Open-Spaced Ridged Hydrogel Scaffolds Containing TiO2-Self-Assembled Monolayer of Phosphonates Promote Regeneration and Recovery Following Spinal Cord Injury
Abstract
1. Introduction
2. Results
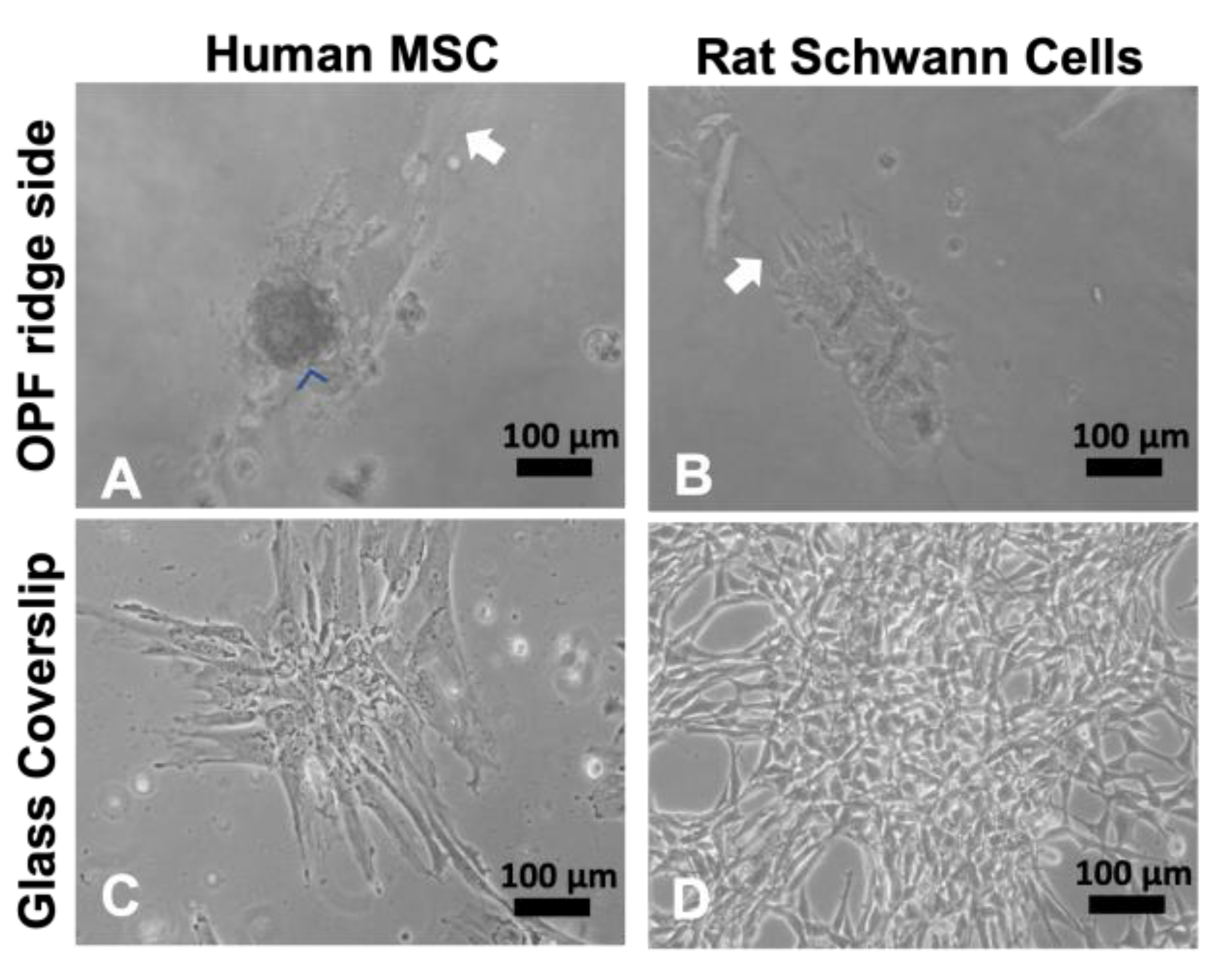
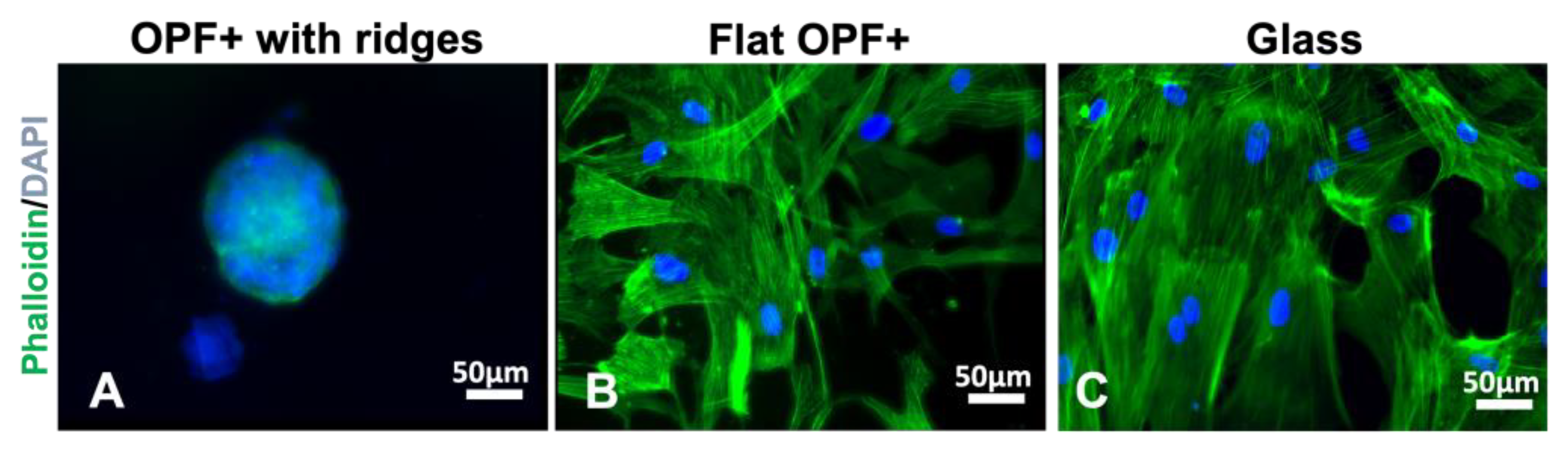
2.1. Cultured Mesenchymal Stromal Cells form Spheres on Ridged Surfaces
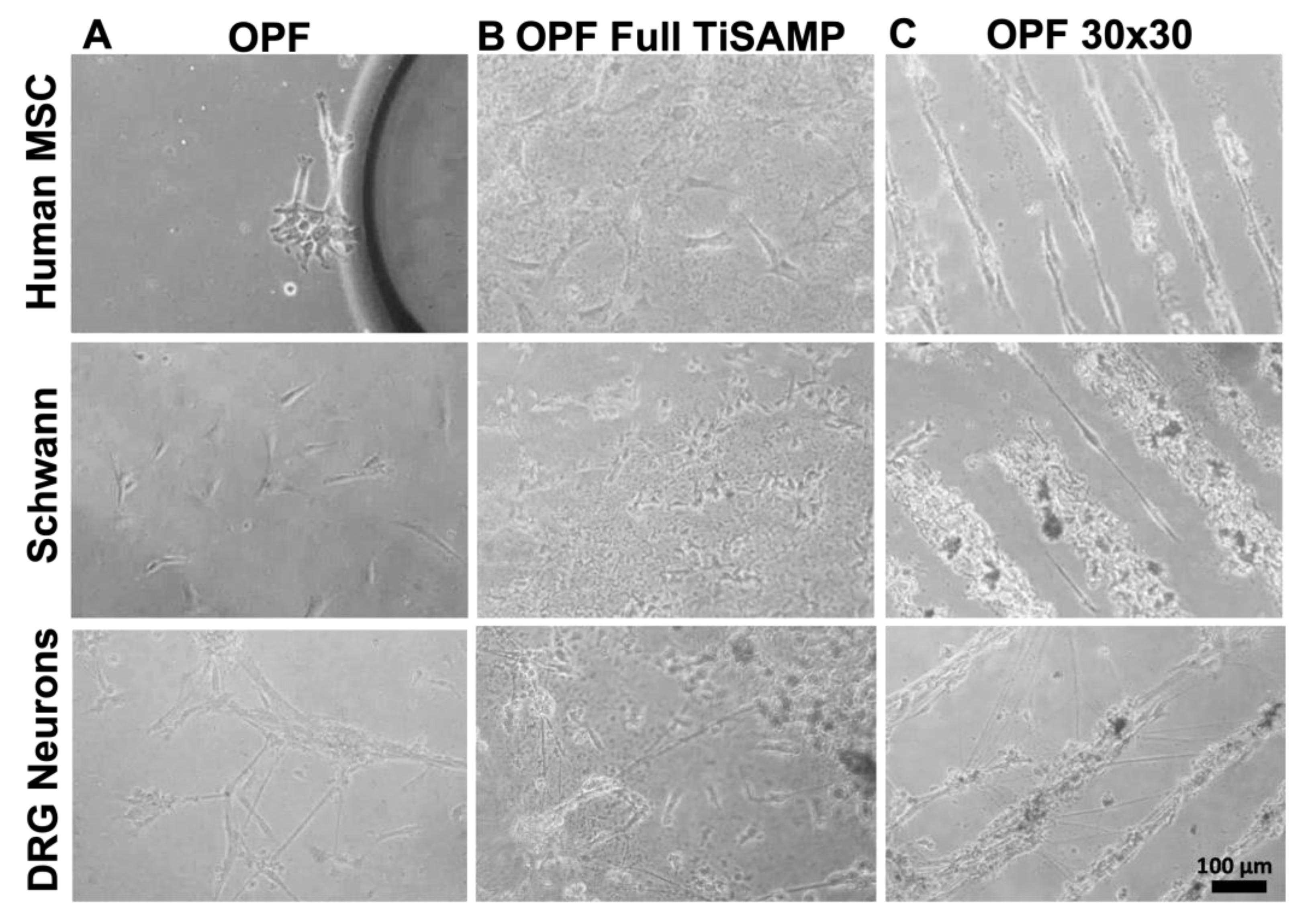
2.2. Mesenchymal Stromal Cells, Schwann Cells, and DRG Neurons Attach to TiSAMP and Extend along the Chemical Pattern
2.3. Rolled Scaffolds Have Equal Number of Hemopoietic Cells, Microglia, and Other Cell Types as Multichannel Scaffolds
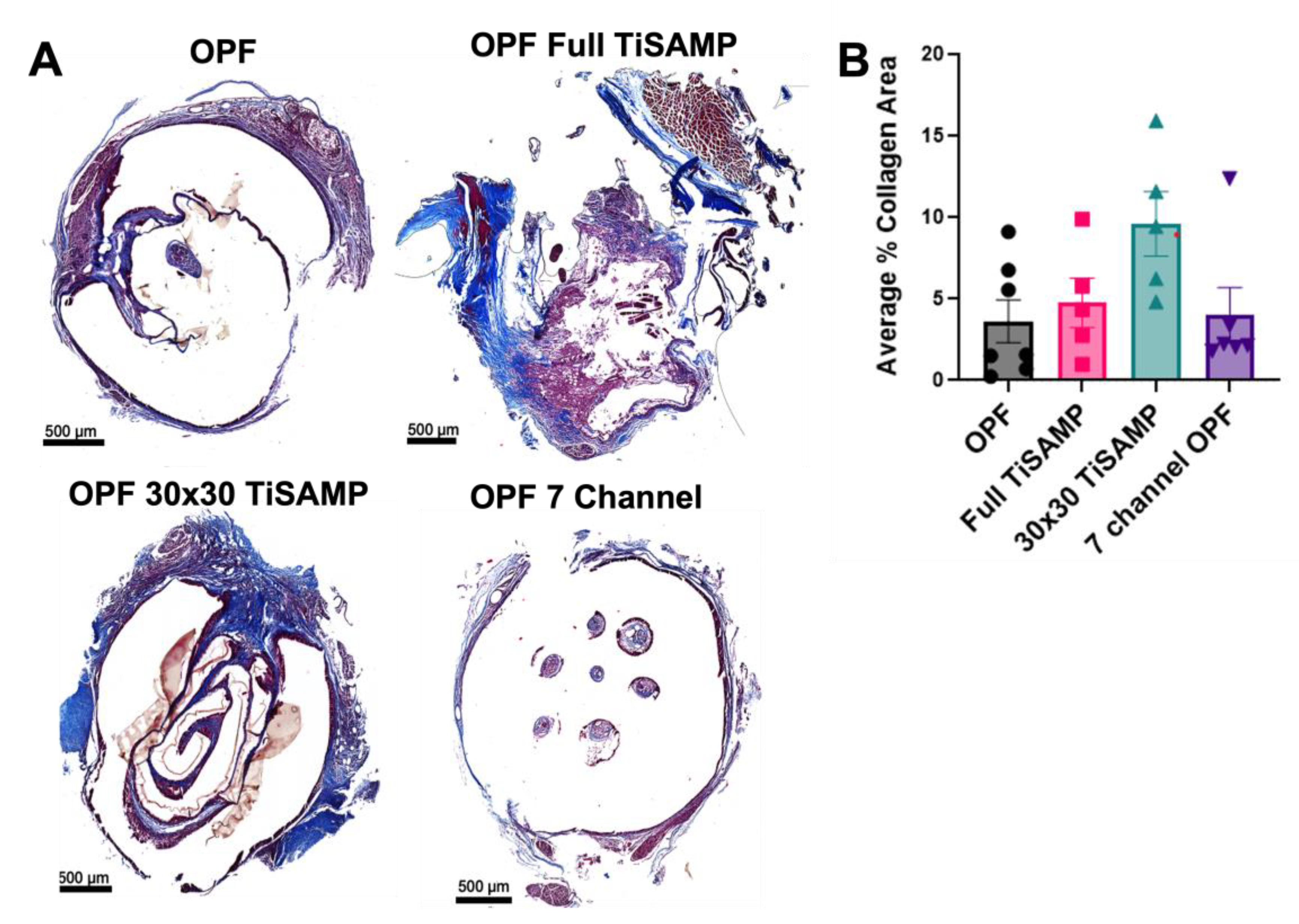
2.4. Rolled Scaffolds Have Equivalent Deposits of Collagen, Laminin, and Fibronectin as Those in Multichannel Scaffolds
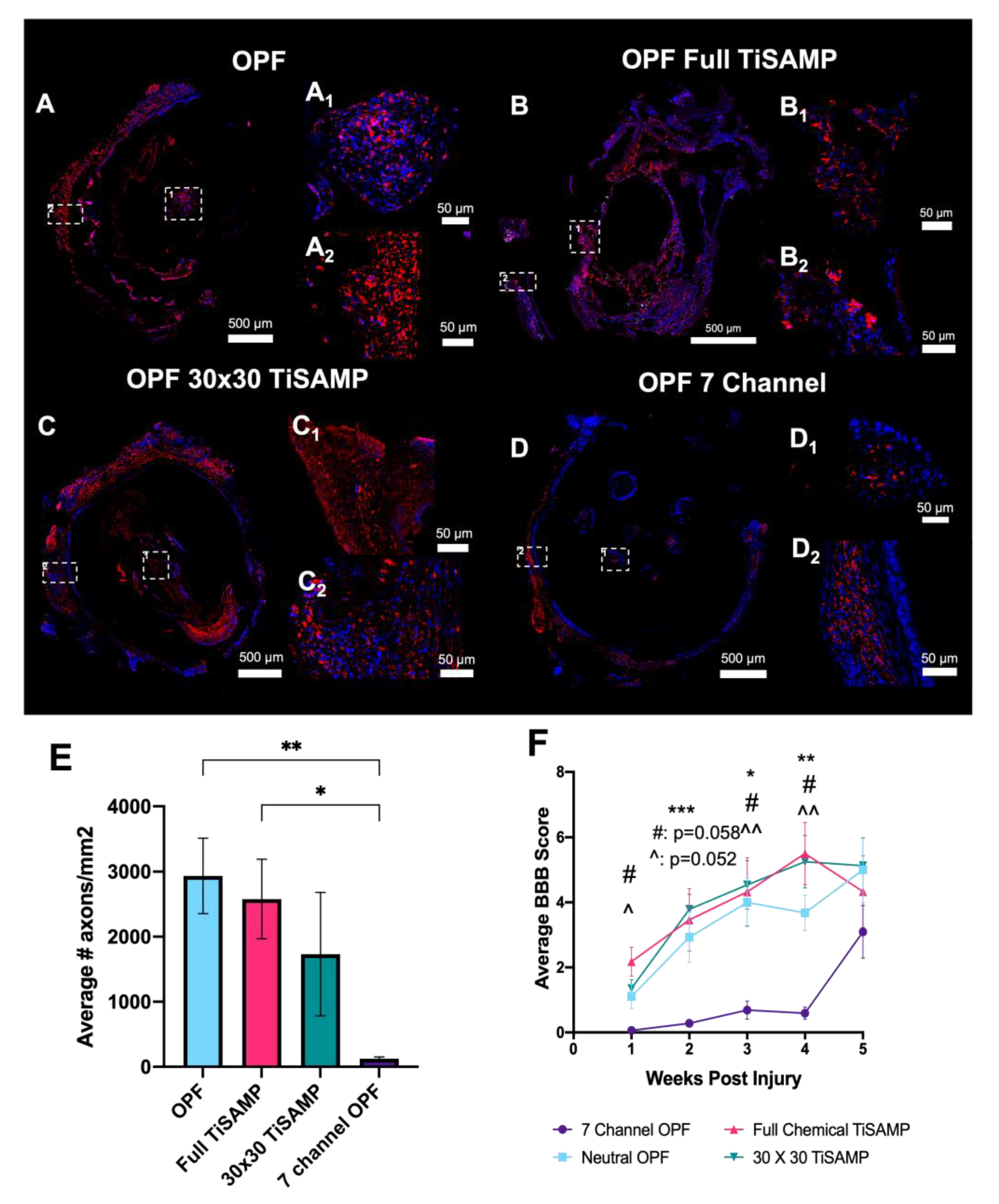
2.5. Implantation of Rolled Scaffolds Results in More Axon Growth through the Scaffold and Improved Hindlimb Function Compared to Those of Multichannel Scaffolds
3. Discussion
4. Conclusions
5. Methods
5.1. OPF Sheet Fabrication
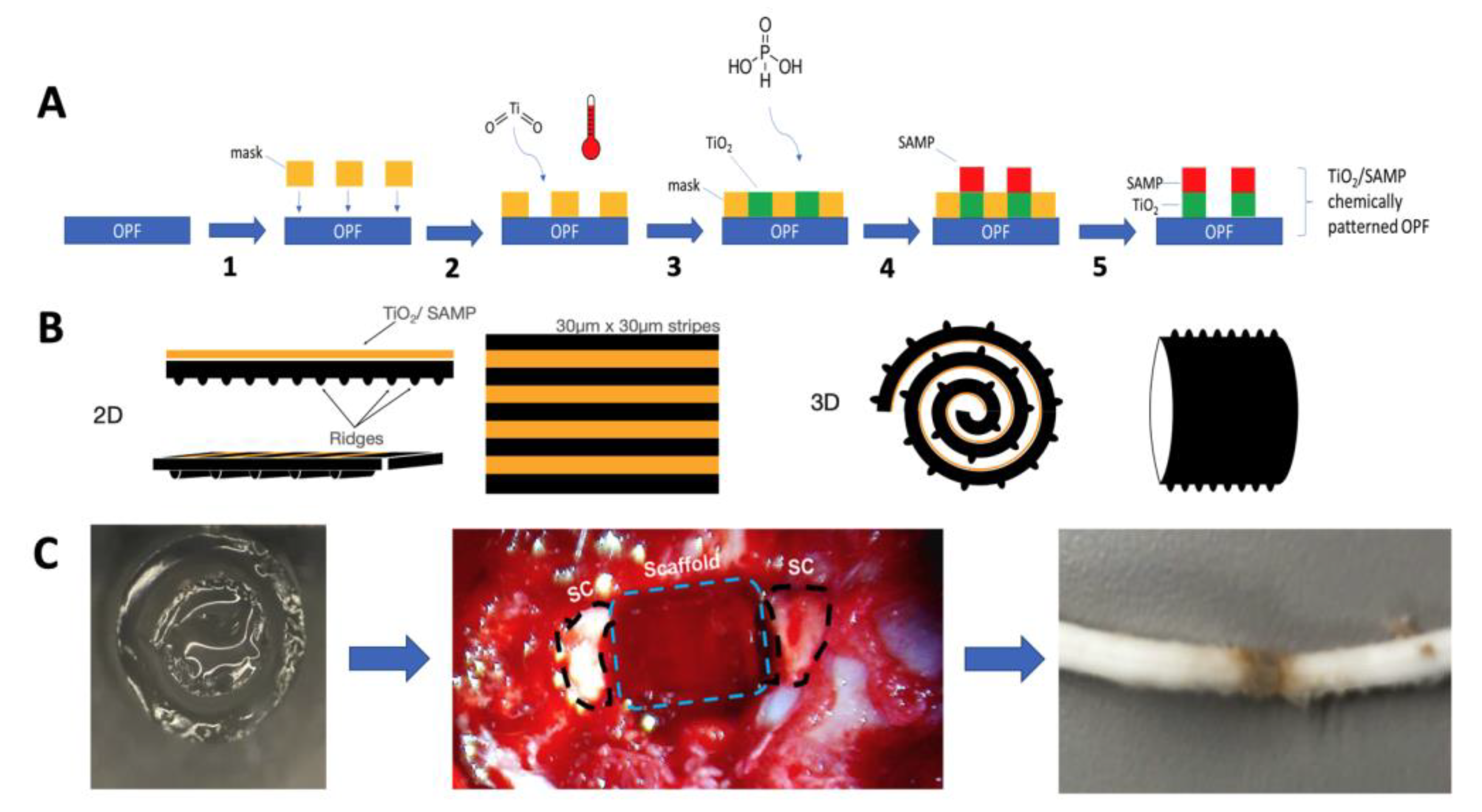
5.2. OPF Polymer Chemical Micropatterning
5.3. Human MSC Isolation and Culture
5.4. Rat Schwann Cell Isolation and Culture
5.5. Co-Culture hMSC and Schwann Cells
5.6. Disassociated Rat Dorsal Root Ganglion Neurons
5.7. OPF Preparation and Cell Seeding
5.8. Immunocytochemistry of Mincultured Cells on Scaffolds
5.9. Image Acquisition for Scaffold Sheets
5.10. Analysis of Laminin and Fibronectin Deposit by Schwann Cells and MSCs on Scaffolds in Culture
5.11. Scaffold Preparation and Surgical Implantation
5.12. Basso, Beattie, and Bresnahan (BBB) Locomotor Score
5.13. Tissue Preparation and Immunohistochemistry
5.14. Trichrome Staining
5.15. Image Acquisition
5.16. Axon Count
5.17. Quantification of Laminin and Fibronectin Components of the ECM in Implanted Scaffolds
5.18. Machine Learning Analysis of Immune Cell Infiltration and Fibrotic Scarring
5.19. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siddiqui, A.M.; Ahuja, C.S.; Tator, C.H.; Fehlings, M.G. Chapter 3: Spinal cord protective and regenerative therapies. In Neurotrauma and Critical Care of the Spine, 2nd ed.; Jallo, J., Vaccaro, A., Eds.; Thieme: New York, NY, USA, 2018; pp. 12–30. [Google Scholar]
- Madigan, N.N.; McMahon, S.; O’Brien, T.; Yaszemski, M.J.; Windebank, A.J. Current tissue engineering and novel therapeutic approaches to axonal regeneration following spinal cord injury using polymer scaffolds. Respir. Physiol. Neurobiol. 2009, 169, 183–199. [Google Scholar] [CrossRef] [PubMed]
- Putra, R.U.; Basri, H.; Prakoso, A.T.; Chandra, H.; Ammarullah, M.I.; Akbar, I.; Syahrom, A.; Kamarul, T. Level of Activity Changes Increases the Fatigue Life of the Porous Magnesium Scaffold, as Observed in Dynamic Immersion Tests, over Time. Sustainability 2023, 15, 823. [Google Scholar] [CrossRef]
- Madigan, N.N.; Windebank, A.J. Spinal cord injury. In Principles of Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1047–1091. [Google Scholar]
- Wong, D.Y.; Leveque, J.C.; Brumblay, H.; Krebsbach, P.H.; Hollister, S.J.; Lamarca, F. Macro-architectures in spinal cord scaffold implants influence regeneration. J. Neurotrauma 2008, 25, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.J.; Friedman, J.A.; Lewellyn, E.B.; Mantila, S.M.; Krych, A.J.; Ameenuddin, S.; Knight, A.M.; Lu, L.; Currier, B.L.; Spinner, R.J.; et al. Multiple-channel scaffolds to promote spinal cord axon regeneration. Biomaterials 2006, 27, 419–429. [Google Scholar] [CrossRef]
- Stokols, S.; Sakamoto, J.; Breckon, C.; Holt, T.; Weiss, J.; Tuszynski, M.H. Templated agarose scaffolds support linear axonal regeneration. Tissue Eng. 2006, 12, 2777–2787. [Google Scholar] [CrossRef] [PubMed]
- Krych, A.J.; Rooney, G.E.; Chen, B.; Schermerhorn, T.C.; Ameenuddin, S.; Gross, L.; Moore, M.J.; Currier, B.L.; Spinner, R.J.; Friedman, J.A.; et al. Relationship between scaffold channel diameter and number of regenerating axons in the transected rat spinal cord. Acta Biomater. 2009, 5, 2551–2559. [Google Scholar] [CrossRef]
- Koffler, J.; Zhu, W.; Qu, X.; Platoshyn, O.; Dulin, J.N.; Brock, J.; Graham, L.; Lu, P.; Sakamoto, J.; Marsala, M. Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nat. Med. 2019, 25, 263. [Google Scholar] [CrossRef]
- Winter, C.C.; Katiyar, K.S.; Hernandez, N.S.; Song, Y.J.; Struzyna, L.A.; Harris, J.P.; Cullen, D.K. Transplantable living scaffolds comprised of micro-tissue engineered aligned astrocyte networks to facilitate central nervous system regeneration. Acta Biomater. 2016, 38, 44–58. [Google Scholar] [CrossRef]
- Agrawal, L.; Saidani, M.; Guillaud, L.; Terenzio, M. Development of 3D culture scaffolds for directional neuronal growth using 2-photon lithography. Mater. Sci. Eng. C 2021, 131, 112502. [Google Scholar] [CrossRef]
- Bryant, S.J.; Cuy, J.L.; Hauch, K.D.; Ratner, B.D. Photo-patterning of porous hydrogels for tissue engineering. Biomaterials 2007, 28, 2978–2986. [Google Scholar] [CrossRef]
- Siddiqui, A.M.; Brunner, R.; Harris, G.M.; Miller, A.L., 2nd; Waletzki, B.E.; Schmeichel, A.M.; Schwarzbauer, J.E.; Schwartz, J.; Yaszemski, M.J.; Windebank, A.J.; et al. Promoting Neuronal Outgrowth Using Ridged Scaffolds Coated with Extracellular Matrix Proteins. Biomedicines 2021, 9, 479. [Google Scholar] [CrossRef]
- Miller, C.; Jeftinija, S.; Mallapragada, S. Micropatterned Schwann cell-seeded biodegradable polymer substrates significantly enhance neurite alignment and outgrowth. Tissue Eng. 2001, 7, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Di Luca, A.; de Wijn, J.R.; van Blitterswijk, C.A.; Camarero-Espinosa, S.; Moroni, L. Tailorable surface morphology of 3D scaffolds by combining additive manufacturing with thermally induced phase separation. Macromol. Rapid Commun. 2017, 38, 1700186. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Kim, K.-H.; Lee, Y.-M.; Giannobile, W.V.; Seol, Y.-J. 3D Printed, Microgroove Pattern-Driven Generation of Oriented Ligamentous Architectures. Int. J. Mol. Sci. 2017, 18, 1927. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.W.; Lim, K.; Bandini, S.B.; Harris, G.M.; Spechler, J.A.; Arnold, C.B.; Fardel, R.; Schwarzbauer, J.E.; Schwartz, J. Controlling the Surface Chemistry of a Hydrogel for Spatially Defined Cell Adhesion. ACS Appl. Mater. Interfaces 2019, 11, 15411–15416. [Google Scholar] [CrossRef]
- Harris, G.M.; Madigan, N.N.; Lancaster, K.Z.; Enquist, L.W.; Windebank, A.J.; Schwartz, J.; Schwarzbauer, J.E. Nerve Guidance by a Decellularized Fibroblast Extracellular Matrix. Matrix Biol. 2017, 60–61, 176–189. [Google Scholar] [CrossRef]
- Ratner, B.D. A pore way to heal and regenerate: 21st century thinking on biocompatibility. Regen. Biomater. 2016, 3, 107–110. [Google Scholar] [CrossRef]
- Homsy, C.A. Bio-Compatibility in selection of materials for implantation. J. Biomed. Mater. Res. 1970, 4, 341–356. [Google Scholar] [CrossRef]
- Silva, M.; Cyster, L.; Barry, J.; Yang, X.; Oreffo, R.; Grant, D.; Scotchford, C.; Howdle, S.; Shakesheff, K.; Rose, F. The effect of anisotropic architecture on cell and tissue infiltration into tissue engineering scaffolds. Biomaterials 2006, 27, 5909–5917. [Google Scholar] [CrossRef]
- Thevenot, P.T.; Nair, A.M.; Shen, J.; Lotfi, P.; Ko, C.-Y.; Tang, L. The effect of incorporation of SDF-1α into PLGA scaffolds on stem cell recruitment and the inflammatory response. Biomaterials 2010, 31, 3997–4008. [Google Scholar] [CrossRef]
- Mountziaris, P.M.; Mikos, A.G. Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Eng. Part B Rev. 2008, 14, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.; Zhong, Y.; Hu, H.; Shao, D.; Haag, R.; Schirner, M.; Lee, J.; Sullenger, B.; Leong, K.W. Design of therapeutic biomaterials to control inflammation. Nat. Rev. Mater. 2022, 7, 557–574. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Agrawal, D.K.; Thankam, F.G. Biomaterials-driven sterile inflammation. Tissue Eng. B Rev. 2022, 28, 22–34. [Google Scholar] [CrossRef]
- Zindel, J.; Kubes, P. DAMPs, PAMPs, and LAMPs in immunity and sterile inflammation. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 493–518. [Google Scholar] [CrossRef]
- Dadsetan, M.; Szatkowski, J.P.; Yaszemski, M.J.; Lu, L. Characterization of photo-cross-linked oligo[poly(ethylene glycol) fumarate] hydrogels for cartilage tissue engineering. Biomacromolecules 2007, 8, 1702–1709. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.K.; Knight, A.M.; Madigan, N.N.; Gross, L.; Dadsetan, M.; Nesbitt, J.J.; Rooney, G.E.; Currier, B.L.; Yaszemski, M.J.; Spinner, R.J.; et al. Comparison of polymer scaffolds in rat spinal cord: A step toward quantitative assessment of combinatorial approaches to spinal cord repair. Biomaterials 2011, 32, 8077–8086. [Google Scholar] [CrossRef] [PubMed]
- Hakim, J.S.; Esmaeili Rad, M.; Grahn, P.J.; Chen, B.K.; Knight, A.M.; Schmeichel, A.M.; Isaq, N.A.; Dadsetan, M.; Yaszemski, M.J.; Windebank, A.J. Positively Charged Oligo[Poly(Ethylene Glycol) Fumarate] Scaffold Implantation Results in a Permissive Lesion Environment after Spinal Cord Injury in Rat. Tissue Eng. A 2015, 21, 2099–2114. [Google Scholar] [CrossRef]
- Rooney, G.E.; Knight, A.M.; Madigan, N.N.; Gross, L.; Chen, B.; Giraldo, C.V.; Seo, S.; Nesbitt, J.J.; Dadsetan, M.; Yaszemski, M.J.; et al. Sustained Delivery of Dibutyryl Cyclic Adenosine Monophosphate to the Transected Spinal Cord Via Oligo [(Polyethylene Glycol) Fumarate] Hydrogels. Tissue Eng A 2011, 17, 1287–1302. [Google Scholar] [CrossRef]
- Runge, M.B.; Dadsetan, M.; Baltrusaitis, J.; Ruesink, T.; Lu, L.; Windebank, A.J.; Yaszemski, M.J. Development of Electrically Conductive Oligo(polyethylene glycol) Fumarate-Polypyrrole Hydrogels for Nerve Regeneration. Biomacromolecules 2010, 11, 2845–2853. [Google Scholar] [CrossRef]
- Madigan, N.N.; Chen, B.K.; Knight, A.M.; Rooney, G.E.; Sweeney, E.; Kinnavane, L.; Yaszemski, M.J.; Dockery, P.; O’Brien, T.; McMahon, S.S.; et al. Comparison of cellular architecture, axonal growth, and blood vessel formation through cell-loaded polymer scaffolds in the transected rat spinal cord. Tissue Eng. A 2014, 20, 2985–2997. [Google Scholar] [CrossRef]
- Siddiqui, A.M.; Oswald, D.; Papamichalopoulos, S.; Kelly, D.; Summer, P.; Polzin, M.; Hakim, J.; Schmeichel, A.M.; Chen, B.; Yaszemski, M.J.; et al. Defining spatial relationships between spinal cord axons and blood vessels in hydrogel scaffolds. Tissue Eng. A 2021, 27, 648–664. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.M.; Islam, R.; Cuellar, C.A.; Silvernail, J.L.; Knudsen, B.; Curley, D.E.; Strickland, T.; Manske, E.; Suwan, P.T.; Latypov, T.; et al. Newly regenerated axons via scaffolds promote sub-lesional reorganization and motor recovery with epidural electrical stimulation. NPJ Regen. Med. 2021, 6, 66. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.K.; Madigan, N.N.; Hakim, J.S.; Dadsetan, M.; McMahon, S.S.; Yaszemski, M.J.; Windebank, A.J. GDNF Schwann cells in hydrogel scaffolds promote regional axon regeneration, remyelination and functional improvement after spinal cord transection in rats. J. Tissue Eng. Regen. Med. 2018, 12, e398–e407. [Google Scholar] [CrossRef] [PubMed]
- Carnicer-Lombarte, A.; Chen, S.-T.; Malliaras, G.G.; Barone, D.G. Foreign Body Reaction to Implanted Biomaterials and Its Impact in Nerve Neuroprosthetics. Front. Bioeng. Biotechnol. 2021, 9, 271. [Google Scholar] [CrossRef] [PubMed]
- Hakim, J.S.; Rodysill, B.R.; Chen, B.K.; Schmeichel, A.M.; Yaszemski, M.J.; Windebank, A.J.; Madigan, N.N. Combinatorial tissue engineering partially restores function after spinal cord injury. J. Tissue Eng. Regen. Med. 2019, 13, 857–873. [Google Scholar] [CrossRef] [PubMed]
- Krupa, P.; Siddiqui, A.M.; Grahn, P.J.; Islam, R.; Chen, B.K.; Madigan, N.N.; Windebank, A.J.; Lavrov, I.A. The Translesional Spinal Network and Its Reorganization after Spinal Cord Injury. Neuroscientist 2022, 28, 163–179. [Google Scholar] [CrossRef]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in regenerative medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef]
- Gombotz, W.R.; Wang, G.H.; Horbett, T.A.; Hoffman, A.S. Protein adsorption to poly(ethylene oxide) surfaces. J. Biomed. Mater. Res. 1991, 25, 1547–1562. [Google Scholar] [CrossRef]
- Liu, S.; Xie, Y.Y.; Wang, B. Role and prospects of regenerative biomaterials in the repair of spinal cord injury. Neural Regen. Res. 2019, 14, 1352–1363. [Google Scholar]
- Li, G.; Che, M.T.; Zhang, K.; Qin, L.N.; Zhang, Y.T.; Chen, R.Q.; Rong, L.M.; Liu, S.; Ding, Y.; Shen, H.Y.; et al. Graft of the NT-3 persistent delivery gelatin sponge scaffold promotes axon regeneration, attenuates inflammation, and induces cell migration in rat and canine with spinal cord injury. Biomaterials 2016, 83, 233–248. [Google Scholar] [CrossRef]
- Huang, L.; Wang, Y.; Zhu, M.; Wan, X.; Zhang, H.; Lei, T.; Blesch, A.; Liu, S. Anisotropic Alginate Hydrogels Promote Axonal Growth across Chronic Spinal Cord Transections after Scar Removal. ACS Biomater. Sci. Eng. 2020, 6, 2274–2286. [Google Scholar] [CrossRef] [PubMed]
- Gros, T.; Sakamoto, J.S.; Blesch, A.; Havton, L.A.; Tuszynski, M.H. Regeneration of long-tract axons through sites of spinal cord injury using templated agarose scaffolds. Biomaterials 2010, 31, 6719–6729. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cheng, T.; Chen, Y.; Gao, F.; Guan, F.; Yao, M. A chitosan-based thermosensitive scaffold loaded with bone marrow-derived mesenchymal stem cells promotes motor function recovery in spinal cord injured mice. Biomed. Mater. 2020, 15, 035020. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.Z.; Wang, D.H.; Cherng, J.H.; Wang, Y.W.; Fan, G.Y.; Liou, N.H.; Liu, J.C.; Chou, C.H. A Collagen-Based Scaffold for Promoting Neural Plasticity in a Rat Model of Spinal Cord Injury. Polymers 2020, 12, 2245. [Google Scholar] [CrossRef] [PubMed]
- Kushchayev, S.V.; Giers, M.B.; Hom Eng, D.; Martirosyan, N.L.; Eschbacher, J.M.; Mortazavi, M.M.; Theodore, N.; Panitch, A.; Preul, M.C. Hyaluronic acid scaffold has a neuroprotective effect in hemisection spinal cord injury. J. Neurosurg. Spine 2016, 25, 114–124. [Google Scholar] [CrossRef]
- Cao, Z.; Yao, S.; Xiong, Y.; Zhang, Z.; Yang, Y.; He, F.; Zhao, H.; Guo, Y.; Wang, G.; Xie, S.; et al. Directional axonal regrowth induced by an aligned fibrin nanofiber hydrogel contributes to improved motor function recovery in canine L2 spinal cord injury. J. Mater. Sci. Mater. Med. 2020, 31, 40. [Google Scholar] [CrossRef]
- Jarrah, R.; Sammak, S.E.; Onyedimma, C.; Ghaith, A.K.; Moinuddin, F.M.; Bhandarkar, A.R.; Siddiqui, A.; Madigan, N.; Bydon, M. The Role of Alginate Hydrogels as a Potential Treatment Modality for Spinal Cord Injury: A Comprehensive Review of the Literature. Neurospine 2022, 19, 272–280. [Google Scholar] [CrossRef]
- Libro, R.; Bramanti, P.; Mazzon, E. The combined strategy of mesenchymal stem cells and tissue-engineered scaffolds for spinal cord injury regeneration. Exp. Ther. Med. 2017, 14, 3355–3368. [Google Scholar] [CrossRef]
- Subramanian, A.; Krishnan, U.M.; Sethuraman, S. Development of biomaterial scaffold for nerve tissue engineering: Biomaterial mediated neural regeneration. J. Biomed. Sci. 2009, 16, 108. [Google Scholar] [CrossRef]
- Conova, L.; Vernengo, J.; Jin, Y.; Himes, B.T.; Neuhuber, B.; Fischer, I.; Lowman, A.; Vernengo, J.; Jin, Y.; Himes, B.T.; et al. A pilot study of poly(N-isopropylacrylamide)-g-polyethylene glycol and poly(N-isopropylacrylamide)-g-methylcellulose branched copolymers as injectable scaffolds for local delivery of neurotrophins and cellular transplants into the injured spinal cord. J. Neurosurg. Spine 2011, 15, 594–604. [Google Scholar] [CrossRef]
- Sun, F.; Shi, T.; Zhou, T.; Dong, D.; Xie, J.; Wang, R.; An, X.; Chen, M.; Cai, J. 3D Poly(Lactic-co-glycolic acid) Scaffolds for Treating Spinal Cord Injury. J. Biomed. Nanotechnol. 2017, 13, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Raynald; Shu, B.; Liu, X.B.; Zhou, J.F.; Huang, H.; Wang, J.Y.; Sun, X.D.; Qin, C.; An, Y.H. Polypyrrole/polylactic acid nanofibrous scaffold cotransplanted with bone marrow stromal cells promotes the functional recovery of spinal cord injury in rats. CNS Neurosci. Ther. 2019, 25, 951–964. [Google Scholar] [CrossRef]
- Li, H.Y.; Führmann, T.; Zhou, Y.; Dalton, P.D. Host reaction to poly(2-hydroxyethyl methacrylate) scaffolds in a small spinal cord injury model. J. Mater. Sci. Mater. Med. 2013, 24, 2001–2011. [Google Scholar] [CrossRef] [PubMed]
- Altinova, H.; Hammes, S.; Palm, M.; Gerardo-Nava, J.; Achenbach, P.; Deumens, R.; Hermans, E.; Führmann, T.; Boecker, A.; van Neerven, S.G.A.; et al. Fibroadhesive scarring of grafted collagen scaffolds interferes with implant-host neural tissue integration and bridging in experimental spinal cord injury. Regen. Biomater. 2019, 6, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Chedly, J.; Soares, S.; Montembault, A.; Von Boxberg, Y.; Veron-Ravaille, M.; Mouffle, C.; Benassy, M.-N.; Taxi, J.; David, L.; Nothias, F. Physical chitosan microhydrogels as scaffolds for spinal cord injury restoration and axon regeneration. Biomaterials 2017, 138, 91–107. [Google Scholar] [CrossRef]
- Zamani, F.; Amani-Tehran, M.; Latifi, M.; Shokrgozar, M.; Zaminy, A. Promotion of spinal cord axon regeneration by 3D nanofibrous core–sheath scaffolds. J. Biomed. Mater. Res. A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2014, 102, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Badner, A.; Siddiqui, A.M.; Fehlings, M.G. Spinal cord injuries: How could cell therapy help? Expert. Opin. Biol. Ther. 2017, 17, 529–541. [Google Scholar] [CrossRef]
- Moinuddin, F.M.; Yolcu, Y.U.; Wahood, W.; Siddiqui, A.M.; Chen, B.K.; Alvi, M.A.; Goyal, A.; Nesbitt, J.J.; Windebank, A.J.; Yeh, J.C.; et al. Early and sustained improvements in motor function in rats after infusion of allogeneic umbilical cord-derived mesenchymal stem cells following spinal cord injury. Spinal Cord 2020, 59, 319–327. [Google Scholar] [CrossRef]
- Schinköthe, T.; Bloch, W.; Schmidt, A. In Vitro Secreting Profile of Human Mesenchymal Stem Cells. Stem Cells Dev. 2008, 17, 199–206. [Google Scholar] [CrossRef]
- Buron, F.; Perrin, H.; Malcus, C.; Héquet, O.; Thaunat, O.; Kholopp-Sarda, M.N.; Moulin, F.T.; Morelon, E. Human Mesenchymal Stem Cells and Immunosuppressive Drug Interactions in Allogeneic Responses: An In Vitro Study Using Human Cells. Transplant. Proc. 2009, 41, 3347–3352. [Google Scholar] [CrossRef]
- Redondo-Castro, E.; Cunningham, C.; Miller, J.; Martuscelli, L.; Aoulad-Ali, S.; Rothwell, N.J.; Kielty, C.M.; Allan, S.M.; Pinteaux, E. Interleukin-1 primes human mesenchymal stem cells towards an anti-inflammatory and pro-trophic phenotype in vitro. Stem Cell Res. Ther. 2017, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Leuning, D.G.; Beijer, N.R.; Fossé, N.A.; Vermeulen, S.; Lievers, E.; Kooten, C.; Rabelink, T.J.; de Boer, J. The cytokine secretion profile of mesenchymal stromal cells is determined by surface structure of the microenvironment. Sci. Rep. 2018, 8, 7716. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Chi, G.; Li, P.; Lv, S.; Xu, J.; Xu, Z.; Xia, Y.; Tan, Y.; Xu, J.; Li, L.; et al. Effects of Matrix Stiffness on the Morphology, Adhesion, Proliferation and Osteogenic Differentiation of Mesenchymal Stem Cells. Int. J. Med. Sci. 2018, 15, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Peng, J.; Guo, Q.; Zhang, L.; Li, Z.; Zhao, B.; Sui, X.; Wang, Y.; Xu, W.; Lu, S. Improvement of peripheral nerve regeneration in acellular nerve grafts with local release of nerve growth factor. Microsurgery 2009, 29, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Bunge, M.B.; Wood, P.M. Realizing the maximum potential of Schwann cells to promote recovery from spinal cord injury. Handb. Clin. Neurol. 2012, 109, 523–540. [Google Scholar] [PubMed]
- Gu, Y.; Ji, Y.; Zhao, Y.; Liu, Y.; Ding, F.; Gu, X.; Yang, Y. The influence of substrate stiffness on the behavior and functions of Schwann cells in culture. Biomaterials 2012, 33, 6672–6681. [Google Scholar] [CrossRef]
- Poitelon, Y.; Lopez-Anido, C.; Catignas, K.; Berti, C.; Palmisano, M.; Williamson, C.; Ameroso, D.; Abiko, K.; Hwang, Y.; Gregorieff, A.; et al. YAP and TAZ control peripheral myelination and the expression of laminin receptors in Schwann cells. Nat. Neurosci. 2016, 19, 879–887. [Google Scholar] [CrossRef]
- Urbanski, M.M.; Kingsbury, L.; Moussouros, D.; Kassim, I.; Mehjabeen, S.; Paknejad, N.; Melendez-Vasquez, C.V. Myelinating glia differentiation is regulated by extracellular matrix elasticity. Sci. Rep. 2016, 6, 33751. [Google Scholar] [CrossRef]
- Harris, G.M.; Raitman, I.; Schwarzbauer, J.E. Chapter 5—Cell-derived decellularized extracellular matrices. In Methods in Cell Biology; Mecham, R.P., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 143, pp. 97–114. [Google Scholar]
- Novoseletskaya, E.; Grigorieva, O.; Nimiritsky, P.; Basalova, N.; Eremichev, R.; Milovskaya, I.; Kulebyakin, K.; Kulebyakina, M.; Rodionov, S.; Omelyanenko, N.; et al. Mesenchymal Stromal Cell-Produced Components of Extracellular Matrix Potentiate Multipotent Stem Cell Response to Differentiation Stimuli. Front. Cell Dev. Biol. 2020, 8, 555378. [Google Scholar] [CrossRef]
- Liesi, P. Extracellular matrix and neuronal movement. Experientia 1990, 46, 900–907. [Google Scholar] [CrossRef]
- Calof, A.L.; Reichardt, L.F. Response of purified chick motoneurons to myotube conditioned medium: Laminin is essential for the substratum-binding, neurite outgrowth-promoting activity. Neurosci. Lett. 1985, 59, 183–189. [Google Scholar] [CrossRef] [PubMed]
- García-Alonso, L.; Fetter, R.D.; Goodman, C.S. Genetic analysis of Laminin A in Drosophila: Extracellular matrix containing laminin A is required for ocellar axon pathfinding. Development 1996, 122, 2611–2621. [Google Scholar] [CrossRef] [PubMed]
- Pankov, R.; Yamada, K.M. Fibronectin at a glance. J. Cell Sci. 2002, 115, 3861–3863. [Google Scholar] [CrossRef]
- Willis, C.M.; Crocker, S.J. The Mosaic of Extracellular Matrix in the Central Nervous System as a Determinant of Glial Heterogeneity. In Composition and Function of the Extracellular Matrix in the Human Body; Travascio, F., Ed.; InTech: London, UK, 2016. [Google Scholar]
- King, V.R.; Henseler, M.; Brown, R.A.; Priestley, J.V. Mats made from fibronectin support oriented growth of axons in the damaged spinal cord of the adult rat. Exp. Neurol. 2003, 182, 383–398. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, B.; Coykendall, K.; Li, Y.; Moon, A.; Priyadarshani, P.; Yao, L. Repair of injured spinal cord using biomaterial scaffolds and stem cells. Stem Cell Res. Ther. 2014, 5, 91. [Google Scholar] [CrossRef]
- Yang, F.; Murugan, R.; Wang, S.; Ramakrishna, S. Electrospinning of nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials 2005, 26, 2603–2610. [Google Scholar] [CrossRef]
- Arguchinskaya, N.V.; Isaeva, E.V.; Kisel, A.A.; Beketov, E.E.; Lagoda, T.S.; Baranovskii, D.S.; Yakovleva, N.D.; Demyashkin, G.A.; Komarova, L.N.; Astakhina, S.O.; et al. Properties and Printability of the Synthesized Hydrogel Based on GelMA. Int. J. Mol. Sci. 2023, 24, 2121. [Google Scholar] [CrossRef] [PubMed]
- Tran, K.A.; Partyka, P.P.; Jin, Y.; Bouyer, J.; Fischer, I.; Galie, P.A. Vascularization of self-assembled peptide scaffolds for spinal cord injury repair. Acta Biomater. 2020, 104, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.D.; Lavik, E.B.; Qu, X.; Park, K.I.; Ourednik, J.; Zurakowski, D.; Langer, R.; Snyder, E.Y. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc. Natl. Acad. Sci. USA 2002, 99, 3024–3029. [Google Scholar] [CrossRef]
- Lazaridou, M.; Bikiaris, D.N.; Lamprou, D.A. 3D Bioprinted Chitosan-Based Hydrogel Scaffolds in Tissue Engineering and Localised Drug Delivery. Pharmaceutics 2022, 14, 1978. [Google Scholar] [CrossRef]
- Chiang, C.E.; Fang, Y.Q.; Ho, C.T.; Assuncao, M.; Lin, S.j.; Wang, Y.C.; Blocki, A.; Huang, C.C. Bioactive Decellularized Extracellular Matrix Derived from 3D Stem Cell Spheroids under Macromolecular Crowding Serves as a Scaffold for Tissue Engineering. Adv. Healthcare Mater. 2021, 10, 2100024. [Google Scholar] [CrossRef] [PubMed]
- Dadsetan, M.; Knight, A.M.; Lu, L.; Windebank, A.J.; Yaszemski, M.J. Stimulation of neurite outgrowth using positively charged hydrogels. Biomaterials 2009, 30, 3874–3881. [Google Scholar] [CrossRef] [PubMed]
- Staff, N.P.; Madigan, N.N.; Morris, J.; Jentoft, M.; Sorenson, E.J.; Butler, G.; Gastineau, D.; Dietz, A.; Windebank, A.J. Safety of intrathecal autologous adipose-derived mesenchymal stromal cells in patients with ALS. Neurology 2016, 87, 2230–2234. [Google Scholar] [CrossRef] [PubMed]
- Krull, A.A.; Setter, D.O.; Gendron, T.F.; Hrstka, S.C.L.; Polzin, M.J.; Hart, J.; Dudakovic, A.; Madigan, N.N.; Dietz, A.B.; Windebank, A.J.; et al. Alterations of mesenchymal stromal cells in cerebrospinal fluid: Insights from transcriptomics and an ALS clinical trial. Stem Cell Res. Ther. 2021, 12, 187. [Google Scholar] [CrossRef] [PubMed]
- Olson, H.E.; Rooney, G.E.; Gross, L.; Nesbitt, J.J.; Galvin, K.E.; Knight, A.; Chen, B.; Yaszemski, M.J.; Windebank, A.J. Neural stem cell- and schwann cell-loaded biodegradable polymer scaffolds support axonal regeneration in the transected spinal cord. Tissue Eng. A 2009, 15, 1797–1805. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.K.; Knight, A.M.; de Ruiter, G.C.W.; Yaszemski, M.J.; Currier, B.L.; Windebank, A.J. Axon regeneration through scaffold into distal spinal cord after transection. J. Neurotrauma 2009, 26, 1759–1771. [Google Scholar] [CrossRef] [PubMed]
- Conti, A.M.; Fischer, S.J.; Windebank, A.J. Inhibition of axonal growth from sensory neurons by excess nerve growth factor. Ann. Neurol. 1997, 42, 838–846. [Google Scholar] [CrossRef]
- Podratz, J.L.; Windebank, A.J. NGF rescues DRG neurons in vitro from oxidative damage produced by hemodialyzers. Neurotoxicology 2005, 26, 343–350. [Google Scholar] [CrossRef]
- Engvall, E.; Earwicker, D.; Haaparanta, T.; Ruoslahti, E.; Sanes, J. Distribution and isolation of four laminin variants; tissue restricted distribution of heterotrimers assembled from five different subunits. Cell Regul. 1990, 1, 731–740. [Google Scholar] [CrossRef]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef]
- Bera, K.; Schalper, K.A.; Rimm, D.L.; Velcheti, V.; Madabhushi, A. Artificial intelligence in digital pathology—New tools for diagnosis and precision oncology. Nat. Rev. Clin. Oncol. 2019, 16, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Signaevsky, M.; Prastawa, M.; Farrell, K.; Tabish, N.; Baldwin, E.; Han, N.; Iida, M.A.; Koll, J.; Bryce, C.; Purohit, D.; et al. Artificial intelligence in neuropathology: Deep learning-based assessment of tauopathy. Lab. Investig. 2019, 99, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Lujan, G.; Quigley, J.C.; Hartman, D.; Parwani, A.; Roehmholdt, B.; Van Meter, B.; Ardon, O.; Hanna, M.G.; Kelly, D.; Sowards, C.; et al. Dissecting the Business Case for Adoption and Implementation of Digital Pathology: A White Paper from the Digital Pathology Association. J. Pathol. Informatics 2021, 12, 17. [Google Scholar] [CrossRef]
- Doupe, P.; Faghmous, J.; Basu, S. Machine Learning for Health Services Researchers. Value Heal. 2019, 22, 808–815. [Google Scholar] [CrossRef]
- Rashidi, H.H.; Tran, N.K.; Betts, E.V.; Howell, L.P.; Green, R. Artificial Intelligence and Machine Learning in Pathology: The Present Landscape of Supervised Methods. Acad. Pathol. 2019, 6, 2374289519873088. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Luo, Y.; Liu, L.; Lao, J.; Shao, Y.; Zhang, M.; Zhang, C.; Sun, M.; Shen, L. Automated diagnosis and quantitative analysis of plus disease in retinopathy of prematurity based on deep convolutional neural networks. Acta Ophthalmol. 2019, 98, e339–e345. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddiqui, A.M.; Thiele, F.; Stewart, R.N.; Rangnick, S.; Weiss, G.J.; Chen, B.K.; Silvernail, J.L.; Strickland, T.; Nesbitt, J.J.; Lim, K.; et al. Open-Spaced Ridged Hydrogel Scaffolds Containing TiO2-Self-Assembled Monolayer of Phosphonates Promote Regeneration and Recovery Following Spinal Cord Injury. Int. J. Mol. Sci. 2023, 24, 10250. https://doi.org/10.3390/ijms241210250
Siddiqui AM, Thiele F, Stewart RN, Rangnick S, Weiss GJ, Chen BK, Silvernail JL, Strickland T, Nesbitt JJ, Lim K, et al. Open-Spaced Ridged Hydrogel Scaffolds Containing TiO2-Self-Assembled Monolayer of Phosphonates Promote Regeneration and Recovery Following Spinal Cord Injury. International Journal of Molecular Sciences. 2023; 24(12):10250. https://doi.org/10.3390/ijms241210250
Chicago/Turabian StyleSiddiqui, Ahad M., Frederic Thiele, Rachel N. Stewart, Simone Rangnick, Georgina J. Weiss, Bingkun K. Chen, Jodi L. Silvernail, Tammy Strickland, Jarred J. Nesbitt, Kelly Lim, and et al. 2023. "Open-Spaced Ridged Hydrogel Scaffolds Containing TiO2-Self-Assembled Monolayer of Phosphonates Promote Regeneration and Recovery Following Spinal Cord Injury" International Journal of Molecular Sciences 24, no. 12: 10250. https://doi.org/10.3390/ijms241210250
APA StyleSiddiqui, A. M., Thiele, F., Stewart, R. N., Rangnick, S., Weiss, G. J., Chen, B. K., Silvernail, J. L., Strickland, T., Nesbitt, J. J., Lim, K., Schwarzbauer, J. E., Schwartz, J., Yaszemski, M. J., Windebank, A. J., & Madigan, N. N. (2023). Open-Spaced Ridged Hydrogel Scaffolds Containing TiO2-Self-Assembled Monolayer of Phosphonates Promote Regeneration and Recovery Following Spinal Cord Injury. International Journal of Molecular Sciences, 24(12), 10250. https://doi.org/10.3390/ijms241210250





