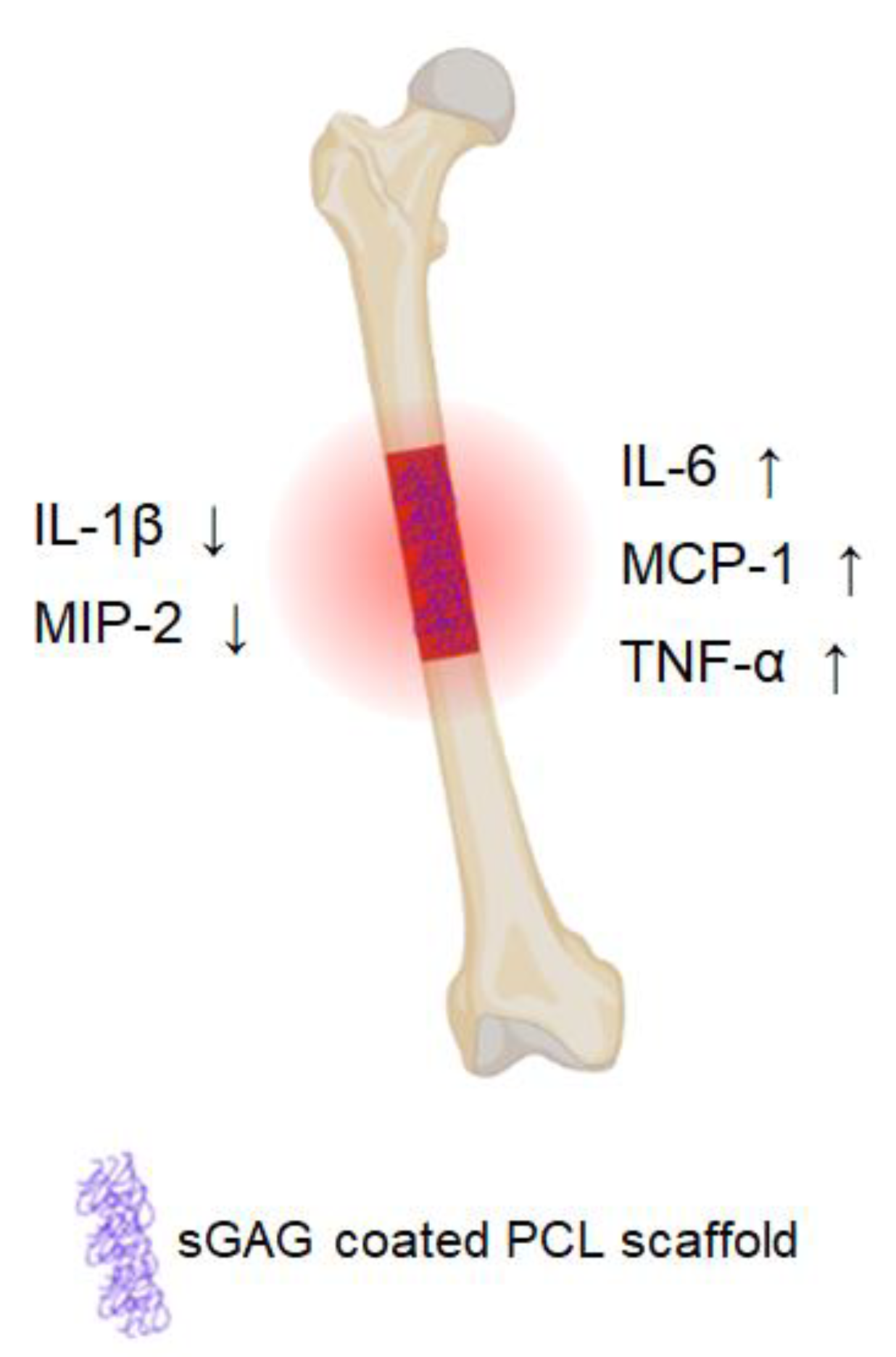
Microdialysis Reveals Anti-Inflammatory Effects of Sulfated Glycosaminoglycanes in the Early Phase of Bone Healing
Abstract
1. Introduction
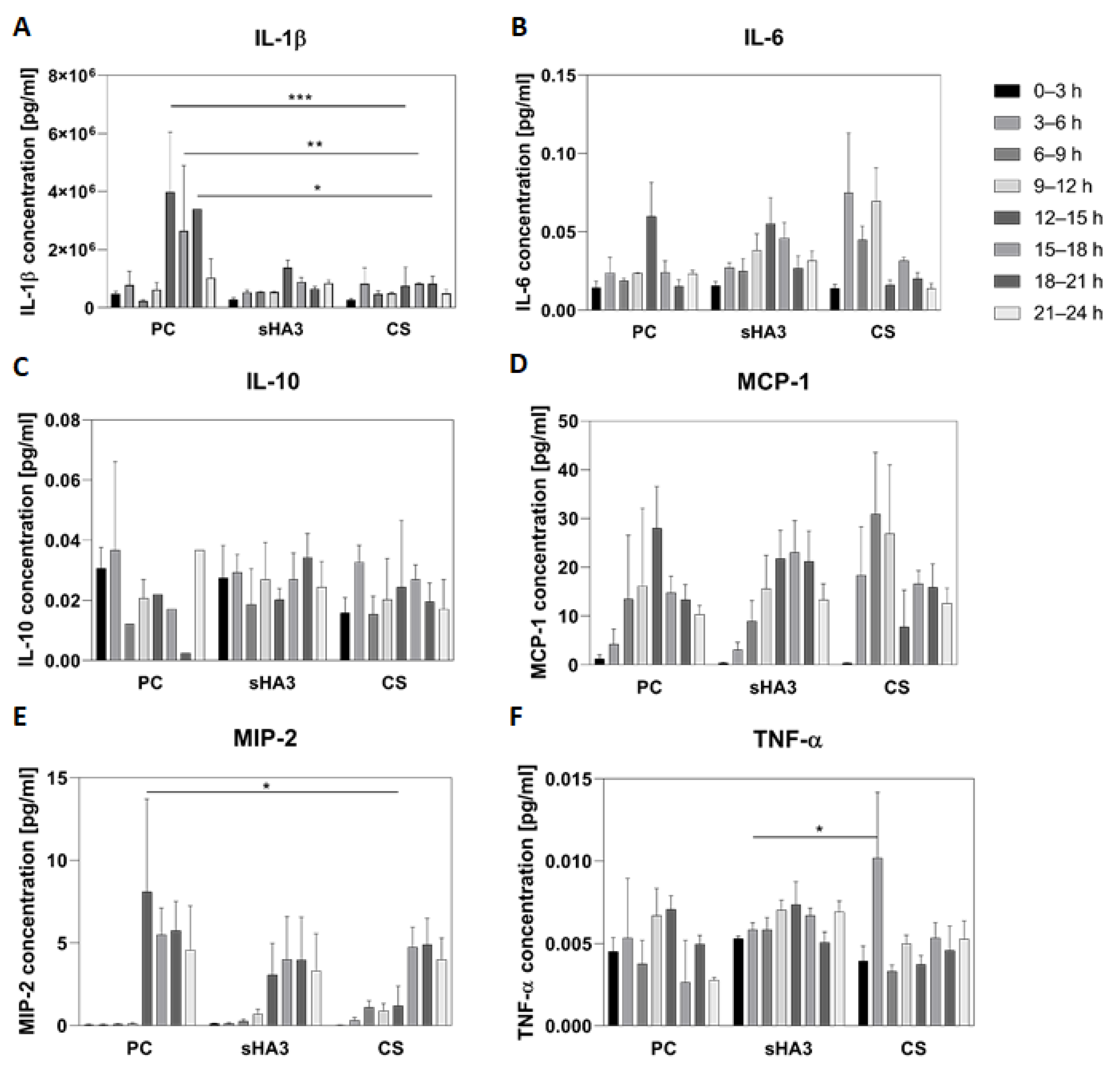
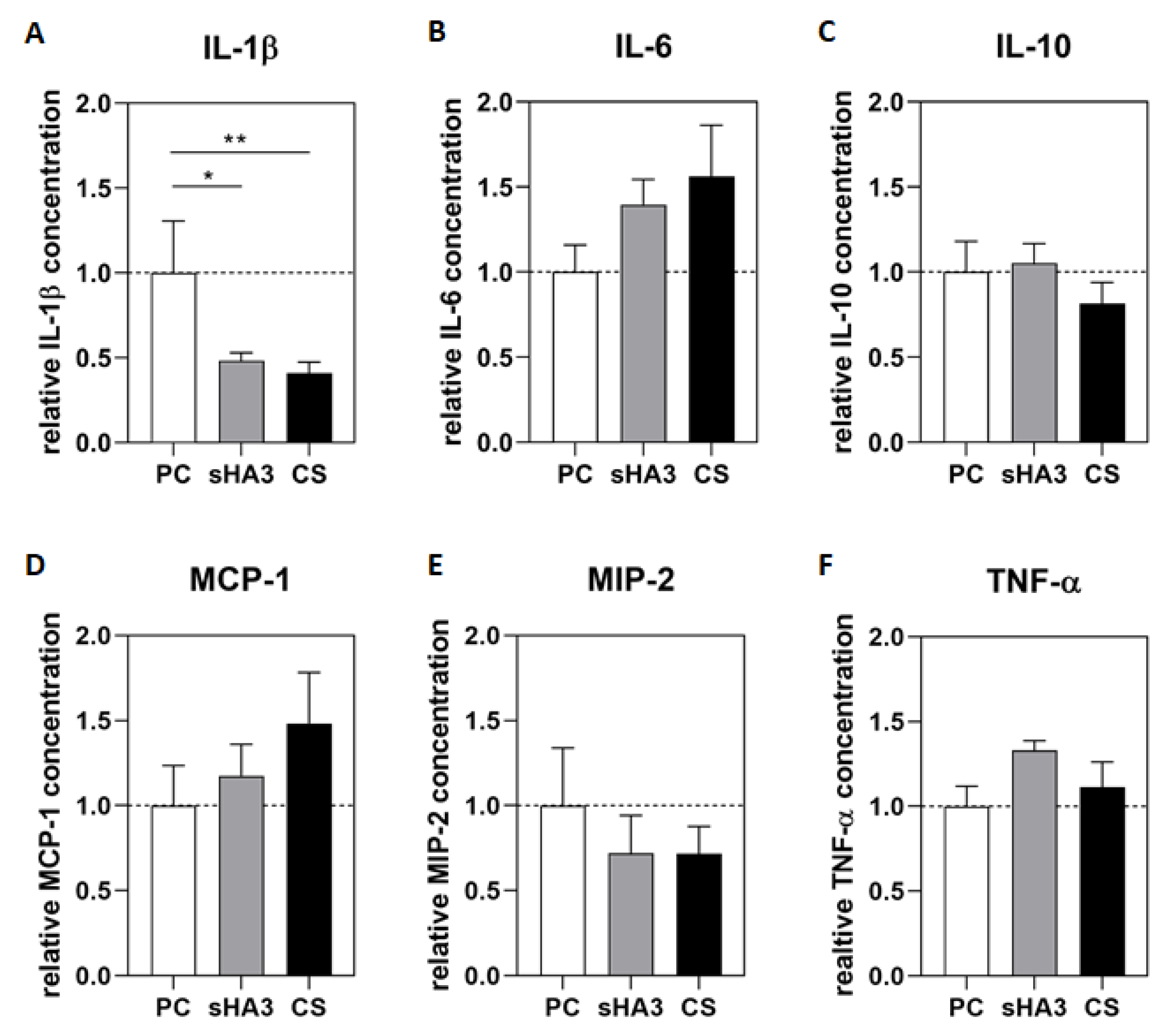
2. Results
3. Discussion
4. Materials and Methods
4.1. sGAG Synthesis
4.2. Preparation and Coating of the PCL Scaffolds
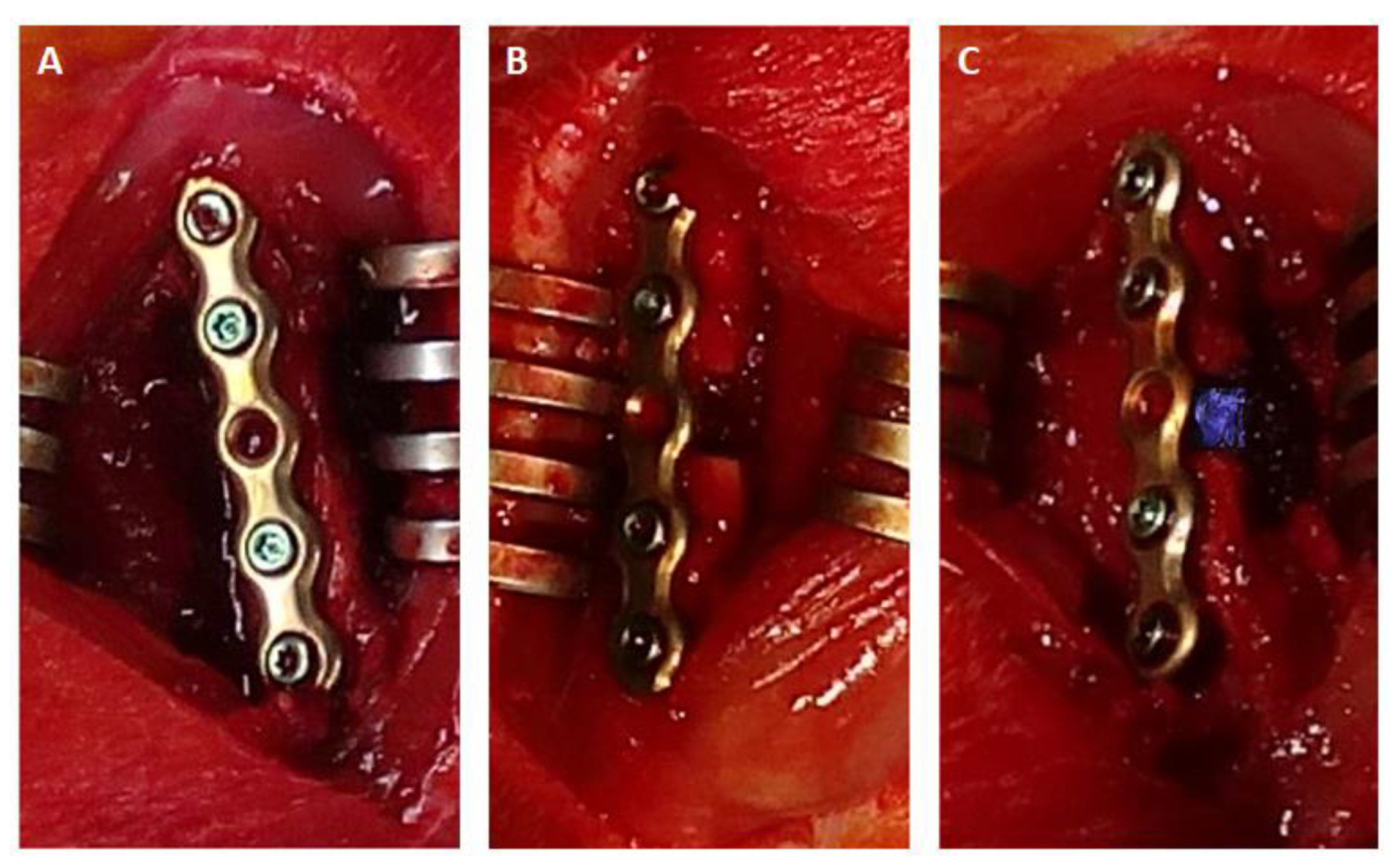
4.3. Animal Surgery
4.4. Microdialysis
4.5. Multiplex ELISA
4.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rothe, R.; Schulze, S.; Neuber, C.; Hauser, S.; Rammelt, S.; Pietzsch, J. Adjuvant Drug-Assisted Bone Healing: Part I—Modulation of Inflammation. Clin. Hemorheol. Microcirc. 2020, 73, 381–408. [Google Scholar] [CrossRef] [PubMed]
- Schulze, S.; Rothe, R.; Neuber, C.; Hauser, S.; Ullrich, M.; Pietzsch, J.; Rammelt, S. Men Who Stare at Bone: Multimodal Monitoring of Bone Healing. Biol. Chem. 2021, 402, 1397–1413. [Google Scholar] [CrossRef] [PubMed]
- Chaparro, O.; Linero, I. Regenerative Medicine: A New Paradigm in Bone Regeneration. In Advanced Techniques in Bone Regeneration; Zorzi, A.R., de Miranda, J.B., Eds.; IntechOpen Limited: London, UK, 2016; ISBN 978-953-51-2538-9. [Google Scholar]
- Schmidt-Bleek, K.; Kwee, B.J.; Mooney, D.J.; Duda, G.N. Boon and Bane of Inflammation in Bone Tissue Regeneration and Its Link with Angiogenesis. Tissue Eng. Part B Rev. 2015, 21, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Rammelt, S.; Illert, T.; Bierbaum, S.; Scharnweber, D.; Zwipp, H.; Schneiders, W. Coating of Titanium Implants with Collagen, RGD Peptide and Chondroitin Sulfate. Biomaterials 2006, 27, 5561–5571. [Google Scholar] [CrossRef] [PubMed]
- Kovtun, A.; Bergdolt, S.; Wiegner, R.; Radermacher, P.; Huber-Lang, M.; Ignatius, A. The Crucial Role of Neutrophil Granulocytes in Bone Fracture Healing. Eur. Cell Mater. 2016, 32, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Förster, Y.; Bernhardt, R.; Hintze, V.; Möller, S.; Schnabelrauch, M.; Scharnweber, D.; Rammelt, S. Collagen/Glycosaminoglycan Coatings Enhance New Bone Formation in a Critical Size Bone Defect—A Pilot Study in Rats. Mater. Sci. Eng. C 2017, 71, 84–92. [Google Scholar] [CrossRef]
- Picke, A.-K.; Salbach-Hirsch, J.; Hintze, V.; Rother, S.; Rauner, M.; Kascholke, C.; Möller, S.; Bernhardt, R.; Rammelt, S.; Pisabarro, M.T.; et al. Sulfated Hyaluronan Improves Bone Regeneration of Diabetic Rats by Binding Sclerostin and Enhancing Osteoblast Function. Biomaterials 2016, 96, 11–23. [Google Scholar] [CrossRef]
- Förster, Y.; Schulze, S.; Penk, A.; Neuber, C.; Möller, S.; Hintze, V.; Scharnweber, D.; Schnabelrauch, M.; Pietzsch, J.; Huster, D.; et al. The Influence of Different Artificial Extracellular Matrix Implant Coatings on the Regeneration of a Critical Size Femur Defect in Rats. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 116, 111157. [Google Scholar] [CrossRef]
- Vogel, S.; Arnoldini, S.; Möller, S.; Schnabelrauch, M.; Hempel, U. Sulfated Hyaluronan Alters Fibronectin Matrix Assembly and Promotes Osteogenic Differentiation of Human Bone Marrow Stromal Cells. Sci. Rep. 2016, 6, 36418. [Google Scholar] [CrossRef]
- Hempel, U.; Preissler, C.; Vogel, S.; Möller, S.; Hintze, V.; Becher, J.; Schnabelrauch, M.; Rauner, M.; Hofbauer, L.C.; Dieter, P. Artificial Extracellular Matrices with Oversulfated Glycosaminoglycan Derivatives Promote the Differentiation of Osteoblast-Precursor Cells and Premature Osteoblasts. Biomed. Res. Int. 2014, 2014, 938368. [Google Scholar] [CrossRef]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The Extracellular Matrix at a Glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed]
- Franz, S.; Rammelt, S.; Scharnweber, D.; Simon, J.C. Immune Responses to Implants—A Review of the Implications for the Design of Immunomodulatory Biomaterials. Biomaterials 2011, 32, 6692–6709. [Google Scholar] [CrossRef] [PubMed]
- Förster, Y.; Gao, W.; Demmrich, A.; Hempel, U.; Hofbauer, L.C.; Rammelt, S. Monitoring of the First Stages of Bone Healing with Microdialysis. Acta Orthop. 2013, 84, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Förster, Y.; Schmidt, J.R.; Wissenbach, D.K.; Pfeiffer, S.E.M.; Baumann, S.; Hofbauer, L.C.; von Bergen, M.; Kalkhof, S.; Rammelt, S. Microdialysis Sampling from Wound Fluids Enables Quantitative Assessment of Cytokines, Proteins, and Metabolites Reveals Bone Defect-Specific Molecular Profiles. PLoS ONE 2016, 11, e0159580. [Google Scholar] [CrossRef]
- Dinarello, C.A. Overview of the IL-1 Family in Innate Inflammation and Acquired Immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef]
- Liu, H.; Li, D.; Zhang, Y.; Li, M. Inflammation, Mesenchymal Stem Cells and Bone Regeneration. Histochem. Cell Biol. 2018, 149, 393–404. [Google Scholar] [CrossRef]
- Thomas, M.V.; Puleo, D.A. Infection, Inflammation, and Bone Regeneration: A Paradoxical Relationship. J. Dent. Res. 2011, 90, 1052–1061. [Google Scholar] [CrossRef]
- Lohmann, N.; Schirmer, L.; Atallah, P.; Wandel, E.; Ferrer, R.A.; Werner, C.; Simon, J.C.; Franz, S.; Freudenberg, U. Glycosaminoglycan-Based Hydrogels Capture Inflammatory Chemokines and Rescue Defective Wound Healing in Mice. Sci. Transl. Med. 2017, 9, eaai9044. [Google Scholar] [CrossRef]
- Neuber, C.; Schulze, S.; Förster, Y.; Hofheinz, F.; Wodke, J.; Möller, S.; Schnabelrauch, M.; Hintze, V.; Scharnweber, D.; Rammelt, S.; et al. Biomaterials in Repairing Rat Femoral Defects: In Vivo Insights from Small Animal Positron Emission Tomography/Computed Tomography (PET/CT) Studies. Clin. Hemorheol. Microcirc. 2019, 73, 177–194. [Google Scholar] [CrossRef]
- Stadlinger, B.; Hintze, V.; Bierbaum, S.; Möller, S.; Schulz, M.C.; Mai, R.; Kuhlisch, E.; Heinemann, S.; Scharnweber, D.; Schnabelrauch, M.; et al. Biological Functionalization of Dental Implants with Collagen and Glycosaminoglycans—A Comparative Study. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 331–341. [Google Scholar] [CrossRef]
- Kessler, B.; Rinchai, D.; Kewcharoenwong, C.; Nithichanon, A.; Biggart, R.; Hawrylowicz, C.M.; Bancroft, G.J.; Lertmemongkolchai, G. Interleukin 10 Inhibits Pro-Inflammatory Cytokine Responses and Killing of Burkholderia Pseudomallei. Sci. Rep. 2017, 7, 42791. [Google Scholar] [CrossRef]
- Cheng, A.; Vantucci, C.E.; Krishnan, L.; Ruehle, M.A.; Kotanchek, T.; Wood, L.B.; Roy, K.; Guldberg, R.E. Early Systemic Immune Biomarkers Predict Bone Regeneration after Trauma. Proc. Natl. Acad. Sci. USA 2021, 118, e2017889118. [Google Scholar] [CrossRef]
- Wood, S.; Jayaraman, V.; Huelsmann, E.J.; Bonish, B.; Burgad, D.; Sivaramakrishnan, G.; Qin, S.; DiPietro, L.A.; Zloza, A.; Zhang, C.; et al. Pro-Inflammatory Chemokine CCL2 (MCP-1) Promotes Healing in Diabetic Wounds by Restoring the Macrophage Response. PLoS ONE 2014, 9, e91574. [Google Scholar] [CrossRef]
- Schlundt, C.; Schell, H.; Goodman, S.B.; Vunjak-Novakovic, G.; Duda, G.N.; Schmidt-Bleek, K. Immune Modulation as a Therapeutic Strategy in Bone Regeneration. J. Exp. Orthop. 2015, 2, 1. [Google Scholar] [CrossRef]
- Gibon, E.; Lu, L.Y.; Nathan, K.; Goodman, S.B. Inflammation, Ageing, and Bone Regeneration. J. Orthop. Transl. 2017, 10, 28–35. [Google Scholar] [CrossRef]
- Ishikawa, M.; Ito, H.; Kitaori, T.; Murata, K.; Shibuya, H.; Furu, M.; Yoshitomi, H.; Fujii, T.; Yamamoto, K.; Matsuda, S. MCP/CCR2 Signaling Is Essential for Recruitment of Mesenchymal Progenitor Cells during the Early Phase of Fracture Healing. PLoS ONE 2014, 9, e104954. [Google Scholar] [CrossRef]
- Maruyama, M.; Rhee, C.; Utsunomiya, T.; Zhang, N.; Ueno, M.; Yao, Z.; Goodman, S.B. Modulation of the Inflammatory Response and Bone Healing. Front. Endocrinol. 2020, 11, 386. [Google Scholar] [CrossRef]
- Ono, T.; Takayanagi, H. Osteoimmunology in Bone Fracture Healing. Curr. Osteoporos. Rep. 2017, 15, 367–375. [Google Scholar] [CrossRef]
- Wang, Q.; Shu, C.; Su, J.; Li, X. A Crosstalk Triggered by Hypoxia and Maintained by MCP-1/MiR-98/IL-6/P38 Regulatory Loop between Human Aortic Smooth Muscle Cells and Macrophages Leads to Aortic Smooth Muscle Cells Apoptosis via Stat1 Activation. Int. J. Clin. Exp. Pathol. 2015, 8, 2670–2679. [Google Scholar]
- Qin, C.-C.; Liu, Y.-N.; Hu, Y.; Yang, Y.; Chen, Z. Macrophage Inflammatory Protein-2 as Mediator of Inflammation in Acute Liver Injury. World J. Gastroenterol. 2017, 23, 3043–3052. [Google Scholar] [CrossRef]
- Lee, R.S.B.; Hamlet, S.M.; Ivanovski, S. The Influence of Titanium Surface Characteristics on Macrophage Phenotype Polarization during Osseous Healing in Type I Diabetic Rats: A Pilot Study. Clin. Oral. Implant. Res. 2017, 28, e159–e168. [Google Scholar] [CrossRef] [PubMed]
- Diab, A.; Abdalla, H.; Li, H.L.; Shi, F.D.; Zhu, J.; Höjberg, B.; Lindquist, L.; Wretlind, B.; Bakhiet, M.; Link, H. Neutralization of Macrophage Inflammatory Protein 2 (MIP-2) and MIP-1alpha Attenuates Neutrophil Recruitment in the Central Nervous System during Experimental Bacterial Meningitis. Infect. Immun. 1999, 67, 2590–2601. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Kim, T.-S.; Choi, Y.; Lorenzo, J. Osteoimmunology: Cytokines and the Skeletal System. BMB Rep. 2008, 41, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Kunze, R.; Rösler, M.; Möller, S.; Schnabelrauch, M.; Riemer, T.; Hempel, U.; Dieter, P. Sulfated Hyaluronan Derivatives Reduce the Proliferation Rate of Primary Rat Calvarial Osteoblasts. Glycoconj. J. 2010, 27, 151–158. [Google Scholar] [CrossRef] [PubMed]
- van der Smissen, A.; Hintze, V.; Scharnweber, D.; Moeller, S.; Schnabelrauch, M.; Majok, A.; Simon, J.C.; Anderegg, U. Growth Promoting Substrates for Human Dermal Fibroblasts Provided by Artificial Extracellular Matrices Composed of Collagen I and Sulfated Glycosaminoglycans. Biomaterials 2011, 32, 8938–8946. [Google Scholar] [CrossRef] [PubMed]
- Möller, S.; Schmidtke, M.; Weiss, D.; Schiller, J.; Pawlik, K.; Wutzler, P.; Schnabelrauch, M. Synthesis and Antiherpetic Activity of Carboxymethylated and Sulfated Hyaluronan Derivatives. Carbohydr. Polym. 2012, 90, 608–615. [Google Scholar] [CrossRef]
- Hempel, U.; Hintze, V.; Möller, S.; Schnabelrauch, M.; Scharnweber, D.; Dieter, P. Artificial Extracellular Matrices Composed of Collagen I and Sulfated Hyaluronan with Adsorbed Transforming Growth Factor Β1 Promote Collagen Synthesis of Human Mesenchymal Stromal Cells. Acta Biomater. 2012, 8, 659–666. [Google Scholar] [CrossRef]
- Rentsch, C.; Rentsch, B.; Heinemann, S.; Bernhardt, R.; Bischoff, B.; Förster, Y.; Scharnweber, D.; Rammelt, S. ECM Inspired Coating of Embroidered 3D Scaffolds Enhances Calvaria Bone Regeneration. BioMed. Res. Int. 2014, 2014, 1–15. [Google Scholar] [CrossRef]
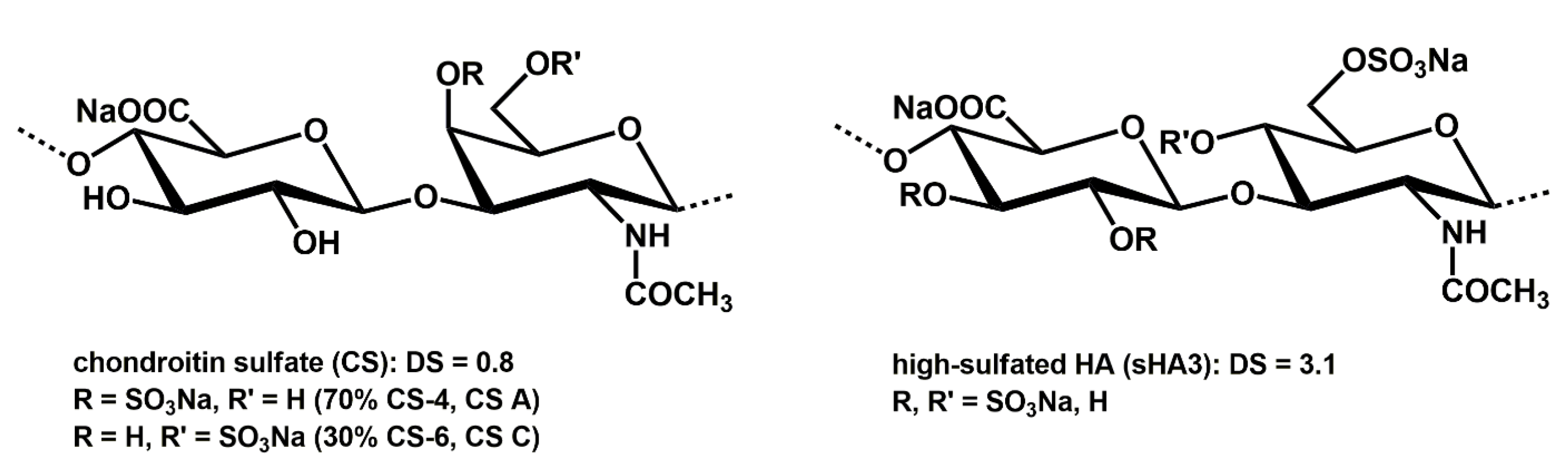
| Sample | CS | sHA3 |
|---|---|---|
| DS | 0.8 | 3.1 |
| Mn [g mol−1] | 18,653 (38,540) | 34,753 (46,845) |
| Mw [g mol−1] | 21,592 (60,434) | 51,268 (80,810) |
| PD | 1.6 | 1.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulze, S.; Neuber, C.; Möller, S.; Pietzsch, J.; Schaser, K.-D.; Rammelt, S. Microdialysis Reveals Anti-Inflammatory Effects of Sulfated Glycosaminoglycanes in the Early Phase of Bone Healing. Int. J. Mol. Sci. 2023, 24, 2077. https://doi.org/10.3390/ijms24032077
Schulze S, Neuber C, Möller S, Pietzsch J, Schaser K-D, Rammelt S. Microdialysis Reveals Anti-Inflammatory Effects of Sulfated Glycosaminoglycanes in the Early Phase of Bone Healing. International Journal of Molecular Sciences. 2023; 24(3):2077. https://doi.org/10.3390/ijms24032077
Chicago/Turabian StyleSchulze, Sabine, Christin Neuber, Stephanie Möller, Jens Pietzsch, Klaus-Dieter Schaser, and Stefan Rammelt. 2023. "Microdialysis Reveals Anti-Inflammatory Effects of Sulfated Glycosaminoglycanes in the Early Phase of Bone Healing" International Journal of Molecular Sciences 24, no. 3: 2077. https://doi.org/10.3390/ijms24032077
APA StyleSchulze, S., Neuber, C., Möller, S., Pietzsch, J., Schaser, K.-D., & Rammelt, S. (2023). Microdialysis Reveals Anti-Inflammatory Effects of Sulfated Glycosaminoglycanes in the Early Phase of Bone Healing. International Journal of Molecular Sciences, 24(3), 2077. https://doi.org/10.3390/ijms24032077







