Identification of Long Noncoding RNAs That Exert Transcriptional Regulation by Forming RNA–DNA Triplexes in Prostate Cancer
Abstract
1. Introduction
2. Results
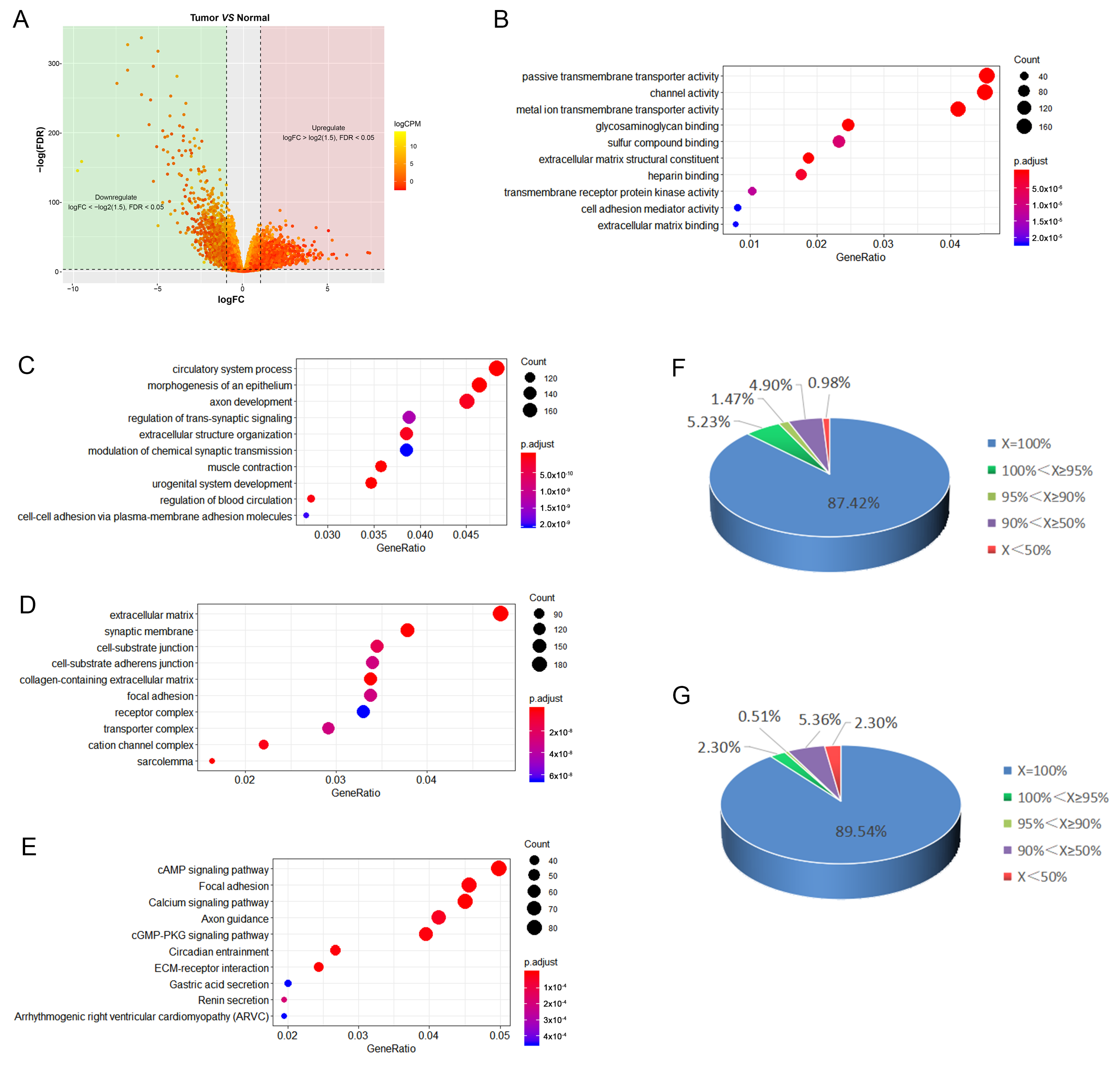
2.1. Differential Expression Profiles in PCa Samples
2.2. Correlation Analysis for mRNAs and lncRNA Genes Using Gene Expression Data
2.3. Prediction of Potential Triplex-Forming lncRNAs and Triplex Structure in the Promoter Region of Co-Expression Genes
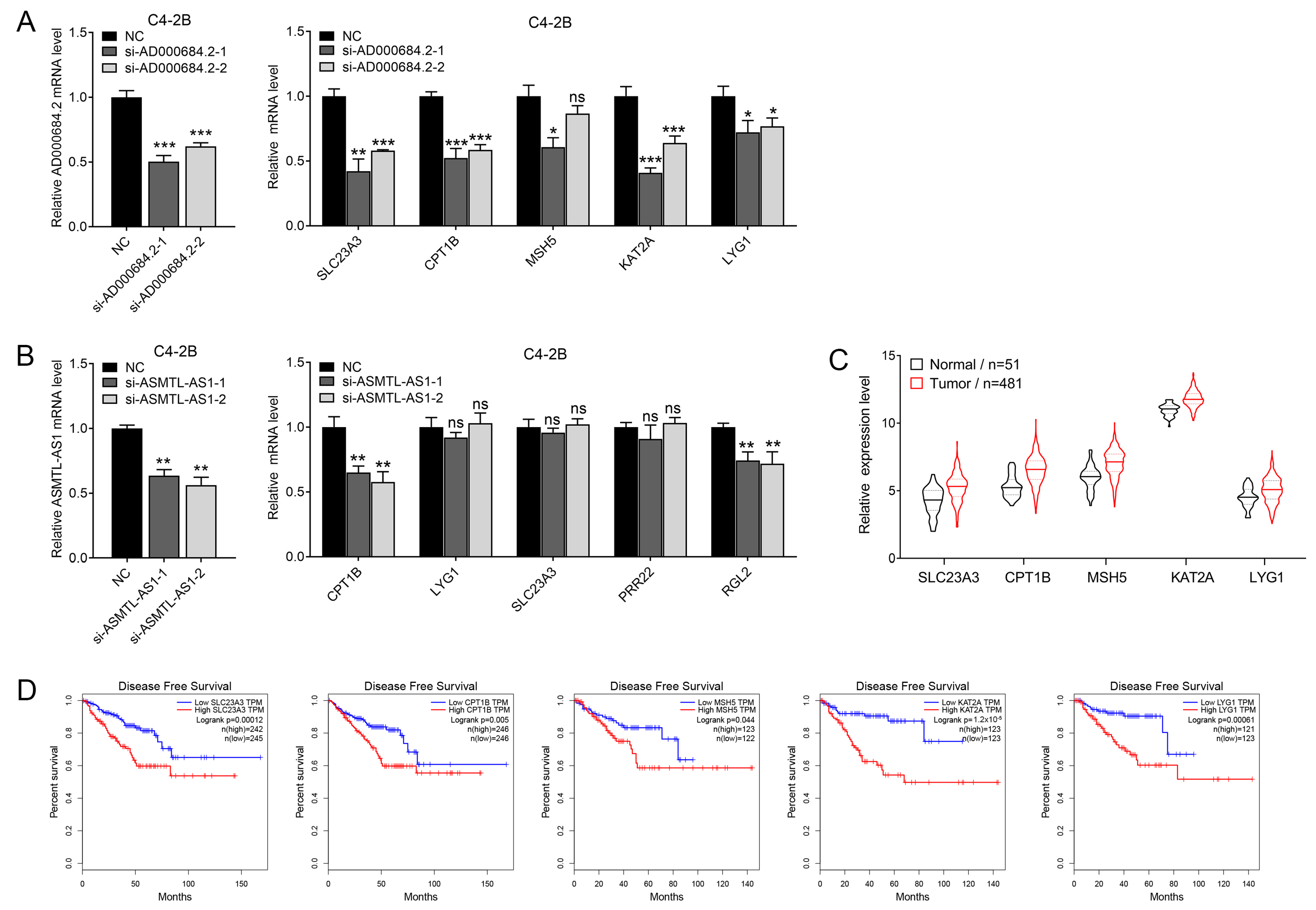
2.4. AD000684.2 and ASMTL-AS1 Are Highly Expressed in PCa
2.5. AD000684.2 Positively Regulates the Expression of Correlated Genes
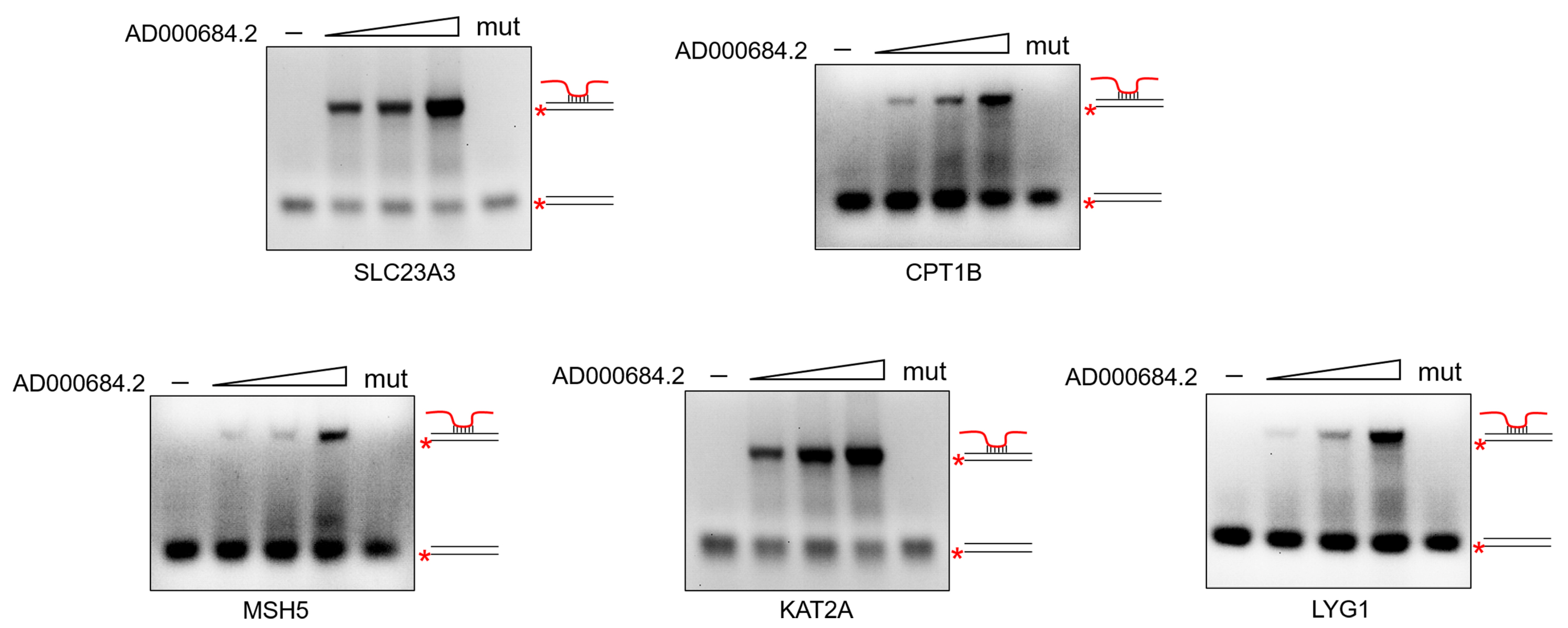
2.6. AD000684.2 Forms RNA–DNA Triplexes with the Promoter Region of Regulated Genes
2.7. AD000684.2 Promotes Cell Proliferation and Motility, as Well as Inhibits Cell Apoptosis, in PCa Cell Lines
3. Discussion
4. Materials and Methods
4.1. Computational Analysis
4.1.1. RNA-Seq Data Acquisition of PCa
4.1.2. Significantly Differential Gene Expression Analysis
4.1.3. COX Proportional Hazards Regression Analysis
4.1.4. Pearson Correlation Coefficient between Differentially Expressed lncRNAs and mRNAs in PCa
4.1.5. Acquisition of the lncRNA Sequences and Promoter Sequences of Genes
4.1.6. In Silico Prediction of RNA–DNA Triplexes
4.2. Experimental Validation
4.2.1. In Vitro Transcription
4.2.2. Electrophoretic Mobility Shift Assay (EMSA)
4.2.3. Cell Culture
4.2.4. RNA Interference and Transfection
4.2.5. Cell Proliferation Assay
4.2.6. Annexin V–FITC Apoptosis Assay
4.2.7. Transwell Migration Assay
4.2.8. Wound Healing Assay
4.2.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, S.V.; Vickers, A.J. Screening for Prostate Cancer. Med. Clin. N. Am. 2020, 104, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Lu, H.; Liu, J.; Wu, S.; Kim, P.; Zhou, X. lncRNAfunc: A knowledgebase of lncRNA function in human cancer. Nucleic Acids Res 2022, 50, D1295–D1306. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, T.; Zhou, W.; Li, J.; Li, X.; Wang, Q.; Jin, X.; Yin, J.; Chen, L.; Zhang, Y.; et al. Pan-cancer characterization of immune-related lncRNAs identifies potential oncogenic biomarkers. Nat. Commun. 2020, 11, 1000. [Google Scholar] [CrossRef]
- Singh, N.; Ramnarine, V.R.; Song, J.H.; Pandey, R.; Padi, S.K.R.; Nouri, M.; Olive, V.; Kobelev, M.; Okumura, K.; McCarthy, D.; et al. The long noncoding RNA H19 regulates tumor plasticity in neuroendocrine prostate cancer. Nat. Commun. 2021, 12, 7349. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, M. lnCaNet: Pan-cancer co-expression network for human lncRNA and cancer genes. Bioinformatics 2016, 32, 1595–1597. [Google Scholar] [CrossRef]
- Zhang, H.; Bian, C.; Tu, S.; Yin, F.; Guo, P.; Zhang, J.; Song, X.; Liu, Q.; Chen, C.; Han, Y. Integrated analysis of lncRNA-miRNA-mRNA ceRNA network in human aortic dissection. BMC Genom. 2021, 22, 724. [Google Scholar] [CrossRef]
- Wu, R.; Hu, W.; Chen, H.; Wang, Y.; Li, Q.; Xiao, C.; Fan, L.; Zhong, Z.; Chen, X.; Lv, K.; et al. A Novel Human Long Noncoding RNA SCDAL Promotes Angiogenesis through SNF5-Mediated GDF6 Expression. Adv. Sci. 2021, 8, e2004629. [Google Scholar] [CrossRef]
- Engreitz, J.M.; Haines, J.E.; Perez, E.M.; Munson, G.; Chen, J.; Kane, M.; McDonel, P.E.; Guttman, M.; Lander, E.S. Local regulation of gene expression by lncRNA promoters, transcription and splicing. Nature 2016, 539, 452–455. [Google Scholar] [CrossRef]
- Felsenfeld, G.; Rich, A. Studies on the formation of two- and three-stranded polyribonucleotides. Biochim. Biophys. Acta 1957, 26, 457–468. [Google Scholar] [CrossRef]
- Li, Y.; Syed, J.; Sugiyama, H. RNA-DNA Triplex Formation by Long Noncoding RNAs. Cell Chem. Biol. 2016, 23, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yang, X.; Huang, W.; Ma, Y.; Ke, H.; Zou, L.; Yang, Q.; Jiao, B. Single-cell profiling of long noncoding RNAs and their cell lineage commitment roles via RNA-DNA-DNA triplex formation in mammary epithelium. Stem Cells 2020, 38, 1594–1611. [Google Scholar] [CrossRef] [PubMed]
- Senturk Cetin, N.; Kuo, C.C.; Ribarska, T.; Li, R.; Costa, I.G.; Grummt, I. Isolation and genome-wide characterization of cellular DNA:RNA triplex structures. Nucleic Acids Res. 2019, 47, 2306–2321. [Google Scholar] [CrossRef]
- He, S.; Zhang, H.; Liu, H.; Zhu, H. LongTarget: A tool to predict lncRNA DNA-binding motifs and binding sites via Hoogsteen base-pairing analysis. Bioinformatics 2015, 31, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Jalali, S.; Singh, A.; Maiti, S.; Scaria, V. Genome-wide computational analysis of potential long noncoding RNA mediated DNA:DNA:RNA triplexes in the human genome. J. Transl. Med. 2017, 15, 186. [Google Scholar] [CrossRef]
- Grote, P.; Herrmann, B.G. The long non-coding RNA Fendrr links epigenetic control mechanisms to gene regulatory networks in mammalian embryogenesis. Rna Biol. 2013, 10, 1579–1585. [Google Scholar] [CrossRef]
- O’Leary, V.B.; Smida, J.; Buske, F.A.; Carrascosa, L.G.; Azimzadeh, O.; Maugg, D.; Hain, S.; Tapio, S.; Heidenreich, W.; Kerr, J.; et al. PARTICLE triplexes cluster in the tumor suppressor WWOX and may extend throughout the human genome. Sci. Rep. 2017, 7, 7163. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, Q.; Li, J.; Zhang, S.; Cao, Y.; Xia, X.; Cai, D.; Tan, J.; Chen, J.; Han, J.J. KCNQ1OT1 promotes genome-wide transposon repression by guiding RNA-DNA triplexes and HP1 binding. Nat. Cell Biol. 2022, 24, 1617–1629. [Google Scholar] [CrossRef]
- Zhang, Y.; Long, Y.; Kwoh, C.K. Deep learning based DNA:RNA triplex forming potential prediction. BMC Bioinform. 2020, 21, 522. [Google Scholar] [CrossRef]
- Beckedorff, F.C.; Ayupe, A.C.; Crocci-Souza, R.; Amaral, M.S.; Nakaya, H.I.; Soltys, D.T.; Menck, C.F.; Reis, E.M.; Verjovski-Almeida, S. The intronic long noncoding RNA ANRASSF1 recruits PRC2 to the RASSF1A promoter, reducing the expression of RASSF1A and increasing cell proliferation. PLoS Genet. 2013, 9, e1003705. [Google Scholar] [CrossRef]
- Yari, H.; Jin, L.; Teng, L.; Wang, Y.; Wu, Y.; Liu, G.Z.; Gao, W.; Liang, J.; Xi, Y.; Feng, Y.C.; et al. LncRNA REG1CP promotes tumorigenesis through an enhancer complex to recruit FANCJ helicase for REG3A transcription. Nat. Commun. 2019, 10, 5334. [Google Scholar] [CrossRef] [PubMed]
- Martianov, I.; Ramadass, A.; Serra Barros, A.; Chow, N.; Akoulitchev, A. Repression of the human dihydrofolate reductase gene by a non-coding interfering transcript. Nature 2007, 445, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Blume, S.W.; Meng, Z.; Shrestha, K.; Snyder, R.C.; Emanuel, P.D. The 5’-untranslated RNA of the human dhfr minor transcript alters transcription pre-initiation complex assembly at the major (core) promoter. J. Cell Biochem. 2003, 88, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Ding, H.; Li, Y.; Xue, D.; Liu, Y. LncRNA TINCR is associated with clinical progression and serves as tumor suppressive role in prostate cancer. Cancer Manag. Res. 2018, 10, 2799–2807. [Google Scholar] [CrossRef]
- Jiang, M.; Cheng, Y.; Wang, D.; Lu, Y.; Gu, S.; Wang, C.; Huang, Y.; Li, Y. Transcriptional network modulated by the prognostic signature transcription factors and their long noncoding RNA partners in primary prostate cancer. Ebiomedicine 2021, 63, 103150. [Google Scholar] [CrossRef]
- Robinson, M.D.; Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010, 11, R25. [Google Scholar] [CrossRef]
- Chen, Y.; Lun, A.T.; Smyth, G.K. From reads to genes to pathways: Differential expression analysis of RNA-Seq experiments using Rsubread and the edgeR quasi-likelihood pipeline. F1000Research 2016, 5, 1438. [Google Scholar] [CrossRef]
- Lund, S.P.; Nettleton, D.; McCarthy, D.J.; Smyth, G.K. Detecting differential expression in RNA-sequence data using quasi-likelihood with shrunken dispersion estimates. Stat. Appl. Genet. Mol. Biol. 2012, 11. [Google Scholar] [CrossRef]
- Lun, A.T.; Chen, Y.; Smyth, G.K. It’s DE-licious: A Recipe for Differential Expression Analyses of RNA-seq Experiments Using Quasi-Likelihood Methods in edgeR. Methods Mol. Biol. 2016, 1418, 391–416. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate—A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.C.; Schlesinger, F.; Davis, C.A.; Zhang, Y.; Li, R.H.; Salit, M.; Gingeras, T.R.; Oliver, B. Synthetic spike-in standards for RNA-seq experiments. Genome Res. 2011, 21, 1543–1551. [Google Scholar] [CrossRef] [PubMed]


| OS 1 | DFS 1 | |||
|---|---|---|---|---|
| log FC > 0 | log FC < 0 | log FC > 0 | log FC < 0 | |
| HR > 1 | 60 | 3 | 166 | 4 |
| HR < 1 | 5 | 16 | 0 | 5 |
| Motif Type | Number of Potential Triplex-Forming lncRNAs | Number of Potential Triplex Target mRNAs | Number of Potential Triplex-Forming Sites (PTSs) |
|---|---|---|---|
| R (purine motif) | 236 | 1116 | 7839 |
| Example: NDP | TFO 1: 3′–GAGAGAcAGAGAGAGAGAGAGAGAGGGAG–5′ | ||
| ||||||*||||||*||||||||||||||| | |||
| TTS 1: 5′–GAGAGAGAGAGAGGGAGAGAGAGAGGGAG–3′ | |||
| 3′–CTCTCTCTCTCTCCCTCTCTCTCTCCCTC–5′ | |||
| Y (pyrimidine motif) | 195 | 1169 | 12,678 |
| Example: CAV1 | TFO: 3′–TTTGTGTGTGTGTTTTTTTGTGTGTGT–5′ | ||
| ||||||||||||||||||*||||||||||| | |||
| TTS: 3′–AGAGACAGAGACAGAAACAGAGAGAGA–5′ | |||
| 5′–TCTCTGTCTCTGTCTTTGTCTCTCTCT–3′ | |||
| M (purine–pyrimidine or mixed motif) | 188 | 938 | 9214 |
| Example: COL6A1 | TFO: 3′–TTTGTGTGTGTGTTTTTTTGTGTGTGT–5′ | ||
| |*|||*|||||*|*|||*||||||||| | |||
| TTS: 3′–AGAGACAGAGACAGAAACAGAGAGAGA–5′ | |||
| 5′–TCTCTGTCTCTGTCTTTGTCTCTCTCT–3′ | |||
| Total | 619 2 | 3223 3 | 29,731 |
| Ranking | The lncRNA Symbol | lncRNA Ensemble | Number of Potential Triplex Target mRNAs | Percentage of Positively Correlated mRNAs 1 |
|---|---|---|---|---|
| 1 | ADAMTS9-AS2 | ENSG00000241684 | 692 | 98.55 |
| 2 | MBNL1-AS1 | ENSG00000229619 | 595 | 99.33 |
| 3 | HCG11 | ENSG00000228223 | 553 | 98.73 |
| 4 | AC107959.1 | ENSG00000245025 | 497 | 100.00 |
| 5 | LINC00654 | ENSG00000205181 | 390 | 98.72 |
| 6 | AF111167.2 | ENSG00000259319 | 390 | 100.00 |
| 7 | LINC01679 | ENSG00000237989 | 374 | 100.00 |
| 8 | AC004846.1 | ENSG00000258376 | 373 | 100.00 |
| 9 | NR2F1-AS1 | ENSG00000237187 | 328 | 100.00 |
| 10 | BOLA3-AS1 | ENSG00000225439 | 300 | 99.67 |
| 11 | MEF2C-AS1 | ENSG00000248309 | 298 | 100.00 |
| 12 | LINC00900 | ENSG00000246100 | 279 | 100.00 |
| 13 | DNM3OS | ENSG00000230630 | 272 | 100.00 |
| 14 | AP002884.1 | ENSG00000250303 | 267 | 100.00 |
| 15 | ACTA2-AS1 | ENSG00000180139 | 227 | 100.00 |
| 16 | AC080013.1 | ENSG00000240207 | 224 | 100.00 |
| 17 | LINC00857 | ENSG00000237523 | 221 | 100.00 |
| 18 | FENDRR | ENSG00000268388 | 215 | 100.00 |
| 19 | CASC15 | ENSG00000272168 | 175 | 100.00 |
| 20 2 | AF165147.1 | ENSG00000232855 | 164 | 100.00 |
| lncRNA Symbol | Start | End | TFOs (5′–3′) 1 | Score 2 |
|---|---|---|---|---|
| AD000684.2 | 464 | 482 | AAAGGGGAAGtAAGcAAA | 16 |
| AD000684.2 | 498 | 528 | AAGAGAAGAcAGAAGAcAGAGAGAGAGGGA | 28 |
| AD000684.2 | 496 | 526 | GtAAGAGAAGAcAGAAGAcAGAGAGAGAGG | 27 |
| AD000684.2 | 495 | 525 | GGtAAGAGAAGAcAGAAGAcAGAGAGAGAG | 27 |
| AD000684.2 | 494 | 524 | AGGtAAGAGAAGAcAGAAGAcAGAGAGAGA | 27 |
| AD000684.2 | 493 | 523 | AAGGtAAGAGAAGAcAGAAGAcAGAGAGAG | 27 |
| AD000684.2 | 492 | 522 | GAAGGtAAGAGAAGAcAGAAGAcAGAGAGA | 27 |
| AD000684.2 | 491 | 521 | GGAAGGtAAGAGAAGAcAGAAGAcAGAGAG | 27 |
| AD000684.2 | 490 | 520 | GGGAAGGtAAGAGAAGAcAGAAGAcAGAGA | 27 |
| AD000684.2 | 489 | 519 | GGGGAAGGtAAGAGAAGAcAGAAGAcAGAG | 27 |
| AD000684.2 | 488 | 518 | AGGGGAAGGtAAGAGAAGAcAGAAGAcAGA | 27 |
| AD000684.2 | 486 | 516 | GcAGGGGAAGGtAAGAGAAGAcAGAAGAcA | 26 |
| AD000684.2 | 484 | 514 | AAGcAGGGGAAGGtAAGAGAAGAcAGAAGA | 27 |
| Triplex Motif | TFO Start | TFO End | Duplex Gene | TTS Start | TTS End | Score |
|---|---|---|---|---|---|---|
| TFO: 3′–AGAAGACAGAAGAGAATGGA–5′ | 494 | 514 | SLC23A3 | 1244 | 1264 | 16 |
| ||||||*||*||||*|*||| | ||||||
| TTS: 5′–AGAAGAGAGGAGAGGATGGA–3′ | ||||||
| 3′–TCTTCTCTCCTCTCCTACCT–5′ | ||||||
| TFO: 5′–GACAGAGAGAGAGGG–3′ | 512 | 527 | CPT1B | 599 | 614 | 12 |
| ||*|||**||||||| | ||||||
| TTS: 3′–GACAGACGGAGAGGG–5′ | ||||||
| 5′–CTGTCTGCCTCTCCC–3′ | ||||||
| TFO: 5′–AGAAGACAGAGAGAGA–3′ | 508 | 524 | MSH5 | 1234 | 1250 | 13 |
| ||*|||**||||||| | ||||||
| TTS: 3′–AGGAGAAAGGGAGAGA–5′ | ||||||
| 5′–TCCTCTTTCCCTCTCT–3′ | ||||||
| TFO: 5′–AGAAGACAGAGAGAG–3′ | 506 | 521 | KAT2A | 961 | 976 | 13 |
| |||*||*|||||||| | ||||||
| TTS: 3′–AGAGGAGAGAGAGAG–5′ | ||||||
| 5′–TCTCCTCTCTCTCTC–3′ | ||||||
| TFO: 3′–AGGGAGAGAGAGACAGAAG–5′ | 509 | 528 | LYG1 | 2726 | 2742 | 16 |
| ||*|||||||||**||||| | ||||||
| TTS: 5′–AGAGAGAGAGAGTGAGAAG–3′ | ||||||
| 3′–TCTCTCTCTCTCACTCTTC–5′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.; Lu, Y.; Chen, Q.; Cheng, Y.; Ma, Y.; Huang, Y.; Qiu, M.; Li, Y. Identification of Long Noncoding RNAs That Exert Transcriptional Regulation by Forming RNA–DNA Triplexes in Prostate Cancer. Int. J. Mol. Sci. 2023, 24, 2035. https://doi.org/10.3390/ijms24032035
Liang Y, Lu Y, Chen Q, Cheng Y, Ma Y, Huang Y, Qiu M, Li Y. Identification of Long Noncoding RNAs That Exert Transcriptional Regulation by Forming RNA–DNA Triplexes in Prostate Cancer. International Journal of Molecular Sciences. 2023; 24(3):2035. https://doi.org/10.3390/ijms24032035
Chicago/Turabian StyleLiang, Yugang, Yali Lu, Qin Chen, Yihang Cheng, Yunsheng Ma, Yan Huang, Minyan Qiu, and Yao Li. 2023. "Identification of Long Noncoding RNAs That Exert Transcriptional Regulation by Forming RNA–DNA Triplexes in Prostate Cancer" International Journal of Molecular Sciences 24, no. 3: 2035. https://doi.org/10.3390/ijms24032035
APA StyleLiang, Y., Lu, Y., Chen, Q., Cheng, Y., Ma, Y., Huang, Y., Qiu, M., & Li, Y. (2023). Identification of Long Noncoding RNAs That Exert Transcriptional Regulation by Forming RNA–DNA Triplexes in Prostate Cancer. International Journal of Molecular Sciences, 24(3), 2035. https://doi.org/10.3390/ijms24032035






