Octafluoro-Substituted Phthalocyanines of Zinc, Cobalt, and Vanadyl: Single Crystal Structure, Spectral Study and Oriented Thin Films
Abstract
1. Introduction
2. Results and Discussion
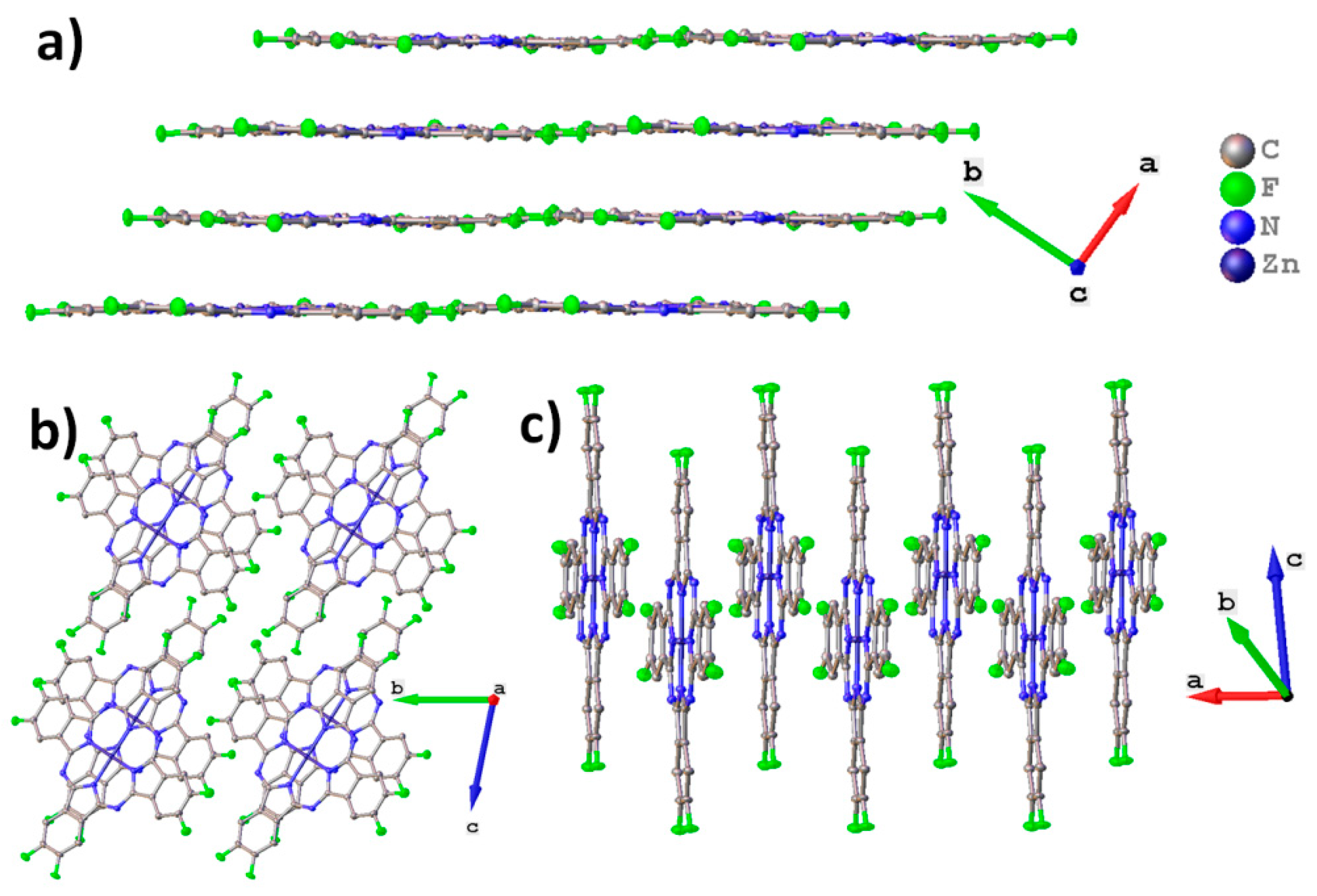
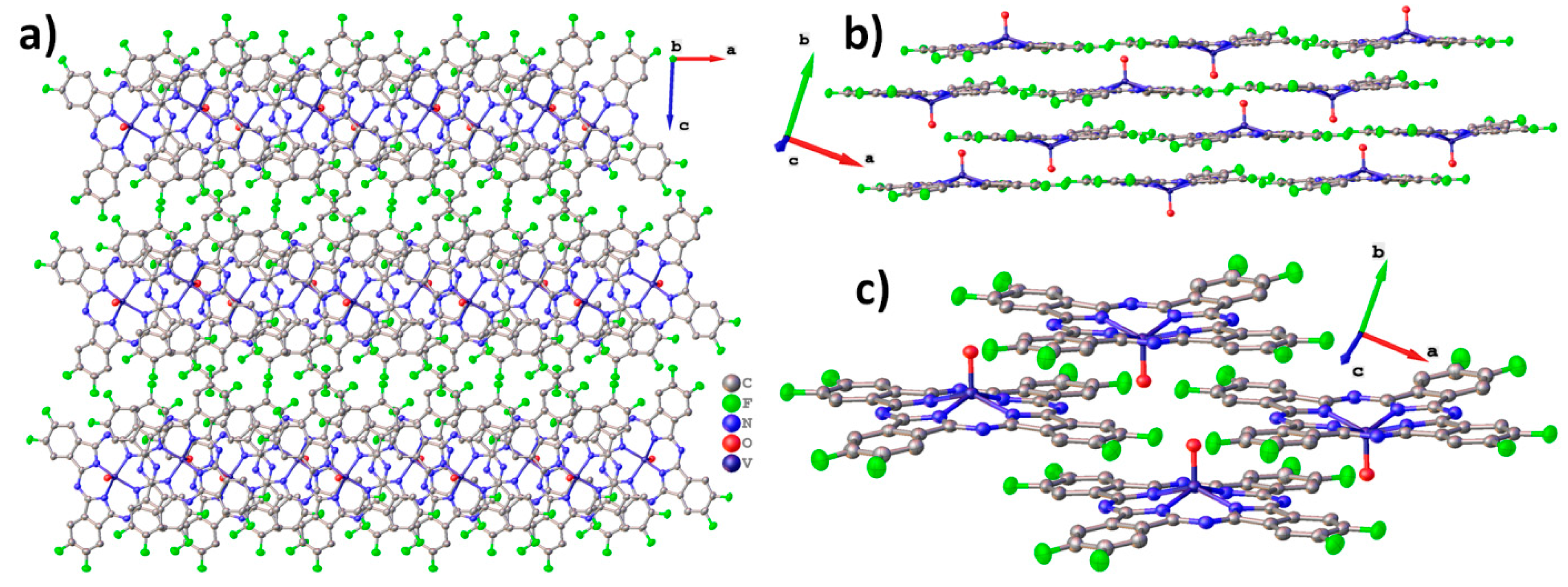
2.1. MPcF8 Crystal Structures
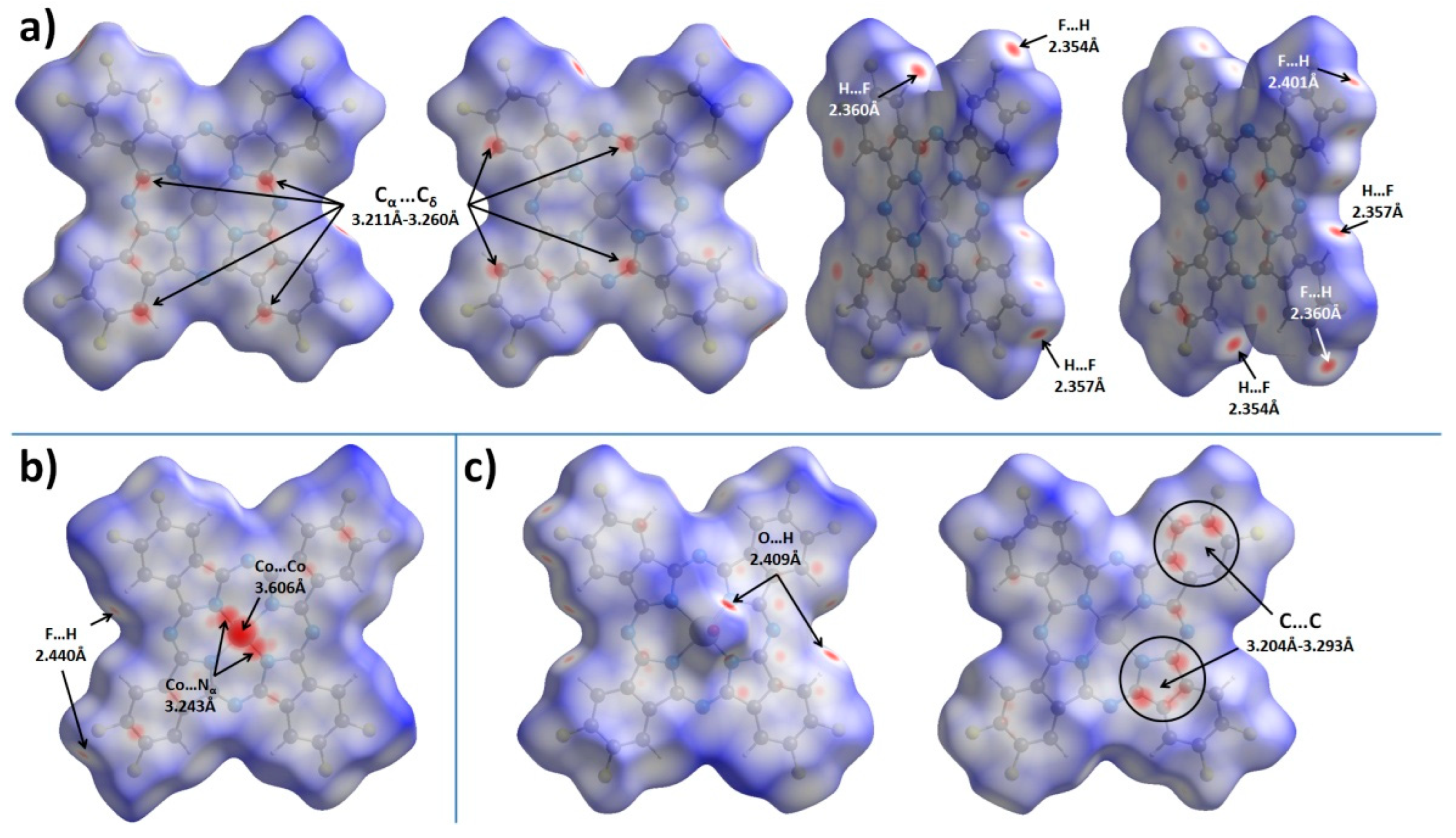
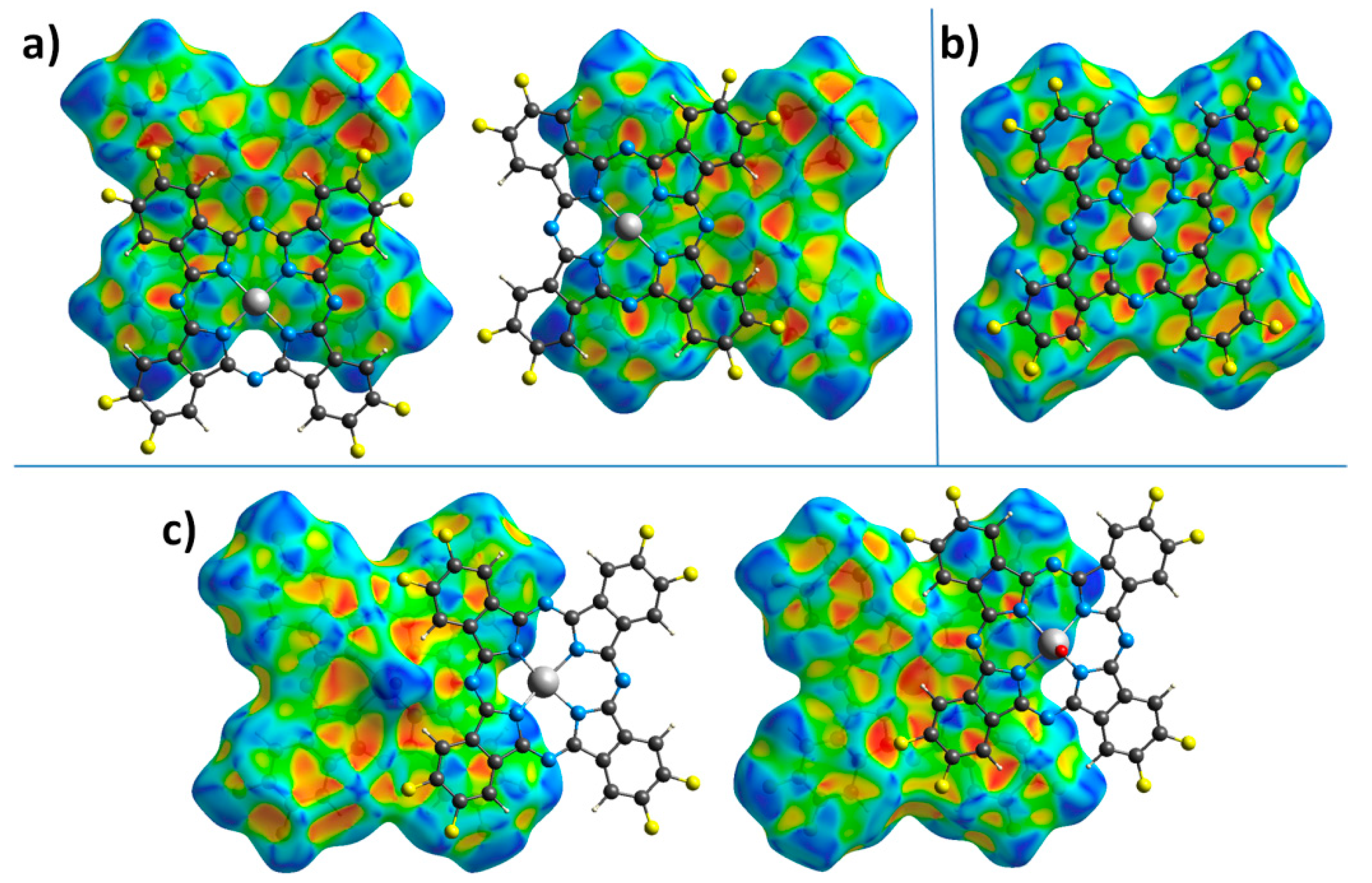
2.2. Hirshfeld Surface Analysis
2.3. Vibrational Spectra of MPcF8
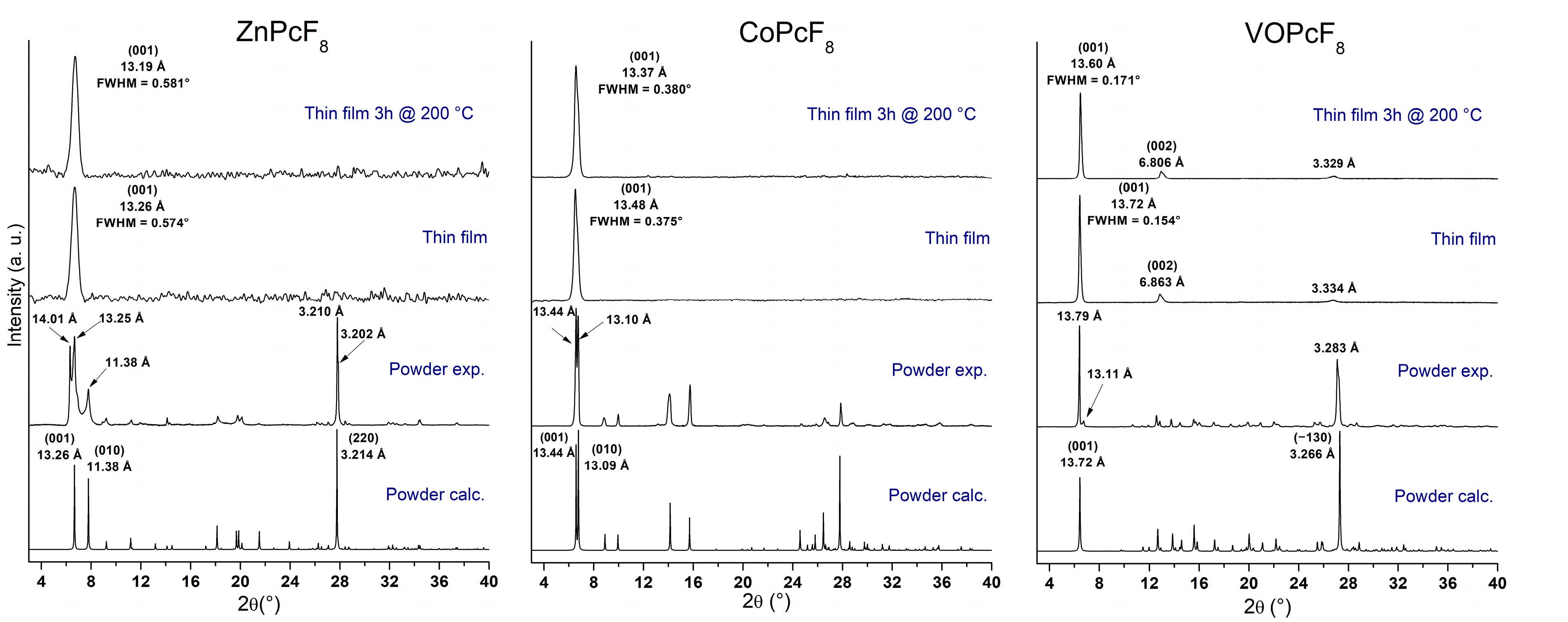
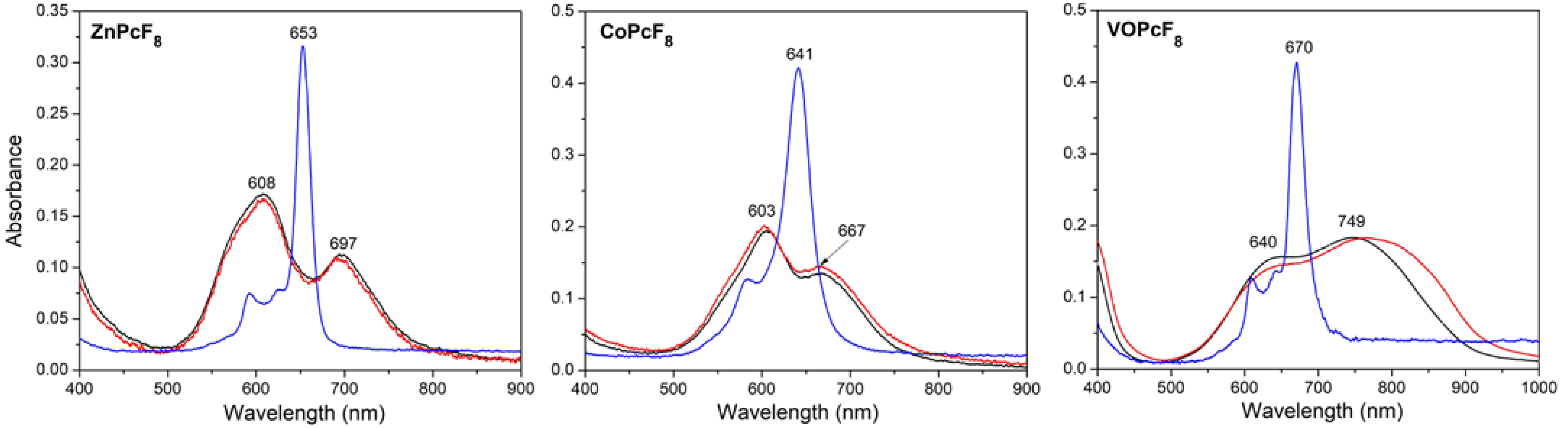
2.4. Study of MPcF8 Powders and Thin Films by XRD and UV-vis Spectroscopy
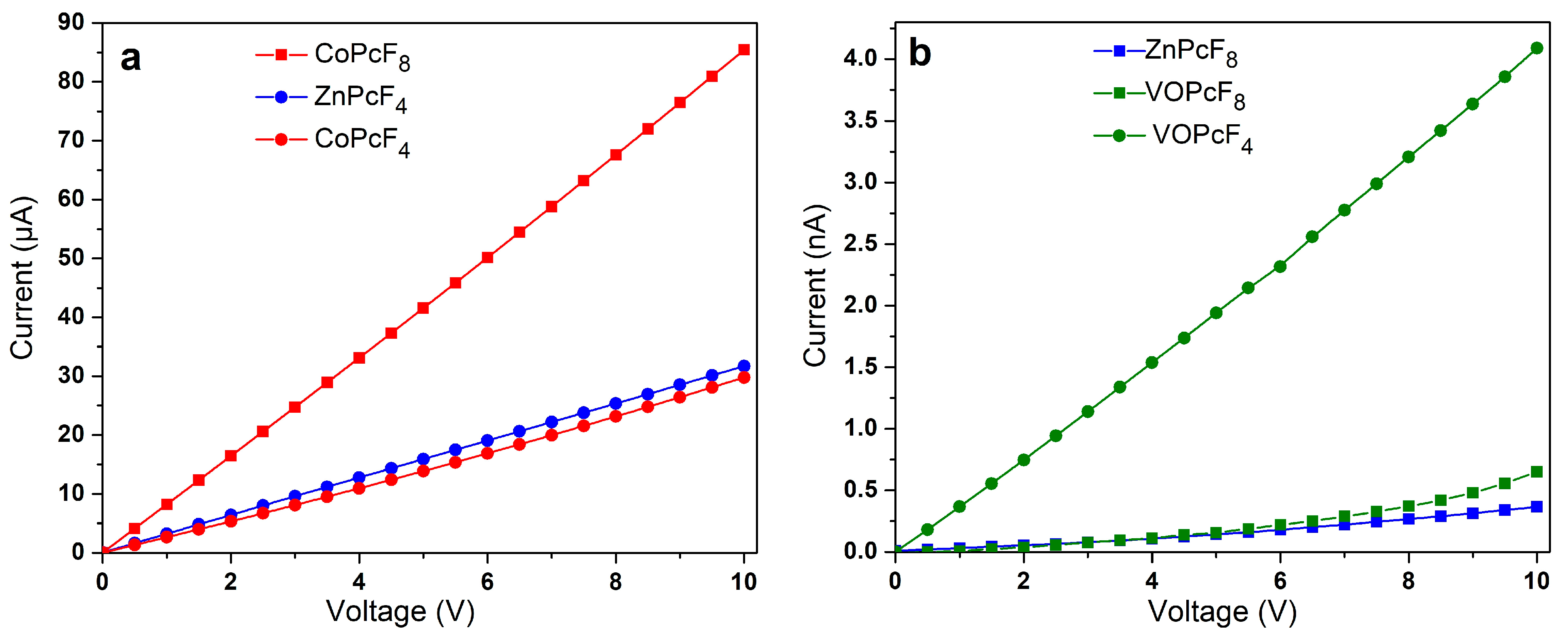
2.5. Study of The Conductivity of MPcF8 Films
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gümrükçü Köse, G.; Karaoğlan, G.K.; Erdağ Maden, Y.; Koca, A. Novel silicon phthalocyanine photosensitizers containing carboxylic acid based axial anchoring groups: Electrochemistry, spectroelectrochemistry, and dye sensitized solar cell performance. Dyes Pigments 2022, 207, 110686. [Google Scholar] [CrossRef]
- Şenoğlu, S.; Özer, M.; Dumludağ, F.; Acar, N.; Salih, B.; Bekaroğlu, Ö. Synthesis, characterization, DFT study, conductivity and effects of humidity on CO2 sensing properties of the novel tetrakis-[2-(dibenzylamino)ethoxyl] substituted metallophthalocyanines. Sensors Actuators, B Chem. 2020, 310, 127860. [Google Scholar] [CrossRef]
- Bunin, D.A.; Martynov, A.G.; Safonova, E.A.; Tsivadze, A.Y.; Gorbunova, Y.G. Robust route toward cationic phthalocyanines through reductive amination. Dyes Pigments 2022, 207, 110768. [Google Scholar] [CrossRef]
- Yahya, M.; Nural, Y.; Seferoğlu, Z. Recent advances in the nonlinear optical (NLO) properties of phthalocyanines: A review. Dye. Pigment. 2022, 198, 109960. [Google Scholar] [CrossRef]
- Lukyanets, E.A. Phthalocyanines as Photosensitizers in the Photodynamic Therapy of Cancer. J. Porphyr. Phthalocyanines 1999, 3, 424–432. [Google Scholar] [CrossRef]
- de Oliveira, M.S.; Farias, E.A.d.O.; de Sousa, A.M.S.; Dionísio, N.A.; Teixeira, P.R.S.; Teixeira, A.S.; do, N.M.; da Silva, D.A.; Eiras, C. Composite films based on copper nanoparticles and nickel phthalocyanine as electrochemical sensors for serotonin detection. Surfaces and Interfaces 2021, 25, 101245. [Google Scholar] [CrossRef]
- Zheng, B.D.; Ye, J.; Zhang, X.Q.; Zhang, N.; Xiao, M.T. Recent advances in supramolecular activatable phthalocyanine-based photosensitizers for anti-cancer therapy. Coord. Chem. Rev. 2021, 447, 214155. [Google Scholar] [CrossRef]
- Szymczak, J.; Rebis, T.; Kotkowiak, M.; Wicher, B.; Sobotta, L.; Tykarska, E.; Mielcarek, J.; Kryjewski, M. Regioisomers of magnesium(II) phthalocyanine bearing menthol substituents–Synthesis, spectral, electrochemical and computational studies. Dyes Pigments 2021, 191, 109357. [Google Scholar] [CrossRef]
- Ivanova, V.; Klyamer, D.; Krasnov, P.; Kaya, E.N.; Kulu, I.; Tuncel Kostakoğlu, S.; Durmuş, M.; Basova, T. Hybrid materials based on pyrene-substituted metallo phthalocyanines as sensing layers for ammonia detection: Effect of the number of pyrene substituents. Sensors Actuators B Chem. 2023, 375, 132843. [Google Scholar] [CrossRef]
- Robin Nxele, S.; Nkhahle, R.; Nyokong, T. The composites of asymmetric Co phthalocyanines-graphitic carbon nitride quantum dots-aptamer as specific electrochemical sensors for the detection of prostate specific antigen: Effects of ring substituents. J. Electroanal. Chem. 2021, 900, 115730. [Google Scholar] [CrossRef]
- Chen, Y.; Yao, Q.; Qu, S.; Shi, W.; Li, H.; Chen, L. Enhanced thermoelectric performance of phthalocyanine complexes/single-walled carbon nanotube hybrids by tuning the types of metal coordination ions. Compos. Commun. 2021, 27, 100891. [Google Scholar] [CrossRef]
- Zeinidenov, A.K.; Aimukhanov, A.K.; Kambar, D.S.; Ilyassov, B.R.; Zavgorodniy, A.V. Effects of phthalocyanine nanostructure on photovoltaic performance of its polymer composite thin films. Mater. Chem. Phys. 2021, 267, 124680. [Google Scholar] [CrossRef]
- Klyamer, D.D.; Sukhikh, A.S.; Trubin, S.V.; Gromilov, S.A.; Morozova, N.B.; Basova, T.V.; Hassan, A.K. Tetrafluorosubstituted Metal Phthalocyanines: Interplay between Saturated Vapor Pressure and Crystal Structure. Cryst. Growth Des. 2020, 20, 1016–1024. [Google Scholar] [CrossRef]
- Basova, T.; Semyannikov, P.; Plyashkevich, V.; Hassan, A.; Igumenov, I. Volatile phthalocyanines: Vapor pressure and thermodynamics. Crit. Rev. Solid State Mater. Sci. 2009, 34, 180–189. [Google Scholar] [CrossRef]
- Matumoto, A.; Hoshino, N.; Akutagawa, T.; Matsuda, M. N-type semiconducting behavior of copper octafluorophthalocyanine in an organic field-effect transistor. Appl. Sci. 2017, 7, 1111. [Google Scholar] [CrossRef]
- Bao, Z.; Lovinger, A.J.; Brown, J. New air-stable n-channel organs thin film transistors. J. Am. Chem. Soc. 1998, 120, 207–208. [Google Scholar] [CrossRef]
- Ling, M.-M.; Bao, Z.; Erk, P. Air-stable n-channel copper hexachlorophthalocyanine for field-effect transistors. Appl. Phys. Lett. 2006, 89, 163516. [Google Scholar] [CrossRef]
- Lessard, B.H. The Rise of Silicon Phthalocyanine: From Organic Photovoltaics to Organic Thin Film Transistors. ACS Appl. Mater. Interfaces 2021, 13, 31321–31330. [Google Scholar] [CrossRef]
- Gai, S.; Wang, B.; Wang, X.; Zhang, R.; Miao, S.; Wu, Y. Ultrafast NH3 gas sensor based on phthalocyanine-optimized non-covalent hybrid of carbon nanotubes with pyrrole. Sensors Actuators B Chem. 2022, 357, 131352. [Google Scholar] [CrossRef]
- Carvalho da Silva, V.N.; Farias, E.A.d.O.; Araújo, A.R.; Xavier Magalhães, F.E.; Neves Fernandes, J.R.; Teles Souza, J.M.; Eiras, C.; Alves da Silva, D.; Hugo do Vale Bastos, V.; Teixeira, S.S. Rapid and selective detection of dopamine in human serum using an electrochemical sensor based on zinc oxide nanoparticles, nickel phthalocyanines, and carbon nanotubes. Biosens. Bioelectron. 2022, 210, 114211. [Google Scholar] [CrossRef]
- Bouvet, M.; Guillaud, G.; Leroy, A.; Maillard, A.; Spirkovitch, S.; Tournilhac, F.-G. Phthalocyanine-based field-effect transistor as ozone sensor. Sensors Actuators B Chem. 2001, 73, 63–70. [Google Scholar] [CrossRef]
- Sukhikh, A.S.; Klyamer, D.D.; Parkhomenko, R.G.; Krasnov, P.O.; Gromilov, S.A.; Hassan, A.K.; Basova, T.V. Effect of fluorosubstitution on the structure of single crystals, thin films and spectral properties of palladium phthalocyanines. Dye. Pigment. 2018, 149, 348–355. [Google Scholar] [CrossRef]
- Sukhikh, A.; Bonegardt, D.; Klyamer, D.; Basova, T. Effect of non-peripheral fluorosubstitution on the structure of metal phthalocyanines and their films. Dyes Pigments 2021, 192, 109442. [Google Scholar] [CrossRef]
- Klyamer, D.; Sukhikh, A.; Nikolaeva, N.; Morozova, N.; Basova, T. Vanadyl phthalocyanine films and their hybrid structures with Pd nanoparticles: Structure and sensing properties. Sensors 2020, 20, 1893. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.M.; Song, H.J.; Hwang, I.C.; Kim, K.S.; Choi, H.C. Single crystal structure of copper hexadecafluorophthalocyanine (F16CuPc) ribbon. Chem. Commun. 2010, 46, 231–233. [Google Scholar] [CrossRef]
- Jiang, H.; Ye, J.; Hu, P.; Wei, F.; Du, K.; Wang, N.; Ba, T.; Feng, S.; Kloc, C. Fluorination of metal phthalocyanines: Single-crystal growth, efficient N-channel organic field-effect transistors, and structure-property relationships. Sci. Rep. 2014, 4, 7573. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Wang, S.; Li, X. Synthesis, spectral characterization of CuPcF16 and its application in organic thin film transistors using p-6p as inducing layer. J. Phys. Chem. Solids 2012, 73, 589–592. [Google Scholar] [CrossRef]
- Klyamer, D.; Sukhikh, A.; Gromilov, S.; Krasnov, P.; Basova, T. Fluorinated metal phthalocyanines: Interplay between fluorination degree, films orientation, and ammonia sensing properties. Sensors 2018, 18, 2141. [Google Scholar] [CrossRef]
- Dong, N.; Wu, X.-M.; Dang, H.-Q.; Liu, D.-Y.; Zhang, Q.; Wei, J.; Yin, S.-G. Improved Performance of Phthalocyanine Derivative Field-Effect Transistors by Inserting a Para-Quarterphenyl as the Inducing Layer. Chinese Phys. Lett. 2014, 31, 058501. [Google Scholar] [CrossRef]
- Bouvet, M.; Gaudillat, P.; Kumar, A.; Sauerwald, T.; Schüler, M.; Schütze, A.; Suisse, J.M. Revisiting the electronic properties of Molecular Semiconductor–Doped Insulator (MSDI) heterojunctions through impedance and chemosensing studies. Org. Electron. 2015, 26, 345–354. [Google Scholar] [CrossRef]
- Kumar, A.; Meunier-Prest, R.; Bouvet, M. Organic heterojunction devices based on phthalocyanines: A new approach to gas chemosensing. Sensors 2020, 20, 4700. [Google Scholar] [CrossRef] [PubMed]
- Kuprikova, N.M.; Klyamer, D.D.; Sukhikh, A.S.; Krasnov, P.O.; Mrsic, I.; Basova, T.V. Fluorosubstituted lead phthalocyanines: Crystal structure, spectral and sensing properties. Dyes Pigments 2020, 173, 107939. [Google Scholar] [CrossRef]
- Bonegardt, D.; Klyamer, D.; Sukhikh, A.; Krasnov, P.; Popovetskiy, P.; Basova, T. Fluorination vs. Chlorination: Effect on the Sensor Response of Tetrasubstituted Zinc Phthalocyanine Films to Ammonia. 2021, 9, 137. [Google Scholar] [CrossRef]
- Shao, X.; Wang, S.; Li, X.; Su, Z.; Chen, Y.; Xiao, Y. Single component p-, ambipolar and n-type OTFTs based on fluorinated copper phthalocyanines. Dyes Pigments 2016, 132, 378–386. [Google Scholar] [CrossRef]
- Brinkmann, H.; Kelting, C.; Makarov, S.; Tsaryova, O.; Schnurpfeil, G.; Wöhrle, D.; Schlettwein, D. Fluorinated phthalocyanines as molecular semiconductor thin films. Phys. Status Solidi Appl. Mater. Sci. 2008, 205, 409–420. [Google Scholar] [CrossRef]
- Peisert, H.; Knupfer, M.; Fink, J. Electronic structure of partially fluorinated copper phthalocyanine (CuPcF4) and its interface to Au(1 0 0). Surf. Sci. 2002, 515, 491–498. [Google Scholar] [CrossRef]
- Jiang, H.; Hu, P.; Ye, J.; Li, Y.; Li, H.; Zhang, X.; Li, R.; Dong, H.; Hu, W.; Kloc, C. Molecular Crystal Engineering: Tuning Organic Semiconductor from p-type to n-type by Adjusting Their Substitutional Symmetry. Adv. Mater. 2017, 29, 1605053. [Google Scholar] [CrossRef]
- Konarev, D.V.; Troyanov, S.I.; Kuzmin, A.V.; Nakano, Y.; Ishikawa, M.; Faraonov, M.A.; Khasanov, S.S.; Litvinov, A.L.; Otsuka, A.; Yamochi, H.; et al. The Salts of Copper Octafluoro- and Hexadecafluorophthalocyanines Containing [CuII(F8Pc)4–]2– Dianions and [CuF16Pc]− Monoanions. Inorg. Chem. 2017, 56, 1804–1813. [Google Scholar] [CrossRef]
- Oison, V.; Koudia, M.; Abel, M.; Porte, L. Influence of stress on hydrogen-bond formation in a halogenated phthalocyanine network. Phys. Rev. B–Condens. Matter Mater. Phys. 2007, 75, 1–6. [Google Scholar] [CrossRef]
- Giovanelli, L.; Bocquet, F.C.; Amsalem, P.; Lee, H.-L.; Abel, M.; Clair, S.; Koudia, M.; Faury, T.; Petaccia, L.; Topwal, D.; et al. Interpretation of valence band photoemission spectra at organic-metal interfaces. Phys. Rev. B 2013, 87, 35413. [Google Scholar] [CrossRef]
- Ballirano, P.; Caminiti, R.; Ercolani, C.; Maras, A.; Orrù, M.A. X-ray powder diffraction structure reinvestigation of the α and β forms of cobalt phthalocyanine and kinetics of the α → β phase transition. J. Am. Chem. Soc. 1998, 120, 12798–12807. [Google Scholar] [CrossRef]
- Ziolo, R.F.; Griffiths, C.H.; Troup, J.M. Crystal structure of vanadyl phthalocyanine, phase II. J. Chem. Soc. Dalt. Trans. 1980, 2300–2302. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Klyamer, D.D.; Sukhikh, A.S.; Gromilov, S.A.; Kruchinin, V.N.; Spesivtsev, E.V.; Hassan, A.K.; Basova, T.V. Influence of fluorosubstitution on the structure of zinc phthalocyanine thin films. Macroheterocycles 2018, 11, 304–311. [Google Scholar] [CrossRef]
- Klyamer, D.D.; Basova, T.V.; Krasnov, P.O.; Sukhikh, A.S. Effect of fluorosubstitution and central metals on the molecular structure and vibrational spectra of metal phthalocyanines. J. Mol. Struct. 2019, 1189, 73–80. [Google Scholar] [CrossRef]
- Tackley, D.R.; Dent, G.; Smith, W.E. IR and Raman assignments for zinc phthalocyanine from DFT calculations. Phys. Chem. Chem. Phys. 2000, 2, 3949–3955. [Google Scholar] [CrossRef]
- Tackley, D.R.; Dent, G.; Smith, W.E. Phthalocyanines: Structure and vibrations. Phys. Chem. Chem. Phys. 2001, 3, 1419–1426. [Google Scholar] [CrossRef]
- Basova, T.V.; Kiselev, V.G.; Schuster, B.E.; Peisert, H.; Chassé, T. Experimental and theoretical investigation of vibrational spectra of copper phthalocyanine: Polarized single-crystal Raman spectra, isotope effect and DFT calculations. J. Raman Spectrosc. 2009, 40, 2080–2087. [Google Scholar] [CrossRef]
- Cranston, R.R.; Lessard, B.H. Metal phthalocyanines: Thin-film formation, microstructure, and physical properties. RSC Adv. 2021, 11, 21716–21737. [Google Scholar] [CrossRef]
- Fronk, M.; Bräuer, B.; Zahn, D.R.T.; Salvan, G. Temperature dependence of the optical anisotropy of vanadyl phthalocyanine films. Thin Solid Films 2008, 516, 7916–7920. [Google Scholar] [CrossRef]
- Kerp, H.R.; Westerduin, K.T.; Van Veen, A.T.; Van Faassen, E.E. Quantification and effects of molecular oxygen and water in zinc phthalocyanine layers. J. Mater. Res. 2001, 16, 503–511. [Google Scholar] [CrossRef]
- van Faassen, E.E.; Schlettwein, D. Energy Migratoin and Light-Induced Charge Transfer at Phthalocyanine Surfaces. In Handbook of Photochemistry and Photobiology, Vol. 3: Supramolecular Photochemistry; Nalwa, H.S., Ed.; American Scientific: Los Angeles, CA, USA, 2003; pp. 355–409. [Google Scholar]
- Klyamer, D.D.; Sukhikh, A.S.; Krasnov, P.O.; Gromilov, S.A.; Morozova, N.B.; Basova, T.V. Thin films of tetrafluorosubstituted cobalt phthalocyanine: Structure and sensor properties. Appl. Surf. Sci. 2016, 372, 79–86. [Google Scholar] [CrossRef]
- Ji, X.; Zou, T.; Gong, H.; Wu, Q.; Qiao, Z.; Wu, W.; Wang, H. Cobalt phthalocyanine nanowires: Growth, crystal structure, and optical properties. Cryst. Res. Technol. 2016, 51, 154–159. [Google Scholar] [CrossRef]
- Yamashita, A.; Maruno, T.; Hayashi, T. Absorption spectra of organic-molecular-beam-deposited vanadyl- and titanylphthalocyanine. J. Phys. Chem. 2002, 97, 4567–4569. [Google Scholar] [CrossRef]
- Zhang, X.F.; Xi, Q.; Zhao, J. Fluorescent and triplet state photoactive J-type phthalocyanine nano assemblies: Controlled formation and photosensitizing properties. J. Mater. Chem. 2010, 20, 6726–6733. [Google Scholar] [CrossRef]
- Chiu, F. A Review on Conduction Mechanisms in Dielectric Films. Adv. Mater. Sci. Eng. 2014, 1, 578168 . [Google Scholar] [CrossRef]
- Jiang, H.; Hu, P.; Ye, J.; Ganguly, R.; Li, Y.; Long, Y.; Fichou, D.; Hu, W.; Kloc, C. Hole Mobility Modulation in Single-Crystal Metal Phthalocyanines by Changing the Metal–π/π–π Interactions. Angew. Chemie–Int. Ed. 2018, 57, 10112–10117. [Google Scholar] [CrossRef]
- Stuzhin, P.A. Fluorinated phthalocyanine and their analogues. In Fluorine in Heterocyclic Chemistry: Volume 1: 5-Membered Heterocycles and Macrocycles; Nenajdenko, V., Ed.; Springer International Publishing: New York, NY, USA, 2014; Volume 1, pp. 621–681. ISBN 9783319043463. [Google Scholar]
- Spesivtsev, E.V.; Rykhlitskii, S.V.; Shvets, V.A. Development of methods and instruments for optical ellipsometry at the Institute of Semiconductor Physics of the Siberian Branch of the Russian Academy of Sciences. Optoelectron. Instrum. Data Process. 2011, 47, 419–425. [Google Scholar] [CrossRef]
- Kruchinin, V.N.; Klyamer, D.D.; Spesivtsev, E.V.; Rykhlitskii, S.V.; Basova, T.V. Optical Properties of Thin Films of Zinc Phthalocyanines Determined by Spectroscopic Ellipsometry. Opt. Spectrosc. 2018, 125, 1019–1024. [Google Scholar] [CrossRef]
- Bruker AXS Inc. Bruker Advanced X-ray Solutions; Bruker AXS Inc: Madison, WI, USA, 2004. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]



| Compound | ZnPcF8 | CoPcF8 | VOPcF8 |
|---|---|---|---|
| Empirical formula | C32H8F8N8Zn | C32H8F8N8Co | C32H8F8N8OV |
| Formula weight | 721.83 | 715.39 | 723.40 |
| Temperature/K | 150 | 150 | 150 |
| Crystal system | triclinic | triclinic | triclini |
| Space group | P−1 | P−1 | P−1 |
| a/Å | 8.2156(5) | 3.6061(2) | 9.0821(5) |
| b/Å | 11.5255(7) | 13.0280(7) | 10.3447(5) |
| c/Å | 14.0379(8) | 13.4901(7) | 13.9293(8) |
| α/° | 78.693(2) | 84.097(2) | 98.578(2) |
| β/° | 73.840(2) | 89.055(2) | 92.165(2) |
| γ/° | 89.047(2) | 82.976(2) | 92.909(2) |
| Volume/Å3 | 1250.92(13) | 625.67(6) | 1291.01(12) |
| Z | 2 | 1 | 2 |
| ρcalcg/cm3 | 1.916 | 1.899 | 1.861 |
| μ/mm−1 | 1.087 | 0.791 | 0.492 |
| Radiation | MoKα (λ = 0.71073) | MoKα (λ = 0.71073) | MoKα (λ = 0.71073) |
| 2Θ range for data collection/° | 3.082 to 61.038 | 3.036 to 51.362 | 4.594 to 56.68 |
| Reflections collected | 15,790 | 9899 | 20,656 |
| Independent reflections | 7629 [Rint = 0.0271, Rsigma = 0.0399] | 2377 [Rint = 0.0489, Rsigma = 0.0476] | 6423 [Rint = 0.0375, Rsigma = 0.0443] |
| Data/restraints/parameters | 7629/0/442 | 2377/0/223 | 6423/0/451 |
| Goodness-of-fit on F2 | 1.030 | 1.063 | 1.039 |
| Final R indexes [I > = 2σ (I)] | R1 = 0.0354, wR2 = 0.0886 | R1 = 0.0539, wR2 = 0.1314 | R1 = 0.0518, wR2 = 0.1393 |
| Final R indexes [all data] | R1 = 0.0551, wR2 = 0.0978 | R1 = 0.0760, wR2 = 0.1427 | R1 = 0.0762, wR2 = 0.1552 |
| Largest diff. peak/hole/e Å−3 | 0.50/−0.35 | 1.05/−0.36 | 1.22/−0.39 |
| Number in CCDC | 2177575 | 2177576 | 2177577 |
| Distances | ZnPcF8 | CoPcF8 | VOPcF8 |
|---|---|---|---|
| between layers, Å | 3.173 | N/A | 3.382 |
| between molecules, Å | 3.217 | 3.299 | 3.411/3.354 |
| dnorm (min/max), Å | −0.1495/1.3175 | −0.1985/1.3833 | −0.1477/1.4484 |
| metal…metal, Å | 4.867 | 3.606 | 5.871 |
| C…C/C…N close contacts, Å | 3.211−3.260 < 3.235> | 3.271−3.275 < 3.273> | 3.204−3.352 < 3.288> |
| O…H close contacts, Å | N/A | N/A | 2.409−2.505 < 2.457> |
| F…H close contacts, Å | 2.354−2.401 < 2.368> | 2.440 | 2.404−2.509 < 2.451> |
| F…F close contacts, Å | N/A | N/A | 2.864 |
| π…π interactions (angle/distance/shift) | 2.603°/3.232 Å/0.777 Å 1.219°/3.230 Å/0.786 Å | 0°/3.251−3.357 Å/1.318−1.561 Å | 4.454°/3.424 Å/1.123 Å 3.995°/3.228 Å/1.068 Å |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukhikh, A.; Klyamer, D.; Bonegardt, D.; Basova, T. Octafluoro-Substituted Phthalocyanines of Zinc, Cobalt, and Vanadyl: Single Crystal Structure, Spectral Study and Oriented Thin Films. Int. J. Mol. Sci. 2023, 24, 2034. https://doi.org/10.3390/ijms24032034
Sukhikh A, Klyamer D, Bonegardt D, Basova T. Octafluoro-Substituted Phthalocyanines of Zinc, Cobalt, and Vanadyl: Single Crystal Structure, Spectral Study and Oriented Thin Films. International Journal of Molecular Sciences. 2023; 24(3):2034. https://doi.org/10.3390/ijms24032034
Chicago/Turabian StyleSukhikh, Aleksandr, Darya Klyamer, Dmitry Bonegardt, and Tamara Basova. 2023. "Octafluoro-Substituted Phthalocyanines of Zinc, Cobalt, and Vanadyl: Single Crystal Structure, Spectral Study and Oriented Thin Films" International Journal of Molecular Sciences 24, no. 3: 2034. https://doi.org/10.3390/ijms24032034
APA StyleSukhikh, A., Klyamer, D., Bonegardt, D., & Basova, T. (2023). Octafluoro-Substituted Phthalocyanines of Zinc, Cobalt, and Vanadyl: Single Crystal Structure, Spectral Study and Oriented Thin Films. International Journal of Molecular Sciences, 24(3), 2034. https://doi.org/10.3390/ijms24032034