Protective Effects of Dietary Luteolin against Mercuric Chloride-Induced Lung Injury in Mice: Involvement of AKT/Nrf2 and NF-ΚB Pathways. Food Chem. Toxicol. 2018, 113, 296–302. [Google Scholar] [CrossRef]
- Liu, X.; Meng, J. Luteolin Alleviates LPS-Induced Bronchopneumonia Injury in Vitro and in Vivo by down-Regulating MicroRNA-132 Expression. Biomed. Pharmacother. 2018, 106, 1641–1649. [Google Scholar] [CrossRef]
- Park, E.J.; Kim, Y.M.; Kim, H.J.; Chang, K.C. Luteolin Activates ERK1/2− and Ca2+-Dependent HO-1 Induction That Reduces LPS-Induced HMGB1, INOS/NO, and COX-2 Expression in RAW264.7 Cells and Mitigates Acute Lung Injury of Endotoxin Mice. Inflamm. Res. 2018, 67, 445–453. [Google Scholar] [CrossRef]
- He, J.; Gu, D.; Wu, X.; Reynolds, K.; Duan, X.; Yao, C.; Wang, J.; Chen, C.-S.; Chen, J.; Wildman, R.P.; et al. Major Causes of Death among Men and Women in China. N. Engl. J. Med. 2005, 353, 1124–1134. [Google Scholar] [CrossRef]
- Jang, Y.J.; Aravinthan, A.; Hossain, M.A.; Kopalli, S.R.; Kim, B.; Kim, N.S.; Kang, C.W.; Kim, J.H. Effect of Korean Red Ginseng through Comparative Analysis of Cardiac Gene Expression in Db/Db Mice. J. Ginseng Res. 2021, 45, 450–455. [Google Scholar] [CrossRef]
- Jennings, R.B.; Ganote, C.E. Structural Changes in Myocardium during Acute Ischemia. Circ. Res. 1974, 35, 156–172. [Google Scholar] [CrossRef]
- Zhao, L.; Zhou, Z.; Zhu, C.; Fu, Z.; Yu, D. Luteolin Alleviates Myocardial Ischemia Reperfusion Injury in Rats via Siti1/NLRP3/NF-ΚB Pathway. Int. Immunopharmacol. 2020, 85, 106680. [Google Scholar] [CrossRef]
- Shen, P.; Deng, X.; Chen, Z.; Ba, X.; Qin, K.; Huang, Y.; Huang, Y.; Li, T.; Yan, J.; Tu, S. SIRT1: A Potential Therapeutic Target in Autoimmune Diseases. Front. Immunol. 2021, 12, 5012. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Gao, S.; Bu, Y.; Zheng, X. Luteolin Protects Cardiomyocytes Cells against Lipopolysaccharide-Induced Apoptosis and Inflammatory Damage by Modulating Nlrp3. Yonsei Med. J. 2022, 63, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Donahoe, S.M.; Stewart, G.C.; McCabe, C.H.; Mohanavelu, S.; Murphy, S.A.; Cannon, C.P.; Antman, E.M. Diabetes and Mortality Following Acute Coronary Syndromes. JAMA 2007, 298, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Rubler, S.; Dlugash, J.; Yuceoglu, Y.Z.; Kumral, T.; Branwood, A.W.; Grishman, A. New Type of Cardiomyopathy Associated with Diabetic Glomerulosclerosis. Am. J. Cardiol. 1972, 30, 595–602. [Google Scholar] [CrossRef]
- Li, L.; Luo, W.; Qian, Y.; Zhu, W.; Qian, J.; Li, J.; Jin, Y.; Xu, X.; Liang, G. Luteolin Protects against Diabetic Cardiomyopathy by Inhibiting NF-ΚB-Mediated Inflammation and Activating the Nrf2-Mediated Antioxidant Responses. Phytomedicine 2019, 59, 152774. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, C.; Zhu, H.; Wang, S.; Zhou, Y.; Zhao, J.; Xia, Y.; Li, D. Luteolin Modulates SERCA2a via Sp1 Upregulation to Attenuate Myocardial Ischemia/Reperfusion Injury in Mice. Sci. Rep. 2020, 10, 15407. [Google Scholar] [CrossRef]
- Liu, D.; Luo, H.; Qiao, C. SHP-1/STAT3 Interaction Is Related to Luteolin-Induced Myocardial Ischemia Protection. Inflammation 2022, 45, 88–99. [Google Scholar] [CrossRef]
- Fernando, S.M.; Rochwerg, B.; Seely, A.J.E. Clinical Implications of the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). CMAJ 2018, 190, E1058–E1059. [Google Scholar] [CrossRef]
- Wu, B.; Song, H.; Fan, M.; You, F.; Zhang, L.; Luo, J.; Li, J.; Wang, L.; Li, C.; Yuan, M. Luteolin Attenuates Sepsis–Induced Myocardial Injury by Enhancing Autophagy in Mice. Int. J. Mol. Med. 2020, 45, 1477–1487. [Google Scholar] [CrossRef]
- Gentile, D.; Fornai, M.; Pellegrini, C.; Colucci, R.; Benvenuti, L.; Duranti, E.; Masi, S.; Carpi, S.; Nieri, P.; Nericcio, A.; et al. Luteolin Prevents Cardiometabolic Alterations and Vascular Dysfunction in Mice with HFD-Induced Obesity. Front. Pharmacol. 2018, 9, 1094. [Google Scholar] [CrossRef]
- Abu-Elsaad, N.; El-Karef, A. The Falconoid Luteolin Mitigates the Myocardial Inflammatory Response Induced by High-Carbohydrate/High-Fat Diet in Wistar Rats. Inflammation 2018, 41, 221–231. [Google Scholar] [CrossRef]
- Frostegård, J. Immunity, Atherosclerosis and Cardiovascular Disease. BMC Med. 2013, 11, 117. [Google Scholar] [CrossRef]
- Zhou, Y.; Little, P.J.; Cao, Y.; Ta, H.T.; Kamato, D. Lysophosphatidic Acid Receptor 5 Transactivation of TGFBR1 Stimulates the MRNA Expression of Proteoglycan Synthesizing Genes XYLT1 and CHST3. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118848. [Google Scholar] [CrossRef]
- Wu, Y.T.; Chen, L.; Tan, Z.B.; Fan, H.J.; Xie, L.P.; Zhang, W.T.; Chen, H.M.; Li, J.; Liu, B.; Zhou, Y.C. Luteolin Inhibits Vascular Smooth Muscle Cell Proliferation and Migration by Inhibiting TGFBR1 Signaling. Front. Pharmacol. 2018, 9, 1059. [Google Scholar] [CrossRef]
- Chen, G.; Xu, H.; Wu, Y.; Han, X.; Xie, L.; Zhang, G.; Liu, B.; Zhou, Y.C. Myricetin Suppresses the Proliferation and Migration of Vascular Smooth Muscle Cells and Inhibits Neointimal Hyperplasia via Suppressing TGFBR1 Signaling Pathways. Phytomedicine 2021, 92, 153719. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zheng, L.; Yang, B.; Wang, X.; Ying, Y. Luteolin Attenuates Atherosclerosis via Modulating Signal Transducer and Activator of Transcription 3-Mediated Inflammatory Response. Drug Des. Dev. Ther. 2019, 13, 3899–3911. [Google Scholar] [CrossRef]
- Li, J.; Dong, J.Z.; Ren, Y.L.; Zhu, J.J.; Cao, J.N.; Zhang, J.; Pan, L.L. Luteolin Decreases Atherosclerosis in LDL Receptor-Deficient Mice via a Mechanism Including Decreasing AMPK-SIRT1 Signaling in Macrophages. Exp. Ther. Med. 2018, 16, 2593–2599. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, M.; Yu, H.; Yuan, G.; Luo, L.; Xu, X.; Xu, Y.; Sui, X.; Leung, E.L.H.; Wu, Q. The Role and Mechanisms of Action of Natural Compounds in the Prevention and Treatment of Cancer and Cancer Metastasis. Front. Biosci. Landmark 2022, 27, 192. [Google Scholar] [CrossRef]
- Franza, L.; Carusi, V.; Nucera, E.; Pandolfi, F. Luteolin, Inflammation and Cancer: Special Emphasis on Gut Microbiota. BioFactors 2021, 47, 181–189. [Google Scholar] [CrossRef]
- Nooreldeen, R.; Bach, H. Current and Future Development in Lung Cancer Diagnosis. Int. J. Mol. Sci. 2021, 22, 8661. [Google Scholar] [CrossRef]
- Jain, D.; Roy-Chowdhuri, S. Advances in Cytology of Lung Cancer. In Proceedings of the Seminars in Diagnostic Pathology; Elsevier: Amsterdam, The Netherlands, 2021; Volume 38, pp. 109–115. [Google Scholar]
- Yu, Q.; Zhang, M.; Ying, Q.; Xie, X.; Yue, S.; Wei, Q.; Bai, Z.; Ma, L. Decrease of AIM2 Mediated by Luteolin Contributes to Non-Small Cell Lung Cancer Treatment. Cell Death Dis. 2019, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, R.; Tian, J.; Song, M.; Zhao, R.; Liu, K.; Zhu, F.; Shim, J.; Dong, Z.; Lee, M. Targeting LIMK1 with Luteolin Inhibits the Growth of Lung Cancer in Vitro and in Vivo. J. Cell. Mol. Med. 2021, 25, 5560–5571. [Google Scholar] [CrossRef] [PubMed]
- Masraksa, W.; Tanasawet, S.; Hutamekalin, P.; Wongtawatchai, T.; Sukketsiri, W. Luteolin Attenuates Migration and Invasion of Lung Cancer Cells via Suppressing Focal Adhesion Kinase and Non-Receptor Tyrosine Kinase Signaling Pathway. Nutr. Res. Pract. 2020, 14, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Shen, T.; Yang, N.; Wang, M.; Hsieh, W.; Chuang, C.; Lai, C.; Chang, Y. Luteolin Reduces Aqueous Extract PM2. 5-Induced Metastatic Activity in H460 Lung Cancer Cells. Int. J. Med. Sci. 2022, 19, 1502–1509. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Xiong, J.; Zhou, Y.; Wu, Y.; Song, Y.; Wang, N.; Chen, L.; Zhang, J. Luteolin Enhances TRAIL Sensitivity in Non-Small Cell Lung Cancer Cells through Increasing DR5 Expression and Drp1-Mediated Mitochondrial Fission. Arch. Biochem. Biophys. 2020, 692, 108539. [Google Scholar] [CrossRef]
- Yang, X.; Feng, Y.; Liu, Y.; Ye, X.; Ji, X.; Sun, L.; Gao, F.; Zhang, Q.; Li, Y.; Zhu, B.; et al. Fuzheng Jiedu Xiaoji Formulation Inhibits Hepatocellular Carcinoma Progression in Patients by Targeting the AKT/CyclinD1/P21/P27 Pathway. Phytomedicine 2021, 87, 153575. [Google Scholar] [CrossRef]
- Im, E.; Yeo, C.; Lee, E.-O. Luteolin Induces Caspase-Dependent Apoptosis via Inhibiting the AKT/Osteopontin Pathway in Human Hepatocellular Carcinoma SK-Hep-1 Cells. Life Sci. 2018, 209, 259–266. [Google Scholar] [CrossRef]
- Lee, Y.; Kwon, Y.H. Regulation of Apoptosis and Autophagy by Luteolin in Human Hepatocellular Cancer Hep3B Cells. Biochem. Biophys. Res. Commun. 2019, 517, 617–622. [Google Scholar] [CrossRef]
- Yang, P.-W.; Lu, Z.-Y.; Pan, Q.; Chen, T.-T.; Feng, X.-J.; Wang, S.-M.; Pan, Y.-C.; Zhu, M.-H.; Zhang, S.-H. MicroRNA-6809-5p Mediates Luteolin-Induced Anticancer Effects against Hepatoma by Targeting Flotillin 1. Phytomedicine 2019, 57, 18–29. [Google Scholar] [CrossRef]
- Feng, X.; Rong, L.; Wang, R.; Zheng, X.; Zhang, L.; Zhang, L.; Lin, Y.; Wang, X.; Li, Z. Luteolin and Sorafenib Combination Kills Human Hepatocellular Carcinoma Cells through Apoptosis Potentiation and JNK Activation. Oncol. Lett. 2018, 16, 648–653. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Kang, K.A.; Piao, M.J.; Hyun, Y.J.; Zhen, A.X.; Cho, S.J.; Ahn, M.J.; Yi, J.M.; Hyun, J.W. Luteolin Promotes Apoptotic Cell Death via Upregulation of Nrf2 Expression by DNA Demethylase and the Interaction of Nrf2 with P53 in Human Colon Cancer Cells. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef]
- Zuo, Q.; Wu, R.; Xiao, X.; Yang, C.; Yang, Y.; Wang, C.; Lin, L.; Kong, A.N. The Dietary Flavone Luteolin Epigenetically Activates the Nrf2 Pathway and Blocks Cell Transformation in Human Colorectal Cancer HCT116 Cells. J. Cell. Biochem. 2018, 119, 9573–9582. [Google Scholar] [CrossRef]
- Yoo, H.S.; Won, S.B.; Kwon, Y.H. Luteolin Induces Apoptosis and Autophagy in HCT116 Colon Cancer Cells via P53-Dependent Pathway. Nutr. Cancer 2022, 74, 677–686. [Google Scholar] [CrossRef]
- Jang, C.H.; Moon, N.; Oh, J.; Kim, J.S. Luteolin Shifts Oxaliplatin-Induced Cell Cycle Arrest at G0/G1 to Apoptosis in Hct116 Human Colorectal Carcinoma Cells. Nutrients 2019, 11, 770. [Google Scholar] [CrossRef]
- Song, Y.; Yu, J.; Li, L.L.; Wang, L.; Dong, L.; Xi, G.; Lu, Y.J.; Li, Z. Luteolin Impacts Deoxyribonucleic Acid Repair by Modulating the Mitogen-Activated Protein Kinase Pathway in Colorectal Cancer. Bioengineered 2022, 13, 10998–11011. [Google Scholar] [CrossRef]
- Potočnjak, I.; Šimić, L.; Gobin, I.; Vukelić, I.; Domitrović, R. Antitumor Activity of Luteolin in Human Colon Cancer SW620 Cells Is Mediated by the ERK/FOXO3a Signaling Pathway. Toxicol. In Vitro 2020, 66, 104852. [Google Scholar] [CrossRef]
- Yao, Y.; Rao, C.; Zheng, G.; Wang, S. Luteolin Suppresses Colorectal Cancer Cell Metastasis via Regulation of the MiR-384/Pleiotrophin Axis. Oncol. Rep. 2019, 42, 131–141. [Google Scholar] [CrossRef]
- Jang, C.H.; Moon, N.; Lee, J.; Kwon, M.J.; Oh, J.; Kim, J.S. Luteolin Synergistically Enhances Antitumor Activity of Oxaliplatin in Colorectal Carcinoma via AMPK Inhibition. Antioxidants 2022, 11, 626. [Google Scholar] [CrossRef]
- Cai, J.; Chen, H.; Lu, M.; Zhang, Y.; Lu, B.; You, L.; Zhang, T.; Dai, M.; Zhao, Y. Advances in the Epidemiology of Pancreatic Cancer: Trends, Risk Factors, Screening, and Prognosis. Cancer Lett. 2021, 520, 1–11. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Chen, L.; Li, H. The Dietary Compound Luteolin Inhibits Pancreatic Cancer Growth by Targeting BCL-2. Food Funct. 2018, 9, 3018–3027. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Naiki-Ito, A.; Suzuki, S.; Inaguma, S.; Komura, M.; Nakao, K.; Naiki, T.; Kachi, K.; Kato, A.; Matsuo, Y. DPYD, down-Regulated by the Potentially Chemopreventive Agent Luteolin, Interacts with STAT3 in Pancreatic Cancer. Carcinogenesis 2021, 42, 940–950. [Google Scholar] [CrossRef] [PubMed]
- Moeng, S.; Son, S.W.; Seo, H.A.; Lee, J.S.; Kim, C.K.; Kuh, H.J.; Park, J.K. Luteolin-Regulated MicroRNA-301-3p Targets Caspase-8 and Modulates TRAIL Sensitivity in PANC-1 Cells. Anticancer Res. 2020, 40, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Bhugul, P.A.; Fan, G.; Ye, T.; Huang, S.; Dai, S.; Chen, B.; Zhou, M. Luteolin Inhibits Pancreatitis-induced Acinar-ductal Metaplasia, Proliferation and Epithelial-mesenchymal Transition of Acinar Cells. Mol. Med. Rep. 2018, 17, 3681–3689. [Google Scholar] [CrossRef]
- Wu, H.-T.; Lin, J.; Liu, Y.-E.; Chen, H.-F.; Hsu, K.-W.; Lin, S.-H.; Peng, K.-Y.; Lin, K.-J.; Hsieh, C.-C.; Chen, D.-R. Luteolin Suppresses Androgen Receptor-Positive Triple-Negative Breast Cancer Cell Proliferation and Metastasis by Epigenetic Regulation of MMP9 Expression via the AKT/MTOR Signaling Pathway. Phytomedicine 2021, 81, 153437. [Google Scholar] [CrossRef]
- Cao, D.; Zhu, G.-Y.; Lu, Y.; Yang, A.; Chen, D.; Huang, H.-J.; Peng, S.-X.; Chen, L.-W.; Li, Y.-W. Luteolin Suppresses Epithelial-Mesenchymal Transition and Migration of Triple-Negative Breast Cancer Cells by Inhibiting YAP/TAZ Activity. Biomed. Pharmacother. 2020, 129, 110462. [Google Scholar] [CrossRef]
- Huang, L.; Jin, K.; Lan, H. Luteolin Inhibits Cell Cycle Progression and Induces Apoptosis of Breast Cancer Cells through Downregulation of Human Telomerase Reverse Transcriptase. Oncol. Lett. 2019, 17, 3842–3850. [Google Scholar] [CrossRef]
- Feng, J.; Zheng, T.; Hou, Z.; Lv, C.; Xue, A.; Han, T.; Han, B.; Sun, X.; Wei, Y. Luteolin, an Aryl Hydrocarbon Receptor Ligand, Suppresses Tumor Metastasis in Vitro and in Vivo. Oncol. Rep. 2020, 44, 2231–2240. [Google Scholar] [CrossRef]
- Wu, H.-T.; Liu, Y.-E.; Hsu, K.-W.; Wang, Y.-F.; Chan, Y.-C.; Chen, Y.; Chen, D.-R. MLL3 Induced by Luteolin Causes Apoptosis in Tamoxifen-Resistant Breast Cancer Cells through H3K4 Monomethylation and Suppression of the PI3K/AKT/MTOR Pathway. Am. J. Chin. Med. 2020, 48, 1221–1241. [Google Scholar] [CrossRef]
- Casili, G.; Ardizzone, A.; Lanza, M.; Gugliandolo, E.; Portelli, M.; Militi, A.; Cuzzocrea, S.; Esposito, E.; Paterniti, I. Treatment with Luteolin Improves Lipopolysaccharide-Induced Periodontal Diseases in Rats. Biomedicines 2020, 8, 442. [Google Scholar] [CrossRef]
- Balci Yuce, H.; Toker, H.; Yildirim, A.; Tekin, M.B.; Gevrek, F.; Altunbas, N. The Effect of Luteolin in Prevention of Periodontal Disease in Wistar Rats. J. Periodontol. 2019, 90, 1481–1489. [Google Scholar] [CrossRef]
- Huang, W.-C.; Liou, C.-J.; Shen, S.-C.; Hu, S.; Hsiao, C.-Y.; Wu, S.-J. Luteolin Attenuates IL-1β-Induced THP-1 Adhesion to ARPE-19 Cells via Suppression of NF-ΚB and MAPK Pathways. Mediators Inflamm. 2020, 2020, 9421340. [Google Scholar] [CrossRef]
- Liu, X.-B.; Liu, F.; Liang, Y.-Y.; Yin, G.; Zhang, H.-J.; Mi, X.-S.; Zhang, Z.-J.; So, K.-F.; Li, A.; Xu, Y. Luteolin Delays Photoreceptor Degeneration in a Mouse Model of Retinitis Pigmentosa. Neural Regen. Res. 2021, 16, 2109. [Google Scholar] [CrossRef]
- Mizrachi, T.; Vaknin-Dembinsky, A.; Brenner, T.; Treinin, M. Neuroinflammation Modulation via A7 Nicotinic Acetylcholine Receptor and Its Chaperone, RIC-3. Molecules 2021, 26, 6139. [Google Scholar] [CrossRef]
- Reale, M.; Costantini, E. Cholinergic Modulation of the Immune System in Neuroinflammatory Diseases. Diseases 2021, 9, 29. [Google Scholar] [CrossRef]
- Dhapola, R.; Hota, S.S.; Sarma, P.; Bhattacharyya, A.; Medhi, B.; Reddy, D.H.K. Recent Advances in Molecular Pathways and Therapeutic Implications Targeting Neuroinflammation for Alzheimer’s Disease. Inflammopharmacology 2021, 29, 1669–1681. [Google Scholar] [CrossRef]
- Kou, J.; Shi, J.; He, Y.; Hao, J.; Zhang, H.; Luo, D.; Song, J.; Yan, Y.; Xie, X.; Du, G. Luteolin Alleviates Cognitive Impairment in Alzheimer’s Disease Mouse Model via Inhibiting Endoplasmic Reticulum Stress-Dependent Neuroinflammation. Acta Pharmacol. Sin. 2022, 43, 840–849. [Google Scholar] [CrossRef]
- Zhou, W.; Hu, M.; Hu, J.; Du, Z.; Su, Q.; Xiang, Z. Luteolin Suppresses Microglia Neuroinflammatory Responses and Relieves Inflammation-Induced Cognitive Impairments. Neurotox. Res. 2021, 39, 1800–1811. [Google Scholar] [CrossRef]
- Nakagawa, T. Luteolin Ameliorates Depression-like Behaviors by Suppressing ER Stress in a Mouse Model of Alzheimer’s Disease. Biochem. Biophys. Res. Commun. 2022, 588, 168–174. [Google Scholar]
- Ahmad, S.; Jo, M.H.; Ikram, M.; Khan, A.; Kim, M.O. Deciphering the Potential Neuroprotective Effects of Luteolin against Aβ1–42-Induced Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 9583. [Google Scholar] [CrossRef]
- Abbas, H.; El Sayed, N.S.; Youssef, N.A.H.A.; ME Gaafar, P.; Mousa, M.R.; Fayez, A.M.; Elsheikh, M.A. Novel Luteolin-Loaded Chitosan Decorated Nanoparticles for Brain-Targeting Delivery in a Sporadic Alzheimer’s Disease Mouse Model: Focus on Antioxidant, Anti-Inflammatory, and Amyloidogenic Pathways. Pharmaceutics 2022, 14, 1003. [Google Scholar] [CrossRef] [PubMed]
- Elsheikh, M.A.; El-Feky, Y.A.; Al-Sawahli, M.M.; Ali, M.E.; Fayez, A.M.; Abbas, H. A Brain-Targeted Approach to Ameliorate Memory Disorders in a Sporadic Alzheimer’s Disease Mouse Model via Intranasal Luteolin-Loaded Nanobilosomes. Pharmaceutics 2022, 14, 576. [Google Scholar] [CrossRef] [PubMed]
- Jayatunga, D.P.W.; Hone, E.; Fernando, W.B.; Garg, M.L.; Verdile, G.; Martins, R.N. A Synergistic Combination of DHA, Luteolin, and Urolithin a against Alzheimer’s Disease. Front. Aging Neurosci. 2022, 14, 780602. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, D.S.; Kang, S.; Kim, H.J. The Combination of Luteolin and L-Theanine Improved Alzheimer Disease–like Symptoms by Potentiating Hippocampal Insulin Signaling and Decreasing Neuroinflammation and Norepinephrine Degradation in Amyloid-β–Infused Rats. Nutr. Res. 2018, 60, 116–131. [Google Scholar] [CrossRef] [PubMed]
- Facchinetti, R.; Valenza, M.; Bronzuoli, M.R.; Menegoni, G.; Ratano, P.; Steardo, L.; Campolongo, P.; Scuderi, C. Looking for a Treatment for the Early Stage of Alzheimer’s Disease: Preclinical Evidence with Co-Ultramicronized Palmitoylethanolamide and Luteolin. Int. J. Mol. Sci. 2020, 21, 3802. [Google Scholar] [CrossRef]
- Raza, C.; Anjum, R. Parkinson’s Disease: Mechanisms, Translational Models and Management Strategies. Life Sci. 2019, 226, 77–90. [Google Scholar] [CrossRef]
- Hayes, M.T. Parkinson’s Disease and Parkinsonism. Am. J. Med. 2019, 132, 802–807. [Google Scholar] [CrossRef]
- Reudhabibadh, R.; Binlateh, T.; Chonpathompikunlert, P.; Nonpanya, N.; Prommeenate, P.; Chanvorachote, P.; Hutamekalin, P. Suppressing Cdk5 Activity by Luteolin Inhibits MPP+-Induced Apoptotic of Neuroblastoma through Erk/Drp1 and Fak/Akt/GSK3β Pathways. Molecules 2021, 26, 1307. [Google Scholar] [CrossRef]
- Elmazoglu, Z.; Yar Saglam, A.S.; Sonmez, C.; Karasu, C. Luteolin Protects Microglia against Rotenone-Induced Toxicity in a Hormetic Manner through Targeting Oxidative Stress Response, Genes Associated with Parkinson’s Disease and Inflammatory Pathways. Drug Chem. Toxicol. 2020, 43, 96–103. [Google Scholar] [CrossRef]
- Brotini, S. Palmitoylethanolamide/Luteolin as Adjuvant Therapy to Improve an Unusual Case of Camptocormia in a Patient with Parkinson’s Disease: A Case Report. Innov. Clin. Neurosci. 2021, 18, 12. [Google Scholar]
- Chen, L.-Y.; Cheng, H.-L.; Kuan, Y.-H.; Liang, T.-J.; Chao, Y.-Y.; Lin, H.-C. Therapeutic Potential of Luteolin on Impaired Wound Healing in Streptozotocin-Induced Rats. Biomedicines 2021, 9, 761. [Google Scholar] [CrossRef]
- Liang, K.-L.; Yu, S.-J.; Huang, W.-C.; Yen, H.-R. Luteolin Attenuates Allergic Nasal Inflammation via Inhibition of Interleukin-4 in an Allergic Rhinitis Mouse Model and Peripheral Blood from Human Subjects with Allergic Rhinitis. Front. Pharmacol. 2020, 11, 291. [Google Scholar] [CrossRef]
- Dong, J.; Xu, O.; Wang, J.; Shan, C.; Ren, X. Luteolin Ameliorates Inflammation and Th1/Th2 Imbalance via Regulating the Tlr4/Nf-Κb Pathway in Allergic Rhinitis Rats. Immunopharmacol. Immunotoxicol. 2021, 43, 319–327. [Google Scholar] [CrossRef]
- Woo, J.-H.; Jang, D.S.; Choi, J.-H. Luteolin Promotes Apoptosis of Endometriotic Cells and Inhibits the Alternative Activation of Endometriosis-Associated Macrophages. Biomol. Ther. 2021, 29, 678. [Google Scholar] [CrossRef]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for Functional Genomics Data Sets—Update. Nucleic Acids Res. 2013, 41, D991–D995. [Google Scholar] [CrossRef]
- Jo, M.; Yi, Y.-S.; Cho, J.Y. Archidendron Lucidum Exerts Anti-Inflammatory Effects by Targeting PDK1 in the NF-κ B Pathway. Am. J. Chin. Med. 2020, 48, 429–444. [Google Scholar] [CrossRef]
- Hwang, S.-H.; Lorz, L.R.; Yi, D.-K.; Noh, J.K.; Yi, Y.-S.; Cho, J.Y. Viburnum Pichinchense Methanol Extract Exerts Anti-Inflammatory Effects via Targeting the NF-ΚB and Caspase-11 Non-Canonical Inflammasome Pathways in Macrophages. J. Ethnopharmacol. 2019, 245, 112161. [Google Scholar] [CrossRef]
- Choi, E.; Kim, M.-Y.; Cho, J.Y. Anti-Inflammatory Activities of Canarium Subulatum Guillaumin Methanol Extract Operate by Targeting Src and Syk in the NF-ΚB Pathway. J. Ethnopharmacol. 2019, 238, 111848. [Google Scholar] [CrossRef]
- Kim, H.G.; Choi, S.; Lee, J.; Hong, Y.H.; Jeong, D.; Yoon, K.; Yoon, D.H.; Sung, G.-H.; Lee, S.; Hong, S. Src Is a Prime Target Inhibited by Celtis Choseniana Methanol Extract in Its Anti-Inflammatory Action. Evid.-Based Complement. Altern. Med. 2018, 2018, 3909038. [Google Scholar] [CrossRef]
- Diniz, L.R.L.; Elshabrawy, H.A.; Souza, M.T.S.; Duarte, A.B.S.; Madhav, N.; de Sousa, D.P. Renoprotective Effects of Luteolin: Therapeutic Potential for COVID-19-Associated Acute Kidney Injuries. Biomolecules 2022, 12, 1544. [Google Scholar] [CrossRef]
- Zhu, P.; Chen, L.; Zhao, Y.; Gao, C.; Yang, J.; Liao, X.; Liu, D.; Yang, B. A Novel Host-Guest Complex Based on Biotin Functionalized Polyamine-β-Cyclodextrin for Tumor Targeted Delivery of Luteolin. J. Mol. Struct. 2021, 1237, 130339. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Feng, W.; Yuan, Q.; Qi, X.; Chen, S.; Yao, P.; Dai, Q.; Xia, P.; Zhang, D. Folic Acid-Modified ROS-Responsive Nanoparticles Encapsulating Luteolin for Targeted Breast Cancer Treatment. Drug Deliv. 2021, 28, 1695–1708. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, A.; Ito, J.; Parida, I.S.; Syoji, N.; Fujii, T.; Takahashi, H.; Nakagawa, K. Improving Water Dispersibility and Bioavailability of Luteolin Using Microemulsion System. Sci. Rep. 2022, 12, 11949. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Wang, C.; Jiang, S.; Yang, T.; Wu, X. Glycosylation of Luteolin in Hydrophilic Organic Solvents and Structure-Antioxidant Relationships of Luteolin Glycosides. RSC Adv. 2022, 12, 18232–18237. [Google Scholar] [CrossRef]

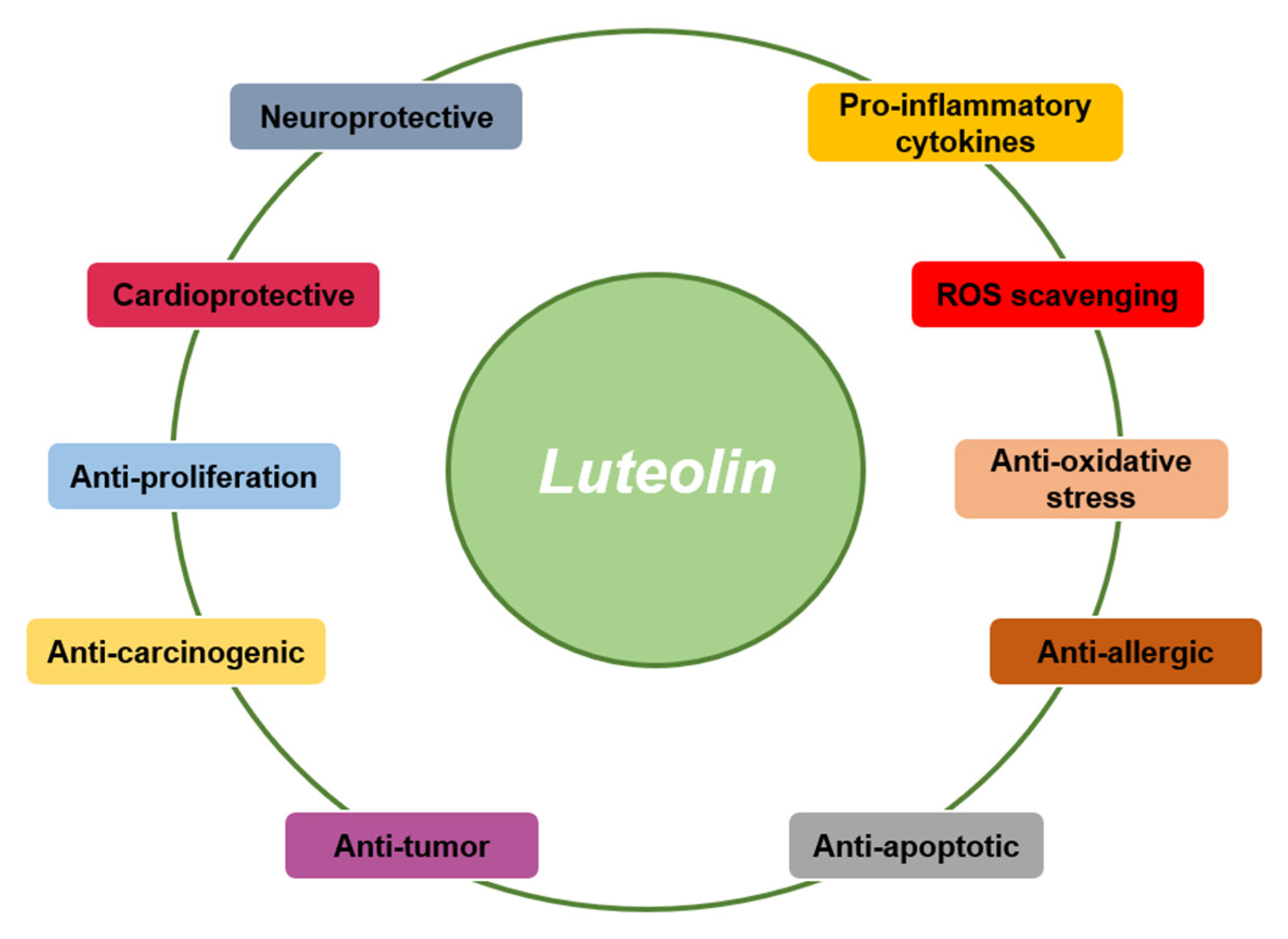

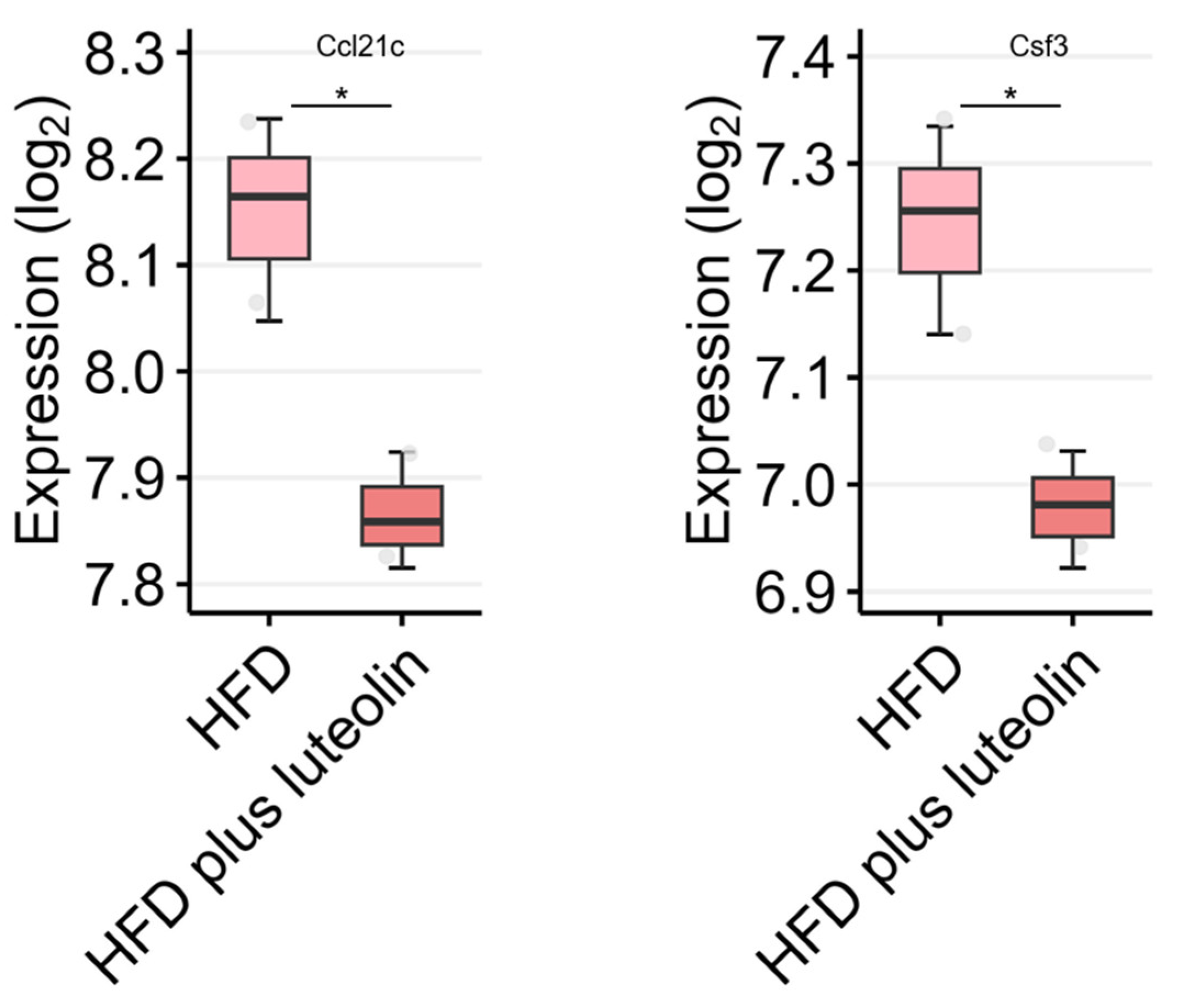
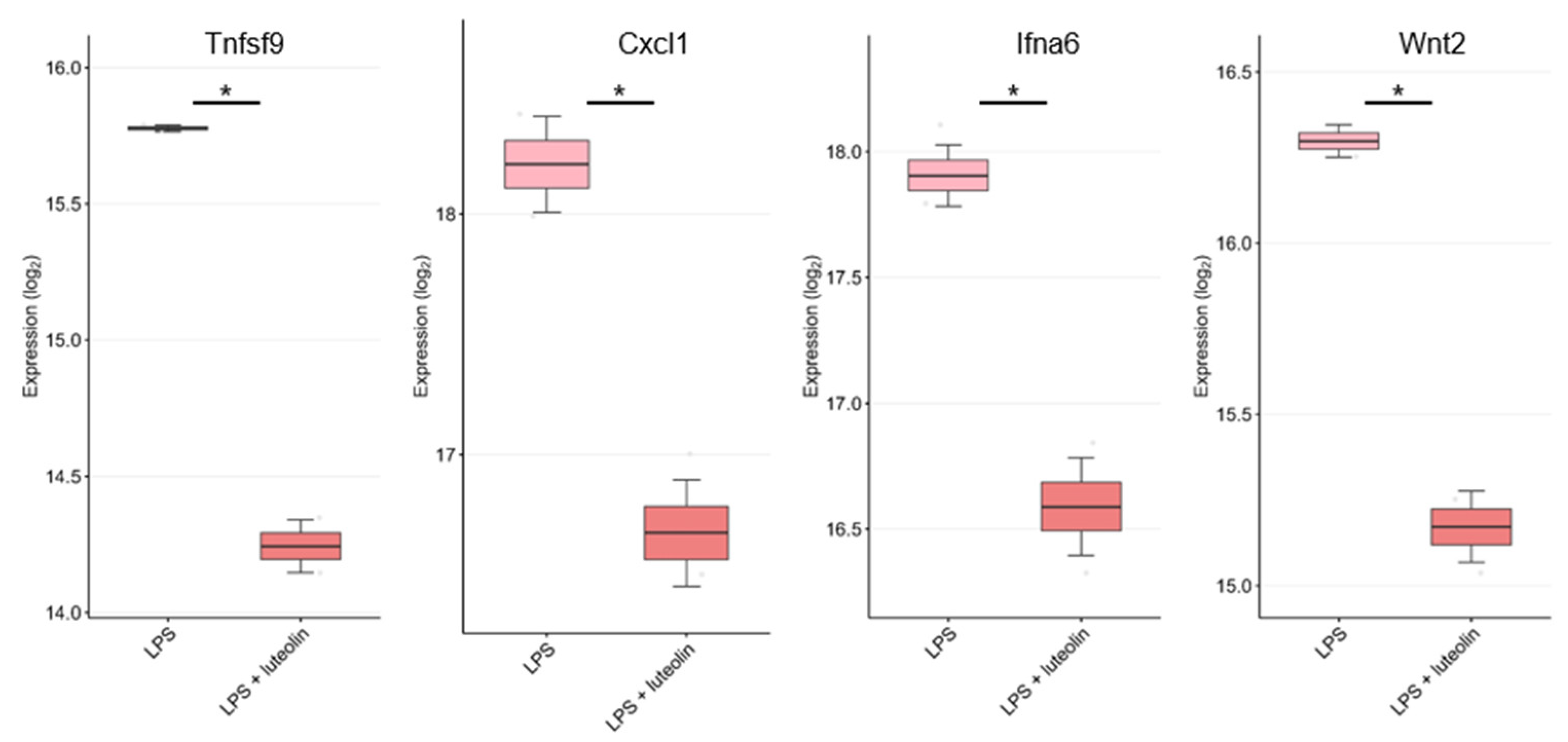

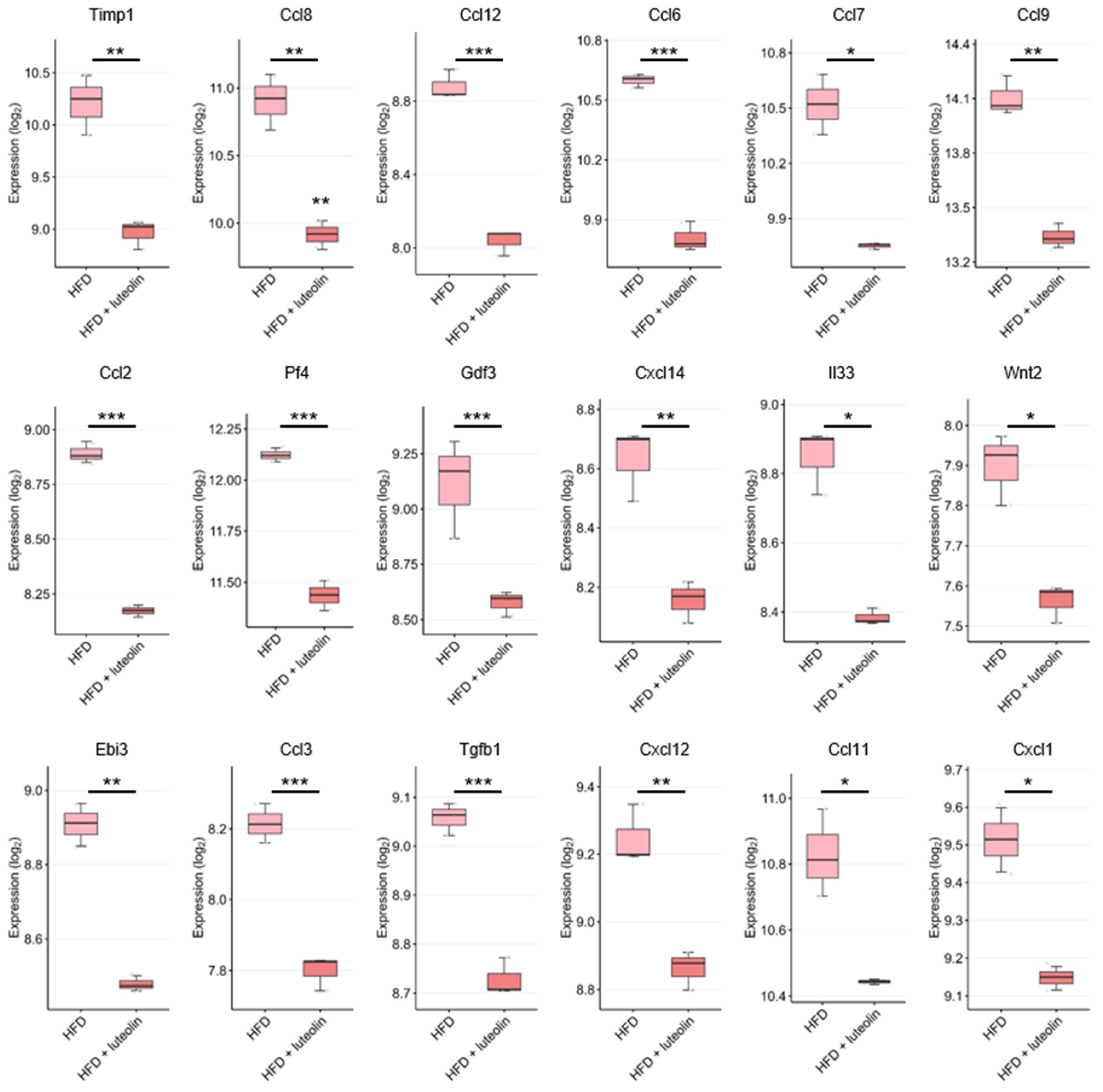
| Disease Type | Disease | Mechanism | Test Type | Refs. |
|---|---|---|---|---|
| Inflammatory Diseases | Gastritis |
| in vitro | [23] |
| in vitro and in vivo | [24,25,139,140,141,142] | ||
| Arthritis |
| in vitro | [27,28] | |
| in vitro and in vivo | [20] | ||
| Asthma |
| in vitro | [43,143] | |
| in vitro and in vivo | [33,36] | ||
| Renal damage |
| in vivo | [48,49,50,51] | |
| Lung injury |
| in vitro and in vivo | [57,58] | |
| in vitro | [53,55,56] | ||
| Cardiovascular Diseases (CVDs) | Myocardial Infarction (MI) |
| in vitro | [64] |
| in vivo | [67,68,69,71,73] | ||
| Atherosclerosis (AS) |
| in vivo | [78,79] | |
| Tumors | Lung cancer |
| in vitro and in vivo | [84,85] |
| in vitro | [86,87,88] | ||
| Hepatocellular carcinoma (HCC) |
| in vitro | [90,91,92] | |
| Colorectal Cancer (CRC) |
| in vitro | [95,96,97,98,99,100,101] | |
| Pancreatic cancer |
| in vitro | [106,107] | |
| in vivo | [104,105] | ||
| Neurodegenerative disorders | Alzheimer’s disease (AD) |
| in vitro and in vivo | [121] |
| in vivo | [120,123,125] | ||
| Parkinson’s disease (PD) |
| in vitro | [131,132] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Kim, M.-Y.; Cho, J.Y. Immunopharmacological Activities of Luteolin in Chronic Diseases. Int. J. Mol. Sci. 2023, 24, 2136. https://doi.org/10.3390/ijms24032136
Huang L, Kim M-Y, Cho JY. Immunopharmacological Activities of Luteolin in Chronic Diseases. International Journal of Molecular Sciences. 2023; 24(3):2136. https://doi.org/10.3390/ijms24032136
Chicago/Turabian StyleHuang, Lei, Mi-Yeon Kim, and Jae Youl Cho. 2023. "Immunopharmacological Activities of Luteolin in Chronic Diseases" International Journal of Molecular Sciences 24, no. 3: 2136. https://doi.org/10.3390/ijms24032136
APA StyleHuang, L., Kim, M.-Y., & Cho, J. Y. (2023). Immunopharmacological Activities of Luteolin in Chronic Diseases. International Journal of Molecular Sciences, 24(3), 2136. https://doi.org/10.3390/ijms24032136








